Abstract
Objective
To determine whether patients in the community with lumbosacral radiculoplexus neuropathy (LRPN) have milder neuropathy than referral patients, we characterized the outcomes and survival of population-based compared to referral-based LRPN cohorts.
Background
Previously, we found that the incidence of LRPN is 4.16/100,000/y, a frequency greater than other inflammatory neuropathies. The survival of patients with LRPN is uncharacterized.
Methods
Sixty-two episodes in 59 patients with LRPN were identified over 16 years (2000–2015). Clinical findings were compared to previous referral-based LRPN cohorts. Survival data were compared to those of age- and sex-matched controls.
Results
At LRPN diagnosis, median age was 70 years, median Neuropathy Impairment Score (NIS) 22 points, 92% had pain, 95% had weakness, 23% were wheelchair-bound, and median modified Rankin Scale score (mRS) was 3 (range 1–4). At last follow-up, median NIS improved to 17 points (p < 0.001) with 56% having ≥4 points improvement, 16% were wheelchair-bound, and median mRS was 2. Compared to referral-based LRPN cohorts, community patients with LRPN had less impairment, less bilateral disease (37% vs 92%), and less wheelchair usage (23% vs 49%). LRPN survival was 86% at 5 years and 55% at 10 years. Compared to age- and sex-matched controls, patients with LRPN had 76% increased risk of death (p = 0.016). In multivariate analysis, diabetes, age, stroke, chronic kidney disease, peripheral artery disease, and coronary artery disease were significant mortality risk factors but LRPN was not.
Conclusion
LRPN is a painful, paralytic, asymmetric, monophasic, sometimes bilateral pan-plexopathy that improves over time but leaves patients with impairment. Although having LRPN increases mortality, this increase is probably due to comorbidities (diabetes) rather than LRPN itself.
Lumbosacral radiculoplexus neuropathy (LRPN) is an immune-mediated neuropathy that typically starts unilaterally with pain, followed by weakness, usually in proximal slightly more than distal lower extremity segments.1-3 Over time, it spreads to involve initially unaffected leg or thigh, often progresses to the contralateral lower extremity, and sometimes affects upper limb and truncal nerves.3-9
LRPN can be divided into diabetic (DLRPN) and nondiabetic (NDLRPN) subtypes and begins subacutely in an asymmetrical pattern and affects (to variable degrees) lumbosacral nerve roots, lumbosacral plexus, and peripheral nerves (radiculoplexus neuropathy), becoming more widespread with time.1 Nerve conduction studies (NCS) usually show reduced motor and sensory potential amplitudes (axonal loss) and EMG shows denervation and chronic neurogenic changes in muscles innervated by multiple nerve roots and peripheral nerves (including lumbosacral paraspinal muscles).3,4,9-11 LRPN nerve pathology shows active axonal degeneration, nerve ischemia, inflammatory infiltrates, and microvasculitis3,12-15 with upregulation of inflammatory mediators.16 LRPN is considered a variant of nonsystemic vasculitic neuropathy.17
Recently, we found the incidence of LRPN in Olmsted County, Minnesota,6 to be 4.16/100,000/y (almost 3 times the incidence of chronic inflammatory demyelinating polyradiculoneuropathy,18 Guillain-Barré syndrome,19 or inflammatory brachial plexus neuropathy20 in the same population). Diabetic patients were 8 times more likely to develop LRPN than were nondiabetic patients.6
Herein we systematically characterize the outcomes of Olmsted County, Minnesota, residents with LRPN and compare these findings to previously published referral-based cohorts of DLRPN3 and NDLRPN.10 Furthermore, we present long-term survival data of patients with LRPN compared to survival data for age- and sex-matched control patients.
Methods
This study was conducted as part of the LRPN epidemiology study performed on the Olmsted County, Minnesota, population that determined the incidence of LRPN and its association with diabetes.6 This study used the facilities of the Rochester Epidemiology Project (REP). We searched for all possible cases of LRPN during a 16-year period (from January 1, 2000, through December 31, 2015) with an Olmsted County address using the central diagnostic index at Mayo Clinic and the REP. A total of 59 patients with LRPN with 62 neuropathy episodes were identified (this is the same cohort that we are studying here). Details about REP21 and our study inclusion criteria can be found in detail elsewhere.6 Briefly, LRPN is defined as (1) clinical symptoms of lower limb pain, weakness, and sensory loss in keeping with LRPN with neurologic examination findings beyond a single nerve or root distribution; (2) NCS/EMG in keeping with an axonal disorder of lumbosacral segments involving at least 2 nerves from at least 2 different root levels with paraspinal denervation accepted; and (3) evaluation by a neurologist and exclusion of other structural and nonstructural causes. Three control patients without LRPN living in Olmsted County were matched to each patient with LRPN based on age and sex. This control group served as a reference cohort for the prevalence of diabetes in the previous study and in the current study was used for survival analysis and mortality risk factors.
Neuropathy Characteristics, Severity, and Disability
Data were extracted from the medical records. The Neuropathy Impairment Score (NIS) was extracted from the Mayo Clinic neurologic examinations. The NIS comprises scores of muscle weakness (range 0–192), decrease of muscle stretch reflexes (range 0–20), and abnormality of 4 sensory modalities (touch, pin, vibration, and proprioception) in the toes and fingers (range 0–32); the total NIS score may vary from 0 to 244.22 The level of disability of the neuropathy was graded using the modified Rankin Scale score (mRS) and by whether patients used gait aids (cane, walker, or wheelchair). The mRS ranges from 0 (no symptoms) to 6 (dead).23 We only extracted mRS scores from neurologic clinical visits. We determined the neurologic symptoms and findings on neurologic examination and the nerve segments affected from 3 different time points: at onset, at diagnosis, and at last neurologic follow-up. We categorized the temporal profile from symptom onset to nadir as hyperacute (<24 hours), rapidly progressive (1 day–1 month), and subacute to chronic (>1 month).
