ABSTRACT
Child undernutrition is a major public health challenge that is persistent and disproportionately prevalent in low- and middle-income countries. Undernourished children face adverse health, economic, and social consequences that can be intergenerational. The first 1000 days of life, from conception until the child's second birthday, constitute the period of greatest vulnerability to undernutrition. The transition process from milk-based diets to solid, semi-solid, and soft food and liquids other than milk, referred to as complementary feeding (CF), occurs between the age of 6 mo and 2 y. CF practices that do not meet the WHO's guiding principles and are lacking in both quality and quantity increase susceptibility to undernutrition, restrict growth, and jeopardize child development and survival. The gut microbiota develops toward an adult-like configuration within the first 2–3 y of life. Recent studies suggest that significant changes in the gut microbial composition and functional capacity occur during the CF period, but these studies were conducted in high-income countries. Research in low- and middle-income countries, on the other hand, has implicated a disrupted gut microbiota in child undernutrition, and animal experiments reveal the potential for a causal relation. Given the growing body of evidence for a plausible role of the gut microbiota in the link between CF and undernutrition, microbiota-targeted complementary food may be a promising treatment modality for undernutrition management. The aims of this paper are to review the evidence for the relation between CF and undernutrition and to highlight the potential of the gut microbiota to be a promising target in this relation. Our summary of the current state of the knowledge in this area provides a foundation for future research and helps inform the design of interventions targeting the gut microbiota to combat child undernutrition and promote healthy growth.
Keywords: malnutrition, child growth, first 1000 days, infant and young child feeding, solid food, gut microbiome, low- and middle-income countries
Complementary feeding interventions targeting the gut microbiota are promising for undernutrition management among children in low- and middle-income countries.
Introduction
Undernutrition refers to insufficient or imbalanced intake of energy and nutrients necessary to maintain growth and good health (1). Child undernutrition manifests in 3 forms—stunting, wasting, and underweight (1)—which are defined using weight and height (length) measurements in the index child compared with age- and sex-specific child growth standards by the WHO (2). Despite efforts to eradicate undernutrition, it remains the most common “disease” among children under the age of 5 (3). According to recent 2020 estimates, 144 million (21.3%) children younger than 5 y are stunted and 47 million (6.9%) are wasted (4). Child undernutrition is disproportionately prevalent in low- and middle-income countries (LMICs) with South Asia and sub-Saharan Africa bearing the greatest disease burden (4).
Nearly half of all deaths in children under the age of 5 are attributable to undernutrition (5). Undernourished children are at a higher risk of death from common child illnesses such as diarrhea, pneumonia, and malaria (6), which together topped the list of the leading causes of death among children under 5 in 2015 (7). In the long term, undernutrition is linked to poorer overall health, impairments in intellectual performance, and reduced economic productivity (8). Further, a woman who was undernourished in childhood has higher odds of delivering a malnourished low-birth-weight newborn, rendering undernutrition an intergenerational problem (3). Interventions to combat undernutrition and promote healthy growth and development among children in LMICs are essential to meet the Sustainable Development Goals, namely those related to hunger eradication and reduction of inequalities (9).
Undernutrition is associated with a web of risk factors, with inadequate dietary intake and infectious diseases being proximal factors in LMICs (10). Approximately 100,000 deaths due to undernutrition in children under 5 could be prevented each year if infant and young child feeding (IYCF) was adequate (11). Environmental enteric dysfunction (EED) due to exposure to enteropathogens is another common risk factor in LMICs with poor sanitation and unhygienic conditions (12). Diarrhea, a particularly visible clinical manifestation of EED, is the second leading cause of death among children under 5, contributing to nearly 9% of deaths in 2015 (7). The interaction between inadequate dietary intake and enteric infections may impair gut and immune functions and can lead to and/or exacerbate pre-existing undernutrition (12).
The first 1000 days of life, from conception until the child's second birthday, constitute a critical window for child growth and development when rapid maturation of metabolic, endocrine, neural, and immune systems occurs, according to the Developmental Origins of Health and Disease (13). Birth cohort studies have consistently demonstrated that the first 1000 days are the period of greatest vulnerability to undernutrition (14), and that undernutrition during this period has profound irreversible effects with limited capacity for catch-up growth (15). The high prevalence and burden of child undernutrition confirm the need to scale up interventions during this vulnerable window, including promotion of optimal age-specific IYCF practices (11, 16).
The human gut microbiota, which refers to the ecological community of commensal, symbiotic, and pathogenic micro-organisms in the gastrointestinal tract (17), is established during the first 2–3 y of life (18). A growing body of evidence has linked disruptions in the establishment and maturation of the gut microbiota to child undernutrition (19–22). Gut microbiota disruptions in early life might result from adverse environmental insults, such as suboptimal IYCF, and contribute to lifelong and intergenerational deficits in growth and development (23). Although several studies have examined the effects of breastfeeding on the gut microbiota (24–28) and risk of undernutrition (29, 30), few have focused on the effects of the following stage of IYCF: complementary feeding (CF). The aims of this paper are to review the evidence for the relation between CF and undernutrition and to highlight the potential of the gut microbiota to be a promising target in this relation.
Methods
PubMed was searched using combinations of the following keywords: complementary feeding or complementary food or solid feeding or solid food (SF) and gut microbiome or gut microbiota for the section on the changes in the gut microbiota during the CF period. The search yielded studies in high-income countries (HICs) not LMICs. For the following section on the gut microbiota and undernutrition, we searched for the papers that proposed a novel approach to describe the disrupted gut microbiota as immature. We also reference a number of papers that describe other definitions of a disrupted gut microbiota. In the last section, based on the definition of the immature gut microbiota, we searched for articles on CF interventions that specifically target the gut microbiota for undernutrition management. As for our selection criteria, we included primary research studies if they 1) were conducted in humans (but we describe an illustrative animal study that enhanced our understanding of the human research); 2) collected (the majority of) fecal samples from infants/children during or around the CF period; 3) examined the effects of CF on the gut microbiota rather than the inherent variation in the process of microbial succession associated with age; 4) examined undernutrition in any of its 3 anthropometric forms (stunting, wasting, underweight); and 5) used next-generation sequencing techniques for microbiome analysis.
Current Status of Knowledge
Inadequate CF practices increase susceptibility to undernutrition
The WHO defines CF as “the process starting when breast milk alone is no longer sufficient to meet the nutritional requirements of infants, and therefore other foods and liquids are needed along with breast milk” (31). The transition process from milk-based diets to solid, semi-solid, and soft food and liquids other than milk typically covers the period between 6 mo and 2 y of age (31). Infants have high nutritional needs for their rapid growth, so optimal CF practices are essential to provide key nutrients to maintain healthy growth and development (32). However, unlike the WHO's recommendation for exclusive breastfeeding for the first 6 mo (33), there is no single recommendation for CF. CF encompasses several dimensions including the age of introduction, amount, energy density, nutrient content, frequency of consumption, food consistency, safe preparation and storage of complementary food, and responsive feeding (34).
