ABSTRACT
Previous meta-analyses that found an inverse association between coffee consumption and metabolic syndrome pooled data from cross-sectional and longitudinal studies, which could lead to potentially misleading conclusions. Hence, this work aimed to reassess this association by analyzing data from the 2 types of studies separately and including recent studies. Online databases including PubMed, Scopus, Embase, The Cumulative Index to Nursing and Allied Health Literature (CINAHL) Plus, and Science Direct were searched for relevant studies published up to July 2020. Both cross-sectional and longitudinal studies were included if published after 1999, reported both effect estimates and CIs, and presented results adjusted for confounding variables. Data of the highest coffee consumption level in each study, as well as those of medium consumption levels in studies with ≥3 consumption categories, were pooled using random-effect models, with sex-stratified and sex-adjusted results being analyzed separately. Results were obtained based on data from 13 cross-sectional studies involving 280,803 participants and 2 longitudinal studies involving 17,014 participants. The overall sex-adjusted association of the highest consumption level was not significant (n = 9 studies; OR: 0.88; 95% CI: 0.70, 1.10; I2: 91.5%) and the 2 longitudinal studies both yielded no association. Subgroup analysis revealed inverse associations in both males and females, as well as in Caucasians with medium coffee consumption (n = 4 studies, OR: 0.88; 95% CI: 0.84, 0.93; I2: 0%). Although residual confounding could affect the results of this meta-analysis, our findings suggested with a low certainty that coffee consumption may not be associated with metabolic syndrome, a finding that is different from those of previous meta-analyses and could be due to variation in characteristics of study participants. More longitudinal studies are also needed to further assess the temporal association between coffee consumption and metabolic syndrome. This meta-analysis was registered at https://www.crd.york.ac.uk/prospero as CRD42018110650.
Keywords: coffee, metabolic syndrome, adult, Asian, Caucasian, meta-analysis
Introduction
Metabolic syndrome is defined as the co-occurrence of multiple metabolic abnormalities, including central obesity, high blood pressure, hyperglycemia, and dyslipidemia (1). Both the International Federation of Diabetes (IDF) (1, 2) and the National Cholesterol Education Program – Adult Treatment Panel III (NCEP ATPIII) (3) have issued their own definition in this regard. Having these risk factors at the same time would lead to greater risks of developing cardiovascular diseases (CVDs) and type 2 diabetes (4). Metabolic syndrome is not only a significant threat to global public health (5) but also a financial burden to both the healthcare systems and patients due to the need for multiple medications (6). Therefore, there is a need to identify affordable and effective preventative agents for metabolic syndrome.
Coffee consumption has been shown to be associated with a lower risk of type 2 diabetes (7) and CVDs (8), both of which are likely to appear in people with metabolic syndrome (4). Two meta-analyses (9, 10) published in 2016 found an inverse association between coffee consumption and metabolic syndrome. Nonetheless, ORs and HRs were pooled in both meta-analyses, whereas the 2 were indeed statistically not interchangeable. As stated by Tierney et al. (11), OR provides a snapshot of the association at a certain time point, whereas HR would take into account both the number and timing of event occurrence. Theoretically, the 2 should not be pooled, yet this has often been done in previous meta-analyses to increase the power of the pooled effect estimates.
In previous meta-analyses, the longitudinal studies included (12, 13) did not find significant results between coffee consumption and the onset of metabolic syndrome, questioning whether a temporal association exists between the 2 variables. In addition, new studies (14–17) have been published after the completion of these meta-analyses, necessitating an update of the overall association. Hence, the research question of this work is whether habitual coffee consumption is associated with a lower likelihood of having metabolic syndrome in human adults, when compared with nonhabitual coffee consumption. Effect estimates from cross-sectional and longitudinal studies were pooled separately when assessing the association.
Methods
Data sources and searches
All studies related to metabolic syndrome and coffee published in English between 1999 and July 2020 were identified from different search engines including PubMed, Scopus, Embase, The Cumulative Index to Nursing and Allied Health Literature (CINAHL) Plus, and Science Direct. Both longitudinal and cross-sectional studies were included. The search terms included “adult,” “metabolic syndrome,” “syndrome x,” “MetS,” “insulin resistance syndrome,” “coffee,” and “caffeine.” The complete search strategies used for each database are shown in the Supplemental Methods. Reference lists of the retrieved articles were manually searched for additional studies. This analysis was registered at https://www.crd.york.ac.uk/prospero as CRD42018110650.
Study selection
Study selection was done through screening of titles, abstracts, and full texts of the articles. Studies meeting the following criteria were included in this analysis: 1) published after 1999, as this was the year the WHO first defined metabolic syndrome (18), 2) involved adults aged ≥18 y, 3) published in English, 4) reported both the effect estimates (i.e. HRs or ORs) and the measure of variance (i.e. 95% CI or SE) or provided adequate data to estimate both, 5) coffee consumption should be 1 of the exposure variables and metabolic syndrome should be 1 of the outcome variables, and 6) presented results adjusted for covariates. If >1 study based on the same cohort was published, only the results of the most recent study were included in the analysis. For studies with missing data, authors were contacted wherever possible for data collection. Unpublished studies and abstracts were excluded. The screening of studies and data extraction were performed by the first and second authors (TWHT and CHW) independently, then cross-checked among the 2. Disputes were resolved through discussion or involvement of a third researcher (JCYL).
Data extraction and assessment of quality of evidence
Risk of bias of the included studies was assessed using the Risk Of Bias In Nonrandomized Studies of Interventions (ROBINS-I) tool (19). Only studies with an overall rating of “low” or “moderate” were included. The following data were extracted from each included study: author, title, year published, location of study, study design (longitudinal/cross-sectional), sample size, gender ratio of participants, mean age or age range of participants, diagnostic criteria of metabolic syndrome, multivariate-adjusted OR or HR of having metabolic syndrome and 95% CI for each coffee-consumption category, covariates adjusted for in each study, and the ethnicity of participants. The certainty of each outcome was assessed according to the Grading of Recommendations Assessment, Development and Evaluation (GRADE) handbook (20) and the findings were communicated using the informative statements published by the GRADE Working Group (21).
Data synthesis and analysis
Two sets of results are presented, 1 involved the pooled estimates of the overall highest consumption category from all studies, whereas the other involved the pooled estimates only from studies with 3 or more coffee consumption levels, which enabled the comparison of effects between medium and high coffee consumption. Three consumption categories were generated, namely low, medium, and high (7). If the amount of consumption was reported in a range, the midpoint of the upper and lower limits was used as the mean consumption value. If the upper limit of the highest consumption level was not provided, the mean consumption value was set at 25% higher than the lower limit. For levels without the lower limit, the mean consumption value was set at 50% below the upper limit (7). For studies with 3 levels, the coffee consumption levels were classified into low, medium, and high consumption categories accordingly. For studies with >3 coffee consumption levels, the highest and the lowest coffee consumption levels were classified as the high and low consumption categories, respectively (7). Then, 1 consumption level that was 1) not the highest or lowest level, and 2) with a mean consumption value closest to the midpoint of the overall highest and the overall lowest consumption categories was selected as the medium consumption category.
Sex-adjusted and sex-stratified effect estimates were pooled separately in this analysis. For the medium, high, and the overall highest consumption category, effect estimates were pooled using a random-effect model with the restricted maximum-likelihood estimator, with the lowest coffee consumption category as the reference group. Statistical heterogeneity in each consumption level was determined by the I2 statistic, with 25, 50, and 75% as the cut-offs for low, moderate, and significant heterogeneity, respectively (10). Publication bias analyses was not conducted as <10 studies were included in all tests in this meta-analysis, as advised by the Cochrane Handbook (22). Sensitivity analyses were conducted to explore the sources of heterogeneity, by conducting subgroup analysis and by removing 1 study at a time. Studies that explained the heterogeneity, changed the significance of the effect, or modified the effect size by >10% when removed were considered influential. Subgroup analysis was conducted by stratifying the included studies according to sex and the definition of metabolic syndrome. After assessing the screened studies, analysis of studies stratified by the ethnicities of participants was also performed.
