Abstract
Objective
Little is known about the prevalence of continued opioid use following aneurysmal subarachnoid hemorrhage (aSAH) despite guidelines recommending their use during the acute phase of disease. We sought to determine prevalence of opioid use following aSAH and test the hypothesis that acute pain and higher inpatient opioid dose increased outpatient opioid use.
Methods
We reviewed consecutively admitted patients with aSAH from November 2015 through September 2019. We retrospectively collected pain scores and daily doses of analgesics. Pain burden was calculated as area under the pain-time curve. Univariate and multivariable regression models determined risk factors for continued opioid use at discharge and outpatient follow-up.
Results
We identified 234 patients with aSAH with outpatient follow-up. Continued opioid use was common at discharge (55% of patients) and follow-up (47% of patients, median 63 [interquartile range 49–96] days from admission). Pain burden, craniotomy, and racial or ethnic minority status were associated with discharge opioid prescription in multivariable analysis. At outpatient follow-up, pain burden (odds ratio [OR] 1.88, 95% confidence interval [CI] 1.5–2.4), depression (OR 3.1, 95% CI 1.1–8.8), and racial or ethnic minority status (OR 2.1, 95% CI 1.1–4.0) were independently associated with continued opioid use; inpatient opioid dose was not.
Conclusion
Continued opioid use following aSAH is prevalent and related to refractory pain during acute illness, but not inpatient opioid dose. More efficacious analgesic strategies are needed to reduce continued opioid use in patients following aSAH.
Classification of Evidence
This study provides Class II evidence that continued opioid use following aSAH is associated with refractory pain during acute illness but not hospital opioid exposure.
The worst headache of one’s life may herald the onset of aneurysmal subarachnoid hemorrhage (aSAH). The headache is severe and persistent. Guidelines recommend opioids for analgesia, despite a general consensus that opioids should be avoided in other types of acute headache.1,2 Little is known about the prevalence of chronic opioid use following aSAH.
The United States continues to face an opioid crisis, with excessive and increasing costs in lives lost, burden of disability, and dollars directed to justice system and health care approaches for combating this epidemic. Opioid use disorder usually begins with a prescription for an acute medical condition.3 Severe and refractory pain after acute injury increases the risk of chronic opioid use. Other risk factors include lower socioeconomic status, race, increased inpatient opioid dose, and history of depression.4,5
We sought to determine the prevalence of and risk factors for continued opioid use after aSAH. We hypothesized that increased acute pain and increased receipt of opioids in the intensive care unit (ICU) would be associated with continued opioid use at time of discharge as well as outpatient follow-up.
Methods
Classification of Evidence
The primary research question was whether the experience of acute pain or receipt of opioids while in the ICU would increase rates of continued opioid use at time of discharge and outpatient follow-up. This study provides Class II evidence that continued opioid use following aSAH is associated with refractory pain during acute illness but not increased hospital opioid dose.
Study Design
We reviewed consecutive patients with aSAH from November 2015 through September 2019 identified in the prospective observational Recovery After Cerebral Hemorrhage (ReACH) database (NCT04189471).
Patient Population
We included only patients with an aneurysmal pattern of subarachnoid hemorrhage (SAH). We excluded patients with perimesencephalic SAH and SAH due to other etiologies including trauma, arteriovenous malformations, and reversible cerebral vasoconstriction syndrome. Patients without follow-up appointments in our medical system were also excluded.
Electronic medical records were retrospectively reviewed for substance use history and toxicology screen as well as baseline clinical and radiographic characteristics.
Pain Management
Initial pain control consisted of acetaminophen and opioids as needed with increasing dose or frequency at the bedside clinician's discretion. Adjunctive therapies were added including gabapentin, lidocaine patches, and dexamethasone.
Pain Assessment
We retrospectively reviewed the electronic medical records for pain scores and analgesic doses. Pain scores were collected at 2-hour intervals from hospital admission through discharge as per nursing assessment protocols. Pain was rated using the Numeric Rating Scale (NRS) with patient-reported scores ranging from 0 to 10, or in patients unable to self-report, the multidimensional objective pain assessment tool (MOPAT) with physiologic-based scores ranging from 0 to 12.6 MOPAT scores were normalized to the NRS on the basis of clinical categorization for severity (i.e., MOPAT 8–12 equates to NRS 7–10). We reviewed all analgesic agents received by patients in the medication administration reconciliation. For each day of hospitalization, total daily dose for each medication was calculated. Opioids were converted using a standard opioid equivalence chart and calculated as total daily oral morphine equivalents.
