Abstract
Objective
To determine whether IV metoclopramide 20 mg + diphenhydramine 25 mg (M + D) was more efficacious than IV placebo for acute moderate or severe posttraumatic headache in the emergency room.
Methods
We conducted this randomized, double-blind, placebo-controlled, parallel-group study in 2 urban emergency departments (EDs). Participants who experienced head trauma and presented to our EDs within 10 days with a headache fulfilling criteria for acute posttraumatic headache were included. We randomized participants in a 1:1 ratio to M + D or placebo. Participants, caregivers, and outcome assessors were blinded to assignment. The primary outcome was improvement in pain on a scale of 0 to 10 between baseline and 1 hour after treatment.
Results
This study was completed between August 2017 and March 2020. We screened 414 patients for participation and randomized 160: 81 to M + D and 79 to placebo. Baseline characteristics were comparable between the groups. All enrolled participants provided primary outcome data. Patients receiving placebo reported mean improvement of 3.8 (SD 2.6), while those receiving M + D improved by 5.2 (SD 2.3), for a difference favoring metoclopramide of 1.4 (95% confidence interval [CI] 0.7–2.2, p < 0.01). Adverse events were reported by 35 of 81 (43%) patients who received metoclopramide and 22 of 79 (28%) of patients who received placebo (95% CI 1–30 for difference of 15%, p = 0.04).
Conclusion
M + D was more efficacious than placebo with regard to relief of posttraumatic headache in the ED.
Trial Registration Information
ClinicalTrials.gov Identifier: NCT03220958.
Classification of Evidence
This study provides Class I evidence that for patients with acute moderate or severe posttraumatic headache, IV M + D significantly improved pain compared to placebo.
Nearly 1.5 million patients present to US emergency departments (EDs) annually after head trauma.1 These patients commonly complain of headache.2 Posttraumatic headache and the postconcussive symptoms that accompany the headache may linger for weeks, months, or years and lead to diminished quality of life for many patients.3 Acute posttraumatic headaches are thought likely to respond to the same medications as primary headache disorders such as migraine and tension-type headaches, but this hypothesis has never been formally tested in a randomized clinical trial.4 We completed a randomized, double-blind, placebo-controlled study of IV metoclopramide + diphenhydramine for acute moderate or severe posttraumatic headache. Our primary hypothesis was that metoclopramide + diphenhydramine would improve headache more than placebo 1 hour after medication administration, as measured on a validated pain scale of 0 to 10. Secondarily, we assessed whether metoclopramide + diphenhydramine was associated with sustained headache relief and relief of postconcussive symptoms 1 hour and 1 week after medication administration.
Methods
Overview
This was a parallel-group, randomized, double-blind, ED-based, placebo-controlled study of IV metoclopramide + diphenhydramine for acute posttraumatic headache. Patients were enrolled during an ED visit and followed up by telephone 48 hours and 7 days later. The primary research question was as follows: among patients in the ED with acute posttraumatic headache, would IV metoclopramide + diphenhydramine provide more relief of headache, as measured by improvement in 0 to 10 pain scale between baseline and 1 hour, than IV normal saline placebo? This study provides Class 1 evidence to address this research question. This study was performed in the EDs of Montefiore Medical Center in the Bronx, NY. These are academic, high-volume EDs with a combined census of 180,000 visits. Salaried, trained, bilingual (English and Spanish), technician-level research associates staff these EDs 24 h/d, 7 d/wk.
Standard Protocol Approvals, Registrations, and Patient Consents
The Albert Einstein College of Medicine Institutional Review Board approved the study. The trial was registered at ClinicalTrials.gov (NCT03220958). All patients provided written consent.
