ABSTRACT
A protective role for vitamin K in cardiovascular disease (CVD), a leading cause of morbidity and mortality, has been proposed because vitamin K–dependent proteins, such as matrix Gla (γ-carboxyglutamic acid) protein (MGP), are present in vascular tissue. MGP functions as a vascular calcification inhibitor—but only when it is carboxylated, which requires vitamin K. There is more than one naturally occurring form of vitamin K. Phylloquinone (vitamin K1) is found in plant-based foods, whereas menaquinones (vitamin K2) are a class of vitamin K compounds found in animal-based and fermented foods. Phylloquinone and menaquinones are capable of carboxylating MGP and other vitamin K–dependent proteins. In rodent models, high intakes of either phylloquinone or menaquinone reduced vascular calcification. Evidence of the relative importance of phylloquinone and menaquinone to CVD in humans is limited and controversial. In some observational studies, higher dietary menaquinone intake, but not phylloquinone intake, was associated with less coronary artery calcification (a subclinical manifestation of CVD) and a lower risk for clinical CVD events. These findings have led to claims that menaquinones have unique cardiovascular health benefits compared with phylloquinone. However, this claim is not supported by the results of the limited number of intervention trials conducted to date. The purpose of this review is to evaluate the strengths and limitations of the available evidence regarding the role of vitamin K in vascular calcification, CVD, and mortality.
Keywords: vitamin K, phylloquinone, menaquinone, matrix Gla protein, vascular calcification, cardiovascular disease, mortality
This review critically evaluates the current scientific evidence about the role of vitamin K in cardiovascular disease.
There has been a recent plethora of claims regarding the role of vitamin K in protecting against cardiovascular disease (CVD), a worldwide leading cause of morbidity and mortality. Proposed >3 decades ago, this putative protective role is based on the presence of vitamin K–dependent (VKD) proteins in vascular tissue, where they are involved in inhibiting vascular calcification (1). While grounded in biological plausibility, there was a paucity of rigorous studies to support any claims linking low vitamin K status to CVD risk when the last US dietary recommendations for vitamin K were evaluated in 2001 (2). Results from recent studies, using a variety of designs, have led to inconsistent interpretations, some of which are contradictory to our current understanding of vitamin K metabolism. As has been noted with numerous topics in nutrition (3), there is a need to evaluate the evidence relative to appropriate scientific standards. The purpose of this review is to evaluate many of the recent claims regarding vitamin K's role in vascular calcification, CVD, and mortality in the context of available scientific evidence.
Forms of Vitamin K
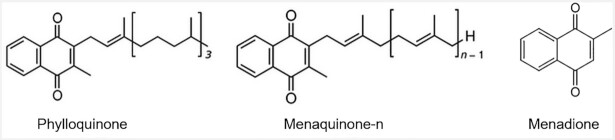
There are multiple naturally occurring forms of vitamin K. Structurally, these forms share a common 2-methyl-1,4-napthoquinone structure but differ in the length and saturation of their prenylated side chain (Figure 1). Phylloquinone (vitamin K1), the plant-based form, has a saturated side chain of 4 isoprenoid units. Menaquinones (vitamin K2) have an unsaturated side chain and variations in length differentiate the forms of menaquinones. While there are 10 menaquinone forms (menaquinone-4–menaquinone-13) found in the food supply, menaquinones are frequently referred to collectively as vitamin K2 (4), which had led to some misunderstanding about their origin and function (5). Menaquinone-4, which is a metabolite of phylloquinone and possibly other menaquinone forms (6–8), has an unsaturated side chain containing 4 isoprenoid units. Some animal-based foods contain menaquinone-4 because menadione in animal feed is also converted to menaquinone-4 (5, 9) (Table 1). Menadione is sometimes referred to as vitamin K3, and has the 2-methyl-1,4-napthoquinone structure but lacks a side chain and does not have vitamin K–related activity unless prenylated to menaquinone. The menaquinone-4 contents of foods, therefore, vary regionally, depending on the menadione content of the feed fed to livestock. Menaquinones-5 through -13 are synthesized by some bacteria and are present in some fermented foods, including dairy, meat, and fermented vegetables (5, 10–13). The type of bacteria used in food processing influences the form and quantity of menaquinones in fermented foods. For example, natto, traditionally consumed in some regions of Japan, is rich in menaquinone-7 because the bacteria used to produce natto (Natto Bacillus) synthesize menaquinone-7. The menaquinone content of dairy foods also varies based on the bacteria used in the fermentation process (5). Bacterially synthesized menaquinones are also found in the gut microbiome. However, the majority of menaquinones synthesized in the gut are in the colon, which lacks bile salts needed for absorption, so the contribution of intestinally derived menaquinones to overall vitamin K nutritional status is uncertain (5). Of the menaquinones, menaquinone-4 and menaquinone-7 are the most-studied forms with respect to human health and disease but significant knowledge gaps persist regarding their role in CVD and other health outcomes.
FIGURE 1.
Molecular structures of vitamin K.
TABLE 1.
Vitamin K contents of foods
| Vitamin K form and food | Amount per serving |
|---|---|
| Phylloquinone 1 | |
| Spinach, raw | 121 µg/25 g |
| Kale, raw | 80 µg/20 g |
| Green leaf lettuce | 45.5 µg/36 g |
| Lo mein, meatless | 25.4 µg/200 g |
| Mayonnaise | 23 µg/14 g |
| Canola oil | 10 µg/14 g |
| Olive oil | 8.4 µg/14 g |
| Macaroni and cheese | 7.1 µg/230 g |
| Menaquinone-41 | |
| Pepperoni | 35.4 µg/85 g |
| Hot dog, 5-inch | 17.1 µg/hot dog |
| Beef, cooked | 0.17 µg/85 g |
| Chicken, cooked | 35 µg/85 g |
| Cheddar cheese | 2 µg/17g |
| Mozzarella cheese, part skim | 1.1 µg/28 g |
| Menaquinone-72 | |
| Natto | 360 µg/40g |
Data from USDA Agricultural Research Service. USDA food-composition databases. Available from: https://ndb.nal.usda.gov/ndb/nutrients/index.
Data from reference 19.
Dietary Vitamin K Intakes
The USDA Food Data Central provides phylloquinone quantities for nearly 16,000 foods in the US food supply and menaquinone-4 contents for just >600 foods (14). The Dutch food-composition database provides values for phylloquinone, total menaquinones (sum of menaquinone-4–menaquinone-13) and total vitamin K (phylloquinone plus total menaquinone) contents of 400 foods (15). Food-composition databases in the United Kingdom, France, and Germany include phylloquinone only, while the databases in Canada, Denmark, and Sweden do not specify whether vitamin K includes phylloquinone, menaquinones, or both (5). Databases in some countries do not provide information about the quantity of vitamin K in foods (16, 17). Comparing vitamin K contents of foods using food-composition databases from different countries can be problematic because the analytical methods used to quantify the vitamin K forms, especially menaquinones, in food and other matrices can vary from laboratory to laboratory. Participation in external quality assurance programs is therefore essential to ensure assay standardization (18). Although more is known about the phylloquinone content of the food supply, knowledge about dietary vitamin K overall would be enhanced by a more thorough evaluation of menaquinones, as well as phylloquinone, in foods, especially in regions of the world for which this information is not currently available.
Understanding the bioavailability and metabolism of phylloquinone and menaquinones is critical to understanding their contribution to CVD and other health conditions. Stable isotope tracers can be used safely in human studies to obtain insight into the dynamics of nutrient metabolism and distribution (20, 21). Through the use of stable isotopes it has been shown that the bioavailability of phylloquinone has high interindividual variation (22) and varies by food matrix and meal composition (23). Some have suggested that some menaquinone forms are more bioavailable than phylloquinone based on studies that measured the phylloquinone and menaquinone concentrations in blood over 48–96 h postingestion (24, 25). However, these studies did not use stable isotopes. To the best of our knowledge, no study has examined menaquinone bioavailability in humans using stable isotopes. This represents an important gap in our understanding of vitamin K bioavailability and metabolism. Yet, it continues to be perpetuated in the scientific literature that some menaquinones have superior bioavailability compared with phylloquinone (4, 26, 27). In the absence of any study evaluating relative bioavailability of all known dietary vitamin K forms using stable isotopes, this assumption is of concern.
The US Institute of Medicine and the European Food Safety Authority have set an Adequate Intake (AI) for vitamin K because both institutes concluded that there are insufficient data to establish an Estimated Average Requirement, which is needed to establish a RDA (by the Institute of Medicine) or a Population Reference Intake (by the European Food Safety Authority) (2, 28). The Institute of Medicine's AI for all age groups is based on median phylloquinone intakes reported in NHANES III (1988–1994). For women and men aged ≥19 y the respective AIs are 90 µg/d and 120 µg/d (2). The European Food Safety Authority set an AI of 1 µg phylloquinone/(kg body weight · d) for all age and sex groups or 70 µg/d for most adults (28), which is consistent with recommendations in Australia and New Zealand (29). As described below, vitamin K is essential for hemostasis, and the AIs set by the Institute of Medicine and European Food Safety Authority are based on amounts assumed to support normal blood coagulation. Abnormal vitamin K–related coagulopathies are rare in the general population since normal coagulation is maintained when vitamin K intakes are as low as 10 µg/d (2, 28, 30, 31). However, it is uncertain if the current AIs are sufficient to meet all vitamin K needs of extrahepatic tissues (31).
The primary dietary sources of phylloquinone are green-leafy vegetables and vegetable oils, but a recent analysis of NHANES (2011–2012) indicates mixed dishes (e.g., pasta and rice dishes) are also significant contributors (32) (Table 1). In the United States, 55% of men and 34% of women between 20 and 30 y old did not meet the recommended vitamin K AI, while among adults >70 y old, 68% of men and 44% of women did not meet the AI (32). In Ireland, 60% of adults aged 18–35 y did not meet the European Food Safety Authority's AI, whereas among adults aged ≥65 y this prevalence was 48% (33). In the United Kingdom, population vitamin K intakes decreased from 1986–1987 to 2000–2001, which was attributed to a decrease in leafy-green vegetables over that same time period (34). Overall, there are a limited number of studies that have systematically evaluated vitamin K intakes at the population level. Additional research is needed to identify regions at risk for low vitamin K intake and obtain a better understanding of contributors to vitamin K intakes globally.
Current US dietary recommendations do not consider menaquinones because at the time the recommendations were established (in 2001), the USDA food-composition database (now Food Data Central) contained only the phylloquinone content of foods. While menaquinone contents of foods are being incorporated into some databases (14, 15), current knowledge about the menaquinone contents of the food supply is still quite limited. In their most recent report, the European Food Safety Authority did not find sufficient evidence to set a separate dietary recommendation for menaquinone intake (28). Until menaquinones have been systematically quantified in the food supply and more is known about the dietary menaquinone intakes in the population and their relative bioavailability, it is premature to suggest 1) menaquinone supplements are needed to secure adequate vitamin K intake (35) and 2) menaquinone intakes require a separate dietary recommendation (4).
