Abstract
Objective
To develop evidence-informed, expert consensus research diagnostic criteria for traumatic encephalopathy syndrome (TES), the clinical disorder associated with neuropathologically diagnosed chronic traumatic encephalopathy (CTE).
Methods
A panel of 20 expert clinician-scientists in neurology, neuropsychology, psychiatry, neurosurgery, and physical medicine and rehabilitation, from 11 academic institutions, participated in a modified Delphi procedure to achieve consensus, initiated at the First National Institute of Neurological Disorders and Stroke Consensus Workshop to Define the Diagnostic Criteria for TES, April, 2019. Before consensus, panelists reviewed evidence from all published cases of CTE with neuropathologic confirmation, and they examined the predictive validity data on clinical features in relation to CTE pathology from a large clinicopathologic study (n = 298).
Results
Consensus was achieved in 4 rounds of the Delphi procedure. Diagnosis of TES requires (1) substantial exposure to repetitive head impacts (RHIs) from contact sports, military service, or other causes; (2) core clinical features of cognitive impairment (in episodic memory and/or executive functioning) and/or neurobehavioral dysregulation; (3) a progressive course; and (4) that the clinical features are not fully accounted for by any other neurologic, psychiatric, or medical conditions. For those meeting criteria for TES, functional dependence is graded on 5 levels, ranging from independent to severe dementia. A provisional level of certainty for CTE pathology is determined based on specific RHI exposure thresholds, core clinical features, functional status, and additional supportive features, including delayed onset, motor signs, and psychiatric features.
Conclusions
New consensus diagnostic criteria for TES were developed with a primary goal of facilitating future CTE research. These criteria will be revised as updated clinical and pathologic information and in vivo biomarkers become available.
Chronic traumatic encephalopathy (CTE) is a neurodegenerative disease associated with exposure to repetitive head impacts (RHIs), including those sustained in contact and collision sports.1-3 The diagnosis of CTE is confirmed only by neuropathologic examination demonstrating a unique pattern of hyperphosphorylated tau (p-tau) deposition.4,5 Descriptions of the clinical features of CTE are based on retrospective reports about deceased individuals with neuropathologically diagnosed CTE6 and include nonspecific cognitive, neuropsychiatric, and motor impairments, progressing to functional dependence and dementia.7 Preliminary diagnostic schemas for the clinical syndrome associated with CTE neuropathology7-10 have recognized deficiencies.11,12 These include the 2014 research diagnostic criteria, termed traumatic encephalopathy syndrome (TES).7 There is a need for evidence-informed, expert consensus diagnostic criteria to facilitate research and help close important knowledge gaps12 on the epidemiology, risk factors, and course of CTE, as well as to enable clinical trials for treatment and prevention.13 The development of consensus diagnostic criteria for the clinical features of CTE is one of the aims of the Diagnostics, Imaging, and Genetics Network for the Objective Study and Evaluation of CTE (DIAGNOSE CTE) Research Project, funded by the National Institute of Neurological Disorders and Stroke (NINDS; U01NS093334). In April 2019, the First NINDS Consensus Workshop to Define the Diagnostic Criteria for TES was held in Phoenix, AZ, for a multidisciplinary panel of 20 clinician-scientists and 7 observers, initiating a modified Delphi process to achieve consensus. This article describes the methodology used and the resulting NINDS Consensus Diagnostic Criteria for TES.
Methods
The previously proposed research diagnostic criteria for TES (table e-1, doi.org/10.5061/dryad.f1vhhmgvk), published in 2014,7 were used as a starting point and initial organizing structure for the development of new consensus criteria. The NINDS Consensus Diagnostic Criteria for TES were generated as a result of a multistep process that included (1) selecting expert consensus panelists; (2) selecting nonvoting observers; (3) assessing reliability, sensitivity, and specificity of the 2014 TES criteria7 used in the DIAGNOSE CTE Research Project (DIAGNOSE CTE) and in the NINDS-funded Understanding Neurologic Injury and Traumatic Encephalopathy (UNITE) study (U01NS086659 and U54NS115266)14; (4) literature review of all reported cases with pathology consistent with CTE and descriptions of clinical features; (5) review of published and unpublished clinicopathologic data from the UNITE study to examine predictive validity of clinical variables in relation to pathologically confirmed CTE; (6) training of the expert panelists, external to the DIAGNOSE CTE study team, who were less familiar with use of the 2014 TES criteria; (7) convening an in-person consensus workshop of the panelists and observers to present a review of CTE literature, clinicopathologic correlation findings, and interrater reliability of the 2014 TES criteria used in the UNITE and DIAGNOSE CTE studies; (8) obtaining consensus using a modified Delphi procedure, started during the in-person consensus workshop, and continuing online for subsequent rounds; and (9) inviting comments from stakeholders, including representatives of interested professional organizations, foundations, patient/family advocacy groups, and industry (table e-2, doi.org/10.5061/dryad.f1vhhmgvk).
Expert Consensus Panel and Observers
The expert consensus panel consisted of 20 clinician-scientists from 11 academic centers around the United States, representing a variety of disciplines (neurology, neuropsychology, neurosurgery, psychiatry, and physical medicine and rehabilitation) and areas of clinical and research expertise (traumatic brain injury [TBI], sports concussion, and neurodegenerative disorders) (see Appendix 1). Fourteen of the 20 panelists were investigators in DIAGNOSE CTE. These panelists, the DIAGNOSE CTE leadership team, and NINDS Program Officials provided recommendations for additional panelists who were senior thought leaders not affiliated with DIAGNOSE CTE and who could provide diversity of expertise and perspectives. Seven individuals were selected, and all agreed to participate. One of the 7 had a last-minute conflict and was unable to attend the consensus workshop. The resulting 20 consensus panel members participated in the workshop and were voting participants in the modified Delphi consensus process. Each panel member participated in all rounds of the modified Delphi consensus.
The nonvoting observers included 3 representatives from NINDS (including the Institute Director and 2 Program Directors), as well as the lead biostatistician, co–principal investigators, and External Advisory Board Chair for DIAGNOSE CTE. Their role was to provide guidance, criticism, and commentary on the goals, process, and emerging consensus diagnostic criteria during each of the modified Delphi rounds.
Reliability, Sensitivity, and Specificity of the 2014 Research Diagnostic Criteria for TES
The 2014 TES criteria were used in the UNITE and DIAGNOSE CTE projects to diagnose deceased and living study subjects, respectively. Diagnoses were adjudicated through multidisciplinary diagnostic consensus conferences for both studies. Interrater reliability, sensitivity, and specificity for the TES criteria were calculated. Interrater reliability was assessed with intraclass correlations to account for multiple raters with inconsistent participation.
Literature Review of Reported Cases of CTE
Two reviewers from the DIAGNOSE CTE research team who were not on the expert consensus panel (M.L.M. and E.M.F.) screened abstracts (n = 513) obtained from a PubMed search using relevant search terms and by cross-referencing bibliographies of CTE literature reviews, using defined inclusion and exclusion criteria to identify all reported cases with descriptions of clinical features and neuropathology available at the time of the search, January 15, 2019. The search yielded 40 articles between 1957 and 2019, with information on 229 cases (tables e-3 and e-4, doi.org/10.5061/dryad.f1vhhmgvk).
Predictive Validity of Pathologically Diagnosed CTE From the UNITE Study
Clinicopathologic Data
The UNITE project relies on donations to a brain bank and retrospective review of clinical histories from detailed, systematized interviews of informants.14 The vast majority of donors had contact sport exposure, mostly American football. Data from 298 brain donors in the UNITE study were analyzed with respect to a number of predictor variables including source of exposure; years of contact sport exposure; cognitive, behavioral, mood, motor, and other clinical features; course; age at symptom onset; and multivariable prediction models. Outcome variables were the presence or absence of neuropathologic diagnosis of CTE (using the NINDS/National Institute of Biomedical Imaging and Bioengineering [NIBIB] diagnostic criteria5), with or without evidence of other neurodegenerative disorders, and categorized according to CTE stage (I–IV, increasing with severity).15 Analyses were based on a subset of the full UNITE sample available at the time. The results of these data analyses are beyond the scope of this article and will be reported in a separate publication.
