Abstract
Neurostimulation provides a new dimension in the treatment of neurologic disorders. For patients with drug-resistant epilepsy, the Responsive Neurostimulation (RNS) System (NeuroPace, Inc.) provides treatment of seizures with a closed-loop device that continuously records brain activity and provides stimulation designed to reduce seizure frequency over time. The presence of a chronic implanted device that can provide an electrographic record of neural activity provides great opportunities for treatment of seizure disorders and neuroscience research. However, our experience with this device indicates that a number of ethical and clinical challenges arise, and these issues may be applicable to neurotechnology developed for other disease states in the future. We present clinical scenarios based on cases from our center that present clinical or ethical dilemmas. The dilemmas revolve around 4 core themes: (1) electroclinical correlation and dissociation; (2) patient concerns about device capabilities; (3) clinician opportunities and burdens; and (4) data ownership and access. Developing a framework for understanding these issues will be critical as closed-loop neuromodulation is applied to a growing range of neuropsychiatric disorders.
Introduction
Neurologic disorders, mental illness, addiction, and chronic pain are collectively the leading cause of disability worldwide, and developing novel treatments for these disorders is an ethical and policy priority. Global governments and private funders have invested billions in initiatives to advance our circuit-level understanding of the brain and develop neuromodulation techniques. One goal of this research is developing closed-loop neuromodulation systems that sense neural activity and deliver stimulation in response. This would represent a significant advance from open-loop neuromodulation systems—such as deep brain stimulation for movement disorders, vagus nerve stimulation for epilepsy, or spinal cord stimulation for pain—which deliver periodic stimulation without a neural control signal.
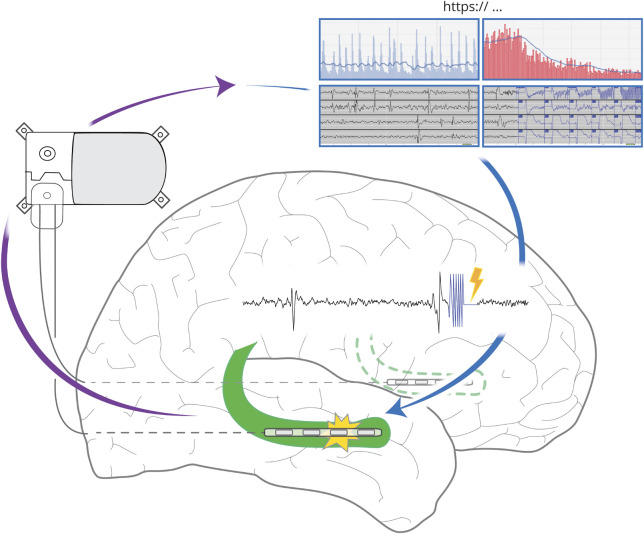
Currently, 1 closed-loop brain-responsive neuromodulation system is in routine clinical practice in the United States: the Responsive Neurostimulation (RNS) System (NeuroPace, Inc.). This device is indicated for patients with drug-resistant focal epilepsy who have 1 or 2 seizure foci and are not surgical resection candidates. The RNS System comprises a cranially implanted neurostimulator connected to intracranial leads that continuously monitor for incipient seizures, and deliver electrical impulses to reduce seizures, through acute seizure termination and/or chronic neuromodulatory effects. Patients upload stored neurostimulator data to a secure online repository for clinicians' review (figure).
Figure. Responsive Neurostimulation (RNS) System.
An implanted neurostimulator is connected to two four-electrode leads placed intracranially at the seizure focus/foci (bilateral hippocampi, green, shown for example). The neurostimulator continuously senses brain activity, and in response to detection of epileptiform activity, electrical counterstimulation (lightning bolt) is delivered through the electrodes to inhibit seizures. Electrocorticograms (ECoG) and counts of detected epileptiform discharges and electrographic seizures are stored on the neurostimulator until they are uploaded to an online data bank. Clinicians can access these time-stamped data to review ECoG and other electrographic metrics, including activity trends.
