ABSTRACT
Vitamin A (VA) is an essential nutrient often lacking in the diets of people in developing countries. Accurate biomarkers of VA status are vital to inform public health policy and monitor interventions. The relative dose-response (RDR) and modified-RDR (MRDR) tests are semi-quantitative screening tests for VA deficiency that have been used in Demographic and Health Surveys and VA intervention studies. A systematic review and meta-analysis of sensitivity and specificity were conducted to summarize the physiological evidence to support the RDR tests as methods to assess VA status and investigate the impact of different pathological and physiological states on the tests. A total of 190 studies were screened for inclusion, with 21 studies comparing the RDR tests with the gold-standard biomarker, liver VA concentration (68% and 80% sensitivity and 85% and 69% specificity for the RDR and MRDR, respectively). Nearly all studies with VA interventions in VA-deficient populations demonstrated a response of the tests to VA intake that would be expected to improve VA status. The impacts of chronic liver disease, protein malnutrition, age, pregnancy and lactation, infection and inflammation, and various other conditions were examined in 51 studies. The RDR and MRDR tests were reported to have been used in 39 observational studies, and the MRDR has been used in at least 6 national micronutrient surveys. The RDR and MRDR are sensitive tests for determining population VA status and assessing VA interventions. Although they are robust to most physiological and pathological states, caution may be warranted when using the tests in neonates, individuals with chronic liver disease, and those with protein or iron malnutrition. Research on further improvements to the tests to increase accessibility, such as sampling breast milk instead of blood or using intramuscular doses in subjects with malabsorption, will allow wider adoption. This review was registered with PROSPERO as CRD42019124180.
Keywords: Demographic and Health Surveys, humans, modified relative dose-response, nutritional status, retinol
Introduction
Vitamin A (VA) is required for human growth, immunity, and vision. Ideally, everyone would satisfy their VA requirements with provitamin A carotenoids from plant sources and preformed VA from animal products; however, VA deficiency (VAD) and, in some cases, hypervitaminosis A currently cause and exacerbate disease in vulnerable populations. Therefore, accurate methods to assess VA status remain necessary to monitor at-risk populations and to determine the efficacy and effectiveness of public health interventions (1), especially when programs overlap (2).
VA exposure and status can be measured in many ways, including dietary intake, total body VA stores (TBS), and VA balance. The biomarker(s) chosen will depend on the desired outcomes of public health questions and resources available (1). Hepatic VA (retinol + retinyl esters) concentration, as micrograms or micromoles of VA/gram liver [also known as total liver VA reserves (TLRs)] is the “gold standard” for VA status, because hepatocytes and stellate cells contain >90% of TBSs in healthy adults with adequate stores (1). In addition to directly measuring TLRs by liver biopsy, TBS and TLRs can be estimated using VA tracers in retinol isotope dilution (RID) tests (3). Because it is difficult and generally unethical to obtain hepatic biopsies, and stable isotope work is relatively expensive, time consuming, and requires specialized equipment, other indirect indicators that accurately reflect TBS and TLRs are required for population surveys. Two common indicators of VAD are circulating (serum or plasma) retinol (R) concentration, and the focus of this review, the relative dose-response (RDR) and modified-RDR (MRDR) tests, herein referred to as RDRs. The RDRs have been used during all major life stages including infancy (4–21), childhood (22–61), lactation (4, 6, 62–66), pregnancy (66–71), and old age (72–74) in the United States and at least 30 other countries.
Serum R concentration is a straightforward measurement usually determined by HPLC and is reported in micrograms per deciliter or micromoles per liter (1). The RDR is the relative change in serum R 4–7 h after administering a dose of retinyl ester expressed as a percentage (1). The MRDR value is the molar ratio of 3,4-didehydroretinol (DR) to R (DR:R) in serum 4–7 h after administering 3,4-didehydroretinyl acetate (DRAc) (1). The RDRs have decades of use in micronutrient surveys associated with Demographic and Health Surveys (DHSs), clinical trials, and interventions to characterize the prevalence of VAD and measure intervention outcomes, and yet there has been debate over these tests’ usefulness since their inception (75–79). Furthermore, the influence of inflammation and disease, other micronutrient deficiencies, differences in analytical methodology, and the relation of serum R to TLR must be considered. In this review, we examine the utility of RDRs and the impact of these factors.
Current Status of Knowledge
Reference ranges for biomarkers of VA status
When considering TLRs, whether measured by biopsy/necropsy or estimated by RID, deficiency is defined as ≤0.1 μmol VA/g, adequate stores >0.1 to <0.7 μmol VA/g, high stores ≥0.7 to <1 μmol VA/g, and a proposed cutoff for (subclinical) hypervitaminosis is ≥1 μmol VA/g (1). Clinical toxicity was originally proposed at >10 μmol VA/g until more data were obtained (1); however, pathological liver histology has been observed at values as low as 3 μmol VA/g in human cadavers (80). These cutoffs were confirmed in an animal model, where significant hepatic fibrosis and cirrhosis were observed with TLRs <0.1 μmol/g, hyperplasia at 0.7 μmol/g, and mild pathology at 1.5 μmol/g (81). Following guidelines set by the WHO, serum R is deficient at concentrations <0.7 μmol/L, with a population considered to have a serious public health concern when 20% of the population has serum R concentrations below this value (82).
Serum retinol lacks sensitivity and specificity to predict TLRs and intervention response
While the focus of this review is the RDRs, serum or plasma R concentrations have been used extensively as an indicator of VA status and some consider it to be the standard to which RDRs should be compared (83). Serum R concentrations are maintained homeostatically, except when hepatic VA reserves are essentially depleted (84). For example, in otherwise healthy humans dying suddenly of unnatural causes, Underwood et al. (85) found no association between serum R and measured hepatic VA concentrations over the range of 0.038–2.3 μmol/g (n = 50). Suthutvoravoot and Olson (86) found no correlation (r = 0.068) between plasma R and measured hepatic VA concentrations (range: 0.028–11 μmol/g; n = 84). Of the 3 cases with plasma R concentrations <0.35 μmol/L, only 1 had a deficient TLR (0.073 μmol VA/g), while the others had adequate and hypervitaminotic TLRs (0.20 and 2.8 μmol VA/g liver). In a recent study of 27 US cadavers, although there was no correlation between serum R and TLRs, serum R was sensitive to VAD but suffered from poor specificity (80). Because serum R and directly measured TLRs are often not correlated, and because serum R responds to external factors such as the acute phase response (87) and protein malnutrition (88) and can remain inaccurate (compared with RID) despite correction for inflammation (89), mismatches between serum R and RDR tests cannot be definitively attributed to inaccuracies in the RDR tests. Therefore, only gold-standard hepatic biopsy- or RID-derived TLRs should be considered in evaluating these tests.
Biological basis of RDRs
The liver is responsible for homeostatically controlling circulating R concentrations by producing and secreting holo-retinol-binding protein (RBP; also commonly referred to as RBP4 in the literature) (90). This plasma protein carries 1 R molecule and circulates in the blood bound to transthyretin (TTR; also known as prealbumin) (91), which prevents its loss through glomerular filtration (92). During VAD, apo-RBP accumulates in the liver due to insufficient ligand, even before serum R decreases, and reaches a steady-state maximum when liver VA is completely depleted (93–95). Early work by Goodman and colleagues, both in vitro and in rats, demonstrated that RBP synthesis is independent of VA status, whereas secretion of the holo-protein is controlled by VA status (93–97). Newly ingested VA entering the hepatocytes from intestine-derived chylomicra will bind to accumulated apo-RBP and be secreted back into the bloodstream to maintain serum R (94). The serum response to a dose of R or DR in RDR tests is therefore determined by the extent of hepatic apo-RBP accumulation and thus VA status. The accumulation of apo-RBP in deficiency and rapid release of holo-RBP after ingestion of VA provides the biological framework for RDR tests.
RDR test
The original RDR test measured VA status by stimulating and quantifying the binding and secretion of accumulated hepatic RBP by administering a dose of retinyl acetate or palmitate and drawing blood before the dose and 5 h after to measure the plasma R change. The RDR value, expressed as a percentage, is calculated as [(A5−A0)/A5] ⋅ 100, where A5 is the plasma (serum) R concentration 5 h postdosing and A0 is the concentration just prior to dosing (98). The RDR cutoff for VAD was defined as 14% based on a healthy control group (99) but was later increased to 20% in consideration of the CV in the response measurements (25).
Rarely, some studies [e.g., (100)] use the formula [(A5−A0)/A0] ⋅ 100. The initial reasoning for A5 as the denominator was the assumption that holo-RBP release will return the subjects’ serum R concentration to their VA-replete homeostatic concentration, and thus the RDR value is the deviation from “normal” (10). This is likely untrue because negative RDR values (a decrease in serum R postdose) are common, with some below −40% (101). Regardless, a denominator of A5 remains the standard because the cutoff values are based on this formula and using A0 would require recalculation without materially improving the test.
The RDR test requires 2 blood samples per individual, which can dissuade use and participation because of logistics. Additionally, an accurate RDR value is dependent on the correct absolute determination of R concentration in both serum samples, which may be influenced by sample integrity (e.g., hemolysis) and analytical precision. These shortcomings led to the development of the MRDR test by Tanumihardjo and Olson (102), which uses a single blood sample ∼5 h after administering DRAc and a ratio of chemically similar analytes extracted and quantified simultaneously, which is more robust to analytical variation.
MRDR test
The MRDR test replaces oral retinyl ester with DRAc (vitamin A2-acetate) for the challenge dose. In most humans, serum DR concentration is negligible; therefore, baseline measurements are not necessary, unless the population consumes high amounts of freshwater fish liver (103). DRAc is synthesized (104), stored dissolved in vegetable oil, and stable at −20° to +2°C for ≥18 mo (42). The structure of DR differs from R by a double bond in the 3–4 position on the β-ionone ring. This structural difference allows the 2 compounds to be readily resolved by reverse-phase HPLC equipped with a single or multi-wavelength UV-visible or photodiode array detector [described in (105)].
As with retinyl esters, DR esters are taken up by hepatocytes from chylomicra and hydrolyzed to form DR, which binds accumulated apo-RBP for secretion into blood. To perform the test, a blood sample is collected between 4 and 7 h postdosing (26, 42, 105). As little as 100–250 μL serum can be analyzed for DR and R using HPLC (105), which is dependent on the analytical platform. Larger volumes are sometimes needed if the group being studied has predominantly VA-adequate individuals (where low DR is expected), or automation dictates a larger volume be left behind in the injector as dead volume.
MRDR values (DR:R in the serum 4–6 h after dosing with DRAc) ≥0.060 are considered VAD (1, 29). Historically, values between 0.030 and 0.060 were considered VA-marginal because they aligned with low dietary intake in American children (26), but these marginal values were observed in Indonesian children even after treatment with large VA supplements (27, 29). Values ≤0.030 were unambiguously considered VA-adequate in all groups studied.
Originally, the DRAc dose given to children was based on body weight (0.35 μmol DRAc/kg for children <6 y), while that of adults was standardized (8.8 μmol DRAc). Standard doses of 5.3 μmol DRAc for children <6 y and 7.0 μmol DRAc for children between 6 and 12 y were proposed, because, while dose size affects response, variations in body weight only accounted for 5–7% of the variation in MRDR values (69, 106). Therefore, basing the dose on body weight needlessly changes the dose size and adds complexity in dispensing doses (42). Research performed in large (500–600 g) VA-deficient rats suggested that a smaller standard dose would be appropriate in infants and children <2 y of age (107). Therefore, a dose of 3.5 μmol DRAc was adopted and has been used in Bangladeshi infants (108) and micronutrient surveys associated with DHSs (e.g., Uganda 2015–2016 DHS in children 12–23 mo of age).
Analytical methods
Sample analysis
Various methods exist to analyze R changes in the RDR test, including column chromatography and spectrophotofluorometry (98) and HPLC (15). RBP concentration in the RBP-RDR has been analyzed by immunodiffusion (7) and ELISA (19). There are other methods for measuring these analytes (109), but the main concern in the various forms of the RDR is minimizing variation between the 2 blood samples to avoid differences due to anything but hepatic accumulation and release of RBP. Conversely, in analyzing the MRDR test by reverse-phase HPLC, nearly all analytical variation is accounted for by measuring a relative ratio of analytes (although care must be taken to generate accurate calibration curves with pure standards for both analytes). The method was optimized to resolve the analytes and minimize serum volume (105).
Reproducibility
Studies that have investigated the reproducibility of RDRs have been performed in children (60, 110), adults (79, 111), and the elderly (72, 73), and predominantly show concordance between the test and retest. However, the most consistent value is the prevalence of positive tests in a group rather than the actual response value or individual positive/negative status because of the risk of false positives and negatives and the variation in response among individuals of VA-sufficient and VA-deficient status.
Sampling time
R appears to peak in serum later in older adults (6–7 h) (72); however, large variations in R occurred over the time course—for example, 1 subject had an RDR of −30% at 5 h but positive 21% at 6 h. Among individuals in other studies who would eventually have positive RDR or MRDR responses, the positive values were measured any time from 4 to 12 h in VA-deficient and VA-sufficient Indonesian women (112), 5 to 8 h in healthy and anorexic women (100), 3 to 15 h in children with chronic liver disease (CLD) (43), and 5 to 12 h in Indonesian children (27). Thus, the current recommendation of 4–7 h in humans is likely adequate for general use.
Dose size
Dosing based on body weight was replaced by recommendations for age groups. Studies examining the effect of different dose sizes on the RDR found the following: 1) a smaller dose is too low to elicit a response, whereas a larger dose produces results (29); 2) a larger dose elicits a larger response, which could potentially require a different cutoff, but otherwise does not affect the test (42, 113, 114); and 3) no difference in response among dose sizes (115). The conclusion stemming from these data is to use the standard dose (large enough to elicit a response) for the age group under investigation, and to use the same dose in follow-up tests or in further studies to maintain generalizability. The recommendation for the MRDR test is 3.5, 5.3, 7.0, and 8.8 μmol DRAc based on age (<2 y, 2–6 y, 6–12 y, and adults, respectively) in all population health studies. For the RDR test, 3.5 μmol was recommended (1, 29, 116).
RBP-RDR
Using the change in serum RBP instead of R concentration was not representative of the RDR test in most studies (16, 43, 73, 117); however, it may have been responding to a change in VA status due to bronchopulmonary dysplasia (BPD) (20) and was 82% reproducible when retested 7 d after the first test, similar to the RDR (79). The discrepancy between serum RBP and R concentrations was noted previously to be due to apo-RBP in circulation (118), as well as measurement error. In some cases, RBP concentrations have been reported to be lower than R concentrations [e.g., (119)]; however, circulating unbound R would indicate pathology if valid (120), rather than representing analytic variation.
Intramuscular-RDR
Intramuscular-RDR tests have been used in patients with impaired VA absorption due to CLD (22, 43) and preterm infants (15, 16, 121), and appropriately represented TLR by biopsy (22). It was used in 2 children with cholestatic CLD who did not have a positive oral RDR (likely due to biliary atresia) but did have a positive intramuscular-RDR (43). The progression to the MRDR from the RDR test followed a desire to decrease the number of blood draws (122); therefore, intramuscular-RDR should be used only when malabsorption or other difficulties in oral dosing are present.
Breast milk–MRDR
Preliminary evidence in VA-sufficient and VA-deficient lactating sows, and VA-sufficient US women, has suggested that measuring DR:R in breast milk is correlated with that in serum (112, 123, 124), and responded to an intervention in VA-deficient Indonesian women, albeit with a smaller difference among intervention groups for milk MRDR (P = 0.045) (65) compared with serum MRDR (P = 0.003) (125). The impetus to decrease invasiveness from 2 blood collections to 1 in the MRDR could further use milk during lactation, which may be advantageous for some investigators.
Systematic review methodology
Justification
This review informs potential adopters of RDRs of the scientific evidence supporting its use in population surveys, determined predominantly from animal models. Furthermore, the impacts of physiological, developmental, and pathological conditions are discussed to describe the challenges that might arise when using RDR in diverse populations. Included literature was sorted into 3 separate arms for this systematic review: 1) a sensitivity/specificity analysis of diagnostic ability of RDR tests to qualitatively determine VA deficiency [TLR ≤0.1 μmol VA/g; (1)], 2) sensitivity of RDR tests to interventions to improve VA status, and 3) changes in RDR values in response to disease or inflammatory, nutritional, developmental, or physiological influences.
Protocol
The protocol was registered with PROSPERO (registration #CRD42019124180). The article search was conducted in consultation with a research librarian at University of Wisconsin–Madison to include animal and human studies without date or language restrictions. Literature searches were conducted using PubMed (Primary), Web of Science, CINAHL, and Agricola using the following search terms: (“relative-dose-response” OR “relative dose response”) AND (“retinol” or “vitamin A”). The last complete search was 18 February 2019. Additional searches were conducted using reference lists from identified articles and reviews and bibliographies of book chapters.
Eligibility criteria
Included articles had to describe primary research and must have been described by ≥1 of 3 categories using the following criteria—category 1: animal or human studies that included measurement of both liver VA concentration and RDR and/or MRDR values; category 2: studies that performed RDRs in human subjects before and after interventions or compared 2 groups receiving different VA interventions; category 3: human and animal studies that investigated the impact on RDRs of conditions such as lactation or normal childhood development and/or pathology, including but not limited to, infection, inflammation, or nutrient deficiency. Studies that did not fit into the previous 3 categories but described cross-sectional surveys and governmental micronutrient surveys associated with DHSs were summarized to provide an aggregate of these data.
Data collection
The first author compiled data independently. For sensitivity and specificity, data collected were species, liver VA concentration, and RDR or MRDR values for each subject/animal (if available). Some studies were included as group means with measures of variation as noted if individual data were not available; others were estimated using axes in Photoshop (Adobe, Inc.) from figures where data were not listed. Unpublished data were included where available. For systematic review (all categories), the primary data of interest were RDR or MRDR values and changes, interventions performed, any physiological/pathological considerations, and species or human region of origin. VA interventions using provitamin A carotenoids were quantified as retinol activity equivalents (RAE), with conversion factors (μg equivalent:1 μg retinol) of 2:1 supplemental β-carotene, 12:1 β-carotene, or 24:1 α-carotene and β-cryptoxanthin in a food matrix (103). Other data collected were length of time between dose administration and blood draw, age, and number of subjects/animals. Risk of bias is discussed below.
Meta-analysis
Sensitivity and specificity were calculated for the meta-analysis in category 1 on available data. These calculations use cutoffs of ≤0.10 μmol VA/g liver and ≥20% RDR or ≥0.060 MRDR value. Sensitivity is the percentage of gold-standard (liver biopsy or RID)–identified VAD cases that were correctly identified by RDR or MRDR (true positives) and specificity is the percentage of gold-standard–identified non-VAD cases correctly identified as non-VAD by RDR or MRDR (true negatives). All available data were plotted without assessing consistency or bias.
Results
The flowchart of study search, selection, and data collection is shown in Figure 1. The search yielded 423 records (190 excluding duplicates): 22 articles (21 studies) were assigned to category 1, including 7 human studies, 6 rat studies, 2 calf studies, and 6 swine studies; 44 human VA intervention articles (39 studies) were assigned to category 2, including 15 studies in children; and 56 articles (51 studies) were assigned to category 3, including studies covering CLD, protein and other nutrient malnutrition, lactation, pregnancy and parity, infection, anorexia, oral contraceptive use, and RBP mutations. Finally, 41 articles (39 studies) describing observational studies and 6 micronutrient surveys associated with DHSs were reported.
FIGURE 1.
PRISMA flow chart describing identification, screening, eligibility, and inclusion of studies. MRDR, modified relative dose-response; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses; RDR, relative dose-response; TLR, total liver reserves.