Vitamin K Function
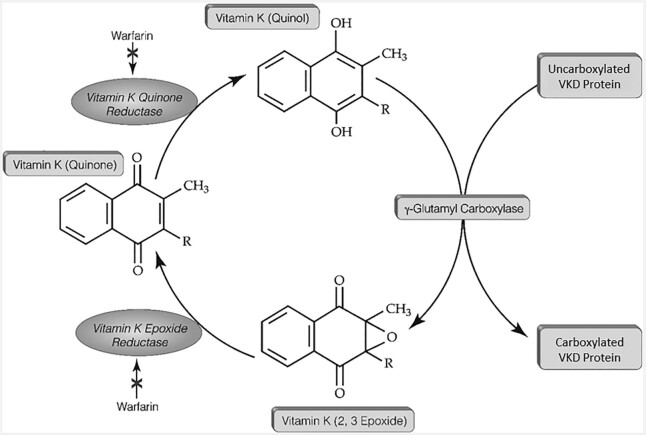
Vitamin K is required for the carboxylation and consequent activation of a family of proteins referred to as VKD proteins. A vitamin K cycle is responsible for VKD protein carboxylation (Figure 2). The γ-glutamyl carboxylase converts clusters of specific glutamic acid (Glu) residues to carboxylated Glu [Gla (γ-carboxyglutamic acid)] using reduced vitamin K that becomes oxidized to an epoxide form. Vitamin K epoxide is then reduced by the vitamin K epoxide reductase (VKORC1) to regenerate reduced vitamin K for continuous carboxylation. This vitamin K cycle is essential to hemostasis, which is maintained by VKD factors that either promote or attenuate blood clotting [factors II (prothrombin), VII, IX, and X and proteins C, S, and Z, respectively]. Anticoagulant drugs have been developed to suppress clotting, for example, warfarin and acenocoumaral, which inhibit VKORC1 to decrease overall carboxylation (36).
FIGURE 2.
A vitamin K cycle drives the carboxylation of VKD proteins. VKD, vitamin K–dependent.
The efficacy of phylloquinone and individual menaquinones in supporting VKD protein carboxylation is poorly understood. Tissues express both phylloquinone and menaquinones, at varying levels, which makes it challenging to understand the impact of individual forms. The only defined system to address vitamin K dependence is a biochemical test showing that both phylloquinone and some menaquinones serve as cofactors for the carboxylase (37). The relative efficiency of these forms in supporting carboxylase activity is unknown. Additional unknowns are the ability of the VKORC1 to reduce phylloquinone and menaquinones, as required for carboxylation, and how efficiently these forms are recycled between VKORC1 and the carboxylase. It has been suggested that certain VKD proteins require phylloquinone and other VKD proteins require menaquinones (26, 38). This conjecture is based on the high concentrations of phylloquinone present in liver where the VKD clotting factors are synthesized (36). Menaquinones are also abundant in liver (39). Currently, there is no evidence that the VKD clotting proteins are exclusively phylloquinone dependent, while extrahepatic VKD proteins are exclusively menaquinone dependent. In vivo studies indicate that both phylloquinone and menaquinone forms are capable of carboxylating VKD clotting factors—for example, as shown by the dose-dependent reduction in the international normalized ratio in warfarin-treated adults by phylloquinone and menaquinone-7 (24). However, when menadione was given to patients to reverse warfarin-induced anticoagulation, it was ineffective (40).
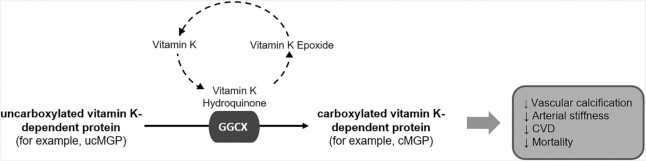
Matrix Gla protein (MGP) and Gla-rich protein (GRP) are VKD proteins found in vascular (and other) tissues where they function as calcification inhibitors, but only when they are carboxylated, which requires vitamin K. It is through this mechanism that vitamin K is thought to be involved in reducing vascular calcification, which is indicative of subclinical CVD (Figure 3). MGP and GRP in atherosclerotic arterial tissue are primarily uncarboxylated (nonfunctional), whereas in healthy tissue these proteins are primarily carboxylated (functional) (41, 42). Osteocalcin (OC) is a VKD protein that is synthesized by osteoblasts, providing the basis for the hypothesis that vitamin K protects against bone loss (43). Vascular calcification and bone remodeling share common mechanisms. OC, which is not normally expressed in vascular tissue, is expressed during vascular calcification (44, 45). Growth arrest specific-6 (Gas6) has also been proposed to have a role in vascular calcification and cardiac health (46, 47), but little is known about the relevance of Gas6 carboxylation to cardiovascular outcomes. With respect to CVD, MGP has been the most studied VKD protein, so this review will focus primarily on MGP.
FIGURE 3.
Potential mode of vitamin K action in CVD and mortality. CVD, cardiovascular disease; GGCX, γ-glutamyl carboxylase; cGMP, carboxylated matrix Gla protein; ucMGP, uncarboxylated matrix Gla protein.
Biomarkers of MGP Function
Multiple forms of MGP are detectable in circulation that differ based on the carboxylation and phosphorylation status of the protein (48). Carboxylation is required for MGP to bind calcium, but the functional consequence of MGP phosphorylation is uncertain. Antibodies directed against different isoforms of MGP are available that detect the different circulating MGP forms (48, 49). However, only the dephosphorylated-uncarboxylated (dp-ucMGP) form decreases in response to vitamin K supplementation and increases in response to vitamin K antagonism (warfarin) (48), consistent with what is understood about the vitamin K cycle (Figure 2). Assays that measure total ucMGP (t-ucMGP; uncarboxylated MGP measured independent of the phosphorylation status) (48, 49) and dephosphorylated carboxylated MGP (dp-cMGP) have also been developed (48, 49). t-ucMGP assayed using a commercially available ELISA (catalog no. CSB-EC013789HU; Cusabio) decreased in response to menaquinone-7 supplementation in hemodialysis patients (49). However, t-ucMGP measured using an in-house ELISA did not change in response to menaquinone-4 or menaquinone-7 supplementation, or vitamin K antagonism with warfarin in healthy adults (48). dp-cMGP is also reported to decrease (48) or not change (50) in response to vitamin K supplementation and not change in response to warfarin treatment (48). These disparate results are inconsistent with the biochemistry of vitamin K and draw into question the validity of these measures.
The dual antibody ELISA to measure plasma dp-ucMGP was developed by VitaK (Maastricht, Netherlands). In 2010, VitaK entered into a research agreement with Immunodiagnostic Systems, Ltd. (IDS, Bolden, UK) (51), and IDS automated and commercialized the assay. As of October 2018, VitaK is no longer a business entity, but the assay to measure plasma dp-ucMGP is available from IDS. The pre-commercialization and IDS assays are both dual antibody ELISAs directed against the same amino acid sequences (48, 52). However, some differences between the 2 have presented challenges when comparing plasma dp-ucMGP concentrations between studies that used the different assay platforms. Pre-commercialization, the assay's lower limit of detection was 21 pmol/L (48), while the IDS assay's lower limit of detection is 300 pmol/L (53). Griffin et al. (53) measured dp-ucMGP in healthy Irish Caucasian adults using the IDS assay and reported 39% had concentrations <300 pmol/L, with a median (range) of 318 (<300–698) pmol/L. Based on these data, the investigators indicated a reference interval of <300–532 pmol/L for healthy adults. These adults were not taking prescription or over-the-counter medications during the week preceding recruitment, were free of prediabetes or diabetes, and had not been diagnosed with cardiac, thyroid, liver or metabolic bone disease, or anemia (53). In general population–based cohorts in Denmark, Switzerland, and Czechoslovakia, the mean plasma dp-ucMGP concentrations ranged from 430 to 570 pmol/L (54–56). Yet, 77% of adults in a Dutch population–based cohort had plasma dp-ucMGP <300 pmol/L (57), while the prevalence of plasma dp-ucMGP <300 pmol/L was 7% in a Danish population–based cohort (55). There is not yet an established threshold of plasma dp-ucMGP that indicates an increased risk for CVD and/or mortality. However, based on the available evidence from observational studies conducted in general population–based cohorts, the threshold is >400 pmol/L (54, 58, 59). dp-ucMGP tends to be higher in clinical populations. For example, in patients with kidney disease, plasma dp-ucMGP typically exceeds 1000 pmol/L (60). Based on the available evidence, the relevance of discriminating dp-ucMGP concentrations <300 pmol/L is uncertain.
Both phylloquinone and menaquinone-7 supplementation reduce plasma dp-ucMGP (61–68), but the magnitude of the response of dp-ucMGP to phylloquinone or menaquinone-7 supplementation is highly variable (Table 2). Reductions in plasma dp-ucMGP ranging from 17% to 66% were reported in response to 360 µg/d menaquinone-7 supplementation for 6 wk to 6 mo (61–66). This variability may be related to differences in study participants and/or duration. However, in 2 separate menaquinone-7 dose–response studies conducted in hemodialysis patients, the response of dp-ucMGP to menaquinone-7 supplementation was highly variable, even within similar patient populations over similar time periods. For example, in 2 separate studies of hemodialysis patients, plasma dp-ucMGP was reported to decrease by 18% in response to 40 μg/d of menaquinone-7 supplementation for 8 wk (63) and by 17% in response to 360 µg/d menaquinone-7 supplementation given 3 times/wk (64). The different dosing regimens (daily vs. 3×/wk) could explain why dp-ucMGP changed similarly in response to
TABLE 2.
Change in circulating dp-ucMGP concentrations in response to vitamin K supplementation1
| Vitamin K supplement form and dose | Design and duration | Change in dp-ucMGP | |||||
|---|---|---|---|---|---|---|---|
| Country | Participants | Baseline dp-ucMGP,2 pmol/L | Absolute, pmol/L | Percentage | Reference | ||
| Phylloquinone (500 µg/d) (with 400 IU cholecalciferol/d and 600 mg Ca/d) | RCT, 3 y | United States | 452 older men and postmenopausal women, 60–80 y old | 485 ± 267 | −450 ± 281 | −79% | (67) |
| Menaquinone-7, 180 μg/d or 360 μg/d | RCT, 12 wk | Netherlands | 60 healthy men and postmenopausal women, 40–65 y old | 360 μg/d: 391 ± 118;180 μg/d: 401 ± 131 | 360 μg/d: −182;180 μg/d: −125 | 360 μg/d: −46%;180 μg/d: −31% | (61) |
| Menaquinone-7; low dose: 10, 20, or 45 μg/d; high dose: 90, 180, or 360 μg/d | RCT, 12 wk | Netherlands | 42 healthy men and women, 18–45 y old | Low dose: 454 (166–621);high dose: 414 (175–697) | Low dose: −52;high dose: −177 | Low dose: −11%;high dose: −40% | (62) |
| Menaquinone-7, 40, 135, or 360 μg/d | RCT, 6 wk | Germany | 50 hemodialysis patients | 40 μg/d: 3750 (1453–7481);135 μg/d: 2754 (954–6223);360 μg/d: 2930 (566–5068) | 40 μg/d: −101 (−3169 to 1118);135 μg/d: 730 (−2755 to 438);360 μg/d: −978 (−3844 to 438) | 40 μg/d: −18%;135 μg/d: −37%;360 μg/d: −61% | (63) |
| Menaquinone-7, 180 μg/d | RCT, 3 y | Netherlands | 244 healthy postmenopausal women | 511 ± 236 | −188 ± 157 | −32% | (69) |
| Menaquinone-7, 360, 720, or 1080 µg 3×/wk | RCT, 8 wk | Belgium | 165 hemodialysis patients | 360 μg/d: 2872 (123–7539);720 μg/d: 2783 (500–7569);1080 μg/d: 3205 (857–7813) | 360 μg/d: −566;720 μg/d: −962;1080 μg/d: −1487 | 360 μg/d: −17%;720 μg/d: −33%;1080 μg/d: −46% | (64) |
| Menaquinone-7, 360 µg/d | Single-arm intervention, 8 wk | Lebanon | 60 renal transplant patients | −55% | (65) | ||
| Phylloquinone, 2000 µg/d | RCT, 1 y | Germany | 99 patients with asymptomatic or mildly symptomatic aortic valve calcification | 432 ± 149 | −189 | −54% | (68) |
| Menaquinone-7, 360 µg/d | RCT, 4 wk | Lebanon | 50 hemodialysis patients | 3179 (1825–4340) | −2884 | −86% | (70) |
| Menaquinone-7, 360 µg/d | RCT, 6 mo | Netherlands | 68 men and women with T2D, mean age 69 y | 613 (513–684) pmol/L | −197 | −32% | (66) |
| Menaquinone-7, 400 µg/d | RCT, 1 y | UK | 159 patients with stage 3b-5 CKD | 1187 | −2053 | −17% | (71) |
CKD, chronic kidney disease; dp-ucMGP, dephosphorylated uncarboxylated matrix Gla protein; RCT, randomized controlled trial; T2D, type 2 diabetes.