Hands-on Experience With 2014 Research Diagnostic Criteria for TES
Mock diagnostic sessions with the 2014 TES criteria,7 using 3 representative cases, were conducted with the external panelists before the NINDS consensus workshop to familiarize them with the criteria and the nature of their use in the research setting.
NINDS Consensus Workshop
On April 15, 2019, the First NINDS Consensus Workshop to Define the Diagnostic Criteria for TES was convened in Phoenix, AZ. The primary aim of the workshop was to begin the process to develop evidence-informed, expert consensus diagnostic criteria for TES. We identified 2 primary goals at the start of the consensus workshop: (1) to improve on previously proposed research diagnostic criteria for TES by maintaining adequate sensitivity and increasing the specificity for underlying CTE pathology, defined by the NINDS and NIBIB consensus criteria5; and (2) to develop a criteria structure amenable to future updates, without the need for complete reorganization.
Panel members agreed that biomarker development for CTE was not sufficiently mature to be included in the criteria at this time. Instead, the criteria would be based solely on clinical information, using RHI exposure history, symptom profiles, and clinical course specific to pathologically confirmed cases of CTE. It was agreed that the TES criteria will be revised in future NINDS consensus workshops based on updated research on biomarkers, neuropathology, clinical features, and reliability and validity of the new criteria.
A summary of evidence gathered before the meeting was provided to the panel and observers, consisting of (1) data on interrater reliability using the 2014 TES criteria7; (2) the literature review of all reported cases with clinical information and descriptions of pathology consistent with CTE; and (3) predictive validity using unpublished data from the UNITE study. All published literature, data extraction summaries, and unpublished analyses were made available to the panel and observers.
The panel discussed and agreed on the following parameters of a modified Delphi procedure to obtain consensus: all voting and commentary would be anonymous; a threshold of ≥80% agreement would be required for consensus on each diagnostic component; and there would be a limit of 4 rounds to achieve consensus.
Modified Delphi Consensus Process
The modified Delphi process follows an iterative procedure commonly used to achieve expert group consensus in medical science and other fields using rounds of voting, with summary of comments from each participant provided anonymously to all participants. It is a useful means of employing expert opinion when available evidence is incomplete.16
The first round of the modified Delphi process occurred at the NINDS consensus workshop. Three subsequent rounds were performed online. Following each round, panelist voting results and comments from each of the panelists and observers were provided to all participants anonymously. These were then used to restructure and revise the criteria by the lead organizers (D.K. and R.S.). The revised criteria were further reviewed and edited by a core subcommittee of panelists (D.D., C.B., and J.M.) before distributing to the full panel for the next round. All panelists and observers were blinded to the identities of the respondents, except the project manager (M.M.) who was not a voting panelist and who maintained the anonymity throughout the entire process, including publication of this report.
Results
Summary of Data Presented at the NINDS Consensus Workshop
Interrater Reliability, Sensitivity, and Specificity of the 2014 Research Diagnostic Criteria for TES7
The 2014 research diagnostic criteria for TES7 had high interrater reliability when used in the UNITE and DIAGNOSE CTE studies. Intraclass correlation for UNITE was 0.77 (n = 293 donors; n = 12 raters) and for DIAGNOSE CTE, 0.93 (n = 153 participants; n = 16 raters), suggesting good agreement for both studies. The 2014 criteria were evaluated with respect to sensitivity and specificity in predicting a pathologic diagnosis of CTE in the UNITE study (CTE, n = 224; no CTE, n = 74). Sensitivity was high (97.32); specificity was low (20.27). Similar results were found when limiting the sample to only those with pure CTE (i.e., pathology meeting CTE criteria without evidence of other neurodegenerative disease diagnoses) or no neurodegenerative pathologies (pure CTE, n = 135; no CTE, n = 40); sensitivity was 97.04 and specificity was 22.50. Although a diagnosis of TES was highly predictive of a pathologic diagnosis of CTE, the confidence intervals were notably wide (CTE vs no CTE, OR = 10.65, 95% CI: 3.32–34.17; pure CTE vs no CTE: OR = 11.00, 95% CI: 2.76–43.81).
Given the high sensitivity and low specificity of the 2014 TES criteria, the panel concluded that the new consensus TES criteria should be structured to provide a greater level of specificity in diagnosing individuals with underlying CTE pathology.
Literature Review of RHI Exposure of Pathologically Confirmed CTE
At the time of this review, the majority of cases with neuropathology consistent with CTE described in the literature involved American football players (73.4%) and boxers (15.7%). There are substantially fewer cases in the literature related to other causes of RHI, including military veterans exposed to blast and other injuries, other contact and collision sports exposure, and victims of domestic violence (table e-5, doi.org/10.5061/dryad.f1vhhmgvk). There is a strong relationship of exposure to RHI as defined by the years of contact and collision sport participation with both the presence and severity of CTE pathology.4,17,18 A recent study that carefully adjusted for brain bank selection bias found that the odds of having CTE doubled for every 2.6 years of football played, and deceased football players diagnosed with CTE were 1/10 as likely to have played fewer than 4.5 years and were 10 times more likely to have played greater than 14.5 years.1 A threshold of 11 years of play maximized the sensitivity and specificity of predicting a pathologic diagnosis of CTE.
Data supporting a definitive association between CTE and TBI—mild to severe, single or multiple—independent of exposure to RHI are limited. There are a small number of autopsy cases of CTE or CTE-like pathology reported in individuals without a history of RHI.19-22 However, history of RHI is often incomplete, and the accuracy of the pathologic diagnosis in some cases may have been compromised.23 For instance, low levels of tau—even at the depths of the cerebral sulci—due to other conditions, such as age-related tau astrogliopathy or primary age-related tauopathy, have been misidentified as CTE.23-26 In contrast, a study from a large neurodegenerative disease brain bank found that nearly one-third of cases with a history of contact sport participation (predominantly amateur football) had evidence of CTE (7/21 at stages III to IV); none (0/198) without a contact sports exposure history had evidence of CTE, including 33 individuals with a history of a single TBI.27
Literature Review of Clinical Features of Pathologically Confirmed CTE
Cognitive Features
The literature review indicated that cognitive features are among the most common clinical problems identified in the histories of individuals diagnosed with CTE at autopsy. The cognitive domains most affected (reported in more than 60% of the cases) are episodic memory, attention, and executive functioning, but other domains, such as language and visuospatial functions, may also be affected (table e-1, doi.org/10.5061/dryad.f1vhhmgvk). Dementia was identified in more than half the cases.
Behavioral Features
Severe behavioral dysregulation problems were identified in more than 40% of the cases of autopsy identified CTE, including violent, impulsive, or explosive behavior. Other descriptions of abnormal behavior included socially inappropriate behavior, aggression, rage, short fuse, and lack of behavioral control (table e-7, doi.org/10.5061/dryad.f1vhhmgvk).
Mood/Affect and Other Psychiatric Features
Changes in mood and affect, anxiety symptoms, and paranoid delusions were frequently reported in histories obtained from individuals with pathologically diagnosed CTE. The most common features (in at least 30% of individual cases) were depression, anxiety, hopelessness, and apathy (table e-8, doi.org/10.5061/dryad.f1vhhmgvk). The panel considered the high base rate of anxiety and mood disorders in the general population in interpreting these data.
Clinical Course
The natural history of clinical problems was characterized by progressive decline in the vast majority (95%) of cases (n = 200; table e-9, doi.org/10.5061/dryad.f1vhhmgvk).
Motor Features
Motor problems were identified in a large proportion of cases. The most common problems involved gait and balance (51%). Dysarthria (23.5%) and signs of parkinsonism (up to 28%) were also reported (table e-10, doi.org/10.5061/dryad.f1vhhmgvk).
Predictive Validity Analyses From the UNITE Study
The following summarizes several key findings that informed the consensus process. The number of years of contact and collision sport exposure (in particular, American football) was strongly associated with a pathologic diagnosis of CTE. Of the clinical variables, cognitive dysfunction, particularly in the domains of attention, episodic memory, executive function, and language, was significantly associated with CTE. Diagnosis of dementia, based on the Functional Activities Questionnaire28 (score ≥9) was also significantly related to a pathologic diagnosis of CTE. The panelists considered the possible selection biases and confounds associated with the UNITE study design in interpreting the predictive validity data.
