Abstract
Objective
To identify the causative gene in a large unsolved family with genetic epilepsy with febrile seizures plus (GEFS+), we sequenced the genomes of family members, and then determined the contribution of the identified gene to the pathogenicity of epilepsies by examining sequencing data from 2,772 additional patients.
Methods
We performed whole genome sequencing of 3 members of a GEFS+ family. Subsequently, whole exome sequencing data from 1,165 patients with epilepsy from the Epi4K dataset and 1,329 Australian patients with epilepsy from the Epi25 dataset were interrogated. Targeted resequencing was performed on 278 patients with febrile seizures or GEFS+ phenotypes. Variants were validated and familial segregation examined by Sanger sequencing.
Results
Eight previously unreported missense variants were identified in SLC32A1, coding for the vesicular inhibitory amino acid cotransporter VGAT. Two variants cosegregated with the phenotype in 2 large GEFS+ families containing 8 and 10 affected individuals, respectively. Six further variants were identified in smaller families with GEFS+ or idiopathic generalized epilepsy (IGE).
Conclusion
Missense variants in SLC32A1 cause GEFS+ and IGE. These variants are predicted to alter γ-aminobutyric acid (GABA) transport into synaptic vesicles, leading to altered neuronal inhibition. Examination of further epilepsy cohorts will determine the full genotype–phenotype spectrum associated with SLC32A1 variants.
Genetic epilepsy with febrile seizures plus (GEFS+), originally called generalized epilepsy with febrile seizures plus,1,2 is a genetic epilepsy syndrome with a broad phenotypic spectrum. Members of a family carrying the same causative variant of major effect may have phenotypes ranging from febrile seizures (FS) to developmental and epileptic encephalopathies (DEEs) including myoclonic-atonic epilepsy (MAE) and Dravet syndrome (DS).2–5 Approximately 40% of affected individuals in GEFS+ families have FS and a further 20% have febrile seizures plus (FS+), where seizures also occur beyond the typical age range associated with FS, with or without fever. The remaining 40% have more complex phenotypes, with other seizure types in addition to FS, or have DEEs.2,6 A small proportion of affected individuals in GEFS+ families do not have FS, but have other seizure types such as generalized tonic-clonic, absence, or focal seizures, including idiopathic generalized epilepsies (IGEs), highlighting that GEFS+ and IGE overlap within families.2 Various genes have been implicated in GEFS+,6 but the underlying gene in approximately 70% of GEFS+ families is yet to be identified.2
We report here the identification of variants in SLC32A1 in 8 families with GEFS+ or IGEs. SLC32A1 codes for the vesicular γ-aminobutyric acid (GABA) transporter (VGAT), also called the vesicular inhibitory amino acid transporter (VIAAT), which transports the inhibitory neurotransmitters GABA and glycine into synaptic vesicles.7 The dysfunction of a gene critical for inhibitory neurotransmission is consistent with the pathogenesis of generalized epilepsies.
Methods
Standard Protocol Approvals, Registrations, and Patient Consents
The study was approved by the Human Research Ethics Committees of Austin Health (Melbourne, Australia), the University of Adelaide, and the Adelaide Women's and Children's Health Network (Australia), or the referring hospital's ethics committee. Written informed consent was obtained from all participants or their parents or legal guardians in the case of minors or individuals with intellectual disability.
Study Design
We first characterized the phenotypes and performed whole genome sequencing in family A, which comprised 8 affected individuals with GEFS+. Following identification of a candidate variant in SLC32A1, we searched for further patients in whole exome sequence data from the Epi4K and Epi25 datasets, as well as by resequencing SLC32A1 in participants with GEFS+ phenotypes (figure 1).
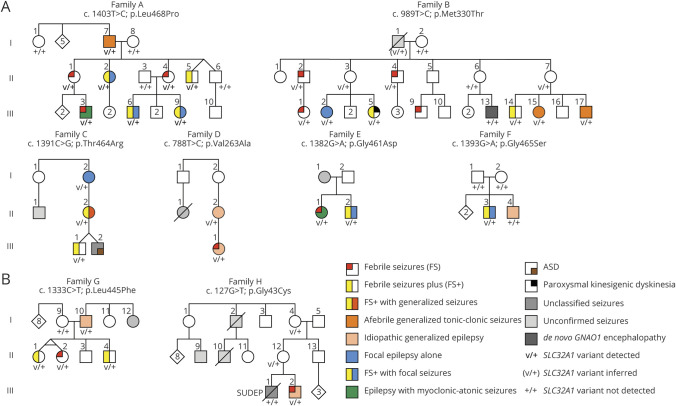
Figure 1. Pedigrees of Families With SLC32A1 Variants.