Furthermore, the potential nonlinear dose-response relation between coffee consumption and odds of having metabolic syndrome was also assessed. The 1-stage approach as detailed by Crippa et al. (23) was used to estimate the dose-response curve using restricted cubic splines with 3 knots at the 25th, 50th, and 75th percentiles. Nonlinearity was tested by assessing the P value of the coefficient at the second spline. All analyses were conducted using the R software version 3.6.2 (24), the package metafor (25), and the package dosresmeta (26). For all statistical tests, P < 0.05 was regarded as statistically significant.
Results
Search result
The article inclusion flow chart is shown in Supplemental Figure 1. The literature search from online databases revealed 1084 articles and 4 articles were retrieved from hand searching, yielding a total of 1088 articles. After removing duplicates and screening by titles and abstracts, 29 articles remained for full-text review, of which 14 articles were excluded based on the inclusion criteria—the references of the excluded articles are shown in the Supplemental Methods. A total of 15 articles, consisting of 13 cross-sectional studies and 2 longitudinal studies, met the eligibility criteria for this systematic review. However, among the 2 included longitudinal studies, 1 reported sex-adjusted results (12), whereas the other reported sex-stratified results (13). Therefore, effect estimates from these 2 studies could not be pooled, and only those of the 13 cross-sectional studies were pooled in the meta-analysis. All included studies had an overall rating of low or moderate risk of bias (Supplemental Table 1).
Characteristics of included cross-sectional studies
The characteristics of the identified cross-sectional studies are shown in Table 1. Among the 13 cross-sectional studies, involving a total of 280,803 participants, 7 provided results adjusted for sex only (14, 15, 27–31), 2 provided both sex-adjusted and sex-stratified results (16, 32), 2 provided only sex-stratified results (17, 33), and 2 included only males in the analyses (34, 35). Three studies (27, 34, 35) presented results for 2 consumption levels only and 1 study presented results in per cup increment (31), thus the results from these studies were included only in the overall highest category. The sex-adjusted OR in the overall highest category was pooled using data of 9 studies (14–16, 27–32), whereas data of the medium and high consumption categories, as well as the dose-response analysis, were pooled from 7 of them (14–16, 28–30, 32). The OR of males in the overall highest category was pooled using data from 6 studies (16, 17, 32–35), whereas data of the medium and high consumption categories, as well as the dose-response analysis, were derived from 4 studies (16, 17, 32, 33). The OR of females in the overall highest category was pooled from data of 4 studies (16, 17, 32, 33), all of which also provided data for the medium consumption category and the dose-response analysis. None of the attempts of contacting authors for extra information was successful. No attempt was made to request sex-stratified results from study authors who published sex-adjusted data, and sex-adjusted data from authors who published sex-stratified data.
TABLE 1.
Summary of cross-sectional studies examining the association between coffee consumption and metabolic syndrome in adults
| Authors (y) | Population | Country | Age, y | Diagnostic criteria1 | Dietary assessment tool | Cases/total participants, n/total n | Effect estimate (95% CI) | Confounders adjusted |
|---|---|---|---|---|---|---|---|---|
| Grosso et al. (2014) (27)2 | Participants in a study on dietary habits and risk of CVD | Italy | 50.2 | IDF 2006 | FFQ (1 espresso cup = 35 mL) | 313/1889 | <1 cup/d: ref; ≥1 cup/d: 0.43 (0.27, 0.70) | Age, sex, BMI, education level, socioeconomic status, total energy intake, smoking status, alcohol intake, physical activity level, adherence to Mediterranean diet, caffeine intake, and source of caffeine |
| Grosso et al. (2015) (32) | Participants in the Polish arm of the HAPIEE study | Poland | 45–69 | IDF 2006 | FFQ (1 cup = 150 mL) | 2461/8821 | <1 cup/d: ref; 1–2 cups/d: 0.92 (0.82, 1.04); >2 cups/d: 0.75 (0.66, 0.86) | Age, sex, education level, occupation, physical activity, smoking status, alcohol intake, total energy intake, and tea intake |
| Kim et al. (2014) (30) | Participants in the KNHANES 2007–2011 without self-reported CVD and T2DM | South Korea | 19–65 | NCEP ATPIII | FFQ (1 cup/d = 133.3 mL/d) | Case: not reported; total participants: 17,953 | <1 cup/wk: ref; 1–6 cups/wk: 1.11 (0.90, 1.38); 1 cup/d: 1.28 (1.07, 1.53); 2 cups/d: 1.23 (1.02, 1.49); ≥3 cups/d: 1.25 (1.02, 1.53) | Age, sex, smoking status, physical activity, alcohol intake, total energy intake, education level, and income |
| Kim and Je (2018) (14) | Participants in the KNHANES 2012–2015 without self-reported dyslipid-emia, CVD, and T2DM | South Korea | 19–65 | IDF 2009 | FFQ (did not report serve size) | 1300/8387 | <1 cup/d: ref; 1–2 cups/d: 1.03 (0.84, 1.27); 3–4 cups/d: 0.75 (0.58, 0.97); ≥5 cups/d: 0.90 (0.64, 1.25) | Age, sex, income, education level, smoking status, alcohol, total energy intake, survey year, physical activity, BMI, sleep duration, intakes of soda, green tea, vegetables, red and white meat, legumes, fruit, whole grains, fish, nuts, alcohol, and dairy products |
| Micek et al. (2018) (16) | Participants of the Second National Multicenter Health Survey in Poland | Poland | ≥20 | IDF 2009 | FFQ (1 cup = 200 g) | 2116/5146 | 0 cup/d: ref; 0–1 cup/d: 0.91 (0.77, 1.08); 1–2 cups/d: 0.83 (0.72, 0.97); >2 cups/d: 0.83 (0.67, 1.03) | Age, sex, education level, occupation, physical activity, smoking status, alcohol intake, total energy intake, and tea consumption |
| Nordestgaard et al. (2015) (28) | Participants in the Copenhagen General Population Study | Denmark | ≥20 | Slightly modified from IDF 20093 | FFQ (did not report serve size) | 26,046/82,740 | 0 cup/d: ref; 0.1–1 cup/d: 0.91 (0.86, 0.97); 1.1–2 cups/d: 0.89 (0.84, 0.94); 2.1–3 cups/d: 0.88 (0.83, 0.93); 3.1–4 cups/d: 0.83 (0.78, 0.89); 4.1–5 cups/d: 0.84 (0.79, 0.90); >5 cups/d: 0.89 (0.83, 0.95) | Age, sex, smoking status, physical activity, and use of antihypertensive and lipid-lowering medication |
| Stutz et al. (2018) (15) | Participants of the Finnish Diabetic Nephropathy Study | Finland | 46.7 | IDF 2009 | FFQ (did not report serve size) | 670/1040 | <1 cup/d: ref; ≥1– <3 cups/d: 1.56 (0.86, 2.83); ≥3 to <5 cups/d: 1.76 (1.02, 3.06); ≥5 cups/d: 2.13 (1.17, 3.87) | Age, sex, energy intake, alcohol intake, physical activity, and smoking status |
| Takami et al. (2013) (29) | Participants of the J-MICC without histories of stroke or ischemic heart disease | Japan | 35–70 | NCEP ATPIII | FFQ (did not report serve size) | 114/577 | <1.5 cups/d: ref; ≥1.5–3 cups/d: 0.56 (0.33, 0.95); ≥3 cups/d: 0.55 (0.31, 0.94) | Age, sex, total energy intake, physical activity, and smoking and drinking habits |
| Matsuura et al. (2012) (33) | Adults of Kansai area, Japan | Japan | 20–65 | Japanese criteria | FFQ (did not report serve size) | Male: 372/2335; female: 32/948 | Male: 0 cup/d: ref; 1–3 cups/d: 0.85 (0.59, 1.20); ≥4 cups/d: 0.61 (0.39, 0.95); female: 0 cup/d: ref; 1–3 cups/d: 0.74 (0.29, 1.90); ≥4 cups/d: 0.48 (0.11, 2.09) | Age, alcohol intake, smoking status, and physical activity |
| Chang et al. (2012) (34)2,4 | Ambulatory males resided in Tianliao township, Taiwan | China | 65–98 | NCEP ATPIII | FFQ (did not report serve size) | 132/361 | Once/wk: ref; ≥ once/wk: 0.92 (0.27, 3.14) | Age, BMI, uric acid level, HOMA index, hsCRP level, occupation, lived with partner, literacy, alcohol and tea intake, smoking habit, and physical activity |
| Yen et al. (2006) (35)2,4 | Male participants of the Keelung Community-based Integrated Screening program | China | 30–79 | NCEP ATPIII modified for Asian subjects5 | FFQ (did not report serve size) | Case: not reported; total participants: 19,839 | Never: ref; ≥ once/wk: 1.02 (1.00, 1.05) | Age, betel-liquid chewing status, education level, physical activity, occupation, smoking habit, alcohol intake, dietary intake, family history of hypertension, CVD, and diabetes |
| Driessen et al. (2009) (31)2 | Participants of the Amsterdam Health and Growth Study | Netherlands | 36 | NCEP ATPIII | FFQ (1 cup = 125 mL) | 37/368 | Per cup increment: 0.96 (0.82, 1.13)6 | Sex, physical activity, total energy intake, smoking habit, alcohol intake |
| Shin et al. (2019) (17) | Participants of the Health Examinees Gem Study | South Korea | 40–69 | NCEP ATPIII | FFQ (did not report serve size) | Male: 12,701/43,682; female: 21,338/86,738 | Male: 0 cups/d: ref; <1 cup/day: 0.87 (0.80, 0.93); 1 cup/d: 0.85 (0.78, 0.93); 2–3 cups/d: 0.85 (0.78, 0.91); ≥4 cups/d: 0.79 (0.70, 0.90); female: 0 cups/d: ref; <1 cup/day: 0.94 (0.89, 0.99); 1 cup/d: 0.83 (0.78, 0.89); 2–3 cups/d: 0.87 (0.82, 0.93); ≥4 cups/d: 0.70 (0.62, 0.78) | Age, BMI, education, smoking status, alcohol drinker status, sugar intake with coffee or tea, total energy intake |
BP, blood pressure; CVD, cardiovascular disease; HAPIEE, Health, Alcohol and Psychosocial factors in Eastern Europe study; hsCRP, high sensitivity C-reactive protein; IDF, International Diabetes Federation; J-MICC, Japan Multi-Institutional Collaborative Cohort Study; KNHANES, Korean National Health and Nutrition Examination Survey; NCEP ATPIII, National Cholesterol Education Program – Adult Treatment Panel III; T2DM, type 2 diabetes mellitus; TG, triglycerides; WC, waist circumference.