Study Population
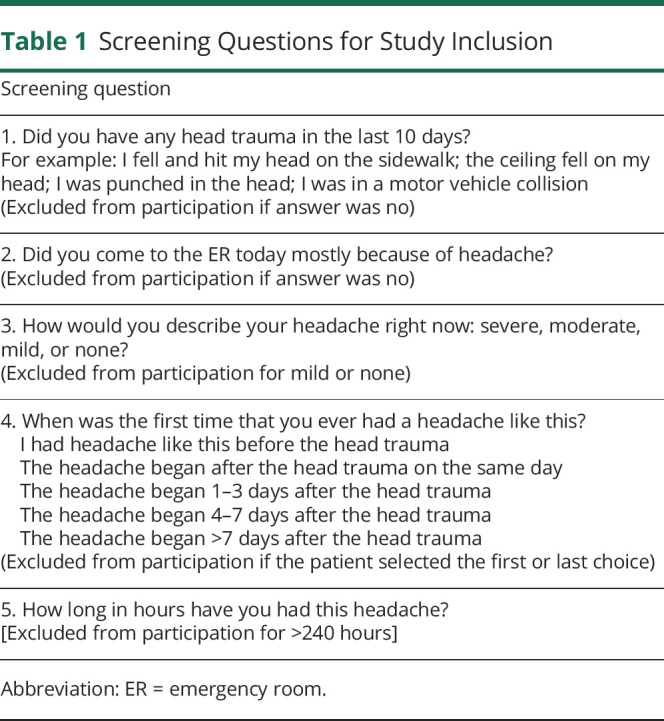
We included adults (≥18 years of age) who met International Classification of Headache Disorders criteria for acute posttraumatic headache.5 These are as follows: (1) traumatic injury to the head has occurred; (2) headache has developed within 7 days of injury to the head; and (3) headache is not better accounted for by another diagnosis (e.g., migraine or tension-type headache) at the time of the initial evaluation. For the patient to be enrolled, the headache had to be rated as moderate or severe in intensity at the time of initial evaluation. Patients were excluded from participation if >10 days had elapsed since the head trauma, if the headache had already been treated with an antidopaminergic medication, or for investigational medication contraindications, known investigational medication allergies, or pregnancy. Research associates used a standardized script to ascertain whether the patient met selection criteria (table 1). If eligibility was uncertain because of the patient's responses, the principal investigator determined whether to include the patient in the study by adjudicating whether the posttraumatic headache was sufficiently different from headaches the patient had experienced previously. The primary determining factor of whether the patient had posttraumatic headache was the temporal relation between the head trauma and the headache. If the patient had similar headaches before the head trauma, we determined that the patient had a primary headache disorder and excluded the patient from participation.
Table 1.
Screening Questions for Study Inclusion
Intervention
Participants were randomized in a 1:1 ratio to 1 of the following 2 study arms. Arm 1 was metoclopramide 20 mg + diphenhydramine 25 mg IV drip over 15 minutes. Diphenhydramine was coadministered to prevent subjective restlessness, a not infrequent side effect of higher doses of metoclopramide.6 Metoclopramide is known to be efficacious for both migraine7 and tension-type headache8 and thus was thought to be plausibly beneficial for posttraumatic headache. The combination of metoclopramide + diphenhydramine had resulted in good outcomes in pilot work conducted at our institution.9 We chose a 20-mg dose of metoclopramide because we did not wish to miss efficacy due to underdosing. The 20-mg doses of metoclopramide have been well tolerated by patients with migraine.10,11 Arm 2 was placebo, a normal saline IV drip over 15 minutes.
Assignment was concealed. The research pharmacist determined assignment according to a random-number sequence generated at randomization.com. This random-number sequence was maintained in a secure location in the pharmacy, inaccessible to research staff or clinicians. Randomization occurred in blocks of 4. Participants, clinicians, and research personnel were blinded. The pharmacist stocked a secured medication cabinet in the ED with sequentially numbered research packages that were used in sequential order. Each research package contained 2 vials. For the active treatment, 1 vial contained metoclopramide mixed into 2 mL normal saline, and the other contained diphenhydramine also mixed into 2 mL saline. For placebo, both vials contained just normal 2 mL saline. Both medication solutions appear identical to normal saline. A clinical nurse placed the contents of the vials into a 100-mL bag of normal saline and administered this as an IV infusion over 15 minutes.
Participants were allowed to receive additional medication for headache or associated symptoms 1 hour after administration of the investigational medication or placebo.
Measures
As a primary measure of headache intensity, we assessed pain using a 0 to 10 scale on which 0 signifies no pain and 10 signifies the worst pain imaginable. Using this scale, we assessed headache intensity at baseline and 1 hour later. We also used the ordinal pain scale recommended for headache research by the International Headache Society on which the headache is described as severe, moderate, mild, or none. We used this scale to assess headache intensity at baseline, 1 hour, 2 hours, and 48 hours. Participants' satisfaction with the medication was assessed by asking them if they would want to receive the same medication during a subsequent visit to the ED for posttraumatic headache. Answer choices to this question were “yes,” “no,” and “not sure.” During the follow-up phone calls, we asked participants to describe how frequently they were experiencing headache using one of the following descriptors: never, rarely, sometimes, often, or always. We also determined the number of days with headache the patient had experienced during the 7 days after ED discharge. For the purpose of this tabulation, a new day began when the patient awoke to begin activities for the day and ended when the patient went to sleep after ceasing all activities for the day. Finally, we assessed postconcussive symptomatology using the Post Concussion Symptom Scale (PCSS) from the Sport Concussion Assessment Tool (SCAT), a commonly used instrument.12 On this instrument, patients rate 22 different postconcussive symptoms using a 0 to 6 scale on which 0 signifies the absence of that symptom and 6 signifies severe symptoms. The research associates collected all data verbally using a script. In the ED, this was done in a face-to-face interview. The 48-hour and 7-day data were collected by telephone.
