Abstract
Objective
To quantify the association between early neurologic recovery, practice pattern variation, and endotracheal intubation during established status epilepticus, we performed a secondary analysis within the cohort of patients enrolled in the Established Status Epilepticus Treatment Trial (ESETT).
Methods
We evaluated factors associated with the endpoint of endotracheal intubation occurring within 120 minutes of ESETT study drug initiation. We defined a blocked, stepwise multivariate regression, examining 4 phases during status epilepticus management: (1) baseline characteristics, (2) acute treatment, (3) 20-minute neurologic recovery, and (4) 60-minute recovery, including seizure cessation and improving responsiveness.
Results
Of 478 patients, 117 (24.5%) were intubated within 120 minutes. Among high-enrolling sites, intubation rates ranged from 4% to 32% at pediatric sites and 19% to 39% at adult sites. Baseline characteristics, including seizure precipitant, benzodiazepine dosing, and admission vital signs, provided limited discrimination for predicting intubation (area under the curve [AUC] 0.63). However, treatment at sites with an intubation rate in the highest (vs lowest) quartile strongly predicted endotracheal intubation independently of other treatment variables (adjusted odds ratio [aOR] 8.12, 95% confidence interval [CI] 3.08–21.4, model AUC 0.70). Site-specific variation was the factor most strongly associated with endotracheal intubation after adjustment for 20-minute (aOR 23.4, 95% CI 6.99–78.3, model AUC 0.88) and 60-minute (aOR 14.7, 95% CI 3.20–67.5, model AUC 0.98) neurologic recovery.
Conclusions
Endotracheal intubation after established status epilepticus is strongly associated with site-specific practice pattern variation, independently of baseline characteristics, and early neurologic recovery and should not alone serve as a clinical trial endpoint in established status epilepticus.
Trial Registration Information
ClinicalTrials.gov Identifier: NCT01960075.
Status epilepticus (SE) is a common neurologic emergency,1-5 requiring immediate intervention to avoid increasingly refractory seizures, cardiopulmonary complications, and the potential for irreversible neuronal injury.6-8 While providers may perform endotracheal intubation to induce anesthetic coma and to facilitate definitive seizure control, intubation may alternatively be performed to secure the airway for imaging, transport, and management of hypoxemia, hypoventilation, or loss of airway protective reflexes.9-11
Clinically, the concern for airway compromise may influence practice patterns during SE management. First-line benzodiazepines are commonly underdosed,12 potentially due to perceived risks of respiratory failure, despite risk being greatest among untreated patients. In clinical trials, intubation remains an endpoint defined as treatment failure,13-15 independently of its indication, in part because evaluating neurologic activity is constrained by concomitant sedation or neuromuscular blockade. Accordingly, clinical trials aiming to develop new therapeutics to improve the early management of SE are challenging, considering that intubation rates have nearly doubled over a decade.16 In the recent Established Status Epilepticus Treatment Trial (ESETT), second-line antiseizure medication yielded seizure cessation with improvement in consciousness in only 50%, while 20% of ESETT patients failed the primary endpoint due to undergoing intubation, regardless of the indication.17,18
We hypothesized that the risk of intubation is partially explained by baseline patient factors and practice pattern variation, independently of posttreatment clinical and neurologic recovery. We therefore performed a secondary analysis within the ESETT cohort, examining 4 phases of established SE management: (1) baseline patient characteristics, (2) acute treatment factors, (3) 20-minute recovery, and (4) 60-minute recovery.
Methods
Study Design and Setting
Details of the ESETT study design have previously been published.18 In brief, patients ≥2 years of age with benzodiazepine-refractory convulsive SE were randomized in a 1:1:1 ratio to a 10-minute infusion of fosphenytoin (FOS) 20 mg/kg (maximum 1.5 g), levetiracetam (LEV) 60 mg/kg (maximum 4.5 g), or valproic acid (VPA) 40 mg/kg (maximum 3 g). Presence or absence of seizure activity was recorded at 20 and 60 minutes after initiation of the investigational protocol, as was the presence or absence of improving responsiveness. Enrollment was halted for futility after a preplanned interim analysis17 but later extended to evaluate the possibility of a most effective treatment in children18; we evaluated the final overall cohort of 478 patients enrolled at 58 hospital emergency departments across the United States between November 2015 and December 2018. Of 58 sites, 25 sites enrolled only adults and 19 sites enrolled only children.18
Standard Protocol Approvals, Registrations, and Patient Consents
Patients were enrolled under Exception From Informed Consent for Emergency Research.19 The study was approved by the Food and Drug Administration and institutional review boards of all participating sites. ESETT was registered at ClinicalTrials.gov (NCT01960075).
Candidate Risk Factors
We examined factors influencing the decision to intubate patients with established SE available to clinicians during 4 phases of care: phase 1, baseline patient characteristics available at presentation and before study drug initiation; phase 2, acute treatment factors, including study drug allocation and site-specific risk of intubation, at each institution; phase 3, 20-minute recovery (from time of study drug initiation); and phase 4, 60-minute recovery.
