ABSTRACT
Personalized and precision nutrition aim to examine and improve health on an individual level, and this requires reconsideration of traditional dietary interventions or behavioral study designs. The limited frequency of measurements in group-level human nutrition trials cannot be used to infer individual responses to interventions, while in behavioral studies, retrospective data collection does not provide an accurate measure of how everyday behaviors affect individual health. This review introduces the concept of N-of-1 study designs, which involve the repeated measurement of a health outcome or behavior on an individual level. Observational designs can be used to monitor a participant's usual health or behavior in a naturalistic setting, with repeated measurements conducted in real time using an Ecological Momentary Assessment. Interventional designs can introduce a dietary or behavioral intervention with predictors and outcomes of interest measured repeatedly either during or after 1 or more intervention and control periods. Due to their flexibility, N-of-1 designs can be applied to both short-term physiological studies and longer-term studies of eating behaviors. As a growing number of disease markers can be measured outside of the clinic, with self-reported data delivered via electronic devices, it is now easier than ever to generate large amounts of data on an individual level. Statistical techniques can be utilized to analyze changes in an individual or to aggregate data from sets of N-of-1 trials, enabling hypotheses to be tested on a small number of heterogeneous individuals. Although their designs necessitate extra methodological and statistical considerations, N-of-1 studies could be used to investigate complex research questions and to study underrepresented groups. This may help to reveal novel associations between participant characteristics and health outcomes, with repeated measures providing power and precision to accurately determine an individual's health status.
Keywords: N-of-1, precision nutrition, personalized nutrition, Ecological Momentary Assessment, self-report measures, study design, review
Introduction
N-of-1 studies, in which patients are studied on an individual level, have been used in medical research for over 100 years (1). However, in the intervening time, research on a group level has largely been favored, often through analyses of data from randomized controlled trials (RCTs) or repeated crossover trials. The goal of an RCT is typically to see if 1 intervention (whether that be a drug, diet, or behavioral intervention) performs significantly better than another intervention or placebo/control by beneficially affecting a health outcome on a group level. Yet, it is generally accepted that not all individuals respond to diets, foods, or supplements in the same way (2), and responses on the individual level are not able to be accurately identified from a standard RCT (3).
An example of a result from a randomized, parallel-arm nutrition study is shown in Figure 1 below, in a study where 202 healthy participants aged 40–65 years completed one of three 12-wk dietary interventions (4). The figure shows systolic blood pressure (SBP) changes after consuming: 1) refined-grain products (control diet); 2) wholegrain wheat products; and 3) wholegrain wheat and oat products (4). Although there are statistically significant reductions in SBP after both types of wholegrain consumption at a group level, there is also a large variation in responses within each group, including in responses to the refined-grain control diet. This highlights the fact that there is a large degree of random variability between successive measurements, both within and between participants. Indeed, biological and analytical variability together can be greater than 50% for some clinical outcomes (5), meaning it can be difficult to ascertain whether a particular participant really responded to the treatment, particularly on the basis of a single measurement before and after a study (3). Furthermore, participants with a particularly high or low value at baseline are more likely to show a greater change away from this value, representing a regression to the mean effect (6). However, within an N-of-1 design, a disease marker can be monitored repeatedly, both with and without nutrition intervention. This provides a measure of both the variability of a disease marker and consistency of a participant's response to an intervention (7).
FIGURE 1.
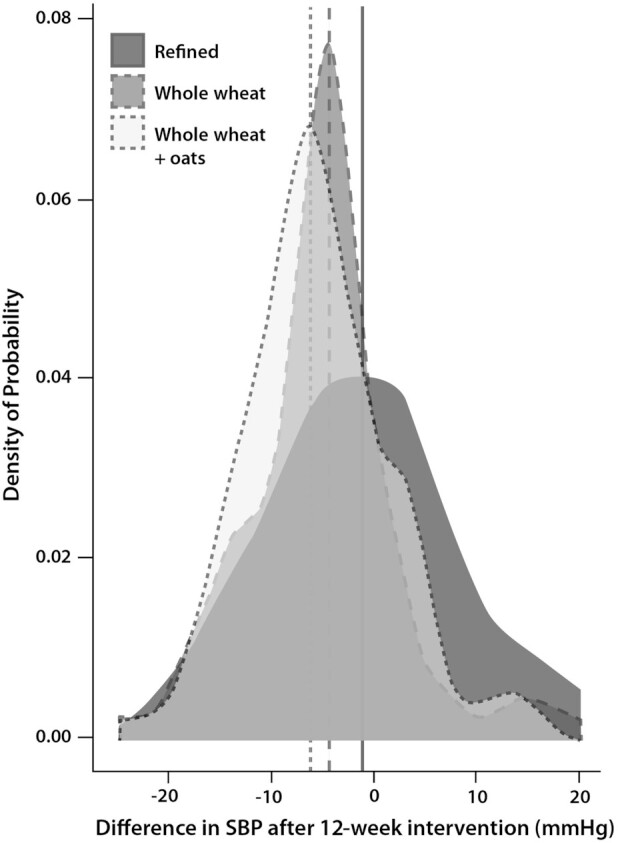
Distribution of difference in SBP between the start and end of a 12-wk intervention across 3 dietary intervention groups (n = 202): two wholegrain interventions [“whole wheat” (n = 71) and “whole wheat + oats” (n = 68)] and a control group not provided with whole grains [“refined” (n = 63)]. The dashed lines represent a mean reduction in SBP by intervention group. Data from a study by Tighe et al. (4) were obtained from Frank Thies (University of Aberdeen). SBP, systolic blood pressure.
What is an N-of-1 study?