IDF 2006 criteria consisted of the following: central obesity (WC ≥90 cm for men and ≥80 cm for women) and any 2 of the following: high TG (TG >1.7 mmol/L, or specific treatment of this lipid abnormality), low HDL cholesterol (HDL cholesterol <1.03 mmol/L for men, <1.29 mmol/L for women, or treatment of low HDL cholesterol), high BP (systolic BP >130 or diastolic BP >85 mmHg, or treatment of diagnosed hypertension), and high fasting glucose level (fasting plasma glucose >5.6 mmol/L, or previously diagnosed T2DM or require treatment of T2DM). IDF 2009 criteria consisted of the following: ≥3 of the following criteria: abdominal obesity (WC ≥90 cm for men and ≥80 cm for women), low HDL cholesterol (<1.0 mmol/L for men and <1.3 mmol/L for women), high TG (TG ≥1.7 mmol/L), high fasting glucose level (fasting plasma glucose ≥5.6 mmol/L), and high BP (systolic BP ≥130 or diastolic BP ≥85 mmHg). NCEP ATPIII criteria: ≥3 of the following: abdominal obesity (WC ≥90 cm for men and ≥80 cm for women), high fasting glucose level (fasting glucose ≥5.6 mmol/L), high TG (TG ≥1.7 mmol/L), low HDL cholesterol (HDL-cholesterol <1.0 mmol/L for men and <1.3 mmol/L for women), and high BP (systolic BP ≥130 mmHg or diastolic BP ≥85 mmHg). Japanese criteria: abdominal obesity (WC ≥85 cm in men or ≥90 cm in women) and ≥2 of the following components: high BP (systolic BP 130 ≥mmHg, diastolic BP ≥ 85 mmHg, or use of antihypertensive medication), dyslipidemia (TG ≥1.7 mmol/L or HDL cholesterol <1.03 mmol/L), and high fasting plasma glucose level (fasting plasma glucose level ≥6.1 mmol/L or use of antidiabetic medication).
Did not provide data for the medium consumption category.
The cut-offs of each individual condition were modified as follows: central obesity: WC ≥94 cm for men and ≥80 cm in women. High BP: systolic BP ≥130 mmHg or diastolic BP ≥85 mmHg or antihypertensive treatment. High TG: nonfasting plasma TG ≥1.7 mmol/L. Low HDL cholesterol: HDL cholesterol <1.0 mmol/L for men and <1.3 mmol/L for women. High glucose level: registry-based diagnosis of diabetes or self-reported diabetes or use of antidiabetic medication or nonfasting baseline glucose levels >11 mmol/L.
Coffee consumption is not the main exposure variable in this study.
This set of criteria is the same as NCEP ATPIII listed above, except the value of high fasting glucose was set at ≥6.1 mmol/L.
Not reported in the published article. Obtained from Marventano et al. (10)
Six included studies involved Caucasian participants (15, 16, 27, 28, 31, 32), whereas the other 7 involved Asian participants (14, 17, 29, 30, 33–35). All studies except 1 (30) did not explicitly state the types of coffee consumed during data collection. Three studies excluded participants with histories of CVD from the analyses (14, 29, 30) and 2 further excluded those with reported diabetes (14, 30). One study only included participants diagnosed with type 1 diabetes (15). Common covariates included in these studies were sex, age, education status, alcohol consumption, and physical activity. The definitions of metabolic syndrome adapted in these studies were mainly from the IDF (1, 2) and the NCEP ATP III (3). One study provided 2 sets of metabolic syndrome prevalence data based on 2 different diagnostic criteria (29), and data based on the NCEP ATP III criteria was used in the analysis.
Meta-analysis results of cross-sectional studies
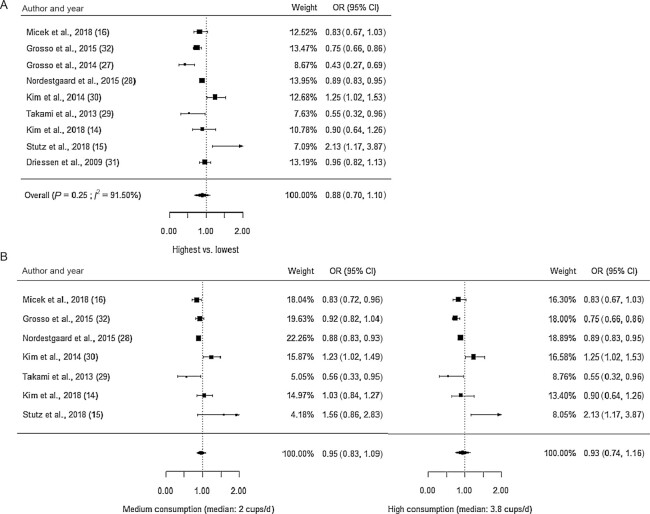
The findings of the meta-analysis are summarized in Table 2. After pooling all sex-adjusted ORs (Figure 1a), coffee consumption may not be associated with the odds of having metabolic syndrome (n = 9 studies, OR: 0.88; 95% CI: 0.70, 1.10) and the result was highly heterogeneous (I2: 92%). When only including studies with 3 or more consumption levels (n = 7 studies), the ORs of both medium and high consumption were also statistically nonsignificant (Figure 1b), suggesting little to no difference in the odds of having metabolic syndrome. The nonsignificant association between coffee consumption and metabolic syndrome after adjustment for sex was of low certainty. The result of the dose-response analysis (Figure 2) showed that the association between coffee consumption and the odds of having metabolic syndrome was nonlinear (n = 7 studies, P for nonlinearity = 0.002) after adjusting for sex.
TABLE 2.