(A) Pedigrees of the 6 families with SLC32A1 variants with consistent evidence of pathogenicity. (B) Pedigrees of the 2 families in which other variants of uncertain significance were identified. All individuals with available DNA were tested and are indicated on the pedigrees by the presence of a result. Heterozygosity for the variant in each family is indicated by “v/+.”
Clinical Data and Sample Collection
Clinical data were obtained by interview using seizure questionnaires,8,9 neurologic examination, and review of medical records, EEG, and imaging data. Epilepsy Phenome/Genome Project phenotype records were sourced for positive cases identified from Epi4K sequencing.
Saliva (using Oragene kits) or peripheral blood samples were obtained from participants. Genomic DNA was extracted from blood samples using the QIAamp DNA Maxi kit (Qiagen, Hilden, Germany). DNA was extracted from saliva samples using the Oragene kit (DNA Genotek, Ottawa, Canada).
Genome-Wide Sequencing and Variant Analysis
Genome sequencing was performed at the Kinghorn Centre for Clinical Genomics, Sydney, Australia. Libraries were prepared using the HyperPrep Library Preparation kit (KAPA Biosystems, Wilmington, MA). Sequencing was performed on the Illumina HiSeq X Ten (San Diego, CA) to minimum 27 × mean coverage. Sequence reads were mapped to hg19 with BWA-MEM (0.7.15-r1140) and variants were called using GATK (3.7-0-gcfedb67) best practice guidelines.10 All variants were annotated for allele frequency, clinical significance, locus identity, and likely pathogenicity using ANNOVAR.11 Variants present in multiple individuals within family A were matched between annotation files using custom scripts.12 Filtering was performed using criteria we defined previously,12 retaining coding and canonical splice site variants with a predicted effect on protein translation, a low minor allele frequency in population databases (ExAC,13 1,000 Genomes, ESP6500, UK10K, Wellderly v1.0), and in silico pathogenicity predictions of PolyPhen-2 damaging or probably damaging, SIFT damaging and CADD_PHRED scaled score >20. Exome sequencing was performed and data were analyzed as previously described.14,15 The analysis methods used, cohorts, and numbers of patients analyzed are summarized in the flowchart in figure 2.
Figure 2. Flowchart of Methods Used in the Study.
Flowchart showing methods and cohorts used to identify SLC32A1 variants in patients with epilepsy and the numbers of patients analyzed in each cohort. Phenotypes in bold are the generalized epilepsy phenotypes associated with SLC32A1 variants. FS = febrile seizures; FS+ = febrile seizures plus; GEFS+ = genetic epilepsy with febrile seizures plus; IGE = idiopathic generalized epilepsy.
Sanger Sequencing
PCR amplification was performed using standard methods. Sanger sequencing was performed using BigDye Terminator v3.1 (Applied Biosystems, Foster City, CA). Sequencing reactions performed for variant validation and genotyping of family members were analyzed by the Australian Genome Research Facility (Adelaide, Australia). Additional patients were screened by Sanger sequencing using BigDye Terminator 3.1 and an ABI 3730 sequencer (Applied Biosystems). Primer sequences are available from the authors on request.
In Silico Pathogenicity Analysis
Data for SLC32A1 population missense variants were downloaded from the full gnomAD v2.1.1 dataset16 (gnomad.broadinstitute.org/gene/ENSG00000101438) on 17 July 2019. Pathogenicity prediction scores were calculated using PolyPhen-2 (genetics.bwh.harvard.edu/pph2/), PROVEAN Human Protein Batch (provean.jcvi.org/index.php), which outputs both PROVEAN and SIFT scores, and CADD (cadd.gs.washington.edu/score).
Data Availability
Variants identified in this study have been submitted to ClinVar. Clinical data for the patients are available from Dryad (table 2, doi.org/10.5061/dryad.s7h44j15s).
Results
SLC32A1 Is the Top Candidate Gene in Family A
Family A (figure 1A) is a GEFS+ family of English ancestry consisting of 8 individuals with confirmed seizures, previously included as family AA in Zhang et al.2 Two individuals had FS alone and 4 had FS+, 3 of whom also had focal seizures. Of the remaining 2 affected individuals, one had MAE and the other had afebrile generalized tonic-clonic seizures (GTCS).