RNS is the vanguard for neuromodulation devices that are promising but pose ethical and clinical challenges. Here, we present clinical scenarios based on cases from our center that may inform thinking about ethical issues relevant not only in epilepsy but also in other neuropsychiatric domains where clinical devices are under development.
Electroclinical Correlation and Dissociation
A 56-year-old man with bitemporal seizure foci and hippocampal RNS electrodes reported a prolonged period of seizure freedom and sought clearance to resume driving. The state law required the patient to be seizure-free for a minimum of 3 months. Although his last known clinical seizure was outside that interval, his RNS data included several recordings of electrographic seizures within the previous 3 months, many during daytime.
An 18-year-old college student with RNS and epilepsy reported transient cognitive impairment during a final examination, resulting in poor performance. Her professor requested that the epileptologist interrogate the RNS recordings to provide an explanation for her academic performance that would justify retesting.
Closed-loop neuromodulation systems such as RNS can create and store recordings of patients' neural states, generating objective records of illness that may be interpreted as corroborating or discrediting patients' own reports. Patients might wish to use recorded information to prove that they were having a seizure or were seizure-free during some time interval—effectively, as an electrographic alibi. Patients may even seek to shift responsibility for their actions to the manufacturers of closed-loop devices, arguing that these companies share liability for adverse events that occur during medium- or high-risk activities that would be undertaken only if RNS data indicated that they were seizure-free. However, such interpretations raise several problems in the case of systems, like RNS, that are not designed for such uses. First, spatial sampling is quite limited, as the RNS neurostimulator can accommodate a maximum of 2 leads with 4 electrodes each (figure). The absence of detectable local epileptiform activity does not exclude the possibility of broader network dysfunction. Conversely, the presence of local epileptiform activity does not demonstrate that global function is necessarily impaired. Second, temporal sampling of RNS recordings is constrained by the device's limited storage capacity. Most patients have more epileptiform activity than can be stored, so predetermined settings are used to determine what activity is captured and how long it is preserved. Therefore, the absence of stored epileptiform activity does not exclude the possibility of a seizure during the time interval of interest—the signal processing algorithm may have failed to record a seizure, or epileptiform activity may have been recorded but later overwritten. Third, the clinical significance of RNS detections varies between patients. Electrographic changes that can prove disabling for some patients may be irrelevant to the clinical presentations of others.
Closed-loop device recordings may be perceived by government agencies, employers, and other authorities as more valid than patients' own reports. In many jurisdictions, including the United States and United Kingdom, regulations require a period of seizure freedom (often with physician verification) before a patient can resume driving after a focal seizure with impaired awareness. Traditionally, such physician verification has been based on patients' own reports of seizure frequency that may be unreliable.1 In the first anecdote above, the electrographic evidence of seizure activity is at odds with the patient's report of clinical seizure freedom. This electroclinical dissociation produces a dilemma for the neurologist: although preventing the patient from driving (based on the additional information provided by the device) may contribute to the safety of the patient and other drivers, the patient is being held to a different standard than patients without RNS who present seemingly reliable accounts of epilepsy in remission. Thus, the RNS patient may feel penalized for consenting to a treatment modality that also provides diagnostic data.
Physiologic data from implanted medical devices have already proven useful in police investigations and criminal prosecutions. In 1 case, an Ohio man was indicted for arson and insurance fraud after his house burned down, in part because data obtained by police from his cardiac pacemaker were held to be incompatible with his reported exertion in rescuing valuables from the fire.2 Neural recordings from patients with epilepsy could have particular evidentiary value in determinations of culpability: patients might not be held criminally responsible for behaviors produced by a seizure (which are not voluntary acts in a legal sense) or for behaviors performed in a postictal state that interferes with awareness of their acts (the legal doctrine of mens rea). However, in cases to date in which epilepsy has been invoked as a defense,3 there have been no electrographic data from the time of an alleged crime. To evaluate such claims, courts and expert witnesses have had to rely on circumstantial evidence regarding the plausibility of seizure as a mechanism to explain a defendant's behavior. If a patient with RNS is accused of a crime, and if the epoch of interest was recorded and stored, the recorded data could provide more direct evidence of whether the patient was in fact seizing at or around the time of the crime.