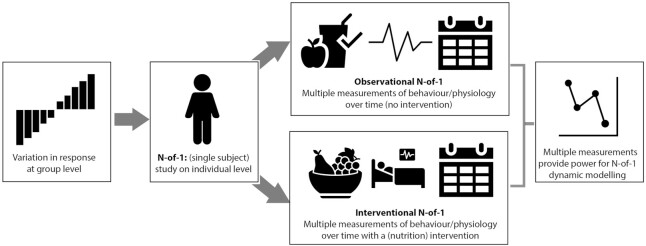
N-of-1 studies are designed to measure or observe 1 person multiple times, with repetition providing statistical power (8). There are 2 broad classes of N-of-1 studies, both of which could be usefully applied to research questions within human nutrition, as shown in Figure 2. Observational N-of-1 studies monitor a participant over time and do not introduce a treatment or intervention. During the period of study, multiple measurements or observations are taken, which could include measurements of a disease marker, behavior, or mood (8). Alternatively, N-of-1 studies can include 1 or more intervention periods. There are many types of interventional N-of-1 designs, depending on the aims of the study. For example, a single intervention period can be sandwiched between 2 observation periods (an ABA design) or 2 different treatments could be repeatedly assigned in a randomized fashion. Both can investigate the variability of treatment responses on an individual level, with the latter able to identify which treatment may be better for that person (9, 10).
FIGURE 2.
Overview of an N-of-1 study. To examine a participant on an individual level, an N-of-1 study can be employed; this can take the form of an observational or interventional design. Both forms enable collection of multiple measurements to provide power for statistical analysis.
An N-of-1 study necessarily works with time-series data from collecting repeated measurements over time (11). In an N-of-1 trial, autocorrelation between successive data points—that is, that a given measurement will tend to be similar to 1 or more measurements preceding it—must be controlled for (12). While outcomes can show temporal trends, such as periodicity and seasonality effects (13), if an intervention is included within an N-of-1 study, its effects on an outcome can also vary with time. For both dietary and behavioral interventions, it will take time from the start of the intervention for the outcome to be maximally affected; similarly, any effects are likely to remain for some time after the intervention has been withdrawn (carry-over effect), meaning the inclusion of washout periods may be necessary (12). Several statistical analysis approaches can be used to account for these effects and to determine whether an intervention has led to significant changes in an outcome on an individual level. One such technique is dynamic modelling, where lagged variables are included within a regression model to control for the effect of the past on a given measurement (11). Information on other analysis approaches, such as Bayesian inference, can be found elsewhere (12).
A strength of dynamic modelling over other time-series methods is that it can adjust for autocorrelation by including the response from more than 1 past occasion within a model (e.g., 7 days before), not just the 1 immediately preceding it; this has been shown to lead to better model estimates (11). Dynamic modelling can be applied to the analysis of both observational and interventional N-of-1 designs, as time-varying covariates can be included in a model to highlight the presence or absence of an intervention, as well as the inclusion of covariates depicting exogenous conditions that vary necessarily (e.g., day of the week) and endogenous conditions that vary at the individual level (e.g., hours of sleep). Just as with a standard regression model, the type of dynamic model applied will depend on the nature of the outcome; for instance, a continuous outcome like blood pressure would necessitate linear dynamic regression, while a binary outcome such as consumption (yes or no) of a particular food would utilize logistic dynamic regression (11), provided relevant statistical assumptions are met.
If the goal of an N-of-1 study is to understand a treatment response or behavior on an individual level only, measures can be adapted to suit the needs of the participant and the interest of the researchers (11). This means N-of-1 studies can be tailored to the individual level: for instance, through designing and delivering individually tailored questionnaires (14) or by adapting a treatment regimen over time depending on how the participant responds to different interventions (15). Participants are likely to retain interest in a study where the measures are adapted to them (8) or where they are aware that the results will be applied to delivering a targeted treatment regimen for them in the future (16). This is a further strength of N-of-1 designs, as low compliance and dropouts are often an issue within group-based studies (17). N-of-1 designs can therefore be highly flexible and may be used to address a potentially limitless number of research questions (8). Conversely, if the same experimental design is followed, multiple N-of-1 studies can be aggregated to determine group-level effects. This has the benefit of requiring a lower number of observations to achieve the same statistical power as traditional group designs (such as RCTs) (1, 9), meaning N-of-1 designs could help save researchers time and resources (9). For participants that are recruited, resources can be used to study each person in more detail and over a longer time frame. Aggregation of N-of-1 studies can be useful for determining whether the results obtained are generalizable, given multiple N-of-1 studies with the same measures have been undertaken (11).
In this review, both observational and interventional N-of-1 studies will be discussed, with a consideration of how different N-of-1 designs can be applied to nutrition research, particularly within the growing fields of personalized and precision nutrition.
Current Status of Knowledge
Observational N-of-1 studies in nutrition
Observational N-of-1 studies are used to measure an individual's health, behavior, or feelings over time, without the introduction of a treatment or intervention (8). This means a single observation period is typically used, in contrast to an interventional design that may alternate treatment and observation (control) periods, or 2 or more different treatments. During the period of observation, repeated measurements of behavior or health can be collected in a naturalistic setting, such as via Ecological Momentary Assessment (EMA). EMA enables the collection of real-time data, minimizing the retrospective recall bias that can occur if asking participants to recall their feelings or actions some time afterwards (8). This can include behavioral assessments (e.g., questionnaires via smartphone) (18), as well as objective markers of health that can be collected away from a research center, such as continuous glucose monitoring (19) or measuring activity via wrist-worn devices, which can also monitor heart-rate or sleep patterns (20). Examples of recently published and ongoing N-of-1 studies in nutrition, including several with physiological measurements, are shown in Table 1.
TABLE 1.