Phase 1 baseline factors evaluated as candidate covariates included demographic characteristics such as age, sex, race and ethnicity, history of epilepsy, acute precipitant of SE, baseline dose of first-line benzodiazepine, calculated as total weight-standardized lorazepam equivalents before study drug initiation, the duration of seizures or SE before emergency department arrival and enrollment, and standardized values of individual baseline vital signs. The z scores for heart rate, respiratory rate, and weight were derived by standardizing those values with the means and SDs of each participant's age cohort. While weight standardized by age was included, height was not reliably ascertained in the emergency setting, and accordingly, body mass index was not available as a covariate. Blood pressure was standardized on the basis of the participant's age and sex cohorts. Given that both high and low blood pressure and respiratory rates have been associated with severity in other emergency conditions,20 we evaluated statistical differences in these parameters as the absolute value of the z score while examining for associations between endotracheal intubation and individual or multiple vital signs in exploratory analyses. Because all patients in this cohort presented with generalized convulsive seizures and because coma and arousal were not reassessed until after treatment in this time-sensitive clinical trial, these components of the Status Epilepticus Severity Score21 were similar for the patients included in the study. The remaining subscore elements (age and seizure history) were thus examined as individual covariates. Data sufficient to reconstruct pediatric severity scores22 were not recorded in this cohort.
Phase 2 treatment factors evaluated as candidate covariates were site-specific intubation risk; intention-to-treat allocation to FOS, LEV, or VPA as the second-line agent study drug; and additional antiseizure medications administered within 30 minutes of second-line agent initiation. To avoid conflating benzodiazepines administered for seizure control with those administered for anesthetic induction before intubation, we excluded benzodiazepines administered either within 5 minutes of intubation or within 5 minutes of an anesthetic (propofol, ketamine, or etomidate) or midazolam administered after initial first-line therapy used an alternative benzodiazepine agent (i.e., midazolam after initial use of lorazepam or diazepam). Site-specific intubation risk was defined by ranking sites (quartiles) based on the site-specific proportion of patients undergoing intubation during the initial 120 minutes (calculated via posterior β distribution given a prior β distribution [0.25, 0.75]); this variable was in addition ascertained in a funnel plot for outliers beyond the 95% and 99% confidence intervals (CIs) for site intubation risk expected for the site-specific enrollment volume at each site and risk for the association between patient age, site-specific intubation risk, and site type (pediatric emergency department, adult emergency department, or general emergency department).
Phase 3 early treatment response candidate covariates included the presence of clinically apparent seizures at 20 minutes after second-line study drug administration, improved responsiveness to verbal or noxious stimulation at 20 minutes after second-line agent study drug administration, and the presence of cardiovascular adverse events (life-threatening hypotension or cardiac arrhythmia; censoring adverse events occurring after intubation). At the time of intubation, the presence of clinical seizures was right-censored after intubation such that patients who were seizing and then intubated before the 20-minute assessment were considered to have clinically apparent seizures at 20 minutes. Similarly, the assessment of improved responsiveness was right-censored at the time of intubation, and patients who were intubated before 60 minutes were considered not to have improved responsiveness at 60 minutes. Specifying these variables in this manner was intended as a conservative procedure to prevent overestimation of the effect of baseline patient characteristics and treatment factors at this stage.
Phase 4 late treatment response candidate covariates included clinically apparent seizures at 60 minutes after second-line agent study drug administration and responsiveness to verbal or noxious stimulation at 60 minutes. Patients met enrollment criteria for continued seizures. As above, the presence of clinical seizures was right-censored at the time of intubation such that patients who were previously seizing and then intubated before the 60-minute assessment were considered to have clinically apparent seizures at 60 minutes. Similarly, responsiveness was right-censored at the time of intubation, and patients who were intubated before 60 minutes were considered not to have improved responsive at 60 minutes. Specifying these variables in this manner was intended as a conservative procedure to prevent overestimation of the effect of baseline patient characteristics and treatment factors at this stage.
Clinical Outcome
The clinical outcome evaluated in this analysis was the binary occurrence of intubation after ESETT study drug initiation within a 120-minute time window, prespecified to limit inclusion of intubation events temporally remote from the measured covariates. To mitigate against recall bias, we evaluated treatment responses that were evaluated in real time before the decision to intubate was made.
Statistical Analysis
The primary analysis was the multivariate association of endotracheal intubation with baseline patient characteristics, treatment factors, and early recovery, available to providers at 4 phases in SE management. A stepwise multiple logistic model was performed in blocks according to information available at each of these 4 phases of care (phase 1–4). For each phase, variables were selected for the final models via Akaike informationcriterion–optimal variable selection in each iteration of 3-fold cross-validation. Before fitting of the multiple logistic model for phase 1, missing baseline data were imputed sequentially for each missing variable, assuming a joint distribution for the variables, using a fully conditional specification regression approach with all other baseline (phase 1) covariates in the model and performing 20 burn-in iterations and a single imputed dataset. Features selected during multivariate logistic regression at each phase of the model and demonstrating a significant independent association with intubation were carried forward as candidates for feature selection at subsequent phases (but not forced in as variables at subsequent phases). The R2 and area under the receiver operating curve (AUC) for the computed model at each phase were reported in addition to the multivariate-adjusted odds ratio (aOR) and 95% CI for variables meeting criteria for inclusion within each phase. This process was repeated to calculate all 4 models representing each phase of care. Bivariable and multivariable analyses were performed with SAS version 9.4; figures were generated with SAS version 9.4 or JMP Pro 15.0.0 (SAS Institute Inc, Cary, NC). Bivariate p values for binary variables were calculated from the χ2 test or Fisher exact test and from the Wilcoxon rank-sum test for nonparametric measures in exploratory analyses.