Such potential legal applications of RNS data can be distinguished from cases in which neural data have not been admitted as evidence in court. In 2 separate cases in 2012, United States vs. Semrau and Maryland vs. Gary Smith, defense attorneys attempted to enter fMRI-based lie-detection tests as evidence for the defendants' truthfulness, and, in both cases, courts excluded these data as not meeting legal standards for scientific evidence. These standards require that scientific methods are generally accepted by the scientific community, and many cognitive neuroscientists have argued that fMRI is not ready to for use in real-world lie detection.4 For instance, in many study paradigms, participants are instructed to lie (rather than spontaneous dishonesty), and fMRI techniques are highly susceptible to countermeasures by motivated subjects. In contrast, RNS recordings would likely meet legal standards for the admissibility of scientific evidence because they can provide contemporaneous data to epochs of interest, ECoG interpretation is a scientifically accepted method for identifying a seizure, and RNS is less vulnerable to countermeasures.
Although RNS data may be legally admissible, there is a danger that the availability of such data may excessively narrow the search for etiologic factors for behavior. Even in the absence of a discrete seizure, epilepsy may be associated with psychiatric comorbidities that may underlie behavioral disturbances, mitigating responsibility.5 In such cases, RNS data demonstrating normal electrographic background at the time of an alleged crime could be interpreted as a straightforward indicator of guilt—obscuring other relevant factors to culpability.
These cases highlight the neurologist's dual responsibilities to the patient and to society and the interest that authorities may take in patients' recorded neurophysiologic data. A neurologist facing electroclinical dissociation in an RNS patient who wishes to resume driving, operate heavy machinery, or engage in other high-risk activities must decide whether the patient's clinical reports of seizure freedom warrant loosening of safety restrictions that would affect those beyond just the patient. Such electroclinical dissociation may be still more consequential in envisioned psychiatric applications of closed-loop neuromodulation. For example, if electrographic signatures predictive of imminent suicidality were identified, might involuntary civil commitment be considered for patients exhibiting such neural biomarkers, even if they otherwise denied suicidal intent? Broader consideration is needed about whether and to what extent neurophysiologic data from RNS or newer closed-loop devices in development should be used in judicial or administrative proceedings, particularly when patients' liberty or public safety is at stake. The neurologic community should engage with legal scholars and other stakeholders in deliberating about responsible applications and limitations of such data to inform the work of courts and regulators in these new domains of potential evidence.
Patient Concerns About Device Capabilities
A 51-year-old man implanted with RNS expressed concern that his treating epileptologist would be able to “read my thoughts,” particularly during intimate activities with his spouse.
A 35-year-old woman declined evaluation for RNS implantation, stating she did not want to be a guinea pig for a device she considered experimental.
Closed-loop devices such as RNS have the capability to record, store, and transmit patients' neural data. RNS patients may be aware of ongoing neuroscientific research using electrophysiologic data to decode mentation from brain activity—sometimes reported in the popular press as mind reading. The ramifications of such technologies for personal liberty and privacy are recurring themes in popular science fiction programs, such as Black Mirror. In this context, patients may fear that RNS data could give their neurologist access to their inner thoughts and experiences, a concern that may not have been anticipated preoperatively when seizure control was the primary issue. In fact, given the spatial and temporal sparseness noted above, RNS does not confer the ability to infer network-level or other emergent properties that require coordination of multiple cortical and subcortical structures.
In the case of RNS, patients' concerns may be allayed by carefully explaining the current limitations of the device. However, advances in signal processing algorithms and our fundamental understanding of the brain may result in evolving device capabilities. If implanted in a region associated with higher-order cognitive functions or mood, future research might elucidate electrographic signatures associated with distinct cognitive or emotional states.6 In addition, recent work has revealed that RNS-captured epileptiform activity demonstrates multiday periodicity that modulates seizure risk and may eventually allow for seizure prediction.7,8 Personalized seizure prediction could be empowering for some patients, but anxiety provoking and debilitating for others. In a recent study of 6 patients implanted with an experimental implanted seizure-prediction device, 1 patient experienced the predictions as an intrusive reminder of her illness that undermined her sense of self-control and made her depressed.9 Careful validation and regulatory approval of such novel applications will be necessary before they can be introduced into clinical practice. For devices that allow wireless uploading of new software, such as decoding algorithms and stimulation parameters, patients should have the ability to opt out of emerging applications after discussing risks and benefits.