Outcomes
The primary outcome was improvement on a previously validated 0 to 10 pain scale between medication administration and 1 hour later.13 Important secondary outcomes were as follows: sustained headache relief for 48 hours, defined as achieving a headache intensity of mild or none in the ED and maintaining that level for 48 hours without the use of any additional analgesic medication; and SCAT PCSS scores 1 hour after medication administration and 7 days later. We also report the following a priori outcomes: (1) headache intensity 1 hour after medication administration using the descriptors severe, moderate, mild, or none (for the purpose of statistical analysis, we dichotomized the results into none/mild vs moderate/severe); (2) use of additional medication in the ED for treatment of headache; (3) whether the patient would want the same medication for posttraumatic headache during a subsequent visit (for the purpose of statistical analysis, we dichotomize the results into no/not sure vs yes); (4) worst headache intensity since ED discharge using the descriptors severe, moderate, mild, or none (for the purpose of statistical analysis, we dichotomized the results into none/mild vs moderate/severe); (5) number of days with headache during the week after ED discharge; and (6) frequency of headache during the week after ED discharge, dichotomized for the purpose of statistical analysis into never/rarely vs sometimes/often/always.
Analysis
We report baseline characteristics with mean and SD, median and interquartile range, or percent as appropriate. The primary outcome is reported as mean 1-hour improvement in each group with SD. We calculated the between-group difference in mean 1-hour improvement and report this with 95% confidence intervals (CIs). If the 95% CI did not cross 0, the result was considered statistically significant. We also compared these results with a Student t test and report the p value. We report frequencies of all dichotomous secondary outcomes as percents with between-group differences and also report the 95% CIs. We compared these results statistically with a χ2 test and report the p value. We report SCAT PCSS scores as median (interquartile range) and compare overall differences between groups by reporting mean (SD) and the 95% CI of the between-group difference. We also compare these results with a Student t test and report the p value.
We assessed blinding by asking study participants during the telephone follow-up at 48 hours whether they thought they received the active medication or placebo.
For the sample size calculation, we reviewed the results of a recent migraine trial involving metoclopramide, which demonstrated a mean improvement in 0 to 10 score of 5.1 and an SD of 2.8.11 Using these values and assuming a normal distribution, a 2-sided α = 0.05, and a previously validated minimum clinically important difference between groups of 1.3,13 we calculated the need for 74 patients in each group. We intended to enroll an additional 10% to account for protocol violations and missing data, giving a total target sample size of 162. When the coronavirus disease 2019 (COVID-19) pandemic caused the cessation of clinical research at our institution, we had enrolled 160 patients. The data monitoring committee decided to end enrollment at that point because we had been able to obtain 100% of the primary outcome data. Our Institutional Review Board approved this decision.
Data Availability
Baseline and outcome data will be posted at ClinicalTrials.gov. Deidentified individual patient data, the study protocol, and the statistical analysis plan are available from the principal investigator by request.
Results
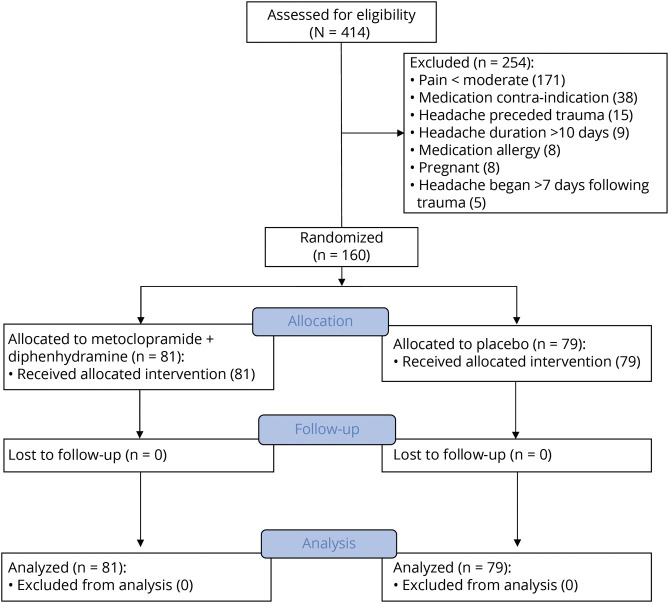
Enrollment began in August 2017 and continued for 32 months until clinical research was halted in March 2020 because of COVID-19. During this time, 414 patients were screened for eligibility, and 160 were enrolled and randomized (figure 1).