In exploratory analyses, we first examined the association between individual or combinations of baseline vital signs (standardized as z scores) and the occurrence of intubation within 120 minutes of study drug initiation. Second, we explored the association between site-specific variation in intubation rates and unique characteristics of these sites. To evaluate for the presence of outliers, we assessed for those sites in which the variation in intubation rate exceeded 95% and 99% CIs according to enrollment volume. We specifically examined for an association between age group and intubation rate, stratified by presentation to a dedicated adult, dedicated pediatric, or general emergency department. We in addition examined association between site-specific variation in intubation rates and baseline benzodiazepine dosing (total lorazepam equivalents, milligrams per kilogram) and subsequent length of stay, restricting these analyses to sites enrolling at least 15 participants. Third, among patients undergoing intubation, we measured the bivariate association between the prospectively documented reason for intubation and age both as a continuous variable and across the age groups in which efficacy was previously examined within the ESETT cohort (2–17, 18–65, and >65 years).18 Finally, we examined the unadjusted association between intubation and discharge outcomes, including the odds of requiring admission to an intensive care unit (ICU), the mean difference in ICU and hospital lengths of stay, the odds of discharge within 30 days, and the odds of all-cause in-hospital mortality.
Data Availability
A complete deidentified dataset, including individual participant data and a data dictionary defining each field in the dataset, will be made available within 1 year after publication of the primary ESETT results. Dataset requests using the National Institute of Neurological Disorders and Stroke Data Request Form should be sent to the National Institute of Neurological Disorders and Stroke Clinical Research Liaison (CRLiaison@ninds.nih.gov).
Results
Of 478 individuals meeting criteria for inclusion, 229 (47.9%) were children <18 years of age and 53 (11.1%) were adults >65 years of age. One hundred seventeen individuals (24.5%) met the analysis endpoint of intubation within 120 minutes (table 1), representing 84.2% of the 139 patients intubated within the first 24 hours (figure 1). At the 20-minute assessment, 32 participants (6.7%) had been intubated. At the 60-minute assessment, 99 participants (20.7%) had been intubated.
Table 1.
Patient Characteristics by Intubation Status (Performed or Attempted Within 120 Minutes)
Figure 1. Time to Intubation.

One hundred seventeen (24.5%) patients met the prespecified analysis endpoint of endotracheal intubation within 120 minutes of Established Status Epilepticus Treatment Trial (ESETT) study drug initiation (inset, red line), after which events were censored (y-axis signifies the proportion of participants free of endotracheal intubation). This prespecified time point captured treatment events proximal to the measured covariates while capturing 84.2% of the 139 patients undergoing endotracheal intubation over the 24 hours during which events were recorded.
In the bivariate analyses (table 1), phase 1 baseline patient characteristics significantly associated with intubation included increasing age, Black race, non-Hispanic ethnicity, and the precipitant of SE. Phase 2 acute treatment factors associated with intubation included quartile of site-specific intubation risk and randomization to FOS (compared to the VPA reference group). Bivariate analysis demonstrated that in addition to 20- and 60-minute measures of neurologic recovery, cardiovascular adverse events after study drug administration were significantly associated with intubation. In a post hoc analysis evaluating the influence of night and weekend timing on intubation, we found no association of intubation with a patient having presented during nighttime, weekend, or nonbusiness hours.
Detailed analysis of baseline vital signs (figure 2) revealed nearly complete overlap in the distribution of individual baseline vital sign parameters between patients who were and those who were not intubated (all p values not significant), as well as nearly complete overlap of multivariate clusters of baseline vital signs between patients undergoing and those not undergoing subsequent intubation, except for individual outlier patients with the combination of tachypnea, tachycardia, and a third abnormal vital sign or alternatively the singular occurrence of profound hypoxia (figure 2B).
Figure 2. Individual or Combinations of Baseline Vital Signs Do Not Differentiate Which Patients Undergo Subsequent ETI.
(A) Individual baseline vital signs are shown as overlapping violin plots for variables including heart rate (HR), respiratory rate (RR), systolic blood pressure (SBP), diastolic blood pressure (DBP), and oxygen saturation (Sao2). Violin plots depict symmetric kernel densities around a common vertical axis, where the kernel density estimated the probability density function at each point, providing a continuous analog of the univariate histogram. (B) Multivariable combinations of baseline vital signs are displayed as colored contour plots depicting clusters of 3 vital signs (x, y, and color) represented 2-dimensionally as individual patients (black dots). There is nearly complete overlap in baseline vital signs between the population of patients managed with and without subsequent intubation, with differences driven primary by individual patient outliers presenting with the combination of multiple baseline vital sign abnormalities (e.g., tachypnea, tachycardia, and a third abnormal vital sign; black circles) or the rare and isolated occurrence of profound hypoxia (red circle). Contour lines computed with Delaunay triangulation separated distinct bivariate combinations of standardized vital signs, assigning a color for the representative value of a third standardized vital sign for the subpopulation of patients between contour lines. All vital signs are standardized as age-specific z scores. ETI = endotracheal intubation.