NINDS Consensus Diagnostic Criteria for TES
None of the components of the 2014 research diagnostic criteria for TES7 were retained in their original form after the first round of voting. Based on initial discussion and on the first round of voting, the criteria were divided into 6 sections to be voted on separately. Consensus was reached after 4 rounds of the modified Delphi process. There was some variability in the final percentage of agreement for each of the 6 approved sections achieved by round 4, with 80% approval for 2 of the 6 sections, 85% for 2 of the sections, and 90% for 2 of the sections. There were no panelists who disapproved of all sections; 2 panelists disapproved of 3 sections, though not the same sections.
There were a number of areas of concern raised by panelists over the course of the modified Delphi process that led to modifications necessary to reach consensus. These areas included defining baseline functioning with respect to onset of clinical features; defining delayed onset; removing clinical features with high population base rates from the core clinical criteria; defining clinical impairment based on objective data, but when not available or inconclusive, relying on clinical judgment based on subjective reports; assuring qualifications of clinicians reporting clinical features; accounting for biases introduced with the preponderance of data from American football clinicopathologic studies; addressing the lack of data for exposure thresholds other than from male American football players; and proposing provisional levels of certainty given the limitations of available empirical data.
Primary Criteria
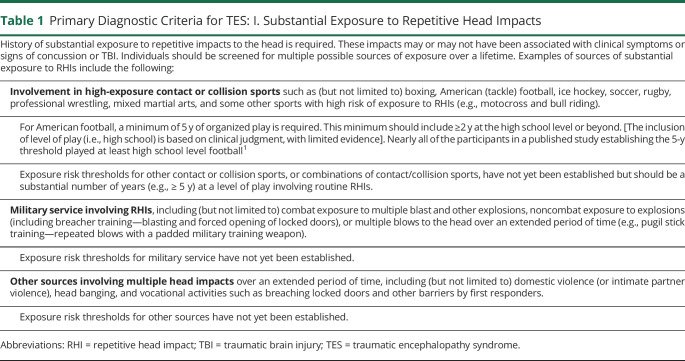
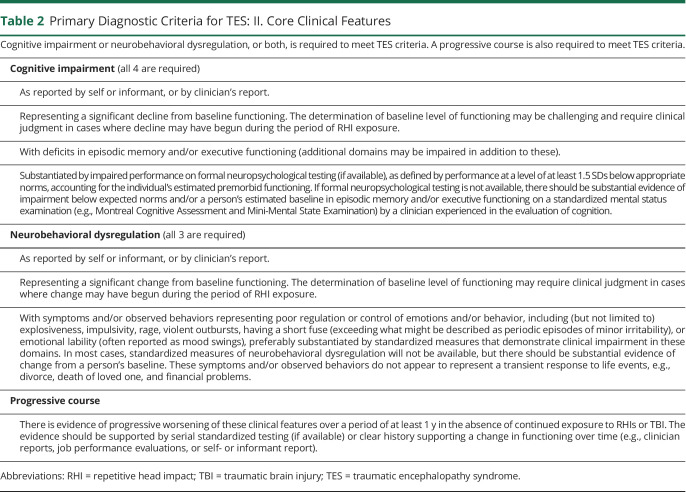
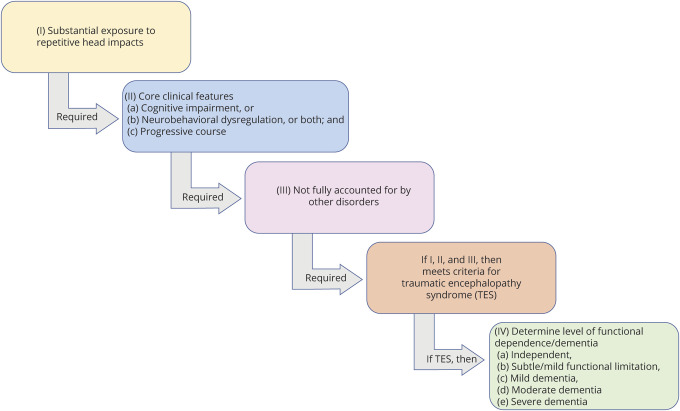
Use of the NINDS Consensus Diagnostic Criteria for TES involves a stepwise process (tables 1–4 and figure 1). The primary criteria are divided into 4 parts: (1) substantial exposure to RHIs; (2) core clinical features; (3) not fully accounted for by other disorders; and (4) level of functional dependence/dementia. Fulfilling the first 3 primary criteria is required for a diagnosis of TES. If these 3 criteria are met, the individual's level of functioning is graded, including assessing for dementia. Tables 1–4 provide the specific wording of the consensus-approved criteria; the following provides a summary of each component.
Table 1.
Primary Diagnostic Criteria for TES: I. Substantial Exposure to Repetitive Head Impacts
Table 2.
Primary Diagnostic Criteria for TES: II. Core Clinical Features
Table 3.
Primary Diagnostic Criteria for TES: III. Not Fully Accounted for by Other Disorders
Table 4.
Primary Diagnostic Criteria for TES: IV. Level of Functional Dependence/Dementia
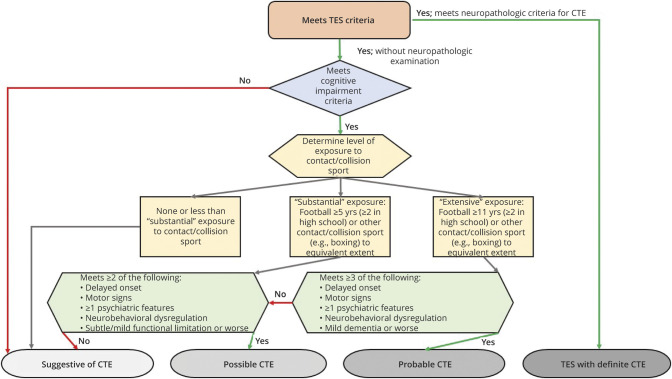
Figure 1. Stepwise Process for Using the NINDS Consensus Diagnostic Criteria for Traumatic Encephalopathy Syndrome.
The first step is to determine whether the individual meets criteria for substantial exposure to repetitive head impacts (table 1). If yes, core clinical features (table 2) are determined, and if criteria are met, those features must be not fully accounted for by other disorders (table 3). If yes, the TES criteria are met and the final step is to determine level of functional dependence/dementia (table 4). NINDS = National Institute of Neurological Disorders and Stroke; TES = traumatic encephalopathy syndrome.
Substantial exposure to RHIs (table 1) refers to a history of multiple impacts to the head, with or without clinical symptoms or signs of concussion or TBI, including exposure to high exposure contact or collision sports, military service involving exposure to repetitive blasts, or other sources, such as domestic violence, head banging, and vocational activities.
The majority of research in this area has been conducted with American football players. It is for this reason that the most specific criteria are provided for individuals with that source of exposure. The inclusion of a minimum number of years for American football is based on initial existing data. The specific thresholds may be modified in the future as additional data are available. Specific thresholds for sources of exposure other than American football, and for women, are not yet known and will be provided in future modifications to these criteria. At this time, a single moderate-severe TBI does not meet the TES exposure criteria; further research is required to assure that this is an appropriate exclusion.
Core clinical features (table 2) requires cognitive impairment (involving episodic memory and/or executive functioning) or neurobehavioral dysregulation (including explosiveness, impulsivity, rage, violent outbursts, and emotional lability), or both, representing a change from baseline, and a progressive course, to meet TES criteria.
The not fully accounted for by other disorders criterion (table 3) excludes cognitive deficits or neurobehavioral dysregulation fully accounted for by preexisting, established, or acquired neurodegenerative disorders or nondegenerative nervous system, medical, or psychiatric disorders and conditions. Comorbid diagnosis of another neurodegenerative disease, substance use disorder, posttraumatic stress disorder (PTSD), or mood or anxiety disorders does not exclude TES. The determination if other conditions more fully account for the core clinical features often may require extensive evaluation.
For those meeting criteria for TES, level of functional dependence and dementia (table 4) is graded according to descriptions of the following levels: independent, subtle/mild functional limitation, mild dementia, moderate dementia, or severe dementia.
