Abstract
Background
It has been suggested that changes to the immune system could be a factor in age-related conditions such as Alzheimer's disease. Our objective was to examine the association between past exposure to conventional vaccines and risk of Alzheimer's disease.
Methods
We analyzed data from a representative community sample of subjects 65 years of age or older participating in the Canadian Study of Health and Aging, a prospective cohort study of dementia. Screening and clinical evaluations were done at both baseline and follow-up. Past exposure to vaccines was assessed at baseline by means of a self-administered questionnaire.
Results
Of the 4392 eligible subjects who were cognitively unimpaired and for whom vaccine information was available at baseline (in 1991–1992) and who completed follow-up 5 years later (in 1996–1997), 527 were diagnosed as having cognitive impairment or dementia other than Alzheimer's disease and were excluded from these analyses. Of the remaining subjects, 3682 were cognitively unimpaired at follow-up and 183 were newly diagnosed as having Alzheimer's disease. After adjustment for age, sex and education, past exposure to vaccines against diphtheria or tetanus, poliomyelitis and influenza was associated with lower risk for Alzheimer's disease (odds ratio [OR] 0.41, 95% confidence interval [CI] 0.27–0.62; OR 0.60, 95% CI 0.37–0.99; and OR 0.75, 95% CI 0.54–1.04 respectively) than no exposure to these vaccines.
Interpretation
Past exposure to vaccines against diphtheria or tetanus, poliomyelitis and influenza may protect against subsequent development of Alzheimer's disease.
The causes of Alzheimer's disease are unknown. Among the many hypotheses that have been raised is the possibility that conventional infectious agents, in conjunction with changes in the immune system, play a role.1,2,3 Evidence for a relation between viral infection and development of Alzheimer's disease comes from the neuroinflammation and apoptosis that are known to occur in this disease.4 Furthermore, changes to the immune system have been implicated in age-related conditions such as Alzheimer's disease.5,6 We analysed the association between past exposure to conventional vaccines and risk of Alzheimer's disease for subjects in the Canadian Study of Health and Aging (CSHA), a multicentre prospective study of dementia in a representative community sample of elderly Canadians.
Methods
Details of the CSHA have been published elsewhere.7,8 Briefly, 9008 subjects 65 years of age or older, randomly selected from the general population living in the community in 1991–1992 (CSHA-1), were screened for dementia with the Modified Mini-Mental State (3MS) examination.9 Subjects who screened positive (score of 77% or less) and a random sample of those who screened negative underwent standardized clinical and neuropsychological evaluations. Preliminary diagnoses of dementia and Alzheimer's disease, according to the criteria in the revised third edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-III-R)10 and the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer's Disease and Related Disorders Associations,11 were made independently by the physician and the neuropsychologist, who subsequently determined the definitive diagnosis by consensus. Follow-up was carried out 5 years later, in 1996–1997, according to the same diagnostic process (CSHA-2). At that time, dementia and Alzheimer's disease were diagnosed according to the more recent DSM-IV criteria.12 Ethical approval for both phases of the study was obtained from ethics review boards in all participating centres.
Exposure to vaccines was assessed at baseline for cognitively unimpaired subjects who agreed to complete a self-administered risk factor questionnaire. Respondents were asked whether they had ever received vaccinations for tetanus, diphtheria, poliomyelitis or influenza. For the purposes of our analyses, vaccines against tetanus and diphtheria were considered together, as they are usually given simultaneously in vaccination programs.
Multivariate logistic regression models were used to calculate odds ratios, with adjustment for age (continuous variable), sex and years of education (continuous variable). Other potential confounders that we considered included current smoking (yes or no), regular alcohol consumption (yes or no), family history of dementia (yes or no), performance scores in basic and instrumental activities of daily living (ADL and IADL; continuous variables), number of antecedents of chronic diseases (continuous variable) and perceived health status (poor or good).
