ABSTRACT
Data on the association of nut intake with risk of cancer and its mortality are conflicting. Although previous meta-analyses summarized available findings in this regard, some limitations may distort their findings. Moreover, none of these meta-analyses examined the dose-response associations of total nut intake with the risk of specific cancers as well as associations between specific types of nuts and cancer mortality. Therefore, this study aimed to summarize available findings on the associations of total nut (tree nuts and peanuts), tree nut (walnuts, pistachios, macadamia nuts, pecans, cashews, almonds, hazelnuts, and Brazil nuts), peanut (whole peanuts without considering peanut butter), and peanut butter consumption with risk of cancer and its mortality by considering the above-mentioned points. We searched the online databases until March 2020 to identify eligible articles. In total, 43 articles on cancer risk and 9 articles on cancer mortality were included in the current systematic review and meta-analysis. The summary effect size (ES) for risk of cancer, comparing the highest with lowest intakes of total nuts, was 0.86 (95% CI: 0.81, 0.92, P < 0.001, I2 = 58.1%; P < 0.01), indicating a significant inverse association. Such a significant inverse association was also seen for tree nut intake (pooled ES: 0.87, 95% CI: 0.78–0.96, P < 0.01, I2 = 15.8%; P = 0.28). Based on the dose-response analysis, a 5-g/d increase in total nut intake was associated with 3%, 6%, and 25% lower risks of overall, pancreatic, and colon cancers, respectively. In terms of cancer mortality, we found 13%, 18%, and 8% risk reductions with higher intakes of total nuts, tree nuts, and peanuts, respectively. In addition, a 5-g/d increase in total nut intake was associated with a 4% lower risk of cancer mortality. In conclusion, our findings support the protective association between total nut and tree nut intake and the risk of cancer and its mortality.
Keywords: nuts, peanuts, cancer, mortality, meta-analysis
Introduction
The prevalence of cancer is increasing at an alarming rate (1); such that in 2015, it was the second leading cause of death and resulted in over 8.7 million deaths worldwide (2). Cancer is associated with high disability and early mortality, as well as imposing a high economic burden on health care systems (3, 4). Therefore, finding appropriate approaches to prevent cancer is urgently required.
Several approaches including dietary modifications have been proposed for this purpose (4–6). Nuts are key components of healthy dietary patterns. This food group is a rich source of vitamins B-6 and E, folate, selenium, fiber, monounsaturated fatty acids (MUFAs) and polyunsaturated fatty acids (PUFAs), and many polyphenols (7). Nuts have recently been hypothesized to exhibit antitumor and cancer-chemopreventive properties because of their compounds that exert antioxidant, anti-inflammatory, and immune-modulating activities (8). However, data on the associations between nut consumption and the risk of cancer and its mortality are conflicting (9–60). In the Netherlands Cohort Study, tree nut and peanut consumption was inversely associated with the risk of gastric and esophageal cancers (21). In addition, in a prospective study in Taiwan, peanut consumption was associated with a reduced risk of colorectal cancer (34). In contrast, findings from the Nurses’ Health Study revealed no significant association between frequent nut consumption and risk of colorectal cancer (33). Regarding cancer mortality, Bonaccio et al. (54) reported an inverse association between nut consumption and risk of cancer mortality, whereas 2 studies did not find any significant association in this regard (9, 33).
Two previously published meta-analyses summarized available findings on the link between total nut intake and overall risk of cancer (61, 62); however, several limitations may distort their findings. For instance, in the meta-analysis of Long et al. (61), the authors did not include an eligible study (31) and included an ineligible study in which the intake of fatty acids (from nuts) was investigated in relation to cancer incidence (63). Furthermore, in the meta-analysis of Wu et al. (62), the authors included studies in which a combination of fruit, nut, seed, and legume intake, rather than nut intake alone, was considered as the exposure variable (64, 65). None of these meta-analyses examined the dose-response associations of total and specific types of nut intake with the risk of overall and specific types of cancers. In terms of cancer mortality, there are 2 meta-analyses (66, 67); however, both did not include an eligible study (58) and the meta-analysis of Aune et al. (66) missed another study that met the required criteria for inclusion (60). Furthermore, both meta-analyses on cancer mortality did not examine the dose-response associations between different types of nuts and cancer mortality.