Genome sequencing was performed on 3 affected individuals (A-II-1, A-III-3, and A-III-6) from family A. After filtering, 53 heterozygous variants were identified that were common to all 3 individuals and had minor allele frequencies of <0.01 in Wellderly v1.0, <0.005 in ESP6500, 1,000 Genomes, and UK10K, and <0.001 in ExAC. Of these, 39 were missense or other protein-altering variants. Eighteen of these were absent in the ExAC database (7 were also absent in gnomAD 2.1.1). Three of the 18 variants were predicted to be probably or possibly damaging by PolyPhen-2 HumVar, damaging by SIFT, and had CADD_PHRED scores greater than 20. Only 1 of these 3 was predicted to be probably damaging by PolyPhen-2 HumVar and was also predicted to be damaging by PROVEAN. This variant was NC_000020.10:g.37357107T>C, leading to a missense change in SLC32A1, NM_080552.3:c.1403T>C; NP_542119.1:p.(Leu468Pro). The variant was validated by Sanger sequencing, segregated in all 8 affected family members, and was absent in unaffected family members (figure 1A; family A).
Identification of Other Patients With SLC32A1 Variants
To determine whether variants in SLC32A1 occur in other patients with epilepsy, we interrogated data from 1,329 patients (165 DEE, 423 focal epilepsy, 56 FS/FS+, 452 IGE, 233 lesional epilepsy) who were exome sequenced as part of the Epi25 project.15 Six rare variants in SLC32A1 were identified, including 3 in families with generalized epilepsies (table). Four variants were identified from exome data from 1,165 individuals with familial epilepsies (640 IGE and 525 non-acquired focal epilepsy) in the Epi4K project (table).14 Previous analyses of exome sequencing data from these individuals did not identify pathogenic variants in any epilepsy-associated genes. Targeted resequencing of SLC32A1 was also performed in 278 unrelated patients: 225 with FS and 53 with other phenotypes in the GEFS+ spectrum. Variants identified in patients with generalized epilepsies were validated and available family members of these patients were genotyped. All variants identified in patients with generalized epilepsies were absent from the gnomAD dataset.
Table.
Details of Variants Identified in SLC32A1 in Genetic Epilepsy With Febrile Seizures Plus (GEFS+) and Idiopathic Generalized Epilepsies (IGEs)
Phenotypes of Other Families Carrying Potentially Pathogenic SLC32A1 Variants
We identified strong candidate variants in 7 additional unrelated individuals from the Epi4K and Epi25 IGE cohorts.14,15 In 5 families (figure 1A), both the segregation and in silico pathogenicity prediction data (table) supported pathogenicity. Family B, with the SLC32A1 variant c.989T>C; p.(Met330Thr), is a large GEFS+ family of English ancestry consisting of 11 individuals with epilepsy. One individual (B-III-13), who did not carry the SLC32A1 variant, had a profound DEE caused by a de novo, previously described recurrent pathogenic variant in GNAO1.17 This individual has been previously described18,19 (patient T2502318 and patient 1119) and was subsequently found to be mosaic for the GNAO1 variant.20 Of the remaining 10 family members, 4 had FS, 2 had FS+, 1 had focal epilepsy, 2 had afebrile GTCS, and 1 had unclassified epilepsy. One individual (B-III-5) had paroxysmal kinesigenic dyskinesia in addition to FS+. This individual had been tested for pathogenic variants in PRRT2, which causes the epilepsy–movement disorder syndrome of infantile convulsions and choreoathetosis, and includes both afebrile and febrile infantile seizures and paroxysmal kinesigenic dyskinesia, with none being found.21
Family C (c.1391C>G; p.[Thr464Arg]) is a 3-generation GEFS+ pedigree. Three individuals had the following phenotypes: FS+, FS+ with generalized seizures, and focal seizures alone. Family D (c.788T>C; p.[Val263Ala]) consists of a mother–daughter pair who had unclassified IGE and juvenile myoclonic epilepsy (JME), respectively. The daughter also had FS. Family E (c.1382G>A; p.[Gly461Asp]) consists of 2 siblings: 1 had mild MAE, including FS; the other had FS+ and focal seizures. Family F (c.1393G>A; p.[Gly465Ser]) consists of 2 brothers: the proband carrying the variant had FS+ and focal seizures; his brother had childhood absence epilepsy but did not carry the variant. The variant arose de novo as it was not detected in either parent. Parentage was confirmed by the analysis of 10 highly variable microsatellite and short tandem repeat markers.
In 2 families (figure 1B), variants were identified but the evidence for pathogenicity was conflicting. Family G (c.1333C>T; p.[Leu445Phe]) is a GEFS+ family consisting of 3 siblings, including monozygotic twins, with FS or FS+, and their father, who had JME. Whereas the variant segregated in all 4 affected individuals, in silico pathogenicity prediction data for this variant did not support pathogenicity (table), in contrast to the other variants identified in patients with generalized epilepsies. Family H (c.127G>T; p.[Gly43Cys]) consists of 2 brothers: 1, who carried the variant, had juvenile absence epilepsy and FS; the other, who had unclassified seizures and sudden unexpected death in epilepsy, did not carry the SLC32A1 variant. The variant was also present in their unaffected mother and maternal grandmother. Screening of 278 unrelated patients with GEFS+ or FS did not identify additional cases with SLC32A1 variants.