Ancillary Testing
NCS/EMG were performed on all patients at the time of LRPN diagnosis using Mayo Clinic laboratory normal values24 and using Nicolet Viking EDX (Natus Neurology, Madison, WI) and Cadwell Sierra Summit (Cadwell Industries, Inc., Kennewick, WA) machines. NCS/EMG studied were selected by the performing electromyographer according to patients' symptoms and findings. Pelvic (lumbosacral plexus) MRI was performed in 31 patients and independently re-reviewed by an experienced peripheral nerve radiologist (B.M.H.), masked to the clinical findings. Lumbosacral plexus abnormalities were classified as abnormal by T2-weighted signal hyperintensity, nerve enlargement, and postgadolinium contrast enhancement. T2-signal abnormality was determined by comparison with normal adjacent skeletal muscle on T2–fat-saturated images with increased relative signal in the nerve considered abnormal. Nerve enlargement was determined with comparison to perceived normal nerves on the MRI. When postgadolinium fat-saturated images were available, the nerves were evaluated for enhancement, with any enhancement considered abnormal.
Survival
All patients with LRPN, even those without neurologic follow-up, continued to live in Olmsted County, and had ongoing medical care provided there by either Olmsted Medical Center (OMC) or by Mayo Clinic after the diagnosis of LRPN was made. Therefore, survival data could be extracted from the LRPN and control patients from Mayo Clinic or OMC medical charts. Data cutoff determined was April 30, 2018. Comorbidities were recorded at the time of diagnosis of LRPN and at the time of closest medical visit for the matched control patient. These data were used to estimate mortality risk factors.
Statistics
Categorical variables are presented as n (%) and differences between groups were compared using χ2/Fisher exact test. Continuous variables are summarized as median (range) and comparison between the groups was done using Kruskal-Wallis test. Group statistics for matched pairs were tested with Wilcoxon signed-rank test. Overall survival was defined as time from diagnosis to death or last contact with a Mayo Clinic or OMC provider. Survival rates after diagnosis were estimated using the Kaplan-Meier method and compared using the log-rank test. Univariate and multivariable Cox-proportional hazard model were used to access the effect of comorbidities on survival differences. SAS (version 9.3) and JMP (version 14, SAS Institute) were used for all statistical analysis. All the tests were 2-sided and p value <0.05 was considered significant.
Standard Protocol Approvals, Registrations, and Patient Consents
The study participants signed informed consent forms authorizing use of their medical records for research and the institutional review boards approved the study protocol.
Data Availability
Fully anonymized data will be shared by request from any qualified investigator.
Results
Demographics and Neuropathy Characteristics
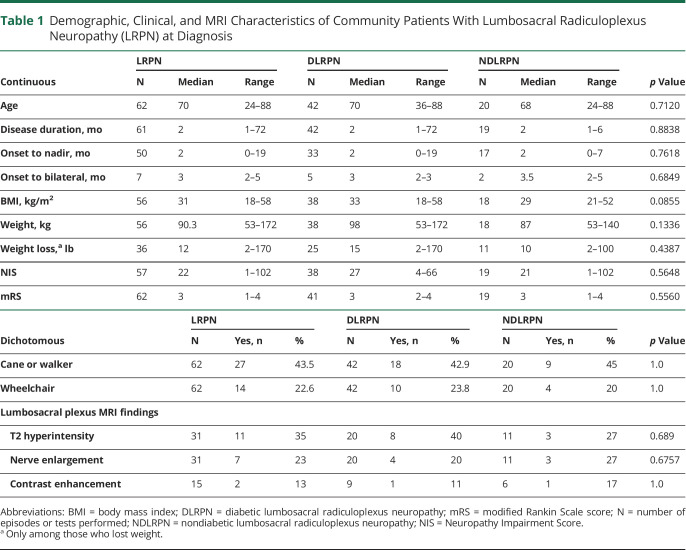
As we previously reported, 59 patients with 62 LRPN episodes were identified from Olmsted County, Minnesota, over a 16-year period.6 The median age of patients with LRPN was 70 (24–88) years, and 56% (n = 33) were male (table 1). At time of diagnosis, pain occurred in 57/62 (91.8%) episodes, weakness in 59/62 (95%) episodes, and the disease was bilateral in 23/62 (37.1%) cases. Thirty-nine patients with LRPN had diabetes, all of whom had type 2 diabetes, except for 2 patients who had type 1 diabetes.
Table 1.
Demographic, Clinical, and MRI Characteristics of Community Patients With Lumbosacral Radiculoplexus Neuropathy (LRPN) at Diagnosis
New data extracted from the population-based LRPN cohort shows that at time of diagnosis, tingling occurred in 34/57 (60%), sensory loss (numbness) in 33/57 (56%), and autonomic symptoms in 12/57 (21%). LRPN started bilaterally in 16/62 episodes (25.8%) and in another 7/46 (15.2%) episodes there was progression to bilateral disease by time of the LRPN diagnosis. In 3 patients, the contralateral limb only started to be affected after the original limb started to improve. The temporal profile from symptom onset to nadir was hyperacute in 2/62 (3.2%) episodes, rapidly progressive in 15/62 (24.2%), subacute to chronic in 41/62 (66.1%), and unclear in 4/62 episodes (6.5%). In 50/57 (87.7%) episodes, the pain was reported to be severe (usually burning, lancinating, or deep aching) or producing contact allodynia (normal touch perceived as pain), requiring opioids for pain control in 35/57 (61.4%) episodes. More than half of the patients (35/62, 56.4%) had both proximal (hip/thigh) and distal (foot/leg) lower extremity weakness (evidence of both upper and lower lumbosacral plexus involvement—pan-plexopathy). Weight loss and use of some form of gait aid (67.6%) was common (table 1). There were no significant demographic or neuropathy characteristic differences between the patients with DLRPN and NDLRPN (table 1).