Summary and GRADE assessments (20) of the pooled effect estimates of the association between coffee consumption and metabolic syndrome1
| Outcome | No. of studies, n2 | Design | Risk of bias | Inconsistency | Indirectness | Imprecision | Pooled OR (95% CI)3 | P of overall effect3 | I 2 , % 4 | Certainty of evidence |
|---|---|---|---|---|---|---|---|---|---|---|
| Odds of having metabolic syndrome adjusted for sex | Overall highest: 9; medium and high: 7 | Cross-sectional | Low | Serious inconsistency5 | No serious indirectness | No serious imprecision | Overall highest: 0.88 (0.70, 1.10); medium: 0.95 (0.83, 1.09); high: 0.93 (0.74, 1.16) | Overall highest: 0.25; medium: 0.45, high: 0.52 | Overall highest: 91.5; medium: 77.6; high: 88.6 | Low |
| Odds of having metabolic syndrome in males | Overall highest: 6; medium and high: 4 | Cross-sectional | Low | No serious inconsistency | No serious indirectness | No serious imprecision | Overall highest: 0.84 (0.72, 0.98); medium: 0.85 (0.80, 0.91); high: 0.78 (0.71, 0.86) | Overall highest: 0.02; medium: <0.001; high: <0.001 | Overall highest: 0; medium: 0; high: 0 | Low |
| Odds of having metabolic syndrome in females | Medium and high: 4 | Cross-sectional | Low | No serious inconsistency | No serious indirectness | No serious imprecision | Medium: 0.88 (0.83, 0.93); high: 0.72 (0.65, 0.79) | Medium: <0.001; high: <0.001 | Medium: 0; high: 0 | Low |
| Odds of having metabolic syndrome in Caucasians | Overall highest: 6; medium and high: 4 | Cross-sectional | Low | Serious inconsistency5 | No serious indirectness | No serious imprecision | Overall highest: 0.86 (0.64, 1.17); medium: 0.88 (0.84, 0.93); high: 0.95 (0.68, 1.33) | Overall highest: 0.35; medium: <0.001; high: 0.77 | Overall highest: 94.7; medium: 0; high: 94.1 | Very low |
| Odds of having metabolic syndrome in Asians | Medium and high: 3 | Cross-sectional | Unclear | Serious inconsistency5 | No serious indirectness | No serious imprecision | Medium: 0.95 (0.64, 1.42); high: 0.90 (0.58, 1.40) | Medium: 0.81; high: 0.65 | Medium: 85.1; high: 79.7 | Very low |
| Odds of having metabolic syndrome as defined by the IDF 2009 criteria (1) | Medium and high: 4 | Cross-sectional | Low | No serious inconsistency | No serious indirectness | No serious imprecision | Medium: 0.89 (0.84, 0.93); high: 0.89 (0.84, 0.95) | Medium: <0.001; high: <0.001 | Medium: 0; high: 0 | Low |
| Odds of having metabolic syndrome as defined by the IDF 2006 criteria (2) | 2 | Cross-sectional | Unclear | Serious inconsistency5 | No serious indirectness | Serious imprecision | 0.60 (0.35, 1.02) | 0.059 | 79.4 | Very low |
| Odds of having metabolic syndrome as defined by the NCEP ATPIII criteria (3) | Overall highest: 3; medium and high: 2 | Cross-sectional | Unclear | Serious inconsistency5 | No serious indirectness | Serious imprecision | Overall highest: 0.93 (0.62, 1.39); medium: 0.86 (0.40, 1.86); high: 0.86 (0.39, 1.93) | Overall highest: 0.73; medium: 0.71, high: 0.72 | Overall highest: 87.1; medium: 86.7; high: 86.5 | Very low |
GRADE, Grading of Recommendations Assessment, Development and Evaluation; IDF, International Diabetes Federation; NCEP ATP III, National Cholesterol Education Program – Adult Treatment Panel III.
Publication bias was not assessed according to the Cochrane handbook (22) as all outcomes included <10 studies.
Overall highest included all studies. Medium and high only included studies with data from 3 or more consumption levels.
Both effect estimates and P were obtained using random-effect models. P < 0.05 was regarded as statistically significant.
I 2 >0% and ≤25% depict no heterogeneity; >25% and ≤50% depict small heterogeneity; >50% and ≤75% depict moderate heterogeneity; >75% depicts high heterogeneity.
Serious inconsistency due to the high heterogeneity in the results.
FIGURE 1.
Forest plots with effect estimates from the random-effect meta-analysis of coffee consumption and metabolic syndrome, adjusted for sex. A) The pooled ORs of the highest consumption category using data from all studies; B) the pooled ORs of the medium and high coffee consumption with data from studies with >3 consumption levels.
FIGURE 2.
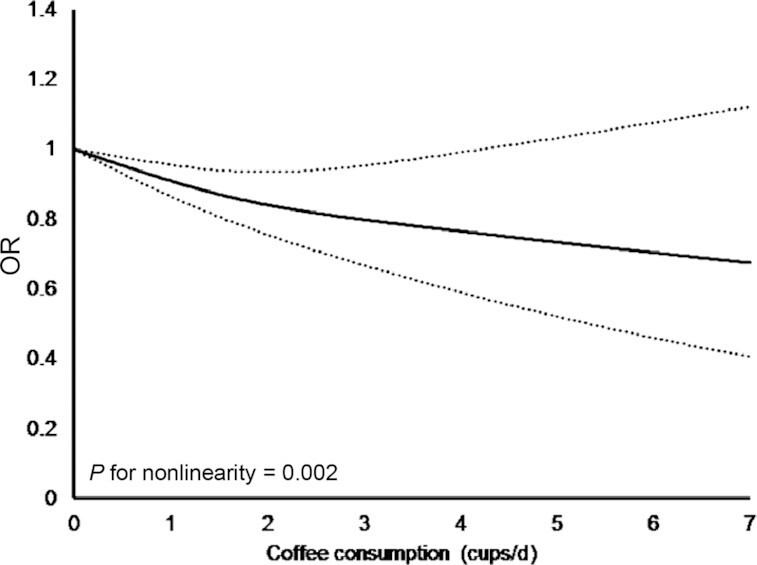
Dose-response curve from the random-effect meta-analysis of coffee consumption and metabolic syndrome, adjusted for sex. Solid line depicts estimated OR and dashed lines depict 95% CI.
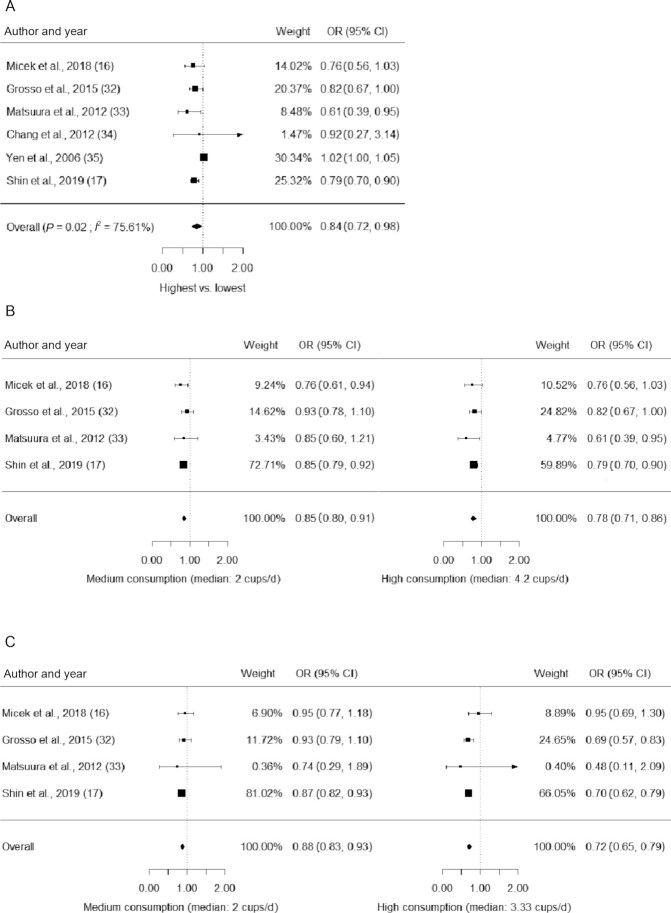
Results from sex-stratified data are shown in Figure 3. Male participants in the highest consumption category may have lower odds of having metabolic syndrome (n = 6 studies; OR: 0.84; 95% CI: 0.72, 0.98; P = 0.02) and the results were highly heterogenous (I2: 76%). When including only studies with 3 or more response levels in men, the results suggested that both medium (n = 4 studies; OR: 0.85; 95% CI: 0.80, 0.91; P < 0.001; I2: 0%) and high coffee consumption (n = 4 studies; OR: 0.78; 95% CI: 0.71, 0.86; P < 0.001; I2: 0%) may be associated with lower odds of having metabolic syndrome, respectively. Similarly, females with medium (n = 4 studies; OR: 0.88; 95% CI: 0.83, 0.93; P < 0.001; I2: 0%) and high coffee consumption (n = 4 studies; OR: 0.72; 95% CI: 0.65, 0.79; P < 0.001; I2: 0%) may have significantly lower odds of having metabolic syndrome than those in the reference category. The findings regarding the sex-stratified results were of low certainty. Results of the dose-response analysis (Figure 4) showed that the association between coffee consumption and the odds of having metabolic syndrome was nonlinear in males (n = 4 studies, P for nonlinearity < 0.001) but not in females (n = 4 studies, P for nonlinearity = 0.10).