In 2003 and 2005, the WHO published detailed guiding principles covering the CF dimensions among breastfed (34) and non-breastfed infants (35), respectively. The guiding principles have had an important impact on policies especially in LMICs and have increased attention to nutrition and growth during the CF period (36). Afterwards, in 2008, the WHO used 10 existing datasets from Africa, Asia, and Latin America to define and validate a set of 8 core and 7 optional indicators to assess IYCF based on the guiding principles (37). Five of the 8 core indicators focus on specific dimensions of CF including age of introduction, energy and nutrient content, and frequency of consumption of the complementary food (Table 1), while the remaining 10 indicators relate to breastfeeding. Other dimensions of CF such as adequate consistency and texture of food and responsive feeding were deemed too complex to assess and require more work to develop valid and reliable indicators (37).
TABLE 1.
Indicators to assess complementary feeding adequacy1
| Indicators | Definition | Notes |
|---|---|---|
| 1. Timely introduction to solid, semi-solid, or soft foods | Proportion of infants 6–8 mo of age who receive solid, semi-solid or soft foods | — |
| 2. Minimum diet diversity | Proportion of children 6–23 mo of age who receive foods from 4 or more of the 7 food groups | The 7 food groups are: grains, roots, and tubers; legumes and nuts; dairy products; flesh foods; eggs; vitamin A–rich fruits and vegetables; other fruits and vegetables. |
| 3. Minimum meal frequency | Proportion of children 6–23 mo of age who receive solid, semi-solid, or soft foods (but also including milk feeds for non-breastfed children) the minimum number of times or more according to the infant's age | The minimum number of times is defined as: 2 times/d for 6–8 mo-old breastfed infants; 3 times/d for 9–23-mo-old breastfed children; 4 times/d for 6–23-mo-old non-breastfed children. |
| 4. Minimum acceptable diet | Proportion of children 6–23 mo of age who had at least the minimum dietary diversity and the minimum meal frequency | This is a summary or composite indicator of the previous 2 indicators. |
| 5. Consumption of iron-rich or iron-fortified foods | Proportion of children 6–23 mo of age who receive an iron-rich food or iron-fortified food that is specially designed for infants and young children, or that is fortified in the home | Suitable iron-rich or iron-fortified foods include flesh foods, commercially fortified foods specially designed for infants and young children that contain iron, or foods fortified in the home with a micronutrient powder containing iron or a lipid-based nutrient supplement containing iron. |
1All indicators are assessed based on the child's dietary intake in the day preceding the survey. Source: WHO (37).
The indicators have been used to assess CF practices worldwide, especially in LMICs. The first published application of the indicators appeared in the country profiles by the WHO using 2002–2008 Demographic and Health Survey (DHS) data from 46 countries (38). Most countries had grossly inadequate CF practices, especially those in South Asia and sub-Saharan Africa with the highest prevalence and burden of child undernutrition (4). In a more recent analysis of the UNICEF global database of national‐level household surveys of 85 countries conducted between 2010 and 2016, CF practices were still poor worldwide and were the poorest in the aforementioned 2 regions (39). Specifically, <50% of children in South Asia and sub-Saharan Africa achieved minimum meal frequency, <25% achieved minimum diet diversity, and <15% achieved a minimum acceptable diet.
Other than assessing CF practices, the WHO indicators have been used to examine the associations between CF and undernutrition susceptibility in different populations in LMICs (40–46), demonstrating the applicability of the indicators for their intended purposes and in populations for whom they were originally developed (37). In Malawi, for example, a sub-Saharan African country severely burdened by child undernutrition (47), 13- to 23-mo-old children who did not meet the minimum meal frequency or the minimum acceptable diet indicators were more likely to be underweight (45). In Bangladesh, which has one of the highest burdens of child undernutrition worldwide (48) and where CF practices remain grossly inadequate (49), untimely introduction of complementary food and failure to meet the minimum diet diversity indicator were associated with stunting among 6- to 23-mo-old children (46). An association between the consumption of a diverse diet and lower likelihood of stunting was also noted in a pooled analysis of 74,548 children aged 6–23 mo using the 2010–2014 DHS from 39 LMICs (50). In addition, consumption of animal-source food, which is not currently a WHO indicator but has emerged as a critical dimension related to protein quality (36), was inversely associated with stunting after adjustment for socioeconomic and other covariates (50).
CF interventions that are effective at reducing undernutrition in LMICs are a high priority (51). Systematic reviews and meta-analyses assessing the effectiveness of CF interventions are valuable, yet variations in study populations, type of interventions, reported outcomes, and grade quality of the reviewed studies render calculation of pooled estimates challenging. Heidkamp (52) synthesized the evidence in 2017 from recent systematic reviews and meta-analyses of the impact of CF interventions on linear and ponderal growth of children aged 6–23 mo in LMICs (53–55). The systematic reviews of interventions with CF education alone (53, 55) and those of interventions with provision of food and other supplements with or without education (54, 55) revealed modest effects on height and weight gain. In another review, CF education and supplementary feeding interventions did not have significant effects on stunting and wasting prevalence, whereas CF provision had significant positive effects on the prevalence of stunting (56). Other systematic reviews have noted similar small and mixed effects of CF interventions on child growth in LMICs (57, 58).
The associations between the multiple dimensions of CF and undernutrition coupled with the limited catch-up growth following CF interventions in undernourished children highlight the complexity of this relation. Further, adequate CF to maintain child growth and development depends not only on what, when, and how children are fed but also on the extent to which their body is capable of efficiently digesting, absorbing, and utilizing these foods (23), 3 functions in which the gut microbiota plays a critical role.
Significant changes in the gut microbiota of children occur during the CF period
The gut microbiota develops and matures toward an adult-like configuration during the first 2–3 y of life (18). Gut microbiota development has prominent effects on long-term health (23) and is affected by several factors, including mode of delivery, antibiotic exposure, and IYCF (24, 25, 27). While the differential effects of milk-based diets (breast vs. formula vs. mixed feeding) on the infant microbiota have been examined (24–28), less research has been conducted on the effects of CF (59). Further, some studies have suggested that breastfeeding in terms of exclusivity, amount, duration, and cessation is the main driver of the changes in the gut microbiota during the CF period rather than CF itself (24, 25). The following section reviews the studies (Table 2) examining the association of CF with the changes in the gut microbiota and addresses whether the aforementioned breastfeeding-related variables influence these changes.
TABLE 2.