Category 1
The RDR tests qualitatively predict VA status (TLR ≤0.10 or >0.10 μmol VA/g) because the amount of challenge dose (either R or DR) released into blood is dependent on the amount of accumulated RBP that, in turn, is dependent on VA liver content. We examined studies that reported hepatic VA by biopsy or at necropsy, mostly in rats, pigs, and cattle, or biopsy and estimation by RID in humans, and RDR or MRDR values (Table 1). These studies were plotted together as RDR (Figure 2A) and MRDR (Figure 2B) values against TLR.
TABLE 1.
Studies comparing the RDRs with a gold-standard biomarker of VA status1
| First author, year (reference) | Country or animal breed | Group | Age | n | Dose-response test | TLR method |
|---|---|---|---|---|---|---|
| Loerch, 1979 (98) | Sherman | Rats fed varying amounts of VA | Weanling | 59 | RDR2 | Necropsy |
| Underwood, 1980 (128) | Sherman | Rats on a VA-sufficient diet and rice protein | Weanling | 3 | RDR | Necropsy |
| Sherman | Rats on a VA-deficient diet and rice protein | Weanling | 4 | RDR | Necropsy | |
| Sherman | Rats on a VA-deficient diet and casein protein | Weanling | 4 | RDR | Necropsy | |
| Russell, 1983 (101) | USA | Adults with chronic liver disease | 45–65 y | 26 | RDR2 | Biopsy |
| Amedee-Manesme, 1984 (129) | France | Adult surgical patients with generally normal liver function | 22–87 y | 12 | RDR | Biopsy |
| Amedee-Manesme, 1987 (22) | France | Children with chronic liver disease | 0.3–8 y | 12 | i.m.-RDR | Biopsy |
| Amedee-Manesme, 1988 (23) | France | Children with chronic liver disease | 3–13 y | 2 | i.m.-RDR | Biopsy |
| Zachman, 1991 (130) | Sprague-Dawley | Rats | 3 wk | 36 | i.m.-RDR2 | Necropsy (RP only) |
| Boner, 1997 (113) | Holstein | Calves | Neonates | 11 | RDR | Biopsy |
| Ribaya-Mercado, 1999 (74) | Guatemala | Adults | 60–81 y | 26 | RDR3 | RID |
| Hammell, 2000 (114) | Holstein | Calves given 0 IU VA/d | 28 d | 13–14 | RDR2,3 | Necropsy |
| Holstein | Calves given 1700 IU VA/d | 28 d | 13–14 | RDR2,3 | Necropsy | |
| Holstein | Calves given 34,000 IU VA/d | 28 d | 13–14 | RDR2,3 | Necropsy | |
| Holstein | Calves given 68,000 IU VA/d | 28 d | 13–14 | RDR2,3 | Necropsy | |
| Santana, 2016 (126) | Brazil | Adults with chronic liver disease | 24–68 y | 41 | RDR2,3 | Biopsy (free retinol only) |
| Tanumihardjo, 1988 (102) | Sprague-Dawley | Rats fed varying amounts of VA | Weanling | 22 | MRDR2 | Necropsy |
| Tanumihardjo, 1990 (122) | Sprague-Dawley | Rats fed varying amounts of VA | Weanling | 24 | MRDR2 | Necropsy |
| Valentine, 2004 (105) | Large White/Landrace crossbreed | Piglets fed VA-deficient diet | Weanling | 10 | MRDR | Necropsy |
| Valentine, 2004; Tanumihardjo, 2011 (131, 132) | Large White/Landrace crossbreed | Piglets fed VA-deficient diet | Weanling | 7 | MRDR | Necropsy |
| Valentine, 2005 (133) | Large White/Landrace crossbreed | Piglets from sows fed varying amounts of VA | Weanling | 18 | MRDR | Necropsy |
| Surles, 2006 (123) | Large White/Landrace crossbreed | Lactating sows on VA-sufficient diet | 3.1 ± 0.9 y | 5 | MRDR | Necropsy |
| Surles, 2007 (134) | Large White/Landrace crossbreed | Piglets 10 d after a high-dose VA supplement | 28 d | 64 | MRDR | Necropsy |
| Escaron, 2009 (107) | Sprague-Dawley | Rats fed varying amounts of VA and dietary fat | 91 d | 16 | MRDR | Necropsy |
| Surles, 2011 (124) | Large White/Landrace crossbreed | Lactating sows following 3 parities of low-VA diet | 2.5 ± 0.3 y | 7 | MRDR | Necropsy |
| Newton, 2016 (24) | Ghana | Children with MRDR test and RID 5 mo later | 8.1–8.7 y | 19 | MRDR | RID |
MRDR, modified relative dose-response; RDR, relative dose-response; RID, retinol isotope dilution; RP, retinyl palmitate; TLR, total liver vitamin A reserves; VA, vitamin A.
Values reported graphically in the source; their coordinates were estimated using the axes for this review.
Reported as group values rather than as individual values in Figure 2.
FIGURE 2.
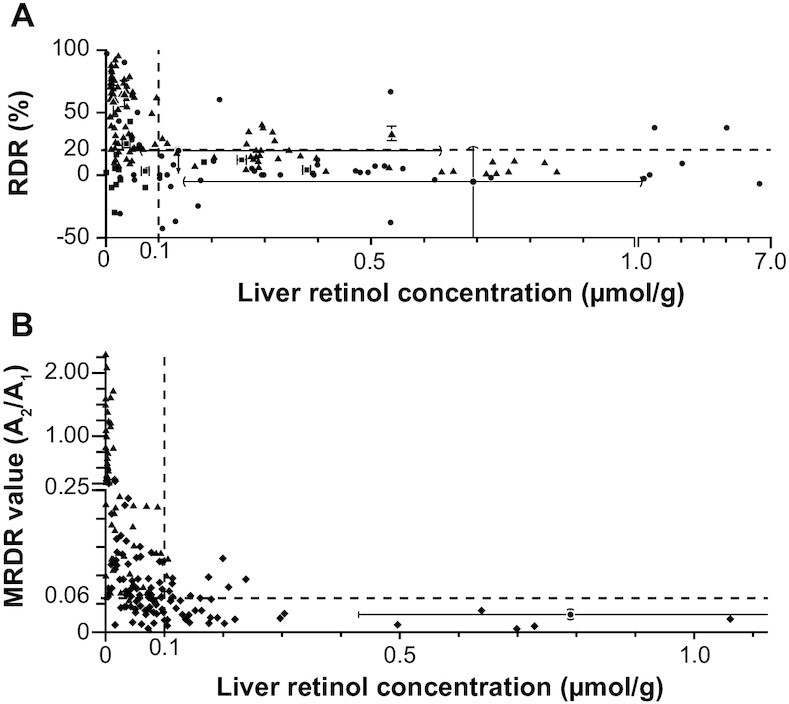
Comparison of the RDR tests with vitamin A TLR, the gold standard for vitamin A status. See Table 1 for more details about individual studies. (A) RDR test value versus TLR. (B) MRDR test value versus TLR. In both panels, accepted cutoffs for deficiency are plotted as dashed lines at an RDR value of 20%, an MRDR value of 0.060, and TLR of 0.1 μmol vitamin A/g (1). The symbols represent individual or group values, where triangles represent rats, squares represent calves, diamonds represent swine, and circles represent humans. For studies where only a group value was available (see Table 1), the SD is plotted with flat end-caps, range is plotted with round end-caps, and an arrow is used for 1 study (126) with unknown group RDR where all points were reported to have an RDR value <20% (i.e., normal). MRDR, modified relative dose-response; RDR, relative dose-response; TLR, total liver reserves.
The RDR had a sensitivity of 68% (82/121) and specificity of 85% (108/127) using all subjects/animals (n = 248) from 10 studies, excluding an 11th study in hepatitis C patients (126) because free retinol was reported rather than TLR (which includes free retinol and retinyl esters). Alternatively, if all subjects are assumed VA-sufficient because the lowest free retinol concentration was 0.067 μmol/g and free retinol accounts for 3–9% of TLRs in humans (127), specificity improves to 89% (149/168) because no subject had a positive RDR.
By the same method, the MRDR had a sensitivity of 80% (111/139) and specificity of 69% (24/35) in 174 subjects/animals in 10 studies. These numbers exclude 1 MRDR study performed in humans, which had a 5-mo VA intervention between RID and MRDR (24), so only the control group was used to avoid changes in status due to the intervention, but the time period likely introduced inaccuracy. Because all 19 individuals were VA-adequate by RID and 2 positive MRDR subjects were not in the final group, including this study improves specificity to 80% (43/54). With the exclusion of 2 MRDR studies using 7 and 5 lactating sows (TLR = 0.23 ± 0.05 and 0.73 ± 0.21 μmol VA/g, respectively) given 35 μmol DRAc (123, 124), specificity increases to 83% (35/42). Four lactating sows with a mean TLR of 0.21 ± 0.03 μmol VA/g had positive MRDR values, which may indicate a difference in response to VA demands during lactation.
Category 2
Studies that examined RDR values before and after an intervention or compared RDR among different intervention groups are described in Table 2. Due to logistics, human studies with gold-standard VA status hepatic measurements and RDR are few; therefore, intervention changes were considered secondary evidence. Lack of change in RDR values can result from either no change in VA status or insensitivity (135).
TABLE 2.
Studies using the RDRs to monitor VA status changes due to an intervention1
| First author, year (reference) | Country | Group | Age | EAR, μg RAE/d | Treatment | Time point | Treatment, average μg RAE/d | n | Test used | Dose-response test value2 | VAD, % |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mobarhan, 1981 (99) | USA | Males | 46–69 y | 625 | Daily VA supplements | Baseline | 8 | RDR | 21 ± 9% | 503 | |
| 4 wk | 10,000 | 8 | RDR | 5 ± 2%†† | 133 | ||||||
| Flores, 1984; Campos, 1987 (25, 136) | Brazil | Children | 1.5–7 y | 210–275 | 200,000-IU VA supplement | Baseline | 57 | RDR | 19 ± 23% | 37 | |
| 30 d | 2000 | 42 | RDR | — | 0 | ||||||
| 120 d | 500 | 31 | RDR | — | 0 | ||||||
| 180 d | 333 | 29 | RDR | 7 ± 10% | 10 | ||||||
| 210–275 | 200,000-IU VA supplement, chickenpox at day 120 | Baseline | 36 | RDR | 20 ± 26% | 38 | |||||
| 30 d | 2000 | 30 | RDR | — | 0 | ||||||
| 120 d | 500 | 31 | RDR | — | 10 | ||||||
| 180 d | 333 | 31 | RDR | 38 ± 22% | 74*** | ||||||
| Amedee-Manesme, 1987 (22) | France | One child | 15 mo | 210 | 33-mg VA supplement | Baseline | 1 | RDR | Positive | — | |
| 2 mo | 550 | 1 | RDR | Negative | — | ||||||
| Amatayakul, 1989 (137) | Thailand | Women | 18–35 y | 500 | 38-mg VA supplement | Baseline | 39 | RDR | Range: −25–29% | 2.5 | |
| 30 d | 1267 | 39 | RDR | Range: −29–17% | 0 | ||||||
| Tanumihardjo, 1990 (26) | USA | Children | 3.7–6.2 y | 210–275 | 52.4-μmol VA supplement | Baseline | 3 | MRDR | 0.018, 0.031, 0.014 | 0 | |
| 14 d | 1070 | 3 | MRDR | 0.011, 0.019, 0.008 | 0 | ||||||
| Stoltzfus, 1993 (4) | Indonesia | Mothers | <50 y | 885–900 | Placebo | Baseline | 71 | RDR | — | 9.2 | |
| 2.5 mo | 0 | 69 | RDR | — | 10 | ||||||
| 5.5 mo | 0 | 72 | RDR | — | 3.0 | ||||||
| 104-μmol VA supplement | Baseline | 69 | RDR | — | 4.3 | ||||||
| 2.5 mo | 425 | 70 | RDR | — | 9.0 | ||||||
| 5.5 mo | 193 | 67 | RDR | — | 1.5 | ||||||
| Infants | 7–21 d | 4004 | Mothers receiving placebo | 5.5 mo | 0 | 64 | RDR | — | 23 | ||
| 4004 | Mothers receiving a VA supplement | 5.5 mo | —5 | 67 | RDR | — | 10* | ||||
| Humphrey, 1994 (28) | Indonesia | Children | 12–59 mo | 210–275 | 105-μmol VA supplement | Baseline | 174 | RDR | — | 19.5 | |
| 3 mo | 335 | 123 | RDR | — | 8.9 | ||||||
| 6 mo | 168 | 137 | RDR | — | 9.5 | ||||||
| 210–275 | 210-μmol VA supplement | Baseline | 170 | RDR | — | 21 | |||||
| 3 mo | 670 | 126 | RDR | — | 8.7 | ||||||
| 6 mo | 335 | 134 | RDR | — | 3.1* | ||||||
| Tanumihardjo, 1994 (29) | Indonesia | Children | 0.7–6.5 y | 275–5004 | 157–315 μmol VA in 1 or 2 supplements | Baseline | 8 | RDR | 18 ± 11% | 63 | |
| Baseline | 8 | MRDR | 0.08 ± 0.05 | 75 | |||||||
| 2 wk | 3207–6435 | 8 | MRDR | 0.04 ± 0.02† | 13 | ||||||
| Azaïs-Braesco, 1995 (73) | France | Elderly adults | 83 ± 6.1 y | 500–625 | 20,000 IU VA/d | Baseline | 5 | RDR | 15.7, 18.8, 20.3, 28.7, 31.0% | 60 | |
| 3 wk | 6000 | 5 | RDR | 2.8, 16.1, 21.6, 2.6, 15.6% | 20 | ||||||
| de Pee, 1995, 1997 (62, 138) | Indonesia | Lactating women | 900 | Daily placebo wafer | Baseline | 49 | MRDR | 0.096 [0.04–0.13] | 687 | ||
| 12 wk | 68 | 49 | MRDR | Change: +0.029 (0.01–0.4) | — | ||||||
| Daily 100–150 g vegetables | Baseline | 47 | MRDR | 0.096 [0.05–0.15] | 687 | ||||||
| 12 wk | 2088 | 47 | MRDR | Change: −0.019 (−0.03–0)* | — | ||||||
| Daily βC-enriched wafer | Baseline | 52 | MRDR | 0.076 [0.04–0.11] | 687 | ||||||
| 12 wk | 2208 | 52 | MRDR | Change: −0.039 (−0.05 to −0.01)††† | — | ||||||
| Manorama, 1996, 1997 (30, 139) | India | Children | 7.6 ± 1.0 y | 275–445 | Daily red palm oil | Baseline | 12 | MRDR | 0.07 ± 0.08 | 25 | |
| 2 mo | 2008 | 12 | 0.02 ± 0.01† | 0 | |||||||
| Daily VA supplement | Baseline | 11 | 0.09 ± 0.08 | 44 | |||||||
| 2 mo | 600 | 10 | 0.02 ± 0.01† | 0 | |||||||
| Rahman, 1996 (5) | Bangladesh | Infants | ∼73 ± ∼23 d | 4004 | Placebo | 12 wk | 0 | 28 | RDR | — | 82 |
| Three 15-mg VA doses over 12 wk | 12 wk | 536 | 33 | RDR | — | 61‡ | |||||
| Tanumihardjo, 1996 (63) | Indonesia | Lactating women | 24.7 ± 6.3 y | 900 | Daily VA supplement | Baseline | 23 | MRDR | 0.100 ± 0.054 | 74 | |
| 35 d | 2402 | 23 | MRDR | 0.040 ± 0.021††† | 13 | ||||||
| Tanumihardjo, 1996 (31) | Indonesia | Children | ∼3.3 ± ∼1.3 y | 210–275 | Placebo, not dewormed | Baseline | 50 | MRDR | 0.056 ± 0.032 | 36 | |
| 4 wk | 0 | 50 | MRDR | 0.050 ± 0.031 | 22 | ||||||
| 210–275 | Placebo, dewormed before the study | Baseline | 51 | MRDR | 0.059 ± 0.045 | 35 | |||||
| 3 wk | 0 | 51 | MRDR | 0.057 ± 0.037 | 33 | ||||||
| 210–275 | Placebo, dewormed during study | Baseline | 52 | MRDR | 0.065 ± 0.059 | 40 | |||||
| 4 wk | 0 | 52 | MRDR | 0.057 ± 0.054 | 35 | ||||||
| 210–275 | 210-μmol VA supplement, not dewormed | Baseline | 49 | MRDR | 0.060 ± 0.062 | 37 | |||||
| 4 wk | 2145 | 49 | MRDR | 0.033 ± 0.014 | 2 | ||||||
| 210–275 | 210-μmol VA supplement, dewormed before the study | Baseline | 54 | MRDR | 0.056 ± 0.018 | 33 | |||||
| 3 wk | 2860 | 54 | MRDR | 0.036 ± 0.018 | 13 | ||||||
| 210–275 | 210-μmol VA supplement, dewormed during the study | Baseline | 52 | MRDR | 0.054 ± 0.038 | 31 | |||||
| 4 wk | 2145 | 52 | MRDR | 0.029 ± 0.018 VA main effect*** | 6 | ||||||
| Raghuramulu, 1998 (32) | India | Children | 1–5 y | 210–275 | 200,000 IU VA divided into 1–4 doses | Baseline | 11 | RDR | — | 263 | |
| 4–10 d | 6000–15,000 | 11 | RDR | — | 293 | ||||||
| Ribaya-Mercado, 1999 (74) | Guatemala | Elderly adults | 60–81 y | 500–625 | Daily VA supplement | Baseline | 9 | 7-h RDR | −16.9 ± 10.1% | — | |
| 32 d | 800 | 9 | 7-h RDR | −6.2 ± 2.6% | — | ||||||
| Rice, 1999; Filteau, 1999, (6, 140) | Bangladesh | Lactating women | ∼20–∼30 y | 885–900 | Placebo | Baseline | 35 | RDR | 3.2%9 (2.6–3.8%) | 14 | |