To evaluate the impact of excluding from the analyses subjects who died during follow-up, we tried to estimate the probability of dementia for this group from various sources (specifically, death certificates; information from proxies about diagnosis of a memory problem, Alzheimer's disease or senile dementia before death; and predictive regression models estimating the probability that a deceased person had dementia before dying, based on 71 subjects who died 2 to 5 months after a diagnostic evaluation).7
Results
Of the 7740 eligible subjects who were cognitively unimpaired at baseline, information on past exposure to vaccines was available for 6211 (80%). Of these, 1172 died before CSHA-2, 374 refused to participate in CSHA-2 and 273 were lost to follow-up. Of the remaining 4392 subjects, 527 were diagnosed as having cognitive impairment or dementia other than Alzheimer's disease and were excluded from these analyses, leaving 3682 subjects who remained cognitively normal at CSHA-2 (the controls) and 183 subjects with a new diagnosis of probable or possible Alzheimer's disease (the cases).
We compared subjects who had Alzheimer's disease at follow-up with those who remained cognitively unimpaired. Those with Alzheimer's disease were older (median 81 v. 72), comprised more women (65% v. 60%) and had completed fewer years of education (median 10 v. 11 years) (Table 1). The reported rates of vaccination against diphtheria or tetanus and for poliomyelitis were lower for subjects with Alzheimer's disease than for controls, but there was no difference in the reported rate of vaccination against influenza (Table 1).
Table 1
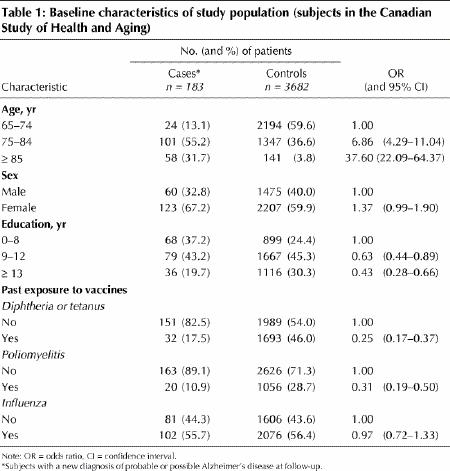
For each type of vaccine, subjects who reported at least one vaccination were at lower risk for Alzheimer's disease than those who had never been exposed, after adjustment for age, sex and education (Table 2). Vaccines against diphtheria or tetanus and against poliomyelitis were associated with statistically significantly lower risk of Alzheimer's disease (60% and 40% lower respectively). Exposure to influenza vaccine was also related to a lower risk of Alzheimer's disease, but the association did not reach statistical significance. Additional adjustment for smoking, alcohol use, family history of dementia, ADL and IADL, chronic diseases and perceived health status yielded similar results (Table 2).
Table 2
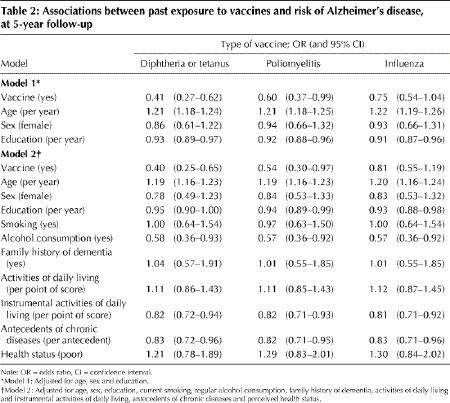
Analyses including people who had died, incorporating estimates of dementia in this group (as described above), produced similar results (not shown).
Interpretation
According to this analysis of data from a large-scale longitudinal study of elderly Canadians, vaccination against diphtheria or tetanus, poliomyelitis or influenza was associated with lower risk for Alzheimer's disease than no vaccination.
The analysis had some limitations. First, rates of reporting of vaccination were relatively low (28% for poliomyelitis, 45% for diphtheria or tetanus and 56% for influenza), which probably reflects some degree of underreporting. Routine vaccination against diphtheria in infancy and childhood has been in place in Canada since 1930.13 Routine vaccination against tetanus started in 1948, with the introduction of the combined diphtheria– pertussis– tetanus vaccine for school programs.14 For vaccination against poliomyelitis, the inactivated vaccine was introduced in 1952, and the trivalent oral vaccine has been used since 1962.13 Widespread influenza vaccination started in the 1970s. However, none of these vaccines has ever been compulsory. Second, because of the self-reported nature of the risk factor questionnaire, we cannot exclude the possibility of recall bias. Moreover, no information was available about the timing of vaccination or the number of doses of vaccine received. Nevertheless, because of the prospective nature of the study, whereby questionnaires were completed at baseline by cognitively unimpaired subjects, before any onset of Alzheimer's disease, any misclassification of exposure should be nondifferential and would tend to produce bias toward the null hypothesis, rather than to generate false-positive results.