Some patients express concern about being a guinea pig when new medical technologies are deployed, regardless of whether the treatment in question has been approved by regulatory authorities for clinical use. These patients want assurance that their treatment is geared solely toward clinical goals; they do not want to be unwilling research subjects. In fact, these assurances may be undermined by the iterative nature of RNS therapy; frequent adjustments to detection settings and stimulation parameters are often needed to optimize treatment. The process may feel experimental to the RNS patient. This may be a particular risk when treating patients from racial, ethnic, or socioeconomic groups that have historically experienced discrimination in the medical setting or have been enrolled in medical research without informed consent. Cultural sensitivity is critical, and patients' perspectives should be elicited in the presurgical diagnostic and consent process.
Clinician Opportunities and Burdens
The caregiver for a 58-year-old woman with RNS emails her epileptologist multiple times each week inquiring, “How are we looking?” and expecting commensurate review and interpretation of electrographic data from the device.
Closed-loop devices like RNS enable the collection of large amounts of neural data from patients over long periods. If patients download data from their RNS devices and upload them to secure online data banks frequently, a wealth of data is available for clinician review. However, the capability for real-time electrocorticographic (ECoG) recording does not guarantee real-time interpretation, and epileptologists may struggle to manage the endless streams of data from these devices. Still, patients who upload their data on a daily basis may expect their clinicians to review their data on similar intervals. Currently, it is unknown at what intervals data review actually changes management or outcomes; expert opinion supports adjusting RNS parameters every few months to avoid obscuring cause-effect relationships. However, independent of parameter adjustments, patients might derive reassurance from knowing that their neurologist is monitoring their recordings more frequently, alleviating anxiety about subclinical seizure burden, or encouraging behavioral changes that reduce seizure risk. Furthermore, switching to a more efficacious anticonvulsant may cause a reduction in RNS seizure detection rates within a week,10 so more frequent RNS surveillance may be indicated for some patients. Urgent ECoG interpretation may also be needed when RNS patients experience abrupt changes in behavior, mood, or cognition. Indeed, RNS data have been used to attribute psychiatric symptoms to epileptic vs nonepileptic etiologies.11 In our own practice, we have used RNS data from 1 patient to rule out seizure as the explanation for an event later diagnosed as a TIA. Conversely, clinicians inexperienced with RNS may need assistance when interpreting MRIs, CTs, or EEGs with RNS artifacts. However, demands for frequent surveillance of RNS data in a large cohort could readily lead to clinician burnout and may limit the scalability of this treatment for the large population of patients with drug-resistant epilepsy.
Alleviating these demands on clinicians may require a new generation of closed-loop devices or automated tools that provide alerts for critical events, such as a sudden increase in electrographic seizure frequency. Such tools may eventually provide information to clinicians and patients that is predictive rather than retrospective—for example, providing data about upcoming seizure risk, if not outright seizure prediction.7,8 If these tools are developed by the device manufacturers, careful independent scrutiny will be needed to ensure that proprietary software provides an unbiased view of a patient's data independent of any commercial interests.
The reversibility of closed-loop neuromodulation (compared with resective surgery) presents both opportunities and dilemmas in clinical decision making. Although RNS is approved as a therapeutic intervention, its ability to detect and record electrographic events over long timescales makes it attractive as a diagnostic technique. A recent case series12 described patients implanted with bilateral mesial temporal RNS leads—for presumed bilateral seizure onsets—who had chronic recordings revealing either strictly unilateral or predominantly unilateral seizure onsets, enabling subsequent unilateral resection with a good outcome. This might suggest an off-label strategy of using RNS to collect chronic intracranial recordings before making an irreversible decision regarding unilateral resection. However, the desire to temporize by obtaining chronic diagnostic data may delay a resection that should have been performed sooner, exposing patients to additional seizures. Yan and Ibrahim13 have addressed the ethical challenges of choosing between resection and RNS. They invoke the need for shared decision making, including discussion of the following: (1) risks and benefits of each treatment choice (palliation with lower morbidity for RNS vs possible seizure freedom but greater risk of deficit with resection); (2) long-term outcomes (which are more established with resection); (3) reversibility and timing (RNS does not preclude future resection, whereas resection is irreversible); and (4) access to the technology necessary for RNS (such as reliable internet access). The authors provide a useful decision-making model to help guide the choice between RNS and resection for patients and clinicians.