List of ongoing and recently published N-of-1 trials relating to nutrition or health behavior
| Title of study/NIH listing | Study status | Patient/participant description | Study design | Statistical analysis method(s) | Intervention(s)/treatment(s) | Primary outcome(s) |
|---|---|---|---|---|---|---|
| Westlake personalized nutrition and health cohort for drug addicts (NCT04105621) | Ongoing | Patients with drug addictions | Observational N-of-1 | Not stated | None | Continuous blood glucose over 2 wk;Change of gut microbiota within 1 year |
| Application of N-of-1 clinical trials in personalized nutrition research: a trial protocol for Westlake N-of-1 trials for macronutrient intake (NCT04125602) (27) | Ongoing | Healthy participants | Repeated, randomized (within-block) crossover N-of-1 (3 blocks) | Bayesian models | High-fat, low-carbohydrate diet;Low-fat, high-carbohydrate diet | Postprandial glycemic responses, including postprandial maximum glucose and 0–24 h area under the curve |
| Coffee and real-time atrial and ventricular ectopy (CRAVE; NCT03671759) | Ongoing | Healthy volunteers | Interventional, randomized in 2-day blocks | Not stated | Caffeine consumption (versus withdrawal) | Change in cardiac ectopy burden (heart rhythm) |
| Measuring individual responses to a whole-grain and nuts intervention to reduce blood pressure in prehypertension (MI-DIET; NCT04326686) | Ongoing | Volunteers with mildly elevated blood pressure | Interventional ABA design1 | Dynamic modelling | Dietary Approaches to Stop Hypertension (DASH) diet with whole grains and nuts provided | Adherence to intervention; Change in blood pressure levels |
| Personalized research on diet in ulcerative colitis and Crohn's disease (PRODUCE; NCT03301311) | Ongoing | Patients aged 7–18 | Repeated, randomized crossover N-of-1 | Individual and population-level (aggregated) analysis | Specific carbohydrate diet;Modified specific carbohydrate diet | Stool frequency and consistency;Pain interference;Gastrointestinal symptoms;Fecal Calprotectin |
| Personalized lifestyle intervention for improving functional health outcomes using N-of-1 tent-umbrella-bucket design (LIFE-HOUSE; NCT04005456) | Ongoing | Patients with a variety of chronic diseases | Crossover N-of-1 (depending on clinical group) | Not stated | Varied interventions including dietary supplements, behavioral change support program, and food plan | Medical Outcome Study Short Form 36 questionnaire;University of Rhode Island Change Assessment questionnaire;Depression Anxiety Stress Scale questionnaire |
| Measuring the effects of caffeine and L-theanine on cognitive performance: a protocol for self-directed, mobile N-of-1 studies (NCT04056650) (28) | Ongoing | Healthy volunteers | Repeated counterbalanced (ABBA or BAAB1) N-of-1 | Linear model with factors for treatment and block | Caffeine;Caffeine + L-theanine | Cognitive function via:1) Remote Associates Test;2) Stroop Test;3) Trail Making Test |
| Self-regulatory processes, motivation to conserve resources and activity levels in people with chronic pain (29) | Published | Patients with chronic pain | Observational N-of-1 | Dynamic regression modelling | None | Motivation to conserve resources;Physical activity;Sedentary time |
| Changes in physical activity during the retirement transition: a series of novel n-of-1 natural experiments (20) | Published | Participants approaching retirement | “Natural” intervention (AB design1) | Dynamic regression modelling | Retirement | Physical activity |
| Tracking snacking in real time: time to look at individualized patterns of behavior (30) | Published | Healthy participants | Observational N-of-1 | Descriptive exploratory analysis;Intra-class correlation coefficients | None | Consumption of high-calorie snack foods |
| The Diabetes Remission Clinical Trial (DiRECT): protocol for a cluster-randomized trial (31) | Complete but with analysis ongoing | Patients with type 2 diabetes mellitus | Interventional and observational N-of-1 (subset of a large, cluster-randomized trial) | Not stated (for N-of-1 component) | Low-calorie food replacement diet versus usual diabetes and obesity management | Adherence to dietary prescription as revealed by Ecological Momentary Assessment |
The number of N-of-1 trials within the field of nutrition is small, highlighting the novelty of N-of-1 designs within this field.
1Pattern of observation periods (A) and intervention periods (B).
Through an observational N-of-1 study, sufficient measurements need to be collected to identify change patterns on an individual level, with the number of repeated measurements representing the sample size of the study (11). This contrasts with traditional observational studies, which aim to investigate population-level trends by following a group of participants over time, with measurements over fewer time points. With an observational N-of-1 study, trends on an individual level are investigated by following individuals for longer periods and taking more frequent measurements. It has been shown that for analysis approaches such as dynamic modelling, 50 measurements are enough for estimating model parameters with precision (11); this can help inform both the measurement frequency and the total length of the study. However, the length of a study can be extended beyond this minimum period. Provided a participant is happy to continue with the study, when appropriate, further information will provide a more accurate representation of their behavior or health status. This approach was used in a study examining physical activity, where each participant extended the data collection period beyond the minimum 2 months (up to 7 months) (20). If the goal of the research is to understand the individual factors associated with beneficial behavioral patterns or improved health outcomes, a larger amount of data will provide greater insight into the participant's usual behavior (8).
Depending on the nature of the study and the outcome of interest, it may be appropriate for participants to collect measurements several times a day for a short-term study; this was carried out in a week-long study that investigated the relationship between snacking, physical activity, and self-regulation (21). For longer studies, the measurement burden could lead to lower compliance, meaning collecting data a single time each day may be more appropriate. Several N-of-1 studies have shown good levels of compliance to daily monitoring over several months (20, 22).
As observational N-of-1 studies employ frequent assessments/measurements over extended periods, such studies would be useful for understanding patterns in a participant's usual behaviors. EMA could be used for collecting ecologically valid data on a participant's eating and purchasing behavior, for example. Due to advances in technology, it is now easier for participants to provide such data, and therefore remain compliant to such studies. For example, a large precision-nutrition study recently had participants photograph foods to assess their dietary intake (23). Within an N-of-1 study, such a method could be combined with a questionnaire delivered to a participant's mobile device on their motivations for choosing the corresponding foods (e.g., taste, health, etc.), for instance. This methodology could improve compliance by reminding participants to record information, thereby yielding accurate estimates of energy intake, while simultaneously examining variation in a participant's motivations to consume different foods. By asking the participant to provide responses shortly after buying or consuming foods, a more accurate picture of their everyday motivations and how these vary over time can be gathered, compared with using a retrospective questionnaire (24).
