Abstract
Objective
To assess whether primary lateral sclerosis (PLS), classified as pure when the EMG is normal, converts to amyotrophic lateral sclerosis (ALS) after longitudinal follow-up.
Methods
Retrospective chart review was performed of patients with pure PLS at Mayo Clinic in Rochester, MN (1990–2016). Inclusion criteria required a normal EMG during the first 4 years of symptoms.
Results
Forty-three patients had pure PLS (25 female, 58%) with a median onset age of 50 years (range 38–78 years) and median follow-up at 9 years’ disease duration (range 4–36 years). The ascending paraparesis phenotype (n = 30, 70%) was most common, followed by hemiparetic onset (n = 9, 21%) and bulbar onset (n = 4, 9%). Among the 30 paraparetic-onset cases, bladder symptoms (n = 18, 60%) and dysarthria (n = 15, 50%) were more common than pseudobulbar affect (n = 9, 30%) and dysphagia (n = 8, 27%). By the last follow-up, 17 of 30 (56%) used a cane and 6 (20%) required a wheelchair. The paraparetic variant, compared with hemiparetic and bulbar onset, had the youngest onset (48 vs 56 vs 60 years, respectively; p = 0.02). Five patients died; 1 patient required a feeding tube; and none required permanent noninvasive ventilation. Two patients developed an idiopathic multisystem neurodegenerative disorder, which surfaced after 19 and 20 years. Two patients developed minor EMG abnormalities. The remainder 39 had persistently normal EMGs.
Conclusions
Pure PLS did not convert to ALS after a median of 9 years’ disease duration follow-up in our study population. The ascending paraparetic phenotype was most common, with earlier onset and frequent bladder involvement. After years of pure PLS, <5% develop a more pervasive neurodegenerative disorder.
Primary lateral sclerosis (PLS) is a neurodegenerative disorder limited to the corticospinal (sometimes corticobulbar) tracts, leading to signs and symptoms of upper motor neuron (UMN) dysfunction.1 By definition, it is nonfamilial and distinct from hereditary spastic paraplegia. It is also typically regarded to be clinically and pathologically distinct from amyotrophic lateral sclerosis (ALS).1,2 The first large case series of PLS, by Stark and Moersch3 in 1945, described a slowly progressive ascending pyramidal course, differing from the rapidly progressive course with lower motor neuron (LMN) involvement of ALS. Diagnostic criteria were refined by Pringle et al.4 and then Gordon et al.5 on the basis of normal EMG studies after 3 and 4 years of isolated UMN findings, respectively. There is ongoing debate about whether PLS is simply a forme fruste of ALS; indeed, transition from PLS to ALS has been reported after many years of relatively slow progression.2,6 Complicating this endeavor has been the recognition of limited LMN findings in patients diagnosed with PLS.7 Some investigators have proposed that such cases likely represent UMN-dominant ALS,5,8 although others consider limited LMN findings on EMG to be consistent with a PLS phenotype.9 Hence, the diagnosis of PLS is often guarded with uncertain long-term implications.
The purpose of this investigation was to better define the long-term outcomes and prognosis of pure PLS via retrospective review of patients evaluated at our institution over the last quarter-century. To assess a neurophysiologically pure PLS cohort, we included only patients with PLS with normal EMGs during the initial 4 years of symptoms.
Methods
The electronic medical records at Mayo Clinic in Rochester from 1990 to 2016 were searched for cases with a clinical diagnosis of probable or possible PLS or spastic paraparesis without a family history. Search terms included PLS, corticospinal degeneration, UMN disease, and hereditary spastic paraparesis.
Medical records were retrospectively reviewed to extract clinically relevant data, including sex, age at onset, age at initial evaluation, first site affected (leg, arm, bulbar), pattern of progression (unilateral or bilateral, site), neuromuscular symptoms, sensory symptoms, pseudobulbar affect, dysarthria, dysphagia, sphincter involvement, gait-aid use, development of additional neurologic symptoms or signs (cognitive impairment, parkinsonism, autonomic failure, cerebellar dysfunction), medications, disease duration at final follow-up, and deceased status. Times to milestones were calculated (e.g., time to gait-aid use). EMG reports were reviewed. MRI of the brain and spine and other available imaging were also reviewed.