To account for the duration and extent to which pain may affect continued opioid use, we calculated a pain burden score for each patient. To do so, we used the trapezoidal method to calculate the cumulative area under the curve for each patient's pain scores during their index ICU stay.7 This area under the curve, divided by the length of ICU stay, resulted in a single integer defined as the pain burden with units of NRS and accounting for variability in patient length of stay. Missing pain scores in the electronic medical record were accounted for using variable time intervals via the trapezoidal method and linear interpolation.
Outcome
Opioid prescription at the time of discharge and medication reconciliation completed at the first outpatient neurosurgical visit were assessed for continued opioid use. If an extensive delay in follow-up occurred (greater than 6 months) and a visit with another physician occurred during the interim, the medication reconciliation from that visit was used instead.
Statistical Analysis
Descriptive statistics and bivariate analyses were performed to compare characteristics of patients who received discharge opioid prescriptions with those who did not. Independent sample t tests were performed for continuous variables, χ2 or Fisher exact tests for categorical variables, and Wilcoxon rank sum test for nonparametric data. Separate multivariable logistic regression models distinguishing patients with continued opioid use from those without at hospital discharge and at outpatient follow-up were then developed using a stepwise backwards elimination method.
Statistical analyses were performed in R (Version 1.2.5001, the R Foundation).
Data Availability
Anonymized study data will be available to qualified investigators from the corresponding author on reasonable request.
Results
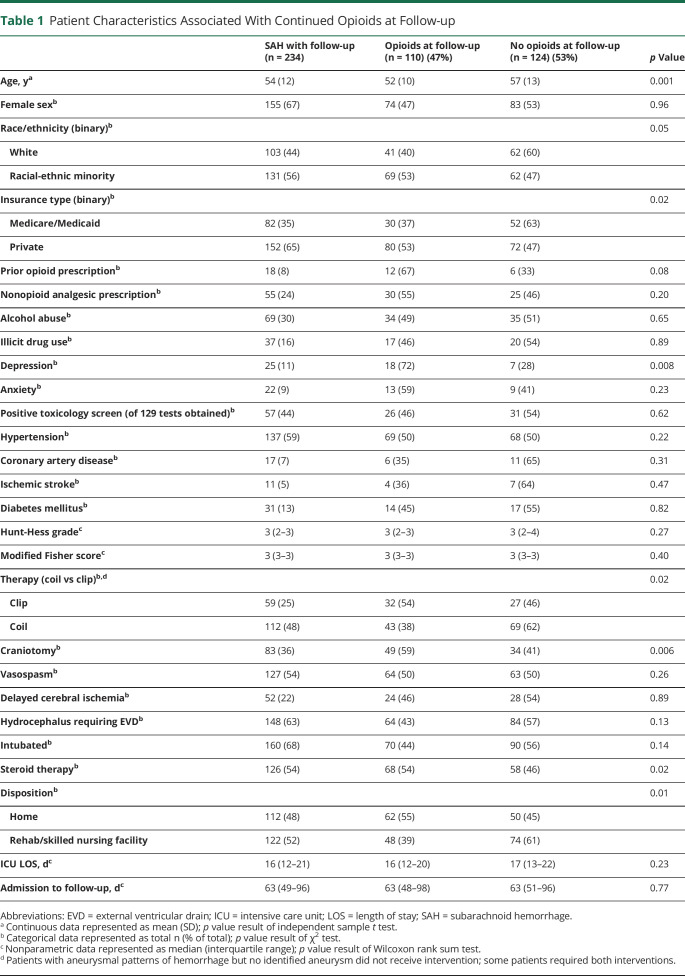
We identified 234 patients with aSAH after excluding 26 patients with perimesencephalic SAH, 45 with other non-aneurysmal SAH, 42 patients with aSAH lacking follow-up, and 3 outlier patients missing >90% of pain scores during prolonged hospitalizations. Median Hunt-Hess score was 3 (interquartile range [IQR] 2–3) with a median ICU length of stay of 16 days (IQR 12–21) and median time to first follow-up of 63 days (IQR 49–96) from admission (table 1). Fifty-five percent of the patients were discharged with a prescription for opioids and 47% endorsed continued use on medication reconciliation at the time of follow-up.