As mentioned previously, it is also possible to measure several objective markers via EMA. In the context of an observational study, this could be used to see whether a participant's usual eating or other health behaviors are associated with improvement or worsening of variable disease markers that can be measured remotely. For example, an assessment of habitual diet could be used to identify those foods that lead to high postprandial glucose levels (23), while variations in sleep duration and quality may be associated with blood pressure fluctuations (25). Within a precision nutrition study, the collection of biomarkers can be used to validate consumption of certain foods or nutrients (17). An observational study can also be used for monitoring acute events, known as event-based monitoring (24). A participant can log an event, such as a headache or allergic response, when it occurs through an app or paper-based diary; the relationship between the event and potential explanatory factors can then be examined. This would help to reveal the dietary or environmental factors that may trigger such an event.
A drawback of EMA is that it necessitates the use of particularly motivated participants, who may be healthier or more engaged with their health as compared to others with shared characteristics (26). However, several features of EMA can motivate high levels of compliance. For example, phone reminders can be used to remind participants to measure their blood pressure, for instance, which could be timed together with other measures, such as a questionnaire, to save time and effort for the participant (24). The times that data are collected can be modified to suit participants; for example, a questionnaire prompt could be delivered after a participant's normal waking time or they could be asked to take a finger-prick blood sample 1 hour after their meal. This can be far more convenient than asking a participant to show up at a research center at a specified time, which may alter their usual activities. To prevent any biases in behavior by delivering prompts at exactly the same time each day, prompting at a random time within an interval (e.g., once any time between 14:00–15:00) can be done to mitigate this (24). It is also possible to deliver personalized questionnaires that are especially relevant to the participant under study (20), which may help retain interest, particularly if the participant will be informed of their results at the end of the study or if the results will be applied to improving the participant's health (e.g., informing a future dietary regimen).
It is important for the researcher to consider how repeated monitoring could influence the behavior under study (24). This is particularly important if participants are aware of their measurements or results during the study (e.g., through use of self-monitoring), which could have an indirect effect on outcomes and potentially mask the effect of any intervention(s). This can partly be mitigated by including an observation period of a sufficient length prior to the intervention, to give participants a chance to get used to monitoring and allow any initial changes to their behavior to revert to normal (8).
Interventional N-of-1 designs
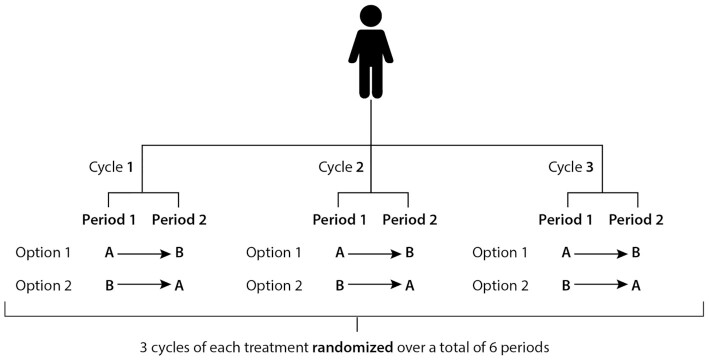
As mentioned previously, there are various N-of-1 designs that incorporate 1 or more intervention periods. A repeated-crossover N-of-1 study is a form of interventional design where an individual is followed over 2 or more “treatment cycles,” as shown in Figure 3 (1). Each cycle is composed of at least 2 periods, depending on the number of treatments or interventions used. The sequence of the periods within a cycle might be planned a priori or randomized. Figure 3 shows an example of where each of 2 treatments is given once per cycle, for a total of 3 cycles, in a random order (within-cycle randomization). This means that there are 8 different combinations possible, given 3 cycles each with 2 periods (1). This type of randomization structure is useful if it is suspected that the treatment effects may be affected by time-related confounders, as it helps these to be spread evenly across both treatments (12). Within-cycle randomization can also be used for comparing the outcome(s) after both treatments, to determine which intervention performed better within each cycle (1). Another approach is complete randomization across all treatment periods, which provides the benefit of a much higher number of potential random sequences (20, where 2 different treatments over 3 cycles are considered); for example, a treatment sequence could be AABBBA (1). The potential for a poorly balanced design such as this means use of this type of randomization should be carefully considered, to ensure the outcome is not affected by time-related confounders; dropouts could also lead to an uneven number of treatment allocations being completed (12). With complete randomization, the average effect of both treatments on the outcome(s) across all cycles would be calculated and compared, rather than within-cycle outcome pairs being compared directly.
FIGURE 3.
Schematic of a repeated-crossover trial with 2 different treatments (A and B). Each treatment is randomized within each cycle, over n cycles (at least 2). In this example, 8 different randomization sequences are possible.
Within nutrition research, one could employ a repeated-crossover design as part of a controlled feeding study; for example, to examine the effect of 2 different calorie-matched breakfasts on postprandial plasma glucose and triglyceride levels after 3 or more separate eating occasions. To use this as an example, a participant could be assessed 6 times over 3 wk, with each eating period separated by at least 1 day to ensure no residual effects of the previous treatment on the next, and with potential confounders monitored and controlled for (e.g., provision of a set meal the preceding evening, and other meal intakes reported via a food diary). In Week 1, breakfast A could be provided on the first occasion, while breakfast B would be provided on the second. In Week 2, this order would either stay the same or be swapped (breakfast B, followed by breakfast A); this would also apply for Week 3. This would utilize the within-cycle randomization approach, as both breakfasts would be provided each week in a random order. In this instance, this would probably be preferable to the complete randomization approach, as it would allow for any potential time-related confounders across the 3 wk to be present across both treatments, which may not occur for complete randomization (e.g., if the sequence AAABBB were used, breakfast B would not be presented until halfway through the second week). An example of such a confounder would be hormonal effects in female participants, which can produce differential metabolic effects throughout the menstrual cycle (32).