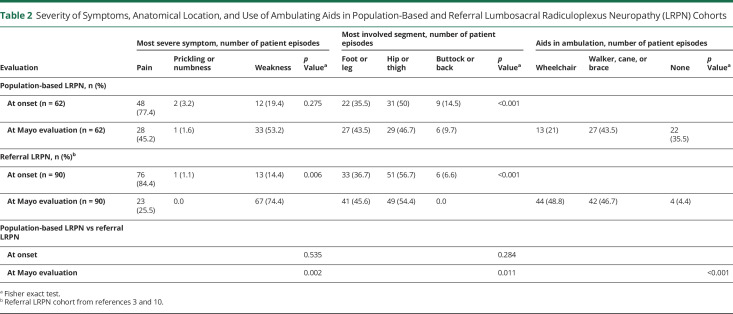
In order to understand whether the disease characteristics are similar in a population-based cohort to what has been previously described, we compared the Olmsted County population-based LRPN cohort to 2 previously published referral-based cohorts evaluated at Mayo Clinic of both DLRPN3 and NDLRPN.10 For simplicity of presentation, we combined the DLRPN and NDLRPN referral-based cohorts' data together into the broader category of LRPN (as we had done for the population-based cohort). The disease began unilaterally in most cases: in the population-based cohort, 46/62 (74.1%) had unilateral onset, whereas in the referral LRPN cohort, 79/90 (87.8%) had unilateral onset (p = 0.051). Having bilateral disease at time of diagnosis was significantly less frequent in the population-based cohort than in the referral-based cohort (23/62 [37.1%] vs 83/90 [92.2%]; p < 0.001). The primary neurologic symptom at disease onset was pain, followed by weakness for both cohorts (table 2). This pattern changed by the time of evaluation and weakness had surpassed pain as the most severe symptom in both cohorts, although having weakness as the most severe symptom was only significant for the referral cohort (p = 0.002) (table 2). The neurologic distribution of involvement was similar for both the population-based and the referral cohorts, with most patients having both hip/thigh and leg/foot segment involvement (pan-plexopathy). Furthermore, in both cohorts, the hip/thigh (upper plexus) segment was the most severely affected segment somewhat more frequently than the foot/leg (lower lumbar plexus) segment and this hip/thigh predominance occurred at both time points (onset and Mayo evaluation) (table 2). Although weight loss occurred in both cohorts, losing more than 10 pounds was significantly more frequent in the referral LRPN cohorts than in the population-based one (70/90 [77.8%] vs 20/62 [32.3%]; p < 0.001). The neurologic impairment was less for the population-based cohort and the median NIS of the population-based cohort was 22 points (1–102), whereas for the LRPN referral cohort the median NIS was 41.0 (6–106.3) (p < 0.001). Gait aids (cane or walker) were used at similar rates for both groups—27/62 (43.5%) in the population-based cohort and 42/90 (46.6%) (p = 1.0) for the referral cohort—but wheelchairs were used less frequently in the population-based cohort (14/62, 22.6%) than in the referral cohort (44/90, 48.9%) (p < 0.001) (table 2). Although LRPN predominantly affected lower limb nerves in all cohorts, occasionally upper limb cervical radiculoplexus neuropathies (RPN) or thoracic radiculopathies occurred. Cervical RPN was more frequently found in the referral cohort (9/90 [10.0%]) than in the population-based cohort (1/62 [1.61%]) (p = 0.049) but thoracic radiculopathy was found in similar frequencies in both groups: 13/90 (14.4%) in the referral and 8/62 (12.9%) in the population-based cohort (p = 1.0).
Table 2.
Severity of Symptoms, Anatomical Location, and Use of Ambulating Aids in Population-Based and Referral Lumbosacral Radiculoplexus Neuropathy (LRPN) Cohorts
Electrodiagnostic Findings
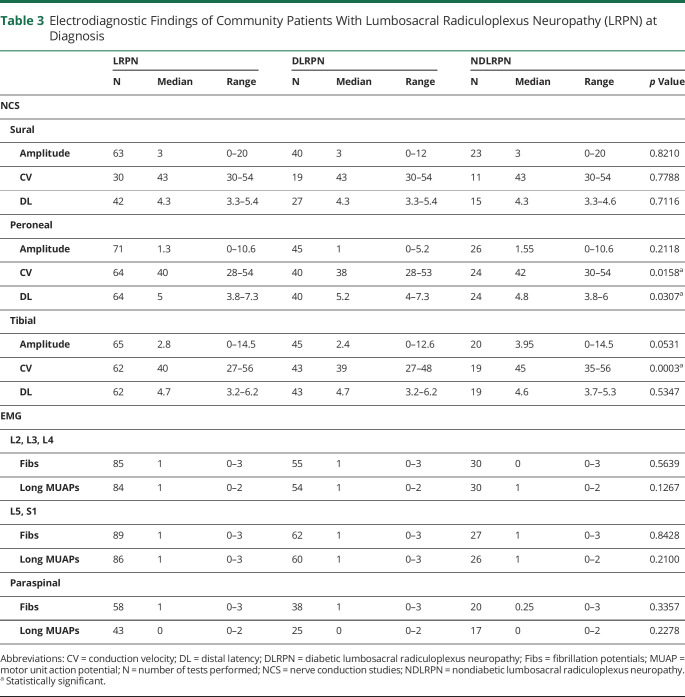
NCS of the population-based LRPN cohort showed low sural sensory nerve action potential amplitudes and low peroneal and tibial compound muscle action potential amplitudes (table 3). Sensory and motor conduction velocities (CVs) and distal latencies were normal or only mildly slow or prolonged. Needle EMG of the population-based LRPN cohort demonstrated involvement of L2–L4 (upper plexus) in 48/62 (77%) and of L5–S1 (lower plexus) myotomes in 57/62 (92%) episodes with fibrillation potentials or long motor unit potentials (MUPs). Neurogenic involvement of both upper and lower plexus myotomes occurred in 44/62 (71%) studies. EMG of lumbosacral paraspinal muscles showed fibrillation potentials in 39/58 (67.2%) and long MUPs in 17/42 (40.4%). Patients with DLRPN had slower peroneal and tibial CVs (p = 0.0166 and p = 0.0003, respectively) and more prolonged peroneal distal motor latencies (p = 0.031) compared to patients with NDLRPN. Overall, the electrodiagnostic findings in the diabetic and nondiabetic cohorts were similar with the diabetic patients having somewhat worse abnormalities.
