Abstract
Background
As COVID-19 has become a pandemic emerging infectious disease it is important to examine whether there was a spatiotemporal clustering phenomenon in the globe during the rapid spread after the first outbreak reported from southern China.
Materials and methods
The open data on the number of COVID-19 cases reported at daily basis form the globe were used to assess the evolution of outbreaks with international air link on the same latitude and also including Taiwan. The dynamic Susceptible-Infected-Recovered model was used to evaluate continental transmission from December 2019 to March 2020 before the declaration of COVID-19 pandemic with basic reproductive number and effective reproductive number before and after containment measurements.
Results
For the initial COVID-19 outbreak in China, the estimated reproductive number was reduced from 2.84 during the overwhelming outbreaks in early January to 0.43 after the strict lockdown policy. It is very surprising to find there were three countries (including South Korea, Iran, and Italy) and the Washington state of the USA on the 38° North Latitude involved with large-scale community-acquired outbreaks since the first imported COVID-19 cases from China. The propagation of continental transmission was augmented from hotspot to hotspot with higher reproductive number immediately before the declaration of pandemic. By contrast, there was not any large community-acquired outbreak in Taiwan.
Conclusion
The propagated spatiotemporal transmission from China to other hotspots may explain the emerging pandemic that can only be exempted by timely border control and preparedness of containment measurements according to Taiwan experience.
Keywords: COVID-19, Pandemic, Containment, Border control, Lockdown
Introduction
When the first outbreak of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) was identified at Huanan (Southern China) Seafood Wholesale Market in December 2019,1 , 2 the first question was asked of whether the emerging COVID-19 is through human-to-human or animal-to-human transmission. As SARS-CoV-2 and Bat CoV might share the same ancestor in that the genome of SARS-CoV-2 showed 96.2% overall sequence identical to Bat CoV RaTG13 and 79.6% sequence identical to SARS-CoV,3 it is therefore speculated that such an outbreak is merely limited to the transmission mode of the latter. However, the first COVID-19 patient, who had no contact with other cases history, was not associated with the seafood market and only 66% patients had direct exposure to seafood market.2 Therefore, human-to-human transmission has been noted among close contacts.1 , 2
Moreover, although evidence has proven that COVID-19 is originated from the seafood market, the spread of COVID-19 could not be stamped out even after the closure of market. Such a kind of human-to-human transmission has been expanded through the rapid transmission from Wuhan city to the rest of China after strict lockdown measures, movement restrictions of Wuhan city, Hubei province and even these control measures were applied to other provinces later. Since then, several countries have set up border control measures, including symptom-based screening, restricting travel to and from China, and self-identified possible exposure via health questionnaires. Despite these containment measures, the spread of COVID-19 from China to other countries has been soon noted. Three major types of SARS-CoV-2 spread from China to Asia, Europe and America were identified by phylogenetic network analysis later on.4 The outbreak of COVID-19 are unlikely to be contained by border control measures, but importation of SARS CoV-2 cases could be further delayed at the early stage of the epidemic.5 International travel restrictions are expected to reduce the spread of COVID-196 but it was spread to around 229 countries till the end of April even after border control measures and travel restriction were adopted by many countries. It should be noted that the size of COVID-19 outbreak was not the same because of difference contained measures implemented in these countries.
From the viewpoint of infectious epidemiology, it is therefore important to document and identify international transmission route before the declaration of COVID-19 pandemic from China to South Korea, Italy, and Iran where COVID-19 cases from three countries seems highly linked according to the chronological order, location, and flights to and from China.6 , 7 COVID-19 cases reported from three countries in early outbreak phase before the evolution into pandemic would provide a new insight into international transmission route for this emerging infection.
The aims of this report are therefore to investigate the early spread of COVID-19 from China to four countries including South Korea, Iran, Italy, and Taiwan through the estimation of the dynamic basic reproduction number (R0) and effective reproduction number (R) after the implementation of containment measurements based on the SIR model in these countries before the declaration of COVID-19 pandemic.