Evidence for Pathogenicity of SLC32A1 Variants
The clear segregation of the variants with the epilepsy phenotypes in the 2 large families A and B provides strong genetic evidence that SLC32A1 variants play a role in the pathogenesis of GEFS+. The combined 2-point LOD score (theta = 0, penetrance = 0.7, variant frequency = 0.001) for families A and B was 4.27, indicating that the cosegregation of the SLC32A1 variants with the phenotypes in these 2 families is unlikely to have occurred by chance.
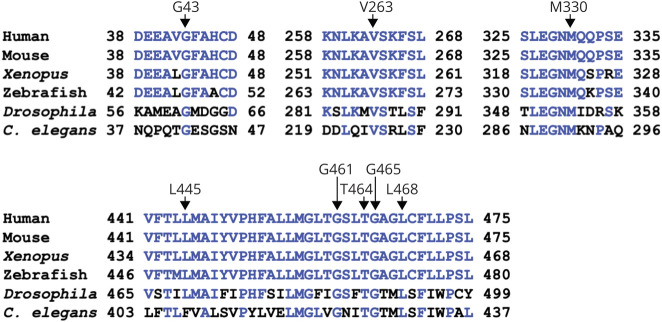
All 8 variants alter amino acid residues that are conserved in the SLC32A1 protein product, VGAT, in vertebrate species and Drosophila. Seven of the 8 residues are also conserved in the ancestral VGAT protein, C elegans unc-4722 (figure 3). Comparison of 4 in silico pathogenicity prediction scores (Poly-Phen-2 HumVar, SIFT, PROVEAN, and CADD_PHRED) for the 8 variants found in patients with GEFS+ or IGE with those calculated for population variants (identified from the entire gnomAD v2.1.1 dataset)16 showed a clear enrichment of variants that are predicted to be damaging in the epilepsy group (figure 4A). The population variants were divided into those present in one gnomAD sample (n = 146) and those present in more than one individual (n = 58). For all 4 prediction scores, the difference between the scores for epilepsy-associated variants and those for both groups of population variants was statistically significant (Mann-Whitney test, p < 0.05), despite the small number of variants from epilepsy patients that were analyzed.
Figure 3. Conservation of Altered Amino Acid Residues.
Partial amino acid alignments (Clustal W method) of vesicular γ-aminobutyric acid transporter sequences from multiple species and C elegans unc-47 (human: NP_542119.1; mouse: NP_033534.2; Xenopus: NP_001079961.1; zebrafish: NP_001074170.1; Drosophila: NP_610938.1; C elegans unc-47: AAB87066) showing conservation of the amino acids altered in genetic epilepsy with febrile seizures plus/idiopathic generalized epilepsy. Residues identical to the human sequence are shown in blue type.
Figure 4. Statistical and Missense Tolerance Analysis of Identified SLC32A1 Variants.
Pathogenicity prediction scores of SLC32A1 variants identified in epilepsy families. (A) Statistical comparison of PolyPhen-2 HumVar, SIFT, PROVEAN, and CADD_PHRED scores for SLC32A1 variants identified in patients with epilepsy and those in the gnomAD database. Variants from gnomAD are divided into 2 groups: those identified only once (gnomAD = 1) and those identified in more than one individual (gnomAD >1). Pathogenicity cutoff scores for SIFT (0.05) and PROVEAN (−2.5) are indicated on the relevant charts. Variants with CADD_PHRED scores above the indicated cutoff score of 23 are the predicted top 0.5% most pathogenic variants. p Values were calculated in Prism 8 (GraphPad Software, San Diego, CA) using the Mann-Whitney test. (B) Missense tolerance ratio chart for SLC32A1 generated using the missense tolerance ratio gene viewer (mtr-viewer.mdhs.unimelb.edu.au/mtr-viewer/). Regions with significant intolerance to missense variation are indicated in red. The positions of the variants are indicated by the arrows. The 6 variants (families A–F) with consistent evidence of pathogenicity are indicated by black arrows and the other 2 (families G and H) by blue arrows. (C) Tolerance landscape plot of SLC32A1 generated using the MetaDome web server (stuart.radboudumc.nl/metadome/). The positions of the variants are indicated by the arrows as in figure 2B.