Values are means ± SEs, means ± SDs, or medians (IQR).
Difference in geometric means between baseline and follow-up.
very different menaquinone-7 doses. It could also reflect the measurement variability in the assay, which then precludes direct comparison across studies in terms of magnitude of change. To our knowledge, the response of dp-ucMGP to supplementation with other menaquinone forms is unknown. Phylloquinone supplementation reduced dp-ucMGP in generally healthy older adults given 500 µg/d for 3 y (67) and in patients with asymptomatic or mildly symptomatic aortic valve calcification given 2000 µg/d for 1 y (68). Collectively, these studies indicate phylloquinone and menaquinone are capable of carboxylating MGP, which contradicts the claim that MGP carboxylation is menaquinone dependent (26, 38). However, differences in study design and supplement dose, combined with concerns about assay consistency, challenge the ability to directly compare the relative change in plasma dp-ucMGP in response to menaquinone-7 or phylloquinone. Until studies designed to directly compare the effects of multiple vitamin K forms on MGP carboxylation are completed, it is premature to claim menaquinone-7 supplementation is most effective at lowering plasma dp-ucMGP, as some have indicated (4, 72).
Vitamin K, CVD, and Mortality
Arterial calcification can manifest as intimal and/or medial. Intimal calcification tends to occur in the coronary arteries [known as coronary artery calcification (CAC)] and other large arteries, and is consistent with atherosclerosis (73). Medial calcification occurs more peripherally and is associated with arterial stiffness (74). Both intimal and medial calcification are associated with an increased risk of clinical CVD and all-cause mortality (75, 76).
Rodent studies
Early in vivo evidence that vitamin K reduces arterial calcification is provided by Schurgers et al. (77), who treated rats with warfarin-induced arterial calcification with either phylloquinone or menaquinone-4. After 6 wk, the rats treated with high doses of phylloquinone or menaquinone-4 had significantly less aortic and carotid artery calcium compared with rats not treated with vitamin K. Treatment with either phylloquinone or menaquinone-4 also improved arterial distensibility, which can be impaired when calcium accumulates in arterial tissue. There were no reported differences in arterial calcium content or distensibility between the rats treated with high doses of phylloquinone or menaquinone-4, suggesting both forms were similarly capable of reducing arterial calcification and improving distensibility.
Observational studies
Multiple scientific reviews focused on vitamin K and CVD have concluded that menaquinones are uniquely cardioprotective (4, 26, 35, 38, 72, 78). This conclusion is based primarily on the results of observational studies that reported higher dietary menaquinone intake was associated with a lower risk for CVD, whereas phylloquinone intake was not (79–81) (Table 3). In one analysis of the European Prospective Investigation into Cancer and Nutrition (PROSPECT-EPIC) cohort, a 10-µg/d increase in energy-adjusted menaquinone intake was associated with 8–9% lower risk for coronary heart disease (CHD) (80). Menaquinones-7 through -9 were identified as key contributors to the lower CHD risk associated with menaquinone intake. Phylloquinone intake was not associated with CHD risk. In a subsequent analysis that included PROSPECT-EPIC participants, neither phylloquinone nor menaquinone intake was associated with all-cause or CVD-specific mortality (82). These studies used food-frequency questionnaires (FFQs) to estimate phylloquinone and menaquinone intakes. While FFQs are commonly used to estimate dietary intakes in population-based studies, they require validation because an FFQ with low validity can lead to erroneous diet–disease associations (83). The relative validity of the Dutch-EPIC FFQ compared with intakes estimated by monthly 24-h dietary recalls over 1 y was low for phylloquinone and menaquinones-4 through -6 (84), which is a limitation to these studies (79–82). The limited characterization of menaquinones in the food supply challenges quantifying menaquinone intake in population-based studies, which further challenges the results of these studies (79–82). Currently, more is known about the menaquinone contents of dairy foods compared with the menaquinone contents of other food groups (5, 10, 12, 85). It is therefore plausible that the reported associations of menaquinone intake with CVD may reflect general health benefits of dairy foods.
TABLE 3.
Observational studies of vitamin K status, CVD, and mortality1
| Participants | Study design | Measurement of vitamin K exposure | Outcome | Results | Reference |
|---|---|---|---|---|---|
| Rotterdam study, 4807 men and women; age 67 ± 8, 60% female | Prospective, 7.2-y follow-up | Phylloquinone and menaquinone intake (µg/d) | Aortic calcification, CHD, mortality | Menaquinone, but not phylloquinone, intake inversely associated with severe aortic calcification, incident CHD, CHD mortality, all-cause mortality | (81) |
| 564 Dutch postmenopausal women; age 67 ± 5 | Cross-sectional | Menaquinone intake (µg/d) | CAC Agatston score | High menaquinone intake associated with less coronary calcification | (79) |
| Prospect-EPIC study, 16,057 Dutch postmenopausal women, mean age 59 ± 6 | Prospective, 8.1-y follow-up | Phylloquinone and menaquinone intake (µg/d) | CVD | Menaquinone, but not phylloquinone, intake inversely associated with CVD risk | (80) |
| 33,289 Men and women from the Dutch EPIC cohort, mean age 48 ± 12, 74% female | Prospective; 16.8-y follow-up | Phylloquinone and menaquinone intake (µg/d) | CVD mortality and all-cause mortality | Neither menaquinone nor phylloquinone intake associated with CVD mortality or all-cause mortality risk | (82) |
| 35,476 Men and women from the Dutch EPIC cohort, mean age 49 ± 12, 74% female | Prospective, 12.1-y follow-up | Phylloquinone and menaquinone intake (µg/d) | Stroke | Neither menaquinone nor phylloquinone intake associated with stroke risk | (93) |
| 7216 Spanish adults from the PREDIMED study, mean age 67 ± 7 | Prospective, 4.8-y follow-up | Phylloquinone and menaquinone intake (µg/d) | All-cause and CVD mortality | Phylloquinone, but not menaquinone, intake inversely associated with all-cause mortality | (86) |
| 72,874 American women from the Nurses’ Health Study, mean age 50 ± 7 y | Prospective, 16-y follow-up | Phylloquinone intake | CHD | Phylloquinone intake not associated with CHD after adjustment for healthy diet and lifestyle | (91) |
| 40,087 American men from the Health Professionals Follow-Up Study, mean age 53 ± 9 y | Prospective, 16-y follow-up | Phylloquinone intake | CHD | Phylloquinone intake not associated with CHD after adjustment for healthy diet and lifestyle | (92) |
| 5296 American men and women from NHANES, age >50 y | Cross-sectional | Phylloquinone intake (µg/d) | Arterial stiffness: pulse pressure | Inadequate dietary phylloquinone intake was associated with higher arterial pulse pressure | (94) |
| 3401 Americans with CKD from NHANES, mean age 61.9 y, 63% female | Prospective, 13.3-y follow-up | Phylloquinone intake (µg/d) | All-cause and CVD mortality | Adequate vitamin K intake associated with reduced risk for all-cause and CVD mortality | (95) |
| 508 Dutch postmenopausal women, age 56 ± 6 y | Cross-sectional | Plasma phylloquinone (nmol/L) | CAC, aortic and mitral valve, aortic artery calcification | Detectable circulating phylloquinone associated with more CAC | (96) |
| 857 American men and women from the MESA, mean age 64 ± 10 y, 45% female | Prospective case-cohort, 2.5-y follow-up | Serum phylloquinone (nmol/L) | CAC progression | Plasma phylloquinone <1.0 nmol/L associated with higher odds of CAC progression in treated hypertensives, but not in those not treated for hypertension | (97) |
| 1061 Older American men and women from Health ABC, mean age 74 ± 5 y, 58% female | Prospective, 12.1-y follow-up | Plasma phylloquinone (nmol/L) | CVD | Plasma phylloquinone <0.2 nmol/L (nondetectable) associated with higher CVD risk in treated hypertensives, but not in those not treated for hypertension | (98) |
| 3891 American men and women from MESA, Health ABC, and the Framingham Offspring, mean age 65 ± 11 y, 55% female | Meta-analysis, prospective, 13.0-y follow-up | Circulating phylloquinone (nmol/L) | CVD and all-cause mortality | Plasma phylloquinone <0.5 nmol/L associated with higher risk of all-cause mortality but not CVD | (99) |
| 200 Dutch postmenopausal women, mean age 67 ± 6 y | Cross-sectional | Plasma dp-ucMGP (pmol/L) | CAC | Borderline positive association between dp-ucMGP and CAC (P = 0.065) | (100) |
| 1087 Czech men and women from the MONICA study, mean age 55 ± 13 y, 53% female | Cross-sectional | Plasma dp-ucMGP (pmol/L) | Arterial stiffness by aortic and distal PWV | Individuals with highest circulating dp-ucMGP (≥641 pmol/L) more likely to elevated aortic PWV; dp-ucMGP was not associated with femoral artery PWV | (56) |
| 1001 Swiss men and women from Kidney Project on Genes in Hypertension (SKIPOGH), mean age 47 ± 17 y, 52% female | Cross-sectional | Plasma dp-ucMGP (pmol/L) | Aortic PWV | Plasma dp-ucMGP positively associated with aortic PWV | (54) |
| 66 American type 2 diabetics, mean age 62 ± 12 y, 9% female | Cross-sectional | Plasma dp-ucMGP (pmol/L) | Carotid-femoral PWV | Plasma dp-ucMGP positively associated with carotid-femoral PWV | (101) |
| 489 Danish adults, mean age 51 ± 13 y, 46% female | Cross-sectional | Plasma dp-ucMGP (pmol/L) | Estimated PWV (ePWV, from mean arterial pressure and age) | Plasma dp-ucMGP positively associated with ePWV | (55) |
| 147 Norwegian patients with symptomatic aortic stenosis, mean age 74 ± 10 y | Prospective, 1.9-y follow-up | Plasma dp-ucMGP (pmol/L) | All-cause mortality | Increased mortality risk in patients with >950 pmol/L dp-ucMGP | (102) |
| 107 Dutch patients with CKD, mean age 67 ± 13 y, 40% female | Prospective, 2.0-y follow-up | Plasma dp-ucMGP (pmol/L) | All-cause mortality | Increased mortality risk in patients with >921 pmol/L dp-ucMGP | (103) |
| 518 Dutch type 2 diabetics in the EPIC cohort, mean age 51 ± 7 y, 82% female | Prospective, 11.2-y follow-up | Plasma dp-ucMGP (pmol/L) | CHD, CVD, peripheral artery disease, heart failure | dp-ucMGP positively associated with CHD, CVD, peripheral artery disease, heart failure risk | (104) |
| 2940 Dutch men and women from the EPIC cohort, mean age 50 ± 12 y, 75% female | Prospective case-cohort, 11.5-y follow-up | Plasma dp-ucMGP (pmol/L) | CVD and stroke | No association between circulating dp-ucMGP and stroke risk or CVD risk | (105) |
| 577 older Dutch men and women from the Longitudinal Aging Study Amsterdam, mean age 59.9 ± 2.9 y, 55% female | Prospective, 5.6-y follow-up | Plasma dp-ucMGP (pmol/L) | CVD | Increased risk of CVD in highest tertile of dp-ucMGP (>400 pmol/L) | (58) |
| 799 Czech patients with stable vascular disease, 65 ± 9 y, 29% female | Prospective, 5.6-y follow-up | Plasma dp-ucMGP (pmol/L) | Mortality | Increased risk of mortality in highest quartile of dp-ucMGP (≥977 pmol/L) | (106) |
| 518 Dutch stable kidney transplant recipients, mean age, 51 ± 12 y, 44% female | Prospective, 9.8-y follow-up | Plasma dp-ucMGP (pmol/L) | Mortality | Increased risk of mortality in highest quartile of dp-ucMGP (≥1535 pmol/L) compared with lowest (<735 pmol/L) | (107) |
| 2318 Flemish adults, mean age 44 ± 18 y, 51% female | Prospective, 14.1-y follow-up | Plasma dp-ucMGP (pmol/L) | Total mortality, CVD mortality, noncancer mortality, coronary events | Total, CVD, and noncancer mortality risk increased in curvilinear manner as dp-ucMGP increased; coronary event risk decreased in curvilinear manner as dp-ucMGP increased | (108) |
| 1061 Older American men and women from Health ABC, mean age 74 ± 5 y, 58% female | Prospective, 12.1-y follow-up | Plasma dp-ucMGP (pmol/L) | CVD | Plasma dp-ucMGP not associated with CVD risk | (98) |