Table 3.
Electrodiagnostic Findings of Community Patients With Lumbosacral Radiculoplexus Neuropathy (LRPN) at Diagnosis
Lumbosacral Plexus MRI Findings
A total of 31 population-based patients with LRPN underwent lumbosacral plexus (pelvis) MRI. The lumbosacral plexus was abnormal in 11/31 (35.5%) patients (figure 1). All 11 abnormal MRIs of lumbosacral plexus had increased T2-weighted signal within the plexus. A total of 7/31 had nerve enlargement and 2/15 had abnormal contrast enhancement (table 4). The distribution of the abnormalities was usually diffusely throughout the plexus (9/11). No differences were found comparing DLRPN vs NDLRPN.
Figure 1. Lumbosacral Plexus MRI Findings.
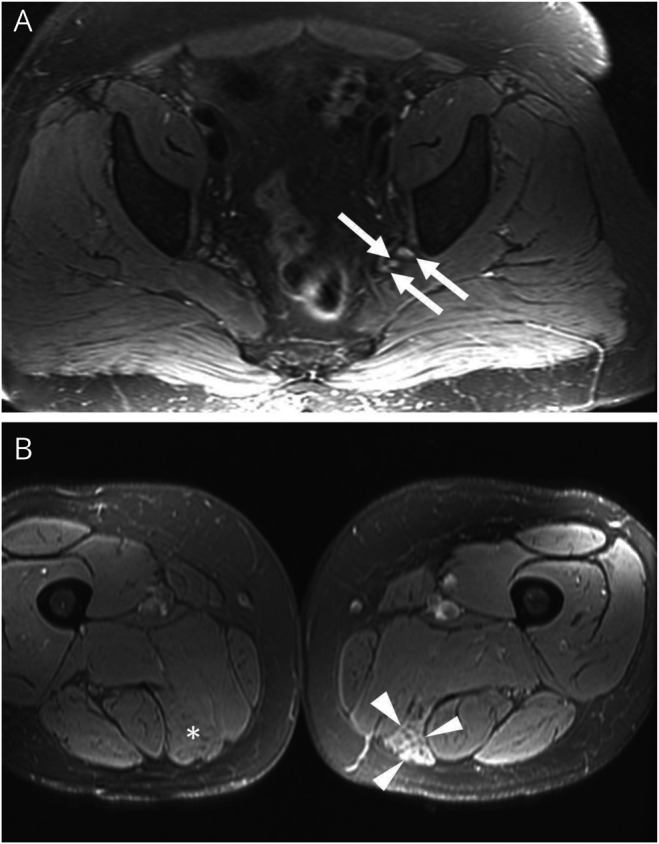
(A) Axial T2-weighted fat-saturated image of the pelvis from a patient with lumbosacral radiculoplexus neuropathy demonstrates mild diffuse enlargement and T2-weighted hyperintensity of the lumbosacral nerves, most notable just proximal to the greater sciatic notch (arrows). These are nonspecific findings that may be associated with nerve inflammation. (B) Axial T2-weighted fat-saturated image of the thighs demonstrates increased intramuscular T2-weighted signal of the left semimembranosus (arrowheads). The left semimembranosus is also mildly atrophic compared to the contralateral thigh on the same image (asterisk). The findings are compatible with denervation change.
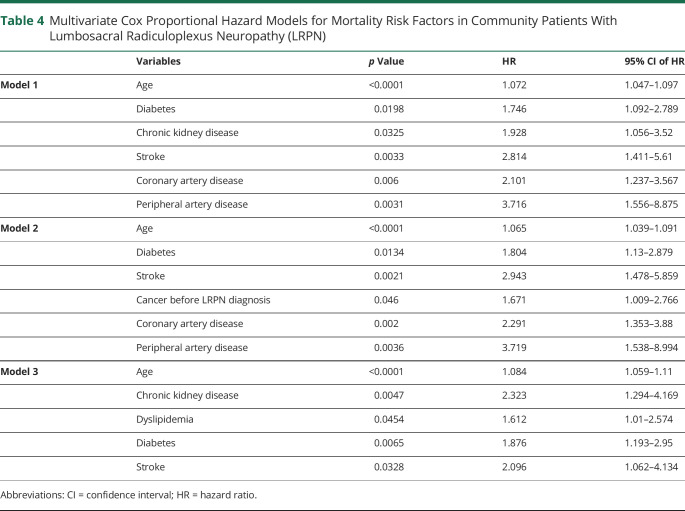
Table 4.