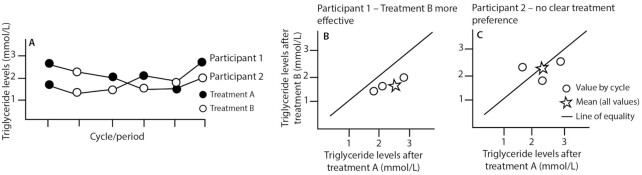
In Figure 4, results from 2 hypothetical N-of-1 trials are shown. Figure 4A shows responses to 2 interventions that are hypothesized to lower triglyceride levels. The results from Participant 1 (Figure 4B) show consistently lower triglyceride values after Treatment B compared with Treatment A within each cycle, with responses to Treatment A being more variable, while responses to Treatment B are more stable. Figure 4B shows that all 3 points lie on the right of the line of equality, indicating that Treatment B is more effective for Participant 1 than Treatment A in the context of this study. In contrast, neither intervention leads to a consistent response in Participant 2 (Figure 4C), with the average of the 3 within-cycle comparisons falling on the line of equality. This suggests that neither treatment is more effective for Participant 2 on the basis of the 3 treatment cycles undertaken. This approach can help to reveal both individual patient heterogeneity in response to treatments and whether there is a clearly “better” treatment overall, through aggregating results from multiple N-of-1 trials, if undertaken (1).
FIGURE 4.
Results from 2 hypothetical repeated-crossover N-of-1 trials using within-cycle randomization, to highlight hypothetical results of a study design with 3 cycles composed of 2 periods each (as shown in Figure 3). These graphs are a modification of those presented in Araujo et al. (1). (A) Triglyceride levels are plotted by cycle and period for 2 participants (labeled as 1 and 2). Note that the colors of the circles (black and white; representing assigned treatment) differ for both participants by treatment period, as each participant has been assigned to a different randomization sequence: results can still be aggregated and compared between individuals, as response to the 2 treatments can be compared by cycle. (B) Triglyceride levels after Treatment A and B for Participant 1, plotted by treatment cycle. Within each cycle, triglyceride levels are consistently lower after Treatment B than A, which suggests Treatment B is more effective for this participant. (C) Triglyceride levels after Treatment A and B for Participant 2, plotted by treatment cycle. Within each cycle, there is no clear association between treatment and triglyceride levels. This suggests neither treatment is effective for consistent triglyceride lowering for this participant.
A repeated-crossover N-of-1 design could feasibly be applied to study the response to a longer-term intervention, such as the effect of 2 alternative nutritional supplements on a more long-term health outcome. However, this would require a series of longer treatment cycles, which could be a considerable burden to a participant. For example, if each intervention took 8 wk to show a stable effect on blood pressure (33), with both interventions administered 3 times, this would result in a study of nearly a year in length. This could potentially lead to patients dropping out or high rates of noncompliance (i.e., forgetting to take the supplement). As a goal of N-of-1 research is to improve compliance, this example could throw the reasoning of doing an N-of-1 study into question. However, it is possible to keep a participant engaged with the study, provided they felt that their role was valued and that the results could be directly applied to improving their health (8, 16). As mentioned previously, an N-of-1 study can employ several tools to improve compliance, such as personalized measures and adapting schedules to suit participants. Especially in the context of a longer study, an important role of the researcher is supporting participants, particularly if the researchers are reliant on the participants collecting their own data or maintaining compliance to an intervention (20). Depending on the nature of the study, it may be appropriate for participants to receive feedback or to be reminded that the more they fulfil the requirements of the study, the more the results can be applied towards improving their health or modifying their behaviors in the future.
Repeated-crossover N-of-1 designs are particularly useful if a researcher is interested in determining the better of 2 interventions for an individual, or even for a group when aggregating results from several N-of-1 trials (1). For example, this type of study would be useful in trials of nutritional supplements, to determine which of 2 combinations of bioactives would be most effective for inclusion in a supplement. The results from a repeated-crossover N-of-1 trial can also reveal within-participant response variability and, if conducted on a number of individuals, can show whether within-participant variability is greater or less than the response variability seen between individuals (10). Indeed, a repeated-crossover N-of-1 trial with analyses on both the individual and group levels is currently being carried out in the context of a nutrition trial examining postprandial glycemic responses to 2 different diets (27).
Alternatively, an adaptive N-of-1 design could be used with repeated-crossover N-of-1 sequences of a longer duration. If it is clear that 1 intervention is superior to another for a particular individual, this design would deliver that intervention to the participant more frequently (15). If the goal of the study is to determine a future diet or treatment for the participant under study, this type of design can help reach a conclusion more quickly, saving extra effort for the participant and time for the researcher (34). For example, if administering a certain diet led to dangerous increases in blood glucose in a diabetic patient who was wearing a continuous glucose monitor, the diet could be terminated early and the participant could be moved to an alternative diet, which may help to stabilize blood glucose levels. This is also a more ethical form of study design, as it prevents a participant from progressing with any treatment that may lead to deleterious effects (15).
Therefore, repeated-crossover N-of-1 trials are versatile and could be applied to both short- and longer-term nutrition intervention settings. They can be similar to typical, group-based crossover trials, where single measurements could be provided at the end of each treatment occasion, with the difference being that each treatment would then be repeated at least once (or used to inform an adaptive treatment regimen). Alternatively, each treatment occasion could be used to study a participant in more detail, with multiple measurements of one, or several, outcomes [if appropriate for the outcome(s) under study] and explanatory variables being investigated in each period.