Inclusion criteria included (1) gradual onset of UMN symptoms and signs after 35 years of age; (2) neurologic examination at our institution by a neurologist; (3) ≥1 normal EMGs performed at our institution at least 4 years after symptom onset; (4) brain and spinal cord MRI scans; (5) no family history; and (6) negative workup for other structural, inflammatory, infectious, autoimmune, or paraneoplastic causes. Exclusion criteria included LMN symptoms or signs within 4 years of symptom onset, except those that can be clearly explained clinically and electrophysiologically by an alternative etiology (including carpal tunnel syndrome, ulnar neuropathy, and chronic radiculopathy); early development of prominent cerebellar, cognitive, parkinsonian, dystonic, or autonomic features; prominent fibrillation or fasciculation potentials on EMG within the first 4 years of symptomatic onset; evidence of demyelination or inflammation on MRI or CSF; and an alternative etiology to explain the UMN signs or symptoms. In addition, all patients were clinically evaluated by a Mayo neuromuscular specialist at least once.
All the included patients must have fulfilled the PLS diagnostic criteria recommended by both Pringle et al.4 and Gordon et al.5 Included patients were subclassified in each group according to clinical phenotypes of lower limb–onset (ascending paraparetic variant), hemibody-onset (unilateral [Mills] variant),10 and bulbar-onset variant.
Statistical Analysis
Descriptive statistical analysis was used. Nominal data are expressed as a percentage. Continuous data (age at onset, age at evaluation, disease duration, time at EMG, age at death, and time to clinical outcomes) were not of normal distribution and are reported as median and range. The p values were calculated with a χ2 test for nominal data and Wilcoxon rank-sum test for nonparametric variables. Statistical significance was set at p < 0.05. The statistical package JMP Pro 14.1.0 version (SAS Institute Inc, Cary, NC) was used.
Standard Protocol Approvals, Registrations, and Patient Consents
This study was approved by the Institutional Review Board of Mayo Clinic Rochester (16-009902). Patients in the study had given consent for review of their medical records for research purposes.
Data Availability
All data derived from this study are included in this article.
Results
Patient Characteristics
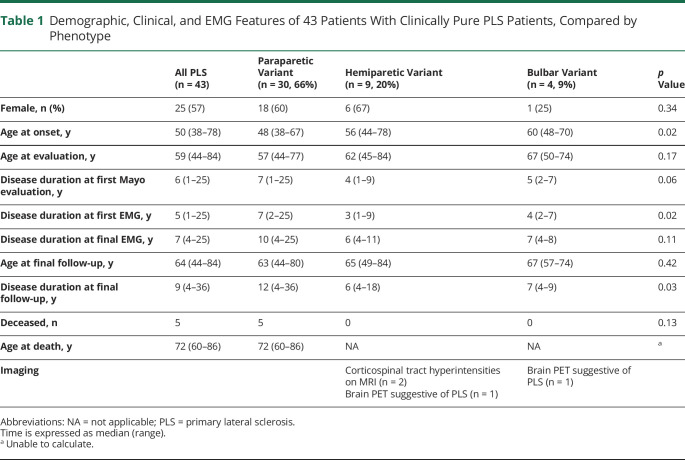
A total of 43 patients (25 female, 58%) fulfilled the inclusion and exclusion criteria for pure PLS. All were White. Twenty-three patients were of non-Latino/non-Hispanic ethnicity; 1 patient was of Latino ethnicity (n = 1); and ethnicity was not available for the remainder (n = 19). The 43 patients had a median age at symptom onset of 50 years (range 38–78 years) and median disease duration of 9 years (range 4–36 years, interquartile range, 6–15 years) at the final follow-up (table 1). The most common presenting symptoms were stiffness (n = 26), subjective weakness (n = 26), and cramps (n = 14), variably accompanying a gait disorder. All patients were documented as having a UMN syndrome most consistent with PLS. A third (n = 13) had incidental finding of impaired vibration sense in the toes, attributed to median age of 62 years. A handful of patients (n = 6) reported subjective sensory symptoms without objective correlate, felt not to detract from the diagnosis of PLS by the assessing physician. Most patients underwent extensive testing to rule out a secondary etiology: MRI of the neuroaxis (n = 42), extensive laboratory evaluation (n = 42) (vitamin B12 [n = 40], sedimentation rate [n = 34], human T-cell leukemia virus 1/2 serology [n = 20], serum copper [n = 17], peroxisomal panel [n = 11], Lyme serology [n = 12], hexosaminidase [n = 8], HIV serology [n = 7], angiotensin-converting enzyme [n = 7], ceruloplasmin [n = 6], antinuclear antigen [n = 6]), CSF studies (n = 33), paraneoplastic antibody analysis (n = 20) (Mayo Clinic laboratories paraneoplastic panel [n = 13], other paraneoplastic panel [n = 4], isolated glutamic acid decarboxylase [GAD] antibodies [n = 1], GAD and amphyphysin [n = 1], GAD and anti–aquaporin-4 antibodies [n = 1]), and/or somatosensory evoked potentials (n = 14). A small number of patients underwent genetic testing (spinocerebellar ataxia panel [n = 3], hereditary spastic paraplegia panel [n = 2], and ALS panel [n = 1]), which was negative.