Further evidence for pathogenicity is provided by missense tolerance ratio scores.23 Six of the 8 variants are in regions of the protein that are highly intolerant to variation (figure 4B). Notably, 5 of the variants are in a region with the highest intolerance to variation seen in the gene, which includes sequence coding for the 7th and 8th transmembrane domains. Similarly, analysis with MetaDome24 indicates that all 8 variants alter residues that are intolerant or highly intolerant to variation (figure 4C). Taken together, the family segregation, pathogenicity prediction, and missense tolerance data indicate that the variants identified in SLC32A1 are likely pathogenic and cause GEFS+, with some individuals having IGE or focal epilepsy. The overlap of IGE and GEFS+ in families is well recognized2 and also observed in these families.
In summary, 8 variants in SLC32A1 were identified in patients or families with generalized epilepsies, including GEFS+ and IGEs (table). The 6 variants identified in families A–F show consistent evidence for pathogenicity from multiple prediction algorithms and segregation data where available. The evidence of pathogenicity for the remaining 2 variants is less compelling.
Discussion
We have identified pathogenic variants in SLC32A1 in GEFS+ and IGE families. SLC32A1 codes for the vesicular GABA and glycine transporter VGAT. The pathogenic mechanism associated with these variants is predicted to be altered GABAergic signaling leading to increased seizure susceptibility. Rare damaging SLC32A1 variants are therefore a genetic determinant for GEFS+ and IGEs.
The 2 large GEFS+ families had persuasive likely pathogenic variants in SLC32A1. The pathogenicity of these variants is supported by the clear segregation of the variants with the epilepsy phenotypes in both families, multiple in silico prediction algorithms, and the absence of these variants in the gnomAD dataset. An additional 4 rare predicted damaging variants were identified in smaller GEFS+ and IGE families (C–F). The variants segregated with the epilepsy phenotype in 3 of these families, where segregation information was available. Although the segregation evidence supporting pathogenicity of these variants is less compelling due to the small size of the families, the concordance of the phenotypes in these families with those in the 2 large families suggests that these SLC32A1 variants are potentially causative. In family G, the segregation of the variant in the 4 affected individuals tested is consistent with pathogenicity but in silico pathogenicity predictions largely are not. In family H, the variant was only present in one of 2 affected individual and 2 unaffected individuals. Thus, the significance of both these variants is uncertain given the ambiguous evidence for pathogenicity.
The variants identified in patients with GEFS+ and IGE are largely predicted to be damaging by in silico pathogenicity prediction tools. Prediction scores for these variants differ significantly from the average pathogenicity prediction scores for SLC32A1 variants from the general population (figure 4A) and all 8 variants alter residues predicted to be intolerant to missense variation (figure 4, B and C). Although a small number of predicted damaging variants in SLC32A1 are listed in the gnomAD database, this is not inconsistent with the apparent role of SLC32A1 variants in the pathogenesis of GEFS+ and IGE. Most individuals from GEFS+ and IGE families have relatively mild self-limited epilepsies. Because the gnomAD dataset only excludes individuals known to have severe pediatric diseases and their first-degree relatives, it is likely to contain data from around 4,000 individuals with FS, based on a 3% incidence in the population,25 and a number of their unaffected relatives. Some variants in SLC32A1 that cause epilepsy would therefore be expected to be present in gnomAD, even if they are a rare cause of FS and related mild epilepsy phenotypes.
SLC32A1 codes for the VGAT protein, which is also referred to as VIAAT. This is the sole molecule known to transport the inhibitory neurotransmitters GABA and glycine into synaptic vesicles7 and is therefore essential for neuronal function. GABAergic inhibition plays the predominant inhibitory role in the brain, while GABA and glycine both play a role in the spinal cord and brainstem.26 Defects of GABAergic transmission are well known to be critical in the pathogenesis of seizures.27
Homozygous VGAT knockout mice have an embryonic lethal phenotype with immobile, stiff embryos.26 Heterozygous VGAT knockout mice have specific defects in sensory processing and glycinergic transmission, without a clear behavioral phenotype. While the mice were not specifically tested for seizures, no phenotypic abnormalities were observed.26,28 It is notable that the observed frequency of loss-of-function (LOF) variants in SLC32A1 is significantly reduced from that expected in the gnomAD v2.1.1 database.16 In contrast, the frequency of missense variants is markedly reduced (observed/expected ratio of 0.63), but this reduction does not reach the threshold for significance. This suggests that LOF variants in the gene are subject to greater negative selection than missense variants.