In stepwise logistic regression (table 2), phase 1 baseline characteristics that independently predicted intubation risk included age, non-Hispanic ethnicity, acute precipitant, and total weight-standardized lorazepam equivalents (AUC 0.63). Baseline diastolic blood pressure met feature selection criteria but was not significantly associated with intubation. The phase 2 model adding acute treatment factors as candidate covariates to factors significant in the phase 1 model demonstrated an increase in the model AUC for predicting intubation (0.70); in this model, age, acute precipitant, and baseline weight-standardized lorazepam equivalents remained significantly associated with intubation, but non-Hispanic ethnicity was no longer associated with intubation after treatment factors entered the model. Of the treatment factors entering the model at phase 2, site-specific risk of intubation was the only covariate remaining significant in the multivariate analysis. Baseline patient characteristics associated with intubation risk did not explain the variation in site-specific risk. Site-specific risk of intubation was also the only baseline or treatment characteristic from phase 1 and phase 2 candidate covariates remaining in these models that adjusted for 20- and 60-minute neurologic recovery. Specifically, treatment at a site within the highest-quartile group of site-specific intubation risk was associated with intubation in the phase 2 model including treatment factors (site-specific risk aOR 8.12, 95% CI 3.08–21.4) and in phase 3 (aOR 23.4, 95% CI 6.99–78.3) and phase 4 (aOR 14.7; 95% CI 3.2–67.5) models adjusting for 20- and 60-minute neurologic recovery.
Table 2.
Multiple Logistic Regression Models Associated With Intubation at Each Phase in the Continuum of SE Management (n = 478)
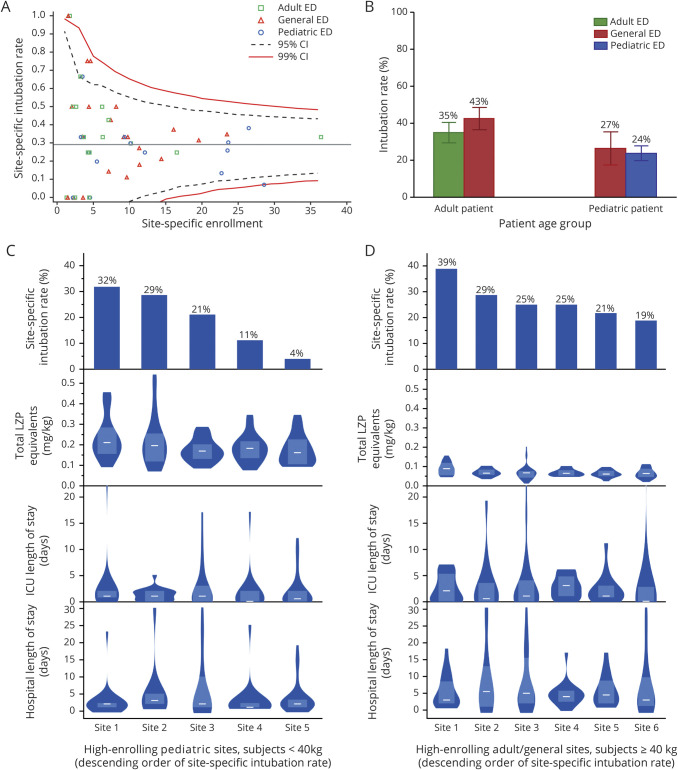
There was significant site-specific variability in intubation rates despite adjustment for enrollment volume (figure 3). Intubation rates were overall lower in children than in adults, but stratifying by age showed no differences in treatment at a dedicated pediatric, dedicated adult, or general ED. Among hospitals enrolling at least 15 patients, site-specific intubation rates varied widely, ranging between 4% and 32% among pediatric sites (analysis restricted to patients <40 kg) and between 19% and 39% among adult and general emergency department sites (analysis restricted to patients ≥40 kg). Weight-standardized doses of total lorazepam equivalents administered before study drug were greater and hospital length of stay was shorter among pediatric patients than adults, but there were no significant associations between site-specific intubation risk and benzodiazepine dosing or length of stay.
Figure 3. Site-Specific Variation and Rate of ETI.
(A, funnel plot) Risk of endotracheal intubation (ETI) includes outliers when adjusted for site enrollment volume. (B) Risk of intubation was significantly lower in pediatric patients than adults but not significantly different when adults or children specifically presented to a center with dedicated pediatric, dedicated adult, or mixed population. (C and D) Among (C) pediatric sites or (D) adult and general emergency department (ED) sites enrolling at least 15 participants, a wide range of site-specific intubation rates were evident, but there was no clear site-specific association between the rate of intubation and the distribution of total weight-standardized lorazepam (LZP) equivalents, intensive care unit (ICU), length of stay (LOS), or hospital LOS. Distributions are shown as violin plots of equal area; white lines represent medians, and shaded boxes represent interquartile ranges. To examine associations between weight-based dosing and site-specific intubation rates, this exploratory analysis restricted the analysis of high-enrolling pediatric ED sites to individuals weighing <40 kg, and at adult and general ED sites to individuals weighing ≥40 kg. CI = confidence interval.