Aside from repeated-crossover trials, there are several other interventional N-of-1 designs that may be useful for addressing research questions within nutrition research. One may wish to study the effects of a single food or behavioral intervention upon 1 or more health outcomes of interest, and potentially, for how long the health effects continue after the intervention. For a disease marker that is unstable over time, such as blood pressure, triglycerides, or glucose, there may be greater merit in collecting several measurements over a single intervention period, than collecting (fewer) single measurements after successive intervention periods. For such markers, which can vary substantially between successive occasions, collecting multiple measurements provides a measure of within-participant variability, which can better inform whether a clinically relevant change in the outcome has occurred (35). A single intervention period may be the only option for an outcome that takes a significant period of time to show a stable treatment effect, where repeated intervention and control periods would not be feasible.
As well as measuring an outcome during an intervention, it is important to repeat any measures of interest with the same frequency during any observation periods. For example, an ABA design would involve an initial observation period (A) for examining the disease marker of interest (and any other factors) at baseline, followed by an intervention period (B) and a follow-up period (A) during which the intervention was withdrawn (8). The length of each period should enable a sufficient number of measurements to be collected (e.g., 50, if analyzing via dynamic modeling) (11). If the researcher is interested in observing effects after the intervention is withdrawn, then the subsequent observation period should be long enough for the treatment to “wash out” (1).
These types of interventional designs have many similarities with observational N-of-1 studies, due to repeated measurements of the outcomes and any other factors of interest. These designs are therefore also appropriate for examining the effect of an intervention on behavioral outcomes. In this instance, the lengths of the intervention and observation period(s) should be based on how long it is anticipated for changes in patterns of behavior to occur and be sustained. For a behavioral intervention that cannot be reversed or withdrawn, an AB design may be most appropriate for comparing behavior or health outcomes prior to and during an intervention (8). However, depending on the nature of the intervention, an ABA design may still be useful if the researcher is interested in monitoring whether any improvements continue or return to baseline after treatment.
Application of N-of-1 studies to nutrition research: considerations and challenges
The two classes of N-of-1 studies—observational and interventional—can be flexibly applied and adapted to research questions in nutrition. Both types of design involve monitoring of a participant over time, and often employ EMA to obtain repeated measurements of health markers, behaviors, or attitudes.
As N-of-1 studies generate a large amount of data on an individual level, they could be particularly useful for application to precision nutrition studies, which often collect large amounts of variable information on individual participants, including physiological, microbiome, and dietary intake data, along with more stable baseline information such as descriptive information and genetics (36). Although including repeated measurements of variable factors over the course of an N-of-1 study increases the participant burden, these measurements can also serve to retain participant interest if the researcher is able to build a profile on the participant that can be shared with them during or at the end of the study. As mentioned previously, repeated measurements of disease markers improve accuracy, providing participants with a better estimate of their “true” value; this can help in determining disease risk (37).
Particularly if studying less stable markers (e.g., blood pressure), there is a chance that different results could be obtained at different time periods; for example, due to seasonality effects (13) or if the participant experienced an acute event, such as a stressful experience, that may have affected their behavior or health for several weeks (38). This would mean that any results obtained would not be an accurate representation of their usual health. However, provided the participant was willing to provide information that might help in the interpretation of their results, this could provide an understanding of how their health can be affected in such circumstances. Conclusions from N-of-1 studies should therefore be interpreted with the consideration that they are not only specific to the individual, but to the time and nature of the study; the latter should also be remembered in the context of interpreting results from group-level trials (3).
N-of-1 studies can also help to address questions of a more behavioral nature within nutrition research. The use of EMA provides a participant the opportunity to respond in real time to factors that may influence their eating behavior or general health, such as where they spent their day, how they felt, and what they were doing (21). This could help in identifying factors that may negatively influence a participant's health. For example, using a daily diet-quality questionnaire may reveal that a participant snacks more on workdays than they thought, or their reporting on a sustained period of high stress might show a subsequent worsening of disease markers. Using the participant to collect their own data is also a useful approach when they are unable to attend a research center for any reason, as self-report measures or outcomes can be collected remotely.
Proposals for N-of-1 trials can face some criticism. There may be an attempt to understand the study from the perspective of a group-level trial, including concerns that there is a lack of statistical power owing to the low number of participants (or a single participant) under study. It should therefore be explained that statistical power is achieved from the number of measurements taken on an individual level (9). Funders or institutes may not see the utility in conducting trials on an individual level or may believe it is not worth the amount of effort and resources required to examine a small number of participants (34). Such criticisms can also affect the interpretation of results from an N-of-1 trial. However, individualized measures or single N-of-1 studies can help to identify variable factors that may affect health or behavior that could otherwise be overlooked, as analysis of time-course data can reveal associations which would be missed if fewer measurements were taken (11). To investigate whether such associations are useful for other, similar patients, aggregation of sets of N-of-1 studies can be useful, particularly if a goal is to determine whether 1 intervention is superior to another.
The field of personalized nutrition has been levelled with the criticism that delivering health advice on an individual level may widen health inequalities (26). Indeed, it is widely known that those of a lower socioeconomic status often have poorer diets (39) and higher burdens of disease (40). However, N-of-1 studies could be applied specifically to help investigate under-represented and heterogeneous individuals. As measures can be adapted to the individual level, factors relevant to the participant under study could be measured to investigate their potential effects upon eating behavior or health. By involving the participant in this process and tailoring the study to their needs, the researcher can identify those factors that are likely to be of greatest relevance (8), while helping the participant comply with and maintain interest in the study. Depending on the research question, the study need not have the strict recruitment criteria common for group-level studies looking for a homogeneous study population, which would rule out many potential participants (34). Comparison of sets of N-of-1 trials, either descriptively or through statistical aggregation, could then help to identify similarities and differences between participant outcomes, and why these may occur.