Summary of the studies examining the changes in the gut microbiota during the CF period1
| Examined variable related to the gut microbiota | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Reference | Study design | Study location | Sample size, n | Age at fecal sample collection | Examined variable related to CF | Examined effect of BF in relation to CF | ɑ-Diversity | Microbial composition | Fecal SCFAs | Functional genes | Relative microbiota maturity2 |
| Thompson et al., 2015 (60) | Prospective cohort | Atlanta, USA | 9 | Periodically between 13 d and 14 mo | Introduction (4–6 mo) | ✓ | ✓ | ✓ | ✗ | ✓3 | ✗ |
| Koenig et al., 2011 (61) | Case study | USA | 1 | Periodically between birth and 2.5 y | Introduction (∼5.5 mo) | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ |
| Pannaraj et al., 2017 (62) | Prospective cohort | California and Florida, USA | 119 | Periodically between 1 and 331 d | Introduction (4–6 mo) and age of introduction (<4 vs. ≥4 mo) | ✓ | ✓ | ✓ | ✗ | ✓3 | ✓ |
| Differding et al., 2020 (64) | Prospective “Nurture” cohort | North Carolina, USA | 67 | At 3 and 12 mo | Age of introduction (≤3 vs. >3 mo) | ✓ | ✓ | ✓ | ✓ | ✗ | ✗ |
| Laursen et al., 2016 and 2017 (65, 66) | Prospective cohort | Denmark | 227 | At 9 and 18 mo | Age of introduction (3–6 mo) and composition | ✓ | ✓ | ✓ | ✗ | ✗ | ✗ |
| Qasem et al., 2017 (69) | Randomized controlled trial | Manitoba, Canada | 87 | Before and after introduction between 4 and 6 mo in 56 infants | Introduction (4–6 mo) and composition | ✓ | ✓ | ✓ | ✗ | ✗ | ✗ |
| Krebs et al., 2013 (70) | Randomized controlled trial | Colorado, USA | 45 | Monthly between 5 and 9 mo in 14 infants | Introduction (5 mo) and composition | ✓ | ✓ | ✓ | ✗ | ✗ | ✗ |
BF, breastfeeding; CF, complementary feeding.
Calculated using a random-forest model which is a machine-learning algorithm to determine a ranked list of all bacterial taxa in the order of age-discriminatory importance.
Predicted functional genes.
Prospective cohort studies with multiple fecal sample collections during the CF period help inform how the introduction to new food components influences the changes in the gut microbial structure and function in early life. Introduction of SF among infants in the United States was associated with increased ɑ-diversity (within sample diversity) as well as changes in the composition of bacterial taxa and predicted function, with observed differences by exclusive breastfeeding status prior to SF introduction (60). Specifically, ɑ-diversity was higher following SF introduction in infants who were not previously exclusively breastfed compared with those who were. SF introduction was associated with overrepresentation of 24 functional genes in exclusively breastfed infants compared with 230 functional genes in non–exclusively breastfed infants. The changes in predicted function following SF introduction were corroborated by a metagenomic analysis of the gut microbiome of a full-term, vaginally delivered, healthy, and exclusively breastfed boy (61). The boy's microbiome was enriched with functional genes characteristic of the adult gut microbiome including genes for vitamin biosynthesis, plant polysaccharide utilization, and breakdown of xenobiotic compounds (foreign substances to the human body; e.g., antibiotics, pharmaceuticals). Further, fecal concentrations of individual SCFAs (acetate, propionate, and butyrate) were higher following introduction of table food. However, since this was a case study of 1 child, it is important to replicate the results among a larger number of infants.
The effect of the age of introduction and the composition of the complementary food on the gut microbiota of infants and children have been also studied. Pannaraj et al. (62) examined the trajectory of microbiota maturity among US infants using a random forest (RF) model, which is a machine-learning algorithm suggested by Subramanian et al. (63) to determine a ranked list of all bacterial taxa in the order of age-discriminatory importance. Early CF (<4 mo) compared with later CF (≥4 mo of age) was associated with a rapid maturation of the gut microbiota and increased predicted function related to xenobiotics biodegradation and metabolism. In the Nurture cohort study, early (≤3 mo) versus late (>3 mo) CF was associated with higher ɑ-diversity and differential abundance of 29 bacterial taxa at 3 and 12 mo of age after adjusting for potential confounders including breastfeeding (64). Early CF was also associated with greater concentrations of fecal butyrate and total SCFA concentrations at 12 mo.
Unlike the studies conducted among infants in the United States, a study by Laursen et al. (65) among Danish infants suggested that the age of SF introduction (3–6 mo) was not associated with ɑ-diversity or microbiota composition at 9 mo. Nevertheless, the duration of exclusive breastfeeding, which was ≤6 mo among all infants, was associated with these microbiota indicators. The authors concluded that exclusive breastfeeding duration, rather than CF timing, was a stronger determinant of the gut microbiota in early life. Studies by Backhed et al. (24) among Swedish infants and Stewart et al. (25) among infants and children in 3 European countries (Germany, Sweden, and Finland) and US states (Colorado, Georgia, and Washington) had a similar conclusion. They argued that breastfeeding cessation rather than SF introduction is the major driver in the development of an adult microbiota. It is worth noting, however, that these studies (24, 25) based their conclusion on the effects of breastfeeding status and did not examine the effect of the CF dimensions, such as age of introduction and composition of SF, on the infants’ microbiota.
To decipher the effect of breastfeeding versus that of CF, Laursen et al. (66) stratified the Danish infants by breastfeeding status at 9 mo. They found that protein and fiber content of SF and progression toward family food were associated with higher ɑ-diversity in both groups of infants, suggesting that these changes were independent of breastfeeding status. Additionally, whereas meat, cheese, and rye bread represented the specific food groups most strongly associated with ɑ-diversity among all infants as well as those no longer breastfed at 9 mo, porridge (primarily oatmeal) was most strongly associated with ɑ-diversity among infants still breastfed. Progression to family food was associated with a decrease in the abundance of several bacterial genera, including Bifidobacterium, and an increase in the abundance of others, including Faecalibacterium and Ruminococcus, among all infants (65). Of note, Bifidobacterium is known for its capacity to utilize lactose and human-milk oligosaccharides in breast milk (67) and has been referred to as a “milk-oriented” taxon in the study by Gehrig et al. (68). Faecalibacterium and Ruminococcus, on the other hand, increase with the addition of dietary fiber or protein to the diet (71, 72) and have been thus referred to as “weaning-phase” taxa. Hence, the changes in the gut microbiota during the CF period are likely to be driven by a combination of breastfeeding- and CF-related variables.
Randomized controlled trials examined the effects of CF on the gut microbiota of infants. Eighty-seven exclusively breastfed infants were randomly assigned to receive either iron-fortified cereal, iron-fortified cereal with fruit, or meat as their first complementary food (69). The age of SF introduction varied by the parents’ preference and ranged between 4 and 6 mo, but all infants consumed their assigned diets for 2–4 wk. In a subset of 56 infants, gut microbiota analysis revealed increased ɑ-diversity following introduction of meat or cereal with fruit, but not cereal alone. No significant changes in gut microbiota composition were noted after introduction of any of the SFs. In another trial, 45 exclusively breastfed 5-mo-old infants were randomly assigned to receive commercially available pureed meats, iron- and zinc-fortified infant cereals, or iron-only fortified infant cereals as the first and primary complementary food through 9–10 mo of age (70). In a subsample of 14 infants, no significant change in ɑ-diversity was observed following introduction of any SF. Changes in gut microbiota composition were apparent among infants introduced to the iron-fortified cereal compared with those introduced to the meat or iron- and zinc-fortified cereal.
The studies in this section suggest that significant changes in the gut microbiota occur during the CF period and are potentially driven by breastfeeding as well as CF. All the studies were conducted in HICs. No similar studies were conducted among LMIC populations, which are the focus of this review, or in the context of undernutrition in which other factors including deficient diets, EED, and poor sanitation might also drive the changes in the gut microbiota during the CF period. Moreover, none have examined the effect of CF adequacy as assessed by the WHO indicators on the gut microbiota. More research is needed to understand 1) the nature and magnitude of the link between CF and the gut microbiota, 2) the extent to which such a link can be beneficial to improve children's growth and nutrition, and 3) the means in which the knowledge gained from items “1” and “2” can translate to the context of undernutrition and LMICs.