Figure 1. CONSORT Flow Diagram.
CONSORT = Consolidated Standards for Reporting Trials.
Baseline characteristics are reported in table 2. The study population was composed predominantly of women. Baseline characteristics were similar between the 2 groups. About one-quarter of the sample had a preexisting recurrent headache disorder. Medication use before ED presentation is reported in table 3.
Table 2.
Baseline Characteristics
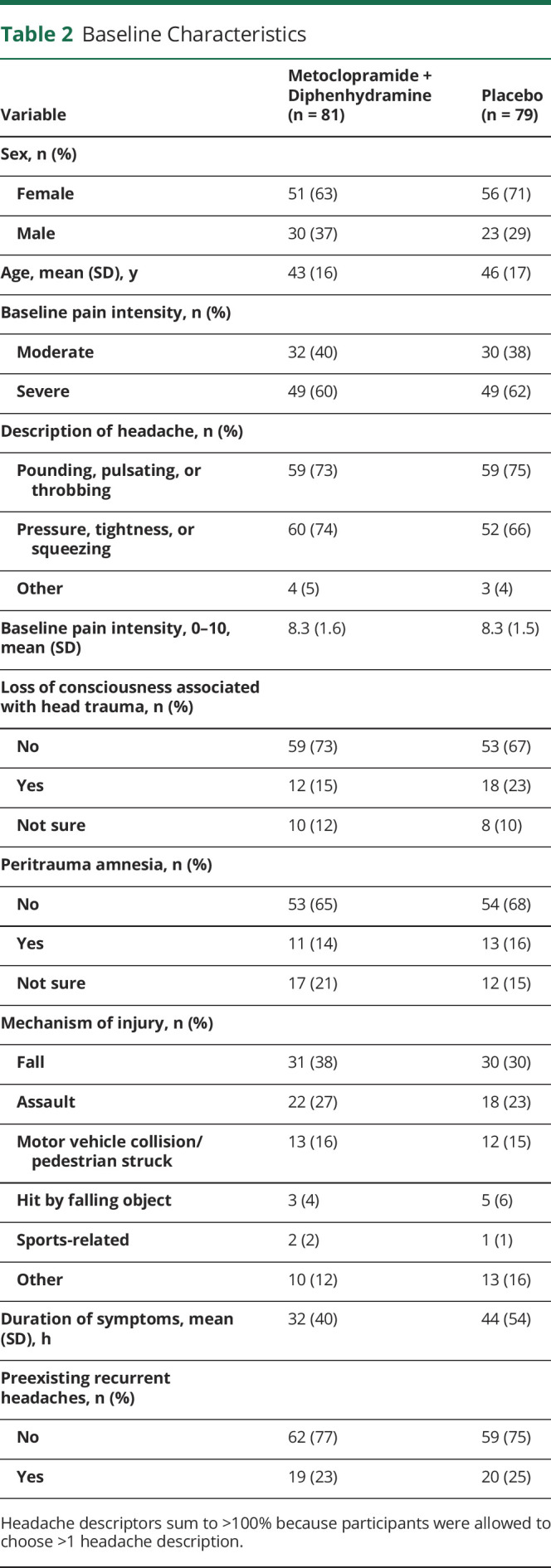
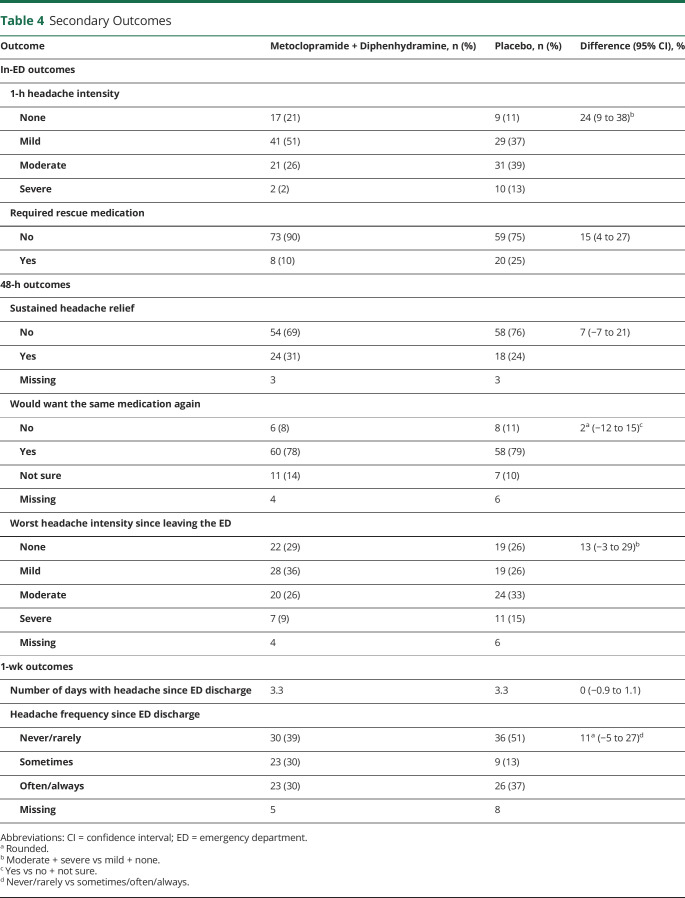
Table 4.