Methods
Data collection
Daily number of infected, recovered and death cases in China were extracted from the published papers from early outbreak of infected cases and open database, Coronavirus disease 2019 (COVID-19) Situation Report provided by World Health Organization (WHO) available after January 21. The study period was from the first reported date of the first case, December 1, 2019 to March 31,2020. The genotyping evolution of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in these countries was also collected in the literatures.8, 9, 10, 11, 12
We also extracted the symptom onset date from the report of Taiwan Center for disease control (CDC) during the same study period described above.
Statistical methods
We applied susceptible-infected-recovered (SIR) deterministic model to fit the confirmed COVID-19 cases for the estimation of R0 and effective reproduction number (R) for each period in China, South Korea, Iran, Italy, and the Washington state of the USA. R0 or R was estimated with function of transmission coefficient (β) and recovery rate (α).14 In addition, overall R for each period was estimated by the sequential Bayesian approach to the empirical data. The conjugated prior assigned Normal distribution for log (β), where β denotes transmission coefficient.
Results
COVID-19 outbreak on 380 latitude
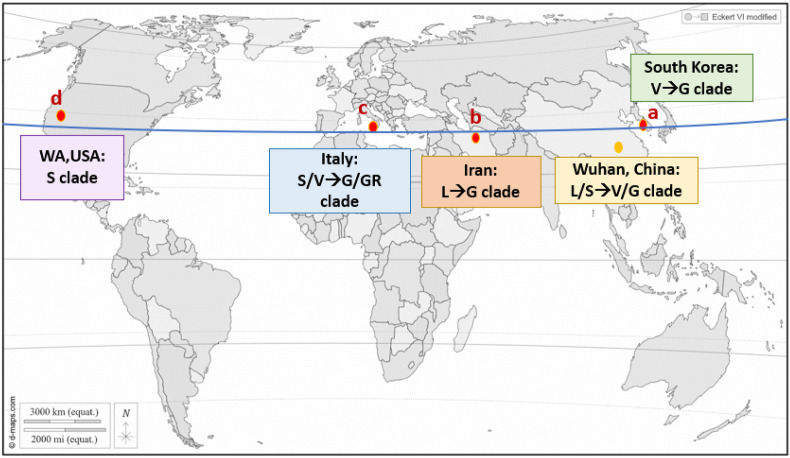
A total of 65,135 confirmed cases were included in our study, from Jan 1, 2019 to Mar 31, 2020. Fig. 1 shows the continents on the 38° north latitude with the early spread of COVID-19 from Wuhan, China, including the major genotyping evolution of SARS-CoV-2. The original strain, detected in the Southern China, is the L clade, and it mutated into the S clade at the beginning of 2020. Then it was followed by clade V and G. The genomic analysis of first outbreak cluster in South Korea on January 19, 2020 belonged to clade V, the mutant type from Wuhan-Hu-1. Gradually, clade G emerged in late March and became the dominant group of infection in May. The original strain in Italy was mainly S or V clade (B.2 cluster), of which V clade was from tourists who traveled from Hubei in China and visited Rome. G or GR clade of the virus became the dominant clade in March. The very early virus introduction to Iran was the L clade (B.4 cluster) in January–February and was suggested to link to China. The viral genome from the first case detected in Washington state of the USA had mutations similar to those found in the Chinese sample which belonged to the S clade.
Figure 1.
The continents on the 38° north latitude (Blue line). (a) South Korea (b) Iran (c) Italy (d) the State of Washington, U.S.
Table 1 illustrates the estimated R0 of four countries during different periods of outbreak to reflect the rapid spread from hotspot to hotspot on the same latitude. Notably, the sequential R0 in the four successive countries during continental transmission were 2.02 (95% CI: 0.84, 4.84) at initial stage from December 1–31, 2019, and it rose up to 6.97 (95% CI: 6.60, 7.36) from Feb.17–25, 2020. This accounted for the declaration of COVID-19 pandemic on March 11, 2020.
Table 1.