Gut microbiota disruptions are associated with, and can even cause, child undernutrition
Disruptions in the gut microbiota in early life are likely to have detrimental effects on children's nutritional status and health later in life. Studies linking the gut microbiota to undernutrition have different definitions for gut microbiota disruptions. One novel approach, described in this section, was proposed by Jeffrey Gordon's team who suggested that undernourished children have immature gut microbiotas (63, 73–75). Such an approach provides potential insight and testable hypotheses on the role of the gut microbiota in child undernutrition and informs the development of promising interventions.
Smith et al. (74) studied 317 Malawian twin pairs during the first 3 y of life, some of whom were discordant for kwashiorkor, a form of severe acute malnutrition (SAM) characterized by peripheral edema, abdominal distension, and hepatic metabolic disruptions (76). Twin studies are especially valuable in gut microbiota research as they help distinguish the effects of genetic from those of environmental factors. Compared with the gut microbiota of the healthy co-twin, the microbiota of the twin with kwashiorkor was immature and resembled that of younger children (74). Even when the twin with kwashiorkor was treated with ready-to-use therapeutic food (RUTF), an energy-dense and micronutrient-enriched paste used in the treatment of SAM, the divergent trajectory of gut microbiota maturation was not corrected.
To further investigate the role of the gut microbiota in undernutrition, Smith et al. (74) transplanted fecal communities from several discordant twin pairs to C57BL/6J germ-free mice, which lack micro-organisms living in and on them. The recipient mice were fed a typical Malawian diet that is nutrient-deficient and low in calorie density for 3 wk. Mice that received transplants from the kwashiorkor-diagnosed twin lost more weight, had overrepresentation of proinflammatory taxa, and exhibited perturbed metabolism of amino acids and carbohydrates compared with mice with fecal transplants from the healthy co-twin. One proposed mechanism in which the gut microbiota might be implicated in kwashiorkor is through the generation of products such as inhibitors of enzymes in the tricarboxylic acid cycle, which can compromise effective energy metabolism and eventually nutritional status. This proof-of-concept study suggests that an immature gut microbiota coupled with a nutrient-deficient diet may be included among the causal risk factors of undernutrition.
Gut microbiota immaturity was further examined in a study by Subramanian et al. (63) among Bangladeshi children younger than 2 y using a RF machine-learning algorithm. The relative abundance of the operational taxonomic units (sequences that are clustered together based on similarity to inform taxonomy) was regressed against the chronological age of each child at the time of fecal sample collection to identify age-discriminatory taxa. Among the 24 most age-discriminatory taxa were the weaning-phase taxa including Faecalibacterium prausnitzii and Ruminococcus sp. and the milk-oriented taxa including Bifidobacterium longum. Two metrics to assess the microbiota maturity of children were subsequently defined using the sparse 24-taxon model. The first metric, relative microbiota maturity, compared the microbiota age of the child with that of healthy children of similar chronologic age. The second, microbiota-for-age z score (MAZ), is similar to the anthropometric z scores used to assess nutritional status such as height-for-age, weight-for-age, and weight-for-height z scores.
Applying the 24-taxon model to the fecal samples of a group of 64 Bangladeshi children aged 6–20 mo with SAM revealed that undernourished children had gut microbiota immaturities and lower MAZ compared with age-matched healthy children (63). The microbiota immaturities were only partially repaired when the children with SAM were treated with RUTF or a locally produced alternative, supporting previous findings that existing nutrition therapies fail to completely repair immature gut microbiotas (74). The RF model among Bangladeshi children was applicable to the population of Malawian twin pairs (73) assessed in the study by Smith et al. (74). F. prausnitzii, Ruminococcus sp., and B. longum were also age-discriminatory in the Malawian sparse 25-taxon model (73). In addition, MAZ among Malawian twins at 12 months was significantly positively correlated with weight-for-height z scores and weight-for-age z scores at 18 mo, suggesting that MAZ may be useful for predicting future ponderal growth.
Although the use of RF models to identify age-discriminatory taxa is helpful, it typically describes a “parts list” as it focuses on the abundance of community members without accounting for the interactions between them (75). Analysis of conserved bacterial taxon–taxon covariance in the gut microbiota of healthy Bangladeshi children younger than 5 y revealed a network of 15 covarying bacteria termed an “ecogroup” (75). F. prausnitzii and B. longum were among the 15 covarying taxa, along with others that were identified as age-discriminatory in the RF model among Bangladeshi children. The ecogroup confirmed the existence of a program of gut microbial community maturation that was completed by the second year of life in healthy Bangladeshi children. The ecogroup further supported findings that undernourished Bangladeshi children had impaired gut community development characterized by perturbed interactions between the 15 covarying taxa.
Other definitions of gut microbiota disruption have been proposed including lower ɑ-diversity and/or higher abundance of pathogenic taxa (77, 78). Different forms of undernutrition have been also studied, ranging from chronic undernutrition (79) to moderate acute malnutrition (MAM) (68) and SAM (63, 73, 74). Further, associations between gut microbiota disruptions and undernutrition have been examined using different microbiota analytic methods [e.g., 16S ribosomal RNA (rRNA) 454 pyrosequencing vs. 16S rRNA Illumina sequencing versus shotgun metagenomic sequencing] in various populations residing in different geographic locations with distinct cultural practices and traditional diets. Such variations in definition, analysis, and study population characteristics possibly explain the contrasting results related to the nature and strength of the association between gut microbiota disruption and undernutrition. All of the studies, however, hint at the potential of the disrupted gut microbiota to be a key player in the web of risk factors of undernutrition. Interestingly, the opposite direction of the relation where child undernutrition may cause gut microbiota disruption has been suggested in a few review papers (20, 21) but has not been not fully studied in children or animal models.
Complementary food targeting the gut microbiota is a promising intervention for undernutrition
With the advancement in our knowledge of the paramount role of gut microbiota disruption in child undernutrition, a number of interventions targeting the gut microbiota have emerged as promising treatment modalities. Prebiotics, probiotics, and synbiotics have gained significant attention following findings of their potential ability to modulate the gut microbiota of undernourished children (80, 81). A systematic review of the effects of probiotics on child growth suggests that probiotics might have more significant effects improving child nutritional status and growth among undernourished children and those living in LMICs (82). There are a number of challenges, however, to implementing probiotic-based approaches, including the need to closely monitor safety, particularly among undernourished children with impaired immune function, and to gain cultural acceptability to supplement children with live micro-organisms (83). Locally produced complementary food that targets gut microbiota immaturities among undernourished children can help overcome such challenges.
Gehrig et al. (68) conducted a pilot randomized controlled trial to compare the efficacy of microbiota-directed complementary food (MDCF) with the traditional rice- and lentil-based ready-to-use supplementary food (RUSF) used in the treatment of MAM. The 1-mo pilot trial tested 3 MDCF formulations with different proportions of 4 ingredients—peanut flour, chickpea flour, soy flour, and banana—among 12- to 18-mo-old Bangladeshi children with MAM. The ingredients were selected based on their growth-promoting effects in gnotobiotic mice and piglets (animals with defined microbial communities) colonized with members of the gut microbiota from undernourished Bangladeshi children. MDCF-1 had all 4 ingredients but at lower concentrations than MDCF-2, and MDCF-3 had chickpea flour and soy flour only. MDCF-1 and RUSF contained milk powder. All MDCF formulations were supplemented with a micronutrient mixture designed to provide 70% of the recommended daily allowances for 12- to 18-mo-old children. The MDCF formulations and RUSF had similar protein-energy ratio, fat-energy ratio, and macro- and micronutrient content and provided 250 kcal/d.