Conclusions
The amount and quality of data gained from an N-of-1 study can be used to identify outcomes and predictors on an individual level, unlike data from traditional, group-based studies. N-of-1 studies can be used to observe a participant's behavior over time, to examine variability in behavior as a response to an intervention, or to determine how long an intervention needs to be administered to see a biologically relevant effect. The design of an N-of-1 study should be considered carefully, with a focus on how often measurements should occur, how they will be taken, and the associated burden on the participant. If the participant is responsible for their own data collection, they should be appropriately supported by the researcher. This can include tailoring or personalizing aspects of the study, if relevant, to maintain compliance and interest in the study, while ensuring sufficient measurements can be collected to fulfil the aims of the research. Therefore, N-of-1 studies need not recruit a large pool of relatively homogeneous participants. When planning an N-of-1 study, it is important to design a statistical analysis plan appropriate for the N-of-1 design used and to consider how missing data will be handled (41) and how any perceived difficulties could be dealt with. Researchers should therefore consult appropriate methodological guides prior to designing N-of-1 studies (12). If designed appropriately, an N-of-1 study is an essential tool for understanding the individual, so should be considered for application to personalized and precision nutrition.
Acknowledgments
The authors’ responsibilities were as follows—TP: wrote the review; RV: structured the review and aided in content development, particularly relating to the statistical analysis of N-of-1 trials; BdR: reviewed the content and figures and supervised the writing process; and all authors: read and approved the final manuscript.
Notes
Supported by Biotechnology and Biological Sciences Research Council (BBSRC) and Unilever R&D, Foods Innovation Centre, Wageningen, The Netherlands [Collaborative Training Partnership (CTP) PhD grant; project reference BB/S506916/1].
Author disclosures: The authors report no conflicts of interest.
Perspective articles allow authors to take a position on a topic of current major importance or controversy in the field of nutrition. As such, these articles could include statements based on author opinions or point of view. Opinions expressed in Perspective articles are those of the author and are not attributable to the funder(s) or the sponsor(s) or the publisher, Editor, or Editorial Board of Advances in Nutrition. Individuals with different positions on the topic of a Perspective are invited to submit their comments in the form of a Perspectives article or in a Letter to the Editor.
Abbreviations used: EMA, Ecological Momentary Assessment; RCT, randomized controlled trial; SBP, systolic blood pressure.
Contributor Information
Tilly Potter, Rowett Institute, University of Aberdeen, United Kingdom.
Rute Vieira, Institute of Applied Health Sciences, University of Aberdeen, United Kingdom.
Baukje de Roos, Rowett Institute, University of Aberdeen, United Kingdom.
References
- 1. Araujo A, Julious S, Senn S. Understanding variation in sets of N-of-1 trials. PLoS One. 2016;11:e0167167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. de Roos B, Brennan L. Personalised interventions–a precision approach for the next generation of dietary intervention studies. Nutrients. 2017;9:847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hecksteden A, Kraushaar J, Scharhag-Rosenberger F, Theisen D, Senn S, Meyer T. Individual response to exercise training–a statistical perspective. J Appl Physiol. 2015;118:1450–9. [DOI] [PubMed] [Google Scholar]
- 4. Tighe P, Duthie G, Vaughan N, Brittenden J, Simpson WG, Duthie S, Mutch W, Wahle K, Horgan G, Thies F. Effect of increased consumption of whole-grain foods on blood pressure and other cardiovascular risk markers in healthy middle-aged persons: a randomized controlled trial. Am J Clin Nutr. 2010;92:733–40. [DOI] [PubMed] [Google Scholar]
- 5. McCormack JP, Holmes DT. Your results may vary: the imprecision of medical measurements. BMJ. 2020;368:m149. [DOI] [PubMed] [Google Scholar]
- 6. Atkinson G, Batterham AM. True and false interindividual differences in the physiological response to an intervention. Exp Physiol. 2015;100:577–88. [DOI] [PubMed] [Google Scholar]
- 7. Senn S. Statistical pitfalls of personalized medicine. Nature. 2018;563:619–21. [DOI] [PubMed] [Google Scholar]
- 8. McDonald S, Quinn F, Vieira R, O'Brien N, White M, Johnston DW, Sniehotta FF. The state of the art and future opportunities for using longitudinal N-of-1 methods in health behaviour research: a systematic literature overview. Health Psychol Rev. 2017;11:307–23. [DOI] [PubMed] [Google Scholar]
- 9. Senn S. Sample size considerations for N-of-1 trials. Stat Methods Med Res. 2019;28:372–83. [DOI] [PubMed] [Google Scholar]
- 10. Senn S, Rolfe K, Julious SA. Investigating variability in patient response to treatment–a case study from a replicate cross-over study. Stat Methods Med Res. 2011;20:657–66. [DOI] [PubMed] [Google Scholar]
- 11. Vieira R, McDonald S, Araújo-Soares V, Sniehotta FF, Henderson R. Dynamic modelling of N-of-1 data: powerful and flexible data analytics applied to individualised studies. Health Psychol Rev. 2017;11:222–34. [DOI] [PubMed] [Google Scholar]
- 12. Kravitz RL, Duan N editors; the The DEcIDE Methods Center N-of-1 Guidance Panel (DeMCN-1) . Design and implementation of N-of-1 trials: a user's guide. Rockville (MD): Effective Health Care Program; 2014. [Google Scholar]
- 13. Marti-Soler H, Gubelmann C, Aeschbacher S, Alves L, Bobak M, Bongard V, Clays E, de Gaetano G, Di Castelnuovo A, Elosua Ret al. Seasonality of cardiovascular risk factors: an analysis including over 230 000 participants in 15 countries. Heart Br Card Soc. 2014;100:1517–23. [DOI] [PubMed] [Google Scholar]
- 14. Kwasnicka D, Naughton F. N-of-1 methods: a practical guide to exploring trajectories of behaviour change and designing precision behaviour change interventions. Psychol Sport Exerc. 2019;101570. [Google Scholar]