Secondary Outcomes
With regard to the primary outcome, patients randomized to placebo reported mean improvement on the 0 to 10 scale of 3.8 (SD 2.6), while those randomized to metoclopramide + diphenhydramine improved by 5.2 (SD 2.3). The 95% CI around this difference of 1.4 was 0.7 to 2.2 (p < 0.01).
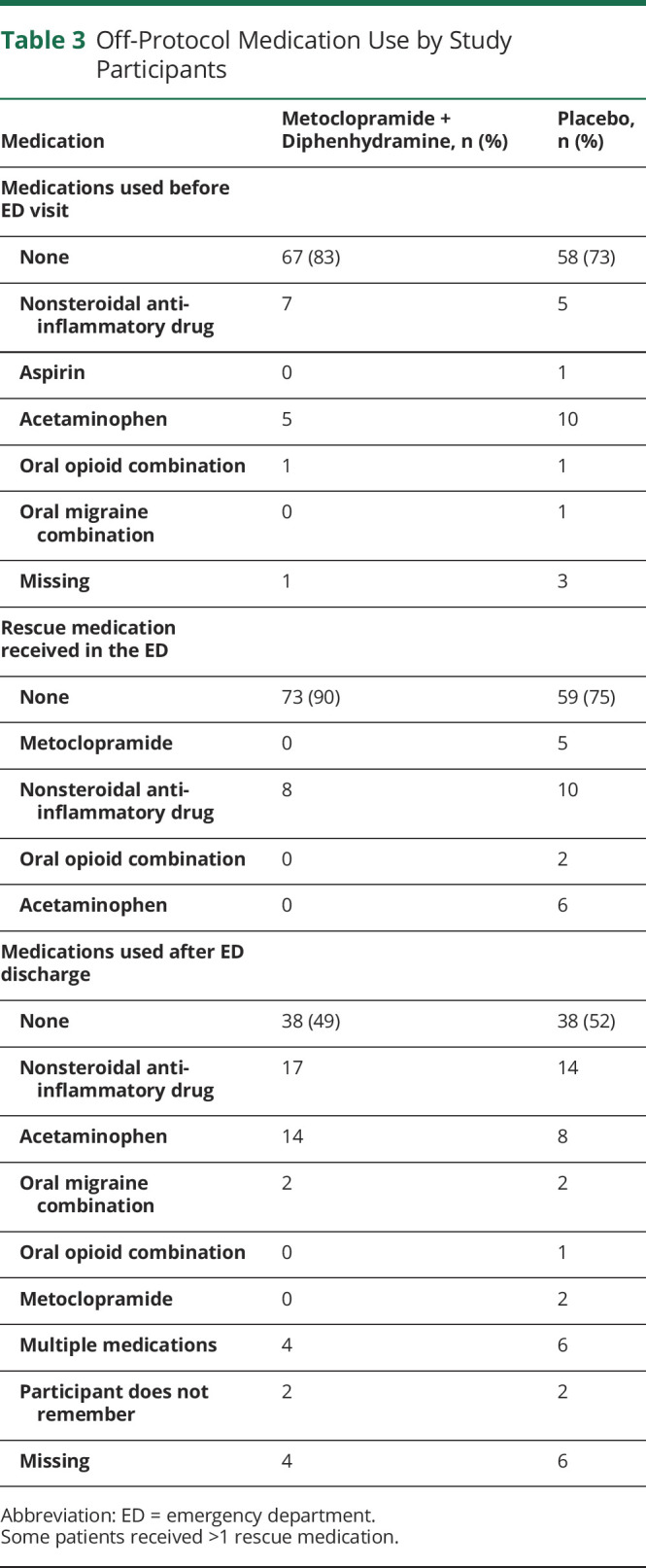
Secondary outcomes are reported in table 4. While in-ED outcomes favored the active medication, there were no differences in headache outcomes during the week after the ED visit. Use of any additional analgesic or headache medication is reported in table 3.
Table 3.