| 2.5 mo | 0 | 35 | RDR | 5.4%9 (4.3–6.9%) | 54 | ||||||
| 5.5 mo | 0 | 36 | RDR | 4.5%9 (3.7–5.5%) | 33 | ||||||
| 8.5 mo | 0 | 31 | RDR | 5.2%9 (3.9–6.9%) | 42 | ||||||
| 885–900 | 7.8-mg βC supplement | Baseline | 35 | RDR | 4.0%9 (3.0–5.3%) | 31 | |||||
| 2.5 mo | 39008 | 36 | RDR | 5.1%9 (4.1–6.4%) | 42 | ||||||
| 5.5 mo | 39008 | 32 | RDR | 3.1%9 (2.4–3.8%) | 19* | ||||||
| 8.5 mo | 39008 | 35 | RDR | 3.9%9 (3.2–4.8%) | 26 | ||||||
| 885–900 | 200,000-IU VA supplement | Baseline | 36 | RDR | 3.2%9 (2.5–3.9%) | 19 | |||||
| 2.5 mo | 2857 | 34 | RDR | 3.8%9 (3.1–4.7%)* | 18** | ||||||
| 5.5 mo | 1299 | 35 | RDR | 3.9%9 (3.1–4.9%) | 31 | ||||||
| 8.5 mo | 840 | 32 | RDR | 4.4%9 (3.7–5.2%) | 28 | ||||||
| Infants | 7–21 d | 4004 | Mothers receiving placebo | 6 mo | —5 | 70 | RDR | 11.8%9 (10.6–13.2%) | 93 | ||
| 4004 | Mothers receiving βC | 6 mo | —5 | 69 | RDR | 10.2%9 (9.0–11.4%) | 58‡ | ||||
| 4004 | Mothers receiving VA | 6 mo | —5 | 69 | RDR | 9.2%9 (8.1–10.5%)** | 60 | ||||
| Tyson, 1999 (7) | USA | Neonates | GA 26.8 ± 1.8 wk | 4004 | Daily ∼1000 IU VA/kg BW | 28 d | ∼200 | 155 | 3-h i.m.-RDR | 7.3%10 [−6.2–49%] | 45 |
| Daily ∼4000 IU VA/kg BW | 28 d | ∼800 | 145 | 3-h i.m.-RDR | 2.9%10 [−10–18%]*** | 2211,*** | |||||
| Solon, 2000 (33) | Philippines | Children | 9.5 ± 2.0 y | 275–445 | Daily placebo bun | 30 wk | 0 | 77 | MRDR | 0.057,12 [0.04–0.07] | 29 |
| Daily VA-fortified bun | 30 wk | 97 | 72 | MRDR | 0.047,11 [0.03–0.06] | 16‡ | |||||
| Ncube, 2001 (64) | Zimbabwe | Lactating women | ∼27 ± ∼7 y | 900 | Placebo | Baseline | 0 | 11 | RDR | 49 ± 18% | 91 |
| 60 d | 0 | 11 | RDR | 42 ± 21% | 73 | ||||||
| Daily 6-mg βC supplement | Baseline | 9 | RDR | 44 ± 22% | 78 | ||||||
| 60 d | 30008 | 9 | RDR | 21 ± 21%† | 33 | ||||||
| Daily papaya supplement | Baseline | 12 | RDR | 35 ± 19% | 58 | ||||||
| 60 d | 5008 | 12 | RDR | 15 ± 33%† | 33 | ||||||
| Daily carrot supplement | Baseline | 11 | RDR | 36 ± 30% | 82 | ||||||
| 60 d | 5008 | 11 | RDR | 25 ± 20% | 64 | ||||||
| Bahl, 2002 (8) | Ghana | Infants | 4004 | Placebo | Baseline | 103 | MRDR | — | 76 | ||
| 4.5 mo | 0 | 93 | MRDR | — | 54 | ||||||
| 7.5 mo | 0 | 61 | MRDR | — | 49 | ||||||
| Maternal 60 mg VA, infant 3 × 7.5 mg VA supplements over 3.5 mo | Baseline | 99 | MRDR | — | 76 | ||||||
| 4.5 mo | >1795 | 94 | MRDR | — | 44 | ||||||
| 7.5 mo | >1075 | 79 | MRDR | — | 46 | ||||||
| India | Infants | 6 wk | 4004 | Placebo | Baseline | 95 | MRDR | — | 91 | ||
| 4.5 mo | 0 | 98 | MRDR | — | 77 | ||||||
| 7.5 mo | 0 | 94 | MRDR | — | 60 | ||||||
| Maternal 60 mg VA, infant 3 × 7.5 mg VA supplements over 3.5 mo | Baseline | 95 | MRDR | — | 87 | ||||||
| 4.5 mo | >1795 | 97 | MRDR | — | 62.9* | ||||||
| 7.5 mo | >1075 | 93 | MRDR | — | 55 | ||||||
| Peru | Infants | 6 wk | 4004 | Placebo | Baseline | — | 91 | MRDR | — | 69 | |
| 4.5 mo | 0 | 117 | MRDR | — | 33 | ||||||
| 7.5 mo | 0 | 111 | MRDR | — | 15 | ||||||
| Maternal 60 mg VA, infant 3 × 7.5 mg VA supplements over 3.5 mo | Baseline | 97 | MRDR | — | 83 | ||||||
| 4.5 mo | >1795 | 117 | MRDR | — | 29 | ||||||
| 7.5 mo | >1075 | 106 | MRDR | — | 15 Overall 4.5 mo,* 7.5 mo NS | ||||||
| Stephensen, 2002 (34) | Peru | Children | 12–50 mo | 210–5004 | Placebo | 2–5 d | 0 | 41 | RDR | — | 34 |
| 150,000–300,000 IU VA over 2 d | 2–5 d | 9000–30,000 | 45 | RDR | — | 16‡ | |||||
| Tanumihardjo, 2002 (70) | Indonesia | Pregnant women | 18–37 y | 885–900 | Placebo | Baseline | 7 | MRDR | 0.032 ± 0.008 | — | |
| 8 wk | 0 | 7 | MRDR | 0.031 ± 0.011 | — | ||||||
| Daily VA supplement | Baseline | 7 | MRDR | 0.043 ± 0.034 | — | ||||||
| 8 wk | 2402 | 7 | MRDR | 0.043 ± 0.044 | — | ||||||
| Daily iron supplement | Baseline | 5 | MRDR | 0.032 + 0.009 | — | ||||||
| 8 wk | 0 | 5 | MRDR | 0.037 ± 0.007 | — | ||||||
| Daily VA and iron supplements | Baseline | 8 | MRDR | 0.042 ± 0.013 | — | ||||||
| 8 wk | 2402 | 8 | MRDR | 0.021 ± 0.015* | — | ||||||
| Ambalavanan, 2003 (9) | USA | Neonates | GA 25 ± 2 wk | 4004 | 500–1500 IU/kg BW daily plus 5000 IU 3 d/wk | 28 d | ∼750–950 | 28 | 2-h i.m.-RDR | 17 ± 33% | 2711 |
| 500–1500 IU/kg BW daily plus 10,000 IU 3 d/wk | 28 d | ∼1400–1600 | 27 | 2-h i.m.-RDR | 14 ± 16% | 4811 | |||||
| 500–1500 IU/kg BW daily plus 15,000 IU 1 d/wk | 28 d | ∼750–950 | 30 | 2-h i.m.-RDR | 27 ± 34% | 5211,‡ | |||||
| Davidsson, 2003 (35) | Cote d'Ivoire | Children | 6–12 y | 275–445 | Single 210-μmol VA supplement | Baseline | 13 | MRDR | 0.156 ± 0.065 | 100 | |
| 39 d | 1540 | 13 | MRDR | 0.125 ± 0.052 | 92 | ||||||
| Wieringa, 2003 (10) | Indonesia | Infants | 4.2 ± 0.5 mo | 4004–5004 | Placebo | 6 mo | 0 | 43 | MRDR | — | 81 |
| Iron supplements 5 d/wk | 6 mo | 0 | 49 | MRDR | — | 51*** | |||||
| Iron and zinc supplements 5 d/wk | 6 mo | 0 | 39 | MRDR | — | 49*** | |||||
| Zinc supplements 5 d/wk | 6 mo | 0 | 48 | MRDR | — | 82 | |||||
| Zinc and 2.4 mg βC 5 d/wk | 6 mo | 8578 | 39 | MRDR | — | 94 | |||||
| 2.4 mg βC 5 d/wk | 6 mo | 8578 | 38 | MRDR | — | 83 | |||||
| Tanumihardjo, 2004 (36) | Indonesia | Children | 3.9 ± 1.3 y | 210–275 | Ascariasis with or without Trichuriasis, single 210-μmol VA supplement and deworming | Baseline | 51 | MRDR | 0.054 ± 0.038 | 31 | |
| 1 mo | 2145 | 51 | MRDR | 0.030 ± 0.018††† | 5.9 | ||||||
| Trichuriasis only, single 210-μmol VA supplement and deworming | Baseline | 29 | MRDR | 0.049 ± 0.040 | 24 | ||||||
| 1 mo | 2145 | 29 | MRDR | 0.031 ± 0.015† | 6.9 | ||||||
| Children | 3.4 ± 1.1 y | 210–275 | Single 210-μmol VA supplement, dewormed prior to baseline | Baseline | 21 | MRDR | 0.023 ± 0.014 | 0 | |||
| 1 mo | 2145 | 21 | MRDR | 0.025 ± 0.013 | 0 | ||||||
| Single 210-μmol VA supplement prior to baseline, dewormed during trial | Baseline | 19 | MRDR | 0.019 ± 0.019 | 0 | ||||||
| 1 mo | 0 | 19 | MRDR | 0.024 ± 0.024 | 0 | ||||||
| Single 210-μmol VA supplement prior to baseline, not dewormed | Baseline | 11 | MRDR | 0.021 ± 0.010 | 0 | ||||||
| 1 mo | 0 | 11 | MRDR | 0.023 ± 0.015 | 0 | ||||||
| van Jaarsveld, 2005 (37) | South Africa | Children | ∼7.3 ± 1.2 y | 275–445 | White sweet potato | Baseline | 0 | 89 | MRDR | 0.038 ± 0.024 | 14 |
| 10.6 wk | 0 | 89 | MRDR | 0.042 ± 0.025 | 18 | ||||||
| Orange sweet potato | Baseline | 89 | MRDR | 0.040 ± 0.028 | 22 | ||||||
| 10.6 wk | 7368 | 89 | MRDR | 0.036 ± 0.019* | 13 | ||||||
| Tchum, 2006 (141) | Ghana | Mothers postpartum | ∼29 ± 6.9 y | 900 | Single 200,000-IU VA supplement postpartum | Baseline | 82 | MRDR | 0.048 ± 0.0377 | 177 | |
| 1 mo | 2143 | 21 | MRDR | 0.026 ± 0.0157,††† | 57 | ||||||
| 3 mo | 719 | 27 | MRDR | 0.031 ± 0.0207,††† | 117 | ||||||
| 5 mo | 429 | 28 | MRDR | 0.023 ± 0.0127,††† | 07 | ||||||
| Two 200,000-IU VA supplements postpartum | Baseline | 85 | MRDR | Treatment group differences NS; values pooled | |||||||
| 1 mo | 4286 | 30 | MRDR | ||||||||
| 3 mo | 1429 | 25 | MRDR | ||||||||
| 5 mo | 857 | 25 | MRDR | ||||||||
| van den Broek, 2006 (71) | Malawi | Pregnant women | 14–28 y | 530–550 | Placebo | Baseline | 0 | 232 | MRDR | — | 2.2 |
| 36–38 wk | 0 | 176 | MRDR | — | 7.5 | ||||||
| Daily 5000 IU VA supplement | Baseline | 234 | MRDR | — | 3.5 | ||||||
| 36–38 wk | 1500 | 174 | MRDR | — | 4.8 | ||||||
| Daily 10,000 IU VA supplement | Baseline | 234 | MRDR | — | 3.0 | ||||||
| 36–38 wk | 3000 | 180 | MRDR | — | 4.2 | ||||||
| Ayah, 2007 (11) | Kenya | Infants | At birth | 4004 | Maternal placebo; infant placebo | 26 wk | 0 | 139 | MRDR | 0.0919 (0.082–0.100) | 76 |
| Maternal 400,000 IU VA; infant placebo | 26 wk | —5 | 140 | MRDR | 0.0829 (0.075–0.088) | 80 | |||||
| Maternal placebo; infant 100,000 IU VA | 26 wk | 357 | 143 | MRDR | 0.0739 (0.067–0.079) | 69 | |||||
| 400,000 IU maternal VA; infant 100,000 IU VA | 26 wk | >3575 | 142 | MRDR | 0.0769 (0.069–0.082) infant VA,*** maternal VA NS | 70 | |||||
| Idindili, 2007 (12) | Tanzania | Infants | 1.41 ± 0.96 mo | 4004 | Maternal 200,000 IU VA; infant 3 x 25,000 IU VA, then 100,000 IU VA at 9 mo | Baseline | 390 | 3-h MRDR | — | 84 | |
| 6 mo | >1345 | 282 | 3-h RDR | — | 47 | ||||||
| 9 mo | >2085 | 269 | 3-h MRDR | — | 40 | ||||||
| Two maternal 200,000 IU VA; infant 3 x 50,000 IU VA, then 100,000 IU VA at 9 mo | Baseline | 390 | 3-h MRDR | — | 82 | ||||||
| 6 mo | >2685 | 293 | 3-h MRDR | — | 43 | ||||||
| 9 mo | >2985 | 278 | 3-h MRDR | — | 41, Dose size NS | ||||||
| Permaesih, 2009; Rosmalina, 2009 (65, 125) | Indonesia | Lactating women | — | 900 | Placebo and placebo cooking oil | Baseline | 34 | MRDR | 0.084 ± 0.041 | — | |
| 100 d | ∼500 | 34 | MRDR | 0.11 ± 0.063 | — | ||||||
| Placebo and VA-fortified cooking oil | Baseline | 30 | MRDR | 0.087 ± 0.045 | — | ||||||
| 100 d | ∼600 | 30 | MRDR | 0.085 ± 0.043* | — | ||||||
| Two 200,000 IU VA supplements and placebo cooking oil | Baseline | 32 | MRDR | 0.080 ± 0.077 | — | ||||||
| 100 d | ∼800 | 32 | MRDR | 0.083 ± 0.052* | — | ||||||
| Two 200,000 IU VA supplements and VA-fortified cooking oil | Baseline | 35 | MRDR | 0.071 ± 0.051 | — | ||||||
| 100 d | ∼1000 | 35 | MRDR | 0.064 ± 0.028* | — | ||||||
| Agne-Djigo, 2012 (13) | Senegal | Infants | ∼6.2 ± 0.4 mo | 4004 | Maternal placebo | Baseline | 347 | MRDR | 0.064 ± 0.0197 | 747 | |
| 14 d | 389 ± 15113 | 19 | MRDR | 0.073 ± 0.017 | 95 | ||||||
| Two maternal 200,000 IU VA supplements; | Baseline | 347 | MRDR | 0.064 ± 0.0197 | 74 | ||||||
| 14 d | 365 ± 21513 | 13 | MRDR | 0.055 ± 0.017* | 54* | ||||||
| Ambrosio, 2012 (38) | Brazil | Children | 1–6 y | 4004 | 6 g dehydrated pumpkin flakes 5 d/wk | Baseline | 97 | RDR | — | 16 | |
| 90 d | 2148 | 97 | RDR | — | 0 | ||||||
| Dougherty, 2012 (39) | USA | Children | ∼7.6 ± ∼2.9 y | 275–445 | Placebo | Baseline | 18 | RDR | 4.4 ± 5.8% | — | |
| 1 y | 0 | 18 | RDR | 3.7 ± 7.2% | — | ||||||
| Daily RDA of VA supplement | Baseline | 23 | RDR | 1.1 ± 10.1% | — | ||||||
| 1 y | 300–600 | 18 | RDR | 2.3 ± 5.6% | — | ||||||
| Daily RDA of VA and 10–20 mg zinc supplement | Baseline | 15 | RDR | 2.9 ± 6.6% | — | ||||||
| 1 y | 300–600 | 12 | RDR | 1.9 ± 6.4% | — | ||||||
| Mactier, 2012 (15) | Scotland | Preterm infants | GA 24–33 wk | 4004 | Placebo | 6 wk | 0 | 32 | 3-h RDR | 14%14 [−22–55%] | 28 |
| 6–12 × 10,000 IU VA i.m | 6 wk | 2143–4286 | 31 | 3-h RDR | 12%14 [−21–36%] | 26 | |||||
| Bresnahan, 2014 (142) | Zambia | Children | 4.5 ± 0.9 y | 210–275 | White maize 6 d/wk | Baseline | 94 | MRDR | 0.030 ± 0.023 | 15 | |
| 70 d | 0 | 86 | MRDR | 0.050 ± 0.025††† | 17 | ||||||
| Orange maize 6 d/wk | Baseline | 99 | MRDR | 0.032 ± 0.021 | 6 | ||||||
| 70 d | 688 | 95 | MRDR | 0.049 ± 0.021††† | 21 | ||||||
| Ahmad, 2020 (108) | Bangladesh | Infants | <48 h | 4004 | Placebo | 15 wk | 0 | 24 | MRDR | 0.035 ± 0.020 | 12.5 |
| Single 50,000 IU VA supplement | 15 wk | 143 | 21 | MRDR | 0.029 ± 0.017 | 9.5 |
VAD was defined as ≥20% RDR or ≥0.060 MRDR unless otherwise noted. BW, body weight; GA, gestational age; MRDR, modified relative dose-response; RDR, relative dose-response; VA, vitamin A; VAD, vitamin A deficiency; βC, β-carotene; —, missing data.
Values are reported as mean ± SD (unless noted otherwise), as available. Significant differences between intervention and placebo denoted by *P <0.05; **P < 0.01; ***P < 0.001. Significant differences from baseline denoted by †P < 0.05; ††P < 0.01; †††P < 0.001. Results trending towards significant differences between intervention and placebo denoted by ‡P < 0.1. Note that these were determined by their respective authors and, in many cases, statistical tests were not performed.
RDR cutoff for deficiency was >14% in this study.
Estimated Average Requirements in infants <12 mo old are unknown so Adequate Intakes (AIs) are listed.
Milk-derived VA intake not measured.
Reported as median [IQR].
Value was reported for entire study population and not separately for each group.
Includes provitamin A carotenoids according to the Institute of Medicine conversion factors of 2:1 (supplemental βC), 12:1 (dietary βC) and 24:1 (dietary α-carotene and β-cryptoxanthin) (103).
Reported as mean (95% CI).
Reported as median [5th percentile–95th percentile].
RDR cutoff for deficiency was >10% in this study.
Estimated from figure in source.
Milk VA intake estimated by deuterium transfer (dose-to-mother approach) (143).
Reported as median [range].
Category 3
Fifty-one studies that investigated interactions of RDR tests with various physiological and pathological states and their major findings are described in Table 3.
TABLE 3.