Table 1.
Demographic, Clinical, and EMG Features of 43 Patients With Clinically Pure PLS Patients, Compared by Phenotype
The ascending paraparetic clinical phenotype was documented in 30 patients (70%), of whom 18 (60%) were female. The hemiparetic phenotype (so-called Mills syndrome) was noted in 9 patients (21%), and 4 patients (9%) had the bulbar-onset variant. The patients with ascending paraparetic phenotype had a significantly younger median age at onset compared to the hemiparetic and bulbar-onset variants (48 years [range 38–67 years] vs 56 years [range 44–78 years] vs 60 years [range 48–70 years], respectively, p = 0.02). The paraparetic phenotype was associated with a median longer disease duration at final follow-up: 12 years (range 4–36 years) compared to 6 years (range 4–11 years) and 7 years (range 4–8 years) for the hemiplegic and bulbar variants, respectively (p = 0.03). The clinical progression of the phenotypic patterns is summarized in table 2. Among the 30 paraparetic-onset cases, there was progression to the upper limbs in 26 (87%) after a median of 7 years’ duration (range 1–26 years). Of the 9 patients with the hemiparetic variant, 4 had onset in 1 upper limb that progressed to the ipsilateral lower limb, while the other 5 had onset in 1 lower limb that ascended to the ipsilateral upper limb.
Table 2.
Progression to Clinical Milestones in 43 Patients With Clinically Pure PLS, Compared by Phenotype
Mobility
There was no difference in the requirements for a gait aid among the 3 variants. In the paraparetic variant, 19 (63%) required a cane and/or a walker by final follow-up, with 6 (20%) using a wheelchair. Among those with the hemiparetic variant, 7 patients required a cane and/or walker (78%), and 3 (33%) used a wheelchair at the last follow-up. In the bulbar variant, 1 patient (25%) required a cane and walker after 7 years, progressing to a wheelchair after 9 years. Median time to use of a cane was faster in the hemiparetic-onset variant compared with the paraparetic- and bulbar-onset variants (1 vs 8 vs 7 years, respectively, p = 0.02). However, time to use of a walker or wheelchair was similar for the 3 variants.
Pseudobulbar Affect
Among those with the paraparetic phenotype, 9 patients (30%) developed pseudobulbar affect at a median of 10 years’ disease duration compared with 1 (11%) patient with hemiparetic phenotype at 6 years. Of the 4 bulbar-onset patients, 1 developed pseudobulbar affective symptoms at 4 years’ disease duration, and a second developed it at 9 years’ disease duration. There was no difference between the phenotypes for time to onset of pseudobulbar affect.
Bulbar Symptoms
In the paraparetic variant, 15 (50%) patients developed dysarthria after a median of 8 years’ duration, and 8 (27%) developed dysphagia after a median of 7 years. In the hemiparetic variant, 5 (56%) developed dysarthria after a median of 5 years’ duration, and 3 (33%) had dysphagia after a median of 8 years. In the bulbar variant, 1 patient reported dysphagia at onset, and 1 patient each developed it at 3 and 4 years’ disease duration after onset. Dysarthria occurred earlier in the bulbar variant (0 vs 8 vs 5 years, p < 0.01) compared with the paraparetic and hemiparetic types. There was no difference for time to onset of dysphagia between the subgroups.
Sphincter Symptoms
In the paraparetic variant, 18 (60%) developed bladder symptoms after a median of 9 years’ disease duration compared with 2 patients in each of the hemiparetic (median 5 years) and bulbar (median 6 years) variants. There was no difference in time to onset of bladder symptoms. The bladder symptoms ranged from isolated frequency or urgency to neurogenic bladder/bowel. Stratified by PLS variant, for the paraparetic onset (n = 18), symptoms ranged from isolated urgency or frequency, recurrent urinary tract infections, and urinary or bowel urge incontinence to neurogenic bladder/bowel in 2 patients. For the hemiparetic-onset variant (n = 2), patients experienced urinary incontinence or incomplete emptying; and for the bulbar-onset variant (n = 2), 1 patient reported urinary frequency, and the other reported bowel/bladder incontinence.