Estimated results on the reproductive number derived by using sequential analysis.
| Reproductive number |
|||
|---|---|---|---|
| Estimate | 95% CI | ||
| 2019-12-1 – 2019-12-31 | 2.02 | (0.84, 4.84) | |
| 2020-1-1 – 2020-1-21 | 2.73 | (1.14, 6.55) | |
| 2020-1-22 – 2020-2-6 | 1.38 | (0.89, 2.14) | |
| 2020-2-7 – 2020-2-16 | 6.95 | (6.02, 8.01) | |
| 2020-2-17 – 2020-2-25 | 6.97 | (6.60, 7.36) | |
| 2020-2-26 – 2020-3-11 | 4.09 | (3.55, 4.71) | |
| 2020-3-12 – 2020-3-30 | 6.10 | (4.79, 7.78) | |
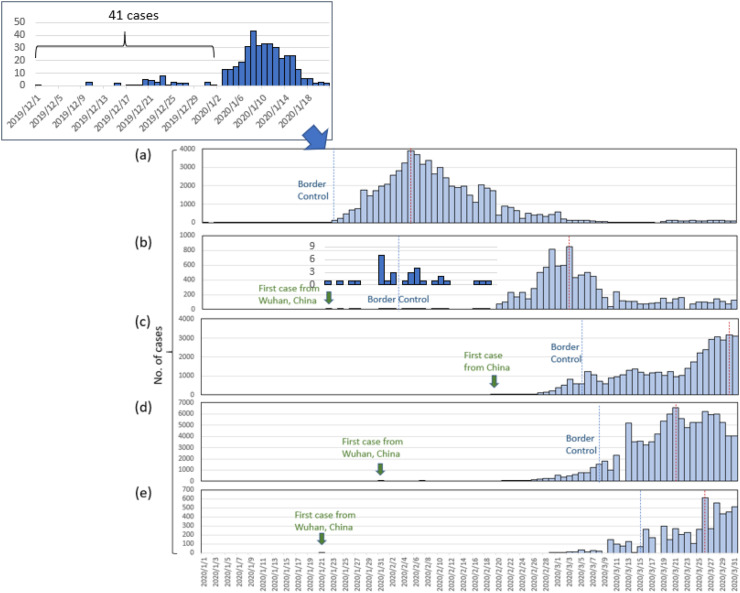
The epidemic curve of confirmed COVID-19 cases with temporal relationships of these countries was shown in Fig. 2 . The epidemic curve according to the reported date and key interventions after the COVID-19 outbreak in Wuhan after December 1, 2019 (Figure S1a). By January 2, 2020, 41 admitted hospital patients had been identified as COVID-19 cases. During the shutdown of Huanan Seafood wholesale market and the lockdown of Wuhan city, an epidemic of confirmed cases emerged due to the subsequent outbreak. Most cases occurred between January 23 and February 19, 2020, with a peak on February 5 (Figure S1a). After a series of lockdown of Wuhan city, Hubei and several other provinces/cities or areas and the universal symptom survey, daily confirmed cases started to decline. During the whole March after March 1, the confirmed cases became no larger than 250 per day.
Figure 2.
Number of confirmed cases by countries or state (a) China (b) South Korea (c) Iran (d) Italy (e) the State of Washington, U.S. Blue dashed line: border control; red dashed line: peak of conformed cases.
The cumulative cases, predicted numbers as well as estimated dynamic effective reproduction number R in China (Figure S1b). The initial estimated R0 was 1.65 and the figure elevated to 2.03 before the lockdown of cities. The peak of R0 rose up to 2.84 during the policy of lockdown of several provinces and cities (from January 23 to February 7). The effective reproduction number declined to 0.85 afterwards on February 7 and keep plummeting to 0.43 after the universal control measure of symptom survey.
The incidence rate for each province/city in mainland China after the implementation of the lockdown of cities and most of measurements are shown in Figure S2. Hubei has the highest incidence among all (114.6 per 105), followed by Zhejiang and Jiangxi. The incidence rate gradually decreases from Hubei to other areas.