Studies using the RDRs in physiological or pathological conditions in humans or animal models1
| First author, year (reference) | Country or animal species | Group | Age | Condition(s) examined | Study design (n at end of study) | n | Main findings |
|---|---|---|---|---|---|---|---|
| Mobarhan, 1981 (99) | USA | Adults | 46–69 y | CLD (cirrhosis), zinc deficiency | RDR pre-/post- VA intervention,2 RDR compared with serum zinc | 8 | RDR changed in response to the intervention in patients with alcoholic cirrhosis and was different according to dark adaptation status. Zinc deficiency was not a limiting factor in the RDR |
| Russell, 1983 (101) | USA | Adults | 45–65 y | CLD (chronic alcoholism, cirrhosis), zinc deficiency | RDR compared with liver biopsy TLR,3 serum zinc | 26 | RDR did not predict TLR. Zinc deficiency was not a limiting factor in the RDR |
| Flores, 1984; Campos, 1987 (25, 136) | Brazil | Children | 18–85 mo | Age, infection (chicken pox), age, malnutrition | RDR pre-/post-VA intervention2 and case/control for infection in children, with 20% of them <75% of Iowan weight-for-age standard | 72 | RDR responded to intervention and then increased drastically in response to infectionWeight-for-age was not correlated with RDR response to intervention |
| Amedee-Manesme, 1987 (22) | France | Children | 2 mo–13 y | CLD (e.g., biliary atresia, portal obstruction, Alagille syndrome), age | i.m.-RDR compared with biopsy TLR3 | 11 | i.m.-RDR predicted VA status |
| i.m.-RDR pre-/post-VA intervention2 | 7 | i.m.-RDR responded to the intervention in CLD patients | |||||
| Amatayakul, 1989 (137) | Thailand | Women | 18–25 y | Oral contraceptive use | RDR pre-/post- VA intervention2 and oral contraceptive compared with intrauterine contraceptive device control | 39 | RDR responded to treatment in the only individual with elevated RDR, not possible to assess contraceptive use effect on RDR |
| Bulux, 1992 (72) | Guatemala | Elderly adults | 60–91 y | Age | RDR 7 d test/retest and time course in elderly adults | 14 | Two high RDR value individuals had a negative RDR 7 d later, and RDR peaked later than expected (6–7 h) |
| Vaisman, 1992 (100) | Israel | Adolescents | 16.3 ± 1.6 | Anorexia | RDR pre-/post-dietary modification for anorexia | 3 | RDR responded to intervention |
| RDR time course | 7 anorexic, 7 healthy | RDR time course was not different between groups | |||||
| Stoltzfus, 1993 (4) | Indonesia | Women | <50 y | Lactation | RDR pre-/post-VA intervention2 in lactating women | 139 | RDR positive prevalence started very low and did not respond to intervention |
| Infants | 7–21 d | Age, breastfeeding | RDR post-maternal VA intervention2 in infants | 131 | RDR was different according to maternal intervention group | ||
| Humphrey, 1994 (28) | Indonesia | Children | 12–59 mo | Age | RDR pre-/post-VA intervention2 in children | 345 | RDR responded to intervention |
| Tanumihardjo, 1994 (106) | Indonesia | Women | 17–41 y | Lactation, body weight | MRDR 1–2 mo test/retest in lactating women | 14 | Positive MRDRs remained positive, 1 negative MRDR remained negative, the other was 0.048 and then 0.060 on retest. Variability increased as the interval increased |
| MRDR time course | 30–33 lactating/time point, 6–8 nonlactating/time point | MRDR was higher in lactating women than nonlactating women at all time points 3–6 h | |||||
| Tanumihardjo, 1994 (29) | Indonesia | Children | 0.7–65 y | Age, malnutrition | MRDR followed by 1.57 μmol RDR 10–17 d later in children with 96% of subjects below 10th percentile of weight-for-age4 | 75 | MRDR (48% positive) did not agree with RDR (10% positive) |
| MRDR followed by 3.5 μmol RDR 3–4 wk later in children, then intervention2 and follow-up MRDR, 59% of subjects below 10th percentile of weight-for-age4 | 47 baseline, 8 follow-up | Preintervention MRDR (12% positive) agreed with RDR (11% positive) and responded to intervention | |||||
| Azaïs-Braesco, 1995 (73) | France | Elderly adults | 83 ± 6.1 y | Age | RDR 3-wk test/retest | 14 | RDR gave the same result in 11/14 individuals |
| Pre-/post-VA intervention | 5 | RDR responded to intervention | |||||
| Wahed, 1995 (41) | Bangladesh | Children | 3–36 mo | Age, malnutrition | MRDR followed by RDR correlation 3 d later in children with low weight-for-age (74% below the 75th percentile weight-for-age)5 | 49 | MRDR (20% positive) did not agree with RDR (60% positive) |
| Manorama, 1996 (30) | India | Children | ∼7.6 ± 0.3 y | Age | MRDR pre-/post-VA intervention in children2 | 21 | MRDR responded to the intervention |
| Tanumihardjo, 1996 (42) | Indonesia | Children | 24–70 mo | Age | MRDR 1 mo crossover test/retest with 5.3 or 8.8 μmol DR in children and time course | 34 | The higher dose increased DR:R but there was no difference in mean DR:R at either time (both doses combined). MRDR can be measured at 4–7 h in children |
| Tanumihardjo, 1996, 2004 (31, 36) | Indonesia | Children | 0.6–6.6 y | Age, infection (trichuriasis, ascariasis) | MRDR pre-/post-VA and/or deworming intervention2 in children | 308 | MRDR was not affected by deworming but responded to VA intervention |
| Tanumihardjo, 1996 (63) | Indonesia | Women | 24.7 ± 6.3 y | Lactation | MRDR 3 × 1 mo retest and pre-/post-VA intervention2 | 23 | MRDR responded to the intervention |
| Boner, 1997 (113) | Holstein | Calves | Neonatal | Age | RDR time course and varying dose size compared with biopsy TLR3 in neonatal calves | 11–16 | RDR value correlated with dose (as varying VA concentrations in 2.3 kg colostrum) but not with TLR at any time |
| de Pee, 1997 (62) | Indonesia | Lactating women | 17–40 y | Lactation | MRDR pre/post-VA or β-carotene intervention2 in lactating women | 265 | MRDR responded to intervention |
| Willumsen, 1997; Filteau, 1998 (87, 144) | South Africa | Children | ∼24 ± 10 mo | Age, inflammation, and immune response (kerosene ingestion) | MRDR in children following kerosene ingestion, and correlation with neopterin | 47 with kerosene ingestion, 45 control | MRDR not different between kerosene ingesters (80% positive) and control (67% positive). MRDR was not correlated with neopterin |
| Raghuramulu, 1998 (32) | India | Children | 1–5 y | Age, malnutrition | RDR pre-/post-intervention2 in children with 26% mild, 66% moderate, and 8% severely undernourished by weight-for-age6 | 49 | RDR did not respond to the intervention by 4–10 d |
| Biesalski, 1999 (145) | Germany | Two German teenagers and their mother | 14, 17, — | Two different RBP mutations | Single RDR in each subject | 3 | RDR was negative in all 3 (homozygous teenagers and heterozygous mother) |
| Ribaya-Mercado, 1999 (74) | Guatemala | Elderly adults | 60–81 y | Age | RDR pre-/post-VA intervention2 in elderly adults | 9 | RDR did not respond to the intervention but mean RDR was negative to begin with |
| RDR compared with RID in elderly adults | 26 | One subject had a false-positive RDR, all subjects were VA-adequate by TLR | |||||
| Rice, 1999 (6); Filteau, 1999 (140) | Bangladesh | Women | 26.6 ± 5.7 y | Lactation | MRDR pre-/post-VA or β-carotene intervention2 in lactating women, correlation with mammary permeability by treatment group | 98–106/time point | MRDR responded to intervention. Mammary permeability was correlated with MRDR but not treatment group |
| Infants | 7–21 d | Breastfeeding, age | MRDR in infants of women in different intervention2 groups | 208 | MRDR responded to the intervention | ||
| Tyson, 1999 (7) | USA | Preterm infants | GA 26.8 ± 1.8 wk | Age | i.m.-RDR post-VA intervention2 in very-low-birth-weight neonates (<1000 g) | 300 | i.m.-RDR responded to the intervention |
| Hammell, 2000 (114) | Holstein | Calves | 28 d | Age | RDR compared to liver biopsy TLR3 with time-course at 20 h postpartum or 28 d, pre-/post-intervention | 53 | RDR was correlated with dose size rather than TLR in neonates, but RDR status correctly correlated with liver stores at 28 d. RDR at 6 and 8 h, but not 4 h, correlated with TLR |
| Solon, 2000 (33) | Philippines | Children | 9.5 ± 2 y | Age | MRDR post-VA intervention2 in children | 149 | MRDR responded to the intervention |
| Ncube, 2001 (64) | Zimbabwe | Women | ∼27 ± 7 y | Lactation | RDR pre-/post-VA intervention2 in lactating women | 43 | MRDR responded to the intervention |
| Bahl, 2002 (8) | Ghana, India, Peru | Infants | 6 wk | Age, breastfeeding | MRDR pre-/post-infant and maternal VA intervention2 in infants | 544 | MRDR responded to the intervention but higher cutoffs (0.09 or 0.012) discriminated between groups more clearly. Maternal and infant supplementation were not examined separately |
| Stephensen, 2002 (34) | Peru | Children | ∼25 mo | Age, infection (pneumonia) | RDR post-VA intervention2 at discharge following pneumonia treatment, correlation with CRP | 86 | RDR responded to intervention in children with low CRP but not high CRP, 2–5 d postintervention |
| Wieringa, 2002, 2003 (10, 146) | Indonesia | Infants | 4.2 ± 0.5 mo | Age, inflammation, iron and zinc nutrition | MRDR post-VA or β-carotene and/or iron and/or zinc intervention2 in infants | 238 | MRDR did not respond to β-carotene or zinc interventions but was improved by iron interventions |
| Tanumihardjo, 2002 (70) | Indonesia | Women | 18–37 y | Pregnancy, iron nutrition | MRDR pre-/post-VA and/or iron intervention in pregnant women | 27 | MRDR only responded to VA + iron intervention |
| Ambalavanan, 2003 (9) | USA | Preterm infants | GA <32 wk | Age, BPD | 2-h i.m.-RDR post-VA intervention2 in very-low-birth-weight neonates receiving VA 3 d/wk, twice the usual dose 3 d/wk, or the usual dose concentrated into 1 d/wk | 27–30/group | i.m.-RDR was not different between groups |
| Davidsson, 2003 (35) | Cote d'Ivoire | Children | 6–12 y | Age | MRDR pre-/post-VA intervention2 in children | 13 | MRDR prevalence did not respond to the intervention |
| Feranchak, 2005 (43) | USA | Children | 0.5–21 y | Choleostatic CLD (biliary atresia, Alagille syndrome, etc.), non-choleostatic CLD (α1-antitrypsin deficiency, autoimmune hepatitis, etc.), age | Oral RDR, then i.m.-RBP-RDR and i.m.-RDR on the following day in choleostatic and non-choleostatic children with CLD, oral RDR time course | 23 choleostatic, 10 non-choleostatic | RBP-RDR at a 9-h time point had no positives but 10 h oral RDR and 9 h i.m.-RDR did. There were no positive RDRs in non-choleostatic CLD. Two children with biliary atresia had no response to oral RDR but did respond to i.m.-RDR. Oral RDR was elevated in i.m.-RDR-positive individuals by 5 h with a maximum at 10 h |
| van Jaarsveld, 2005 (37) | South Africa | Children | 7.3 ± 1.2 y | Age, inflammation | MRDR pre-/post-β-carotene intervention2 in children, correlation with CRP and AGP | 176 | MRDR responded to the intervention. Excluding children with elevated CRP and/or AGP did not affect results |
| Surles, 2006 (123) | Large White/Landrace crossbreed | Sows | 3.1 ± 0.9 y | Lactation | MRDR compared with liver necropsy TLR,3 time course including DR loss to milk | 6 | MRDR was low in VA-sufficient sows; 10–20% of dose is excreted in milk. Milk DR:R was correlated with MRDR |
| van den Broek, 2006 (71) | Malawi | Women | ∼227 [14–30] y | Pregnancy, iron deficiency | MRDR pre-/post-VA intervention2 in mostly anemic pregnant women | 530 | MRDR positive prevalence was very low and did not respond to the intervention |
| Ayah, 2007 (11) | Kenya | Infants | 26 wk | Age, breastfeeding | MRDR post-infant and/or maternal VA intervention2 | 564 | MRDR responded to infant, but not maternal, intervention |
| Idindili, 2007 (12) | Tanzania | Infants | 1.41 ± 0.96 mo | Age, breastfeeding | MRDR post-infant and maternal VA intervention2 | 166 | MRDR responded to intervention but was not different between the 2 high-dose levels. Maternal and infant supplementation were not examined separately |
| Surles, 2007 (134) | Large White/ Landrace crossbreed | Piglets | 28 d | Age | MRDR pre-/ post-VA intervention in young piglets, compared with liver necropsy TLR,3 correlation with parity | 56 | MRDR responded to intervention. The second parity of piglets had a lower TLR, which was reflected by MRDR |
| Permaesih, 2009 (65) | Indonesia | Women | ∼20–30 y | Lactation | MRDR pre-/post-VA intervention2 | 30–35/group | MRDR was lower in treatment groups vs. placebo |
| Astiazaran-Garcia, 2010 (44) | Mexico | Children | 8.9 ± 1.7 y | Age, infection (Giardia lamblia) | MRDR pre-/ post-treatment for G. lamblia in children | 30 | MRDR responded to the treatment |
| Surles, 2011 (124) | Large White/ Landrace crossbreed | Sows | 2.1 ± 0.3 y | Lactation, parity | MRDR and milk DR time course in sows after 2 or 3 parities on VA-free diet, comparison with necropsy TLR3 | 7–8/time point | MRDR was elevated after 3, but not 2, parities on VA-deficient feed, despite sufficient TLR (∼0.2 μmol/g). Milk DR:R was correlated with MRDR |
| Agne-Djigo, 2012 (13) | Senegal | Infants | ∼6 ± 0.4 mo | Age, breastfeeding | MRDR pre-/ post-maternal VA intervention2 with deuterium dose-to-mother (milk intake measured) | 32 | MRDR responded to maternal intervention despite very similar milk retinol concentration and milk intake in treatment and control |
| Ambrosio, 2012 (38) | Brazil | Children | 12–72 mo | Age | RDR pre-/post-VA intervention2 in children | 97 | RDR responded to the intervention |
| Dougherty, 2012 (39) | USA | Children | ∼8 ± 3 y | Age, zinc status, sickle cell anemia | RDR pre-/post-VA and/or zinc intervention2 in children with sickle cell anemia | 49 | RDR was very low before and after the intervention in all groups so comparisons were not possible |
| Mactier, 2012 (15) | Scotland | Preterm infants | 24–33 wk GA | Age | 3-h RDR post-VA intervention2 in preterm infants | 63 | 3-h RDR did not respond to intervention |
| Schmiedchen, 2014 (16) | Germany | Newborn infants | 3 d | Age, low birth weight | i.m.-RDR and RBP-i.m.-RDR 25-d test/retest in low-birth-weight (<1500 g) newborn infants | 63 | i.m.-RDR was not correlated with i.m.-RBP-RDR. RDR decreased over time |
| Bresnahan, 2014 (142) | Zambia | Children | 4.5 ± 0.9 y | Age, inflammation | MRDR pre-/ post-β-carotene intervention2 in children, correlation with CRP and AGP | 181 | MRDR increased in response to low VA study diet. MRDR was not correlated with CRP or AGP |
| Santana, 2016 (126) | Brazil | Adults | 24–68 y | CLD (non-cirrhotic hepatitis C), body weight | RDR compared to liver biopsy free retinol concentration3 in adults with CLD and 49% BMI ≥25 kg/m2 | 43 | All RDRs were negative and free liver retinol was adequate (some subjects just below 0.1 μmol/g). Degree of fibrosis did not affect RDR or free liver retinol |
AGP, α1-glycoprotein; CLD, chronic liver disease; CRP, C-reactive protein; DR, 3,4-didehydroretinol; DR:R, molar ratio of 3,4-didehydroretinol to retinol; GA, gestational age; MRDR, modified relative dose-response; RBP, retinol-binding protein; RDR, relative dose-response; RID, retinol isotope dilution; RP, retinyl palmitate; TLR, total liver reserves; VA, vitamin A.
See Table 2 for more details.
Defined by the WHO (147).
Defined by the National Center for Health Statistics (148).
Defined by Rao et al. (149).
Reported as median [IQR].
Observational studies
Thirty-nine observational (cross-sectional, case/control, and cohort) studies and 6 micronutrient surveys associated with DHSs that used the MRDR are reported in Table 4.
TABLE 4.
Observational studies and micronutrient surveys associated with Demographic and Health Surveys assessing prevalence of VAD using the RDRs1
| Study, year (reference) | Country | Group | Age | n | Dose-response test | Dose-response test values | VAD, % |
|---|---|---|---|---|---|---|---|
| Micronutrient survey, 2017 (150) | Ghana | Children | 6–59 mo | 149 | MRDR | 0.031 ± 0.0232 | 7 |
| Women | 15–49 y | 153 | MRDR | 0.021 ± 0.017 | 5 | ||
| Micronutrient survey (Whitehead R., 2020; unpublished data) | Uganda | Children | 12–23 mo | 88 | MRDR | 0.032 ± 0.026 | 7 |
| Women | 15–49 y | 35 | MRDR | 0.015 ± 0.009 | 0 | ||
| Micronutrient survey, 2016 (151) | Nepal | Children | 6–59 mo | 659 | MRDR | 0.013 ± 0.028 | 4 |
| Women | 15–49 y | 529 | MRDR | 0.010 ± 0.039 | 3 | ||
| Micronutrient survey, 2013 (152) | Guatemala | Children | 12–59 mo | 54 | MRDR | — | 17 |
| Women | 15–49 y | 69 | MRDR | — | 0 | ||
| Micronutrient survey, 2016 (153) | Guatemala | Children | 6–59 mo | 45 | MRDR | 0.04 | 16 |
| Women | 15–49 y | 88 | MRDR | 0.02 | 2.3 | ||
| Micronutrient survey, 2015–2016 (119) | Malawi | Preschool children | 6–59 mo | 76 | MRDR | 0.018 ± 0.001 | 0 |
| School-aged children | 6–14 y | 85 | MRDR | 0.011 ± 0.001 | 0 | ||
| Women | 15–49 y | 96 | MRDR | 0.010 ± 0.001 | 0 | ||
| Molla, 1983 (154) | Bangladesh | Children with acute diarrhea | 5.9 ± 2.2 y | 13 | RDR | 69 ± 7% | — |
| Woodruff, 1987 (14) | USA | Preterm infants | GA 31.5 ± 2.6 wk | 83 | RDR | 28%3 [0–60%] | — |
| Gadomski, 1989 (47) | Guatemala | Children | 3.9 ± 0.4 y | 235 | RDR | — | 8 |
| Shenai, 1990 (19) | USA | Preterm infants without BPD at birth | GA 27 ± 2 wk | 12 | i.m.-RBP-RDR | 51 ± 24% | — |
| Preterm infants without BPD at 28 d | 12 | i.m.-RBP-RDR | 3 ± 3% | — | |||
| Preterm infants with BPD at birth | GA 27 ± 2 wk | 12 | i.m.-RBP-RDR | 70 ± 46% | — | ||
| Preterm infants with BPD at 28 d | 12 | i.m.-RBP-RDR | 13 ± 10% | — | |||
| Flores, 1991 (45) | Brazil | Children | 2–6 y | 243 | RDR | — | 40 |
| Usha, 1991 (155) | India | Children with persistent diarrhea and abnormal CIC | 5–15 mo | 23 | RDR | 88 ± 14% | 100 |
| Children with persistent diarrhea and normal CIC | 5–15 mo | 6 | RDR | 16 ± 12% | 66 | ||
| Landman, 1992 (17) | USA | Preterm infants given VA by i.m. injection daily | 32 d | 10 | i.m.-RDR | 9 ± 16% | — |
| Preterm infants given VA enterally daily | 32 d | 9 | i.m.-RDR | 8 ± 11% | — | ||
| Suharno, 1992 (68) | Indonesia | Pregnant women | 20–35 y | 45 | RDR | 2.5 ± 13% | 9 |
| Duitsman, 1993, 1995 (67, 156) | USA | Pregnant women | 15–37 y | 10 | MRDR | 0.010 ± 0.004 | 0 |
| Flores, 1994 (46) | Brazil | Children | 11–77 mo | 83 | RDR | — | 58 |
| Sovani, 1994 (50) | Indonesia | Children | 13–59 mo | 114 | RDR | — | 29 |
| Rahman, 1995 (49) | Bangladesh | Children with weight-for-age <90%4 | 5–35 mo | 34 | RDR | — | 68 |
| Shenai, 1995 (20) | USA | Preterm infants without BPD at birth | GA 27 ± 2 wk | 8 | i.m.-RBP-RDR | 9 ± 8%5 | — |
| Preterm infants without BPD at 42 d | 8 | i.m.-RBP-RDR | 0 ± 3%5 | — | |||
| Preterm infants with BPD at birth | GA 27 ± 2 wk | 12 | i.m.-RBP-RDR | 13 ± 10%5 | — | ||
| Preterm infants with BPD at 42 d | 12 | i.m.-RBP-RDR | 42 ± 42%5 | — | |||
| Donnen, 1996 (52) | Zaire (now Democratic Republic of Congo) | Children | 33.3 ± 19.5 mo | 79 | RDR | — | 7.6 |
| Kafwembe, 1996 (157) | Zambia | Children | 7–29 mo | 87 | MRDR | — | 78 |
| Makdani, 1996 (48) | Belize | Children | 25–115 mo | 503 | RDR | 6%3 [−20–60%]5 | 176 |
| Paiva, 1996 (158) | Brazil | Male smokers and nonsmokers | 43–74 y | 36 | RDR | 1.8%7 [0–8.7%] | 0 |
| Spannaus-Martin, 1997 (59) | USA | Children | 0.4–6 y | 77 | MRDR | 0.025 ± 0.012 | 0 |
| Fazio-Tirrozzo, 1998 (159) | Malawi | Girls | 10–19 y | 112 | MRDR | — | 60 |
| Silveira, 1999 (160) | Brazil | Adults with HIV/AIDS | 33 ± 9 y | 14 | RDR | — | 28 |
| Farbos, 2000 (53) | Mali | Children | 4–6 y | 228 | MRDR | — | 70 |
| Kassaye, 2001 (55) | Ethiopia | Children | 7.8 ± 0.9 y | 824 | MRDR | 0.05 ± 0.06 | 41 |
| Ncube, 2001 (161) | Zimbabwe | Lactating women | 27 ± 7 y | 43 | RDR | 41 ± 23% | 76 |
| Reyes, 2002 (56) | Mexico | Children | 0.1–5 y | 422 | RDR | — | 42 |
| Schemann, 2002 (58) | Mali | Children | 0.5–6 y | 192 | MRDR | — | 77 |
| De Abreu, 2005 (51) | Brazil | Malnourished children | <10 y | 123 | RDR | — | 11 |
| Healthy children | <10 y | 98 | RDR | — | 2 | ||
| Weinman, 2007 (121) | Brazil | Preterm infants | 28 d | 92 | i.m.-RDR | — | 51 |
| Maciel, 2008 (162) | Brazil | Children with active VL | 8.9 ± 3.8 y | 20 | MRDR | 0.036 ± 0.030 | 15 |
| Children with a history of VL | 32 | MRDR | 0.022 ± 0.018 | 6.3 | |||
| Children with asymptomatic infection | 39 | MRDR | 0.021 ± 0.016 | 5.1 | |||
| Children with no history of VL | 34 | MRDR | 0.019 ± 0.019 | 1.9 | |||
| Custodio, 2009 (163) | Brazil | Children | 5.5–11 y | 103 | RDR | — | 20 |
| Kafwembe, 2009 (54) | Zambia | Children | 0.5–5 y | 353 | MRDR | — | 6.8 |
| de Paula, 2010 (164) | Brazil | Adults with cirrhosis | 53 ± 10 y | 58 | RDR | — | 34 |
| Samba, 2010 (57) | Republic of Congo | Children | 0.5–6 y | 158 | MRDR | — | 30 |
| Fujita, 2011, 2017 (165, 166) | Kenya | Lactating women | 28 ± 7 y | 192 | RDR | — | 176 |
| Hotz, 2012 (167) | Zambia | Children | 2–5 y | 232 | MRDR | 0.051 ± 0.097 | 22 |
| Amaral, 2013 (168) | Brazil | Children with upper respiratory infection | 59 ± 1.6 mo | 69 | MRDR | 0.066 ± 0.045 | 58 |
| Children with upper respiratory infection and wheezing | 46 | MRDR | 0.021 ± 0.021 | 2.2 | |||
| Control children | 39 | MRDR | 0.007 ± 0.006 | 0 | |||
| Arantes Ferreira Peres, 2013 (169) | Brazil | Adults with CLD | 55 ± 9 y | 144 | RDR | — | 34 |
| Samba, 2013 (66) | Republic of Congo | Pregnant or lactating women | — | 82 | MRDR | — | 87 |
| Chaves, 2015 (115) | Brazil | Adults with CLD | 30–81 y | 178 | RDR | — | 50 |
| Soares-Mota, 2015 (170) | Brazil | Adults with Crohn's disease | 35 ± 13 y | 28 | RDR | — | 37 |
| Healthy adults | 33 | RDR | — | 12 |
VAD defined as RDR ≥20% or MRDR ≥0.060. BPD, bronchopulmonary dysplasia; CIC, conjunctival impression cytology; CLD, chronic liver disease; GA, gestational age; MRDR, modified relative dose-response; RBP, retinol-binding protein; RDR, relative dose-response; VA, vitamin A; VAD, vitamin A deficiency; VL, visceral leishmaniasis; —, missing data.