Table 1.
Patient Characteristics Associated With Continued Opioids at Follow-up
Continued Opioid Use at Hospital Discharge
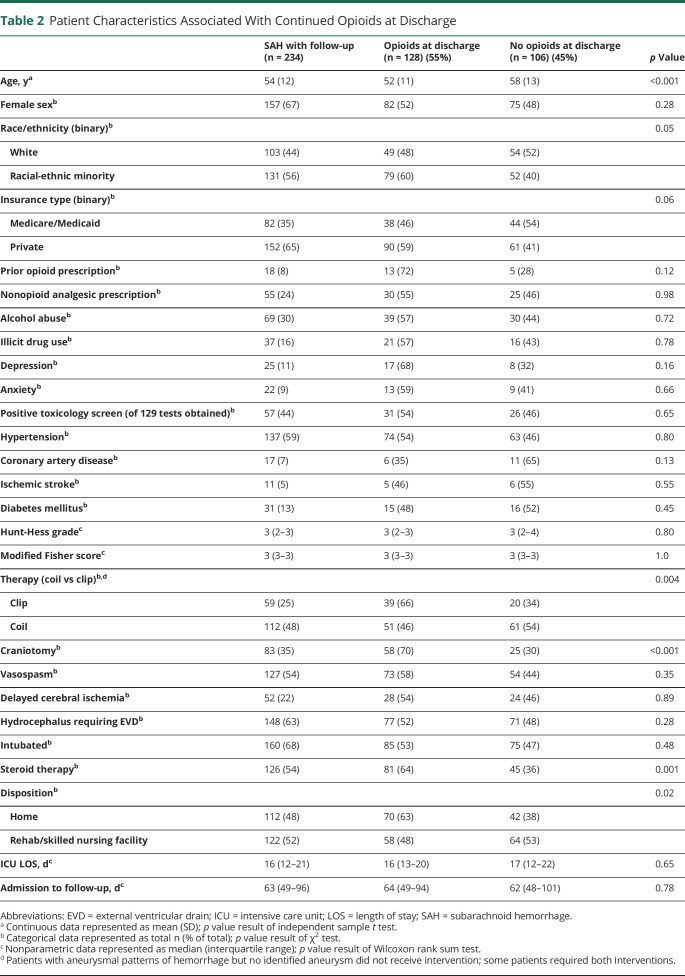
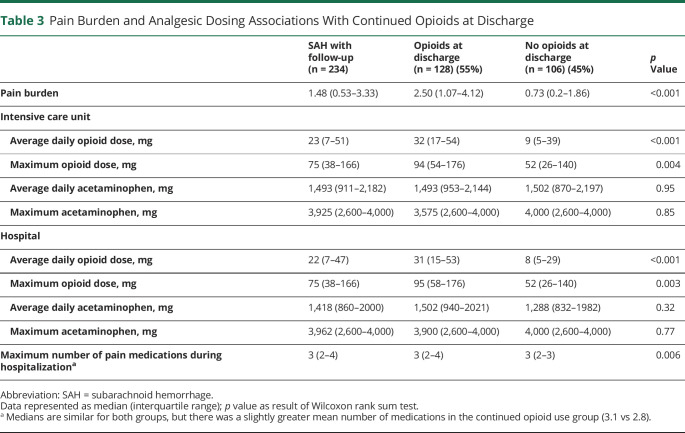
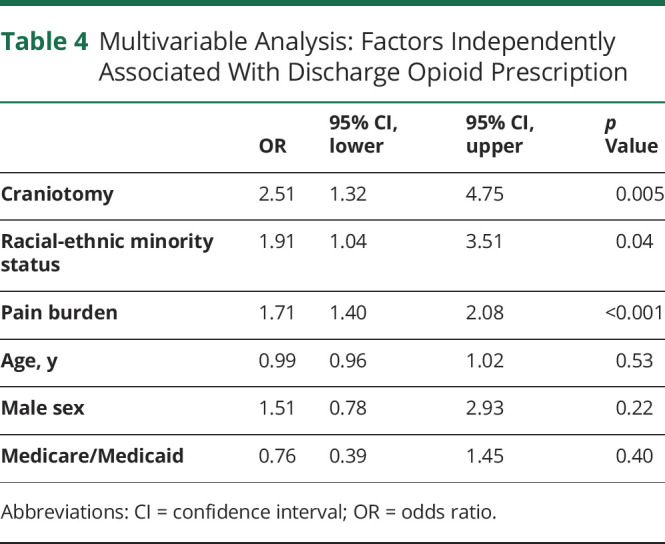
In univariate analysis, younger age, racial-ethnic minority status, craniotomy, adjunctive steroid therapy, and home discharge were all associated with continued opioid use at discharge (table 2). The pain burden for patients who continued to use opioids was significantly greater than in those who no longer required opioids (2.82 NRS vs 0.82 NRS, p < 0.001). Similarly, the maximum number of different analgesic therapies utilized during hospitalization was higher in patients with continued opioid use. We also found a difference in the average and maximum daily opioid dose during both ICU stay and total hospital stay between those who continued to use opioids at discharge and those who did not (table 3). In multivariable analysis, racial-ethnic minority status (odds ratio [OR] 1.9, 95% confidence interval [CI] 1.0–3.5), craniotomy (OR 2.5, 95% CI 1.3–4.8), and pain burden (OR 1.7, 95% CI 1.4–2.1) were associated with continued opioid use at discharge (table 4).