The first confirmed and sporadic cases of COVID-19 occurring during mid-January to the end of January in South Korea were imported from Mainland China that were purported to be associated with religious gathering (Figure S3a). The first outbreak of COVID-19 in South Korea started from end of January to February 19, 2020. The second outbreak started from February 20 due to the church cluster infection. Border control, foreigners from Hubei were not permitted to have entry, was performed on February 2, 2020. By February 26, government of South Korea implemented the universal testing of COVID-19, and the daily reported case grew up rapidly, with a peak of 851 cases on March 3. From March 4, the epidemic curve showed decreasing trend. The R0 surged rapidly during the first outbreak (R0 = 4.52), and remained high throughout the second outbreak (R0 = 1.88). However, the effective reproduction number went below 1 after blocking the buildings (Figure S3b). Assuming that large drive-through screening may take into effect after 10 days, the estimated efficacy of this measure was 29% within 1 week.
For Middle East country, Iran, two deaths were reported on February 19,2020 (Figure S4a). After verification, these two cases were also the first two cases of COVID-19 in Iran. Lockdown, all of Iran's neighboring states have started to put some travel limitations, and border control, closed their borders with Iran, were performed since February 23, 2020. Iran was not recognized as a source spreading coronavirus to other countries until March 9, 2020. Checkpoints were placed between cities to limit travel on March 5. However, people seemed to ignore the travelling guidance. Another outbreak broke out on March 24, 2020, with a peak of daily cases of 3186 and more than 1500 daily cases occurred until the end of March. The epidemic curve gradually declined after March, after the announcement of banning internal travel and social distancing policy. Up to March 31, 2020 there were 44,605 COVID-19 cases in Iran. Figure S4b presents the R0 by different periods of national policies implemented in Iran. Since the rapid case spread-out after the first two cases confirmed, the R0 was high before the travel ban, with the R0 of 13.62 and 5.88. However, the effective reproduction number declined to 1.67 and 1.91 after travel ban.
On 31 January, the first two cases of COVID-19 who came from Wuhan were confirmed in Rome, Italy (Figure S5a). On February 21, 2020, the fourth confirmed case was reported in Lombardy. Since then, extensive testing was performed on everyone who had possibly been in contact with the infected subject. Due to this super spreader causing the big cluster infection in Lombardy, the outbreak in Italy increased dramatically. Border control, all flights to and from China were suspended, was performed on January 31, 2020. Nationwide movement restriction, lockdown, was announced on March 1, 2020. The region of Lombardy with 14 additional northern and central provinces started to be put under lockdown on March 8, 2020. However, the peak appeared on March 21, 2020 with daily confirmed cases of 6557. There were 105,792 COVID-19 cases in Italy up to the end of March, 2020. After the launch of lockdown strategy and shut-down of business activities, the effective reproduction number declined from 2.65 to 1.96 and 1.09, respectively (Figure S5b).
In United States, the first confirmed patient, who returned from Wuhan, China, was reported by the state of Washington on January 21, 2020. Thereafter, the first outbreak was identified in a long-term care facility on February 28, 2020, in King County, Washington. After the second wave of outbreak in this facility, it became the initial epicenter in U.S. with around 129 cases and 23 deaths associated with the outbreak. In the State of Washington of U.S., emergency state was declared on February 29, 2020 when there's an outbreak in a long-term care facility on February 28, 2020, in King County. Mandatory screening for visitors and staff in nursing home on March 9, 2020. All schools were closed from March 17 to April 24, 2020. Lockdown and all sit-down restaurants were closed since March 15, 2020. The stay-at-home order, was announced since March 23, 2020. A total of 5432 confirmed cases were reported until the end of March (Figure S6a). During the outbreak in long-term care facility, the R0 reached 16.7 and 4.5 afterward. However, the effective reproduction number became 2.77 and 2.31 after the announcement of lockdown and school closure on March 15, 2020, and subsequent order of stay-at-home strategy (Figure S6b).