Given the above-mentioned points, a comprehensive meta-analysis is required to consider these issues. Therefore, the current systematic review and meta-analysis of observational studies was conducted to examine the associations between total nut, tree nut, peanut, and peanut butter consumption and risk of cancer and its mortality by summarizing available findings in this regard.
Methods
The current systematic review and meta-analysis were designed, conducted, and reported in adherence to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) (68).
Search strategy
We searched the online databases including PubMed, Scopus, Clarivate Web of Science, and Google Scholar until March 2020 to identify observational studies that examined the association between nut consumption and risk of cancer or its mortality. The keywords used in the search strategy are shown in Supplemental Table 1. No restrictions in terms of publication time or the language of articles were considered. In addition, the reference lists of selected articles were searched to identify studies that might have been missed.
Inclusion criteria
We included studies with the following criteria: 1) observational studies with prospective, case-control, or cross-sectional designs; 2) studies that considered the intake of total nuts (tree nuts and peanuts), tree nuts (walnuts, pistachios, macadamia nuts, pecans, cashews, almonds, hazelnuts, and Brazil nuts), peanuts (whole peanuts without considering peanut butter), and peanut butter as the exposure variable, and risk of cancer or its mortality as the outcome variable; 3) those performed on adults (≥18 y); 4) those studies that reported ORs or RRs or HRs along with 95% CIs for the association between nut consumption and risk of cancer or its mortality. If findings from 1 dataset were published in >1 article, we selected the most recent version; otherwise, the 1 with the greatest number of cases was included.
Exclusion criteria
We excluded letters, comments, short communications, reviews, meta-analyses, ecological studies, and animal studies. Studies that investigated the relation between nut consumption and cancer recurrence were also excluded. Moreover, we did not include studies that assessed a dietary pattern containing a high amount of nuts in relation to cancer.
Data extraction
Required data from each eligible study were extracted by 2 independent investigators, and any disagreements were reconciled by discussion. Any reported ORs or HRs or RRs and corresponding 95% CIs for the association between nut consumption and risk of cancer or its mortality were extracted from each study. In addition to effect sizes (ESs), the following information was extracted: first author's name, year of publication, country of origin, demographic characteristics of participants (age range or mean age and gender), number of participants and cases, duration of follow-up for prospective studies, methods used for dietary assessment and the diagnosis of cancer, and confounding variables adjusted in the statistical analysis. If a study reported its findings based on gender or any other variables, then we considered that study as 2 separate studies.
Quality assessment
The quality of studies included in the current meta-analysis was assessed using the Newcastle Ottawa Scale (NOS), designed for nonrandomized studies (69). According to this scale, a maximum of 9 points would be awarded to each study according to the following parameters: 4 points for selection of participants, 2 points for comparability, and 3 points for the assessment of outcomes. Studies achieving 9 points were considered to provide the highest quality.
Statistical analysis
The ORs, RRs, and HRs (and 95% CIs) were considered the ESs of all studies. To calculate the summary ES for the comparison of the highest versus lowest categories of nut consumption, a random-effects model was used to take between-study heterogeneity into account (70). Using the random-effects model, we also calculated both Q-statistic and I2 values as the indicators of heterogeneity. I2 values of >50% were considered significant between-study heterogeneity (71). In case of significant between-study heterogeneity, we conducted subgroup analysis based on participants’ gender, duration of follow-up, sample size, geographical location, methods used for the assessment of nut intake, type of ES, verification of breast cancer, study design, and adjustment for confounding variables including energy intake and BMI to detect possible sources of heterogeneity. Publication bias was examined using the Egger's regression asymmetry test (72). A trim-and-fill method was used to detect the effect of probable missing studies on the overall effect (73). We also performed a sensitivity analysis using a fixed-effects model in which each study was excluded to examine the influence of that study on the overall estimate.