Medications
Antispasmodics were prescribed in 22 patients, most commonly baclofen (n = 20), followed by tizanidine (n = 7), and cyclobenzaprine (n = 1). Most patients were intolerant of these at low doses because of sleepiness side effect. Four patients were suggested to try intrathecal baclofen, dantrolene, and/or botulinum toxin for severe spasticity. Riluzole was offered to 3 patients and prescribed in 2 patients, and the lack of definite evidence for PLS was discussed with all. None received edaravone. Two patients were prescribed dextromethorphan/quinidine sulfate for pseudobulbar affect.
EMG Studies
All patients had EMGs performed at least 4 years after symptom onset. Sixteen patients had an initial EMG performed within the first 4 years of symptom onset; patients with hemiparetic and bulbar onset were more likely to have earlier initial EMGs compared to those with paraparetic onset (at 3 vs 4 vs 7 years, respectively, p = 0.02). The median time of the final EMG was 7 years from onset (range 4–25 years). At the final follow-up, 2 patients with the bulbar-onset phenotype developed minor denervation changes on the EMG, but they were insufficient for ALS diagnosis. The first patient developed neurogenic changes in right L5-S1 muscles associated with uncompensated denervation on EMG at last follow-up after 7 years, which could have been consistent with a new lumbosacral radiculopathy although an evolving LMN disorder could not be excluded. The second patient developed chronic motor neuropathy/neuronopathy findings on EMG after 7 years’ duration, but it was noted that the absence of fibrillations or fasciculations was atypical for ALS. Two additional patients developed widespread neurogenic changes, accompanied by additional clinical features, which are described below.
Imaging
Two patients with hemiparetic-onset variant had MRI brain imaging suggestive of an UMN degenerative disorder, with bilateral abnormal increased T2 signal involving bilateral corticospinal tracts and progressive atrophy in the left motor strip, without abnormal enhancement. There were also 2 patients with 18F-fluorodeoxyglucose PET/CT brain scans suggestive of PLS, with hypometabolism in the precentral gyrus or the motor strip, without any corresponding abnormality on MRI. The remaining patients with MRI brain and cord imaging studies did not show any apparent stigmata of PLS.
Clinical Outcomes
Two of the 43 patients with PLS presented with a late-developing deviant neurodegenerative condition. The first was a patient with the paraparetic variant, who had symptom onset at age 44 and initially was diagnosed with PLS with a normal EMG. He was followed up clinically over many years. After 19 years’ duration, at 63 years of age, he developed new features of parkinsonism and cerebellar ataxia, accompanied by EMG evidence of chronic right greater than left L5 and S1 radiculopathies with little evidence of active denervation. By 67 years of age, repeat EMG showed progressive changes suggestive of a widespread, longstanding, neurogenic process reflecting disease of anterior horn cells vs a motor-predominant polyradiculopathy. This was diagnosed as an idiopathic multisystem neurodegenerative disorder after extensive negative laboratory, imaging, autonomic, and genetic workup. He was last seen at 36 years of follow-up at 80 years of age.
A second patient had paraparetic-onset symptoms at age 40 years and was evaluated at our institution at age 50 years, with a clinical and EMG diagnosis consistent with PLS. However, at follow-up after 20 years’ disease duration, EMG showed widespread LMN findings in upper and lower limbs and paraspinals. An MRI lumbar spine demonstrated an enlarged arachnoid and dural sac, enlarged CSF space, and fatty degeneration in the lumbar parapinals, suggestive of a cauda equina process. The working diagnosis was an indeterminate neurodegenerative disorder affecting UMNs and LMNs, plus a cauda equina lesion.
Two paraparetic-onset patients developed cognitive symptoms at the final follow-up. The first had mild cognitive slowing after 7 years’ disease duration at 68 years of age, and the other at 69 years of age developed mild impairment in complex, nonverbal reasoning associated with memory difficulties and disinhibited behavior after 25 years disease duration. None of these patients had MRI findings suggestive of an alternative neurodegenerative disease. One patient developed subjective unilateral sensory symptoms over the disease course, without objective correlates on examination, EMG, or imaging, which was felt to be indeterminate.