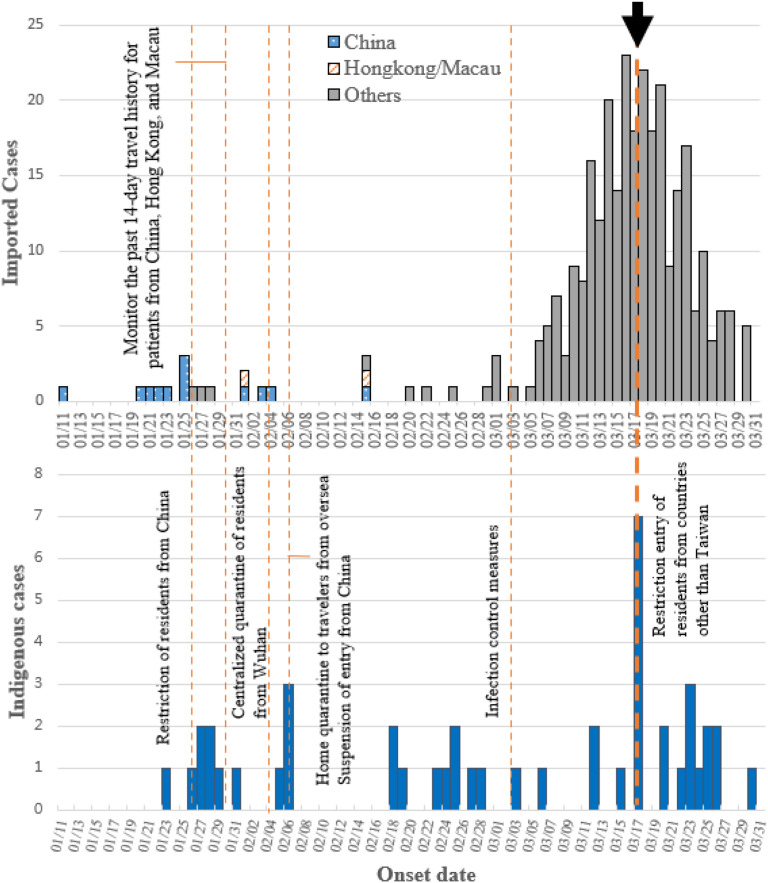
In Taiwan, the imported and domestic cases by the date of symptom onset and key interventions from Jan 11, 2020, which was the onset day of first reported cases from Taiwan, to the end of March (Fig. 3 ). Border control (boarding quarantine) and symptom-based screening were immediately performed for all flights from Wuhan city in Taiwan on December 31, 2019. Furthermore, surveillance system set up, COVID-19 was listed as notifiable disease, on January 15, 2020. In addition, resources allocation, including setting the price of masks, increasing production of masks, and using government funds were taken action by the Central Epidemic Command Center (CECC) in response to COVID-19.13 Hence, among a total of 439 confirmed cases, 348 were imported cases. Five confirmed COVID-19 cases in the family cluster resulted from an imported case from China, and another five confirmed COVID-19 cases in the hospital cluster from the unknown origin were noted in February 2020. Soon after the first case was detected, the government announced the restriction of residents from China on Jan 26, 2020. After several implements of border control (suspension of entry from China), quarantine surveillance (home quarantine to travelers from oversea, centralized quarantine of residents from Wuhan) during Jan 30 and Feb 7, 2020, and the major control strategy of the restriction of entry of residents from China and other countries on Mar 17, the number of imported cases decreased. The first crisis of the potential community outbreak was controlled by contact tracing, quarantine with symptoms screening, isolation, wearing masks in crowded settings, and social distancing.
Figure 3.
The epidemic curves of confirmed cases in Taiwan including (a) Imported COVID-19 cases and (b) domestic COVID-19 cases. Arrow: The major control measure in Taiwan on March 17, 2020.