Multivariate Cox Proportional Hazard Models for Mortality Risk Factors in Community Patients With Lumbosacral Radiculoplexus Neuropathy (LRPN)
Neurologic Follow-up
A total of 44 population-based patients with LRPN had at least one follow-up visit with a neurologist more than 3 months after the LRPN diagnosis. The median time from LRPN diagnosis to last neurologic follow-up was 8 (4–49) months. At last neurologic follow-up, 36/44 (81%) patients had ongoing weakness, 19/44 (43%) pain, 13/41 (31.7%) tingling, and 16/41 (39%) sensory loss. The median time from neuropathy onset to the beginning of neurologic recovery was 2 months (range 0–14) for pain (n = 41) and 3 months (range 1–12) for weakness (n = 29). At last neurologic follow-up, the most severe symptom was weakness in 24/44 (50%), followed by pain in 14/44 (31.8%), and tingling/numbness in 4 (9.1%) patients. At last neurologic follow-up, the most severe anatomical site of involvement was equal for upper lumbar plexus and lower lumbosacral plexus: the hip or thigh was most involved in 19/44 (43.1%), the foot or leg in 19/44 (43.1%), and the buttock or back in 4/44 (9.1%) patients. Only 2 patients were completely symptom-free with normal neurologic examination (both had NDLRPN). At last neurologic follow-up, 7/44 (15.9%) remained wheelchair-bound, 12/44 (27.3%) still needed gait aids (cane or walker) to ambulate, and 25/44 (56.8%) were walking independently. No differences between DLRPN and NDLRPN were seen in severity of symptoms, anatomic location, and use of ambulating aids at onset, diagnosis, or last follow-up.
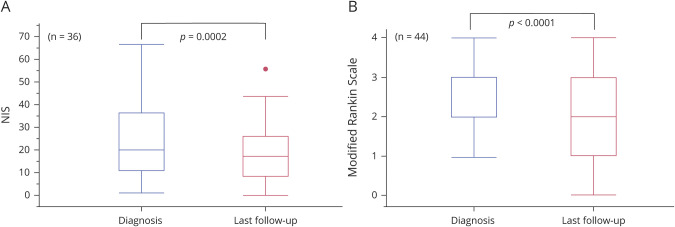
Median NIS at last follow-up neurology visit (available for 36 patients) improved overall from 20 to 17 points (p = 0.0002) (figure 2). A total of 20/36 (55.6%) had improvement of ≥4 NIS points and only 2/36 (5.6%) had worsening of NIS of ≥4 points at last follow-up visit. The median mRS improved from 3 to 2 (0–4) (p < 0.0001) (figure 2). In 16/44 (36.3%), mRS improved 1 point, in 6/44 (13.6%) it improved 2 points, in 21/44 (47.7%) it did not change, and in 1 patient the mRS was worse by 1 point at last neurologic follow-up visit.
Figure 2. Matched Comparison of Neuropathy Impairment Score (NIS) and Modified Rankin Scale Score (mRS) of Community Patients With Lumbosacral Radiculoplexus Neuropathy From Diagnosis to Last Follow-up With Neurologist.
(A) NIS. (B) mRS.
In 16/62 (25.8%) episodes of LRPN, the patients received immunotherapy. IV methylprednisolone was given in 13/16, oral prednisone in 1/16, and IV immunoglobulin in 2/16 episodes. Patients who received immunotherapy had a higher median NIS at time of diagnosis (more impairment) than nontreated patients (27.5 vs 21; p = 0.894) but this difference was not significant. No demographic or neuropathy characteristic was significantly different between treatment and nontreatment groups. The median change in NIS and median change in mRS were not different between treatment vs nontreatment groups. No patient developed severe adverse effects from the immunotherapy.
Long-term Prognosis, Survival, and Mortality Risk Factors
The median time from diagnosis to last follow-up with any community health care provider or to death for population-based patients with LRPN was 7.1 years. At final community follow-up, 6 patients had been diagnosed with an autoimmune disorder that developed after the episode of LRPN: Hashimoto thyroiditis (n = 1), Hashimoto thyroiditis and polymyalgia rheumatica (n = 1), vitiligo (n = 1), bullous pemphigoid (n = 1), Graves disease (n = 1), and CREST scleroderma (n = 1). The patient with CREST (calcinosis, Raynaud phenomenon, esophageal dysmotility, sclerodactyly, and telangiectasia) scleroderma presented with Raynaud, sclerodactyly, dysphagia, and esophagitis 11 years after the diagnosis of LRPN. Interestingly, this patient had a recurrence of LRPN 1 year after the CREST diagnosis and was treated with IV methylprednisolone with marked improvement of her symptoms (this recurrence was not included in our analysis as it happened after the time of study).
Half of the patients with NDLRPN had glucose impairment (10/20) at time of LRPN and 2/20 (10%) of them eventually developed type 2 diabetes (after their episodes of LRPN). No patient with LRPN developed systemic vasculitis. No patient with LRPN was diagnosed with cancer (other than skin) within 5 years of the LRPN episode.