Supportive Features and Provisional Levels of Certainty for CTE Pathology
As objective in vivo biomarkers are developed, refined, and validated for the detection of underlying neuropathologic changes of CTE, it is anticipated that one or more of these biomarkers will be used in conjunction with the Primary Criteria for TES to increase diagnostic specificity (tables 5 and 6). However, before the availability of validated diagnostic biomarkers, the current diagnostic schema provides criteria for Provisional Levels of Certainty for CTE Pathology, aimed at increasing the diagnostic specificity of the TES criteria. If an individual is found to meet criteria for a diagnosis of TES, these additional criteria can be used to provide a description of the level of certainty that there is underlying p-tau pathology meeting current neuropathologic diagnostic criteria for CTE. This method of examining the relationship between antemortem clinical features and postmortem neuropathology to support levels of diagnostic certainty, as well as the terminology and corresponding weightings of sensitivity and specificity, has been used in criteria for diagnosis of other neurodegenerative diseases (e.g., progressive supranuclear palsy, PSP29,30).
Table 5.
Supportive Features Used in Determining Provisional Levels of Certainty for CTE Pathology
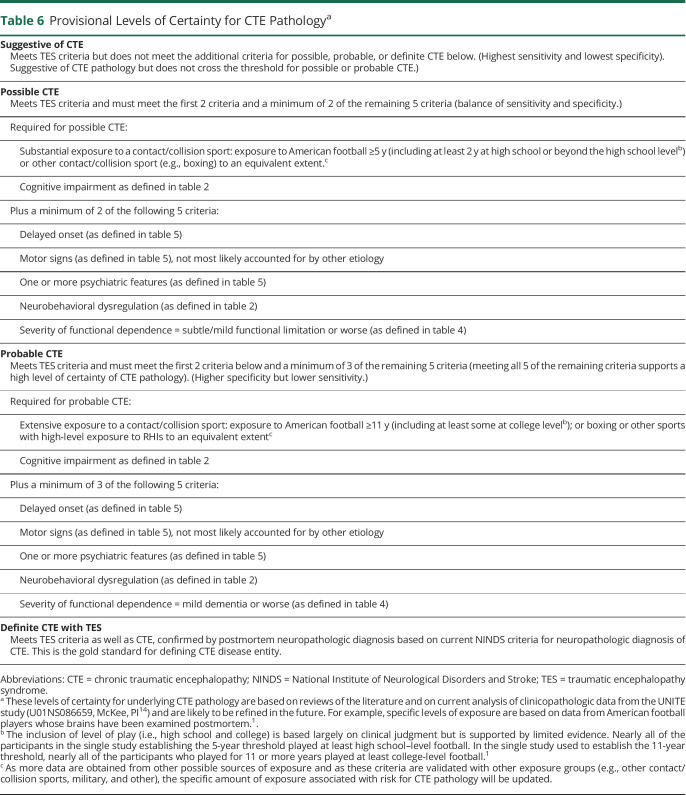
Table 6.
Provisional Levels of Certainty for CTE Pathologya
Determination of Provisional Levels of Certainty for CTE Pathology (suggestive, possible, probable, and definite) is based on a stepwise assessment of source and level of RHI exposure, specific clinical features, and a set of supportive features (figure 2). If an individual meets criteria for TES, and if they meet the core clinical feature of cognitive impairment (but not neurobehavioral dysregulation without cognitive impairment), they are then assessed for the level of exposure to contact/collision sports. The preponderance of evidence used to formulate the current Provisional Levels of Certainty is from exposure to American football and, to a lesser extent, other contact/collision sports. Therefore, individuals without a history of playing contact/collision sports could only meet criteria for suggestive of CTE. If an individual is determined to have had substantial exposure to contact/collision sports, they are then assessed for the level of functional dependence/dementia (table 4) and a set of supportive features (table 5), including delayed onset, motor signs, and psychiatric features. Delayed onset refers to a clearly established, substantial (i.e., several years) period of stable functioning after the RHI exposure ends, before core clinical features become apparent. Motor signs include parkinsonism, other motor signs (e.g., dysarthria, ataxia, and imbalance), or features of motor neuron disease. The inclusion of motor neuron disease as a supportive feature is based on preliminary research indicating an association between motor neuron disease and CTE neuropathology in a small number of individuals with a history of RHI exposure.31,32
Figure 2. Flow Diagram for Determining Provisional Levels of Certainty for CTE Pathology for Research Purposes.
Specific criteria are provided in tables 1–6. These provisional criteria are not meant for clinical diagnostic purposes. Rather, they are meant for research settings by providing a description of the level of certainty (i.e., diagnostic specificity) that an individual's clinical features are due to underlying p-tau pathology meeting the current neuropathologic diagnostic criteria for CTE. CTE = chronic traumatic encephalopathy.
Discussion
Here, we present NINDS Consensus Diagnostic Criteria for TES for use in research settings, developed using a modified Delphi process, informed by available evidence, employing an expert panel of 20 clinician-scientists representing a variety of disciplines and multiple institutions. The panel members and 7 observers initially convened at the First NINDS Consensus Workshop to Define the Diagnostic Criteria for TES. Following 4 rounds of reviewing, anonymous voting, and revising over the subsequent 8 months, a consensus was reached for 4 primary criteria for the diagnosis of TES as well as criteria for supportive features and provisional levels of certainty for CTE pathology.
Our approach in the development of these criteria is similar to the iterative process used in the development of diagnostic criteria for other neurodegenerative diseases.30,33-36 We began with the previously proposed 2014 research diagnostic criteria for TES.7 We then made substantial revisions based on current evidence and an expert consensus process, resulting in the current diagnostic criteria. No sections of the 2014 TES criteria were retained in original form, although some aspects of the general structure and nomenclature were carried into the new criteria. Our plan is to update these consensus diagnostic criteria for TES in future expert consensus efforts, incorporating new evidence into the consensus process, including findings from clinicopathologic validation studies and in vivo diagnostic biomarker research.
At the April 2019 NINDS consensus workshop, panelists and observers agreed that a primary aim should be to improve specificity of the TES diagnostic criteria over the 2014 criteria in identifying cases of presumed CTE based on the current NINDS/NIBIB neuropathologic criteria. This was further underscored through the early rounds of Delphi voting and commentary and was addressed through the following modifications to the TES criteria: (1) requiring substantial exposure to RHI; (2) requiring a progressive course, reflecting the neurodegenerative nature of CTE; (3) clarifying the core clinical features to include only those with higher frequency in CTE but excluding those with high population base rates (e.g., depression and anxiety); and (4) requiring cognitive impairment (i.e., excluding neurobehavioral dysregulation without cognitive impairment) as a necessary criterion for possible and probable CTE, the higher levels of certainty for CTE pathology.
Psychiatric features such as anxiety, depression, apathy, and paranoia were not included as core features but reserved as supportive features that are used in determining levels of certainty for CTE pathology. Although these symptoms have been reported at a high frequency in published cases with confirmed CTE and commonly reported by next of kin of individuals with neuropathologically diagnosed CTE, the inclusion of these psychiatric problems as core clinical features in the previous TES criteria was problematic because of high population base rates37 and the potential for CTE false-positive diagnoses.
In the current consensus TES criteria, the nonspecific mood and behavior features in the 2014 TES criteria have been replaced with a more specific syndrome of what the panelists termed, neurobehavioral dysregulation (i.e., poor regulation or control of emotions and/or behavior, including explosiveness, impulsivity, rage, violent outbursts, having a short fuse, emotional lability) as a core clinical feature (table 2). Aspects of neurobehavioral dysregulation are often observed in primary psychiatric and neurodegenerative conditions, but the specific features of neurobehavioral dysregulation, along with a progressive course and a history of substantial RHI exposure, distinguish neurobehavioral dysregulation in TES from these other disorders. Although impulsivity is one of the possible early features of behavioral variant frontotemporal dementia (bvFTD),34 unlike in TES, the impulsivity in bvFTD is often part of an overall picture of behavioral disinhibition, marked by the loss of social decorum, such as using crude language, telling off color jokes, and being rude without embarrassment.38 Moreover, the remaining diagnostic criteria for bvFTD are distinct from those for TES. Mild behavioral impairment (MBI) is a diagnostic construct in which later onset (age ≥ 50) behavior or personality changes are viewed as early manifestations of neurodegenerative disease, depending on the location and type of underlying neuropathology.39 Although neurobehavioral dysregulation would be consistent with the MBI diagnostic features of emotional dysregulation and impulse dyscontrol, individuals younger than 50 years or who have dementia would not meet diagnostic criteria for MBI. Some features of neurobehavioral dysregulation are also similar to those in idiopathic psychiatric disorders such as intermittent explosive disorder and disruptive mood dysregulation disorder.40 However, these can be distinguished from the neurobehavioral dysregulation of TES because they are primarily disorders with onset in childhood or adolescence that cannot be associated with other conditions, such as brain injury or dementia, and are not progressive through adulthood.40
As with other diagnostic criteria for cognitive and neuropsychiatric disorders,30,34-36,39-42 these consensus criteria for TES include a requirement that the core clinical features cannot be fully accounted for by other preexisting, established, or acquired nondegenerative nervous system, medical, or psychiatric disorders and conditions (table 3). Also similar to other diagnostic schemas,30,34-36,39-42 our criteria state that a comorbid diagnosis of another neurodegenerative disease, substance use disorder, PTSD, mood or anxiety disorder, or a combination of these does not exclude a TES diagnosis, unless they are determined to account for all core clinical features. This determination requires clinical judgment and is best made by experienced clinicians.