| 4275 Dutch men and women in the Prevention of Renal and Vascular End-Stage Disease Study, mean age 53 ± 12 y, 54% female | Prospective, 10-y follow-up | Plasma dp-ucMGP (pmol/L) | CVD mortality, all-cause mortality | Positive J-shape association between plasma dp-ucMGP and CVD mortality and all-cause mortality; cutoff for all-cause mortality was 414 pmol/L and for CVD mortality was 557 pmol/L | (59) |
| Meta-analysis | 7 prospective studies | Plasma dp-ucMGP (pmol/L) | CVD, CVD mortality, all-cause mortality | Plasma dp-ucMGP positively associated with all-cause mortality and cardiovascular mortality risk, but not with CVD risk | (109) |
1CAC, coronary artery calcification; CHD, coronary heart disease; CKD, chronic kidney disease; CVD, cardiovascular disease; dp-ucMGP dephosphorylated uncarboxylated matrix Gla protein; EPIC, European Prospective Investigation into Cancer and Nutrition; MESA, Multi-Ethnic Study of Atherosclerosis; MONICA MonItoring Trends and Determinants in Cardiovascular Disease, PREDIMED Prevention with Mediterranean Diet, PWV, pulse-wave velocity.
In the Prevention with Mediterranean Diet (PREDIMED) cohort, baseline phylloquinone, but not menaquinone, intake was associated with a 36% lower risk for all-cause mortality over 4.8 y of follow-up (86). Neither baseline phylloquinone nor menaquinone intakes were associated with CVD mortality. Longitudinally, participants who increased their phylloquinone or menaquinone intake during follow-up had a 35–37% lower risk for all-cause mortality compared with those who decreased or did not change their intake. Those who increased their phylloquinone intake also had a 38% lower risk of CVD mortality risk (86). The validity of the PREDIMED FFQ for phylloquinone and menaquinone intakes was evaluated relative to four 3-d dietary records, but data quantifying the relative validity are not provided (86). Phylloquinone intakes estimated using FFQs were consistently higher than when estimated using a 5-d diet record (87, 88), which may reflect overreporting of vegetables when an FFQ is used, as previously reviewed (89). In 2 separate US cohorts, phylloquinone intake and plasma phylloquinone concentrations were positively correlated when intakes were <200 µg/d, but not when intakes exceeded 200 µg/d (89, 90). This suggests that an FFQ may not accurately capture phylloquinone intakes in regions where the intake typically exceeds 200 μg/d (79–81, 86). An additional limitation to studies linking vitamin K to CVD, mortality, and/or other health outcomes based on dietary phylloquinone intake is residual confounding. Phylloquinone is found primarily in green-leafy vegetables and plant oils; thus, phylloquinone intakes reflect healthy diets and lifestyles (91, 92). Adjustment for independent healthy diet and/or lifestyle characteristics could minimize this residual confounding, but it is unlikely to be completely eliminated.
When information on nutrient content of the food supply is limited, as is the case with menaquinones, biomarkers of nutrient status are often used as an indicator of intake (110). Some research groups have reported measurement of circulating menaquinones in general and clinic-based populations (111–113). In patients with chronic kidney disease, lower concentrations of menaquinone-4 and menaquinone-7 were associated with significantly higher odds of aortic artery and iliac artery calcification, respectively, and low menaquinone-5 concentrations were found to be associated with lower odds for abdominal aorta calcification (111). However, the assays used in these studies (111–113) were not validated using external quality-assurance programs. External quality-assurance programs are vital to ensure analytical reliability of in-house assays. A vitamin K external quality-assurance scheme (KEQAS) program has been available for circulating phylloquinone assays since 1996 (18). Recently similar schemes for circulating menaquinone-4 and menaquinone-7 have also become available through KEQAS (114), although currently 75% fewer laboratories participate in the menaquinone-4 and -7 schemes compared with the phylloquinone scheme. Quality-assurance schemes for assays that measure other menaquinone forms in circulation are not available. Moving forward, the rigor of studies linking circulating phylloquinone, menaquinone-4, and menaquinone-7 to CVD and other health outcomes will be strengthened by laboratories participating in KEQAS to ensure assay validation. Ideally, KEQAS participation should be reported in all scientific reports of circulating phylloquinone, menaquinone-4, and menaquinone-7 as well.
A limited number of studies have evaluated the association of circulating phylloquinone with CVD and mortality (96–99) (Table 3). Moreover, the laboratory that measured circulating phylloquinone in these studies participates in KEQAS. In a cross-sectional analysis of Dutch postmenopausal women, higher plasma phylloquinone was associated with a higher prevalence of coronary calcium (96). However, the plasma samples in this study were not obtained in a fasted state and triglyceride measures were unavailable. This is a notable limitation because circulating phylloquinone concentrations peak 6–10 h postprandially (115) and circulating phylloquinone is transported on triglyceride-rich lipoproteins (116). In order for circulating phylloquinone to better reflect vitamin K nutritional status and minimize confounding by triglycerides, it is optimal to measure from fasting samples and/or adjust for triglycerides statistically. In 2 US cohorts, low serum phylloquinone (measured from fasting samples) was associated with a higher odds for CAC progression (97) and incident CVD (98) (adjusted for triglycerides) in adults treated for hypertension but not in those not treated for hypertension. However, more recently, this finding was not replicated in a participant-level meta-analysis of 3 US cohorts. In this study, participants with circulating phylloquinone <0.5 nmol/L had a 19% higher risk for all-cause mortality compared with those with concentrations >1.0 nmol/L, while the risk for incident CVD did not significantly differ according to circulating phylloquinone (99). The association of circulating phylloquinone with CVD or mortality did not differ according to hypertension or other CVD risk factors. There were fewer CVD events than deaths in these cohorts, so it is possible the study was underpowered to detect a significant association with incident CVD. Alternatively, the all-cause mortality outcome may better reflect the range of morbidity associated with worse systemic vascular health than incident CVD (99). There is currently no established threshold to define sufficient or insufficient vitamin K status based on circulating phylloquinone. When the Institute of Medicine's recommended intakes are met, circulating phylloquinone approximates 1.0 nmol/L, so concentrations <0.5 nmol/L would reflect intakes that are less than half of the AI (2, 117). Given the overall paucity of data, future research is needed to identify a threshold of circulating phylloquinone below which the putative risk for CVD and/or mortality increases.
The association of the different circulating MGP forms, including plasma dp-ucMGP, with vascular calcification and CVD is variable, as reviewed by Barrett et al. (118). In several population- and clinic-based studies, plasma dp-ucMGP was positively associated with vascular calcification, arterial stiffness, and/or CVD (54, 58, 100, 103, 104, 119), while others reported no association (98, 105, 120) (Table 3). The amount of dp-ucMGP in circulation depends, in part, on the total amount of MGP available (67). Unless dp-ucMGP is corrected for total MGP, it may not only reflect vitamin K status but also the amount of MGP synthesized in vascular (and other) tissues. Because the assays that measure dp-ucMGP and total MGP are on different platforms, plasma dp-ucMGP is rarely corrected for total MGP concentrations. Therefore, it may be inaccurate to attribute associations of dp-ucMGP with CVD and other health outcomes entirely to vitamin K insufficiency.
Intervention Studies
The reported positive association of dp-ucMGP with vascular calcification (54, 58, 100, 103, 104, 119) and reduction in plasma dp-ucMGP with vitamin K supplementation (61–68) provides the basis for the premise that reducing dp-ucMGP with vitamin K supplementation is cardioprotective. Nine randomized controlled trials (RCTs) have tested the effect of vitamin K supplementation on surrogate measures of CVD, as recently reviewed (121). Seven of these trials evaluated vascular or valvular calcification as outcomes and also reported changes in dp-ucMGP (49, 66–68, 69, 122–124). In each of the trials, a significant treatment effect of vitamin K in decreasing concentrations of dp-ucMGP was observed. In an RCT of type 2 diabetics, 360 µg/d menaquinone-7 supplementation for 6 mo reduced plasma dp-ucMGP, as expected, but did not reduce CAC or femoral artery calcification measured using sodium fluoride positron emission tomography (18F-NaF PET) (66). Moreover, these investigators noted a nonsignificant increase in the femoral artery target-to-background ratio evaluated using 18F-NaF PET, indicative of more calcification, in the menaquinone-7–treated group. Despite the randomization, the menaquinone-7 group had more vascular calcification at baseline, which might have influenced the outcome (66). In a 3-y intervention in postmenopausal women, 180 µg/d menaquinone-7 reduced dp-ucMGP and, among women with worse baseline stiffness, improved the arterial stiffness index, but not pulse-wave velocity [PWV; the gold-standard measure of arterial stiffness (125)] (69). The association between change in stiffness index and change in dp-ucMGP was not reported. Similarly, no impact of menaquinone-7 supplementation was found on the change in PWV over 6 mo in elderly Scottish participants (122) or Dutch hemodialysis patients treated with rivaroxaban (123), despite significant reductions in dp-ucMGP concentrations reported in both studies. In an open-label trial of hemodialysis patients, 200 µg/d menaquinone-7 supplementation for 1 y reduced plasma uncarboxylated MGP (ucMGP)but had no effect on aortic calcification (49). Given the high burden of vascular calcification in this patient population, it is possible the menaquinone-7 dose and corresponding reduction in ucMGP was not enough to slow CAC progression (69). In generally healthy older men and women, 500 µg/d phylloquinone supplementation for 3 y reduced plasma dp-ucMGP (by 79%) and slowed CAC progression among participants who were adherent to the intervention. However, the change in dp-ucMGP was not predictive of the change in CAC (67, 124). In an open-label trial, 2000 µg/d phylloquinone for 1 y reduced dp-ucMGP and slowed the progression of aortic valve calcification in mild and moderately symptomatic patients (68). It was not reported whether the change in dp-ucMGP and change in aortic valve calcification were associated. Some have suggested reducing dp-ucMGP with menaquinone-7 supplementation would be “beneficial for the prevention of future cardiovascular events” (72). However, the available evidence, albeit limited, does not support this premise at this time. The results of available phylloquinone trials, which are also limited in number, suggest there may be a beneficial effect of phylloquinone supplementation on vascular or valvular calcification (68, 124). No trials have tested vitamin K supplementation on CVD events or mortality, so clinical relevance remains uncertain. Future studies designed to compare the effects of phylloquinone and menaquinone on vascular calcification and related CVD and mortality are needed to clarify whether the vitamin K forms have differential effects on cardiovascular health. Until such studies are completed, claims that menaquinone-7 is superior to phylloquinone in this regard are misleading (4, 26, 35, 72).