A method suggested by Greenland and Longnecker (74) and Orsini et al. (75) was used to compute the trend from the OR/RR/HR estimates and their CIs across categories of nut consumption. In this method, the distributions of sample size, cases, and the ORs/RRs/HRs with the variance estimates for ≥3 quantitative categories of exposure were required. Therefore, studies with missing data on the sample size and number of cases in each category of exposure, and those with <3 quantitative categories of exposure were excluded from the dose-response analysis. We considered the midpoint of nut consumption in each category as the corresponding OR/RR/HR estimate. For studies that reported the nut consumption as a range, we estimated the midpoint in each category by calculating the mean of the lower and upper bound. When the highest and lowest categories were open-ended, the length of these open-ended intervals was assumed to be the same as that of the adjacent intervals. If a study did not report the midpoint in each category of nut consumption or it was not computable, we excluded that study from the dose-response analysis. A 2-stage random-effects dose-response meta-analysis was applied to examine a possible nonlinear association between nut consumption and risk of cancer and its mortality. This was done through the modeling of nut consumption and restricted cubic splines with 4 knots at fixed percentiles of 5, 35, 65, and 95% of the distribution. Based on the Orsini method (75), we calculated restricted cubic spline models using the generalized least-squares trend estimation method, which takes into account the correlation within each set of reported ORs/RRs/HRs. Then, all the study-specific estimates were combined using the restricted maximum likelihood method in a multivariate random-effects meta-analysis (76). A probability value for nonlinearity was estimated using null hypothesis testing in which the coefficient of the second spline was considered equal to 0. In addition to nonlinear associations, linear dose-response associations between nut consumption and risk of cancer and its mortality were investigated using the 2-stage generalized least-squares trend estimation method. First, study-specific slope lines were estimated, and then, these lines were combined to obtain an overall average slope (75). Study-specific slope lines were combined using a random-effects model. Statistical analyses were conducted using STATA version 14.0 (StataCorp). P < 0.05 was considered statistically significant for all tests, including Cochran's Q test.
Results
Literature search
We identified 2571 articles in our initial search. After exclusion of duplicate articles and those that did not meet the inclusion criteria, we identified 61 full-text articles of potentially relevant studies. After full-text review, we excluded an additional 4 studies because they considered a combination of intakes of nuts, legume, fruits, seeds, or pulses, not nut intake alone, as the exposure variable (64, 77, 78, 65). Two studies assessed the intake of fatty acids from nuts in relation to the risk of cancer and therefore were excluded (63, 79). We also excluded the study of Berkey et al. (80) in which the risk of benign breast disease was estimated rather than breast cancer. Also, we found 4 duplicate articles (11, 59, 81, 82), of them, 2 were published on the Nurses’ Health Study II (NHS II) (11, 81) and 2 on the Netherlands Cohort Study (59, 82). Since these articles assessed similar exposure and outcome variables, we included only the 1 with higher quality or with the most number of cases for each dataset (11, 59) and excluded the duplicate articles (81, 82). Moreover, we found other articles published on the same datasets, but since they assessed different types of nuts or outcome variables (i.e. different cancers), we included them in this meta-analysis. After the above-mentioned exclusions, 52 articles including 34 articles from prospective studies (9–17, 19–34, 52–60), 17 articles from case-control studies (35–51), and 1 article with both prospective and case-control datasets (18) were enrolled for the current meta-analysis. Among them, 43 articles reported ESs for cancer risk (9–51) and 9 articles reported estimates for risk of cancer mortality (52–60). Dietary intakes of total nuts (n = 38), tree nuts (n = 7), peanuts (n = 14), and peanut butter (n = 8) were assessed among 43 publications on cancer risk. Out of 9 publications on cancer mortality, all assessed total nut intake (52–60), 2 assessed tree nut intake (53, 59), 3 articles considered peanut consumption (57–59), and 3 articles assessed peanut butter intake as the exposure variable (57–59). Data on other specific types of nuts including walnuts (n = 2) and hazelnuts (n = 1) were not sufficient for a meta-analysis. A flow diagram of the study selection is shown in Figure 1.