At the end of the trial, all children had improved weight-for-height z scores regardless of the treatment group (n = 14–17 children per group) (63). Children randomly assigned to MDCF-2, compared with MDCF-3, had a significantly greater increase in midupper arm circumference, an independent diagnostic criterion of MAM (84). Further, MDCF-2 induced an increase in the relative abundance of several weaning-phase taxa including F. prausnitzii and a decrease in that of milk-oriented taxa such as B. longum compared with the 3 other treatments. However, there was no improvement in MAZ with any of the treatments, which the authors attributed to the possible confounding by unexpectedly high baseline microbiota maturity scores in the children with MAM in this study (63). MDCF-2 was uniquely associated with covariation of the ecogroup taxa towards a more complete community repair (75). MDCF-2 also increased the abundance of proteins that are higher in plasma from healthy children and that are positively correlated with height-for-age z scores, and it reduced the concentrations of proteins elevated in plasma of undernourished children and those that are negatively correlated with height-for-age z scores (63). Therefore, despite the small group size and the short length of the study, MDCF-2 elicited a marked shift in the microbiota profiles and plasma proteome toward those of healthy children.
Findings from this pilot trial informed the design and implementation of a proof-of-concept efficacy trial, whose protocol has been recently published (85) with similar aims but among a larger group of 248 Bangladeshi children with MAM for a longer 3-mo period. Another study protocol has been published for the MALINEA (Malnutrition et Infection de l’Enfance en Afrique) project, which aims to compare the effects of 3 strategies of re-nutrition for MAM on the gut microbiota of 840 children aged 6–24 mo old in 4 African countries (Madagascar, Niger, Central African Republic, and Senegal) (86). The children are randomly assigned to receive enriched flour alone, enriched flour with prebiotics (combination of inulin and fructo-oligosaccharides), or enriched flour coupled with a 4-d antibiotic treatment (azithromycin) for 12 wk. It is hoped that results from these studies will serve as a stepping stone for the development of complementary food targeting the microbiota of undernourished children for sustainable benefits on child growth.
Conclusions and Future Research
The persistent high prevalence of child undernutrition in LMICs and its long-term health and societal costs are a call for action. This review paper provides evidence supporting a plausible relation between CF, the gut microbiota, and child undernutrition (Figure 1). An association between inadequate CF and child undernutrition was suggested in numerous studies in different LMICs. In contrast, studies examining the changes in the gut microbial composition and function during the CF period were largely conducted in HICs. Further, a disrupted gut microbiota has been associated with, and causally linked to, child undernutrition. Indeed, the direction of the causality is not fully examined and potentially exists in both directions depending on genetic and environmental conditions. The current state of knowledge supporting the potential of the gut microbiota to be a key player in the relation between CF and undernutrition has ignited interest in developing microbiota-directed interventions during the CF period for undernutrition management.
FIGURE 1.
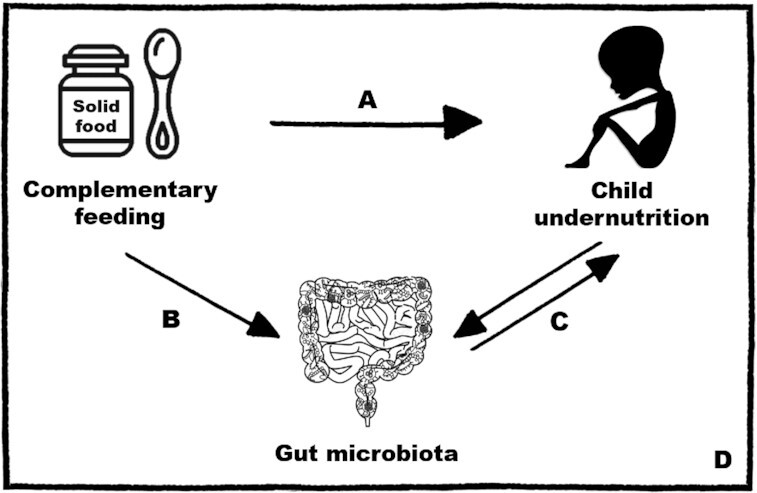
Summary of the plausible links between CF, the gut microbiota, and child undernutrition. (A) Inadequate CF is associated with child undernutrition. These associations were mostly examined in LMICs. CF interventions often have limited effects on child growth, hinting at the complexity of the relation between CF and undernutrition. (B) Significant changes in the gut microbiota occur during the CF period. The introduction of new food components and the age of introduction and composition of the complementary food influence the gut microbiota. However, evidence in this area originates solely from HICs. (C) Gut microbiota disruptions are associated with, and causally linked to, child undernutrition. A bidirectional relation is plausible where a disrupted gut microbiota can cause undernutrition and vice versa. (D) Interventions for undernutrition targeting the gut microbiota during the CF period are promising. More research is needed to further our knowledge of the interplay between CF, the gut microbiota, and undernutrition as this can help better inform the design of interventions with long-lasting benefits to combat child undernutrition and promote health and well-being. CF, complementary feeding; HIC, high-income country; LMIC, low- and middle-income country.
More research is needed to further our knowledge of the interplay between the different dimensions of CF and the gut microbiota, especially among populations in LMICs, and to inform an update of the WHO guiding principles and indicators of IYCF that were published over a decade ago (22). Research is also necessary to decipher the mechanisms involved in the association between inadequate CF and undernutrition, including those related to the microbial communities residing in the child's gut. Studies using animal models and those conducted among children in LMICs can address how CF and the gut microbiota influence growth and will contribute to the development of an updated algorithm of the risk factors of undernutrition. The potential of the disrupted gut microbiota as a key player in this algorithm is evident. Such a holistic view of the risk factors of undernutrition can help better inform the design of interventions to create persistent beneficial effects on the maturation process of the gut microbiota and child growth. This, in turn, can help decrease the prevalence and burden of undernutrition and promote health and well-being.
Acknowledgments
The authors’ responsibilities were as follows—RFC: conceived the research aims, searched and collected the literature, and wrote the draft manuscript; MRF and T-WLC: provided valuable input for the write-up of the manuscript; and all authors: read and approved the final manuscript.
Notes
The authors reported no funding received for this study.
Author disclosures: The authors report no conflicts of interest.
Abbreviations used: CF, complementary feeding; DHS, Demographic and Health Survey; EED, environmental enteric dysfunction; HIC, high-income country; IYCF, infant and young child feeding; LMIC, low- and middle-income country; MAM, moderate acute malnutrition; MAZ, microbiota-for-age z score; MDCF, microbiota-directed complementary food; RF, random forest; rRNA, ribosomal RNA; RUSF, ready-to-use supplementary food; RUTF, ready-to-use therapeutic food; SAM, severe acute malnutrition; SF, solid food.