Based on the available evidence, lowering plasma dp-ucMGP does not appear to correspond to reductions in arterial calcification (49, 66, 67, 124). This suggests the mechanisms underlying vitamin K's role in vascular calcification are not fully explained by plasma dp-ucMGP. Arterial calcification is a complex process and it is possible that a single measure obtained from plasma does not reflect what is happening at the tissue level. There are alternate mechanisms through which vitamin K may be involved in cardiovascular health [e.g., inflammation (126–128)], which would not be captured by plasma dp-ucMGP. There are also limitations to the sole use of dp-ucMGP as a biomarker of vitamin K status, as reviewed in detail elsewhere (89), that should be considered. The concentration of plasma dp-ucMGP that reflects suboptimal function has not been established (129), so it is unclear how low dp-ucMGP should be to confer protective effects. Some have described dp-ucMGP as “the best single biomarker of vitamin K deficiency” (130). There is currently no single biomarker of vitamin K status that is considered a gold standard. Circulating phylloquinone and dp-ucMGP both change in response to vitamin K intakes but appear to reflect different aspects of vitamin K metabolism and function with respect to cardiovascular health. The scientific evidence on vitamin K and CVD and mortality would be strengthened by studies that estimate vitamin K status using multiple vitamin K biomarkers, or biomarkers in combination with dietary intake (89).
Conclusions
The biological plausibility for a protective role of vitamin K in CVD and mortality is based on the presence of VKD proteins in vascular tissue that inhibit vascular calcification—when they are carboxylated, which requires vitamin K. Phylloquinone and menaquinone forms are capable of carboxylating VKD proteins. Recent claims have been made that menaquinones, particularly menaquinone-7, have “profound” cardiovascular health benefits, compared with phylloquinone (4, 26). This claim is based primarily on the results of observational studies (79–81), which are inherently limited and have not been fully supported by intervention trials (49, 66, 69, 122, 123). While the results of 2 trials suggest a beneficial effect for phylloquinone (68, 124), there are limitations to these trials that preclude claiming a cardioprotective role for phylloquinone at this time. Not unlike nutrition research overall (131), vitamin K research is facing challenges because conclusions have been drawn that are not based on rigorously designed studies. Science is self-correcting. Current misconceptions about vitamin K can be addressed with rigorously designed and reproducible studies that are designed to test and compare the effects of different vitamin K forms on cardiovascular health and other health outcomes.
Acknowledgments
The authors’ responsibilities were as follows—MKS: compiled the data and drafted the manuscript and had responsibility for the final content; KLB, GF, XF, RMH, and SLB: revised and edited the manuscript and contributed to the content; and all authors: read and approved the final manuscript.
Notes
Supported by USDA Agricultural Research Service cooperative agreement 58-1950-7-707, NIH National Institute of Diabetes and Digestive and Kidney Diseases R01DK111392, and the Canadian Institute of Health Research (CIHR).
Author disclosures: The authors report no conflicts of interest.
Any opinions, findings, conclusions, or recommendations expressed in this publication are those of the authors and do not necessarily reflect the views of the USDA, NIH, or CIHR.
Perspective articles allow authors to take a position on a topic of current major importance or controversy in the field of nutrition. As such, these articles could include statements based on author opinions or point of view. Opinions expressed in Perspective articles are those of the author and are not attributable to the funder(s) or the sponsor(s) or the publisher, Editor, or Editorial Board of Advances in Nutrition. Individuals with different positions on the topic of a Perspective are invited to submit their comments in the form of a Perspectives article or in a Letter to the Editor.
Abbreviations used: AI, Adequate Intake; CAC, coronary artery calcification; CHD, coronary heart disease; CVD, cardiovascular disease; dp-cMGP, dephosphorylated carboxylated matrix Gla protein; dp-ucMGP, dephosphorylated uncarboxylated matrix Gla protein; FFQ, food-frequency questionnaire; Gas-6, growth arrest specific-6; Gla, γ-carboxyglutamic acid; GRP, Gla-rich protein; KEQAS, vitamin K external quality assurance scheme; MGP, matrix Gla protein; MONICA, MonItoring Trends and Determinants in Cardiovascular Disease; PREDIMED, Prevention with Mediterranean Diet; OC, osteocalcin; PWV, PROSPECT-EPIC European Prospective Investigation into Cancer and Nutrition; pulse-wave velocity; RCT, randomized controlled trial; t-ucMGP, total uncarboxylated matrix Gla protein; ucMGP, uncarboxylated matrix Gla protein; VKD, vitamin K–dependent; VKORC1, Vitamin K epoxide reductase; 18F-NaF PET, 18F sodium fluoride positron emission tomography.
Contributor Information
M Kyla Shea, Tufts University USDA Human Nutrition Research Center on Aging, Boston, MA, USA.
Kathleen L Berkner, Department of Cardiovascular and Metabolic Sciences, Cleveland Clinic Lerner College of Medicine at CWRU, Cleveland Clinic, Cleveland, OH, USA.
Guylaine Ferland, Département de Nutrition, Université de Montréal, Montreal, Quebec, Canada.
Xueyan Fu, Tufts University USDA Human Nutrition Research Center on Aging, Boston, MA, USA.
Rachel M Holden, Department of Medicine, Queen's University, Kingston, Ontario, Canada.
Sarah L Booth, Tufts University USDA Human Nutrition Research Center on Aging, Boston, MA, USA.
References
- 1. Berkner KL, Runge KW. The physiology of vitamin K nutriture and vitamin K-dependent protein function in atherosclerosis. J Thromb Haemost. 2004;2(12):2118–32. [DOI] [PubMed] [Google Scholar]
- 2. Institute of Medicine Food and Nutrition Board . Dietary Reference Intakes for vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. Washington (DC: ): National Academies Press; 2002. [PubMed] [Google Scholar]
- 3. Brown AW, Altman DG, Baranowski T, Bland JM, Dawson JA, Dhurandhar NV, Dowla S, Fontaine KR, Gelman A, Heymsfield SBet al. Childhood obesity intervention studies: a narrative review and guide for investigators, authors, editors, reviewers, journalists, and readers to guard against exaggerated effectiveness claims. Obes Rev. 2019;20(11):1523–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Akbulut AC, Pavlic A, Petsophonsakul P, Halder M, Maresz K, Kramann R, Schurgers L.. Vitamin K2 needs an RDI separate from vitamin K1. Nutrients. 2020;12(6):1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Walther B, Karl JP, Booth SL, Boyaval P.. Menaquinones, bacteria, and the food supply: the relevance of dairy and fermented food products to vitamin K requirements. Adv Nutr. 2013;4(4):463–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Al-Rajabi A, Booth SL, Peterson JW, Choi SW, Suttie JW, Shea MK, Miao B, Grusak MA, Fu X. Deuterium-labeled phylloquinone has tissue-specific conversion to menaquinone-4 among Fischer 344 male rats. J Nutr. 2012;142(5):841–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Okano T, Shimomura Y, Yamane M, Suhara Y, Kamao M, Sugiura M, Nakagawa K. Conversion of phylloquinone (vitamin K1) into menaquinone-4 (vitamin K2) in mice: two possible routes for menaquinone-4 accumulation in cerebra of mice. J Biol Chem. 2008;283(17):11270–9. [DOI] [PubMed] [Google Scholar]
- 8. Suhara Y, Wada A, Tachibana Y, Watanabe M, Nakamura K, Nakagawa K, Okano T. Structure-activity relationships in the conversion of vitamin K analogues into menaquinone-4: substrates essential to the synthesis of menaquinone-4 in cultured human cell lines. Bioorg Med Chem. 2010;18(9):3116–24. [DOI] [PubMed] [Google Scholar]
- 9. Elder SJ, Haytowitz DB, Howe J, Peterson JW, Booth SL. Vitamin K contents of meat, dairy, and fast food in the u.s. Diet. J Agric Food Chem. 2006;54(2):463–7. [DOI] [PubMed] [Google Scholar]
- 10. Fu X, Harshman SG, Shen X, Haytowitz DB, Karl JP, Wolfe BE, Booth SL. Multiple vitamin K forms exist in dairy foods. Curr Dev Nutr. 2017;1(6):e000638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fu X, Shen X, Finnan EG, Haytowitz DB, Booth SL.. Measurement of multiple vitamin K forms in processed and fresh-cut pork products in the U.S. food supply. J Agric Food Chem. 2016;64(22):4531–5. [DOI] [PubMed] [Google Scholar]
- 12. Manoury E, Jourdon K, Boyaval P, Fourcassie P. Quantitative measurement of vitamin K2 (menaquinones) in various fermented dairy products using a reliable high-performance liquid chromatography method. J Dairy Sci. 2013;96(3):1335–46. [DOI] [PubMed] [Google Scholar]
- 13. Kaneki M, Hodges SJ, Hosoi T, Fujiwara S, Lyons A, Crean SJ, Ishida N, Nakagawa M, Takechi M, Sano Yet al. Japanese fermented soybean food as the major determinant of the large geographic difference in circulating levels of vitamin K2: possible implications for hip-fracture risk. Nutrition. 2001;17(4):315–21. [DOI] [PubMed] [Google Scholar]
- 14. USDA . Food Data Central. [Internet]. 2019. [Accessed 2020 Jul 15]. Available from: https://fdc.nal.usda.gov/. [Google Scholar]
- 15. National Institute for Public Health and the Environment Ministry of Health, Welfare and Sport . Dutch Food Composition Database. [Internet]. 2019. [Accessed 2020 Jul 15]. Available from: https://www.rivm.nl/en/dutch-food-composition-database. [Google Scholar]
- 16. Food Standards Australia New Zealand . Australian Food Composition Database. [Internet].[Accessed 2020 Jul 15]. Available from: https://www.foodstandards.gov.au/science/monitoringnutrients/ausnut. [Google Scholar]