Data reported as mean ± SD (unless noted otherwise).
Reported as median [range].
Defined by the National Center for Health Statistics (148).
Estimated from figure in source.
RDR cutoff for deficiency was >0.14 in this study.
Reported as mean [range].
Discussion
Liver biopsy and RID demonstrate that RDRs are useful biomarkers of group VAD
When analyzing the totality of reported RDR values and gold-standard VA status comparisons in reference to TLRs or RID, both RDR tests demonstrate a clear relation between VA status and response. In the MRDR plot, no subject/animal with a TLR >0.3 μmol VA/g liver indicated a false positive, although little is known about the response of MRDR during hypervitaminosis or toxicity. Furthermore, most false positives and false negatives are in a small region of TLR (∼0.05–0.15 μmol VA/g liver), which lends support to the use of MRDR as a population indicator. To illustrate this point, if a country's survey reports an area with 20–50% positive MRDR tests, even if most of the tests happened to be false positives between 0.10 and 0.15 (unlikely), one could assume that the population VA status is deficient because the “optimal” range is 0.4–0.7 μmol/g liver (1), and interventions to raise VA status are needed. Conversely, if only a small proportion (<5%) had elevated MRDR values, it could be determined with some certainty (given the exclusion of other factors that may interfere with the test) that the population is mostly composed of VA-adequate individuals and VA interventions are working or not needed. The sensitivity and specificity values reported above were calculated using the cutoff for deficiency at TLR ≤0.10 μmol/g recommended by experts in 2016 (1). The Institute of Medicine's 2001 minimally acceptable cutoff of 0.07 μmol/g improves sensitivity for RDR to 74% (78/106) and MRDR to 87% (97/112) with specificities of 85% (120/142) and 60% (37/62). The higher sensitivity is because of the region of uncertainty between 0.05 and 0.15 μmol/g, which is likely affected by individual variation in requirements and response. Finally, it is important to note that the individual response is not absolutely correlated with VA status except for extreme responses during severe VAD and lack of response in predominantly adequate groups; therefore, RDRs are generally not appropriate for clinical diagnosis resolved at the individual level in areas with a high level of VAD.
Interventions with large VA intakes provide evidence for the usefulness of RDR tests
Biomarkers are useful for defining the true VA status of a group, eliminating the need for invasive procedures like biopsy, but this strength means that most human studies using the RDRs assume accuracy. In order to evaluate the evidence for the MRDR beyond the gold-standard studies above, we included intervention studies and evaluated whether a change in VA status distribution should be expected based on the daily VA intake, and determined if this was reflected by the test. In the absence of a highly controlled or monitored diet with precisely known VA content in the majority of studies, we calculated the supplemental VA given, either as daily intake or the total amount of VA given divided by the period between RDR tests, and compared it with the Estimated Average Requirement for the study group. This approach is limited by the contribution of diet; however, by comparing whether a change was seen in the control group during the same period, it is possible to evaluate the test given these other factors.
Two conclusions emerge from these results. First, no change was observed among studies in subjects who start with a low prevalence of VAD [0–10% (4, 27, 71, 74, 137)] and children who had recently received a high-dose supplement (36) unless the study was long enough to allow low-/zero-VA intake groups to become deficient if the intervention replaces rather than augments a normal diet (142). The inability to affect low prevalence even with very high doses of VA likely indicates these few tests are false positives as seen in category 1 or reflect some other issue like tracer malabsorption or RBP deficiency. Second, VA interventions in the remaining studies almost categorically resulted in a decrease in the prevalence of positive RDR tests (4–8, 11, 12, 22, 25, 28–31, 33, 34, 36–38, 63, 64, 73, 99, 125, 138, 141). Note that Rahman et al. (5) and Stephensen et al. (34) saw trending large decreases in RDR in intervention versus placebo (P = 0.06 and 0.08, respectively). Among the groups that did not see a change, one repeated the test only 4–10 d after the first test (32) and other tests were performed in low-birth-weight infants in their first few weeks of life (discussed in more detail below) (9, 15). In both cases, this may not have been long enough for RBP to differentially accumulate among groups. Furthermore, Stephensen et al. (34) performed RDR 2–5 d following administration of a large VA dose, which should have depleted RBP, and the prevalence of deficiency was greatly decreased in the treatment group. This result is ambiguous because it is possible that they depleted accumulated hepatic RBP in this group and prevented a response. It has been recommended to wait 2–4 wk following a test or large VA dose before performing another test (29) and to avoid the test in the neonatal period (113, 114).
Another study did not see changes in MRDR with VA supplementation unless iron was supplemented (discussed below) (70). Similarly, Dougherty et al. (39) did not see an improvement in RDR in children with sickle cell anemia, which may indicate an iron component. Two more studies with no improvement used provitamin A carotenoids (6, 10), which have variable bioefficacy based on genetic and dietary factors as well as VA status (171, 172). Rice et al. (6) observed a change at a later time point and other studies using provitamin A carotenoids did see changes [e.g., (30, 64)]. Finally, a single study did not observe improvement in MRDR with supplementation (35): only 1 of 13 children dropped below the threshold for deficiency following a dose equivalent to 1540 μg RAE/d over 39 days. Likely, these children were extremely VA deficient and not able to build stores during the intervention. In summary, in studies that started with a significant prevalence of VAD, nearly all demonstrated an improvement in dose-response when supplemented with VA, lending support to RDRs as biomarkers of VA status.
RDRs are useful throughout most life stages and pathologies but may be affected by CLD and iron and protein nutrition
Disease and inflammation affect VA metabolism, which may affect RDRs. Several explanations exist for an increase, decrease, or lack of change. In the absence of gold-standard TLR, reliable determinations of the effect of infection or nutritional deficiency (other than VA) on RDRs must have examined a change in values before and after correction of a disease or deficiency while monitoring VA intake, or examine the interaction of physiological/pathological conditions with the response of the tests to VA intervention.
Chronic liver disease
CLD has been theorized to affect RDR tests by impacting RBP synthesis and affecting fat absorption and fat-soluble retinoid uptake in the intestine (affecting both underlying VA status and dose-response tests) (101). An appropriate decrease in RDR to interventions was seen in RDR-positive subjects with CLD (99), meaning that RBP synthesis is not prevented during CLD. Among 3 studies, RDR was not sensitive to deficient TLR (101), intramuscular-RDR was sensitive to deficient TLR (22), and in the third study subjects were not deficient (126), limiting information about sensitivity. Oral dose-response tests are not well supported during CLD, but intramuscular-RDR will bypass malabsorption in choleostatic CLD (22, 43). Furthermore, 1 issue in the Russell et al. study (101) was false positives—that is, more RBP released than expected, which would indicate that insufficient RBP is not the issue and VA metabolism is impacted.
Protein-energy malnutrition and anorexia
Large oral or intramuscular R doses yielded smaller RBP releases in low-protein diets compared with control in rats (173), and in human protein malnutrition, with or without energy malnutrition, than in energy malnutrition alone (174), indicating decreased RBP production or accumulation. This decreased RBP, however, was shown both in circulating and postdose RBP (174), and the percentage change in the RDR value was ∼250% in all groups, indicating that the RDRs could still be functional. Interestingly, the rat studies demonstrated a difference in peak RBP concentration postdose not only by protein content but also protein quality (amino acid makeup) when comparing soybean or rice protein. A similar rat study comparing rice with or without VA fortification and VA-free casein as dietary protein confirmed that, even when incomplete protein causes decreased growth and lowers the absolute rise in retinol following an oral VA dose, the RDR can discriminate between the VA-fortified group and controls (128). It should be noted that the VA-supplemented group had a mean RDR value of 33% despite no deficiency, which could mean that the absolute RDR cutoff might be affected in protein malnutrition.
VA interventions in subjects with low weight-for-age (i.e., energy malnutrition leading to stunting) led to reductions in positive RDR tests (25, 29); however, instances of nonresponse to intervention (32) or poor correlation between RDR and MRDR (29, 41) have also occurred, potentially with a dose-size interaction (29) or the time since last test or large VA dose (29, 41). Protein malnutrition may lengthen the time required to regenerate RBP or the amount of retinyl ester required to stimulate a response, in addition to limiting its concentration. Additional care is needed when applying RDR tests to malnourished individuals by using larger doses and avoiding situations where RBP might have been recently depleted by another test or VA dose.
Body weight and obesity
Other than undernutrition, the influence of body weight on the RDRs has not been rigorously explored, especially in overweight and obese individuals. Santana et al. (126) compared RDR with liver biopsy in CLD with a group of whom 49% were overweight or obese, but there were no deficient patients or positive RDR tests. Large adipose stores could interact with the tracer as a sink or storage site (175, 176), produce apo-RBP (118), or signal for increased hepatic RBP production (177). Some research in large (∼300 kg) lactating sows indicated that their serum DR kinetics are similar to lactating humans, although the exact TLR that correlated with positive MRDR may not be identical. Therefore, further human research in overweight/obese populations is needed to characterize parameters of response during obesity given the continued worldwide increase in prevalence.
Zinc and iron deficiency
Iron and zinc interact bidirectionally with VA status through multiple pathways, including VA and mineral absorption, RBP synthesis, cellular differentiation, signaling pathways, and the immune system (178–180). In several studies, however, RDRs were unchanged by zinc supplementation (10, 39). There does appear to be an iron component: VA alone did not improve MRDR status in pregnant women but a combination of VA and iron did (70). Another study did not demonstrate an effect of VA on MRDR in mostly anemic pregnant women, but the initial prevalence of positive MRDR tests was low (<5%) (71). Similarly, β-carotene did not improve MRDR but iron did (10). The RDR test was also used to investigate sickle cell disease response to VA and zinc interventions in children (39). RDR was low before and after the intervention, which could either mean that VA status was adequate or that sickle cell anemia, potentially through disrupted iron status, impairs the function of the RDR test.
Age
Neonates
Work performed in calves demonstrated that the RDR test administered shortly after birth reflects recent VA intake but not TLR (113, 114); however, by 28 d the RDR reflected TLR. RBP may not accumulate quickly in the livers of neonates according to VA status. Multiple intramuscular-VA intervention and case/control studies in low-birth-weight preterm infants (variably defined as infants born weighing <1000, 1200, 1300, or 1500 g), tested in the first month of life by intramuscular-RDR or intramuscular-RBP-RDR have been performed, with varying results. The use of intramuscular technique, with variable RDR times (2–5 h) is because these infants are typically fed parenterally, and intramuscular-RDR may peak earlier (3 h) (174) because it avoids the lag associated with intestinal uptake and secretion. Furthermore, preterm birth is often associated with complex health issues. VA status was investigated with oral- and intramuscular-RDR and intramuscular-RBP-RDR tests during BPD, which is treated with intramuscular VA. These studies compared RDR the day after birth with that at 28 d. Data in calves suggested that the initial RDR was not accurate; therefore, later time points might be more appropriate, representing a gap in validation in infants to determine the usefulness of early RDR tests. Regardless, the RDR test has demonstrated differences in infants with and without BPD or various methods of feeding or management (17, 19–21). These used the same quantity of VA in all groups, so results should be interpreted with caution. A cutoff value of 10% derived from control groups in 2 studies (7, 19) has been maintained consistently in the RDR in low-birth-weight infant literature [e.g., (9, 15)]. Tyson et al. (7) provided differing amounts of VA and demonstrated that the RDR value as well as the prevalence of neonates with intramuscular-RDR >10% responded appropriately to VA intervention by day 28. Ambalavanan et al. (9) performed a similar study with different VA dosing regimens but did not see a difference in intramuscular-RDR value or positive prevalence between the high-dose group and standard regimen by day 28. Finally, Mactier et al. (15) performed a large VA intervention in neonates and did not see a response between intervention and control. Unfortunately, among the studies available with the RDR test, some supplemented different quantities of VA while others examined BPD against non-BPD controls, but no study utilized a 2 × 2 factorial approach. In summary, these studies did not maintain consistent methodology.
Infants and children
Studies in infants tend to focus on milk transfer of VA and are covered below. Many of the studies in categories 1 and 2 and their respective tables were performed in children because they are vulnerable to VAD (181); therefore, the utility of the RDR tests in children is well documented.
Elderly
In 2 VA interventions in older adults, the RDR test responded to intervention in VA-deficient subjects (73), but not when the population was VA sufficient (mean RDR of −16.9% ± 10.1%) (74). In that study, comparison with TLR determined by RID demonstrated that all were VA sufficient so the 1 positive RDR value (of 26 subjects) was likely false.
Maternal/infant concerns
Pregnancy
Pregnant women are assumed to have a slightly higher Estimated Average Requirement (50 μg RAE/d) to contribute VA to the fetus but otherwise have requirements similar to nonpregnant women (103); however, this does not mean that VA (or DR) metabolism, other than utilization, is not impacted. Two studies used MRDR to assess VA interventions in pregnancy and demonstrated an interaction with iron status (70, 71), which is known to worsen during pregnancy but is difficult to measure because of hemodilution (182). Alongside iron supplementation, however, VA did cause MRDR to improve and in the other study, VAD was low, so iron may be a relevant effector in pregnancy.
Lactation
VA metabolism during lactation, evaluated in genetic knockouts (183) and tracer studies (124, 184), appears to be a complex and dynamic balance of chylomicron- and RBP-delivered VA secretion into milk. The loss of R or DR dose to milk, which is estimated to be anywhere from 10% to 40% (185), and potentially altered RBP secretion, must be considered along with the potential for lactation to cause true positives due to sacrificing maternal VA for the infant.
Repeated MRDR tests did not change appreciably over 0.5–3.25 mo in Indonesian lactating women (106), although the MRDR value was higher in lactating women from lower socioeconomic status compared with higher-educated nonlactating women over a time-course study (106). Similarly, among lactating women with low initial prevalence of positive RDRs (≤10%), there was little change over 3 or 6 mo regardless of whether or not VA was supplemented (4). Lactating women with significant amounts of deficiency at baseline had decreases in positive RDR or MRDR prevalence with VA or β-carotene supplementation but not placebo (6, 64, 138) or increases in positive MRDR with placebo and no change in VA intervention (125), which is strong evidence that the RDRs remain effective in lactation. Two studies in lactating sows consuming VA-deficient feed demonstrated that 3 parities with low VA intake led to positive MRDR values; however, these sows had a low mean TLR of 0.2 μmol/g (123, 124). Whether the issue was that lactation leads to a different TLR cutoff for deficiency to provide VA to milk, that lactation caused false positives, or that swine have a different TLR cutoff in general, remains unclear.
Breastfeeding
Infants are born with low VA stores and must receive VA from milk or formula (186). Currently, WHO guidance suggests that maternal postpartum VA supplementation is not recommended to improve infant mortality (187); however, prior RDR studies have included VA supplementation to the mother, the infant, or both. Most studies do not quantify the exact amount of VA transferred to the child, which is not easy without using a tracer. Agne-Djigo et al. (13) used a deuterated water technique called “dose-to-mother,” which measures milk volume consumption (143), and multiplied consumption by a static milk VA concentration to estimate VA delivered from milk from mothers given 400,000 IU VA or placebo. By this technique, infant VA intake was similar (389 ± 151 μg RAE/d in the maternal placebo group and 365 ± 125 μg RAE/d in the intervention group) but MRDR value and prevalence of positives was significantly decreased in the infants (95% positive in the placebo group, 54% positive in the intervention group), indicating that milk VA transfer is a dynamic system. In swine with litters of 7–12 piglets, 22% of a 35-μmol α-retinol dose (a tracer representing chylomicron delivery of VA because it cannot bind RBP) was distributed to offspring, ∼2% per piglet (184). Translated to the infant study, and given the 400,000 IU (120,000 μg) dose, a mean infant body weight of 7.3 kg, and an assumed liver fraction of body weight of 4%, 2% of the dose transferred would add a minimum of 8 μmol, or 0.03 μmol VA/g liver if all dose was stored, which is significant but still an underestimation since this does not include secondary transfer of the dose to milk by RBP in the mother, or account for the single child compared with a litter of piglets.
In studies that did not quantify dose transfer to the infant, response to maternal VA supplementation was mixed, with some studies showing an effect (4, 6), others showing only infant supplementation was effective (11), and some did not separate the 2 in a multifactorial approach (8, 12). However, the data, especially when evaluating infant supplementation where effects on VA status are more likely, indicate that RDR and MRDR are properly responding to changes in VA status.
Inflammation, infection, and the acute phase response
RBP is a negative acute phase protein, decreasing in times of infection and inflammation (188). Infection can deplete VA stores, and VA-deficient individuals are more susceptible to infection than individuals with optimal status (189). The MRDR test, which is a ratio of DR to R bound to circulating RBP, would be affected by the same factor in both the numerator and denominator, theoretically remaining unchanged, whereas the RDR test has a higher likelihood of an inflammation effect given 2 different sampling times.