Contributor Information
Rana F Chehab, Department of Nutrition Science, Purdue University, West Lafayette, IN, USA.
Tzu-Wen L Cross, Department of Nutrition Science, Purdue University, West Lafayette, IN, USA.
Michele R Forman, Department of Nutrition Science, Purdue University, West Lafayette, IN, USA.
References
- 1. World Health Organization . Malnutrition 2020. [Internet]. Available from: http://www.who.int/news-room/fact-sheets/detail/malnutrition (accessed June 2020). [Google Scholar]
- 2. World Health Organization . The WHO child growth standards. [Internet]. Available from: https://www.who.int/childgrowth/en/ (accessed 2020 Feb 12). [Google Scholar]
- 3. Ahmed T, Hossain M, Sanin KI. Global burden of maternal and child undernutrition and micronutrient deficiencies. Ann Nutr Metab. 2012;61(Suppl 1):8–17. [DOI] [PubMed] [Google Scholar]
- 4. United Nations Children’s Fund (UNICEF), World Health Organization, International Bank for Reconstruction and Development/The World Bank . Levels and trends in child malnutrition: key findings of the 2020 edition of the joint child malnutrition estimates. Geneva: World Health Organization; 2020; . Licence: CC BY-NC-SA 3.0 IGO. [Google Scholar]
- 5. Bryce J, Boschi-Pinto C, Shibuya K, Black RE. WHO estimates of the causes of death in children. Lancet. 2005;365(9465):1147–52. [DOI] [PubMed] [Google Scholar]
- 6. Caulfield LE, de Onis M, Blössner M, Black RE. Undernutrition as an underlying cause of child deaths associated with diarrhea, pneumonia, malaria, and measles. Am J Clin Nutr. 2004;80(1):193–8. [DOI] [PubMed] [Google Scholar]
- 7. Liu L, Oza S, Hogan D, Chu Y, Perin J, Zhu J, Lawn JE, Cousens S, Mathers C, Black RE. Global, regional, and national causes of under-5 mortality in 2000–15: an updated systematic analysis with implications for the Sustainable Development Goals. Lancet. 2016;388(10063):3027–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Victora CG, Adair L, Fall C, Hallal PC, Martorell R, Richter L, Sachdev HS. Maternal and child undernutrition: consequences for adult health and human capital. Lancet. 2008;371(9609):340–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. United Nations . Sustainable Development Goals. [Internet]. Available from: https://sustainabledevelopment.un.org/?menu=1300 (accessed June 2020). [Google Scholar]
- 10. UNICEF . Strategy for improved nutrition of children and women in developing countries. New York: UNICEF; 1990. [Google Scholar]
- 11. Bhutta ZA, Das JK, Rizvi A, Gaffey MF, Walker N, Horton S, Webb P, Lartey A, Black RE. Lancet Nutrition Interventions Review Group, the Maternal and Child Nutrition Study Group . Evidence-based interventions for improvement of maternal and child nutrition: what can be done and at what cost?. Lancet. 2013;382(9890):452–77. [DOI] [PubMed] [Google Scholar]
- 12. McCormick BJJ, Lang DR. Diarrheal disease and enteric infections in LMIC communities: how big is the problem?. Trop Dis Travel Med Vaccines. 2016;2:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hoffman, Reynolds RM, Hardy DB. Developmental Origins of Health and Disease: current knowledge and potential mechanisms. Nutr Rev. 2017;75(12):951–70. [DOI] [PubMed] [Google Scholar]
- 14. Victora CG, de Onis M, Hallal PC, Blossner M, Shrimpton R. Worldwide timing of growth faltering: revisiting implications for interventions. Pediatrics. 2010;125(3):e473–80. [DOI] [PubMed] [Google Scholar]
- 15. Leroy JL, Frongillo EA, Dewan P, Black MM, Waterland RA. Can children catch up from the consequences of undernourishment? Evidence from child linear growth, developmental epigenetics, and brain and neurocognitive development. Adv Nutr. 2020;11(4):1032–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Badham J. Ensuring optimal breastfeeding and improvements in complementary feeding to improve infant and young child nutrition in developing countries. Matern Child Nutr. 2013;9:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lederberg J, McCray AT. Ome SweetOmics—a genealogical treasury of words. The Scientist. 2001;15(7):8. [Google Scholar]
- 18. Yatsunenko T, Rey FE, Manary MJ, Trehan I, Dominguez-Bello MG, Contreras M, Magris M, Hidalgo G, Baldassano RN, Anokhin APet al. Human gut microbiome viewed across age and geography. Nature. 2012;486(7402):222–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gordon JI, Dewey KG, Mills DA, Medzhitov RM. The human gut microbiota and undernutrition. Sci Transl Med. 2012;4(137):137ps12. [DOI] [PubMed] [Google Scholar]
- 20. Hoffman DJ, Taddei CR, Doak CM. Microbiome, growth retardation and metabolism: are they related?. Ann Hum Biol. 2017;44(3):201–7. [DOI] [PubMed] [Google Scholar]
- 21. Kane AV, Dinh DM, Ward HD. Childhood malnutrition and the intestinal microbiome. Pediatr Res. 2015;77(1–2):256–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Velly H, Britton RA, Preidis GA. Mechanisms of cross-talk between the diet, the intestinal microbiome, and the undernourished host. Gut Microbes. 2017;8(2):98–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Robertson RC, Manges AR, Finlay BB, Prendergast AJ. The human microbiome and child growth—first 1000 days and beyond. Trends Microbiol. 2019;27(2):131–47. [DOI] [PubMed] [Google Scholar]
- 24. Backhed F, Roswall J, Peng Y, Feng Q, Jia H, Kovatcheva-Datchary P, Li Y, Xia Y, Xie H, Zhong Het al. Dynamics and stabilization of the human gut microbiome during the first year of life. Cell Host Microbe. 2015;17(5):690–703. [DOI] [PubMed] [Google Scholar]
- 25. Stewart CJ, Ajami NJ, O'Brien JL, Hutchinson DS, Smith DP, Wong MC, Ross MC, Lloyd RE, Doddapaneni H, Metcalf GAet al. Temporal development of the gut microbiome in early childhood from the TEDDY study. Nature. 2018;562(7728):583–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ho NT, Li F, Lee-Sarwar KA, Tun HM, Brown BP, Pannaraj PS, Bender JM, Azad MB, Thompson AL, Weiss STet al. Meta-analysis of effects of exclusive breastfeeding on infant gut microbiota across populations. Nat Commun. 2018;9(1):4169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Martin R, Makino H, Cetinyurek Yavuz A, Ben-Amor K, Roelofs M, Ishikawa E, Kubota H, Swinkels S, Sakai T, Oishi Ket al. Early-life events, including mode of delivery and type of feeding, siblings and gender, shape the developing gut microbiota. PLoS One. 2016;11(6):e0158498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Levin AM, Sitarik AR, Havstad SL, Fujimura KE, Wegienka G, Cassidy-Bushrow AE, Kim H, Zoratti EM, Lukacs NW, Boushey HAet al. Joint effects of pregnancy, sociocultural, and environmental factors on early life gut microbiome structure and diversity. Sci Rep. 2016;6:31775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Charbonneau MR, O'Donnell D, Blanton LV, Totten SM, Davis JC, Barratt MJ, Cheng J, Guruge J, Talcott M, Bain JRet al. Sialylated milk oligosaccharides promote microbiota-dependent growth in models of infant undernutrition. Cell. 2016;164(5):859–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Davis JC, Lewis ZT, Krishnan S, Bernstein RM, Moore SE, Prentice AM, Mills DA, Lebrilla CB, Zivkovic AM. Growth and morbidity of Gambian infants are influenced by maternal milk oligosaccharides and infant gut microbiota. Sci Rep. 2017;7:40466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. World Health Organization . Appropriate complementary feeding. 2019; [Internet]. Available from: https://www.who.int/elena/titles/complementary_feeding/en/ (accessed 2019 Oct 30). [Google Scholar]