- 17. Lupianez-Barbero A, Gonzalez Blanco C, de Leiva Hidalgo A. Spanish food composition tables and databases: need for a gold standard for healthcare professionals. Endocrinol Diabetes Nutr. 2018;65(6):361–73. [DOI] [PubMed] [Google Scholar]
- 18. Card DJ, Shearer MJ, Schurgers LJ, Harrington DJ.. The external quality assurance of phylloquinone (vitamin K(1)) analysis in human serum. Biomed Chromatogr. 2009;23(12):1276–82. [DOI] [PubMed] [Google Scholar]
- 19. Kamao M, Suhara Y, Tsugawa N, Uwano M, Yamaguchi N, Uenishi K, Ishida H, Sasaki S, Okano T. Vitamin K content of foods and dietary vitamin K intake in Japanese young women. J Nutr Sci Vitaminol. 2007;53(6):464–70. [DOI] [PubMed] [Google Scholar]
- 20. Davies PSW. Stable isotopes: their use and safety in human nutrition studies. Eur J Clin Nutr. 2020;74(3):362–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kim IY, Suh SH, Lee IK, Wolfe RR. Applications of stable, nonradioactive isotope tracers in in vivo human metabolic research. Exp Mol Med. 2016;48:e203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ellis JL, Fu X, Al Rajabi A, Grusak MA, Shearer MJ, Naumova EN, Saltzman E, Barger K, Booth SL. Plasma response to deuterium-labeled vitamin K intake varies by TG response, but not age or vitamin k status, in older and younger adults. J Nutr. 2019;149(1):18–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jones KS, Bluck LJ, Wang LY, Stephen AM, Prynne CJ, Coward WA.. The effect of different meals on the absorption of stable isotope-labelled phylloquinone. Br J Nutr. 2009;102(8):1195–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Schurgers LJ, Teunissen KJ, Hamulyak K, Knapen MH, Vik H, Vermeer C. Vitamin K-containing dietary supplements: comparison of synthetic vitamin K1 and natto-derived menaquinone-7. Blood. 2007;109(8):3279–83. [DOI] [PubMed] [Google Scholar]
- 25. Schurgers LJ, Vermeer C. Differential lipoprotein transport pathways of K-vitamins in healthy subjects. Biochim Biophys Acta. 2002;1570(1):27–32. [DOI] [PubMed] [Google Scholar]
- 26. Halder M, Petsophonsakul P, Akbulut AC, Pavlic A, Bohan F, Anderson E, Maresz K, Kramann R, Schurgers L. Vitamin K: double bonds beyond coagulation insights into differences between vitamin K1 and K2 in health and disease. IJMS. 2019;20(4):896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Simes DC, Viegas CSB, Araujo N, Marreiros C. Vitamin K as a diet supplement with impact in human health: current evidence in age-related diseases. Nutrients. 2020;12(1):138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA) . Dietary Reference Values for vitamin K. [Internet]. 2017. [Accessed 2020 Jul 20]. Available from: http://www.efsa.europa.eu/sites/default/files/consultation/170113.pdf. [Google Scholar]
- 29. Nutrient Reference Values for Australia and New Zealand [Internet]. In: Australian Government National Health and Medical Research Council. 2014. [Accessed 2020 Jul 20]. Available from: https://www.nrv.gov.au/nutrients/vitamin-k. [Google Scholar]
- 30. Ferland G, Sadowski JA, O'Brien ME. Dietary induced subclinical vitamin K deficiency in normal human subjects. J Clin Invest. 1993;91(4):1761–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. McCann JC, Ames BN. Vitamin K, an example of triage theory: is micronutrient inadequacy linked to diseases of aging?. Am J Clin Nutr. 2009;90(4):889–907. [DOI] [PubMed] [Google Scholar]
- 32. Harshman SG, Finnan EG, Barger KJ, Bailey RL, Haytowitz DB, Gilhooly CH, Booth SL. Vegetables and mixed dishes are top contributors to phylloquinone intake in US adults: data from the 2011–2012 NHANES. J Nutr. 2017;147(7):1308–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hayes A, Hennessy A, Walton J, McNulty BA, Lucey AJ, Kiely M, Flynn A, Cashman KD. Phylloquinone intakes and food sources and vitamin K status in a nationally representative sample of Irish adults. J Nutr. 2016;146(11):2274–80. [DOI] [PubMed] [Google Scholar]
- 34. Thane CW, Bolton-Smith C, Coward WA. Comparative dietary intake and sources of phylloquinone (vitamin K1) among British adults in 1986–7 and 2000–1. Br J Nutr. 2006;96(6):1105–15. [DOI] [PubMed] [Google Scholar]
- 35. Maresz K. Proper calcium use: vitamin K2 as a promoter of bone and cardiovascular health. Integr Med (Encinitas). 2015;14(1):34–9. [PMC free article] [PubMed] [Google Scholar]
- 36. Suttie JW. Vitamin K in health and disease. Boca Raton (FL): CRC Press, Taylor and Francis Group; 2009. [Google Scholar]
- 37. Buitenhuis HC, Soute BA, Vermeer C. Comparison of the vitamins K1, K2 and K3 as cofactors for the hepatic vitamin K-dependent carboxylase. Biochim Biophys Acta. 1990;1034(2):170–5. [DOI] [PubMed] [Google Scholar]
- 38. Schwalfenberg GK. Vitamins K1 and K2: the emerging group of vitamins required for human health. J Nutr Metab. 2017;2017:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Suttie JW. The importance of menaquinones in human nutrition. Annu Rev Nutr. 1995;15:399–417. [DOI] [PubMed] [Google Scholar]
- 40. Card DJ, Shearer MJ, Schurgers LJ, Gomez K, Harrington DJ. What's in a name? The pharmacy of vitamin K. Br J Haematol. 2016;174(6):989–90. [DOI] [PubMed] [Google Scholar]
- 41. Schurgers LJ, Teunissen KJ, Knapen MH, Kwaijtaal M, van DR, Appels A, Reutelingsperger CP, Cleutjens JP, Vermeer C.. Novel conformation-specific antibodies against matrix gamma-carboxyglutamic acid (Gla) protein: undercarboxylated matrix Gla protein as marker for vascular calcification. Arterioscler Thromb Vasc Biol. 2005;25(8):1629–33. [DOI] [PubMed] [Google Scholar]
- 42. Viegas CS, Rafael MS, Enriquez JL, Teixeira A, Vitorino R, Luis IM, Costa RM, Santos S, Cavaco S, Neves Jet al. Gla-rich protein acts as a calcification inhibitor in the human cardiovascular system. Arterioscler Thromb Vasc Biol. 2015;35(2):399–408. [DOI] [PubMed] [Google Scholar]
- 43. Shea MK, Booth SL. Vitamin K's role in age-related bone loss: a critical review. In: Holick MF, NievesJW,editors. Nutrition in bone health. New York: Springer; 2015. p. 471–86. [Google Scholar]
- 44. Wu M, Rementer C, Giachelli CM. Vascular calcification: an update on mechanisms and challenges in treatment. Calcif Tissue Int. 2013;93(4):365–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Rashdan NA, Sim AM, Cui L, Phadwal K, Roberts FL, Carter R, Ozdemir DD, Hohenstein P, Hung J, Kaczynski Jet al. Osteocalcin regulates arterial calcification via altered Wnt signaling and glucose metabolism. J Bone Miner Res. 2020;35(2):357–67. [DOI] [PubMed] [Google Scholar]
- 46. Qiu C, Zheng H, Tao H, Yu W, Jiang X, Li A, Jin H, Lv A, Li H. Vitamin K2 inhibits rat vascular smooth muscle cell calcification by restoring the Gas6/Axl/Akt anti-apoptotic pathway. Mol Cell Biochem. 2017;433(1–2):149–59. [DOI] [PubMed] [Google Scholar]
- 47. Holden RM, Hetu MF, Li TY, Ward EC, Couture LE, Herr JE, Christilaw E, Adams MA, Johri AM. Circulating Gas6 is associated with reduced human carotid atherosclerotic plaque burden in high risk cardiac patients. Clin Biochem. 2019;64:6–11. [DOI] [PubMed] [Google Scholar]
- 48. Cranenburg EC, Koos R, Schurgers LJ, Magdeleyns EJ, Schoonbrood TH, Landewe RB, Brandenburg VM, Bekers O, Vermeer C. Characterisation and potential diagnostic value of circulating matrix Gla protein (MGP) species. Thromb Haemost. 2010;104(4):811–22. [DOI] [PubMed] [Google Scholar]
- 49. Oikonomaki T, Papasotiriou M, Ntrinias T, Kalogeropoulou C, Zabakis P, Kalavrizioti D, Papadakis I, Goumenos DS, Papachristou E. The effect of vitamin K2 supplementation on vascular calcification in haemodialysis patients: a 1-year follow-up randomized trial. Int Urol Nephrol. 2019;51(11):2037–44. [DOI] [PubMed] [Google Scholar]
- 50. Schlieper G, Westenfeld R, Kruger T, Cranenburg EC, Magdeleyns EJ, Brandenburg VM, Djuric Z, Damjanovic T, Ketteler M, Vermeer Cet al. Circulating nonphosphorylated carboxylated matrix gla protein predicts survival in ESRD. JASN. 2011;22(2):387–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Immunodiagnostic systems holdings acquisition and research agreement. FE Fund Information; 2010; [Internet]. [Accessed 2020 Aug 16]. Available from: https://www.investegate.co.uk/immunodiagnostic-sys/rns/acquisition—research-agreem/201009130700075363S/. [Google Scholar]
- 52. Evenepoel P, Claes K, Meijers B, Laurent M, Bammens B, Naesens M, Sprangers B, Pottel H, Cavalier E, Kuypers D. Poor vitamin K status is associated with low bone mineral density and increased fracture risk in end-stage renal disease. J Bone Miner Res. 2019;34(2):262–9. [DOI] [PubMed] [Google Scholar]
- 53. Griffin TP, Islam MN, Wall D, Ferguson J, Griffin DG, Griffin MD, O'Shea PM. Plasma dephosphorylated-uncarboxylated matrix Gla-protein (dp-ucMGP): reference intervals in Caucasian adults and diabetic kidney disease biomarker potential. Sci Rep. 2019;9(1):18452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Pivin E, Ponte B, Pruijm M, Ackermann D, Guessous I, Ehret G, Liu YP, Drummen NE, Knapen MH, Pechere-Bertschi Aet al. Inactive matrix Gla-protein is associated with arterial stiffness in an adult population-based study. Hypertension. 2015;66(1):85–92. [DOI] [PubMed] [Google Scholar]
- 55. Jespersen T, Mollehave LT, Thuesen BH, Skaaby T, Rossing P, Toft U, Jorgensen NR, Corfixen BL, Jakobsen J, Frimodt-Moller Met al. Uncarboxylated matrix Gla-protein: a biomarker of vitamin K status and cardiovascular risk. Clin Biochem. 2020;83:49. [DOI] [PubMed] [Google Scholar]
- 56. Mayer O Jr, Seidlerova J, Wohlfahrt P, Filipovsky J, Vanek J, Cifkova R, Windrichova J, Topolcan O, Knapen MH, Drummen NEet al. Desphospho-uncarboxylated matrix Gla protein is associated with increased aortic stiffness in a general population. J Hum Hypertens. 2016;30(7):418–23. [DOI] [PubMed] [Google Scholar]