To interpret studies examining inflammation, infection, and acute phase response markers, we must define what is expected if the test is affected or unaffected by these conditions. In the absence of TLR measurements, the best study design evaluates the same subjects in a short time period with and without infection, either by curing the infection or having measurements before it was contracted; if the RDRs are the same with and without the disease, it could be said to be robust to infection. If the time period is too long, however, VA stores may change in response to the infection. There were 4 studies with this general design. First, Tanumihardjo et al. specifically designed a study to answer this question in children diagnosed with Ascaris lumbricoides infections with a short intervention and intertest period (3–4 wk, long enough to re-accumulate RBP). The study used different combinations and timings of VA intervention and albendazole, a deworming agent, and demonstrated no difference in MRDR due to deworming, but showed the expected decrease in MRDR values following VA supplementation (31, 36).
Stephensen et al. (34) performed the RDR test in children discharged from the hospital after pneumonia treatment given either VA intervention or placebo. The test was only performed 3 d after the VA dose so there is some concern about RBP accumulation time, especially because supplementation would differentially deplete accumulated RBP among dosed subjects. The investigators determined that the RDR value responded to the VA intervention in children with low C-reactive protein (CRP), a marker of acute phase response, but not in children with high CRP. They concluded that acute phase response caused ∼20% of false positives in the VA-supplemented group who should theoretically all be VA sufficient given their recent high dose, as was seen in the CRP-negative, VA-supplemented subjects (16/17 subjects RDR-negative); however, it could also be an effect of inflammation on the retention of the high-dose supplement. Furthermore, among placebo subjects who did not have RBP depleted by the supplement, the high-CRP group had fewer positive RDR subjects than the low-CRP group, which could indicate false negatives due to inflammation (or VA sufficiency, but inflammation is normally expected to lead to decreased VA stores). Overall, this study could indicate that the RDR test is less sensitive to VAD during the acute phase response but requires a longer time before RDR testing after a large dose to allow RBP accumulation.
Campos et al. (136) unexpectedly encountered chickenpox 120 d into a study with periodic RDR tests following a large VA dose. There was a large increase in positive tests at 180 d in infected (74%) versus uninfected children (10%). This change could be attributed either to a chickenpox-mediated decrease in TLR, which is possible because the infection and increase in RDR occurred over 60 d between 120 and 180 d, or to an acute phase–induced increase in false positives. Similarly, Astiazaran-Garcia et al. (44) performed the MRDR test in children infected with Giardia lamblia before and 6 mo after pharmacological treatment. They noted a decrease in both MRDR values and Giardia at 6 mo, which again could be due to a recovery in VA stores following infection, or a decrease in acute phase–induced false positives; however, Tanumihardjo et al. (31) indicated that, in the short-term, false positives were not an issue because the MRDR does not change simply by removing the infection.
Other studies, which were otherwise excluded from this review because they were observational, have not shown an association of RDRs with acute phase proteins, such as CRP and α1-glycoprotein (AGP) (37, 165, 142, 144, 146, 163, 167).
Other factors
Amatayakul et al. (137) used the RDR test to investigate interactions and the effect of oral contraceptives on VA status. Only 1 individual was RDR positive, which was negative after VA intervention, indicating that the RDR is responsive despite oral contraceptive use.
Mutations in RBP made the RDR test ineffective in humans (145), because RBP could not carry incoming R into the blood. Based on RBP-null mice (190), these subjects likely had elevated liver VA and would be healthy through chylomicron-delivered VA with regular consumption. The exception to this, observed in knockout mice and reproduced in these humans, are ocular symptoms such as night blindness, because the eye is partially reliant on STRA6 (stimulated by retinoic acid 6)-mediated uptake of retinol from RBP for vision (191, 192).
Conclusions and Future Directions
The RDRs, used in a variety of studies, are recognized as reliable measures of population VA status, which is reflected in WHO guidelines suggesting the use of the MRDR (116). The MRDR is a more accessible test than the RDR and is less prone to analysis error. For this reason, the number of micronutrient surveys associated with DHSs using the MRDR in a subset of individuals also being measured for serum R or RBP is increasing, with surveys in Burkina Faso, Guatemala, and Uganda underway. This review provides a valuable reference for investigators seeking to improve their VAD prevalence estimates in surveys. Future studies on the MRDR test should perform the RID test to provide human gold-standard evidence for the MRDR in addition to the large body of liver biopsy and RID evidence already supporting RDRs. Other modifications to the test, such as measuring an early-time-point stable-isotope RDR during the first 5 h of the 14-d RID (193), could provide feedback about population VAD before full results are available. The breast-milk MRDR also provides a promising noninvasive method of VA status assessment in women vulnerable to VAD (65, 112, 125).
Acknowledgments
The authors’ responsibilities were as follows—JS: was responsible for the review design; JS and SAT: were responsible for writing and final content; both authors read and approved the final manuscript.
Notes
The Global Health Institute provided funds during the latter part of the conduct of this systematic review, which was registered with PROSPERO (#CRD42019124180).
Author disclosures: The authors reported no conflicts of interest.
Abbreviations used: BPD, bronchopulmonary dysplasia; CLD, chronic liver disease; CRP, C-reactive protein; DR, 3,4-didehydroretinol; DRAc, 3,4-didehydroretinyl acetate; DR:R, molar ratio of 3,4-didehydroretinol to retinol; GA, gestational age; MRDR, modified-relative dose response; R, retinol; RAE, retinol activity equivalents; RBP, retinol-binding protein; RDR, relative dose-response; RID, retinol isotope dilution; TBS, total body store; TLR, total liver vitamin A reserve; VA, vitamin A; VAD, vitamin A deficiency.
Contributor Information
Jesse Sheftel, Interdepartmental Graduate Program in Nutritional Sciences, University of Wisconsin-Madison, Madison, WI, USA.
Sherry A Tanumihardjo, Interdepartmental Graduate Program in Nutritional Sciences, University of Wisconsin-Madison, Madison, WI, USA.
References
- 1. Tanumihardjo SA, Russell RM, Stephensen CB, Gannon BM, Craft NE, Haskell MJ, Lietz G, Schulze K, Raiten DJ. Biomarkers of Nutrition for Development (BOND)—vitamin A review. J Nutr. 2016;146:1816S–48S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tanumihardjo SA, Kaliwile C, Boy E, Dhansay MA, van Stuijvenberg ME. Overlapping vitamin A interventions in the United States, Guatemala, Zambia, and South Africa: case studies. Ann NY Acad Sci. 2019;1446:102–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sheftel J, Loechl C, Mokhtar N, Tanumihardjo SA. Use of stable isotopes to evaluate bioefficacy of provitamin A carotenoids, vitamin A status, and bioavailability of iron and zinc. Adv Nutr. 2018;9:625–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Stoltzfus RJ, Hakimi M, Miller KW, Rasmussen KM, Dawiesah S, Habicht JP, Dibley MJ. High dose vitamin A supplementation of breast-feeding Indonesian mothers: effects on the vitamin A status of mother and infant. J Nutr. 1993;123:666–75. [DOI] [PubMed] [Google Scholar]
- 5. Rahman MM, Mahalanabis D, Alvarez JO, Wahed MA, Islam MA, Habte D, Khaled MA. Acute respiratory infections prevent improvement of vitamin A status in young infants supplemented with vitamin A. J Nutr. 1996;126:628–33. [DOI] [PubMed] [Google Scholar]
- 6. Rice AL, Stoltzfus RJ, de Francisco A, Chakraborty J, Kjolhede CL, Wahed MA. Maternal vitamin A or beta-carotene supplementation in lactating Bangladeshi women benefits mothers and infants but does not prevent subclinical deficiency. J Nutr. 1999;129:356–65. [DOI] [PubMed] [Google Scholar]
- 7. Tyson JE, Wright LL, Oh W, Kennedy KA, Mele L, Ehrenkranz RA, Stoll BJ, Lemons JA, Stevenson DK, Bauer CRet al. Vitamin A supplementation for extremely-low-birth-weight infants. N Engl J Med. 1999;340. [DOI] [PubMed] [Google Scholar]
- 8. Bahl R, Bhandari N, Wahed MA, Kumar GT, Bhan MK; WHO/CHD Immunization-Linked Vitamin A Group . Vitamin A supplementation of women postpartum and of their infants at immunization alters breast milk retinol and infant vitamin A status. J Nutr. 2002;132:3243–8. [DOI] [PubMed] [Google Scholar]
- 9. Ambalavanan N, Wu TJ, Tyson JE, Kennedy KA, Roane C, Carlo WA. A comparison of three vitamin A dosing regimens in extremely-low-birth-weight infants. J Pediatr. 2003;142:656–61. [DOI] [PubMed] [Google Scholar]
- 10. Wieringa FT, Dijkhuizen MA, West CE, Thurnham DI, Muhilal, van der Meer JW. Redistribution of vitamin A after iron supplementation in Indonesian infants. Am J Clin Nutr. 2003;77:651–7. [DOI] [PubMed] [Google Scholar]
- 11. Ayah RA, Mwaniki DL, Magnussen P, Tedstone AE, Marshall T, Alusala D, Luoba A, Kaestel P, Michaelsen KF, Friis H. The effects of maternal and infant vitamin A supplementation on vitamin A status: a randomised trial in Kenya. Br J Nutr. 2007;98:422–30. [DOI] [PubMed] [Google Scholar]
- 12. Idindili B, Masanja H, Urassa H, Bunini W, van Jaarsveld P, Aponte JJ, Kahigwa E, Mshinda H, Ross D, Schellenberg DM. Randomized controlled safety and efficacy trial of 2 vitamin A supplementation schedules in Tanzanian infants. Am J Clin Nutr. 2007;85:1312–9. [DOI] [PubMed] [Google Scholar]
- 13. Agne-Djigo A, Idohou-Dossou N, Kwadjode KM, Tanumihardjo SA, Wade S. High prevalence of vitamin A deficiency is detected by the modified relative dose-response test in six-month-old Senegalese breast-fed infants. J Nutr. 2012;142:1991–6. [DOI] [PubMed] [Google Scholar]
- 14. Woodruff CW, Latham CB, Mactier H, Hewett JE. Vitamin A status of preterm infants: correlation between plasma retinol concentration and retinol dose response. Am J Clin Nutr. 1987;46:985–8. [DOI] [PubMed] [Google Scholar]
- 15. Mactier H, McCulloch DL, Hamilton R, Galloway P, Bradnam MS, Young D, Lavy T, Farrell L, Weaver LT. Vitamin A supplementation improves retinal function in infants at risk of retinopathy of prematurity. J Pediatr. 2012;160:954. [DOI] [PubMed] [Google Scholar]
- 16. Schmiedchen B, Longardt AC, Buhrer C, Raila J, Loui A, Schweigert FJ. The relative dose response test based on retinol-binding protein 4 is not suitable to assess vitamin A status in very low birth weight infants. Neonatology. 2014;105:155–60. [DOI] [PubMed] [Google Scholar]
- 17. Landman J, Sive A, Heese HD, Van der Elst C, Sacks R. Comparison of enteral and intramuscular vitamin A supplementation in preterm infants. Early Hum Dev. 1992;30:163–70. [DOI] [PubMed] [Google Scholar]
- 18. Khaled MA, Wahed MA, Alvarez JO, Rahman MM, Mahalanabis D, Habte D. Vitamin A status in post supplemented 1-year-old infants using the relative dose-response (RDR) test [abstract]. FASEB J. 1995;9:A2661. [DOI] [PubMed] [Google Scholar]
- 19. Shenai JP, Rush MG, Stahlman MT, Chytil F. Plasma retinol-binding protein response to vitamin A administration in infants susceptible to bronchopulmonary dysplasia. J Pediatr. 1990;116:607–14. [DOI] [PubMed] [Google Scholar]
- 20. Shenai JP, Rush MG, Parker RA, Chytil F. Sequential evaluation of plasma retinol-binding protein response to vitamin A administration in very-low-birth-weight neonates. Biochem Mol Med. 1995;54:67–74. [DOI] [PubMed] [Google Scholar]
- 21. Zachman RD, Samuels DP, Brand JM, Winston JF, Pi JT. Use of the intramuscular relative-dose-response test to predict bronchopulmonary dysplasia in premature infants. Am J Clin Nutr. 1996;63:123–9. [DOI] [PubMed] [Google Scholar]
- 22. Amedee-Manesme O, Mourey MS, Hanck A, Therasse J. Vitamin A relative dose response test: validation by intravenous injection in children with liver disease. Am J Clin Nutr. 1987;46:286–9. [DOI] [PubMed] [Google Scholar]
- 23. Amedee-Manesme O, Luzeau R, Wittepen JR, Hanck A, Sommer A. Impression cytology detects subclinical vitamin A deficiency. Am J Clin Nutr. 1988;47:875–8. [DOI] [PubMed] [Google Scholar]
- 24. Newton S, Owusu-Agyei S, Asante KP, Amoaful E, Mahama E, Tchum SK, Ali M, Adjei K, Davis CR, Tanumihardjo SA. Vitamin A status and body pool size of infants before and after consuming fortified home-based complementary foods. Arch Public Health. 2016;74:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Flores H, Campos F, Araujo RC, Underwood BA. Assessment of marginal vitamin A deficiency in Brazilian children using the relative dose response procedure. Am J Clin Nutr. 1984;40:1281–9. [DOI] [PubMed] [Google Scholar]
- 26. Tanumihardjo SA, Koellner PG, Olson JA. The modified relative-dose-response assay as an indicator of vitamin A status in a population of well-nourished American children. Am J Clin Nutr. 1990;52:1064–7. [DOI] [PubMed] [Google Scholar]
- 27. Tanumihardjo SA, Muhilal, Yuniar Y, Permaesih D, Sulaiman Z, Karyadi D, Olson JA. Vitamin A status in preschool-age Indonesian children as assessed by the modified relative-dose-response assay. Am J Clin Nutr. 1990;52:1068–72. [DOI] [PubMed] [Google Scholar]
- 28. Humphrey JH, Natadisastra G, Muhilal, Friedman DS, Tielsch JM, West KP Jr, Sommer A. A 210-μmol dose of vitamin A provides more prolonged impact on vitamin A status than 105 μmol among preschool children. J Nutr. 1994;124:1172–8. [DOI] [PubMed] [Google Scholar]
- 29. Tanumihardjo SA, Permaesih D, Dahro AM, Rustan E, Muhilal, Karyadi D, Olson JA. Comparison of vitamin A status assessment techniques in children from two Indonesian villages. Am J Clin Nutr. 1994;60:136–41. [DOI] [PubMed] [Google Scholar]
- 30. Manorama R, Brahmam GN, Rukmini C. Red palm oil as a source of beta-carotene for combating vitamin A deficiency. Plant Food Hum Nutr. 1996;49:75–82. [DOI] [PubMed] [Google Scholar]
- 31. Tanumihardjo SA, Permaesih D, Muherdiyantiningsih, Rustan E, Rusmil K, Fatah AC, Wilbur S, Muhilal, Karyadi D, Olson JA. Vitamin A status of Indonesian children infected with Ascaris lumbricoides after dosing with vitamin A supplements and albendazole. J Nutr. 1996;126:451–7. [DOI] [PubMed] [Google Scholar]
- 32. Raghuramulu N, Underwood B, Bhaskaram P, Arunjyothi CS, Reddy V. Vitamin A relative dose response test in undernourished children. Nutr Res. 1998;18:533–41. [Google Scholar]
- 33. Solon FS, Klemm RD, Sanchez L, Darnton-Hill I, Craft NE, Christian P, West KP Jr.. Efficacy of a vitamin A-fortified wheat-flour bun on the vitamin A status of Filipino schoolchildren. Am J Clin Nutr. 2000;72:738–44. [DOI] [PubMed] [Google Scholar]
- 34. Stephensen CB, Franchi LM, Hernandez H, Campos M, Colarossi A, Gilman RH, Alvarez JO. Assessment of vitamin A status with the relative-dose-response test in Peruvian children recovering from pneumonia. Am J Clin Nutr. 2002;76:1351–7. [DOI] [PubMed] [Google Scholar]
- 35. Davidsson L, Adou P, Zeder C, Walczyk T, Hurrell R. The effect of retinyl palmitate added to iron-fortified maize porridge on erythrocyte incorporation of iron in African children with vitamin A deficiency. Br J Nutr. 2003;90:337–43. [DOI] [PubMed] [Google Scholar]
- 36. Tanumihardjo SA, Permaesih D, Muhilal. Vitamin A status and hemoglobin concentrations are improved in Indonesian children with vitamin A and deworming interventions. Eur J Clin Nutr. 2004;58:1223–30. [DOI] [PubMed] [Google Scholar]
- 37. van Jaarsveld PJ, Faber M, Tanumihardjo SA, Nestel P, Lombard CJ, Benade AJ. Beta-carotene-rich orange-fleshed sweet potato improves the vitamin A status of primary school children assessed with the modified-relative-dose-response test. Am J Clin Nutr. 2005;81:1080–7. [DOI] [PubMed] [Google Scholar]
- 38. Ambrosio CLB, Campos F, de Faro ZP, Flores H, Chagas MHD, de Santana RA. Flocos desidratados de abóbora na prevenção da carência de vitamina A em pré-escolares de uma creche [Dehydrated pumpkin flakes for preventing vitamin A deficiency in preschoolers attending a daycare]. Rev Nutr. 2012;25:57–64. (in Portuguese). [Google Scholar]
- 39. Dougherty KA, Schall JI, Kawchak DA, Green MH, Ohene-Frempong K, Zemel BS, Stallings VA. No improvement in suboptimal vitamin A status with a randomized, double-blind, placebo-controlled trial of vitamin A supplementation in children with sickle cell disease. Am J Clin Nutr. 2012;96:932–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bresnahan KA, Tanumihardjo SA. Undernutrition, the acute phase response to infection, and its effects on micronutrient status indicators. Adv Nutr. 2014;5:702–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wahed MA, Alvarez JO, Khaled MA, Mahalanabis D, Rahman MM, Habte D. Comparison of the modified relative dose response (MRDR) and the relative dose response (RDR) in the assessment of vitamin A status in malnourished children. Am J Clin Nutr. 1995;61:1253–6. [DOI] [PubMed] [Google Scholar]
- 42. Tanumihardjo SA, Cheng JC, Permaesih D, Muherdiyantiningsih Rustan E, Muhilal Karyadi D, Olson JA. Refinement of the modified-relative-dose-response test as a method for assessing vitamin A status in a field setting: experience with Indonesian children. Am J Clin Nutr. 1996;64:966–71. [DOI] [PubMed] [Google Scholar]
- 43. Feranchak AP, Gralla J, King R, Ramirez RO, Corkill M, Narkewicz MR, Sokol RJ. Comparison of indices of vitamin A status in children with chronic liver disease. Hepatology. 2005;42:782–92. [DOI] [PubMed] [Google Scholar]
- 44. Astiazaran-Garcia H, Lopez-Teros V, Valencia ME, Vazquez-Ortiz F, Sotelo-Cruz N, Quihui-Cota L. Giardia lamblia infection and its implications for vitamin A liver stores in school children. Ann Nutr Metab. 2010;57:228–33. [DOI] [PubMed] [Google Scholar]