Data Ownership and Access
An RNS patient with a background in software engineering seeks to use their own signal-processing algorithms to analyze their recordings. However, the device manufacturer currently does not provide these data to patients.
An RNS patient requests access to the online clinician portal to “understand my brain waves.” The patient does not have a medical or scientific background and has an anxiety disorder.
Chronic ambulatory ECoG recorded via RNS provides a unique opportunity to study longitudinal brain function in naturalistic settings, which may be of tremendous interest to neuroscientists and commercial parties. Researchers may seek access to the raw data uploaded to the manufacturer's servers rather than the processed data available to clinicians via proprietary websites. At present, the data are owned by the device manufacturer, and using them for research is a challenging endeavor. As RNS becomes more widespread and the collective ECoG library grows, the potential applications for sophisticated machine-learning algorithms will increase. Empowering patients to submit their recordings to such studies and investigators to access these data would be of great scientific interest to the broader epilepsy community. Failing to do so might put the manufacturers of RNS at a competitive advantage but may not serve the greater good. Negotiating this divide will be critical in the future.
It is also conceivable that closed-loop neurostimulator data could be monetized. For example, if RNS data suggested a subpopulation of patients with exclusively nocturnal seizures, might these patients receive targeted marketing for seizure detection devices designed to awaken family members? If multiday periodicity was identified in a patient's seizure pattern, might that patient receive well-timed ads for rescue medications? Although such applications are not currently envisioned for RNS data, new vendors entering the neuromodulation space may develop creative ways of subsidizing device cost and increasing manufacturers' profits. ECoG patterns unique to an epilepsy subpopulation or even a single patient might be used to develop new algorithms or devices, raising questions of consent, intellectual property, and compensation analogous to those encountered posthumously in the case of Henrietta Lacks (the source of the immortalized HeLa cell line).14 Failure of the neurologic community to reach agreement with device manufacturers on these points may invite regulatory or legislative initiatives to either liberate the ECoG data from a proprietary site or protect it from commercial use, presumably under terms less favorable to manufacturers.
Finally, patients may seek access to intracranial electrophysiologic data out of curiosity for personal biometrics, in the same way that people collect information about their sleep and step counts from wearable devices. Currently, RNS data are not accessible to patients in any form. The barriers, proprietary and otherwise, to obtaining these data might be galling to patients who have undergone a highly invasive procedure to have a closed-loop device implanted. Even if raw ECoG data were made available to patients, there is no normal reference range or narrative report to guide interpretation, in contrast to laboratory results or imaging reports, for example. ECoG interpretation is challenging even for seasoned clinicians, and patient expectations about acquiring these skills would need to be calibrated. Finally, patient access to data might prompt more frequent queries to clinicians, adding to the burdens previously discussed. A framework will be necessary to balance patient autonomy with preservation of scientific-medical authority.
Conclusions
Closed-loop neuromodulation devices for epilepsy provide neural recordings, which, despite unprecedented chronicity, have intrinsic limitations and represent clinical reality imperfectly. Neurologists using these data may experience competing responsibilities to their patients and to society. The myriad ethical and clinical implications of closed-loop devices remain unfamiliar to many patients, and misperceptions will inevitably abound. In this era of Big Data, clinicians' bandwidth to review and interpret chronic recordings—Long Data—from these devices may not scale with patients' expectations. Expectation and reality may also diverge for data ownership, as control of the data is highly asymmetric among stakeholders. Epilepsy represents the first test case of closed-loop neuromodulation, and the dilemmas posed here will extend to other applications of this technology. Further challenges—such as questions of autonomy and agency—will arise as closed-loop neuromodulation is applied to motor behavior and mood. Anticipating that closed-loop devices will play ever-increasing roles in clinical practice, neurologists should view the associated challenges as opportunities to advocate for patients, to educate society, and to promote responsible development of future neurotechnology.