- 57. Dekker LH, Vinke PC, Riphagen IJ, Minovic I, Eggersdorfer ML, van den Heuvel E, Schurgers LJ, Kema IP, Bakker SJL, Navis G. Cheese and healthy diet: associations with incident cardio-metabolic diseases and all-cause mortality in the general population. Front Nutr. 2019;6:185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. van den Heuvel EG, van Schoor NM, Lips P, Magdeleyns EJ, Deeg DJ, Vermeer C, den Heijer M. Circulating uncarboxylated matrix Gla protein, a marker of vitamin K status, as a risk factor of cardiovascular disease. Maturitas. 2014;77(2):137–41. [DOI] [PubMed] [Google Scholar]
- 59. Riphagen IJ, Keyzer CA, Drummen NEA, de Borst MH, Beulens JWJ, Gansevoort RT, Geleijnse JM, Muskiet FAJ, Navis G, Visser STet al. Prevalence and effects of functional vitamin K insufficiency: the PREVEND study. Nutrients. 2017;9(12):1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Caluwe R, Verbeke F, De Vriese AS. Evaluation of vitamin K status and rationale for vitamin K supplementation in dialysis patients. Nephrol Dial Transplant. 2020;35(1):23–33. [DOI] [PubMed] [Google Scholar]
- 61. Dalmeijer GW, van der Schouw YT, Magdeleyns E, Ahmed N, Vermeer C, Beulens JW.. The effect of menaquinone-7 supplementation on circulating species of matrix Gla protein. Atherosclerosis. 2012;225(2):397–402. [DOI] [PubMed] [Google Scholar]
- 62. Theuwissen E, Cranenburg EC, Knapen MH, Magdeleyns EJ, Teunissen KJ, Schurgers LJ, Smit E, Vermeer C. Low-dose menaquinone-7 supplementation improved extra-hepatic vitamin K status, but had no effect on thrombin generation in healthy subjects. Br J Nutr. 2012;108(9):1652–7. [DOI] [PubMed] [Google Scholar]
- 63. Westenfeld R, Krueger T, Schlieper G, Cranenburg EC, Magdeleyns EJ, Heidenreich S, Holzmann S, Vermeer C, Jahnen-Dechent W, Ketteler Met al. Effect of vitamin K2 supplementation on functional vitamin K deficiency in hemodialysis patients: a randomized trial. Am J Kidney Dis. 2012;59(2):186–95. [DOI] [PubMed] [Google Scholar]
- 64. Caluwe R, Vandecasteele S, Van Vlem B, Vermeer C, De Vriese AS.. Vitamin K2 supplementation in haemodialysis patients: a randomized dose-finding study. Nephrol Dial Transplant. 2014;29(7):1385–90. [DOI] [PubMed] [Google Scholar]
- 65. Mansour AG, Hariri E, Daaboul Y, Korjian S, El Alam A, Protogerou AD, Kilany H, Karam A, Stephan A, Bahous SA. Vitamin K2 supplementation and arterial stiffness among renal transplant recipients—a single-arm, single-center clinical trial. J Am Soc Hypertens. 2017;11(9):589–97. [DOI] [PubMed] [Google Scholar]
- 66. Zwakenberg SR, de Jong PA, Bartstra JW, van Asperen R, Westerink J, de Valk H, Slart R, Luurtsema G, Wolterink JM, de Borst GJet al. The effect of menaquinone-7 supplementation on vascular calcification in patients with diabetes: a randomized, double-blind, placebo-controlled trial. Am J Clin Nutr. 2019;110(4):883–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Shea MK, O'Donnell CJ, Vermeer C, Magdeleyns EJ, Crosier MD, Gundberg CM, Ordovas JM, Kritchevsky SB, Booth SL. Circulating uncarboxylated matrix Gla protein is associated with vitamin K nutritional status, but not coronary artery calcium, in older adults. J Nutr. 2011;141(8):1529–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Brandenburg VM, Reinartz S, Kaesler N, Kruger T, Dirrichs T, Kramann R, Peeters F, Floege J, Keszei A, Marx Net al. Slower progress of aortic valve calcification with vitamin K supplementation: results from a prospective interventional proof-of-concept study. Circulation. 2017;135(21):2081–3. [DOI] [PubMed] [Google Scholar]
- 69. Knapen MH, Braam LA, Drummen NE, Bekers O, Hoeks AP, Vermeer C.. Menaquinone-7 supplementation improves arterial stiffness in healthy postmenopausal women: double-blind randomised clinical trial. Thromb Haemost. 2015;113(5):1135. [DOI] [PubMed] [Google Scholar]
- 70. Aoun M, Makki M, Azar H, Matta H, Chelala DN. High dephosphorylated-uncarboxylated MGP in hemodialysis patients: risk factors and response to vitamin K2, a pre-post intervention clinical trial. BMC Nephrol. 2017;18(1):191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Witham MD, Lees JS, White MT, Band M, Bell S, Chantler DJ, Ford I, Fulton RL, Kennedy G, Littleford RCet al. Vitamin K supplementation to improve vascular stiffness in CKD: the K4Kidneys Randomized Controlled Trial. JASN. 2020;31(10):2434–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Shioi A, Morioka T, Shoji T, Emoto M.. The inhibitory roles of vitamin K in progression of vascular calcification. Nutrients. 2020;12(2):583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Andrews J, Psaltis PJ, Bartolo BAD, Nicholls SJ, Puri R.. Coronary arterial calcification: a review of mechanisms, promoters and imaging. Trends Cardiovasc Med. 2018;28(8):491–501. [DOI] [PubMed] [Google Scholar]
- 74. Lanzer P, Boehm M, Sorribas V, Thiriet M, Janzen J, Zeller T, St Hilaire C, Shanahan C. Medial vascular calcification revisited: review and perspectives. Eur Heart J. 2014;35(23):1515–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Allison MA, Hsi S, Wassel CL, Morgan C, Ix JH, Wright CM, Criqui MH. Calcified atherosclerosis in different vascular beds and the risk of mortality. Arterioscler Thromb Vasc Biol. 2012;32(1):140–6. [DOI] [PubMed] [Google Scholar]
- 76. Bos D, Leening MJ, Kavousi M, Hofman A, Franco OH, van der Lugt A, Vernooij MW, Ikram MA.. Comparison of atherosclerotic calcification in major vessel beds on the risk of all-cause and cause-specific mortality: the Rotterdam study. Circ Cardiovasc Imaging. 2015;8(12):3843. [DOI] [PubMed] [Google Scholar]
- 77. Schurgers LJ, Spronk HM, Soute BA, Schiffers PM, DeMey JG, Vermeer C.. Regression of warfarin-induced medial elastocalcinosis by high intake of vitamin K in rats. Blood. 2007;109(7):2823–31. [DOI] [PubMed] [Google Scholar]
- 78. Wasilewski GB, Vervloet MG, Schurgers LJ. The bone-vasculature axis: calcium supplementation and the role of vitamin K. Front Cardiovasc Med. 2019;6:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Beulens JW, Bots ML, Atsma F, Bartelink ML, Prokop M, Geleijnse JM, Witteman JC, Grobbee DE, van der Schouw YT. High dietary menaquinone intake is associated with reduced coronary calcification. Atherosclerosis. 2009;203(2):489–93. [DOI] [PubMed] [Google Scholar]
- 80. Gast GC, de Roos NM, Sluijs I, Bots ML, Beulens JW, Geleijnse JM, Witteman JC, Grobbee DE, Peeters PH, van der Schouw YT. A high menaquinone intake reduces the incidence of coronary heart disease. Nutr Metab Cardiovasc Dis. 2009;19(7):504–10. [DOI] [PubMed] [Google Scholar]
- 81. Geleijnse JM, Vermeer C, Grobbee DE, Schurgers LJ, Knapen MH, van dM I, Hofman A, Witteman JC. Dietary intake of menaquinone is associated with a reduced risk of coronary heart disease: the Rotterdam study. J Nutr. 2004;134(11):3100–5. [DOI] [PubMed] [Google Scholar]
- 82. Zwakenberg SR, den Braver NR, Engelen AIP, Feskens EJM, Vermeer C, Boer JMA, Verschuren WMM, van der Schouw YT, Beulens JWJ. Vitamin K intake and all-cause and cause specific mortality. Clin Nutr. 2017;36(5):1294–300. [DOI] [PubMed] [Google Scholar]
- 83. Cade J, Thompson R, Burley V, Warm D. Development, validation and utilisation of food-frequency questionnaires—a review. Public Health Nutr. 2002;5(4):567–87. [DOI] [PubMed] [Google Scholar]
- 84. Zwakenberg SR, Engelen AIP, Dalmeijer GW, Booth SL, Vermeer C, Drijvers J, Ocke MC, Feskens EJM, van der Schouw YT, Beulens JWJ. Reproducibility and relative validity of a food frequency questionnaire to estimate intake of dietary phylloquinone and menaquinones. Eur J Clin Nutr. 2017;71(12):1423–8. [DOI] [PubMed] [Google Scholar]
- 85. Vermeer C, Raes J, van ’t Hoofd C, Knapen MHJ, Xanthoulea S.. Menaquinone content of cheese. Nutrients. 2018;10(4):446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Juanola-Falgarona M, Salas-Salvado J, Martinez-Gonzalez MA, Corella D, Estruch R, Ros E, Fito M, Aros F, Gomez-Gracia E, Fiol Met al. Dietary intake of vitamin K is inversely associated with mortality risk. J Nutr. 2014;144(5):743–50. [DOI] [PubMed] [Google Scholar]
- 87. Presse N, Shatenstein B, Kergoat MJ, Ferland G.. Validation of a semi-quantitative food frequency questionnaire measuring dietary vitamin K intake in elderly people. J Am Diet Assoc. 2009;109(7):1251–5. [DOI] [PubMed] [Google Scholar]
- 88. Feskanich D, Rimm EB, Giovannucci EL, Colditz GA, Stampfer MJ, Litin LB, Willett WC. Reproducibility and validity of food intake measurements from a semiquantitative food frequency questionnaire. J Am Diet Assoc. 1993;93(7):790–6. [DOI] [PubMed] [Google Scholar]
- 89. Shea MK, Booth SL. Concepts and controversies in evaluating vitamin K status in population-based studies. Nutrients. 2016;8(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. McKeown NM, Jacques PF, Gundberg CM, Peterson JW, Tucker KL, Kiel DP, Wilson PW, Booth SL. Dietary and nondietary determinants of vitamin K biochemical measures in men and women. J Nutr. 2002;132(6):1329–34. [DOI] [PubMed] [Google Scholar]
- 91. Erkkila AT, Booth SL, Hu FB, Jacques PF, Manson JE, Rexrode KM, Stampfer MJ, Lichtenstein AH.. Phylloquinone intake as a marker for coronary heart disease risk but not stroke in women. Eur J Clin Nutr. 2005;59(2):196–204. [DOI] [PubMed] [Google Scholar]
- 92. Erkkila AT, Booth SL, Hu FB, Jacques PF, Lichtenstein AH. Phylloquinone intake and risk of cardiovascular diseases in men. Nutr Metab Cardiovasc Dis. 2007;17(1):58–62. [DOI] [PubMed] [Google Scholar]
- 93. Vissers LE, Dalmeijer GW, Boer JM, Monique Verschuren WM, van der Schouw YT, Beulens JW. Intake of dietary phylloquinone and menaquinones and risk of stroke. JAHA. 2013;2(6):e000455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Vaccaro JA, Huffman FG.. Phylloquinone (vitamin K(1)) intake and pulse pressure as a measure of arterial stiffness in older adults. J Nutr Gerontol Geriatr. 2013;32(3):244–57. [DOI] [PubMed] [Google Scholar]