The reason for intubation was ascertained among the 117 patients undergoing intubation within the first 120 minutes after study drug administration as respiratory depression in 41 (35.0%), decreased level of consciousness in 30 (25.6%), and persistent seizure activity in 42 (35.9%) patients (figure 4). Four (3.4%) patients underwent intubation for other cited reasons, including epistaxis, emesis, treatment of ictal-interictal continuum activity, and the combination of continued seizures and respiratory depression (1 each). Respiratory depression was the most common reason for intubation among pediatric patients 2 to 17 years of age (53%) but was the reason for intubation in only 29% of patients 18 to 65 years of age and only 6% of patients >65 years of age. Alternatively, continued seizure activity was the reason cited for intubation in 22% of pediatric patients 2 to 17 years of age but was more common among adults 18 to 65 years of age (40%) and adults >65 years of age (59%) (Fisher exact test, p = 0.007). Overall, patients intubated for respiratory depression were significantly younger (median age 11 years, interquartile range [IQR] 5–55 years) than patients intubated for decreased level of consciousness (median 42 years, IQR 7–64 years, p = 0.05) or continued seizure activity (median 46 years, IQR 17–65 years, p = 0.002). In addition, patients intubated for respiratory depression had a significantly shorter hospital length of stay (median length of stay 4 days, IQR 2–6.25 days) than patients intubated for continued seizure activity (median 7 days, IQR 3–12 days, p = 0.04), although duration of intubation itself was not collected as a primary data variable.
Figure 4. Age and Length of Stay Vary According to the Reason for ETI.
(A and B) Reason for endotracheal intubation (ETI) varied across age groups. Respiratory depression was the most commonly cited reason among patients 2 to 17 years of age (10%, n = 45) but the least commonly cited reason among patients >65 years of age (2%, n = 17). Decreased level of consciousness (LOC) and continued seizure activity were more commonly cited as reasons for endotracheal intubation among patients 18 to 65 or >65 years of age compared with patients 2 to 17 years of age. Patients intubated due to continued seizure activity (median 46 years, interquartile range [IQR] 17–65 years, p = 0.002) or decreased LOC (median 42 years, IQR 7–64 years, p = 0.05) were significantly older (B) than patients intubated due to respiratory depression (median age 11 years, IQR 5–55 years). In addition, hospital length of stay was longer (C) for patients intubated due to continued seizure activity (median 7 days, IQR 3–12 days, p = 0.04) than patients intubated due to respiratory depression (median length of stay 4 days, IQR 2–6.25 days).
Unadjusted associations between intubation and discharge outcomes (table 3) demonstrated that patients intubated in the initial 120 minutes were more likely to be admitted to the ICU (odds ratio 19.7, 95% CI 8.43–45.9) and less likely to be discharged from the hospital within 30 days (odds ratio 0.27, 95% CI 0.13–0.57). Median ICU length of stay was brief for all patients: 3 days (IQR 2–5 days) for patients intubated and 0 days (IQR 0–2 days) for those who were not intubated (mean difference 2.9 days, 95% CI 1.8–4.0). Median hospital length of stay was also longer for patients intubated (5 days, IQR 3–12 days) than those who were not intubated (2 days, IQR 1–4 days; mean difference 5.0 days, 95% CI 3.2–6.7). There was no significant difference in all-cause hospital mortality, although mortality rates were low in both groups.
Table 3.
Unadjusted Associations Between Endotracheal Intubation and Discharge Outcomes
Discussion
In this secondary analysis of a large randomized comparative effectiveness trial of 3 antiseizure medications for benzodiazepine-refractory SE, baseline patient characteristics including age, ethnicity, acute precipitant, baseline vital signs, and baseline benzodiazepine dosing were only weakly associated with endotracheal intubation. At the treatment phase and beyond, site-specific differences in intubation rates had a strong, independent association, even after adjustment for lack of early neurologic recovery. This effect was present despite lack of neurologic recovery being defined conservatively, in order to avoid overestimating effects of phase 1 baseline and phase 2 treatment factors. Site-specific outliers were identified after adjustment for enrollment, including site-specific intubation rates ranging from 4% to 32% at pediatric centers and 19% to 39% among adult centers with high enrollment. While older age was associated with intubation risk, designation as a dedicated pediatric, adult, or general ED site or benzodiazepine dosing did not clearly explain site-specific variation, nor did enrollment volume, door-to-drug time, benzodiazepine dosing, or length of stay as a proxy of disease severity.