All of the patients with the hemiparetic variant retained a diagnosis of clinically pure PLS at the final follow-up.
Five patients (all with the paraparetic variant) were known to have died at median age of 72 years (range 60–86 years) after a median of 28 years’ disease duration (range 20–33 years). Details are available for only 1 of these patients, a man who died of septic shock at 61 years of age after 24 years of PLS with complications of dysphagia requiring a feeding tube at age 49 years, communication device for severe dysarthria at age 50 years, severe progressive spastic quadriparesis limiting mobility and requiring a motorized wheelchair, and neurogenic bladder/bowel with recurrent urinary tract infections. He was treated with riluzole, although the final clinical diagnosis remained consistent PLS. For the remainder 4 deceased patients, the causes of death were not known. Autopsy results were not available.
None of the 43 patients required permanent noninvasive ventilation. However, 3 patients required continuous positive airway pressure therapy, attributed to obstructive sleep apnea in 1 patient, mixed obstructive sleep apnea and PLS in the second, and multifactorial causes (obstructive and central sleep apnea, obesity, and PLS) in the third.
Discussion
We assessed 43 patients with a pure PLS syndrome who were subsequently followed up for a median of 9 years’ total disease duration and confirmed that ALS was not the ultimate outcome in any case (a prior concern).2,6,10 The natural history observed for our cases of pure PLS contrasts with the limited lifespan observed when UMN signs are associated with the distinctive LMN findings of ALS (as defined by El Escorial criteria).11 Pure PLS in our series translated into disability but often with potential for partial compensation.
Of 43 patients, 2 progressive and potentially neurodegenerative conditions surfaced 2 decades after paraparetic-onset PLS (<5%). In 1 patient, a multisystem neurodegenerative disorder became apparent 19 years after PLS onset; in another patient, a mixed indeterminate UMN and LMN syndrome evolved after 20 years of follow-up, with imaging also suggestive of a cauda equina lesion. It can be debated how either of these later conditions relates to PLS. Another patient died of complications of PLS after a protracted course of 24 years due to urosepsis; this was the only patient to require a feeding tube. None of the patients required permanent noninvasive ventilation, and continuous positive airway pressure use in 3 patients appeared to be driven by sleep-disordered breathing rather than PLS. Parenthetically, none of the patients with the hemiparetic variant (n = 9) developed evidence of any more pervasive disorder beyond PLS by the final follow-up to 18 years’ duration. Of the 2 patients with the bulbar-onset variant who developed minor neurogenic changes at last follow-up at 7 years, 1 patients may had an early radiculopathy, and the other might have had an indeterminate neurogenerative disorder similar to those described above. However, follow-up EMG and clinical data beyond 7 years were not available to definitively exclude evolution to ALS in these cases.
We compared our findings to the recently reported outcomes of the prospective Northeast ALS Consortium (NEALS) PLS registry.12 Of 250 patients with PLS in this registry, with a median follow-up of 3 years after study enrollment, 17 (7%) required a feeding tube, 7 (3%) needed permanent assisted ventilation, and there were 18 (7%) deaths. In our cohort, the requirement for feeding tube (2%, 1 of 43) and permanent noninvasive ventilation (0%) was lower, and the number of deaths was higher (12%, 5 of 43), which might be accounted for by longer median follow-up duration and lack of data about end-of-life cares.
PLS may assume any 1 of 3 different patterns of onset and progression, which may not always be recognized. The most common and presumably best known phenotype was the ascending paraparetic form, documented in 70% of cases, followed by hemiparetic (21%) and bulbar (9%) variants. Notably, the hemiparetic variant either could be an ascending form starting in a lower limb, or could start in an upper limb and descend to involve the ipsilateral lower limb. These findings are consistent with other studies reporting that lower limb symptoms or spastic paraparesis were most frequently identified, followed by upper limb onset and then bulbar onset.4,5 A hypothesis for these phenotypes would be largely speculative. The 3 phenotypic patterns for PLS have previously been recognized and have gone without explanation. Neurodegenerative diseases may behave like this, with selective vulnerability of certain CNS regions that are not always consistent. Examples include the posterior cortical atrophy phenotype of Alzheimer disease13 and the different phenotypes seen as progressive supranuclear palsy variants.14
To summarize from our experience, PLS is predominantly a disorder of middle age, which in this series ranged from 38 to 78 years at onset. It affects both sexes (slightly more among women in this series). Although the primary symptoms reflect corticospinal and corticobulbar systems, bladder symptoms were common. While the paraparetic onset pattern is most recognizable, clinicians should be aware of the hemiparetic- and bulbar-onset variants, which appear to reflect the same disease process. The paraparetic-onset variant appears to occur a little earlier in life.