Table 2.
Patient Characteristics Associated With Continued Opioids at Discharge
Table 3.
Pain Burden and Analgesic Dosing Associations With Continued Opioids at Discharge
Table 4.
Multivariable Analysis: Factors Independently Associated With Discharge Opioid Prescription
Continued Opioid Use at Outpatient Follow-up
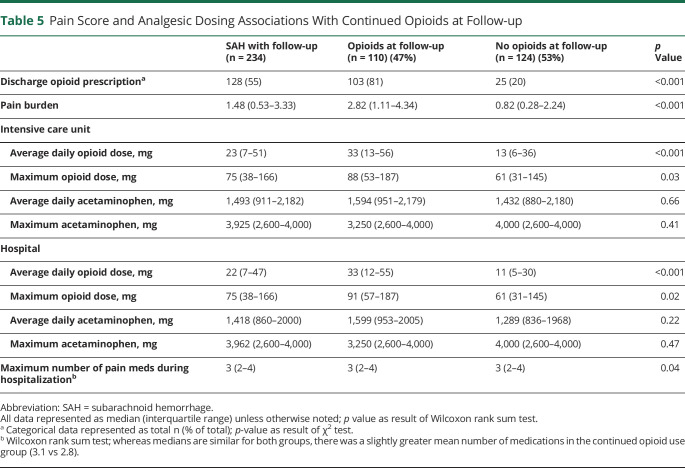
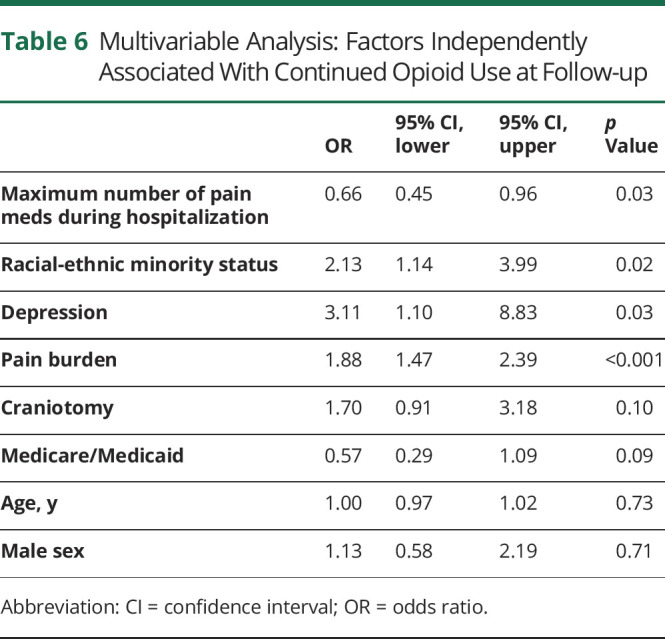
The pain burden for patients who continued to use opioids at outpatient follow-up was significantly greater than in those who no longer used opioids (2.82 NRS vs 0.82 NRS, p < 0.001). Consistent with findings at discharge, both average and maximum daily opioid dose during ICU and hospital stays as well as the maximum number of different analgesic therapies utilized during hospitalization were higher in patients with continued opioid use at outpatient follow-up (table 5). Racial-ethnic minority status (OR 2.1, 95% CI 1.1–4.0), history of depression (OR 3.1, 95% CI 1.1–8.8), and pain burden (OR 1.9, 95% CI 1.5–2.4) were associated with continued opioid use in multivariable analysis, while daily opioid dose during ICU stays and duration of hospitalization were not (table 6).