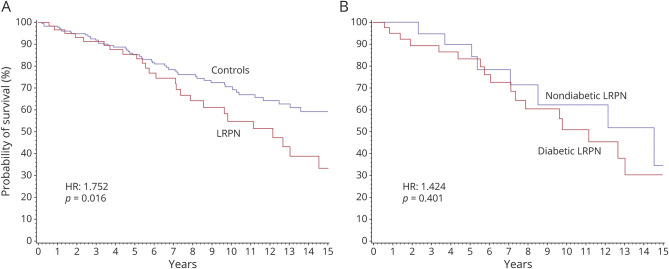
At the time of data cutoff, 27/62 patients with LRPN were deceased (45.8%). The median overall survival among patients with LRPN was 12.2 years. The causes of death included cancer (n = 7), pneumonia (n = 6), myocardial infarction (n = 3), heart failure (n = 2), advanced dementia (n = 1), stroke (n = 1), bowel ischemia (n = 1), ruptured abdominal aortic aneurysm (n = 1), renal failure (n = 1), and unknown (n = 4). No death was directly related to the LRPN. The probability of survival in patients with LRPN at 5 and 10 years was 86% and 55%, respectively. Survival was significantly worse in patients with LRPN compared to age- and sex-matched controls (median survival 12.2 vs ≥17 years; p = 0.0182). Patients with LRPN had a 76% increased risk of death compared to age- and sex-matched controls (p = 0.0164) (figure 3). Although there was a 42% increased risk of death in DLRPN vs NDLRPN, this was not statistically significant and the median survival was not different (11.1 vs 14.5 years; p = 0.401) (figure 3). Mortality risk factors in univariate analysis were female sex (HR 0.547 [95% CI 0.343–0.874]; p = 0.0116), age (HR 1.087 [1.063–1.112]; p < 0.0001), chronic kidney disease (5.418 [3.054–9.612]; p < 0.0001), hypertension (HR 2.296 [1.446–3.646]; p = 0.0004), diabetes (HR 2.512 [1.603–3.937]; p < 0.0001), coronary artery disease (HR 4.998 [3.097–8.066]; p < 0.0001), heart failure (HR 4.035 [2.205–7.383]; p < 0.0001), stroke (HR 3.91 [2.002–7.664]; p < 0.0001), peripheral artery disease (HR 5.94 [2.554–13.818]; p < 0.0001), dyslipidemia (HR 1.86 [1.187–2.914]; p = 0.0068), cancer (HR 3.534 [2.199–5.679]; p < 0.0001), and LRPN (HR 1.756 [1.102–2.799]; p = 0.0179). In multivariate analysis, age, diabetes, and stroke were mortality risk factors in all the 3 statistical models, while chronic kidney disease, coronary artery disease, and peripheral vascular disease were mortality risk factors in 2 models (table 4). Importantly, having had an episode of LRPN was not an independent mortality risk factor in the multivariate analysis. In addition, the survival curves of patients with DLRPN and age- and sex-matched controls with diabetes was not statistically different.
Figure 3. Kaplan-Meier Survival Curves.
(A) Controls (blue) vs patients with lumbosacral radiculoplexus neuropathy (LRPN) (red). (B) Nondiabetic LRPN (blue) vs diabetic LRPN (red). HR = hazard ratio.
Discussion
Although previous studies have characterized the long-term neurologic outcomes of patients with LRPN, they have all used referral-based cohorts and have not studied a population-based setting.3,10 Here, using a population-based cohort, we have shown that LRPN typically presents as an acute to subacute painful lower extremity neuropathy involving the upper lumbar plexus (hip/thigh) slightly more frequently than the lower plexus (leg/foot). Over time, severe pain is followed by weakness so that weakness subsequently becomes the most debilitating problem. LRPN is accompanied by weight loss commonly but less frequently than previously reported. At the time of LRPN diagnosis, most patients have involvement of both the upper and lower lumbosacral plexus (both hip/thigh and foot/leg segments) by both clinical examination and EMG findings producing a pan-plexopathy with proximal and distal lower extremity involvement. In more than one-third of cases (37.1%), the disease involves both lower limbs. Occasionally, coexisting thoracic radiculopathies (12.9%) or cervical radiculoplexus neuropathies (1.6%) are present and approximately one-fourth of patients with LRPN are wheelchair-bound at the time of diagnosis.
Our findings suggest that while the overall clinical features are very similar, there are some differences in the severity and distribution between the population-based LRPN cohort and the referral-based LRPN cohorts (DLRPN and NDLRPN).3,10 In both community and referral cohorts, we find that LRPN starts focally with pain (in the upper slightly more frequently than the lower lumbar plexus), typically followed by weakness most often involving both the upper and lower plexus segments. The most important differences when comparing these cohorts is that community LRPN is not as severe of an illness as is referral-based LRPN, but the overall clinical presentation and distribution of the neuropathy is largely the same. This more mild presentation is demonstrated by community patients with LRPN having significantly lower NIS scores (22.0 vs 43.0), less frequent bilateral disease (37.1% vs 92.2%), less frequent associated weight loss (32.2% vs 77.8%), and less wheelchair use (21.6% vs 48.8%). These differences convey an important message—that patients referred to Mayo Clinic and who are willing to travel for LRPN evaluation are more severely affected. Therefore, the information collected from the population-based cohort provides a better approximation of what an average case of LRPN looks like. In a few of our community cases, LRPN was a mild unilateral lower extremity neuropathy with little impairment that could have been easily misdiagnosed as a structural radiculopathy. Careful electrodiagnostic examination and review of lumbosacral spine MRI are important to differentiate structural radiculopathy from LRPN and to avoid unnecessary spinal surgeries. The presence of allodynia, dense sensation loss, associated weight loss, and weakness involving several lumbosacral myotomes are important clues that may help differentiate LRPN from structure radiculopathy.1,8,25,26 Our study confirms that most LRPN cases improve over time (are monophasic) but some patients are left with problematic pain, weakness, sensory loss, and functional impairment. Complete recovery is rare, occurring in only 2 patients with NDLRPN in our population-based cohort.
Our study showed that one-third of patients with LRPN had an abnormality on lumbosacral plexus MRI. The most common abnormality was T2-weighted signal hyperintensity of the lumbosacral plexus, followed by nerve enlargement. Most often the abnormality was diffuse. These abnormalities are similar but less frequent than previous radiculoplexus neuropathy studies have found. A study using conventional MRI with IV contrast and diffusion tensor imaging (DTI) sequences from lumbar spine to distal thigh27 found increased T2 signal or enlargement in 11/13 patients with DLRPN and mean fractional anisotropy was decreased in DLRPN nerves compared to controls. DTI is not routinely done in our clinical practice. In a study of postsurgical inflammatory neuropathy (many with LRPN), abnormal MRI (mostly increased T2-weighted signal and nerve enlargement) of roots, plexus, or nerves in 17/17 patients was found.28 In diabetic cervical radiculoplexus neuropathy, abnormal brachial plexus MRI was found (mostly increased T2 signal and nerve enlargement) in almost all (47/48) cases.5 The reason we found fewer lumbosacral plexus MRI abnormalities than other studies is the milder involvement of a population-based cohort compared to a tertiary referral-based cohort (as is the case for the symptoms and neurologic examination findings). The findings of increased T2-weighted signal and nerve enlargement are nonspecific and related to inflammation of the nerves; however, the MRI serves an important role in the workup to exclude other causes of the syndrome (infiltrative pathologies).