Transmembrane prediction algorithms and subsequent epitope mapping experiments showed that VGAT has 9 transmembrane domains, a large cytosolic N-terminal domain, and a small C-terminal domain located in the vesicle lumen29 (figure 5). The N-terminal domain contains a signal sequence responsible for synaptic vesicle targeting.30 The 5 variants in families A, C, E, F, and G are located in adjacent predicted transmembrane domains (figure 5). Notably, 4 of these are clustered within 10 amino acid residues in the eighth transmembrane domain, suggesting that this domain is a hotspot for epilepsy-associated variants in VGAT. The variant in family H is located in the vesicle targeting sequence, and the variants in families B and D are located in predicted cytosolic loops (figure 5).
Figure 5. Vesicular γ-Aminobutyric Acid Transporter (VGAT) Structure and Locations of Identified Variants.
Predicted structure of VGAT, adapted from Martens et al.29 The synaptic vesicle targeting motif identified by Santos et al.30 is indicated by the red filled circles. The locations of the 6 variants with consistent evidence of pathogenicity (families A–F) are indicated by black filled circles and the 2 other variants (families G–H) are indicated by blue filled circles.
Given the distinctly different phenotypes observed in knockout mice, the notable clustering of the variants seen in patients with epilepsy, and the apparent tolerability of at least some missense variants in the human population, we speculate that the missense variants associated with seizures in humans may have a more specific effect on VGAT function than just LOF. Given the role of VGAT in loading inhibitory neurotransmitters into synaptic vesicles, this can be hypothesized to be a partial LOF leading to reduced transport of neurotransmitters, particularly GABA, into synaptic vesicles and thus a lower rate of GABA receptor opening leading to reduced neuronal inhibition. Of the well-established GEFS+ genes, variants in GABRG2 directly affect GABA signaling,27 and variants in SCN1A, SCN1B, and STX1B may indirectly affect it. Other genes affecting GABA signaling where there is weaker evidence for a role in GEFS+ and IGE include the GABA receptor subunit genes GABRA1, GABRB2, GABRB3, and GABRD27 and SLC12A5 encoding the chloride pump KCC2,31,32 which is required for effective GABA receptor function.33 Collectively, these findings indicate that genetic variants leading to altered GABAergic transmission are a major cause of GEFS+ and IGEs. The involvement of SLC32A1 variants in the pathogenesis of generalized epilepsies is consistent with this.
We have identified rare missense variants in SLC32A1, coding for the vesicular GABA transporter VGAT, in 8 families with GEFS+ and IGEs. There are multiple lines of evidence supporting the pathogenicity of 6 of these variants. There is ambiguous evidence supporting the pathogenicity of the remaining 2 variants. Although we did not identify further patients in our screening of a cohort of patients with GEFS+ and FS, additional GEFS+ and IGE cohorts should be studied to determine the full genotypic and phenotypic spectrum of SLC32A1 pathogenic variants. This will determine the proportion of GEFS+ and IGE cases resulting from pathogenic variants in the gene. The increasing availability of exome and genome sequencing data from large cohorts of patients with epilepsy will facilitate further understanding of the role of SLC32A1.
Acknowledgment
The authors thank the patients and families for their participation in this research; Joshua Reid and Tim Green (University of Melbourne, Austin Health) for performing DNA extractions; and the Epilepsy Phenome/Genome Project, the Epi4K Consortium, and the Epi25 Collaborative for the provision of clinical and exome data.
Glossary
- DEEs
developmental and epileptic encephalopathies
- DS
Dravet syndrome
- FS
febrile seizures
- FS+
febrile seizures plus
- GABA
γ-aminobutyric acid
- GEFS+
genetic epilepsy with febrile seizures plus
- GTCS
generalized tonic-clonic seizures
- IGE
idiopathic generalized epilepsy
- JME
juvenile myoclonic epilepsy
- LOF
loss-of-function
- MAE
myoclonic-atonic epilepsy
- VGAT
vesicular GABA transporter
- VIAAT
vesicular inhibitory amino acid transporter
Appendix. Authors

Footnotes
Editorial, page 831
Study Funding
This work was supported by grants from the National Health and Medical Research Council of Australia: Program Grant 1091593 to S.F.B., I.E.S., and J.G.; Career Development Fellowship 1085984 to S.E.H.; Senior Research Fellowship 1102971 to M.B.; and Senior Practitioner Fellowship 1104831 to I.E.S. Additional funding was provided by the Independent Research Institute Infrastructure Support Scheme and the Victorian State Government Operational Infrastructure Program.