It is not possible to determine from these analyses the causes of site-specific variation intubation risk. Unmeasured clinical factors such as baseline comorbid conditions also may have affected the decision to perform intubation, given that intubation rate was associated with the acute precipitant of SE. Finally, unmeasured structural factors such as the transport distance from a site's emergency department to the admitting unit or neuroimaging may explain this variation. Because site-specific variation was associated with intubation risk independently of covariates recorded in this clinical trial, future studies, including qualitative research, are required to determine whether site-specific risk is attributable predominantly to unmeasured clinical, structural, or practitioner factors. Trends in intubation rates among patients with SE have increased markedly over time,16 underscoring that practitioner approach may explain part of this site-specific variation. Of note, trends in intubation rates have decreased for other conditions over time, including stroke, profound agitation, and elective surgery,23-25 although intubation rates vary with patient and provider variability in these other conditions as well,23,24,26-28 in some populations with associated interactions with outcome.29
Of note, allocation in ESETT to FOS was associated with an increased risk of intubation when adjusted for several baseline and treatment factors, including age as a continuous variable. The increased rate of intubation among patients receiving FOS raises the consideration of LEV and VPA as preferable second-line agents, particularly in pediatric patients ≥2 years of age.18 However, other large randomized trials have not found similar associations.13,30 Early neurologic recovery variables, including seizure cessation and improved responsiveness at 20 minutes, were also strongly associated with intubation risk, but site-specific heterogeneity was the strongest risk factor.
There were no strong bivariate associations between the decision to perform intubation and individual baseline vital signs, nor did combinations of multiple baseline vital signs discriminate baseline physiologic phenotypes associated with the risk of subsequent intubation. While continuous physiologic data were not recorded, specific adverse events of life-threatening hypotension or arrhythmia during the phase of assessing early neurologic recovery phase were independently associated with subsequent intubation. Still, site-specific risk was associated with intubation independently of such adverse cardiac events.
Limitations in this study include ascertainment of covariates by the study team or care providers who were not blinded to the outcome; this potential for recall bias is mitigated by treatment response being evaluated in real time before the decision to intubate was made. Second, while site-specific risk itself was not associated with other significant baseline characteristics such as seizure precipitant or ethnicity, we did not have available additional data for which site may have served as a proxy, including socioeconomic status or type of insurance. In addition, the acute precipitant of SE was determined by adjudication after hospital discharge; while this information may not always have been known at admission, the highest-risk conditions—metabolic disturbances, stroke, intracerebral hemorrhage, neurologic infections, brain tumors, and toxic exposures—are often diagnosed early after presentation for many patients. Finally, we examined only associations between intubation and outcomes in bivariate models, demonstrating that these associations are similar to associations previously demonstrated.31-34
By quantifying the contribution of practice variation and treatment response on the risk of intubation, our results suggest that the influence of age and site-specific practice variation on the decision to perform intubation may exceed the influence of door-to-drug time or second-line agent choice. Data from the 20- and 60-minute phases of neurologic recovery in addition underscore previous observations that terminating seizures is associated with reduced rates of intubation, independently of benzodiazepine dosing.35 Accordingly, the association itself between total benzodiazepine dosing and intubation is likely the result of confounding by indication, in which the continued occurrence of seizures, itself a risk for subsequent intubation, requires additional doses of benzodiazepines.
Clinically, these results point to opportunities to study the clinical impact of reducing practice variation, for example, by promoting noninvasive airway management strategies.36 Considering that the vast majority of ESETT trial patients lacked baseline vital sign abnormalities, one strategy deserving future evaluation is managing mild initial respiratory depression with basic airway maneuvers before proceeding to intubation. The American Epilepsy Society guidelines recommend interventions stabilizing the airway and breathing in the first 5 minutes by prehospital or emergency personnel, but the potential remains to clarify the continued approach to the airway at later phases of care during neurologic recovery.35
From the standpoint of testing new therapeutic strategies, the design and conduct of future clinical trials for patients with SE may be influenced by our findings. First, without clinical standardization guidelines aimed at minimizing heterogeneity, variability in intubation practice patterns may obscure significant differences between interventions for SE. Second, intubation risk appears to be driven more by continued seizures and failure to show improving responsiveness than by administration of additional benzodiazepines, suggesting that future trials exploring more intensive early therapy for SE may be warranted. Third, patients undergoing intubation, particularly those intubated for respiratory depression, have a relatively short length of stay and a low mortality rate despite higher rates of precipitants including acute brain injuries, tumors, and infections, suggesting that intubation performed for transport, neuroimaging, or progression of an acute brain injury may not itself represent treatment failure.
Both clinical practice and the design of clinical trials testing candidate therapies for SE may benefit from the emergence of rapid EEG monitoring technologies.37 These tools allow emergency practitioners to differentiate patients with depressed level of consciousness who are intubated for airway protection from those intubated for continued seizure activity by directly measuring the neurophysiologic response to treatment.38 In future clinical trials, novel endpoints incorporating an ordinal “desirability of outcome” ranking39 may allow testing the tradeoff between seizure cessation and intubation related to lack of clinical or neurophysiologic improvement. These and other strategies may more precisely define the efficacy of seizure cessation by identifying more biologically based surrogates of clinical outcome.13,17,18,30
Acknowledgment
The authors wish to thank the Neurologic Emergencies Treatment Trial (NETT) and Pediatric Emergency Care Applied Research Network (PECARN) investigators who participated in the ESETT Clinical Trial.