- 32. World Health Organization; UNICEF . Global strategy for infant and young child feeding. Geneva (Switzerland):World Health Organization; 2003. [Google Scholar]
- 33. World Health Organization . The optimal duration of exclusive breastfeeding: a systematic review. Geneva (Switzerland): World Health Organization; 2001. [Google Scholar]
- 34. WHO/PAHO . Guiding principles for complementary feeding of the breastfed child. Washington (DC); Pan American Health Organization; 2003. [Google Scholar]
- 35. World Health Organization . Guiding Principles For Feeding Non-Breastfed Children 6–24 Months of Age. Geneva (Switzerland): World Health Organization; 2005. [Google Scholar]
- 36. Michaelsen KF, Grummer‐Strawn L, Bégin F. Emerging issues in complementary feeding: global aspects. Matern Child Nutr. 2017;13:e12444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. WHO; UNICEF; IFPRI; UCDavis; FANTA; AED; USAID . Indicators for assessing infant and young child feeding practices. Part 1: definitions. Geneva (Switzerland): World Health Organization; 2008. [Google Scholar]
- 38. World Health Organization . Indicators for assessing infant and young child feeding practices. Part 3: country profiles. Geneva (Switzerland): World Health Organization; 2010. [Google Scholar]
- 39. White JM, Bégin F, Kumapley R, Murray C, Krasevec J. Complementary feeding practices: current global and regional estimates. Matern Child Nutr. 2017;13:e12505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Perkins JM, Jayatissa R, Subramanian SV. Dietary diversity and anthropometric status and failure among infants and young children in Sri Lanka. Nutrition. 2018;55–56:76–83. [DOI] [PubMed] [Google Scholar]
- 41. Ahmad I, Khalique N, Khalil S, Urfi, Maroof M. Dietary diversity and stunting among infants and young children: a cross-sectional study in Aligarh. Indian J Community Med. 2018;43(1):34–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sie A, Tapsoba C, Dah C, Ouermi L, Zabre P, Barnighausen T, Arzika AM, Lebas E, Snyder BM, Moe Cet al. Dietary diversity and nutritional status among children in rural Burkina Faso. Int Health. 2018;10(3):157–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Menon P, Bamezai A, Subandoro A, Ayoya MA, Aguayo V. Age-appropriate infant and young child feeding practices are associated with child nutrition in India: insights from nationally representative data. Matern Child Nutr. 2015;11(1):73–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Campbell RK, Aguayo V, Kang Y, Dzed L, Joshi V, Waid J, Gupta SD, Haselow N, West KP Jr. Infant and young child feeding practices and nutritional status in Bhutan. Matern Child Nutr. 2018;14(3):e12580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Walters CN, Rakotomanana H, Komakech JJ, Stoecker BJ. Maternal determinants of optimal breastfeeding and complementary feeding and their association with child undernutrition in Malawi (2015–2016). BMC Public Health. 2019;19(1):1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zongrone A, Winskell K, Menon P. Infant and young child feeding practices and child undernutrition in Bangladesh: insights from nationally representative data. Public Health Nutr. 2012;15(9):1697–704. [DOI] [PubMed] [Google Scholar]
- 47. Doctor HV, Nkhana-Salimu S. Trends and determinants of child growth indicators in Malawi and implications for the Sustainable Development Goals. AIMS Public Health. 2017;4(6):590–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. National Institute of Population Research and Training (NIPORT); Mitra and Associates; ICF International . Bangladesh Demographic and Health Survey 2014. Dhaka, Bangladesh, and Rockville, Maryland, USA: NIPORT, Mitra and Associates, and ICF International; 2016. [Google Scholar]
- 49. Manikam L, Robinson A, Kuah JY, Vaidya HJ, Alexander EC, Miller GW, Singh KK, Dawe V, Ahmed S, Lingam R. A systematic review of complementary feeding practices in South Asian infants and young children: the Bangladesh perspective. BMC Nutr. 2017;3(1):56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Krasevec J, An X, Kumapley R, Begin F, Frongillo EA. Diet quality and risk of stunting among infants and young children in low- and middle-income countries. Matern Child Nutr. 2017;13(Suppl 2):e12430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Dewey KG, Adu‐Afarwuah S. Systematic review of the efficacy and effectiveness of complementary feeding interventions in developing countries. Matern Child Nutr. 2008;4:24–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Heidkamp RA. Evidence for the effects of complementary feeding interventions on the growth of infants and young children in low- and middle-income countries. Nestle Nutr Inst Workshop Ser. 2017;87:89–102. [DOI] [PubMed] [Google Scholar]
- 53. Lassi ZS, Das JK, Zahid G, Imdad A, Bhutta ZA. Impact of education and provision of complementary feeding on growth and morbidity in children less than 2 years of age in developing countries: a systematic review. BMC Public Health. 2013;13(Suppl 3):S13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kristjansson E, Francis DK, Liberato S, Benkhalti Jandu M, Welch V, Batal M, Greenhalgh T, Rader T, Noonan E, Shea Bet al. Food supplementation for improving the physical and psychosocial health of socio-economically disadvantaged children aged three months to five years. Cochrane Database Syst Rev. 2015;3:CD009924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Imdad A, Yakoob MY, Bhutta ZA. Impact of maternal education about complementary feeding and provision of complementary foods on child growth in developing countries. BMC Public Health. 2011;11(Suppl 3):S25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Lassi ZS, Rind F, Irfan O, Hadi R, Das JK, Bhutta ZA. Impact of infant and young child feeding (IYCF) nutrition interventions on breastfeeding practices, growth and mortality in low- and middle-income countries: systematic review. Nutrients. 2020;12(3):722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Panjwani A, Heidkamp R. Complementary feeding interventions have a small but significant impact on linear and ponderal growth of children in low- and middle-income countries: a systematic review and meta-analysis. J Nutr. 2017;147(11):2169s–78s. [DOI] [PubMed] [Google Scholar]