Disclosure
S.E.H., B.M.R., R.V.H., A.E.G., M.C., M.F.B., B.E.G., K.L.H., M.R.S., S.H., E.B.G., P.W.-W., J.T.P., M.B., S.P., E.L.H., M.S.H., M.A.C., and J.G. have no financial disclosures in relation to this work. Ingrid Scheffer serves/has served on the editorial boards of the Annals of Neurology, Neurology®, and Epileptic Disorders; may accrue future revenue on pending patent WO61/010176 (filed: 2008): therapeutic compound; has a patent for SCN1A testing held by Bionomics Inc. and licensed to various diagnostic companies; has a patent on molecular diagnostic/theranostic targets for benign familial infantile epilepsy (BFIE) (PRRT2) 2011904493 and 2012900190 and PCT/AU2012/001321 (TECH ID:2012-009) with royalties paid; has served on scientific advisory boards for UCB, Eisai, GlaxoSmithKline, BioMarin, Nutricia, Rogcon, and Xenon Pharmaceuticals; has received speaker honoraria from GlaxoSmithKline, Athena Diagnostics, UCB, BioMarin, Biocodex, and Eisai; has received funding for travel from Athena Diagnostics, UCB, Biocodex, GlaxoSmithKline, Biomarin, and Eisai; and receives/has received research support from the National Health and Medical Research Council of Australia, Health Research Council of New Zealand, CURE, Australian Epilepsy Research Fund, and NIH/NINDS. Samuel Berkovic reports grants from NHMRC during the conduct of the study; grants from UCB Pharma, grants from Eisai, grants from SciGen, personal fees from Bionomics, and personal fees from Athena Diagnostics outside the submitted work; has a patent on methods of treatment and diagnosis of epilepsy by detecting mutations in the SCN1A gene with royalties paid to Bionomics Inc., licensed to Athena Diagnostics, Genetics Technologies Ltd; a patent on diagnostic and therapeutic methods for EFMR (epilepsy and mental retardation limited to females) with royalties paid to, licensed to Athena Diagnostics; and a patent on gene and mutations thereof associated with seizure and movement disorders (PRRT2) with royalties paid to, licensed to Athena Diagnostics. Go to Neurology.org/Nhttps://n.neurology.org/lookup/doi/10.1212/WNL.0000000000011855 for full disclosures.
References
- 1.Scheffer IE, Berkovic SF. Generalized epilepsy with febrile seizures plus: a genetic disorder with heterogeneous clinical phenotypes. Brain 1997;120:479–490. [DOI] [PubMed] [Google Scholar]
- 2.Zhang YH, Burgess R, Malone JP, et al. Genetic epilepsy with febrile seizures plus: refining the spectrum. Neurology 2017;89:1–10. [DOI] [PubMed] [Google Scholar]
- 3.Singh R, Scheffer IE, Crossland K, Berkovic SF. Generalized epilepsy with febrile seizures plus: a common childhood-onset genetic epilepsy syndrome. Ann Neurol 1999;45:75–81. [DOI] [PubMed] [Google Scholar]
- 4.Singh R, Andermann E, Whitehouse WP, et al. Severe myoclonic epilepsy of infancy: extended spectrum of GEFS+? Epilepsia 2001;42:837–844. [DOI] [PubMed] [Google Scholar]
- 5.Scheffer IE, Harkin LA, Dibbens LM, Mulley JC, Berkovic SF. Neonatal epilepsy syndromes and generalized epilepsy with febrile seizures plus (GEFS+). Epilepsia 2005;46S10:1–47. [DOI] [PubMed] [Google Scholar]
- 6.Myers KA, Scheffer IE, Berkovic SF; the ILAE Genetics Commission. Genetic literacy series: genetic epilepsy with febrile seizures plus. Epileptic Disord 2018;20:232–238. [DOI] [PubMed] [Google Scholar]
- 7.Schiöth HB, Roshanbin S, Hägglund MGA, Fredriksson R. Evolutionary origin of amino acid transporter families SLC32, SLC36 and SLC38 and physiological, pathological and therapeutic aspects. Mol Aspects Med 2013;34:571–585. [DOI] [PubMed] [Google Scholar]
- 8.Reutens D, Howell RA, Gebert KE, Berkovic SF. Validation of a questionnaire for clinical seizure diagnosis. Epilepsia 1992;33:26–30. [DOI] [PubMed] [Google Scholar]
- 9.Nesbitt G, McKenna K, Mays V, et al. The Epilepsy Phenome/Genome Project (EPGP) informatics platform. Int J Med Inform 2013;82:248–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van der Auwera GA, Carneiro MO, Hartl C, et al. From FastQ data to high confidence variant calls: the Genome Analysis Toolkit best practices pipeline. Curr Protoc Bioinformatics 2013;11:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang K, Li M, Hakonarson H. ANNOVAR: functional annotation of genetic variants from high-throughput sequencing data. Nucleic Acids Res 2010;38:e164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Corbett MA, Bellows ST, Li M, et al. Dominant KCNA2 mutation causes episodic ataxia and pharmacoresponsive epilepsy. Neurology 2016;87:1975–1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lek M, Karczewski KJ, Minikel EV, et al. Analysis of protein-coding genetic variation in 60,706 humans. Nature 2016;536:285–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Epi4K Consortium; Epilepsy Phenome/Genome Project. Ultra-rare genetic variation in common epilepsies: a case-control sequencing study. Lancet Neurol 2017;16:135–143. [DOI] [PubMed] [Google Scholar]