As stated earlier, a goal of these new criteria was to improve on the specificity of 2014 criteria for the clinical presentation of CTE, while also assuring adequate sensitivity. Without accurate diagnostic biomarkers, it is difficult to achieve high specificity based on cognitive and neuropsychiatric features alone, especially when so many of these features are shared across multiple disorders. We have attempted to increase specificity of the clinical features of TES by requiring substantial RHI exposure (as defined in table 1) and a progressive course (table 2). It is indeed possible for an individual to meet TES criteria and meet criteria for a comorbid disease or disorder. For example, a 65-year-old former college football player (who played for 7 years) who developed progressive multidomain cognitive impairment (with pronounced episodic memory deficits and executive dysfunction) at age 60 years, emotional lability at age 62 years, and is now functionally dependent in most instrumental activities of daily living (although is engaged in some family and community activities) would meet diagnostic criteria for both TES with Mild Dementia and Diagnostic and Statistical Manual of Mental Disorders-5 Major Neurocognitive Disorder Due to Possible Alzheimer's Disease, with behavioral disturbance. However, if that same individual had a history of several family members with progressive cognitive decline in their early 60s (with postmortem diagnosis of Alzheimer disease in his mother) and he had an amyloid PET, which clearly demonstrated elevated neuritic amyloid plaque deposition, a clinician could suspect that the AD fully accounts for the individual's clinical features. Therefore, the patient in this example would not meet TES criteria because the profile can be fully accounted for by a diagnosis of Major Neurocognitive Disorder Due to Probable Alzheimer's Disease, with behavioral disturbance.
The levels of functional dependence and dementia included in these TES diagnostic criteria are based primarily on accepted and widely used descriptions, such as those for the Clinical Dementia Rating (CDR) Dementia Staging Instrument43 or diagnostic criteria for all-cause dementia and mild cognitive impairment.33,42,44 Although our classification schema has face validity and closely follows the CDR, there is need for research examining the reliability and validity of our new rating.
These NINDS Consensus Diagnostic Criteria for TES will require future research to determine their interrater reliability and predictive validity. Longitudinal studies of individuals with diverse RHI exposure histories and clinical features who receive antemortem TES diagnoses and who, after death, receive postmortem neuropathologic diagnoses are required. Well-designed longitudinal studies of at-risk populations will help address some of the limitations in the presently available evidence, including the absence of information on incidence and prevalence of TES and CTE, and the ascertainment bias1 associated with brain bank studies using retrospective clinical information. Future research will need to incorporate any updates/revisions to CTE neuropathologic diagnostic criteria.
These TES criteria are limited by the lack of existing clinicopathologic data regarding risk for CTE pathology from RHI exposures other than tackle football and, to a lesser extent, boxing. Revisions to these criteria should incorporate findings from future research on risk from other contact and collision sports (e.g., ice hockey, soccer, rugby, mixed martial arts, professional wrestling, motocross, rodeo, bull riding, and race car driving), military service, intimate partner and other domestic physical violence, head banging (e.g., by individuals with developmental disorders), and breaching (e.g., by first responders), as well as newer data on specific exposure risk thresholds for American football. It is possible that the clinical presentation of CTE may differ based on source of RHI exposure, sex, and other variables. At this time, the preponderance of research indicates that CTE pathology (based on the NINDS-NIBIB criteria5) is found only in individuals with a history of repetitive mild brain trauma. However, there are observations of individuals who, following a history of a single moderate-to-severe TBI, demonstrate chronic or progressive cognitive and neuropsychiatric impairment.45,46 Recent research also indicates that in individuals with TBI history, those who also have RHI exposure history have greater cognitive impairment and depression symptoms.47 Future clinicopathologic research should include a full range of injury exposure and severity to facilitate empirical investigation into the appropriateness of the current exclusion of a single (or more) moderate-severe TBI to meet criteria for TES.48
It is unclear whether tau pathology of CTE alone is responsible for the core clinical features of cognitive impairment and neurobehavioral dysregulation seen in individuals with a history of extensive RHI exposure.49 For example, it is not yet known if, and to what extent, the patchy areas of perivascular p-tau deposition at the depths of cerebral sulci seen in early stage CTE have direct clinical correlates. It is possible there are specific lesion loci that, through disconnections with distant cortical areas or deeper gray matter nuclei, result in specific clinical features. In addition, p-tau involvement in specific brainstem nuclei (e.g., locus coeruleus) may result in specific early features. Even in later stage CTE, the relationship between the types and locations of p-tau lesions and cognitive and neuropsychiatric symptoms has not been elucidated. It is possible that some clinical features of TES may be unrelated to p-tau pathology and instead due to other consequences of brain trauma, such as white matter rarefaction50 or the long-term chronic or progressive inflammatory changes to subcortical and deep white matter.51 Moreover, neuropathologic factors unrelated to RHI exposure (e.g., arteriosclerosis) as well as additional medical and psychosocial variables, such as sleep disorders, vascular risk factors, chronic pain, and racial and associated inequities in social determinants of health, may contribute to the clinical features of TES in individuals with substantial RHI exposure or modify the clinical presentation of CTE pathology. Similar to other neurodegenerative diseases, it is also likely that some individuals with CTE pathology, especially earlier stage pathology, may not have any meaningful clinical symptoms.
The Provisional Levels of Certainty for CTE Pathology are stratified based on the relative degrees of sensitivity and specificity and, therefore, could provide guidance for selection of participants for different types of research studies. For example, Suggestive of CTE is weighted toward high sensitivity and low specificity and would be appropriate for studies focusing on early identification and longitudinal evaluation of individuals at risk of developing possible or probable CTE. Possible CTE balances sensitivity and specificity and would be appropriate for descriptive epidemiologic studies. Probable CTE is weighted toward high specificity but low sensitivity and would be appropriate for targeted therapeutic trials and diagnostic biomarker studies for which the exclusion of non-CTE participants is important.
Although there have been preliminary reports of potential fluid52-54 and neuroimaging biomarkers49,55-58 of CTE pathology, they are limited by small sample sizes, a restricted range of symptom severity, a focus on former professional football players, small or no control groups, low specificity, and, most importantly, the lack of postmortem validation. Potential diagnostic biomarkers for CTE tau pathology include tau PET imaging with radiotracers that specifically bind to CTE tau isoforms; and CSF and/or blood analytes for p-tau181 or p-tau217, other CTE tau species, or proteomic (or other -omic) profile signatures of CTE, and others. There are additional potential supportive fluid and imaging biomarkers that may have higher sensitivity-to-specificity ratios that could be used as screening biomarkers. These include fMRI measures of network connectivity, magnetic resonance spectroscopy measures of neurochemical metabolism, structural MRI (e.g., cortical thinning, volumetrics, and evidence of cavum septum pellucidum), and CSF or plasma measures of neurodegeneration (e.g., total tau and neurofilament light chain protein). Further research on individual biomarkers and combinations of biomarkers should improve the accuracy of diagnosis of TES associated with underlying CTE pathology.