- 58. Park JJH, Harari O, Siden E, Dron L, Zannat NE, Singer J, Lester RT, Thorlund K, Mills EJ. Interventions to improve linear growth during complementary feeding period for children aged 6–24 months living in low- and middle-income countries: a systematic review and network meta-analysis. Gates Open Res. 2019;3:1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Davis EC, Wang M, Donovan SM. The role of early life nutrition in the establishment of gastrointestinal microbial composition and function. Gut Microbes. 2017;8(2):143–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Thompson AL, Monteagudo-Mera A, Cadenas MB, Lampl ML, Azcarate-Peril MA. Milk- and solid-feeding practices and daycare attendance are associated with differences in bacterial diversity, predominant communities, and metabolic and immune function of the infant gut microbiome. Front Cell Infect Microbiol. 2015;5:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Koenig JE, Spor A, Scalfone N, Fricker AD, Stombaugh J, Knight R, Angenent LT, Ley RE. Succession of microbial consortia in the developing infant gut microbiome. Proc Natl Acad Sci. 2011;108(Suppl 1):4578–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Pannaraj PS, Li F, Cerini C, Bender JM, Yang S, Rollie A, Adisetiyo H, Zabih S, Lincez PJ, Bittinger Ket al. Association between breast milk bacterial communities and establishment and development of the infant gut microbiome. JAMA Pediatr. 2017;171(7):647–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Subramanian S, Huq S, Yatsunenko T, Haque R, Mahfuz M, Alam MA, Benezra A, DeStefano J, Meier MF, Muegge BDet al. Persistent gut microbiota immaturity in malnourished Bangladeshi children. Nature. 2014;510(7505):417–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Differding MK, Benjamin-Neelon SE, Hoyo C, Ostbye T, Mueller NT. Timing of complementary feeding is associated with gut microbiota diversity and composition and short chain fatty acid concentrations over the first year of life. BMC Microbiol. 2020;20(1):56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Laursen MF, Andersen LB, Michaelsen KF, Molgaard C, Trolle E, Bahl MI, Licht TR. Infant gut microbiota development is driven by transition to family foods independent of maternal obesity. mSphere. Published online 16 June2016.; doi: 10.1128/mSphere.00069-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Laursen MF, Bahl MI, Michaelsen KF, Licht TR. First foods and gut microbes. Front Microbiol. 2017;8:356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. McKeen S, Young W, Mullaney J, Fraser K, McNabb WC, Roy NC. Infant complementary feeding of prebiotics for the microbiome and immunity. Nutrients. 2019;11(2):364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Gehrig JL, Venkatesh S, Chang H-W, Hibberd MC, Kung VL, Cheng J, Chen RY, Subramanian S, Cowardin CA, Meier MF. Effects of microbiota-directed foods in gnotobiotic animals and undernourished children. Science. 2019;365(6449):eaau4732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Qasem W, Azad MB, Hossain Z, Azad E, Jorgensen S, Castillo San Juan S, Cai C, Khafipour E, Beta T, Roberts LJ 2ndet al. Assessment of complementary feeding of Canadian infants: effects on microbiome & oxidative stress, a randomized controlled trial. BMC Pediatr. 2017;17(1):54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Krebs NF, Sherlock LG, Westcott J, Culbertson D, Hambidge KM, Feazel LM, Robertson CE, Frank DN. Effects of different complementary feeding regimens on iron status and enteric microbiota in breastfed infants. J Pediatr. 2013;163(2):416–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Walton GE, Lu C, Trogh I, Arnaut F, Gibson GR. A randomised, double-blind, placebo controlled cross-over study to determine the gastrointestinal effects of consumption of arabinoxylan-oligosaccharides enriched bread in healthy volunteers. Nutr J. 2012;11(1):36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Zhu Y, Lin X, Zhao F, Shi X, Li H, Li Y, Zhu W, Xu X, Li C, Zhou G. Meat, dairy and plant proteins alter bacterial composition of rat gut bacteria. Sci Rep. 2015;5:15220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Blanton LV, Charbonneau R, Salih T, Barratt MJ, Venkatesh S, Ilkaveya O, Subramanian S, Manary MJ, Trehan I, Jorgensen JM. Gut bacteria that prevent growth impairments transmitted by microbiota from malnourished children. Science. 2016;351(6275):aad3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Smith MI, Yatsunenko T, Manary MJ, Trehan I, Mkakosya R, Cheng J, Kau AL, Rich SS, Concannon P, Mychaleckyj JCet al. Gut microbiomes of Malawian twin pairs discordant for kwashiorkor. Science. 2013;339(6119):548–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Raman AS, Gehrig JL, Venkatesh S, Chang HW, Hibberd MC, Subramanian S, Kang G, Bessong PO, Lima AAM, Kosek MNet al. A sparse covarying unit that describes healthy and impaired human gut microbiota development. Science. 2019;365(6449):eaau4735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Ahmed T, Rahman S, Cravioto A. Oedematous malnutrition. Indian J Med Res. 2009;130(5):651–4. [PubMed] [Google Scholar]
- 77. Gough EK, Stephens DA, Moodie EE, Prendergast AJ, Stoltzfus RJ, Humphrey JH, Manges AR. Linear growth faltering in infants is associated with Acidaminococcus sp. and community-level changes in the gut microbiota. Microbiome. 2015;3:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Gupta SS, Mohammed MH, Ghosh TS, Kanungo S, Nair GB, Mande SS. Metagenome of the gut of a malnourished child. Gut Pathog. 2011;3(1):7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Dinh DM, Ramadass B, Kattula D, Sarkar R, Braunstein P, Tai A, Wanke CA, Hassoun S, Kane AV, Naumova ENet al. Longitudinal analysis of the intestinal microbiota in persistently stunted young children in South India. PLoS One. 2016;11(5):e0155405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Sheridan PO, Bindels LB, Saulnier DM, Reid G, Nova E, Holmgren K, O'Toole PW, Bunn J, Delzenne N, Scott KP. Can prebiotics and probiotics improve therapeutic outcomes for undernourished individuals?. Gut Microbes. 2014;5(1):74–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Pekmez CT, Dragsted LO, Brahe LK. Gut microbiota alterations and dietary modulation in childhood malnutrition—the role of short chain fatty acids. Clin Nutr. 2019;38(2):615–30. [DOI] [PubMed] [Google Scholar]
- 82. Onubi OJ, Poobalan AS, Dineen B, Marais D, McNeill G. Effects of probiotics on child growth: a systematic review. J Health Popul Nutr. 2015;34:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Edwards PT, Kashyap PC, Preidis GA. Microbiota on biotics: probiotics, prebiotics, and synbiotics to optimize growth and metabolism. Am J Physiol Gastrointest Liver Physiol. 2020;319(3):G382–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. World Health Organization; UNICEF . WHO child growth standards and the identification of severe acute malnutrition in infants and children: a joint statement by the World Health Organization and the United Nations Children’s Fund. Geneva: World Health Organization; 2009. PMID: 24809116. [PubMed] [Google Scholar]
- 85. Mostafa I, Nahar NN, Islam MM, Huq S, Mustafa M, Barratt M, Gordon JI, Ahmed T. Proof-of-concept study of the efficacy of a microbiota-directed complementary food formulation (MDCF) for treating moderate acute malnutrition. BMC Public Health. 2020;20(1):242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Vray M, Hedible BG, Adam P, Tondeur L, Manirazika A, Randremanana R, Mainassara H, Briend A, Artaud C, von Platen Cet al. A multicenter, randomized controlled comparison of three renutrition strategies for the management of moderate acute malnutrition among children aged from 6 to 24 months (the MALINEA project). Trials. 2018;19(1):666. [DOI] [PMC free article] [PubMed] [Google Scholar]