Appendix. Authors
Contributor Information
Winston Chiong, Email: winston.chiong@ucsf.edu.
Vikram R. Rao, Email: vikram.rao@ucsf.edu.
Study Funding
W.C.'s contributions to this work were supported by the National Institute of Mental Health under Award Number R01MH114860. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. No targeted funding reported for M.H. or V.R.R.
Disclosure
M. Hegde and W. Chiong report no disclosures relevant to this manuscript. V.R. Rao has served as a paid consultant for NeuroPace, Inc., manufacturer of the RNS System, but NeuroPace did not provide any targeted funding or other support for this work. Go to Neurology.org/N for full disclosures.
References
- 1.Elger CE, Hoppe C. Diagnostic challenges in epilepsy: seizure under-reporting and seizure detection. Lancet Neurol 2018;17:279–288. [DOI] [PubMed] [Google Scholar]
- 2.Wootson CR Jr.. Washington Post 2017. Available at: washingtonpost.com/news/to-your-health/wp/2017/02/08/a-man-detailed-his-escape-from-a-burning-house-his-pacemaker-told-police-a-different-story/. Accessed December 19, 2020. [Google Scholar]
- 3.Reuber M, Mackay RD. Epileptic automatisms in the criminal courts: 13 cases tried in England and Wales between 1975 and 2001. Epilepsia 2008;49:138–145. [DOI] [PubMed] [Google Scholar]
- 4.Wagner AD, Bonnie RJ, Casey BJ, et al. fMRI and Lie Detection. MacArthur Foundation Research Network on Law & Neuroscience; 2016. Vanderbilt Law Research Paper No. 17-10 (2016). [Google Scholar]
- 5.Saleh C, Reuber M, Beyenburg S. Epileptic seizures and criminal acts: is there a relationship? Epilepsy Behav 2019;97:15–21. [DOI] [PubMed] [Google Scholar]
- 6.Kirkby LA, Luongo FJ, Lee MB, et al. An amygdala-Hippocampus subnetwork that encodes variation in human mood. Cell 2018;175:1688–1700.e14. [DOI] [PubMed] [Google Scholar]
- 7.Baud MO, Kleen JK, Mirro EA, et al. Multi-day rhythms modulate seizure risk in epilepsy. Nat Commun 2018;9:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Proix T, Truccolo W, Leguia MG, et al. Forecasting seizure risk in adults with focal epilepsy: a development and validation study. Lancet Neurol 2021;20:127–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gilbert F, Cook M, O'Brien T, Illes J. Embodiment and estrangement: results from a first-in-human “intelligent BCI” trial. Sci Eng Ethics 2019;25:83–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Quraishi IH, Mercier MR, Skarpaas TL, Hirsch LJ. Early detection rate changes from a brain-responsive neurostimulation system predict efficacy of newly added antiseizure drugs. Epilepsia 2020;61:138–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Issa Roach AT, Chaitanya G, Riley KO, Muhlhofer W, Pati S. Optimizing therapies for neurobehavioral comorbidities of epilepsy using chronic ambulatory electrocorticography. Epilepsy Behav 2020;102:106814. [DOI] [PubMed] [Google Scholar]
- 12.Hirsch LJ, Mirro EA, Salanova V, et al. Mesial temporal resection following long-term ambulatory intracranial EEG monitoring with a direct brain-responsive neurostimulation system. Epilepsia 2020;61:408–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yan H, Ibrahim GM. Resective epilepsy surgery involving eloquent cortex in the age of responsive neurostimulation: a value-based decision-making framework. Epilepsy Behav 2019;99:106479. [DOI] [PubMed] [Google Scholar]
- 14.Wolinetz CD, Collins FS. Recognition of research participants' need for autonomy: remembering the legacy of Henrietta Lacks. JAMA 2020;324:1027–1028. [DOI] [PubMed] [Google Scholar]