A diagnosis of TES (a clinical syndrome) is not intended as a diagnosis of CTE (a neuropathologic diagnosis). Despite the previous lack of expert consensus or validated diagnostic criteria for the clinical diagnosis of CTE, a recent survey study of former National Football League players found that 108 (of 3,913) respondents reported having been given a diagnosis of CTE by a clinician.59 The authors of that study cautioned that receiving a premortem CTE diagnosis could produce a nocebo effect, leading to additional mental health difficulties, as well as a reduction in the diagnostic evaluation and intervention for treatable conditions. These NINDS Consensus Diagnostic Criteria for TES are meant primarily for research purposes and should be used cautiously in clinical and medicolegal settings, avoiding equivalence with a diagnosis of CTE, and using appropriate care when communicating a diagnosis of TES. Moreover, the Provisional Levels of Certainty for CTE Pathology described herein are intended for research purposes only. We strongly discourage disclosure of individual Levels of Certainty to participants or their health care providers.
With additional clinicopathologic research, including longitudinal studies with antemortem clinical evaluations and postmortem neuropathologic examination, and the development and validation of in vivo diagnostic biomarkers for underlying CTE pathology, we anticipate revising these consensus diagnostic criteria for TES; application of these criteria may then be appropriate for clinical diagnosis. For now, however, it is important for clinicians, researchers, and the public to be appropriately educated that a diagnosis of CTE cannot yet be confirmed during life and that it is imperative for individuals at presumed risk for CTE be properly evaluated for potentially treatable conditions and for relevant comorbidities that may exacerbate or accelerate neurodegeneration.59,60
Consensus diagnostic criteria for TES were developed through a modified Delphi process involving a multidisciplinary panel of experts. The criteria are meant for use in research settings to facilitate investigations into the clinical features associated with CTE pathology and to fill other knowledge gaps, including the development of biomarkers for antemortem diagnosis of CTE. The Primary TES criteria favor diagnostic sensitivity over specificity with regard to predicting CTE neuropathology. To improve specificity, consensus diagnostic criteria for Provisional Levels of Certainty for underlying CTE pathology were developed. The levels of certainty (i.e., suggestive, possible, probable, and definite) are based on exposure thresholds, core and supportive features, and level of functional dependence. As objective in vivo biomarkers are developed and validated for the specific detection of underlying CTE neuropathology, it is anticipated that these criteria will be revised to maximize overall clinical diagnostic accuracy.
Glossary
- bvFTD
behavioral variant frontotemporal dementia
- CTE
chronic traumatic encephalopathy
- DIAGNOSE CTE
Diagnostics, Imaging, and Genetics Network for the Objective Study and Evaluation of CTE
- MBI
Mild behavioral impairment
- NIBIB
National Institute of Biomedical Imaging and Bioengineering
- NINDS
National Institute of Neurological Disorders and Stroke
- PTSD
posttraumatic stress disorder
- RHI
repetitive head impact
- TBI
traumatic brain injury
- TES
traumatic encephalopathy syndrome
- UNITE
Understanding Neurologic Injury and Traumatic Encephalopathy
Appendix. Authors
Footnotes
Editorial, page 835
Podcast: NPub.org/ibkmnc
Contributor Information
Douglas I. Katz, Email: dkatz@bu.edu.
Charles Bernick, Email: bernick@uw.edu.
David W. Dodick, Email: dodick.david@mayo.edu.
Jesse Mez, Email: jessemez@bu.edu.
Megan L. Mariani, Email: mmariani@bu.edu.
Charles H. Adler, Email: cadler@mayo.edu.
Michael L. Alosco, Email: malosco@bu.edu.
Laura J. Balcer, Email: laura.balcer@nyulangone.org.
Sarah J. Banks, Email: sbanks@ucsd.edu.
William B. Barr, Email: william.barr@nyumc.org.
David L. Brody, Email: david.brody@nih.gov.
Robert C. Cantu, Email: rcantu@emersonhosp.org.
Kristen Dams-O'Connor, Email: kristen.dams-o'connor@mountsinai.org.
Yonas E. Geda, Email: yonas.geda@dignityhealth.org.
Barry D. Jordan, Email: bjordan@dhs.lacounty.gov.
Thomas W. McAllister, Email: twmcalli@iupui.edu.
Elaine R. Peskind, Email: peskind@uw.edu.
Ronald C. Petersen, Email: peter8@mayo.edu.
Jennifer V. Wethe, Email: wethe.jennifer@mayo.edu.
Ross D. Zafonte, Email: rzafonte@mgh.harvard.edu.
Éimear M. Foley, Email: 1eimearfoley@gmail.com.
Debra J. Babcock, Email: dbabcock@ninds.nih.gov.
Walter J. Koroshetz, Email: koroshetzw@ninds.nih.gov.
Yorghos Tripodis, Email: yorghos@bu.edu.
Ann C. McKee, Email: amckee@bu.edu.
Martha E. Shenton, Email: shenton@bwh.harvard.edu.
Jeffrey L. Cummings, Email: jcummings@cnsinnovations.com.
Eric M. Reiman, Email: eric.reiman@bannerhealth.com.
Study Funding
National Institute of Neurological Disorders and Stroke (U01NS093334).
Disclosure
D.I. Katz receives royalties from Springer/Demos Publishing for a text book on brain injury; serves as expert witness in legal cases involving brain injury and concussion; receives financial support from the National Institute of Neurological Disorders and Stroke (NINDS); receives a stipend from Encompass Health as program medical director for brain injury and chair of the Annual Neurorehabilitation Conference; and has received honoraria for a keynote address for the HealthSouth Annual Medical Directors Meeting and some grand rounds lectures involving TBI (Harvard, UMass). C. Bernick has received research funding from the Ultimate Fighting Championships, Bellator/Spike TV, Top Rank Promotions, and Haymon Boxing. D.W. Dodick reports the following conflicts within the past 12 months: consulting: AEON, Amgen, Clexio, Cerecin, Ctrl M, Allergan, Alder, Biohaven, Linpharma, Lundbeck, Promius, Eli Lilly, eNeura, Novartis, Impel, Satsuma, Theranica, WL Gore, Nocira, XoC, Zosano, Upjohn (Division of Pfizer), Pieris, Revance, and Equinox; honoraria: CME Outfitters, Curry Rockefeller Group, DeepBench, Global Access Meetings, KLJ Associates, Academy for Continued Healthcare Learning, Majallin LLC, Medlogix Communications, MJH Life Sciences, Miller Medical Communications, Southern Headache Society (MAHEC), WebMD Health/Medscape, Wolters Kluwer, Oxford University Press, Cambridge University Press. Research Support: Department of Defense, NIH, Henry Jackson Foundation, Sperling Foundation, American Migraine Foundation, and Patient-Centered Outcomes Research Institute (PCORI); stock options/shareholder/patents/board of directors: Ctrl M (options), Aural Analytics (options), ExSano (options), Palion (options), Healint (options), Theranica (options), Second Opinion/Mobile Health (options), Epien (options/board), Nocira (options), Matterhorn (shares/board), Ontologics (shares/board), King-Devick Technologies (options/board), and Precon Health (options/board); and patent 17189376.1-1466:vTitle: Botulinum Toxin Dosage Regimen for Chronic Migraine Prophylaxis. J. Mez, M.L. Mariani, C.H. Adler, and M.L. Alosco report no disclosures. L.J. Balcer is Editor-in-Chief, Journal of Neuro-Ophthalmology. S.J. Banks and W.B. Barr report no disclosures. D.L. Brody has served as a paid consultant for Pfizer Inc, Intellectual Ventures, Signum Nutralogix, Kypha Inc, Sage Therapeutics, iPerian Inc, Navigant, Avid Radiopharmaceuticals (Eli Lilly & Co), the St Louis County Public Defender, the United States Attorney's Office, the St Louis County Medical Examiner, GLG, Stemedica, and Luna Innovations; he holds equity in the company Inner Cosmos and receives royalties from sales of Concussion Care Manual (Oxford University Press). Dr. Brody has testified in over 60 medicolegal cases for which he was compensated for his time; he has received research funding from the Department of Defense, NIH, F-prime Foundation, Health South, Bright Focus Foundation, NeuroRx Inc., the Cure Alzheimer's Fund, the National Football League Charities, Pfizer, Burroughs Wellcome Fund, Hope Center at Washington University, and Thrasher Research Fund. R.C. Cantu is Senior Advisor to the National Football League Head Neck & Spine Committee, Vice President and Chair of the Scientific Advisory Committee, National Operating Committee on Standards for Athletic Equipment, and Co-Founder and Medical Director of Concussion Legacy Foundation; he receives royalties from Houghton Mifflin Harcourt and provides legal expert opinion on cases