FIGURE 3.
Forest plot effect estimates from the random-effect meta-analysis of coffee consumption and metabolic syndrome, stratified by sex. A) The pooled ORs of the highest consumption category using data of male participants from all studies; B) the pooled ORs of the medium and high coffee consumption from studies with data from male participants and from >3 consumption levels; C) the pooled ORs of the medium and high coffee consumption from studies with data from female participants and from >3 consumption levels.
FIGURE 4.
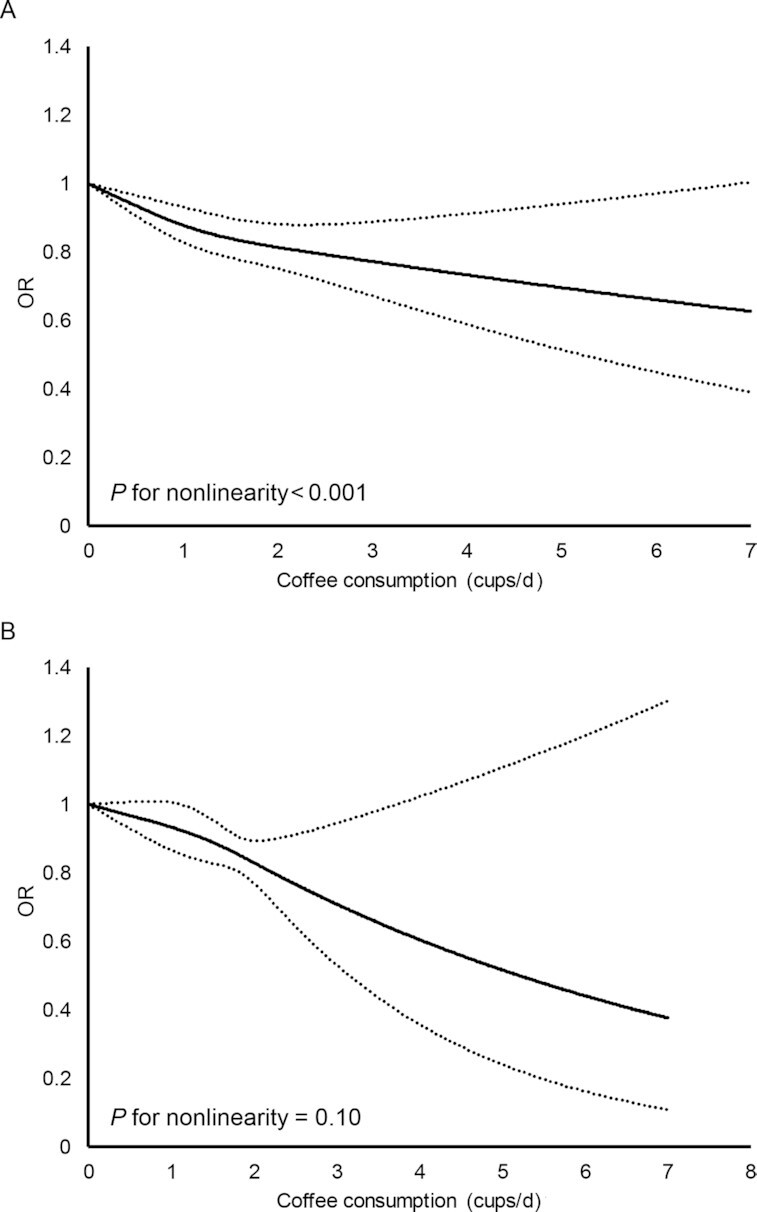
Dose-response curve from the random-effect meta-analysis of coffee consumption and metabolic syndrome in A) males and B) females. Solid lines depict estimated OR and dashed lines depict 95% CI.
Results of sensitivity analysis
Results of the sensitivity analysis revealed that the study by Kim et al. (30) is an influential study. Removing this study led to significant results in both medium (n = 6 studies; OR: 0.89; 95% CI: 0.85–0.93; I2: 0%) and high coffee consumption (OR: 0.85; 95% CI: 0.74, 0.98; I2: 60.0%), whereas removing others did not lead to material differences at any consumption levels. In terms of ethnicities of the participants, medium coffee consumption was associated with a lower prevalence of metabolic syndrome in Caucasian participants (n = 4 studies; OR: 0.88; 95% CI: 0.84, 0.93; I2: 0%), yet the OR for high coffee consumption was not statistically significant. The ORs of both medium and high coffee consumption in Caucasians became statistically significant after excluding the study by Stutz et al. (15), which included only patients with type 1 diabetes (n = 4 studies; medium consumption: OR: 0.89; 95% CI: 0.85, 0.93; I2: 0%; high consumption: OR: 0.85; 95% CI: 0.74, 0.98; I2: 60.0%). Statistically nonsignificant results were observed for Asian participants at both consumption levels. When the included studies were stratified by the diagnosis criteria of metabolic syndrome, only the data pooled from studies that adapted the IDF criteria published in 2009 (1) were statistically significant. All findings in the sensitivity analysis were of very low to low certainty.
Characteristics of longitudinal studies
A total of 2 longitudinal studies were eligible for inclusion. One was conducted in Norway (13) and involved 17,014 participants aged 20–61 y at baseline, with an average follow-up period of 13.8 y. After adjusting for age and baseline examination, coffee consumption was positively associated with the incidence of metabolic syndrome in women but not in men, yet the statistical significance was lost after adjusting for lifestyle variables. The other study was carried out in the USA (12) and the data of 9514 participants were analyzed. The baseline age was 53.6 y and the average follow-up period was 9 y. After adjusting for covariates, coffee consumption was not associated with the incidence of metabolic syndrome. These results showed that coffee consumption may not result in reduced risk of having metabolic syndrome with a low certainty.
Discussion
In this meta-analysis, the pooled, sex-adjusted OR suggested that coffee consumption may not be associated with metabolic syndrome. Nonetheless, sex-stratified analysis showed significant inverse associations in both males and females. Results of subgroup analyses revealed that only moderate coffee consumption in Caucasians, but not Asians, was associated with lower odds of having metabolic syndrome. All findings in the current analysis had a low or very low certainty in the quality of evidence rating.
The nonsignificant overall association observed between coffee consumption and metabolic syndrome does not align with the results of the 2 previous meta-analyses (9, 10), both of which observed a significant inverse association. One reason behind this contradiction might be the presence of methodological differences between this work and the previous ones. First, we analyzed only ORs whereas the previous analyses pooled ORs and HRs in the same analysis. Nonetheless, the 2 measures were different in essence—OR is an effect estimate that measures cross-sectional association, whereas HR is calculated based on the time of the onset of the event, and the 2 are not statistically interchangeable. Second, we analyzed the sex-adjusted and sex-stratified ORs separately, whereas previous analyses pooled these 2 types of studies and reported a single effect estimate. The practice of pooling OR and HR, as well as pooling sex-adjusted OR and sex-stratified OR, could both lead to difficulty in interpreting the resultant effect estimate. In addition, this study adapted a different set of inclusion criteria, thus excluding several studies that were included in previous meta-analyses. For instance, this analysis excluded studies with results not adjusted for lifestyle covariates (36–38), so that the pooled effect estimates were less confounded by these. It is important to assess the effect estimates with and without adjustment for lifestyle variables, which were also associated with metabolic syndrome (4, 39), separately to avoid confounding the association with coffee. All these differences led to changes in the number of included studies and the pooled estimates.