Discussion
This study made use of spatiotemporal cluster infection on the 38 degree of North Latitude to verify continental transmission of SARS-COV-2 from the dawn of the outbreak of COVID-19 in the Wuhan city, China to the clustered infection occurring Korea, Iran, Italy, and the state of Washington in the U.S. on the premise of the transmissibility of 14-day incubation period. In addition, during worldwide spreading, the virus mutated has been found and it might select for dangerous viral pathogens and transmission.15
In our study, the range of R0 of COVID-19 was estimated as 1.65, 2.03, 2.84, 0.85 and 0.43 in the different periods in China, which showed the origin of COVID-19 outbreak in Wuhan city and the spread of SARS-CoV-2 from Hubei to the rest of China and around the world. The range of R0 of COVID-19 before and after typical control measures were 1.88 and 0.26 in Korea, 2.65, and 1.09 in Italy, 1.91 and 0.19 in Iran. Imported COVID-19 cases are the major origin of indigenous cases in these countries. The effectiveness of control measures could be found about 2 weeks later after the implementation of control measures by the change value of effective R. Also, special cluster events accelerated the spread of COVID-19, such as the outbreak of Shincheonji religious organization in Daegu of South Korea, a large proportion of healthcare workers infection in Lombardy of Italy, and large gatherings, Iranian legislative election, in Iran. Hence, not keeping social distancing and not wearing face masks in mass gatherings increase the risk of spreading COVID-19.
The initial border measures, such as on-board body temperature/symptoms screening, self-identify via health questionnaires, home quarantine to travelers from oversea, isolation and so on in response to COVID-19 were effective to control the spread of COVID-19 from Wuhan/Hubei/China or Hong Kong or Macao to Taiwan. Many imported cases were found with symptom-based screening in the airport when they arrived to Taiwan. Most domestic cases were infected by imported cases, and no large-scale community-acquired infection was noted. The effects of border control and travel restriction could decrease the daily rate of exportation, and it also delays the importation of cases into communities in past study.5
Though all flights to and from China were suspended as early as on January 31, first imported COVID-19 patients, originally from Wuhan, arrived in Italy on January 23, and arrived in Rome on 28 January. However, these COVID-19 cases were confirmed in Rome rather than in Italy. The cause responsible for the subsequent community outbreak in Italy was thus the incomplete boarder control measures associated with the lack of travel restriction in countries of European Union. In Iran, there was no active boarder control policy, but the travel across boarder was blocked by the neighbor countries. In South Korea, the travel of foreigners from Hubei were prohibited since February 2, 2019. No strict boarder control measure was taken in South Korea during the period. In summary, the boarder control measures as a response to block the continental spread of COVID-19 in Italy, Iran, and South Korea were lagged behind the surge in pandemic.
Strict lockdown measures and movement restrictions might have contributed to controlling the spread of COVID-19 in China because the dynamics of the reproduction number below the epidemic threshold (<1) was estimated after February 7, 2020. The effect of strict control measures had been reported in China, outside Hubei province and Wuhan.16, 17, 18 Similarly strict lockdown measures were taken in Daegu of South Korea, in Lombardy of Italy and a brief nationwide lockdown in Iran. To compare with the strict lockdown among these countries, the effect of decreasing R was found about two weeks after these measures.
Large-scale screening for COVID-19 to find all cases for isolation, which can decrease infectious period of cases in the community, was the major control policy in South Korea. The outbreak could be under control, even though only local area movement restrict was taken.
The R0, denoting the average number of new infections generated by an infectious person, indicated the risk of an infectious agent with respect to epidemic spread. The R0 value are often associated with 3 primary parameters: the duration of contagiousness after a person becomes infected, the likelihood of infection per contact between a susceptible person and an infectious person, and the contact rate.11 Therefore, the effective reproduction number can also be specified at a particular time, which can be used to trace changes in R as the effectiveness of control measures in this study. The initial R0 was estimated as 1.65–2.84, which is similar to recent reports in China.1 , 17, 18, 19 The previous study noted that the estimated value of effective R could vary with different periods.19 The effective R below 1.0 after February 7, 2020 was also estimated in our study after the policy of lockdown.