Table 5.
Pain Score and Analgesic Dosing Associations With Continued Opioids at Follow-up
Table 6.
Multivariable Analysis: Factors Independently Associated With Continued Opioid Use at Follow-up
Discussion
We found that about half of patients discharged from the hospital following aSAH required opioids at discharge and continued to use opioids over 2 months after admission. Acute pain burden, racial-ethnic minority status, and craniotomy were associated with continued opioid use at discharge, while acute pain burden, racial-ethnic minority status, and a history of depression were associated with continued opioid use at outpatient follow-up. We did not find opioid dose received during ICU stay or total hospital stay to be independently associated with long-term opioid use.
The incidence of continued opioid use in our study far exceeds previously reported rates of long-term use in other ICU and postoperative populations, but is similar to those found in some studies of traumatic brain injury.4,5,8 We speculate that patients with acute brain injury may be particularly vulnerable to central sensitization to nociceptive stimuli, leading to increased opioid use.9
Among the risk factors associated with continued opioid use at discharge in our study, pain burden and craniotomy stand out as potentially modifiable. Receipt of craniotomy was associated with opioid use at discharge, but not at outpatient follow-up. We may have lacked power to show an independent effect of craniotomy at follow-up as others have shown that pain after craniotomy is moderate to severe in up to 76% of patients within the first few days, and nearly 30% of postcraniotomy patients develop chronic headache.10,11 Although not every aneurysm may be amenable to endovascular coiling, strategies to mitigate postcraniotomy pain such as scalp nerve blocks hold promise and likely deserve further attention.12
As for continued opioid use at outpatient follow-up, our data confirm depression as an independent predictor, similar to prior studies of postsurgical populations.4 Future studies may consider tracking outpatient depressive symptoms and antidepressant use as important effect modifiers. Our model demonstrates a trend toward reduced odds of continued opioid use with increased utilization of opioid-sparing analgesics. Further research is necessary to develop effective alternative multimodal analgesic strategies including nonpharmacologic interventions. In other studies of long-term ICU follow-up, there is continued decrement in opioid use over time.5 Additional observation with longer follow-up is needed to determine whether the same holds true for aSAH.
In contrast to prior studies, we did not find an independent association between acute opioid dosage and continued opioid use. This is likely due to a ceiling effect whereby clinicians did not feel comfortable increasing opioid dosing despite poorly controlled pain in order to preserve the neurologic examination in patients with aSAH. Whereas refractory pain and opioid exposure are inextricably linked, our findings suggest that refractory pain is the driving factor in the inpatient setting for continued opioid use at discharge and beyond.
There are important limitations to this single-center analysis, including concern about generalizability to other aSAH patient populations. Although it is presumed that pain described by patients with aSAH most commonly reflects headache, retrospective review of pain scores did not allow us to consistently differentiate pain by location or character. A third key limitation is that our median follow-up time was approximately 2 months, whereas 3 months usually defines chronic opioid use.3 In addition, we relied on outpatient medication reconciliation, which may be prone to inaccuracy, as self-reported opioid use likely underestimates true chronic use.13 Larger, multicenter studies with longer follow-up and access to prescription monitoring databases are required to validate our findings. Nonetheless, our findings highlight an overlooked problem requiring further attention.