regarding the National Collegiate Athletic Association (NCAA), NHL, and others. K. Dams-O’Connor receives financial support from the National Institute of Neurological Disorders and Stroke (NINDS), the National Institute on Aging (NIA), the Department of Defense Congressionally Directed Medical Research Program (DoD CDMRP), the National Institute on Disability, Independent Living and Rehabilitation Research (NIDILRR), and the Patient-Centered Outcomes Research Institute (PCORI). She serves as expert witness in legal cases involving brain injury and concussion and has received honoraria for lectures involving TBI. Y.E. Geda reports grant support from the National Institute on Aging (R01 AG057708). B.D. Jordan reports no disclosures. T.W. McAllister reports grant support from the US Department of Defense and the NCAA. E.R. Peskind serves on scientific advisory boards for Avanir Pharmaceuticals and Acadia Pharmaceuticals, and her research is funded by the Department of Veterans Affairs (VA) Rehabilitation Research and Development Service B77421, VA Clinical Research and Development Service and an anonymous foundation. R.C. Petersen is a consultant for Roche, Inc., Merck, Inc., Biogen, Inc., and Eisai, Inc., and is a member of a data safety monitoring board for Genentech, Inc. J.V. Wethe reports no disclosures. R.D. Zafonte receives royalties from Springer/Demos Publishing for serving as coeditor of the text Brain Injury Medicine; he serves on scientific advisory boards of Oxeia Biopharma, Biodirection, ElMINDA, and Myomo; he also evaluates patients in the MGH Brain and Body-TRUST Program, which is funded by the NFL Players Association (NFLPA). Dr Zafonte serves on the NFLPA Mackey-White Health Committee; his other research was partially supported by: NIDILRR: 90DPTB0017, 90DPTB0011, 90DPBU0001, 90SI5021; Football Players Health Study at Harvard, -225417.5109721.0031- DOD: W81XWH-17-1-0335, NIH U01NS086729, and he serves a Co-PI on a NIH/NINDS-funded T32 on Neurorehabilitation and Neurotechnology. É.M. Foley, D. Babcock, W.J. Koroshetz, Y. Tripodis, A.C. McKee, and M.E. Shenton report no disclosures. J.L. Cummings has provided consultation to Acadia, Actinogen, Alkahest, Alzheon, Annovis Bio, Avanir, Axsome, Biogen, Cassava, Cerecin, Cerevel, Cortexyme, Cytox, EIP Pharma, Eisai, Foresight, GemVax, Genentech, Green Valley, Grifols, Karuna, Merck, Novo Nordisk, Otsuka, Resverlogix, Roche, Samumed, Samus, Signant Health, Suven, and United Neuroscience pharmaceutical and assessment companies. Dr. Cummings has stock options in ADAMAS, Annovis Bio, MedAvante, and BiOasis; he owns the copyright of the Neuropsychiatric Inventory. Dr Cummings is supported by Keep Memory Alive (KMA), NIGMS grant P20GM109025, NINDS grant U01NS093334, and NIA grant R01AG053798. E.M. Reiman is a scientific advisor to Alkahest, Alzheon, Aural Analytics, Denali, Green Valley, MagQ, Takeda, and United Neuroscience; he is also an advisor to Roche/Roche Diagnostics (expenses only); he is a principal investigator of prevention trials that include research agreements with Genentech/Roche and Novartis/Amgen, PET studies that include research agreements with Avid/Lilly, and several NIH and foundation-supported research studies; he is the inventor of a patent owned by Banner Health involving the use of biomarkers to accelerate the evaluation of Alzheimer's disease prevention therapies; he is cofounder and shareholder of AlzPath, a new company, which aims to further develop blood-based biomarkers for AD and related diseases and advance their use in research, drug development and clinical settings; he received relevant support from NINDS grant U01 NS093334 (Stern/Cummings/Reiman/Shenton, PIs) and NIA grant P30 AG019610 (Reiman, PI). R.A. Stern reports consulting fees from Biogen, Inc.; he receives royalties from Psychological Assessment Resources, Inc. for published tests and he holds stock options as a member of the Board of Directors of King-Devick Technologies, Inc.; he is a member of the Medical Science Committee for the NCAA Student-Athlete Concussion Injury Litigation; he received relevant support from NIH grants U01NS093334, P30AG1384623, R01AG062348, R01AG062624, and U54NS115266. Go to Neurology.org/Nhttps://n.neurology.org/lookup/doi/10.1212/WNL.0000000000011850 for full disclosures.
References
- 1.Mez J, Daneshvar DH, Abdolmohammadi B, et al. . Duration of American football play and chronic traumatic encephalopathy. Ann Neurol 2020;87:116–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Corsellis JA, Bruton CJ, Freeman-Browne D. The aftermath of boxing. Psychol Med 1973;3:270–303. [DOI] [PubMed] [Google Scholar]
- 3.Ling H, Morris HR, Neal JW, et al. . Mixed pathologies including chronic traumatic encephalopathy account for dementia in retired association football (soccer) players. Acta Neuropathol 2017;133:337–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McKee AC, Stern RA, Nowinski CJ, et al. . The spectrum of disease in chronic traumatic encephalopathy. Brain 2013;136:43–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McKee AC, Cairns NJ, Dickson DW, et al. . The first NINDS/NIBIB consensus meeting to define neuropathological criteria for the diagnosis of chronic traumatic encephalopathy. Acta Neuropathol 2016;131:75–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stern RA, Daneshvar DH, Baugh CM, et al. . Clinical presentation of chronic traumatic encephalopathy. Neurology 2013;81:1122–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Montenigro PH, Baugh CM, Daneshvar DH, et al. . Clinical subtypes of chronic traumatic encephalopathy: literature review and proposed research diagnostic criteria for traumatic encephalopathy syndrome. Alzheimers Res Ther 2014;6:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jordan BD. The clinical spectrum of sport-related traumatic brain injury. Nat Rev Neurol 2013;9:222–230. [DOI] [PubMed] [Google Scholar]
- 9.Victoroff J. Traumatic encephalopathy: review and provisional research diagnostic criteria. NeuroRehabilitation 2013;32:211–224. [DOI] [PubMed] [Google Scholar]
- 10.Reams N, Eckner JT, Almeida AA, et al. . A clinical approach to the diagnosis of traumatic encephalopathy syndrome: a review. JAMA Neurol 2016;73:743–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Laffey M, Darby AJ, Cline MG, Teng E, Mendez MF. The utility of clinical criteria in patients with chronic traumatic encephalopathy. NeuroRehabilitation 2018;43:431–441. [DOI] [PubMed] [Google Scholar]
- 12.Asken BM, Sullan MJ, DeKosky ST, Jaffee MS, Bauer RM. Research gaps and controversies in chronic traumatic encephalopathy: a review. JAMA Neurol 2017;74:1255–1262. [DOI] [PubMed] [Google Scholar]
- 13.Alosco ML, Stern RA. The long-term consequences of repetitive head impacts: chronic traumatic encephalopathy. Handbook Clin Neurol 2019;167:337–355. [DOI] [PubMed] [Google Scholar]
- 14.Mez J, Solomon TM, Daneshvar DH, et al. . Assessing clinicopathological correlation in chronic traumatic encephalopathy: rationale and methods for the UNITE Study. Alzheimer's Res Ther 2015;7:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alosco ML, Cherry JD, Huber BR, et al. . Characterizing tau deposition in chronic traumatic encephalopathy (CTE): utility of the McKee CTE staging scheme. Acta Neuropathol 2020;140:495–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jorm AF. Using the Delphi expert consensus method in mental health research. Aust N Z J Psychiatry 2015;49:887–897. [DOI] [PubMed] [Google Scholar]
- 17.Mez J, Daneshvar DH, Kiernan PT, et al. . Clinicopathological evaluation of chronic traumatic encephalopathy in players of American football. JAMA 2017;318:360–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cherry JD, Tripodis Y, Alvarez VE, et al. . Microglial neuroinflammation contributes to tau accumulation in chronic traumatic encephalopathy. Acta Neuropathol Commun 2016;4:112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iverson GL, Luoto TM, Karhunen PJ, Castellani RJ. Mild chronic traumatic encephalopathy neuropathology in people with no known participation in contact sports or history of repetitive neurotrauma. J Neuropathol Exp Neurol 2019;78:615–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Puvenna V, Engeler M, Banjara M, et al. . Is phosphorylated tau unique to chronic traumatic encephalopathy? Phosphorylated tau in epileptic brain and chronic traumatic encephalopathy. Brain Res 2016;1630:225–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fournier CN, Gearing M, Upadhyayula SR, Klein M, Glass JD. Head injury does not alter disease progression or neuropathologic outcomes in ALS. Neurology 2015;84:1788–1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ling H, Holton JL, Shaw K, Davey K, Lashley T, Revesz T. Histological evidence of chronic traumatic encephalopathy in a large series of neurodegenerative diseases. Acta Neuropathol 2015;130(06):891–893. [DOI] [PubMed] [Google Scholar]