To compare with SARS, COVOD-19 manifests higher transmissibility because both aerosol and fomite transmission modes with the persistence of SARS-CoV-2 on inanimate surfaces were reported.20 If cluster occurred from imported cases to domestic cases such as the Shincheonji religious events in South Korea, hospital infection in Italy and larger gathers activity in Iran, R would be markedly increased to more than 3. In addition, the higher proportion of nosocomial infection in patients with COVID-19 was found in the early outbreak.21, 22, 23
We believed that not only border measures with detail contact tracing but also public awareness, including wearing the mask, washing hands, and social distancing, slowed down the spread of COVID in the community. In comparison with COVID-19, a total of 664 SARS (Severe Acute Respiratory Syndrome) probable cases and many hospital clusters were reported during the period of March to June, 2003 in Taiwan because of no early border measures and insufficient hospital infection control. Therefore, hospital infection control measures, such as outdoor infection risk assessments, traffic control building, frequently cleaning environment, and auto-waring information by combining information of emigrant and immigration with health insurance card, also played an important role in slowing down the spread of COVID-19 in Taiwan's community.13 , 14 These measures can buy time to coordinate an appropriate public health response to COVID-19 outbreaks in Taiwan. Lockdown measures were never taken because boarder control measures and other public interventions, such as wearing mask, washing hands and social distancing, succeed in Taiwan.
The strength of this study is to quantify the spreading COVID-19 and confinement measures by the estimated dynamic reproduction number in different countries. Furthermore, the uncertainty of the dynamic reproduction number can be estimated by the sequential Bayesian approach, but not in the deterministic SIR model. There are some limitations to this study. Overestimation of the dynamic reproduction number may present in these countries of the 38° North Latitude because the imported cases were not excluded. There was a smaller proportion of imported cases because the outbreak happened in the community of these countries. In addition, the origin of COVID-19 cases is from China, the estimation of dynamic reproduction numbers is unbiased estimated from the global view by the sequential Bayesian approach.
In conclusion, border measures, social distancing and preventing nosocomial infection are key points to prevent the spread of COVID-19 in the community. Of course, the most effective control measure is strict lockdown during the period of outbreak. If public health interventions are earlier performed in Wuhan city, Hubei province and China, the outbreak of COVID-19 should be prevented or minimized in China. In addition, pandemic of COVID-19 would not have occurred worldwide. In the same way, these measures can be effective performed as early as possible, the size of COVID-19 outbreak may be obviously decreased.
Acknowledgements
This study was supported by Ministry of Science and Technology, Taiwan (MOST 109-2327-B-002-009).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jfma.2021.05.008.
Appendix A. Supplementary data
The following is the supplementary data to this article:
References
- 1.Li Q., Guan X., Wu P., Wang X., Zhou L., Tong Y. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med. 2020;382:1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China [published correction appears in Lancet. 2020 Jan 30;:] Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhou P., Yang X.L., Wang X.G., Hu B., Zhang L., Zhang W. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Forster P., Forster L., Renfrew C., Forster M. Phylogenetic network analysis of SARS-CoV-2 genomes. Proc Natl Acad Sci U S A. 2020;117:9241–9243. doi: 10.1073/pnas.2004999117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wells C.R., Sah P., Moghadas S.M., Pandey A., Shoukat A., Wang Y. Impact of international travel and border control measures on the global spread of the novel 2019 coronavirus outbreak. Proc Natl Acad Sci U S A. 2020;117:7504–7509. doi: 10.1073/pnas.2002616117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chinazzi M., Davis J.T., Ajelli M., Gioannini C., Litvinova M., Merler S. The effect of travel restrictions on the spread of the 2019 novel coronavirus (COVID-19) outbreak. Science. 2020;368:395–400. doi: 10.1126/science.aba9757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gardner L. 2020. https://systems.jhu.edu/research/public-health/ncov-model-2 (Update January 31: modeling the spreading risk of 2019-nCoV Johns Hopkins University center for systems science and engineering). updated January 31, 2020. Available from: [Google Scholar]