- 95. Cheung CL, Sahni S, Cheung BM, Sing CW, Wong IC.. Vitamin K intake and mortality in people with chronic kidney disease from NHANES III. Clin Nutr. 2015;34(2):235–40. [DOI] [PubMed] [Google Scholar]
- 96. Dalmeijer GW, van der Schouw YT, Booth SL, de Jong PA, Beulens JW.. Phylloquinone concentrations and the risk of vascular calcification in healthy women. Arterioscler Thromb Vasc Biol. 2014;34(7):1587–90. [DOI] [PubMed] [Google Scholar]
- 97. Shea MK, Booth SL, Miller ME, Burke GL, Chen H, Cushman M, Tracy RP, Kritchevsky SB. Association between circulating vitamin K1 and coronary calcium progression in community-dwelling adults: the Multi-Ethnic Study of Atherosclerosis. Am J Clin Nutr. 2013;98(1):197–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Shea MK, Booth SL, Weiner DE, Brinkley TE, Kanaya AM, Murphy RA, Simonsick EM, Wassel CL, Vermeer C, Kritchevsky SB. Circulating Vitamin K is inversely associated with incident cardiovascular disease risk among those treated for hypertension in the health, aging, and body composition study (Health ABC). J Nutr. 2017;147(5):888–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Shea MK, Barger K, Booth SL, Matuszek G, Cushman M, Benjamin EJ, Kritchevsky SB, Weiner DE. Vitamin K status, cardiovascular disease, and all-cause mortality: a participant-level meta-analysis of 3 US cohorts. Am J Clin Nutr. 2020;111:1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Dalmeijer GW, van der Schouw YT, Vermeer C, Magdeleyns EJ, Schurgers LJ, Beulens JW.. Circulating matrix Gla protein is associated with coronary artery calcification and vitamin K status in healthy women. J Nutr Biochem. 2012;24(4):624–8. [DOI] [PubMed] [Google Scholar]
- 101. Sardana M, Vasim I, Varakantam S, Kewan U, Tariq A, Koppula MR, Syed AA, Beraun M, Drummen NE, Vermeer Cet al. Inactive matrix Gla-protein and arterial stiffness in type 2 diabetes mellitus. Am J Hypertens. 2017;30(2):196–201. [DOI] [PubMed] [Google Scholar]
- 102. Ueland T, Gullestad L, Dahl CP, Aukrust P, Aakhus S, Solberg OG, Vermeer C, Schurgers LJ.. Undercarboxylated matrix Gla protein is associated with indices of heart failure and mortality in symptomatic aortic stenosis. J Intern Med. 2010;268:483. [DOI] [PubMed] [Google Scholar]
- 103. Schurgers LJ, Barreto DV, Barreto FC, Liabeuf S, Renard C, Magdeleyns EJ, Vermeer C, Choukroun G, Massy ZA.. The circulating inactive form of matrix Gla protein is a surrogate marker for vascular calcification in chronic kidney disease: a preliminary report. CJASN. 2010;5(4):568–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Dalmeijer GW, van der Schouw YT, Magdeleyns EJ, Vermeer C, Verschuren WM, Boer JM, Beulens JW.. Matrix Gla protein species and risk of cardiovascular events in type 2 diabetic patients. Diabetes Care. 2013;36(11):3766–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Dalmeijer GW, van der Schouw YT, Magdeleyns EJ, Vermeer C, Verschuren WM, Boer JM, Beulens JW. Circulating desphospho-uncarboxylated matrix gamma-carboxyglutamate protein and the risk of coronary heart disease and stroke. J Thromb Haemost. 2014;12(7):1028–34. [DOI] [PubMed] [Google Scholar]
- 106. Mayer O Jr, Seidlerova J, Bruthans J, Filipovsky J, Timoracka K, Vanek J, Cerna L, Wohlfahrt P, Cifkova R, Theuwissen Eet al. Desphospho-uncarboxylated matrix Gla-protein is associated with mortality risk in patients with chronic stable vascular disease. Atherosclerosis. 2014;235(1):162–8. [DOI] [PubMed] [Google Scholar]
- 107. Keyzer CA, Vermeer C, Joosten MM, Knapen MH, Drummen NE, Navis G, Bakker SJ, de Borst MH.. Vitamin K status and mortality after kidney transplantation: a cohort study. Am J Kidney Dis. 2015;65(3):474–83. [DOI] [PubMed] [Google Scholar]
- 108. Liu YP, Gu YM, Thijs L, Knapen MH, Salvi E, Citterio L, Petit T, Carpini SD, Zhang Z, Jacobs Let al. Inactive matrix Gla protein is causally related to adverse health outcomes: a Mendelian randomization study in a Flemish population. Hypertension. 2015;65(2):463–70. [DOI] [PubMed] [Google Scholar]
- 109. Zhang S, Guo L, Bu C.. Vitamin K status and cardiovascular events or mortality: a meta-analysis. Eur J Prev Cardiol. 2019;26(5):549–53. [DOI] [PubMed] [Google Scholar]
- 110. Potischman N, Freudenheim JL. Biomarkers of nutritional exposure and nutritional status: an overview. J Nutr. 2003;133(Suppl 3):873S–4S. [DOI] [PubMed] [Google Scholar]
- 111. Fusaro M, Noale M, Viola V, Galli F, Tripepi G, Vajente N, Plebani M, Zaninotto M, Guglielmi G, Miotto Det al. Vitamin K, vertebral fractures, vascular calcifications, and mortality: VItamin K Italian (VIKI) dialysis study. J Bone Miner Res. 2012;27(11):2271–8. [DOI] [PubMed] [Google Scholar]
- 112. Dunovska K, Klapkova E, Sopko B, Cepova J, Prusa R. LC-MS/MS quantitative analysis of phylloquinone, menaquinone-4 and menaquinone-7 in the human serum of a healthy population. PeerJ. 2019;7:e7695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Karl JP, Fu X, Dolnikowski GG, Saltzman E, Booth SL. Quantification of phylloquinone and menaquinones in feces, serum, and food by high-performance liquid chromatography-mass spectrometry. J Chromatogr B. 2014;963:128–33. [DOI] [PubMed] [Google Scholar]
- 114. Vitamin K external quality assurance scheme (KEQAS). [Internet]. [Accessed 2020 Sep 7]. Available from: https://www.guysandstthomas.nhs.uk/our-services/human-nutristasis/keqas-participants.aspx. [Google Scholar]
- 115. Novotny JA, Kurilich AC, Britz SJ, Baer DJ, Clevidence BA. Vitamin K absorption and kinetics in human subjects after consumption of 13C-labelled phylloquinone from kale. Br J Nutr. 2010;104(6):858–62. [DOI] [PubMed] [Google Scholar]
- 116. Shearer MJ, Fu X, Booth SL. Vitamin K nutrition, metabolism, and requirements: current concepts and future research. Adv Nutr. 2012;3(2):182–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Booth SL, Martini L, Peterson JW, Saltzman E, Dallal GE, Wood RJ.. Dietary phylloquinone depletion and repletion in older women. J Nutr. 2003;133(8):2565–9. [DOI] [PubMed] [Google Scholar]
- 118. Barrett H, O'Keeffe M, Kavanagh E, Walsh M, O'Connor EM. Is matrix Gla protein associated with vascular calcification? A systematic review. Nutrients. 2018;10(4):415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Rennenberg RJ, de Leeuw PW, Kessels AG, Schurgers LJ, Vermeer C, van Engelshoven JM, Kemerink GJ, Kroon AA. Calcium scores and matrix Gla protein levels: association with vitamin K status. Eur J Clin Invest. 2010;40(4):344–9. [DOI] [PubMed] [Google Scholar]
- 120. Zwakenberg SR, van der Schouw YT, Vermeer C, Pasterkamp G, den Ruijter HM, Beulens JWJ. Matrix Gla protein, plaque stability, and cardiovascular events in patients with severe atherosclerotic disease. Cardiology. 2018;141(1):32–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Vlasschaert C, Goss CJ, Pilkey NG, McKeown S, Holden RM.. Vitamin K supplementation for the prevention of cardiovascular disease: where is the evidence? A systematic review of controlled trials. Nutrients. 2020;12(10):2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Fulton RL, McMurdo ME, Hill A, Abboud RJ, Arnold GP, Struthers AD, Khan F, Vermeer C, Knapen MH, Drummen Net al. Effect of vitamin K on vascular health and physical function in older people with vascular disease—a randomised controlled trial. J Nutr Health Aging. 2016;20(3):325–33. [DOI] [PubMed] [Google Scholar]
- 123. De Vriese AS, Caluwe R, Pyfferoen L, De Bacquer D, De Boeck K, Delanote J, De Surgeloose D, Van Hoenacker P, Van Vlem B, Verbeke F.. Multicenter randomized controlled trial of vitamin K antagonist replacement by rivaroxaban with or without vitamin K2 in hemodialysis patients with atrial fibrillation: the Valkyrie study. JASN. 2020;31(1):186–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Shea MK, O'Donnell CJ, Hoffmann U, Dallal GE, Dawson-Hughes B, Ordovas JM, Price PA, Williamson MK, Booth SL.. Vitamin K supplementation and progression of coronary artery calcium in older men and women. Am J Clin Nutr. 2009;89(6):1799–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Townsend RR. Arterial stiffness: recommendations and standardization. Pulse. 2016;4(Suppl 1):3–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Ohsaki Y, Shirakawa H, Miura A, Giriwono PE, Sato S, Ohashi A, Iribe M, Goto T, Komai M.. Vitamin K suppresses the lipopolysaccharide-induced expression of inflammatory cytokines in cultured macrophage-like cells via the inhibition of the activation of nuclear factor kappaB through the repression of IKKalpha/beta phosphorylation. J Nutr Biochem. 2010;211:120. [DOI] [PubMed] [Google Scholar]
- 127. Reddi K, Henderson B, Meghji S, Wilson M, Poole S, Hopper C, Harris M, Hodges SJ.. Interleukin 6 production by lipopolysaccharide-stimulated human fibroblasts is potently inhibited by naphthoquinone (vitamin K) compounds. Cytokine. 1995;7(3):287–90. [DOI] [PubMed] [Google Scholar]
- 128. Shea MK, Cushman M, Booth SL, Burke GL, Chen H, Kritchevsky SB.. Associations between vitamin K status and haemostatic and inflammatory biomarkers in community-dwelling adults: the Multi-Ethnic Study of Atherosclerosis. Thromb Haemost. 2014;112(3):438–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. van Ballegooijen AJ, Beulens JW.. The role of vitamin K status in cardiovascular health: evidence from observational and clinical studies. Curr Nutr Rep. 2017;6(3):197–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Wei FF, Trenson S, Verhamme P, Vermeer C, Staessen JA. Vitamin K-dependent matrix Gla protein as multifaceted protector of vascular and tissue integrity. Hypertension. 2019;73(6):1160–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Hall KD. Challenges of human nutrition research. Science. 2020;367(6484):1298–300. [DOI] [PubMed] [Google Scholar]