- 45. Flores H, Azevedo MN, Campos FA, Barreto-Lins MC, Cavalcanti AA, Salzano AC, Varela RM, Underwood BA. Serum vitamin A distribution curve for children aged 2–6 y known to have adequate vitamin A status: a reference population. Am J Clin Nutr. 1991;54:707–11. [DOI] [PubMed] [Google Scholar]
- 46. Flores H, Guerra NB, Cavalcanti ACA, Campos F, Azevedo M, Silva MBM. Bioavailability of vitamin A in a synthetic rice premix. J Food Science. 1994;59:371–2. [Google Scholar]
- 47. Gadomski AM, Kjolhede CL, Wittpenn J, Bulux J, Rosas AR, Forman MR. Conjunctival impression cytology (CIC) to detect subclinical vitamin A deficiency: comparison of CIC with biochemical assessments. Am J Clin Nutr. 1989;49:495–500. [DOI] [PubMed] [Google Scholar]
- 48. Makdani D, Sowell AL, Nelson JD, Apgar J, Gunter EW, Hegar A, Potts W, Rao D, Wilcox A, Smith JC. Comparison of methods of assessing vitamin A status in children. J Am Coll Nutr. 1996;15:439–49. [DOI] [PubMed] [Google Scholar]
- 49. Rahman MM, Mahalanabis D, Wahed MA, Islam M, Habte D, Khaled MA, Alvarez JO. Conjunctival impression cytology fails to detect subclinical vitamin A deficiency in young children. J Nutr. 1995;125:1869–74. [DOI] [PubMed] [Google Scholar]
- 50. Sovani I, Humphrey JH, Kuntinalibronto DR, Natadisastra G, Muhilal, Tielsch JM. Response of Bitot's spots to a single oral 100,000- or 200,000-IU dose of vitamin A. Am J Ophthalmol. 1994;118:792–6. [DOI] [PubMed] [Google Scholar]
- 51. De Abreu J, Borno S, Montilla M, Dini E. Anemia y deficiencia de vitamina A en niños evaluados en un centro de atención nutricional de Caracas [Anemia and deficiency of vitamin A in children evaluated in a nutritional attention center from Caracas]. Arch Latinoam Nutr. 2005;55:226–34. (in Spanish). [PubMed] [Google Scholar]
- 52. Donnen P, Brasseur D, Dramaix M, Vertongen F, Ngoy B, Zihindula M, Hennart P. Vitamin A deficiency and protein-energy malnutrition in a sample of pre-school age children in the Kivu Province in Zaire. Eur J Clin Nutr. 1996;50:456–61. [PubMed] [Google Scholar]
- 53. Farbos S, Resnikoff S, Peyramaure F. Urbanisation and vitamin A deficiency in children: comparison between a traditional district and a new settlement in Mali. Eur J Epidemiol. 2000;16:1143–9. [DOI] [PubMed] [Google Scholar]
- 54. Kafwembe EM, Chipipa J, Njunju E, Chilengi R. The vitamin A status of Zambian children in a community of vitamin A supplementation and sugar fortification strategies as measured by the modified relative dose response (MRDR) test. Int J Vitam Nutr Res. 2009;79:40–7. [DOI] [PubMed] [Google Scholar]
- 55. Kassaye T, Receveur O, Johns T, Becklake MR. Prevalence of vitamin A deficiency in children aged 6–9 years in Wukro, northern Ethiopia. Bull World Health Organ. 2001;79:415–22. [PMC free article] [PubMed] [Google Scholar]
- 56. Reyes H, Villalpando S, Perez-Cuevas R, Rodriguez L, Perez-Cuevas M, Montalvo I, Guiscafre H. Frequency and determinants of vitamin A deficiency in children under 5 years of age with pneumonia. Arch Med Res. 2002;33:180–5. [DOI] [PubMed] [Google Scholar]
- 57. Samba C, Gourmel B, Houze P, Malvy D. Assessment of vitamin A status of preschool children in a sub-Saharan African setting: comparative advantage of modified relative-dose response test. J Health Popul Nutr. 2010;28:484–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Schemann JF, Banou AA, Guindo A, Joret V, Traore L, Malvy D. Prevalence of undernutrition and vitamin A deficiency in the Dogon Region, Mali. J Am Coll Nutr. 2002;21:381–7. [DOI] [PubMed] [Google Scholar]
- 59. Spannaus-Martin DJ, Cook LR, Tanumihardjo SA, Duitsman PK, Olson JA. Vitamin A and vitamin E statuses of preschool children of socioeconomically disadvantaged families living in the midwestern United States. Eur J Clin Nutr. 1997;51:864–9. [DOI] [PubMed] [Google Scholar]
- 60. Apgar J, Makdani D, Sowell AL, Gunter EW, Hegar A, Rao D, Smith JC. Reproducibility of relative dose response (RDR) test and serum retinol and retinyl ester concentrations in children after a 2-week interval. J Am Coll Nutr. 1996;15:450–7. [DOI] [PubMed] [Google Scholar]
- 61. Wolde-Gebriel Z, West CE, Gebru H, Tadesse AS, Fisseha T, Gabre P, Aboye C, Ayana G, Hautvast JG. Interrelationship between vitamin A, iodine and iron status in schoolchildren in Shoa Region, central Ethiopia. Br J Nutr. 1993;70:593–607. [DOI] [PubMed] [Google Scholar]
- 62. de Pee S, Yuniar Y, West CE, Muhilal. Evaluation of biochemical indicators of vitamin A status in breast-feeding and non-breast-feeding Indonesian women. Am J Clin Nutr. 1997;66:160–7. [DOI] [PubMed] [Google Scholar]
- 63. Tanumihardjo SA, Muherdiyantiningsih, Permaesih D, Komala Muhilal, Karyadi D, Olson JA. Daily supplements of vitamin A (8.4 μmol, 8000 IU) improve the vitamin A status of lactating Indonesian women. Am J Clin Nutr. 1996;63:32–5. [DOI] [PubMed] [Google Scholar]
- 64. Ncube TN, Greiner T, Malaba LC, Gebre-Medhin M. Supplementing lactating women with pureed papaya and grated carrots improved vitamin A status in a placebo-controlled trial. J Nutr. 2001;131:1497–502. [DOI] [PubMed] [Google Scholar]
- 65. Permaesih D. Efikasi suplementasi dan fortifikasi vitamin A pada minyak goreng terhadap status vitamin A dan faktor imunitas air susu ibu. Dissertation. Bogor (Indonesia): Institut Pertanian Bogor; 2009; (in Indonesian). [Google Scholar]
- 66. Samba C, Tchibindat F, Gourmel B, Houze P, Malvy D. Prevalence of vitamin A deficiency in pregnant and lactating women in the Republic of Congo. J Health Popul Nutr. 2013;31:28–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Duitsman PK, Cook LR, Tanumihardjo SA, Olson JA. Vitamin A inadequacy in socioeconomically disadvantaged pregnant Iowan women as assessed by the modified relative dose response (MRDR) test. Nutr Res. 1995;15:1263–76. [Google Scholar]
- 68. Suharno D, West CE, Muhilal, Logman MH, de Waart FG, Karyadi D, Hautvast JG. Cross-sectional study on the iron and vitamin A status of pregnant women in West Java, Indonesia. Am J Clin Nutr. 1992;56:988–93. [DOI] [PubMed] [Google Scholar]
- 69. Tanumihardjo SA, Suharno D, Permaesih D, Muherdiyantiningsih, Dahro AM, Muhilal Karyadi D, Olson JA. Application of the modified relative dose response test to pregnant Indonesian women for assessing vitamin A status. Eur J Clin Nutr. 1995;49:897–903. [PubMed] [Google Scholar]
- 70. Tanumihardjo SA. Vitamin A and iron status are improved by vitamin A and iron supplementation in pregnant Indonesian women. J Nutr. 2002;132:1909–12. [DOI] [PubMed] [Google Scholar]
- 71. van den Broek NR, White SA, Flowers C, Cook JD, Letsky EA, Tanumihardjo SA, Mhango C, Molyneux M, Neilson JP. Randomised trial of vitamin A supplementation in pregnant women in rural Malawi found to be anaemic on screening by HemoCue. BJOG. 2006;113:569–76. [DOI] [PubMed] [Google Scholar]
- 72. Bulux J, Carranza E, Castaneda C, Solomons NW, Sokoll LJ, Morrow FD, Russell RM. Studies on the application of the relative-dose-response test for assessing vitamin A status in older adults. Am J Clin Nutr. 1992;56:543–7. [DOI] [PubMed] [Google Scholar]
- 73. Azaïs-Braesco V, Moriniere C, Guesne B, Partier A, Bellenand P, Baguelin D, Grolier P, Alix E. Vitamin A status in the institutionalized elderly. Critical analysis of four evaluation criteria: dietary vitamin A intake, serum retinol, relative dose-response test (RDR) and impression cytology with transfer (ICT). Int J Vitam Nutr Res. 1995;65:151–61. [PubMed] [Google Scholar]
- 74. Ribaya-Mercado JD, Mazariegos M, Tang G, Romero-Abal ME, Mena I, Solomons NW, Russell RM. Assessment of total body stores of vitamin A in Guatemalan elderly by the deuterated-retinol-dilution method. Am J Clin Nutr. 1999;69:278–84. [DOI] [PubMed] [Google Scholar]
- 75. Verhoef H, West CE. Validity of the relative-dose-response test and the modified-relative-dose-response test as indicators of vitamin A stores in liver. Am J Clin Nutr. 2005;81:835–9. [DOI] [PubMed] [Google Scholar]
- 76. Tanumihardjo SA, Underwood BA. Utility of the relative-dose-response and modified-relative-dose-response tests as population indicators of vitamin A status. Am J Clin Nutr. 2005;82:1135–7. [DOI] [PubMed] [Google Scholar]
- 77. Verhoef H. Utility of the relative-dose-response and modified-relative-dose-response tests as population indicators of vitamin A status—Reply to SA Tanumihardjo and BA Underwood. Am J Clin Nutr. 2005;82:1137–8. [DOI] [PubMed] [Google Scholar]
- 78. Olson JA. The reproducibility, sensitivity and specificity of the relative dose response (RDR) test for determining vitamin A status. J Nutr. 1991;121:917–20. [DOI] [PubMed] [Google Scholar]
- 79. Solomons NW, Morrow FD, Vasquez A, Bulux J, Guerrero AM, Russell RM. Test-retest reproducibility of the relative dose response for vitamin A status in Guatemalan adults: issues of diagnostic sensitivity. J Nutr. 1990;120:738–44. [DOI] [PubMed] [Google Scholar]
- 80. Olsen K, Suri DJ, Davis C, Sheftel J, Nishimoto K, Yamaoka Y, Toya Y, Welham NV, Tanumihardjo SA. Serum retinyl esters are positively correlated with analyzed total liver vitamin A reserves collected from US adults at time of death. Am J Clin Nutr. 2018;108:997–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Sowa M, Mourao L, Sheftel J, Kaeppler M, Simmons G, Grahn M, Davis CR, von Lintig J, Simon PW, Pixley KVet al. Overlapping vitamin A interventions with provitamin A carotenoids and preformed vitamin A cause excessive liver retinol stores in male Mongolian gerbils. J Nutr.Published online May 26, 2020. doi: 10.1093/jn/nxaa142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. WHO . Serum retinol concentrations for determining the prevalence of vitamin A deficiency in populations. Geneva (Switzerland): World Health Organization; 2011. [Google Scholar]
- 83. Rice AL, Stoltzfus RJ, de Francisco A, Kjolhede CL. Evaluation of serum retinol, the modified-relative-dose-response ratio, and breast-milk vitamin A as indicators of response to postpartum maternal vitamin A supplementation. Am J Clin Nutr. 2000;71:799–806. [DOI] [PubMed] [Google Scholar]
- 84. Underwood BA. Vitamin A in animal and human nutrition. In: Sporn MB, Roberts AB, Goodman DS, editors. The retinoids, Vol 1. Orlando (FL): Academic Press; 1984. [Google Scholar]
- 85. Underwood BA, Siegel H, Weisell RC, Dolinski M. Liver stores of vitamin A in a normal population dying suddenly or rapidly from unnatural causes in New York City. Am J Clin Nutr. 1970;23:1037–42. [DOI] [PubMed] [Google Scholar]
- 86. Suthutvoravoot S, Olson JA. Plasma and liver concentrations of vitamin A in a normal population of urban Thai. Am J Clin Nutr. 1974;27:883–91. [DOI] [PubMed] [Google Scholar]
- 87. Willumsen JF, Simmank K, Filteau SM, Wagstaff LA, Tomkins AM. Toxic damage to the respiratory epithelium induces acute phase changes in vitamin A metabolism without depleting retinol stores of South African children. J Nutr. 1997;127:1339–43. [DOI] [PubMed] [Google Scholar]
- 88. Deshmukh DS, Malathi P, Ganguly J. Studies on metabolism of vitamin A. 5. Dietary protein content and metabolism of vitamin A. Biochem J. 1964;90:98–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Suri DJ, Tanumihardjo JP, Gannon BM, Pinkaew S, Kaliwile C, Chileshe J, Tanumihardjo SA. Serum retinol concentrations demonstrate high specificity after correcting for inflammation but questionable sensitivity compared with liver stores calculated from isotope dilution in determining vitamin A deficiency in Thai and Zambian children. Am J Clin Nutr. 2015;102:1259–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Blaner WS, Li Y, Brun P-J, Yuen JJ, Lee S-A, Clugston RD. Vitamin A absorption, storage and mobilization. In: Asson-Batres MA, Rochette-Egly C, editors. The biochemistry of retinoid signaling II: the physiology of vitamin A—uptake, transport, metabolism and signaling. Dordrecht (Netherlands): Springer; 2016. pp.95–125. [Google Scholar]
- 91. Kanai M, Raz A, Goodman DS. Retinol-binding protein: the transport protein for vitamin A in human plasma. J Clin Invest. 1968;47:2025–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. van Bennekum AM, Wei S, Gamble MV, Vogel S, Piantedosi R, Gottesman M, Episkopou V, Blaner WS. Biochemical basis for depressed serum retinol levels in transthyretin-deficient mice. J Biol Chem. 2001;276:1107–13. [DOI] [PubMed] [Google Scholar]
- 93. Soprano DR, Smith JE, Goodman DS. Effect of retinol status on retinol-binding protein biosynthesis rate and translatable messenger RNA level in rat liver. J Biol Chem. 1982;257:7693–7. [PubMed] [Google Scholar]
- 94. Muto Y, Smith JE, Milch PO, Goodman DS. Regulation of retinol-binding protein metabolism by vitamin A status in the rat. J Biol Chem. 1972;247:2542–50. [PubMed] [Google Scholar]
- 95. Smith JE, Muto Y, Milch PO, Goodman DS. The effects of chylomicron vitamin A on the metabolism of retinol-binding protein in the rat. J Biol Chem. 1973;248:1544–9. [PubMed] [Google Scholar]
- 96. Soprano DR, Soprano KJ, Goodman DS. Retinol-binding protein messenger RNA levels in the liver and in extrahepatic tissues of the rat. J Lipid Res. 1986;27:166–71. [PubMed] [Google Scholar]
- 97. Dixon JL, Goodman DS. Studies on the metabolism of retinol-binding protein by primary hepatocytes from retinol-deficient rats. J Cell Physiol. 1987;130:14–20. [DOI] [PubMed] [Google Scholar]
- 98. Loerch JD, Underwood BA, Lewis KC. Response of plasma levels of vitamin A to a dose of vitamin A as an indicator of hepatic vitamin A reserves in rats. J Nutr. 1979;109:778–86. [DOI] [PubMed] [Google Scholar]
- 99. Mobarhan S, Russell RM, Underwood BA, Wallingford J, Mathieson RD, Al-Midani H. Evaluation of the relative dose response test for vitamin A nutriture in cirrhotics. Am J Clin Nutr. 1981;34:2264–70. [DOI] [PubMed] [Google Scholar]
- 100. Vaisman N, Wolfhart D, Sklan D. Vitamin A metabolism in plasma of normal and anorectic women. Eur J Clin Nutr. 1992;46:873–8. [PubMed] [Google Scholar]
- 101. Russell RM, Iber FL, Krasinski SD, Miller P. Protein-energy malnutrition and liver dysfunction limit the usefulness of the relative dose response (RDR) test for predicting vitamin A deficiency. Hum Nutr Clin Nutr. 1983;37:361–71. [PubMed] [Google Scholar]
- 102. Tanumihardjo SA, Olson JA. A modified relative dose-response assay employing 3,4-didehydroretinol (vitamin A2) in rats. J Nutr. 1988;118:598–603. [DOI] [PubMed] [Google Scholar]
- 103. Institute of Medicine Food and Nutrition Board . Vitamin A. In: Dietary Reference Intakes for vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. Washington (DC): National Academies Press; 2001. pp.82–161. [PubMed] [Google Scholar]
- 104. Barua AB, Ghosh MC. Preparation and properties of 4-oxo-retinoic acid and its methylester. Tetrahedron Lett. 1972;13:1823–5. [Google Scholar]
- 105. Valentine AR, Tanumihardjo SA. Adjustments to the modified relative dose response (MRDR) test for assessment of vitamin A status minimize the blood volume used in piglets. J Nutr. 2004;134:1186–92. [DOI] [PubMed] [Google Scholar]
- 106. Tanumihardjo SA, Muherdiyantiningsih, Permaesih D, Dahro AM, Muhilal, Karyadi D, Olson JA. Assessment of the vitamin A status in lactating and nonlactating, nonpregnant Indonesian women by use of the modified-relative-dose-response (MRDR) test. Am J Clin Nutr. 1994;60:142–7. [DOI] [PubMed] [Google Scholar]
- 107. Escaron AL, Green MH, Tanumihardjo SA. Plasma turnover of 3,4-didehydroretinol (vitamin A2) increases in vitamin A-deficient rats fed low versus high dietary fat. J Lipid Res. 2009;50:694–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Ahmad SM, Huda MN, Raqib R, Qadri F, Alam MJ, Afsar MNA, Peerson JM, Tanumihardjo SA, Stephensen CB. High-dose neonatal vitamin A supplementation to Bangladeshi infants increases the percentage of CCR9-positive Treg cells in infants with lower birthweight in early infancy, and decreases plasma SCD14 concentration and the prevalence of vitamin A deficiency at two years of age. J Nutr. Published online September 16, 2020. 10.1093/jn/nxaa260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Craft NE, Furr HC. Methods for assessment of vitamin A (retinoids) and carotenoids. In: Harrington D, editor. Laboratory assessment of vitamin status. Cambridge (MA): Academic Press; 2019. pp.21–47. [Google Scholar]
- 110. Tanumihardjo SA, Olson JA. The reproducibility of the modified relative dose response (MRDR) assay in healthy individuals over time and its comparison with conjunctival impression cytology (CIC). Eur J Clin Nutr. 1991;45:407–11. [PubMed] [Google Scholar]
- 111. Morrow FD, Guerrero AM, Russell RM, Dallal G, Solomons NW. Test-retest reproducibility of the relative dose response for vitamin A status in Guatemalan adults: issues of diagnostic specificity. J Nutr. 1990;120:745–50. [DOI] [PubMed] [Google Scholar]
- 112. Sheftel J, Bresnahan KA, Fadjarwati T, Tanumihardjo SA. Modified relative dose response values differ between lactating women in the United States and Indonesia. Exp Biol Med (Maywood). 2020;245:797–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Boner MJ. Use of the relative dose response assay to determine the vitamin A status of neonatal calves: effects of season on colostrum quality and immunological parameters. Master's thesis. Brookings (SD): South Dakota State University; 1997. [Google Scholar]
- 114. Hammell DC, Franklin ST, Nonnecke BJ. Use of the relative dose response (RDR) assay to determine vitamin A status of calves at birth and four weeks of age. J Dairy Sci. 2000;83:1256–63. [DOI] [PubMed] [Google Scholar]
- 115. Chaves GV, Peres WA, Goncalves JC, Ramalho A. Vitamin A and retinol-binding protein deficiency among chronic liver disease patients. Nutrition. 2015;31:664–8. [DOI] [PubMed] [Google Scholar]
- 116. WHO . Indicators for assessing vitamin A deficiency and their application in monitoring and evaluating intervention programmes. Geneva (Switzerland): World Health Organization; 1996. [Google Scholar]
- 117. Fujita M, Brindle E, Rocha A, Shell-Duncan B, Ndemwa P, O'Connor KA. Assessment of the relative dose-response test based on serum retinol-binding protein instead of serum retinol in determining low hepatic vitamin A stores. Am J Clin Nutr. 2009;90:217–24. [DOI] [PubMed] [Google Scholar]