- 15. Schork NJ, Goetz LH. Single-subject studies in translational nutrition research. Annu Rev Nutr. 2017;37:395–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Brookes ST, Biddle L, Paterson C, Woolhead G, Dieppe P. “Me's me and you's you”: exploring patients’ perspectives of single patient (N-of-1) trials in the UK. Trials. 2007;8:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kristensen M, Pelletier X, Ross A, Thielecke F. A high rate of non-compliance confounds the study of whole grains and weight maintenance in a randomised intervention trial–the case for greater use of dietary biomarkers in nutrition intervention studies. Nutrients. 2017;9:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bentley KH, Kleiman EM, Elliott G, Huffman JC, Nock MK. Real-time monitoring technology in single-case experimental design research: opportunities and challenges. Behav Res Ther. 2019;117:87–96. [DOI] [PubMed] [Google Scholar]
- 19. Dover AR, Stimson RH, Zammitt NN, Gibb FW. Flash glucose monitoring improves outcomes in a type 1 diabetes clinic. J Diabetes Sci Technol. 2017;11:442–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. McDonald S, Vieira R, Godfrey A, O'Brien N, White M, Sniehotta FF. Changes in physical activity during the retirement transition: a series of novel N-of-1 natural experiments. Int J Behav Nutr Phys Act. 2017;14:167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. McMinn D, Allan JL. The SNAPSHOT study protocol: snacking, physical activity, self-regulation, and heart rate over time. BMC Public Health. 2014;14:1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jamison RN, Raymond SA, Levine JG, Slawsby EA, Nedeljkovic SS, Katz NP. Electronic diaries for monitoring chronic pain: 1-year validation study. Pain. 2001;91:277–85. [DOI] [PubMed] [Google Scholar]
- 23. Berry SE, Valdes AM, Drew DA, Asnicar F, Mazidi M, Wolf J, Capdevila J, Hadjigeorgiou G, Davies R, Al Khatib Het al. Human postprandial responses to food and potential for precision nutrition. Nat Med. 2020;26:964–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shiffman S, Stone AA, Hufford MR. Ecological Momentary Assessment. Annu Rev Clin Psychol. 2008;4:1–32. [DOI] [PubMed] [Google Scholar]
- 25. Calhoun DA, Harding SM. Sleep and hypertension. Chest. 2010;138:434–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chatelan A, Bochud M, Frohlich KL. Precision nutrition: hype or hope for public health interventions to reduce obesity?. Int J Epidemiol. 2019;48:332–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tian Y, Ma Y, Fu Y, Zheng J-S. Application of N-of-1 clinical trials in personalized nutrition research: a trial protocol for Westlake N-of-1 trials for macronutrient intake (WE-MACNUTR). Curr Dev Nutr. 2020;4:nzaa143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Golden E, Johnson M, Jones M, Viglizzo R, Bobe J, Zimmerman N. Measuring the effects of caffeine and L-theanine on cognitive performance: a protocol for self-directed, mobile N-of-1 studies. Front Comput Sci. 2020;2:4. [Google Scholar]
- 29. McMillan G, Dixon D. Self-regulatory processes, motivation to conserve resources and activity levels in people with chronic pain: a series of digital N-of-1 observational studies. Front Psychol. 2020;11:516485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Allan J, Mcminn D, Powell D. Tracking snacking in real time: time to look at individualised patterns of behaviour. Nutr Health. 2019;25:179–84. [DOI] [PubMed] [Google Scholar]
- 31. The Diabetes Remission Clinical Trial (DiRECT): protocol for a cluster randomised trial. BMC Fam Pract. 2016;17:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Draper CF, Duisters K, Weger B, Chakrabarti A, Harms AC, Brennan L, Hankemeier T, Goulet L, Konz T, Martin FPet al. Menstrual cycle rhythmicity: metabolic patterns in healthy women. Sci Rep. 2018;8: 14568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. European Food Safety Authority (EFSA) Panel on Dietetic Products, Nutition and Allergies (EFSA NP); Turck D, Bresson J-L, Burlingame B, Dean T, Fairweather-Tait S, Heinonen M, Hirsch-Ernst KI, Mangelsdorf I, McArdle HJet al. Guidance for the scientific requirements for health claims related to antioxidants, oxidative damage and cardiovascular health (Revision 1). EFSA J. 2018;16:5136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kravitz RL, Duan N, Niedzinski EJ, Hay MC, Subramanian SK, Weisner TS. What ever happened to N-of-1 trials? Insiders’ perspectives and a look to the future. Milbank Q. 2008;86:533–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Swinton PA, Hemingway BS, Saunders B, Gualano B, Dolan E. A statistical framework to interpret individual response to intervention: paving the way for personalized nutrition and exercise prescription. Front Nutr. 2018;5:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Schüssler-Fiorenza Rose SM, Contrepois K, Moneghetti KJ, Zhou W, Mishra T, Mataraso S, Dagan-Rosenfeld O, Ganz AB, Dunn J, Hornburg Det al. A longitudinal big data approach for precision health. Nat Med. 2019;25:792–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Vohra S, Shamseer L, Sampson M, Bukutu C, Schmid CH, Tate R, Nikles J, Zucker DR, Kravitz R, Guyatt Get al. CONSORT extension for reporting N-of-1 trials (CENT) 2015 statement. BMJ. 2015;350:h1738. [DOI] [PubMed] [Google Scholar]
- 38. Rozanski A, Blumenthal JA, Kaplan J. Impact of psychological factors on the pathogenesis of cardiovascular disease and implications for therapy. Circulation. 1999;99:2192–217. [DOI] [PubMed] [Google Scholar]
- 39. Maguire ER, Monsivais P. Socio-economic dietary inequalities in UK adults: an updated picture of key food groups and nutrients from national surveillance data. Br J Nutr. 2015;113:181–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Public Health England . Public health outcomes framework: health equity report. London (UK): Public Health England; 2017. [Google Scholar]
- 41. McDonald S, Vieira R, Johnston DW. Analysing N-of-1 observational data in health psychology and behavioural medicine: a 10-step SPSS tutorial for beginners. Health Psychol Behav Med. 2020;8:32–54. [DOI] [PMC free article] [PubMed] [Google Scholar]