The frequency of gait-aid use was similar for all variants, but gait-aid use occurred earlier for the hemiparetic variant after a median of 1 year compared to 7 years for the paraparetic variant. Unsurprisingly, the bulbar group had earlier onset of dysarthria but did not have earlier onset of either dysphagia or pseudobulbar affective symptoms compared to those with the paraparetic and hemiparetic variants. Urinary symptoms were encountered in a little more than half of the entire PLS group, most commonly in the paraparesis group. This is important for clinicians to be aware of and has been underemphasized in prior studies. None of the patients reported other autonomic symptoms.
The limitations of our study include a relatively small sample size, given that PLS is a rare disorder, and its retrospective design. It is possible that some of the paraparetic-onset cases were due to unrecognized recessive hereditary spastic paraparesis, where a family history may be absent, and limited genetic testing was available at the time of evaluation. However, we think that this is unlikely because no patients developed symptoms suggestive of a dorsal column disorder or neuropathy. This could potentially be addressed in future research efforts using up-to-date genetic screening on patients with PLS. It is conceivable that some patients with shorter follow-up may have subsequently evolved to ALS, although follow-up duration was substantial in most cases (up to 36 years, interquartile range 6–16 years). The findings may also not generalize outside of our White population. The study strengths include uniform data collected from a single neurology department and its associated EMG laboratory with standardized EMG reporting and strict subject inclusion criteria. Prospective research endeavors such as the NEALS PLS registry could help further verify the findings in this study.
These data indicate that PLS is a distinctive clinical disorder and a legitimate neurologic diagnosis. Patients with PLS can be reassured that in the setting of an adequate neurologic workup and sufficient follow-up, they have a diagnosis with limited and well-defined neurologic disability.
An incompletely unresolved question is how PLS should be positioned in the general category of neurodegenerative disease. Neurodegenerative disorders are conventionally defined by their neuropathology signatures (e.g., Lewy bodies, neuritic plaques, and neurofibrillary tangles) or specific protein deposits (e.g., α-synuclein, β-amyloid, or phosphorylated tau). The neuropathology of PLS is limited but had consistently demonstrated degeneration of the motor cortex and descending corticospinal tracts, without features of ALS such as Bunina bodies or ubiquinated cytoplasmic inclusions.1,4,15 This suggests a paucity of microscopic overlap with ALS. However a recent small autopsy case series of PLS reported TAR DNA-binding protein 43 (TDP-43) immunoreactive pathology was found within both motor cortex and preserved LMNs in each case.16 As TDP-43 is the major pathologic protein in most cases of ALS, this would suggest a common proteinopathy of ALS and PLS. However, the only neuropathology case of Mills variant to date was negative for TDP-43 immunohistochemistry.17 The argument appears unresolved as to whether PLS represents a form fruste of ALS (e.g., an aborted form of ALS) vs a distinct motor neuron disease in which the LMN is protected. Thus, our understanding of PLS as a unique condition is still in its infancy.
Glossary
- ALS
amyotrophic lateral sclerosis
- GAD
glutamic acid decarboxylase
- LMN
lower motor neuron
- NEALS
Northeast ALS Consortium
- PLS
primary lateral sclerosis
- TDP-43
TAR DNA-binding protein 43
- UMN
upper motor neuron
Appendix. Authors

Footnotes
Editorial, page 783
CME Course: NPub.org/cmelist
Study Funding
No targeted funding reported.
Disclosure
A. Hassan reports current grant support from IntraBio and previous support from AbbVie and National Center for Advancing Translational Sciences; S.O Mittal reports no disclosures; W.T. Hu reports patents on CSF-based diagnosis of frontotemporal lobar degeneration with TDP-43 inclusions and prognosis of spinal muscular atrophy; licensed technology on serologic analysis of coronavirus disease 2019 to Sigma-Millipore; consulting fees from ViveBio LLC, AARP Inc, Biogen Inc, and Fujirebio Diagnostics Inc; and research support from Fujirebio Diagnostics Inc. K.A. Josephs reports funding from the National Institute on Aging, the National Institute of Neurologic Disorders and Stroke, and the National Institute on Deafness and Other Communication Disorders. E.J. Sorenson reports grants from Cytokinetics, Biogen, and UCB Pharma. J.E. Ahlskog receives royalties from Oxford University Press for 3 recently published books. Go to Neurology.org/Nhttps://n.neurology.org/lookup/doi/10.1212/WNL.0000000000011771 for full disclosures.