- 23.McKee AC, Stein TD, Crary JF, Bieniek KF, Cantu RC, Kovacs GG. Practical considerations in the diagnosis of mild chronic traumatic encephalopathy and distinction from age-related tau astrogliopathy. J Neuropath Exp Neurol 2020;79:921–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Forrest SL, Kril JJ, Wagner S, et al. . Chronic traumatic encephalopathy (CTE) is absent from a European community-based aging cohort while cortical aging-related tau astrogliopathy (ARTAG) is highly prevalent. J Neuropathol Exp Neurol 2019;78:398–405. [DOI] [PubMed] [Google Scholar]
- 25.McKee AC. The neuropathology of chronic traumatic encephalopathy: the status of the literature. Sem Neurol 2020;40:359–369. [DOI] [PubMed] [Google Scholar]
- 26.Liu AK, Goldfinger MH, Questari HE, Pearce RK, Gentleman SM. ARTAG in the basal forebrain: widening the constellation of astrocytic tau pathology. Acta Neuropathol Commun 2016;4:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bieniek KF, Ross OA, Cormier KA, et al. . Chronic traumatic encephalopathy pathology in a neurodegenerative disorders brain bank. Acta Neuropathol 2015;130:877–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pfeffer RI, Kurosaki TT, Harrah CH Jr., Chance JM, Filos S. Measurement of functional activities in older adults in the community. J Gerontol 1982;37:323–329. [DOI] [PubMed] [Google Scholar]
- 29.Respondek G, Kurz C, Arzberger T, et al. . Which ante mortem clinical features predict progressive supranuclear palsy pathology?. Mov Disord 2017;32:995–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hoglinger GU, Respondek G, Stamelou M, et al. . Clinical diagnosis of progressive supranuclear palsy: the movement disorder society criteria. Mov Disord 2017;32:853–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McKee AC, Gavett BE, Stern RA, et al. . TDP-43 proteinopathy and motor neuron disease in chronic traumatic encephalopathy. J Neuropathol Exp Neurol 2010;69:918–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Walt GS, Burris HM, Brady CB, et al. . Chronic traumatic encephalopathy within an amyotrophic lateral sclerosis brain bank cohort. J Neuropathol Exp Neurol 2018;77:1091–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jack CR Jr., Bennett DA, Blennow K, et al. . NIA-AA research framework: toward a biological definition of Alzheimer's disease. Alzheimer's Demen 2018;14:535–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rascovsky K, Hodges JR, Knopman D, et al. . Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain 2011;134:2456–2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McKeith IG, Boeve BF, Dickson DW, et al. . Diagnosis and management of dementia with Lewy bodies: fourth consensus report of the DLB Consortium. Neurology 2017;89:88–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sachdev P, Kalaria R, O'Brien J, et al. . Diagnostic criteria for vascular cognitive disorders: a VASCOG statement. Alzheimer Dis Assoc Disord 2014;28:206–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Iverson GL, Gardner AJ. Risk of misdiagnosing chronic traumatic encephalopathy in men with depression. J Neuropsychiatry Clin Neurosci 2020;32:139–146. [DOI] [PubMed] [Google Scholar]
- 38.Olney NT, Spina S, Miller BL. Frontotemporal dementia. Neurol Clin 2017;35:339–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ismail Z, Smith EE, Geda Y, et al. . Neuropsychiatric symptoms as early manifestations of emergent dementia: provisional diagnostic criteria for mild behavioral impairment. Alzheimer's Demen 2016;12:195–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed. American Psychiatric Association; 2013. [Google Scholar]
- 41.McKeith IG, Ferman TJ, Thomas AJ, et al. . Research criteria for the diagnosis of prodromal dementia with Lewy bodies. Neurology 2020;94:743–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McKhann GM, Knopman DS, Chertkow H, et al. . The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging—Alzheimer's Association Workgroups on Diagnostic Guidelines for Alzheimer's disease. Alzheimer's Demen 2011;7:263–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Morris JC. Clinical Dementia Rating: a reliable and valid diagnostic and staging measure for dementia of the Alzheimer type. Intl Psychogeriatrics 1997;9(suppl 1):173–176. [DOI] [PubMed] [Google Scholar]
- 44.Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med 2004;256:183–194. [DOI] [PubMed] [Google Scholar]
- 45.Wilson L, Stewart W, Dams-O'Connor K, et al. . The chronic and evolving neurological consequences of traumatic brain injury. Lancet Neurol 2017;16:813–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Himanen L, Portin R, Isoniemi H, Helenius H, Kurki T, Tenovuo O. Longitudinal cognitive changes in traumatic brain injury: a 30-year follow-up study. Neurology 2006;66:187–192. [DOI] [PubMed] [Google Scholar]
- 47.Alosco ML, Tripodis Y, Baucom ZH, et al. . The late contributions of repetitive head impacts and TBI to depression symptoms and cognition. Neurology 2020;95:e793–e804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Edlow BL, Keene CD, Perl DP, et al. . Multimodal characterization of the late effects of traumatic brain injury: a methodological overview of the Late Effects of Traumatic Brain Injury Project. J Neurotrauma 2018;35:1604–1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stern RA, Adler CH, Chen K, et al. . Tau positron-emission tomography in former National Football League players. NEJM 2019;380:1716–1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Alosco ML, Stein TD, Tripodis Y, et al. . Association of white matter rarefaction, arteriolosclerosis, and tau with dementia in chronic traumatic encephalopathy. JAMA Neurol 2019;76:1298–1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Filley CM, Kelly JP. White matter and cognition in traumatic brain injury. J Alzheimers Dis 2018;65:345–362. [DOI] [PubMed] [Google Scholar]
- 52.Alosco ML, Tripodis Y, Fritts NG, et al. . Cerebrospinal fluid tau, abeta, and sTREM2 in former National Football League players: modeling the relationship between repetitive head impacts, microglial activation, and neurodegeneration. Alzheimer Demen 2018;14:1159–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stern RA, Tripodis Y, Baugh CM, et al. . Preliminary study of plasma exosomal tau as a potential biomarker for chronic traumatic encephalopathy. J Alzheimer Dis 2016;51:1099–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Muraoka S, Jedrychowski MP, Tatebe H, et al. . Proteomic profiling of extracellular vesicles isolated from cerebrospinal fluid of former National Football League players at risk for chronic traumatic encephalopathy. Front Neurosci 2019;13:1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Alosco ML, Koerte IK, Tripodis Y, et al. . White matter signal abnormalities in former National Football League players. Alzheimer Demen 2018;10:56–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Alosco ML, Tripodis Y, Rowland B, et al. . A magnetic resonance spectroscopy investigation in symptomatic former NFL players. Brain Imaging Behav 2020;14:1419–1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Koerte IK, Hufschmidt J, Muehlmann M, et al. . Cavum septi pellucidi in symptomatic former professional football players. J Neurotrauma 2016;33:346–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dickstein D, Pullman M, Fernandez C, et al. . Cerebral [18 F] T807/AV1451 retention pattern in clinically probable CTE resembles pathognomonic distribution of CTE tauopathy. Translational Psychiat 2016;6:e900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Grashow R, Weisskopf MG, Baggish A, et al. . Premortem chronic traumatic encephalopathy diagnoses in professional football. Ann Neurol 2020;88:106–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Roberts AL, Zafonte RD, Speizer FE, et al. . Modifiable risk factors for poor cognitive function in former American-style football players: findings from the Harvard Football Players Health Study. J Neurotrauma 2020;38:189–195. [DOI] [PMC free article] [PubMed] [Google Scholar]