Off-Protocol Medication Use by Study Participants
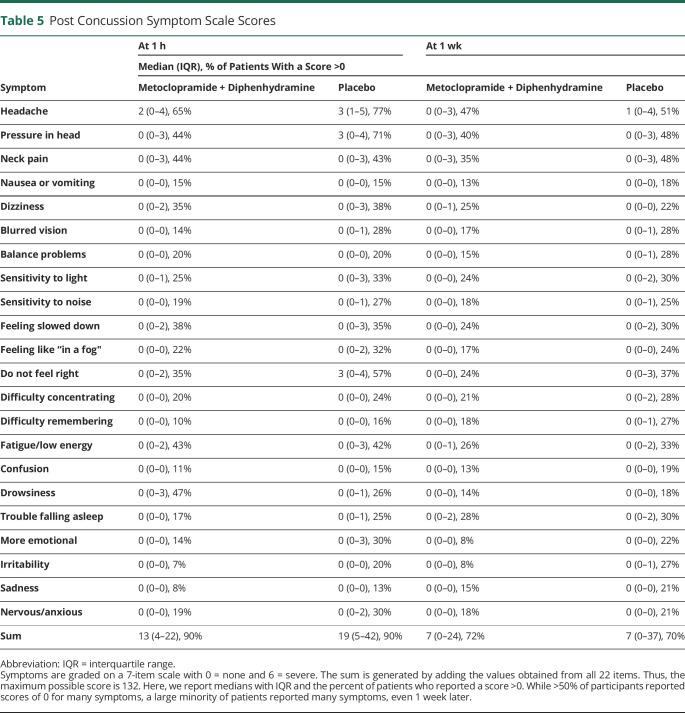
PCSS scores were assessed 1 hour and 1 week after medication administration. At the 1-hour time point, patients treated with metoclopramide had fewer symptoms than those who received placebo. The mean PCSS score in the metoclopramide group was 16 vs 25 in the placebo arm (95% CI 3–15 for difference of 9, p < 0.01) (figure 2). One week later, the mean PCSS score in the metoclopramide group was 14 and 21 in the placebo arm (95% CI 0–15 for difference of 7, p = 0.06). Data on specific symptoms from the PCSS are presented in table 5.
Figure 2. Sport Concussion Assessment Tool PCSS Sums 1 Hour and 1 Week After Treatment.
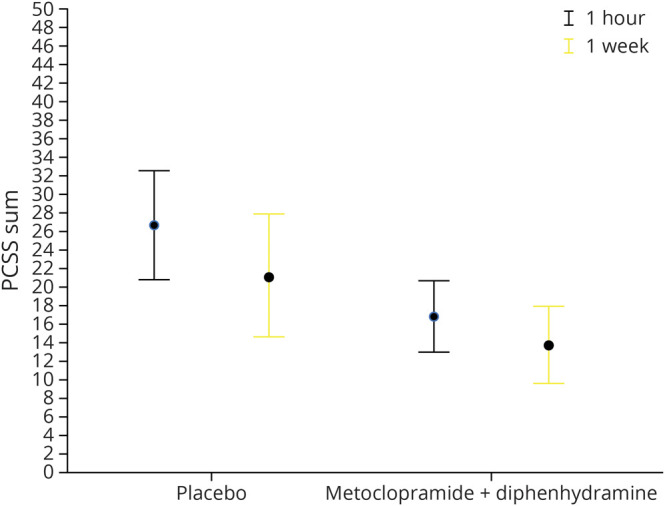
Twenty-two postconcussive symptoms were rated on a 7-item scale with 0 = none and 6 = severe. The sum is generated by adding the values obtained from all 22 items. Thus, the maximum possible score is 132. Patients who received metoclopramide + diphenhydramine reported fewer postconcussive symptoms 1 hour and 1 week after treatment. The figure depicts means with 95% confidence intervals. PCSS = Post Concussion Symptom Scale
Table 5.
Post Concussion Symptom Scale Scores
Adverse events were reported by 35 of 81 (43%) patients who received metoclopramide and 22 of 79 (28%) patients who received placebo (95% CI 1–30 for difference of 15%, p = 0.04). These adverse events include drowsiness among 15 patients receiving metoclopramide + diphenhydramine and 8 patients receiving placebo, akathisia in 5 patients receiving metoclopramide + diphenhydramine, dizziness in 4 patients receiving metoclopramide + diphenhydramine and 1 patient receiving placebo, diarrhea in 2 patients receiving metoclopramide + diphenhydramine and 1 patient receiving placebo, and symptoms consistent with postconcussive syndrome in 7 patients receiving metoclopramide + diphenhydramine and 5 patients receiving placebo. No other symptoms were noted by >2 participants. There were no serious or unexpected adverse events.