- 8.Tang X., Wu C., Li X., Song Y., Yao X., Wu X. On the origin and continuing evolution of SARS-CoV-2. Nat Sci Rev. 2020;7:1012–1023. doi: 10.1093/nsr/nwaa036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim J.M., Park S.Y., Lee D., Kim J.S., Park Y., Gwack J. Genomic investigation of the coronavirus disease-2019 outbreak in the Republic of Korea. Sci Rep. 2021;11:6009. doi: 10.1038/s41598-021-85623-6. Published 2021 Mar 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Di Giallonardo F., Duchene S., Puglia I., Curini V., Profeta F., Cammà C. Genomic epidemiology of the first wave of SARS-CoV-2 in Italy. Viruses. 2020;12:1438. doi: 10.3390/v12121438. Published 2020 Dec 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fattahi Z., Mohseni M., Jalalvand K., Moghadam F.A., Ghaziasadi A., Keshavarzi F. medRxiv; 2020. Two independent introductions of SARS-CoV-2 into the Iranian outbreak. [Google Scholar]
- 12.Bedford T., Greninger A.L., Roychoudhury P., Starita L.M., Famulare M., Huang M.L. Cryptic transmission of SARS-CoV-2 in Washington state. Science. 2020;370:571–575. doi: 10.1126/science.abc0523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang C.J., Ng C.Y., Brook R.H. Response to COVID-19 in Taiwan: big data analytics, new technology, and proactive testing. J Am Med Assoc. 2020;323:1341–1342. doi: 10.1001/jama.2020.3151. [DOI] [PubMed] [Google Scholar]
- 14.Lai C.C., Hsu C.Y., Jen H.H., Yen A.M., Chan C.C., Chen H.H. The bayesian susceptible-exposed-infected-recovered model for the outbreak of COVID-19 on the diamond princess cruise ship [published online ahead of print, 2021 Jan 26] Stoch Environ Res Risk Assess. 2021:1–15. doi: 10.1007/s00477-020-01968-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weber S., Ramirez C., Doerfler W. Signal hotspot mutations in SARS-CoV-2 genomes evolve as the virus spreads and actively replicates in different parts of the world. Virus Res. 2020;289:198170. doi: 10.1016/j.virusres.2020.198170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dietz K. The estimation of the basic reproduction number for infectious diseases. Stat Methods Med Res. 1993;2:23–41. doi: 10.1177/096228029300200103. [DOI] [PubMed] [Google Scholar]
- 17.Zhao S., Lin Q., Ran J., Musa S.S., Yang G., Wang W. Preliminary estimation of the basic reproduction number of novel coronavirus (2019-nCoV) in China, from 2019 to 2020: a data-driven analysis in the early phase of the outbreak. Int J Infect Dis. 2020;92:214–217. doi: 10.1016/j.ijid.2020.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu Y., Gayle A.A., Wilder-Smith A., Rocklöv J. The reproductive number of COVID-19 is higher compared to SARS coronavirus. J Trav Med. 2020;27 doi: 10.1093/jtm/taaa021. taaa021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pan A., Liu L., Wang C., Guo H., Hao X., Wang Q. Association of public health interventions with the epidemiology of the COVID-19 outbreak in Wuhan, China. J Am Med Assoc. 2020;323:1915–1923. doi: 10.1001/jama.2020.6130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van Doremalen N., Bushmaker T., Morris D.H., Holbrook M.G., Gamble A., Williamson B.N. medRxiv; 2020. Aerosol and surface stability of HCoV-19 (SARS-CoV-2) compared to SARS-CoV-1. 2020.03.09.20033217. Published 2020 Mar 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou Q., Gao Y., Wang X., Liu R., Du P., Wang X. Nosocomial infections among patients with COVID-19, SARS and MERS: a rapid review and meta-analysis. Ann Transl Med. 2020;8:629. doi: 10.21037/atm-20-3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Du Q., Zhang D., Hu W., Li X., Xia Q., Wen T. Nosocomial infection of COVID-19: a new challenge for healthcare professionals (Review) Int J Mol Med. 2021;47:31. doi: 10.3892/ijmm.2021.4864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang X., Zhou Q., He Y., Liu L., Ma X., Wei X. Nosocomial outbreak of COVID-19 pneumonia in Wuhan, China. Eur Respir J. 2020;55:2000544. doi: 10.1183/13993003.00544-2020. Published 2020 Jun 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.