Although the sex-adjusted ORs were not statistically significant, the sex-stratified ORs were significant for both male and female participants. This is probably due to the inclusion of a new study with 130,420 participants (17), which observed a significant inverse association between coffee consumption and metabolic syndrome in both sexes. Data from this study, which had a much higher number of participants than the other studies, dominated the pooled effect estimates, thus leading to significant findings.
A significant association between coffee consumption and metabolic syndrome was observed only in Caucasians but not Asians, similar to the results of a previous meta-analysis (10). Among the studies involving Caucasian participants, the study conducted by Stutz et al. (15), involving a group of patients with type 1 diabetes instead of the general population, found a significant positive association between coffee consumption and metabolic syndrome. Excluding this study led to significant ORs in both medium and high coffee consumption in Caucasians. On the other hand, the nonsignificant results in Asians could be due to differences in study design—only 3 studies reported sex-adjusted ORs whereas the other 3 reported ORs for separate sexes, and the small number of studies made it more difficult to detect a significant association. Furthermore, the results between Asian studies also varied—1 Korean study found that coffee consumption was positively associated with metabolic syndrome (30), whereas another Korean study conducted in a different cohort reported nonsignificant ORs (14). With the small number of studies, it is premature to conclude that coffee consumption was not associated with metabolic syndrome in Asians. More epidemiological studies are needed to explore this association in the Asian population.
Pooling data from studies that adapted the IDF 2009 definition (1) led to an inverse significant association between coffee consumption and metabolic syndrome both at medium and high consumption. This was not observed when pooling data from studies that used the IDF 2006 definition (2) and the NCEP ATPIII definition (3). This could be partly due to more studies using the IDF 2009 definition than the other 2. Furthermore, the IDF 2009 definition was less stringent when compared with the other 2, thereby possibly leading to more cases observed. When compared with the IDF 2006 definition, central obesity was not mandatory for diagnosing metabolic syndrome in the IDF 2009 definition. When compared with the NCEP ATPIII definition, which mainly consists of biomarker thresholds, the IDF 2009 definition includes these thresholds as well as the use of medication for individual conditions. Nonetheless, the small number of studies included in this meta-analysis limited the power of the analysis, and this research question should be reassessed in future meta-analyses when more studies are available.
The 2 longitudinal studies included in the current meta-analysis (12, 13) did not observe any significant association between coffee consumption and incidence of metabolic syndrome, which contradicts the findings of cross-sectional studies involving Caucasian participants. Using the technique of Mendelian Randomization (MR), several studies pointed out that there might not be a causal association between coffee consumption and T2DM (40), stroke (41), and metabolic syndrome (42). These studies speculated that residual confounding and reverse causality might explain the consistent, inverse, cross-sectional association between coffee consumption and various health outcomes. Our results appear to be in congruence with these speculations.
The overall nonsignificant and heterogeneous associations between coffee consumption and metabolic syndrome could also be due to important consumption habits that were not considered in previous epidemiological studies. One such habit is the consumption of different types of coffee. Indeed, different types of coffee could substantially vary in the content of biological compounds, which potentially mediate the health benefits associated with coffee consumption. For instance, the manufacturing process of instant coffee and paper-filtered coffee removed most of the cafestol, which is a natural lipid found in coffee beans and is capable of raising serum cholesterol concentrations (43). Angeloni et al. (44) also found that the espresso method was more efficient in extracting chlorogenic acids, which suppress inflammation and benefit blood glucose control (45, 46), from ground coffee beans when compared with the French press method and paper filtering. In a previous Korean population-based study, instant coffee drinkers were more likely to have metabolic syndrome when compared with filtered coffee drinkers (30), which sheds light on the potential variation in the effects resulting from consuming different coffee types when assessing the association with metabolic syndrome. Nonetheless, only 1 (30) of the included studies collected data on the types of coffee consumed. As a result, the role of coffee types could not be further explored in the current meta-analysis.
Another such habit is the addition of milk and/or sugar in coffee. Previous studies found that >50% of coffee consumers in Australia and the USA reported habitual usage of milk or sugar, or both when drinking coffee (47, 48). Nonetheless, studies examining the effect of sugar and milk added to coffee were limited. Excess added sugar consumption was found to contribute to the development of cardiometabolic risk factors (49, 50), whereas dairy consumption was inversely associated with CVDs (51, 52). Hence, the possible health effect of these food items when consumed with coffee warrants further investigation. Furthermore, a previous analysis (47) found that Australians who consumed sugar-sweetened coffee had worse diet quality than those who added milk only or nothing at all, which has important implications as an unhealthy dietary pattern is associated with metabolic syndrome (53).
The differing times of coffee consumption could also contribute to the inconsistent results. Postprandial responses of both glucose and triglycerides were found to be predictive of insulin resistance (54) and vascular anomalies (55), respectively. Moreover, it has long been established that the constituent of a meal will affect the postprandial metabolic responses of the next meal. For example, consuming milk 60 min before a meal led to a lower postprandial glycemic response and smaller glycemic excursion when compared with consuming the 2 together (56). Similarly, the lipid content of the meal directly affected the postprandial lipid profile in circulation (57). Previous studies have investigated the effect of coffee consumption around mealtime—1 acute study found that drinking coffee with sugar added before a nutrient load could reduce the postprandial glycemic responses (58). Another cross-sectional study (59) found that whereas coffee consumption was not associated with lower fasting glucose concentrations in healthy adults, it was associated with lower glucose concentrations 2 h after an oral-glucose-tolerance test (OGTT). Hence, the time of coffee consumption around mealtime and its long-term effect warrants further investigation in future epidemiological studies.
One strength of this study is that ORs and HRs, which are statistically different outcome measurements, were analyzed separately, thus ensuring the results are free from misinterpretation. Moreover, the risk of bias of all screened studies was rigorously assessed by a widely recognized tool (19) and only those with a low or moderate risk of bias were included in the analysis. On the other hand, several limitations should be considered when interpreting the results of this study. First, since the types of coffee consumed were not recorded in most of the included studies, this factor could not be further assessed. Second, all included studies in the meta-analysis are cross-sectional, thus temporality and causation could not be determined, and the possibility of residual confounding could not be ruled out. Third, due to the small number of included studies, publication bias was not assessed according to the Cochrane Handbook (22). Moreover, the results of this study are not generalizable to populations other than Asians and European Caucasians. In addition, the results in this analysis were not corrected for multiple comparison, which could lead to inflation of type I error. Nonetheless, most of the subgroup analyses were specified at the registration stage, except for the ethnicity-stratified analysis, which was data driven. Finally, we acknowledge that all the findings in this work are of low or very low certainty. This is because findings from observational studies were automatically given a “low” grading in the GRADE scale due to the inability to exclude the effect of residual confounding from the study results (20). However, the evidence in this work was generated by a rigorous and systematic protocol, thereby forming the best available evidence in the current body of literature on this topic. Apart from that, our study also addressed a major limitation in previous meta-analyses by separately analyzing cross-sectional and longitudinal studies, thus providing a more accurate effect estimate for the association between coffee consumption and metabolic syndrome.
In conclusion, based on the available evidence, coffee consumption may not be associated with lower odds of having metabolic syndrome with a low certainty. This could be due to the small number of studies available, as well as the high heterogeneity resulting from studies done involving participants of different ethnic groups. Future studies should collect data on coffee types usually consumed and habits of milk and sugar usage, whereas more longitudinal studies are needed to investigate the temporal association between coffee consumption and metabolic syndrome.
Supplementary Material
Acknowledgments
The authors’ contributions were as follows—THTW: formatted the research question, researched the data, analyzed the data, and wrote the manuscript; CHW: formatted the research question, researched the data, analyzed the data, and wrote the manuscript: XZ, YZ, JX, and KCY: analyzed the data, reviewed the manuscript, and contributed to the discussions: JMFW: reviewed the manuscript and contributed to the discussions; JCYL: formatted the research question, reviewed the manuscript, and contributed to the discussions; all authors: read and approved the final manuscript; JCYL: is the guarantor of this work and has primary responsibility of the final content.