In the metoclopramide + diphenhydramine arm, 36 (47%) participants thought they received active medication, 5 (6%) thought they received placebo, and 36 (47%) stated that they did not know. In the placebo arm, 30 (42%) participants thought they received active medication, 5 (7%) thought they received placebo, and 36 (51%) did not know.
Discussion
In this randomized, double blind, placebo-controlled study, the combination of IV metoclopramide and diphenhydramine proved more efficacious than placebo for relief of acute posttraumatic headache 1 hour after medication administration, but this was not sustained beyond the ED visit. The active intervention arm also modestly outperformed the placebo arm with regard to overall postconcussive symptomatology both in the ED and 1 week later. However, more than two-thirds of study participants, regardless of study arm, were unable to obtain sustained relief from their headache, and nearly one-third reported frequent headaches during the week after ED discharge. Medication-related adverse events were common.
We were unable to identify other randomized studies of treatment for acute posttraumatic headache.4 Retrospective and open-label data in adults and children have suggested that metoclopramide and other antidopaminergic antiemetics may be efficacious for this disorder. In an open-label study of 21 adults, nearly two-thirds reported sustained headache relief after receiving metoclopramide + diphenhydramine.9 In a retrospective study from a pediatric ED, 93% of patients who received metoclopramide or prochlorperazine reported ≥50% improvement in pain scores.14 Metoclopramide is a guideline-supported, evidence-based treatment of acute migraine7 and has been shown to be more effective than a nonsteroidal anti-inflammatory drug for tension-type headache.8 However, a specific mechanism of action for antidopaminergic medication–induced alleviation of headache of any type has yet to be described.15
We combined diphenhydramine with metoclopramide to prevent symptoms of restlessness, which frequently occur with higher IV doses of metoclopramide.6 It is less likely that diphenhydramine has independent antiheadache properties.16 We chose a 20-mg dose of metoclopramide because we did not want to miss a clinically important effect due to underdosing. It is unclear if a 10-mg dose of metoclopramide alone would be sufficient or if larger doses would be more efficacious. Among patients with migraine, doses of metoclopramide >10 mg do not increase efficacy.11
We were surprised to see a sustained benefit of the investigational medication on postconcussive symptoms 1 week later. It may be that early and effective treatment of postconcussive symptoms leads to better longer-term results. We had not hypothesized that this would be the case, and the statistical differences were not robust; thus, these results should be interpreted cautiously. In a sample of nonconcussed high school and collegiate athletes, mean scores on the PCSS ranged from 2.5 to 5.9 at various time points, reflecting the baseline population prevalence of migraine and mood disorders, among other ailments.14 Thus, the observed reduction in mean PCSS score from 21 in the placebo arm to 14 in the intervention arm represents a potentially substantial decrease in suffering because it is greater than the variability seen in a nonconcussed population. However, it must be noted that a minimum clinically important difference has not yet been derived for this instrument, and athletes may be dissimilar in important ways from an ED population. The median PCSS score at 1 week was 7 in both groups with an interquartile range from 0 to 24 in the treatment arm and 0 to 37 in the placebo arm, indicating substantial suffering even 1 week later in >25% of the study population.
Future work should determine the optimal dose and duration of treatment with metoclopramide targeted at longer-term outcomes beyond the ED visit. In addition, future work can determine whether early treatment can affect other postconcussive symptoms such as depression, sleep disorders, and anxiety, which negatively affect the lives of many patients with posttraumatic headache. Comparative-effectiveness studies could compare metoclopramide to nonsteroidal anti-inflammatory drugs or migraine-specific medications such as triptans. Case reports indicate that corticosteroids may be efficacious for acute posttraumatic headache.17
Limitations of this work include the following. This study was conducted in 2 EDs in one of the most socioeconomically depressed urban counties in the United States. Because headache outcomes may be associated with access to care, these results may not be generalizable to other populations. Similarly, we studied a cohort of individuals drawn from the general population. Therefore, the generalizability of these data to military and athletic settings is necessarily limited. We administered diphenhydramine along with metoclopramide to minimize symptoms of restlessness, which are a frequent and unpleasant side effect of higher doses of IV metoclopramide. However, diphenhydramine itself has many anticholinergic side effects that could be confused with postconcussive symptoms. We excluded patients for whom >10 days had elapsed since the head trauma occurred. Therefore, these data do not inform management of persistent posttraumatic headache. It must be noted that IV administration itself may have a placebo effect higher than an oral placebo. Therefore, it has to be assumed that the placebo effect observed in this study was higher than would be seen with an oral placebo. Finally, despite the fact that pain intensity in the active intervention group improved compared to the placebo group, both groups wanted to receive the same medication again at a comparable rate. The higher frequency of side effects in the active arm or clinically less significant outcome parameters in this setting might account for these contradictory findings.