- 15.Epi25 Collaborative. Ultra-rare genetic variation in the epilepsies: a whole-exome sequencing study of 17,606 individuals. Am J Hum Genet 2019;105:267–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Karczewski KJ, Francioli LC, Tiao G, et al. The mutational constraint spectrum quantified from variation in 141,456 humans. Nature 2020;581:434–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nakamura K, Kodera H, Akita T, et al. De Novo mutations in GNAO1, encoding a Gαo subunit of heterotrimeric G proteins, cause epileptic encephalopathy. Am J Hum Genet 2013;93:496–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Epi4K Consortium. De novo mutations in SLC1A2 and CACNA1A are important causes of epileptic encephalopathies. Am J Hum Genet 2016;99:287–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kelly M, Park M, Mihalek I, et al. Spectrum of neurodevelopmental disease associated with the GNAO1 guanosine triphosphate-binding region. Epilepsia 2019;60:406–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Myers CT, Hollingsworth G, Muir AM, et al. Parental mosaicism in “de novo” epileptic encephalopathies. N Engl J Med 2018;378:1646–1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scheffer IE, Grinton BE, Heron SE, et al. PRRT2 phenotypic spectrum includes sporadic and fever-related infantile seizures. Neurology 2012;79:2104–2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McIntire SL, Reimer RJ, Schuske K, Edwards RH, Jorgensen EM. Identification and characterization of the vesicular GABA transporter. Nature 1997;389:870–876. [DOI] [PubMed] [Google Scholar]
- 23.Traynelis J, Silk M, Wang Q, et al. Optimizing genomic medicine in epilepsy through a gene-customized approach to missense variant interpretation. Genome Res 2017;27:1715–1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wiel L, Baakman C, Gilissen D, Veltman JA, Vriend G, Gilissen C. MetaDome: pathogenicity analysis of genetic variants through aggregation of homologous human protein domains. Hum Mutat 2019;40:1030–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hauser WA, Annegers JF, Rocca WA. Descriptive epidemiology of epilepsy: contributions of population-based studies from Rochester, Minnesota. Mayo Clin Proc 1996;7:576–586. [DOI] [PubMed] [Google Scholar]
- 26.Wojcik SM, Katsurabayashi S, Guillemin I, et al. A shared vesicular carrier allows synaptic corelease of GABA and Glycine. Neuron 2006;50:575–587. [DOI] [PubMed] [Google Scholar]
- 27.Maljevic S, Møller RS, Reid CA, et al. Spectrum of GABAA receptor variants in epilepsy. Curr Opin Neurol 2019;31:183–190. [DOI] [PubMed] [Google Scholar]
- 28.Yamada MH, Nishikawa K, Kubo K, Yanagawa Y, Saito S. Impaired glycinergic synaptic transmission and enhanced inflammatory pain in mice with reduced expression of vesicular GABA transporter (VGAT). Mol Pharmacol 2012;81:610–619. [DOI] [PubMed] [Google Scholar]
- 29.Martens H, Weston MC, Boulland JL, et al. Unique luminal localization of VGAT-C terminus allows for selective labeling of active cortical GABAergic synapses. J Neurosci 2008;28:13125–13131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Santos MS, Park CK, Foss SM, Li H, Voglmaier M. Sorting of the vesicular GABA transporter to functional vesicle pools by an atypical dileucine-like motif. J Neurosci 2013;33:10634–10646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kahle KT, Merner ND, Friedel P, et al. Genetically encoded impairment of neuronal KCC2 cotransporter function in human idiopathic generalized epilepsy. EMBO Rep 2014;15:766–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Puskarjov M, Seja P, Heron SE, et al. A variant of KCC2 from patients with febrile seizures impairs neuronal chloride extrusion and dendritic spine formation. EMBO Rep 2014;15:723–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schulte JT, Wierenga CJ, Bruining H. Chloride transporters and GABA polarity in developmental, neurological and psychiatric conditions. Neurosci Biobehav Rev 2018;90:260–271. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Variants identified in this study have been submitted to ClinVar. Clinical data for the patients are available from Dryad (table 2, doi.org/10.5061/dryad.s7h44j15s).