- 118. Mills JP, Furr HC, Tanumihardjo SA. Retinol to retinol-binding protein (RBP) is low in obese adults due to elevated apo-RBP. Exp Biol Med (Maywood). 2008;233:1255–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. National Statistical Office; Community Health Sciences Unit [Malawi]; Centers for Disease Control and Prevention; Emory University. Malawi Micronutrient Survey. Atlanta (GA): National Statistical Office, Community Health Sciences Unit [Malawi] , Centers for Disease Control and Prevention, Emory University; 2017. [Google Scholar]
- 120. Smith FR, Goodman DS. Vitamin A transport in human vitamin A toxicity. N Engl J Med. 1976;294:805–8. [DOI] [PubMed] [Google Scholar]
- 121. Weinman AR, Jorge SM, Martins AR, de Assis M, Martinez FE, Camelo JS Jr. Assessment of vitamin A nutritional status in newborn preterm infants. Nutrition. 2007;23:454–60. [DOI] [PubMed] [Google Scholar]
- 122. Tanumihardjo SA, Furr HC, Erdman JW Jr, Olson JA. Use of the modified relative dose response (MRDR) assay in rats and its application to humans for the measurement of vitamin A status. Eur J Clin Nutr. 1990;44:219–24. [PubMed] [Google Scholar]
- 123. Surles RL, Li J, Tanumihardjo SA. The modified-relative-dose-response values in serum and milk are positively correlated over time in lactating sows with adequate vitamin A status. J Nutr. 2006;136:939–45. [DOI] [PubMed] [Google Scholar]
- 124. Surles RL, Hutson PR, Valentine AR, Mills JP, Tanumihardjo SA. 3, 4-Didehydroretinol kinetics differ during lactation in sows on a retinol depletion regimen and the serum:milk 3, 4-didehydroretinol:retinol ratios are correlated. J Nutr. 2011;141:554–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Rosmalina Y, Permaesih D, Fajarwati T, Herman S. Pengaruh pemberian minyak goreng yang difortifikasi vitamin A terhadap cadangan vitamin A tubuh pada ibu nifas [The effects of distribution of vitamin A fortified cooking oil on vitamin A body reserve of breast feeding mothers]. Penel Gizi Makan. 2009;32:138–48. (in Indonesian). [Google Scholar]
- 126. Santana RC, Machado AA, Martinelli AL, Jordao AA, Ramalho LN, Vannucchi H. Assessment of indicators of vitamin A status in non-cirrhotic chronic hepatitis C patients. Braz J Med Biol Res. 2016;49:e4785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Tanumihardjo SA, Furr HC, Amedee-Manesme O, Olson JA. Retinyl ester (vitamin A ester) and carotenoid composition in human liver. Int J Vitam Nutr Res. 1990;60:307–13. [PubMed] [Google Scholar]
- 128. Underwood BA. Effect of protein quantity and quality on plasma response to an oral dose of vitamin A as an indicator of hepatic vitamin A reserves in rats. J Nutr. 1980;110:1635–40. [DOI] [PubMed] [Google Scholar]
- 129. Amedee-Manesme O, Anderson D, Olson JA. Relation of the relative dose response to liver concentrations of vitamin A in generally well-nourished surgical patients. Am J Clin Nutr. 1984;39:898–902. [DOI] [PubMed] [Google Scholar]
- 130. Zachman RD, Chen XM. Intramuscular relative dose response (RDR) determination of liver vitamin A stores in rats. J Nutr. 1991;121:187–91. [DOI] [PubMed] [Google Scholar]
- 131. Valentine AR. Evaluation of the modified relative dose response test and vitamin A supplementation in a swine model. Master's thesis. Madison (WI): University of Wisconsin-Madison; 2004. [Google Scholar]
- 132. Tanumihardjo SA. Vitamin A: biomarkers of nutrition for development. Am J Clin Nutr. 2011;94:658S–65S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Valentine AR, Tanumihardjo SA. One-time vitamin A supplementation of lactating sows enhances hepatic retinol in their offspring independent of dose size. Am J Clin Nutr. 2005;81:427–33. [DOI] [PubMed] [Google Scholar]
- 134. Surles RL, Mills JP, Valentine AR, Tanumihardjo SA. One-time graded doses of vitamin A to weanling piglets enhance hepatic retinol but do not always prevent vitamin A deficiency. Am J Clin Nutr. 2007;86:1045–53. [DOI] [PubMed] [Google Scholar]
- 135. Tanumihardjo SA. Can lack of improvement in vitamin A status indicators be explained by little or no overall change in vitamin A status of humans?. J Nutr. 2001;131:3316–8. [DOI] [PubMed] [Google Scholar]
- 136. Campos FA, Flores H, Underwood BA. Effect of an infection on vitamin A status of children as measured by the relative dose response (RDR). Am J Clin Nutr. 1987;46:91–4. [DOI] [PubMed] [Google Scholar]
- 137. Amatayakul K, Underwood BA, Ruckphaopunt S, Singkamani R, Linpisarn S, Leelapat P, Thanangkul O. Oral contraceptives: effect of long-term use on liver vitamin A storage assessed by the relative dose response test. Am J Clin Nutr. 1989;49:845–8. [DOI] [PubMed] [Google Scholar]
- 138. de Pee S, West CE, Hautvast J, Muhilal, Karyadi D, West CE. Lack of improvement in vitamin A status with increased consumption of dark-green leafy vegetables. Lancet North Am Ed. 1995;346:75–81. [DOI] [PubMed] [Google Scholar]
- 139. Manorama R, Sarita M, Rukmini C. Red palm oil for combating vitamin A deficiency. Asia Pac J Clin Nutr. 1997;6:56–9. [PubMed] [Google Scholar]
- 140. Filteau SM, Rice AL, Ball JJ, Chakraborty J, Stoltzfus R, de Francisco A, Willumsen JF. Breast milk immune factors in Bangladeshi women supplemented postpartum with retinol or beta-carotene. Am J Clin Nutr. 1999;69:953–8. [DOI] [PubMed] [Google Scholar]
- 141. Tchum SK, Tanumihardjo SA, Newton S, de Benoist B, Owusu-Agyei S, Arthur FK, Tetteh A. Evaluation of vitamin A supplementation regimens in Ghanaian postpartum mothers with the use of the modified-relative-dose-response test. Am J Clin Nutr. 2006;84:1344–9. [DOI] [PubMed] [Google Scholar]
- 142. Bresnahan KA, Chileshe J, Arscott S, Nuss E, Surles R, Masi C, Kafwembe E, Tanumihardjo SA. The acute phase response affected traditional measures of micronutrient status in rural Zambian children during a randomized, controlled feeding trial. J Nutr. 2014;144:972–8. [DOI] [PubMed] [Google Scholar]
- 143. International Atomic Energy Agency . Stable isotope technique to assess intake of human milk in breastfed infants. Vienna (Austria): International Atomic Energy Agency; 2010. [Google Scholar]
- 144. Filteau SM, Raynes JG, Simmank K, Wagstaff LA. Vitamin A status does not influence neopterin production during illness or health in South African children. Br J Nutr. 1998;80:75–9. [DOI] [PubMed] [Google Scholar]
- 145. Biesalski HK, Frank J, Beck SC, Heinrich F, Illek B, Reifen R, Gollnick H, Seeliger MW, Wissinger B, Zrenner E. Biochemical but not clinical vitamin A deficiency results from mutations in the gene for retinol binding protein. Am J Clin Nutr. 1999;69:931–6. [DOI] [PubMed] [Google Scholar]
- 146. Wieringa FT, Dijkhuizen MA, West CE, Northrop-Clewes CA, Muhilal. Estimation of the effect of the acute phase response on indicators of micronutrient status in Indonesian infants. J Nutr. 2002;132:3061–6. [DOI] [PubMed] [Google Scholar]
- 147. WHO . Measuring change in nutritional status: guidelines for assessing the nutritional impact of supplementary feeding programmes for vulnerable groups. Geneva (Switzerland): World Health Organization; 1983. [Google Scholar]
- 148. Hamill PV, Drizd TA, Johnson CL, Reed RB, Roche AF, Moore WM. Physical growth: National Center for Health Statistics percentiles. Am J Clin Nutr. 1979;32:607–29. [DOI] [PubMed] [Google Scholar]
- 149. Rao DH, Satyanarayana K, Sastry JG. Growth pattern of well-to-do Hyderabad pre-school children. Indian J Med Res. 1976;64:629–38. [PubMed] [Google Scholar]
- 150. University of Ghana; GroundWork; University of Wisconsin-Madison; KEMRI-WellcomeTrust; UNICEF . Ghana Micronutrient Survey. Accra (Ghana): GroundWork; 2017. [Google Scholar]
- 151. MHP Nepal; New ERA; UNICEF; EU; USAID; CDC . Nepal National Micronutrient Status Survey, 2016. Kathmandu (Nepal): Ministry of Health and Population, Nepal; 2018. [Google Scholar]
- 152. INCAP . Informe del Sistema de Vigilancia Epidemiológica de Salud y Nutrición—SIVESNU–2013, Informe Final. Guatemala City (Guatemala): Instituto de Nutrición de Centro América y Panamá; 2015(in Spanish). [Google Scholar]
- 153. INCAP . Informe del Sistema de Vigilancia Epidemiológica de Salud y Nutrición—SIVESNU–2016, Informe Final. Guatemala City (Guatemala): Instituto de Nutrición de Centro América y Panamá; 2018; (in Spanish). [Google Scholar]
- 154. Molla A, Islam A, Molla AM, Jahan F. Change in serum vitamin A concentration after an oral dose in children with acute diarrhea. J Pediatr. 1983;103:1000–2. [DOI] [PubMed] [Google Scholar]
- 155. Usha N, Sankaranarayanan A, Walia BN, Ganguly NK. Assessment of preclinical vitamin A deficiency in children with persistent diarrhea. J Pediatr Gastroenterol Nutr. 1991;13:168–75. [DOI] [PubMed] [Google Scholar]
- 156. Duitsman PK, Tanumihardjo SA, Cook L, Olson JA. Vitamin A status of low-income pregnant women in Iowa as assessed by the modified relative dose-response. Ann NY Acad Sci. 1993;678:344–5. [DOI] [PubMed] [Google Scholar]
- 157. Kafwembe EM, Sukwa TY, Manyando C, Mwandu D, Chipipa J, Chipaila P. The vitamin A status of Zambian children attending an under five clinic as evaluated by the modified relative dose response (MRDR) test. Int J Vitam Nutr Res. 1996;66:190–6. [PubMed] [Google Scholar]
- 158. Paiva SAR, Godoy I, Vannucchi H, Favaro RMD, Geraldo RRC, Campana AO. Assessment of vitamin A status in chronic obstructive pulmonary disease patients and healthy smokers. Am J Clin Nutr. 1996;64:928–34. [DOI] [PubMed] [Google Scholar]
- 159. Fazio-Tirrozzo G, Brabin L, Brabin B, Agbaje O, Harper G, Broadhead R. A community based study of vitamin A and vitamin E status of adolescent girls living in the Shire Valley, Southern Malawi. Eur J Clin Nutr. 1998;52:637–42. [DOI] [PubMed] [Google Scholar]
- 160. Silveira SA, Figueiredo JF, Jordao Junior A, de Unamuno Mdo R, Rodrigues Md, Vannucchi H. Subnutrição e hipovitaminose A em pacientes com AIDS [Malnutrition and hypovitaminosis A in AIDS patients]. Rev Soc Bras Med Trop. 1999;32:119–24. (in Portugese). [DOI] [PubMed] [Google Scholar]
- 161. Ncube TN, Malaba L, Greiner T, Gebre-Medhin M. Evidence of grave vitamin A deficiency among lactating women in the semi-arid rural area of Makhaza in Zimbabwe: a population-based study. Eur J Clin Nutr. 2001;55:229–34. [DOI] [PubMed] [Google Scholar]
- 162. Maciel BL, Lacerda HG, Queiroz JW, Galvao J, Pontes NN, Dimenstein R, McGowan SE, Pedrosa LF, Jeronimo SM. Association of nutritional status with the response to infection with Leishmania chagasi. Am J Trop Med Hyg. 2008;79:591–8. [PubMed] [Google Scholar]
- 163. Custodio VI, Daneluzzi JC, Custodio RJ, Del Ciampo LA, Ferraz IS, Martinelli CE Jr., Ricco RG, Cupo P, Hering SE, Meirelles MSet al. Vitamin A deficiency among Brazilian school-aged children in a healthy child service. Eur J Clin Nutr. 2009;63:485–90. [DOI] [PubMed] [Google Scholar]
- 164. de Paula TP, Ramalho A, Braulio VB. The effectiveness of relative dose response to retinol intake as an evaluation of vitamin A status of cirrhotic patients. J Hum Nutr Diet. 2010;23:583–9. [DOI] [PubMed] [Google Scholar]
- 165. Fujita M, Lo YJ, Brindle E. Nutritional, inflammatory, and ecological correlates of maternal retinol allocation to breast milk in agro-pastoral Ariaal communities of northern Kenya. Am J Hum Biol. 2017;29:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Fujita M, Shell-Duncan B, Ndemwa P, Brindle E, Lo YJ, Kombe Y, O'Connor K. Vitamin A dynamics in breastmilk and liver stores: a life history perspective. Am J Hum Biol. 2011;23:664–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Hotz C, Chileshe J, Siamusantu W, Palaniappan U, Kafwembe E. Vitamin A intake and infection are associated with plasma retinol among pre-school children in rural Zambia. Public Health Nutr. 2012;15:1688–96. [DOI] [PubMed] [Google Scholar]
- 168. Amaral CT, Pontes NN, Maciel BL, Bezerra HS, Triesta AN, Jeronimo SM, McGowan SE, Dantas VM. Vitamin A deficiency alters airway resistance in children with acute upper respiratory infection. Pediatr Pulmonol. 2013;48:481–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Arantes Ferreira Peres W, Villaca Chaves G, Saraiva Goncalves JC, Ramalho A, Moraes Coelho HS. Assessment of the relative dose-response test as indicators of hepatic vitamin A stores in various stages of chronic liver disease. Nutr Clin Pract. 2013;28:95–100. [DOI] [PubMed] [Google Scholar]
- 170. Soares-Mota M, Silva TA, Gomes LM, Pinto MA, Mendonca LM, Farias ML, Nunes T, Ramalho A, Zaltman C. High prevalence of vitamin A deficiency in Crohn's disease patients according to serum retinol levels and the relative dose-response test. WJG. 2015;21:1614–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Tanumihardjo SA, Palacios N, Pixley KV. Provitamin A carotenoid bioavailability: what really matters?. Int J Vitam Nutr Res. 2010;80:336–50. [DOI] [PubMed] [Google Scholar]
- 172. Borel P, Desmarchelier C. Genetic variations associated with vitamin A status and vitamin A bioavailability. Nutrients. 2017;9:246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Muhilal H, Glover J. Effects of dietary deficiencies of protein and retinol on the plasma level of retinol-binding protein in the rat. Br J Nutr. 1974;32:549–58. [DOI] [PubMed] [Google Scholar]
- 174. Large S, Neal G, Glover J, Thanangkul O, Olson RE. The early changes in retinol-binding protein and prealbumin concentrations in plasma of protein-energy malnourished children after treatment with retinol and an improved diet. Br J Nutr. 1980;43:393–402. [DOI] [PubMed] [Google Scholar]
- 175. Wongsiriroj N, Jiang H, Piantedosi R, Yang KJZ, Kluwe J, Schwabe RF, Ginsberg H, Goldberg IJ, Blaner WS. Genetic dissection of retinoid esterification and accumulation in the liver and adipose tissue. J Lipid Res. 2014;55:104–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Sheftel J, Sowa M, Mourao L, Zoue LT, Davis CR, Simon PW, Tanumihardjo SA. Total adipose retinol concentrations are correlated with total liver retinol concentrations in male Mongolian gerbils, but only partially explained by chylomicron deposition assessed with total α-retinol. Curr Dev Nutr. 2019;3:nzy096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Thompson SJ, Sargsyan A, Lee SA, Yuen JJ, Cai J, Smalling R, Ghyselinck N, Mark M, Blaner WS, Graham TE. Hepatocytes are the principal source of circulating RBP4 in mice. Diabetes. 2017;66:58–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Christian P, West KP Jr.. Interactions between zinc and vitamin A: an update. Am J Clin Nutr. 1998;68:435S–41S. [DOI] [PubMed] [Google Scholar]
- 179. Rosales FJ, Jang JT, Pinero DJ, Erikson KM, Beard JL, Ross AC. Iron deficiency in young rats alters the distribution of vitamin A between plasma and liver and between hepatic retinol and retinyl esters. J Nutr. 1999;129:1223–8. [DOI] [PubMed] [Google Scholar]
- 180. Michelazzo FB, Oliveira JM, Stefanello J, Luzia LA, Rondo PH. The influence of vitamin A supplementation on iron status. Nutrients. 2013;5:4399–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. WHO . Essential nutrition actions: improving maternal, newborn, infant and young child health and nutrition. Geneva (Switzerland): World Health Organization; 2013. [PubMed] [Google Scholar]
- 182. WHO . Assessing the iron status of populations: including literature reviews. Report of a joint World Health Organization/Centers for Disease Control and Prevention technical consultation on the assessment of iron status at the population level, Geneva, Switzerland, 6–8 April 2004. 2nd ed.Geneva (Switzerland): World Health Organization; 2004. [Google Scholar]
- 183. O'Byrne SM, Kako Y, Deckelbaum RJ, Hansen IH, Palczewski K, Goldberg IJ, Blaner WS. Multiple pathways ensure retinoid delivery to milk: studies in genetically modified mice. Am J Physiol Endocrinol Metab. 2010;298:E862–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Dever JT, Surles RL, Davis CR, Tanumihardjo SA. α-Retinol is distributed through serum retinol-binding protein-independent mechanisms in the lactating sow-nursing piglet dyad. J Nutr. 2011;141:42–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Sheftel J, Surles RL, Tanumihardjo SA. Highlight article: retinol isotope dilution accurately predicts liver reserves in piglets but overestimates reserves in lactating sows. Exp Biol Med (Maywood). 2019;244:579–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Olson JA, Gunning DB, Tilton RA. Liver concentrations of vitamin A and carotenoids, as a function of age and other parameters, of American children who died of various causes. Am J Clin Nutr. 1984;39:903–10. [DOI] [PubMed] [Google Scholar]
- 187. WHO . Guideline: vitamin A supplementation in postpartum women. Geneva (Switzerland): World Health Organization; 2011. [PubMed] [Google Scholar]
- 188. Rosales FJ, Ritter SJ, Zolfaghari R, Smith JE, Ross AC. Effects of acute inflammation on plasma retinol, retinol-binding protein, and its mRNA in the liver and kidneys of vitamin A-sufficient rats. J Lipid Res. 1996;37:962–71. [PubMed] [Google Scholar]
- 189. Stephensen CB. Vitamin A, infection, and immune function. Annu Rev Nutr. 2001;21:167–92. [DOI] [PubMed] [Google Scholar]
- 190. Quadro L, Blaner WS, Salchow DJ, Vogel S, Piantedosi R, Gouras P, Freeman S, Cosma MP, Colantuoni V, Gottesman ME. Impaired retinal function and vitamin A availability in mice lacking retinol binding protein. EMBO J. 1999;18:4633–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Vogel S, Piantedosi R, O'Byrne SM, Kako Y, Quadro L, Gottesman ME, Goldberg IJ, Blaner WS. Retinol-binding protein-deficient mice: biochemical basis for impaired vision. Biochemistry. 2002;41:15360–8. [DOI] [PubMed] [Google Scholar]
- 192. Berry DC, Jacobs H, Marwarha G, Gely-Pernot A, O'Byrne SM, DeSantis D, Klopfenstein M, Feret B, Dennefeld C, Blaner WSet al. The STRA6 receptor is essential for retinol-binding protein-induced insulin resistance but not for maintaining vitamin A homeostasis in tissues other than the eye. J Biol Chem. 2013;288:24528–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Tang G, Qin J, Hao LY, Yin SA, Russell RM. Use of a short-term isotope-dilution method for determining the vitamin A status of children. Am J Clin Nutr. 2002;76:413–8. [DOI] [PubMed] [Google Scholar]