One may ask whether there is a fundamental difference between DLRPN and NDLRPN. The clinical neuropathy pattern, severity, disability, MRI findings, and prognosis of DLRPN compared to NDLRPN are essentially the same. The electrophysiologic findings are similar but showed more severe abnormalities in DLRPN vs NDLRPN. The motor nerve conduction velocities are significantly slower in DLRPN compared to NDLRPN. These electrophysiologic differences are also seen in the referral cohorts, with DLRPN having more severe abnormalites.3,10 We interpret these findings to mean that some DLRPN cases have a coexisting diabetic polyneuropathy. These findings, taken together with previous studies, highly suggest that DLRPN and NDLRPN are ultimately part of the same entity (LRPN), and that both conditions are due to a common disease mechanism (ischemic injury and microvasculitis) and both are variants of nonsystemic vasculitic neuropathy.17 However, because LRPN occurs 8 times more commonly in diabetic patients, it is important to continue to classify DLRPN as a form of diabetic neuropathy, and so we advocate separate classification of DLRPN and NDLRPN.6 This separation also seems reasonable as most of our patients with NDLRPN did not eventually become diabetic.
We found that having LRPN is associated with a shorter overall survival. The median survival of 12.2 years is significantly shorter than for age- and sex-matched controls (≥17 years, median value not reached). We found that patients with LRPN have a 76% increased risk of death compared to controls. However, this increased mortality rate is likely due to higher frequency of diabetes and its associated comorbidities in patients with LRPN as LRPN itself was not an independent mortality risk factor in multivariate analysis. Diabetes is a known strong mortality risk factor, and was identified as a risk factor in all 3 multivariate analysis models (table 4) along with patients' age and stroke. A survival study in nonsystemic vasculitic neuropathy performed in 48 patients followed for more than 6 months showed a 21% mortality rate and that the 5-year survival was similar to our LRPN cohort (86% vs 87%).29 One major difference was that no deaths in our community patients with LRPN were considered to be related to the neuropathy, whereas 50% of deaths were deemed to be related to the nonsystemic vasculitic neuropathy in the other study. One possible explanation for this difference is that none of our patients was treated with chronic immunosuppression, in contrast to the nonsystemic vasculitic neuropathy study, where use of immunosuppression was common.
Herein, we confirm that LRPN is a monophasic, lower limb predominant neuropathy starting focally (in the thigh or leg) with pain and progressing to a multifocal neuropathy with weakness (with both thigh and leg affected and sometimes bilateral involvement). Frequently patients need gait aids to walk and have associated weight loss. Compared with referral-based cohorts, we find that community-based LRPN is a milder disease with similar neuropathy characteristics. We advocate continued differentiation and use of the terms DLRPN and NDLRPN as diabetes is an important risk factor in the development of LRPN, but believe that both conditions represent a single disease of LRPN. LRPN is self-limited inflammatory neuropathy that improves over time but often leaves people with problematic functional impairment and ongoing need for walking aids. Although having LRPN increases mortality risk, this increased mortality is probably due to higher prevalence of diabetes and other comorbidities rather than to the LRPN itself.
Glossary
- CV
conduction velocity
- DLRPN
diabetic lumbosacral radiculoplexus neuropathy
- DTI
diffusion tensor imaging
- LRPN
lumbosacral radiculoplexus neuropathy
- mRS
modified Rankin Scale
- MUP
motor unit potential
- NDLRPN
nondiabetic lumbosacral radiculoplexus neuropathy
- NCS
nerve conduction studies
- NIS
Neuropathy Impairment Score
- OMC
Olmsted Medical Center
- REP
Rochester Epidemiology Project
- RPN
radiculoplexus neuropathies
Appendix. Authors
Study Funding
This study used the resources of the Rochester Epidemiology Project, which is supported by the National Institute on Aging of the NIH under award number R01AG034676. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Disclosure
Dr. Pinto, Dr. Ng, Dr. Howe, P. Thapa, and Dr. Laughlin report no disclosures. Dr. P.J. Dyck previously received honoraria for his services as an associate editor of Diabetes and receives honoraria for teaching of the neurologic examination and neurophysiologic tests in pharmaceutical industry trials from Alnylam, Inc., Ionis, Inc., and Eidos, Inc. None of these trials is related to the present report. Dr. P.J.B. Dyck reports no disclosures. Go to Neurology.org/N for full disclosures.