Continued opioid use following aSAH is prominent and associated with acute pain burden, but not acute opioid exposure. Novel analgesic strategies that reduce acute pain burden might reduce chronic opioid use following treatment for aSAH.
Glossary
- aSAH
aneurysmal subarachnoid hemorrhage
- CI
confidence interval
- ICU
intensive care unit
- IQR
interquartile range
- MOPAT
multidimensional objective pain assessment tool
- NRS
Numeric Rating Scale
- OR
odds ratio
- SAH
subarachnoid hemorrhage
Appendix. Authors
Footnotes
Study Funding
No targeted funding reported.
Disclosure
M.N. Jaffa, J.E. Podell, M.C. Smith, A. Foroutan, and A. Kardon, W.-T.W. Chang, M. Motta, G.Y. Parikh, K.N. Sheth, N. Badjatia, M.J. Armahizer, J.M. Simard, and N.A. Morris report no disclosures. Go to Neurology.org/N for full disclosures.
References
- 1.Steiner T, Juvela S, Unterberg A, et al. European Stroke Organization guidelines for the management of intracranial aneurysms and subarachnoid haemorrhage. Cerebrovasc Dis 2013;35(2):93–112. [DOI] [PubMed] [Google Scholar]
- 2.Orr SL, Friedman BW, Christie S, et al. Management of adults with acute migraine in the emergency department: the American Headache Society evidence assessment of parenteral pharmacotherapies. Headache 2016;56(6):911–940. [DOI] [PubMed] [Google Scholar]
- 3.Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain: United States, 2016. MMWR Recomm Rep 2016;65:1–49. [DOI] [PubMed] [Google Scholar]
- 4.Sun EC, Darnall BD, Baker LC, Mackey S. Incidence of and risk factors for chronic opioid use among opioid-naive patients in the postoperative period. JAMA Intern Med 2016;176(9):1286–1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yaffe PB, Green RS, Butler MB, Witter T. Is admission to the intensive care unit associated with chronic opioid use? A 4-year follow-up of intensive care survivors. J Intensive Care Med 2017;32(7):429–435. [DOI] [PubMed] [Google Scholar]
- 6.Weigand DL, Wilson T, Pannullo D, et al. Measuring acute pain over time in the critically ill using the multidimensional objective pain assessment tool (MOPAT). Pain Manag Nurs 2018;19(3):277–287. [DOI] [PubMed] [Google Scholar]
- 7.Hemphill JC III, Barton CW, Morabito D, Manley GT. Influence of data resolution and interpolation method on assessment of secondary brain insults in neurocritical care. Physiol Meas 2005;26:373–383. [DOI] [PubMed] [Google Scholar]
- 8.Adams RS, Thomas CP, Ritter GA, et al. Predictors of postdeployment prescription opioid receipt and long-term prescription opioid utilization among army active duty soldiers. Mil Med 2019;184(1-2):e101–e109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sahbaie P, Irvine K, Liang D, et al. Mild traumatic brain injury causes nociceptive sensitization through spinal chemokine upregulation. Sci Rep 2019;9:19500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thibault M, Girard F, Moumdjian R, Chouinard P, Boudreault D, Ruel M. Craniotomy site influences postoperative pain following neurosurgical procedures: a retrospective study. Can J Anaesth 2007;54:544–548. [DOI] [PubMed] [Google Scholar]
- 11.Rocha-Filho PA, Gherpelli JL, de Siqueira JT, Rabella GD. Post-craniotomy headache: characteristics, behaviour and effect on quality of life in patients operated for treatment of supratentorial intracranial aneurysms. Cephalgia 2008;28(1):41–48. [DOI] [PubMed] [Google Scholar]
- 12.Guilfoyle MR, Helmy A, Duane D, Hutchinson PJA. Regional scalp block for postcraniotomy analgesia. Anesth Analgesia. 2013; 116(5): 1093–1102. [DOI] [PubMed] [Google Scholar]
- 13.Hilario EY, Griffin ML, McHugh RK, et al. Denial of urinalysis-confirmed opioid use in prescription opioid dependence. J Subst Abuse Treat 2015;48(1):85–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized study data will be available to qualified investigators from the corresponding author on reasonable request.