Notes
The authors reported no funding received for this study.
Author disclosures: The authors report no conflicts of interest.
Supplemental Table 1, Supplemental Figure 1, and Supplemental Methods are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/advances.
Abbreviations used: CVD, cardiovascular disease; GRADE, Grading of Recommendations Assessment, Development and Evaluation; IDF, International Diabetes Federation; NCEP ATP III, National Cholesterol Education Program – Adult Treatment Panel III; T2DM, type 2 diabetes mellitus.
Contributor Information
Tommy H T Wong, School of Biological Sciences, The University of Hong Kong, Hong Kong Special Administrative Region, People's Republic of China.
Chi Ho Wong, School of Biological Sciences, The University of Hong Kong, Hong Kong Special Administrative Region, People's Republic of China.
Xiaoyu Zhang, Department of Statistics and Actuarial Science, The University of Hong Kong, Hong Kong Special Administrative Region, People's Republic of China.
Yunpeng Zhou, Department of Statistics and Actuarial Science, The University of Hong Kong, Hong Kong Special Administrative Region, People's Republic of China.
Jinfeng Xu, Department of Statistics and Actuarial Science, The University of Hong Kong, Hong Kong Special Administrative Region, People's Republic of China.
Kam Chuen Yuen, Department of Statistics and Actuarial Science, The University of Hong Kong, Hong Kong Special Administrative Region, People's Republic of China.
Jennifer M F Wan, School of Biological Sciences, The University of Hong Kong, Hong Kong Special Administrative Region, People's Republic of China.
Jimmy C Y Louie, School of Biological Sciences, The University of Hong Kong, Hong Kong Special Administrative Region, People's Republic of China.
References
- 1. Alberti K, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, Fruchart J-C, James WPT, Loria CM, Smith SC. Harmonizing the metabolic syndrome. Circulation. 2009;120(16):1640–5. [DOI] [PubMed] [Google Scholar]
- 2. Alberti K, Zimmet P, Shaw J. Metabolic syndrome—a new world-wide definition. A Consensus Statement from the International Diabetes Federation. Diabet Med. 2006;23(5):469–80. [DOI] [PubMed] [Google Scholar]
- 3. National Cholesterol Education Program (NCEP) Expert Panel . Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) Final Report. Circulation. 2002;106(25):3143. [PubMed] [Google Scholar]
- 4. Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet North Am Ed. 2005;365(9468):1415–28. [DOI] [PubMed] [Google Scholar]
- 5. O'Neill S, O'Driscoll L. Metabolic syndrome: a closer look at the growing epidemic and its associated pathologies. Obes Rev. 2015;16(1):1–12. [DOI] [PubMed] [Google Scholar]
- 6. Grundy SM. Drug therapy of the metabolic syndrome: minimizing the emerging crisis in polypharmacy. Nat Rev Drug Discov. 2006;5:295. [DOI] [PubMed] [Google Scholar]
- 7. Ding M, Bhupathiraju SN, Chen M, van Dam RM, Hu FB. Caffeinated and decaffeinated coffee consumption and risk of type 2 diabetes: a systematic review and a dose-response meta-analysis. Diabetes Care. 2014;37(2):569–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ding M, Bhupathiraju SN, Satija A, van Dam RM, Hu FB. Long-term coffee consumption and risk of cardiovascular disease: a systematic review and a dose-response meta-analysis of prospective cohort studies. Circulation. 2014;129(6):643–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Shang F, Li X, Jiang X. Coffee consumption and risk of the metabolic syndrome: a meta-analysis. Diabetes Metab. 2016;42(2):80–7. [DOI] [PubMed] [Google Scholar]
- 10. Marventano S, Salomone F, Godos J, Pluchinotta F, Del Rio D, Mistretta A, Grosso G. Coffee and tea consumption in relation with non-alcoholic fatty liver and metabolic syndrome: a systematic review and meta-analysis of observational studies. Clin Nutr. 2016;35(6):1269–81. [DOI] [PubMed] [Google Scholar]
- 11. Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lutsey PL, Steffen LM, Stevens J. Dietary intake and the development of the metabolic syndrome. Circulation. 2008;117(6):754–61. [DOI] [PubMed] [Google Scholar]
- 13. Wilsgaard T, Jacobsen BK. Lifestyle factors and incident metabolic syndrome: The Tromos Study 1979–2001. Diabetes Res Clin Pract. 2007;78(2):217–24. [DOI] [PubMed] [Google Scholar]
- 14. Kim Y, Je Y. Moderate coffee consumption is inversely associated with the metabolic syndrome in the Korean adult population. Br J Nutr. 2018;120(11):1279–87. [DOI] [PubMed] [Google Scholar]
- 15. Stutz B, Ahola AJ, Harjutsalo V, Forsblom C, Groop PH. Association between habitual coffee consumption and metabolic syndrome in type 1 diabetes. Nutr Metab Cardiovasc Dis. 2018;28(5):470–6. [DOI] [PubMed] [Google Scholar]
- 16. Micek A, Grosso G, Polak M, Kozakiewicz K, Tykarski A, Puch Walczak A, Drygas W, Kwaśniewska M, Pająk A. Association between tea and coffee consumption and prevalence of metabolic syndrome in Poland—results from the WOBASZ II study (2013–2014). Int J Food Sci Nutr. 2018;69(3):358–68. [DOI] [PubMed] [Google Scholar]
- 17. Shin S, Lim J, Lee H-W, Kim CE, Kim S-A, Lee J-K, Kang D. Association between the prevalence of metabolic syndrome and coffee consumption among Korean adults: results from the Health Examinees study. Appl Physiol Nutr Metab. 2019;44(12):1371–8. [DOI] [PubMed] [Google Scholar]
- 18. World Health Organization . Definition, diagnosis and classification of diabetes mellitus and its complications: report of a WHO consultation. Part 1: diagnosis and classification of diabetes mellitus. Geneva (Switzerland): World Health Organization; 1999. [Google Scholar]
- 19. Sterne JAC, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, Henry D, Altman DG, Ansari MT, Boutron Iet al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Schünemann H, Brożek J, Guyatt G, Oxman A . Grade handbook for grading quality of evidence and strength of recommendations. The GRADE Working Group; 2013. [Google Scholar]
- 21. Santesso N, Glenton C, Dahm P, Garner P, Akl EA, Alper B, Brignardello-Petersen R, Carrasco-Labra A, De Beer H, Hultcrantz Met al. GRADE guidelines 26: informative statements to communicate the findings of systematic reviews of interventions. J Clin Epidemiol. 2020;119:126–35. [DOI] [PubMed] [Google Scholar]
- 22. Higgins J, Green S. Cochrane handbook for systematic reviews of interventions. [Internet] Version 5.1.0 June 2019. Available from: www.handbook.cochrane.org. [Google Scholar]
- 23. Crippa A, Discacciati A, Bottai M, Spiegelman D, Orsini N. One-stage dose-response meta-analysis for aggregated data. Stat Methods Med Res. 2019;28(5):1579–96. [DOI] [PubMed] [Google Scholar]
- 24. R Core Team . R: a language and environment for statistical computing; 2019. [Internet] Available from: https://www.R-project.org/. [Google Scholar]
- 25. Viechtbauer W. Conducting Meta-Analyses in R with the metafor Package. J Stat Soft. 2010;36(3). [Google Scholar]
- 26. Crippa A, Orsini N. [Internet] Multivariate dose-response meta-analysis: the dosresmeta R package. J Stat Softw; Vol 1, Code Snippet 1 (2016) 2016. Available from: doi: 10.18637/jss.v072.c01. [Google Scholar]
- 27. Grosso G, Marventano S, Galvano F, Pajak A, Mistretta A. Factors associated with metabolic syndrome in a Mediterranean population: role of caffeinated beverages. J Epidemiol. 2014;24(4):327–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nordestgaard AT, Thomsen M, Nordestgaard BG. Coffee intake and risk of obesity, metabolic syndrome and type 2 diabetes: a Mendelian randomization study. Int J Epidemiol. 2015;44(2):551–65. [DOI] [PubMed] [Google Scholar]