Glossary
- aOR
multivariate-adjusted odds ratio
- CI
confidence interval
- ESETT
Established Status Epilepticus Treatment Trial
- FOS
fosphenytoin
- ICU
intensive care unit
- IQR
interquartile range
- LEV
levetiracetam
- SE
status epilepticus
- VPA
valproic acid
Appendix 1. Authors

Appendix 2. Coinvestigators
Contributor Information
Collaborators: Hannah Cock, Nathan Fountain, Barbara Dworetzky, Gail Anderson, Jeffrey Buchhalter, Elizabeth Sugar, Alexis Topjian, Peter Gilbert, and Abhi Sathe
Study Funding
Funding provided by the NIH/National Institute of Neurologic Disorders and Stroke (awards U01NS088034, U01NS088023, U01NS056975, U01NS059041, and U01NS073476).
Disclosure
E.S. Rosenthal received personal consulting fees from UCB Pharma, Inc and Ceribell, Inc. J.J. Elm, J. Ingles, A.J. Rogers, T.E. Terndrup, M. Holsti, D.G. Thomas, L. Babcock, P.J. Okada, R.H. Lipsky, J.B. Miller, R.W. Hickey, M.E. Barra, T.P. Bleck, J.C. Cloyd, R. Silbergleit, D.H. Lowenstein, L.D. Coles, J. Kapur, S. Shinnar, and J.M. Chamberlain report no disclosures relevant to the manuscript. Go to Neurology.org/N for full disclosures.
References
- 1.Betjemann JP, Lowenstein DH. Status epilepticus in adults. Lancet Neurol 2015;14:615–624. [DOI] [PubMed] [Google Scholar]
- 2.Towne AR. Epidemiology and outcomes of status epilepticus in the elderly. Int Rev Neurobiol 2007;81:111–127. [DOI] [PubMed] [Google Scholar]
- 3.Shinnar S, Pellock JM, Moshe SL, et al. In whom does status epilepticus occur: age-related differences in children. Epilepsia 1997;38:907–914. [DOI] [PubMed] [Google Scholar]
- 4.DeLorenzo RJ, Hauser WA, Towne AR, et al. A prospective, population-based epidemiologic study of status epilepticus in Richmond, Virginia. Neurology 1996;46:1029–1035. [DOI] [PubMed] [Google Scholar]
- 5.DeLorenzo RJ, Pellock JM, Towne AR, Boggs JG. Epidemiology of status epilepticus. J Clin Neurophysiol 1995;12:316–325. [PubMed] [Google Scholar]
- 6.Trinka E, Kalviainen R. 25 Years of advances in the definition, classification and treatment of status epilepticus. Seizure 2017;44:65–73. [DOI] [PubMed] [Google Scholar]
- 7.Gainza-Lein M, Sanchez Fernandez I, Jackson M, et al. Association of time to treatment with short-term outcomes for pediatric patients with refractory convulsive status epilepticus. JAMA Neurol 2018;75:410–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sutter R, Dittrich T, Semmlack S, Ruegg S, Marsch S, Kaplan PW. Acute systemic complications of convulsive status epilepticus: systematic review. Crit Care Med 2018;46:138–145. [DOI] [PubMed] [Google Scholar]
- 9.Denninghoff KR, Nuno T, Pauls Q, et al. Prehospital intubation is associated with favorable outcomes and lower mortality in ProTECT III. Prehosp Emerg Care 2017;21:539–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vohra TT, Miller JB, Nicholas KS, et al. Endotracheal intubation in patients treated for prehospital status epilepticus. Neurocrit Care 2015;23:33–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Glauser T, Shinnar S, Gloss D, et al. Evidence-based guideline: treatment of convulsive status epilepticus in children and adults: report of the Guideline Committee of the American Epilepsy Society. Epilepsy Curr 2016;16:48–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sathe AG, Tillman H, Coles LD, et al. Underdosing of benzodiazepines in patients with status epilepticus enrolled in established status epilepticus treatment trial. Acad Emerg Med 2019;26:940–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dalziel SR, Borland ML, Furyk J, et al. Levetiracetam versus phenytoin for second-line treatment of convulsive status epilepticus in children (ConSEPT): an open-label, multicentre, randomised controlled trial. Lancet 2019;393:2135–2145. [DOI] [PubMed] [Google Scholar]
- 14.Dalziel SR, Furyk J, Bonisch M, et al. A multicentre randomised controlled trial of levetiracetam versus phenytoin for convulsive status epilepticus in children (protocol): Convulsive Status Epilepticus Paediatric Trial (ConSEPT): a PREDICT study. BMC Pediatr 2017;17:152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lyttle MD, Gamble C, Messahel S, et al. Emergency treatment with levetiracetam or phenytoin in status epilepticus in children: the EcLiPSE study: study protocol for a randomised controlled trial. Trials 2017;18:283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alkhachroum AM, Rubinos C, Chatterjee A, et al. Rates and trends of endotracheal intubation in patients with status epilepticus. Neurohospitalist 2019;9:190–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kapur J, Elm J, Chamberlain JM, et al. Randomized trial of three anticonvulsant medications for status epilepticus. N Engl J Med 2019;381:2103–2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chamberlain JM, Kapur J, Shinnar S, et al. Efficacy of levetiracetam, fosphenytoin, and valproate for established status epilepticus by age group (ESETT): a double-blind, responsive-adaptive, randomised controlled trial. Lancet 2020;395:1217–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.CFR: Code of Federal Regulations Title 21. 50.24. [online]. Available at: accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=50.24. Accessed June 25, 2021. [Google Scholar]