In this randomized clinical trial, IV metoclopramide + diphenhydramine provided superior posttraumatic headache relief and relief of other concussion symptoms compared to placebo 1 hour after medication administration and a marginal improvement in concussion score at 1 week.
Glossary
- CI
confidence interval
- COVID-19
coronavirus disease 2019
- ED
emergency department
- PCSS
Post Concussion Symptom Scale
- SCAT
Sport Concussion Assessment Tool
Appendix. Authors
Footnotes
Class of Evidence: NPub.org/coe
Study Funding
This publication was supported in part by the Harold and Muriel Block Institute for Clinical and Translational Research at Einstein and Montefiore grant support (UL1TR001073).
Disclosure
The authors report no disclosures relevant to the manuscript. Go to Neurology.org/N for full disclosures.
References
- 1.Blyth BJ, Bazarian JJ. Traumatic alterations in consciousness: traumatic brain injury. Emerg Med Clin North Am 2010;28:571–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Seifert TD, Evans RW. Posttraumatic headache: a review. Curr Pain Headache Rep 2010;14:292–298. [DOI] [PubMed] [Google Scholar]
- 3.Evans RW. Posttraumatic headaches in civilians, soldiers, and athletes. Neurol Clin 2014;32:283-303. [DOI] [PubMed] [Google Scholar]
- 4.Larsen EL, Ashina H, Iljazi A, et al. Acute and preventive pharmacological treatment of post-traumatic headache: a systematic review. J Headache Pain 2019;20:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders, 3rd edition. Cephalalgia 2018;38:1–211. [DOI] [PubMed] [Google Scholar]
- 6.Friedman BW, Bender B, Davitt M, et al. A randomized trial of diphenhydramine as prophylaxis against metoclopramide-induced akathisia in nauseated emergency department patients. Ann Emerg Med 2009;53:379–385. [DOI] [PubMed] [Google Scholar]
- 7.Orr SL, Friedman BW, Christie S, et al. Management of adults with acute migraine in the emergency department: the American Headache Society evidence assessment of parenteral pharmacotherapies. Headache 2016;56:911–940. [DOI] [PubMed] [Google Scholar]
- 8.Friedman BW, Adewunmi V, Campbell C, et al. A randomized trial of intravenous ketorolac versus intravenous metoclopramide plus diphenhydramine for tension-type and all nonmigraine, noncluster recurrent headaches. Ann Emerg Med 2013;62:311–318.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Friedman BW, Babbush K, Irizarry E, White D, John Gallagher E. An exploratory study of IV metoclopramide+diphenhydramine for acute post-traumatic headache. Am J Emerg Med 2018;36:285–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Friedman BW, Corbo J, Lipton RB, et al. A trial of metoclopramide vs sumatriptan for the emergency department treatment of migraines. Neurology 2005;64:463–468. [DOI] [PubMed] [Google Scholar]
- 11.Friedman BW, Mulvey L, Esses D, et al. Metoclopramide for acute migraine: a dose-finding randomized clinical trial. Ann Emerg Med 2011;57:475–482,e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chin EY, Nelson LD, Barr WB, McCrory P, McCrea MA. Reliability and validity of the Sport Concussion Assessment Tool-3 (SCAT3) in high school and collegiate athletes. Am J Sports Med 2016;44:2276–2285. [DOI] [PubMed] [Google Scholar]
- 13.Bijur PE, Latimer CT, Gallagher EJ. Validation of a verbally administered numerical rating scale of acute pain for use in the emergency department. Acad Emerg Med 2003;10:390–392. [DOI] [PubMed] [Google Scholar]
- 14.Chan S, Kurowski B, Byczkowski T, Timm N. Intravenous migraine therapy in children with posttraumatic headache in the ED. Am J Emerg Med 2015;33:635–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Charbit AR, Akerman S, Goadsby PJ. Dopamine: what's new in migraine? Curr Opin Neurol 2010;23:275–281. [DOI] [PubMed] [Google Scholar]
- 16.Friedman BW, Cabral L, Adewunmi V, et al. Diphenhydramine as adjuvant therapy for acute migraine: an emergency department-based randomized clinical trial. Ann Emerg Med 2016;67:32–39.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bramley H, Melinosky C, Silvis M, Ross S. Pediatric posttraumatic headache: two cases using steroids as abortive therapy. Pediatr Emerg Care 2012;28:1081–1084. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Baseline and outcome data will be posted at ClinicalTrials.gov. Deidentified individual patient data, the study protocol, and the statistical analysis plan are available from the principal investigator by request.