- 29. Takami H, Nakamoto M, Uemura H, Katsuura S, Yamaguchi M, Hiyoshi M, Sawachika F, Juta T, Arisawa K. Inverse correlation between coffee consumption and prevalence of metabolic syndrome: baseline survey of the Japan Multi-Institutional Collaborative Cohort (J-MICC) Study in Tokushima, Japan. J Epidemiol. 2013;23(1):12–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kim H-J, Cho S, Jacobs DR, Park K. Instant coffee consumption may be associated with higher risk of metabolic syndrome in Korean adults. Diabetes Res Clin Pract. 2014;106(1):145–53. [DOI] [PubMed] [Google Scholar]
- 31. Driessen MT, Koppes LLJ, Veldhuis L, Samoocha D, Twisk JWR. Coffee consumption is not related to the metabolic syndrome at the age of 36 years: the Amsterdam Growth and Health Longitudinal Study. Eur J Clin Nutr. 2009;63(4):536–42. [DOI] [PubMed] [Google Scholar]
- 32. Grosso G, Stepaniak U, Micek A, Topor-Mądry R, Pikhart H, Szafraniec K, Pająk A. Association of daily coffee and tea consumption and metabolic syndrome: results from the Polish arm of the HAPIEE study. Eur J Nutr. 2015;54(7):1129–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Matsuura H, Mure K, Nishio N, Kitano N, Nagai N, Takeshita T. Relationship between coffee consumption and prevalence of metabolic syndrome among Japanese civil servants. J Epidemiol. 2012;22(2):160–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chang C-S, Chang Y-F, Liu P-Y, Chen C-Y, Tsai Y-S, Wu C-H. Smoking, habitual tea drinking and metabolic syndrome in elderly men living in rural community: The Tianliao Old People (TOP) Study 02. PLoS One. 2012;7(6):e38874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yen AM-F, Chiu Y-H, Chen L-S, Wu H-M, Huang C-C, Boucher BJ, Chen TH-H. A population-based study of the association between betel-quid chewing and the metabolic syndrome in men. Am J Clin Nutr. 2006;83(5):1153–60. [DOI] [PubMed] [Google Scholar]
- 36. Wan C-J, Lin L-Y, Yu T-H, Sheu WHH. Metabolic syndrome associated with habitual indulgence and dietary behavior in middle-aged health-care professionals. J Diabetes Investig. 2010;1(6):259–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Dos Santos PR, Ferrari GSL, Ferrari CKB. Diet, sleep and metabolic syndrome among a legal Amazon population, Brazil. Clin Nutr Res. 2015;4(1):41–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hino A, Adachi H, Enomoto M, Furuki K, Shigetoh Y, Ohtsuka M, Kumagae S-I, Hirai Y, Jalaldin A, Satoh Aet al. Habitual coffee but not green tea consumption is inversely associated with metabolic syndrome: an epidemiological study in a general Japanese population. Diabetes Res Clin Pract. 2007;76(3):383–9. [DOI] [PubMed] [Google Scholar]
- 39. Grundy SM. Metabolic syndrome update. Trends Cardiovasc Med. 2016;26(4):364–73. [DOI] [PubMed] [Google Scholar]
- 40. Kwok MK, Leung GM, Schooling CM. Habitual coffee consumption and risk of type 2 diabetes, ischemic heart disease, depression and Alzheimer's disease: a Mendelian randomization study. Sci Rep. 2016;6(1):36500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Qian Y, Ye D, Huang H, Wu DJH, Zhuang Y, Jiang X, Mao Y. Coffee consumption and risk of stroke: a Mendelian randomization study. Ann Neurol. 2020. doi: 10.1002/ana.25693. [DOI] [PubMed] [Google Scholar]
- 42. Nordestgaard AT, Nordestgaard BG. Coffee intake, cardiovascular disease and all-cause mortality: observational and Mendelian randomization analyses in 95 000–223 000 individuals. Int J Epidemiol. 2016;45(6):1938–52. [DOI] [PubMed] [Google Scholar]
- 43. Urgert R, Schulz AG, Katan MB. Effects of cafestol and kahweol from coffee grounds on serum lipids and serum liver enzymes in humans. Am J Clin Nutr. 1995;61(1):149–54. [DOI] [PubMed] [Google Scholar]
- 44. Angeloni G, Guerrini L, Masella P, Bellumori M, Daluiso S, Parenti A, Innocenti M. What kind of coffee do you drink? An investigation on effects of eight different extraction methods. Food Res Int. 2019;116:1327–35. [DOI] [PubMed] [Google Scholar]
- 45. Liang N, Kitts DD. Role of chlorogenic acids in controlling oxidative and inflammatory stress conditions. Nutrients. 2015;8(1):16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Tajik N, Tajik M, Mack I, Enck P. The potential effects of chlorogenic acid, the main phenolic components in coffee, on health: a comprehensive review of the literature. Eur J Nutr. 2017;56(7):2215–44. [DOI] [PubMed] [Google Scholar]
- 47. Wong THT, Sui Z, Rangan A, Louie JCY. Discrepancy in socioeconomic status does not fully explain the variation in diet quality between consumers of different coffee types. Eur J Nutr. 2018;57(6):2123–31. [DOI] [PubMed] [Google Scholar]
- 48. Bouchard DR, Ross R, Janssen I. Coffee, tea and their additives: association with BMI and waist circumference. Obes Facts. 2010;3(6):345–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Cottrell RC. Sugar: an excess of anything can harm. Nature. 2012;483(7388):158. [DOI] [PubMed] [Google Scholar]
- 50. World Health Organization . Guideline: sugars intake for adults and children. Geneva (Switzerland): World Health Organization; 2015. [PubMed] [Google Scholar]
- 51. Drouin-Chartier J-P, Brassard D, Tessier-Grenier M, Côté JA, Labont M-È, Desroches S, Couture P, Lamarche B. Systematic review of the association between dairy product consumption and risk of cardiovascular-related clinical outcomes. Adv Nutr. 2016;7(6):1026–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Dehghan M, Mente A, Rangarajan S, Sheridan P, Mohan V, Iqbal R, Gupta R, Lear S, Wentzel-Viljoen E, Avezum Aet al. Association of dairy intake with cardiovascular disease and mortality in 21 countries from five continents (PURE): a prospective cohort study. Lancet North Am Ed. 2018;392(10161):2288–97. [DOI] [PubMed] [Google Scholar]
- 53. Rodríguez-Monforte M, Sánchez E, Barrio F, Costa B, Flores-Mateo G. Metabolic syndrome and dietary patterns: a systematic review and meta-analysis of observational studies. Eur J Nutr. 2017;56(3):925–47. [DOI] [PubMed] [Google Scholar]
- 54. Blaak EE, Antoine J-M, Benton D, Björck I, Bozzetto L, Brouns F, Diamant M, Dye L, Hulshof T, Holst JJet al. Impact of postprandial glycaemia on health and prevention of disease. Obes Rev. 2012;13(10):923–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kolovou G, Ooi TC. Postprandial lipaemia and vascular disease. Curr Opin Cardiol. 2013;28(4). [DOI] [PubMed] [Google Scholar]
- 56. Sun L, Tan KWJ, Han CMS, Leow MK-S, Henry CJ. Impact of preloading either dairy or soy milk on postprandial glycemia, insulinemia and gastric emptying in healthy adults. Eur J Nutr. 2017;56(1):77–87. [DOI] [PubMed] [Google Scholar]
- 57. Lopes LL, Rocha D, Da Silva A, Peluzio MdCG, Bressan J, Hermsdorff HHM. Postprandial lipid response to high-saturated and high-monounsaturated fat meals in normal-weight or overweight women. J Am Coll Nutr. 2018;37(4):308–15. [DOI] [PubMed] [Google Scholar]
- 58. Louie JCY, Atkinson F, Petocz P, Brand-Miller JC. Delayed effects of coffee, tea and sucrose on postprandial glycemia in lean, young, healthy adults. Asia Pac J Clin Nutr. 2008;17(4):657–62. [PubMed] [Google Scholar]
- 59. Yarmolinsky J, Mueller NT, Duncan BB, Bisi Molina Mdel C, Goulart AC, Schmidt MI. Coffee consumption, newly diagnosed diabetes, and other alterations in glucose homeostasis: a cross-sectional analysis of the longitudinal study of adult health (ELSA-Brasil). PLoS One. 2015;10(5):e0126469. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.