- 20.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med 1985;13:818–829. [PubMed] [Google Scholar]
- 21.Rossetti AO, Logroscino G, Milligan TA, Michaelides C, Ruffieux C, Bromfield EB. Status Epilepticus Severity Score (STESS): a tool to orient early treatment strategy. J Neurol 2008;255:1561–1566. [DOI] [PubMed] [Google Scholar]
- 22.Goncalves JP, Severo M, Rocha C, Jardim J, Mota T, Ribeiro A. Performance of PRISM III and PELOD-2 scores in a pediatric intensive care unit. Eur J Pediatr 2015;174:1305–1310. [DOI] [PubMed] [Google Scholar]
- 23.Marcus RJ, Thompson JP. Anaesthesia for manipulation of forearm fractures in children: a survey of current practice. Paediatr Anaesth 2000;10:273–277. [DOI] [PubMed] [Google Scholar]
- 24.Cole JB, Klein LR, Nystrom PC, et al. A prospective study of ketamine as primary therapy for prehospital profound agitation. Am J Emerg Med 2018;36:789–796. [DOI] [PubMed] [Google Scholar]
- 25.Schonenberger S, Uhlmann L, Hacke W, et al. Effect of conscious sedation vs general anesthesia on early neurological improvement among patients with ischemic stroke undergoing endovascular thrombectomy: a randomized clinical trial. JAMA 2016;316:1986–1996. [DOI] [PubMed] [Google Scholar]
- 26.Yarrow S, Hare J, Robinson KN. Recent trends in tracheal intubation: a retrospective analysis of 97904 cases. Anaesthesia 2003;58:1019–1022. [DOI] [PubMed] [Google Scholar]
- 27.Gravesteijn BY, Sewalt CA, Ercole A, et al. Variation in the practice of tracheal intubation in Europe after traumatic brain injury: a prospective cohort study. Anaesthesia 2020;75:45–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Steinberg JA, Somal J, Brandel MG, et al. Site of occlusion may influence decision to perform thrombectomy under general anesthesia or conscious sedation. J Neurosurg Anesthesiol 2021;33:147–153. [DOI] [PubMed] [Google Scholar]
- 29.Bossers SM, Schwarte LA, Loer SA, Twisk JW, Boer C, Schober P. Experience in prehospital endotracheal intubation significantly influences mortality of patients with severe traumatic brain injury: a systematic review and meta-analysis. PLoS One 2015;10:e0141034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lyttle MD, Rainford NEA, Gamble C, et al. Levetiracetam versus phenytoin for second-line treatment of paediatric convulsive status epilepticus (EcLiPSE): a multicentre, open-label, randomised trial. Lancet 2019;393:2125–2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kowalski RG, Ziai WC, Rees RN, et al. Third-line antiepileptic therapy and outcome in status epilepticus: the impact of vasopressor use and prolonged mechanical ventilation. Crit Care Med 2012;40:2677–2684. [DOI] [PubMed] [Google Scholar]
- 32.Marchi NA, Novy J, Faouzi M, Stahli C, Burnand B, Rossetti AO. Status epilepticus: impact of therapeutic coma on outcome. Crit Care Med 2015;43:1003–1009. [DOI] [PubMed] [Google Scholar]
- 33.Sutter R, De Marchis GM, Semmlack S, et al. Anesthetics and outcome in status epilepticus: a matched two-center cohort study. CNS Drugs 2017;31:65–74. [DOI] [PubMed] [Google Scholar]
- 34.Sutter R, Marsch S, Fuhr P, Kaplan PW, Ruegg S. Anesthetic drugs in status epilepticus: risk or rescue? A 6-year cohort study. Neurology 2014;82:656–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alldredge BK, Gelb AM, Isaacs SM, et al. A comparison of lorazepam, diazepam, and placebo for the treatment of out-of-hospital status epilepticus. N Engl J Med 2001;345:631–637. [DOI] [PubMed] [Google Scholar]
- 36.Racca F, Vianello A, Mongini T, et al. Practical approach to respiratory emergencies in neurological diseases. Neurol Sci 2020;41:497–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vespa PM, Olson DM, John S, et al. Evaluating the clinical impact of rapid response electroencephalography: the DECIDE multicenter prospective observational clinical study. Crit Care Med 2020;48:1249–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cisse FA, Osman GM, Legros B, et al. Validation of an algorithm of time-dependent electro-clinical risk stratification for electrographic seizures (TERSE) in critically ill patients. Clin Neurophysiol 2020;131:1956–1961. [DOI] [PubMed] [Google Scholar]
- 39.Evans SR, Rubin D, Follmann D, et al. Desirability of outcome ranking (DOOR) and response adjusted for duration of antibiotic risk (RADAR). Clin Infect Dis 2015;61:800–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
A complete deidentified dataset, including individual participant data and a data dictionary defining each field in the dataset, will be made available within 1 year after publication of the primary ESETT results. Dataset requests using the National Institute of Neurological Disorders and Stroke Data Request Form should be sent to the National Institute of Neurological Disorders and Stroke Clinical Research Liaison (CRLiaison@ninds.nih.gov).