References
- 1.Dyck PJB, Windebank AJ. Diabetic and nondiabetic lumbosacral radiculoplexus neuropathies: new insights into pathophysiology and treatment. Muscle Nerve 2002;25:477-491. [DOI] [PubMed] [Google Scholar]
- 2.Pirart J. Diabetes mellitus and its degenerative complications: a prospective study of 4,400 patients observed between 1947 and 1973. Diabetes Care 1978;1:252-263. [Google Scholar]
- 3.Dyck PJB, Norell JE, Dyck PJ. Microvasculitis and ischemia in diabetic lumbosacral radiculoplexus neuropathy. Neurology 1999;53:2113-2121. [DOI] [PubMed] [Google Scholar]
- 4.Bastron JA, Thomas JE. Diabetic polyradiculopathy: clinical and electromyographic findings in 105 patients. Mayo Clin Proc 1981;56:725-732. [PubMed] [Google Scholar]
- 5.Massie R, Mauermann ML, Staff NP, et al. Diabetic cervical radiculoplexus neuropathy: a distinct syndrome expanding the spectrum of diabetic radiculoplexus neuropathies. Brain 2012;135:3074-3088. [DOI] [PubMed] [Google Scholar]
- 6.Ng PS, Dyck PJ, Laughlin RS, Thapa P, Pinto MV, Dyck PJB. Lumbosacral radiculoplexus neuropathy: incidence and the association with diabetes mellitus. Neurology 2019;92:e1188–e1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coppack SW, Watkins PJ. The natural history of diabetic femoral neuropathy. Q J Med 1991;79:307-313. [PubMed] [Google Scholar]
- 8.Barohn RJ, Sahenk Z, Warmolts JR, Mendell JR. The Bruns-Garland syndrome (diabetic amyotrophy): revisited 100 years later. Arch Neurol 1991;48:1130-1135. [DOI] [PubMed] [Google Scholar]
- 9.Said G, Lacroix C, Lozeron P, Ropert A, Plante V, Adams D. Inflammatory vasculopathy in multifocal diabetic neuropathy. Brain 2003;126:376-385. [DOI] [PubMed] [Google Scholar]
- 10.Dyck PJB, Norell JE, Dyck PJ. Non-diabetic lumbosacral radiculoplexus neuropathy: natural history, outcome and comparison with the diabetic variety. Brain 2001;124:1197-1207. [DOI] [PubMed] [Google Scholar]
- 11.Evans BA, Stevens JC, Dyck PJ. Lumbosacral plexus neuropathy. Neurology 1981;31:1327. [DOI] [PubMed] [Google Scholar]
- 12.Dyck PJB, Engelstad J, Norell J, Dyck PJ. Microvasculitis in non-diabetic lumbosacral radiculoplexus neuropathy (LSRPN): similarity to the diabetic variety (DLSRPN). J Neuropathol Exp Neurol 2000;59:525-538. [DOI] [PubMed] [Google Scholar]
- 13.Said G, Goulon-Goeau C, Lacroix C, Moulonguet A. Nerve biopsy findings in different patterns of proximal diabetic neuropathy. Ann Neurol 1994;35:559-569. [DOI] [PubMed] [Google Scholar]
- 14.Kelkar P, Masood M, Parry GJ. Distinctive pathologic findings in proximal diabetic neuropathy (diabetic amyotrophy). Neurology 2000;55:83-88. [DOI] [PubMed] [Google Scholar]
- 15.Llewelyn JG, Thomas PK, King RH. Epineurial microvasculitis in proximal diabetic neuropathy. J Neurol 1998;245:159-165. [DOI] [PubMed] [Google Scholar]
- 16.Kawamura N, Dyck PJ, Schmeichel AM, Engelstad JK, Low PA, Dyck PJ. Inflammatory mediators in diabetic and non-diabetic lumbosacral radiculoplexus neuropathy. Acta Neuropathol 2008;115:231-239. [DOI] [PubMed] [Google Scholar]
- 17.Collins MP, Dyck PJB, Hadden RDM. Update on classification, epidemiology, clinical phenotype and imaging of the nonsystemic vasculitic neuropathies. Curr Opin Neurol 2019;32:684-695. [DOI] [PubMed] [Google Scholar]
- 18.Laughlin RS, Dyck PJ, Melton LJ III, Leibson C, Ransom J, Dyck PJB. Incidence and prevalence of CIDP and the association of diabetes mellitus. Neurology 2009;73:39-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kennedy RH, Danielson MA, Mulder DW, Kurland LT. Guillain-Barré syndrome: a 42-year epidemiologic and clinical study. Mayo Clinic Proc 1978;53:93-99. [PubMed] [Google Scholar]
- 20.Beghi E, Kurland LT, Mulder DW, Nicolosi A. Brachial plexus neuropathy in the population of Rochester, Minnesota, 1970-1981. Ann Neurol 1985;18:320-323. [DOI] [PubMed] [Google Scholar]
- 21.Rocca WA, Yawn BP, St Sauver JL, Grossardt BR, Melton LJ III. History of the Rochester Epidemiology Project: half a century of medical records linkage in a US population. Mayo Clinic Proc 2012;87:1202-1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dyck PJB, Gonzalez-Duarte A, Obici L, et al. Development of measures of polyneuropathy impairment in hATTR amyloidosis: from NIS to mNIS+7. J Neurol Sci 2019;405:116424. [DOI] [PubMed] [Google Scholar]
- 23.van Swieten JC, Koudstaal PJ, Visser MC, Schouten HJ, van Gijn J. Interobserver agreement for the assessment of handicap in stroke patients. Stroke 1988;19:604-607. [DOI] [PubMed] [Google Scholar]
- 24.Daube J, Rubin D. Clinical neurophysiology appendix (CD-ROM). In: Daube J, Rubin D, eds. III Clinical Neurophysiology. Oxford: Oxford University Press; 2009. [Google Scholar]
- 25.Said G. Diabetic neuropathy: a review. Nat Clin Pract Neurol 2007;3:331-340. [DOI] [PubMed] [Google Scholar]
- 26.Laughlin RS, Dyck PJ. Diabetic radiculoplexus neuropathies. Handbook Clin Neurol 2014;126:45-52. [DOI] [PubMed] [Google Scholar]
- 27.Hlis R, Poh F, Bryarly M, Xi Y, Chhabra A. Quantitative assessment of diabetic amyotrophy using magnetic resonance neurography: a case-control analysis. Eur Radiol 2019;29:5910-5919. [DOI] [PubMed] [Google Scholar]
- 28.Staff NP, Engelstad J, Klein CJ, et al. Post-surgical inflammatory neuropathy. Brain 2010;133:2866-2880. [DOI] [PubMed] [Google Scholar]
- 29.Collins MP, Periquet MI, Mendell JR, Sahenk Z, Nagaraja HN, Kissel JT. Nonsystemic vasculitic neuropathy: insights from a clinical cohort. Neurology 2003;61:623-630. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Fully anonymized data will be shared by request from any qualified investigator.