References
- 1.Singer MA, Statland JM, Wolfe GI, Barohn RJ. Primary lateral sclerosis. Muscle Nerve 2007;35:291–302. [DOI] [PubMed] [Google Scholar]
- 2.Tartaglia MC, Rowe A, Findlater K, Orange JB, Grace G, Strong MJ. Differentiation between primary lateral sclerosis and amyotrophic lateral sclerosis: examination of symptoms and signs at disease onset and during follow-up. Arch Neurol 2007;64:232–236. [DOI] [PubMed] [Google Scholar]
- 3.Stark FM, Moersch FP. Primary lateral sclerosis: a distinct clinical entity. J Nervous Ment Dis 1945;102:332–337. [Google Scholar]
- 4.Pringle CE, Hudson AJ, Munoz DG, Kiernan JA, Brown WF, Ebers GC. Primary lateral sclerosis: clinical features, neuropathology and diagnostic criteria. Brain 1992;115:495–520. [DOI] [PubMed] [Google Scholar]
- 5.Gordon PH, Cheng B, Katz IB, et al. The natural history of primary lateral sclerosis. Neurology 2006;66:647–653. [DOI] [PubMed] [Google Scholar]
- 6.Bruyn RP, Koelman JH, Troost D, de Jong JM. Motor neuron disease (amyotrophic lateral sclerosis) arising from longstanding primary lateral sclerosis. J Neurol Neurosurg Psychiatry 1995;58:742–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Le Forestier N, Maisonobe T, Piquard A, et al. Does primary lateral sclerosis exist? A study of 20 patients and a review of the literature. Brain 2001;124:1989–1999. [DOI] [PubMed] [Google Scholar]
- 8.Singer MA, Kojan S, Barohn RJ, et al. Primary lateral sclerosis: clinical and laboratory features in 25 patients. J Clin Neuromuscul Dis 2005;7:1–9. [DOI] [PubMed] [Google Scholar]
- 9.Fournier CN, Murphy A, Loci L, et al. Primary lateral sclerosis and early upper motor neuron disease: characteristics of a cross-sectional population. J Clin Neuromuscul Dis 2016;17:99–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jaiser SR, Mitra D, Williams TL, Baker MR. Mills' syndrome revisited. J Neurol 2019;266:667–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brooks BR, Miller RG, Swash M, Munsat TL; World Federation of Neurology Research Group on Motor Neuron Diseases. El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Mot Neuron Disord 2000;1:293–299. [DOI] [PubMed] [Google Scholar]
- 12.Paganoni S, De Marchi F, Chan J, et al. The NEALS primary lateral sclerosis registry. Amyotroph Lateral Scler Frontotemporal Degener 2020;21:74–81. [DOI] [PubMed] [Google Scholar]
- 13.Schott JM, Crutch SJ, Carrasquillo MM, et al. Genetic risk factors for the posterior cortical atrophy variant of Alzheimer's disease. Alzheimers Dement 2016;12:862–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Whitwell JL, Tosakulwong N, Botha H, et al. Brain volume and flortaucipir analysis of progressive supranuclear palsy clinical variants. Neuroimage Clin 2020;25:102152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tan CF, Kakita A, Piao YS, et al. Primary lateral sclerosis: a rare upper-motor-predominant form of amyotrophic lateral sclerosis often accompanied by frontotemporal lobar degeneration with ubiquitinated neuronal inclusions? Report of an autopsy case and a review of the literature. Acta Neuropathol 2003;105:615–620. [DOI] [PubMed] [Google Scholar]
- 16.Mackenzie IRA, Briemberg H. TDP-43 pathology in primary lateral sclerosis. Amyotroph Lateral Scler Frontotemporal Degener 2020;21:52–58. [DOI] [PubMed] [Google Scholar]
- 17.Fernandes PM, Turner MR, Zeidler M, Smith C, Davenport R. Progressive hemiparesis in a 75-year-old man. Pract Neurol 2015;15:63–71. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data derived from this study are included in this article.