It might also be argued that exposure to vaccines is only indirectly associated with Alzheimer's disease, as a potential marker of a healthy lifestyle. However, when we controlled for other baseline characteristics that are also related to lifestyle, the results remained the same (Table 2).
Our findings may result from some artifact related to the limited quality of available data on exposure to vaccines. They are also compatible with the hypothesis that past exposure to specific types of vaccines may be related to a lower risk of Alzheimer's disease in elderly people. As such, they support recent reports suggesting that both aging and Alzheimer's disease may involve changes in immune responses.1,2 Epidemiological and clinical studies seem warranted to explore this hypothesis.
Acknowledgments
The data reported in this article were collected as part of the Canadian Study of Health and Aging. The core study was funded by the Seniors' Independence Research Program, through the National Health Research and Development Program (NHRDP) of Health Canada, project 6606-3954-MC (S). Additional funding was provided by Pfizer Canada Incorporated through the Medical Research Council/Pharmaceutical Manufacturers Association of Canada Health Activity Program, NHRDP project 6603-1417-302 (R), Bayer Incorporated, and British Columbia Health Research Foundation projects 38 (93-2) and 34 (96-1). The study was coordinated through the University of Ottawa and the Division of Aging and Seniors, Health Canada. Danielle Laurin was supported in part by a National Health PhD fellowship (NHRDP project 6605-5228-47) and by the Chair for Geriatric Research at Université Laval. René Verreault is supported by the Chair for Geriatric Research at Université Laval.
Footnotes
This article has been peer reviewed.
Competing interests: None declared.
Correspondence to: Dr. René Verreault, Laval University Geriatric Research Unit, Centre d'hébergement Saint-Augustin du Centre hospitalier affilié universitaire de Québec, 2135 Terrasse Cadieux, Beauport QC G1C 1Z2; fax 418 667-2459; Rene.Verreault@msp.ulaval.ca
References
- 1.Chorsky RL, Yaghmai F, Hill WD, Stopa EG. Alzheimer's disease: a review concerning immune response and microischemia. Med Hypotheses 2001; 56: 124-7. [DOI] [PubMed]
- 2.Armstrong R, Winsper S, Blair J. Hypothesis: Is Alzheimer's disease a metal-induced immune disorder? Neurodegeneration 1995;4:107-11. [DOI] [PubMed]
- 3.Renvoize EB, Awad IO, Hambling MH. A sero-epidemiological study of conventional infectious agents in Alzheimer's disease. Age Ageing 1987;16:311-4. [DOI] [PubMed]
- 4.Cheon MS, Bajo M, Gulesserian T, Cairns N, Lubec G. Evidence for the relation of herpes simplex virus type 1 to Down syndrome and Alzheimer's disease. Electrophoresis 2001;22:445-8. [DOI] [PubMed]
- 5.Effros RB. Ageing and the immune system. Novartis Found Symp 2001;235: 130-9. [DOI] [PubMed]
- 6.Song C, Vandewoude M, Stevens W, De Clerck L, Van der Planken M, Whelan A, et al. Alterations in immune functions during normal aging and Alzheimer's disease. Psychiatry Res 1999;85:71-80. [DOI] [PubMed]
- 7.Canadian Study of Health and Aging Working Group. The incidence of dementia in Canada. Neurology 2000;55:66-73. [PubMed]
- 8.Canadian Study of Health and Aging Working Group. Canadian study of health and aging: study methods and prevalence of dementia. CMAJ 1994;150 (6): 899-913. [PMC free article] [PubMed]
- 9.Teng EL, Chui HC. The Modified Mini-Mental State (3MS) examination. J Clin Psychiatry 1987;48:314-8. [PubMed]
- 10.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 3rd revised ed. Washington: The Association; 1987.
- 11.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS–ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology 1984;34:939-44. [DOI] [PubMed]
- 12.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed. Washington: The Association; 1994.
- 13.National Advisory Committee on Immunization. Canadian immunization guide. 5th ed. Ottawa: Health Canada; 1998.
- 14.Varughese P. Tetanus in Canada 1921–1978. Can Dis Wkly Rep 1980;6:113-8.