FIGURE 1.
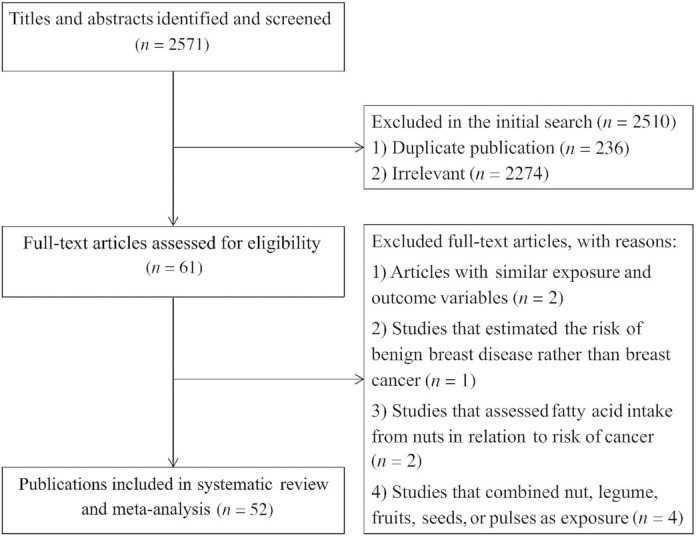
Flow diagram of the study selection.
Characteristics of included studies
The characteristics of included studies are provided in Supplemental Tables 2 and 3. The total number of participants in these studies ranged from 220 to 495,785 people, with an age range between 18 and 87 y. In total, 1,739,414 participants (prospective studies = 1,714,983 and case-control studies = 24,431) were enrolled in the 52 publications included in the current systematic review. In the case of multiple publications of the same dataset, only the 1 with the largest sample size was considered for the calculation. During the follow-up periods, ranging from 4.8 to 30 y, the total number of cancer cases was 64,699 and the number of cancer deaths was 48,038. Six studies included only men (9, 19, 32, 37, 39, 42), and 13 publications involved women (10, 11, 15, 23, 27, 29, 30, 33, 35, 41, 43, 45, 50). The remaining 33 articles were conducted on both genders, in which 4 articles reported ESs separately for men and women (20, 22, 34, 53). All cohort studies, particularly those on cancer mortality, were done on healthy individuals. Out of 52 articles, 16 articles were from the USA, 35 articles from non-USA countries, and 1 study was performed on both Chinese and American populations. Dietary intake of nuts was assessed using FFQs in 46 articles, a research-made questionnaire in 4 publications, and both FFQ and dietary recall in 2 articles. Among the included studies, total nut consumption consisted of peanut and tree nut intake, tree nut consumption was defined as the intake of walnuts, pistachios, macadamia nuts, pecans, cashews, almonds, hazelnuts, and Brazil nuts, and peanut consumption was assessed without considering peanut butter intake. Among the included cohort studies, 29 articles used baseline data of nut intake in their analysis (single measurement), whereas 6 studies considered the average nut intake throughout the follow-up (repeated measurements) as the main exposure (10, 28, 32, 33, 53, 57). In most articles, ESs were adjusted for age (n = 51), BMI (n = 24), smoking (n = 22), alcohol consumption (n = 14), physical activity (n = 14), energy intake (n = 25), and other dietary variables (n = 14). Also, 6 studies controlled their analysis for the background diet of participants (22, 24, 32, 52, 54, 60). The NOS scores of the included studies ranged between 5 and 8. Looking at the variation of NOS score and considering the score of 7 as the median for a total score of NOS, 28 articles had a score of ≥7, defined as the high-quality studies (Supplemental Tables 4 and 5).
Findings from the systematic review
Out of 38 articles on the association between total nut intake and cancer risk, 10 articles indicated an inverse association (10, 13, 14, 18, 21, 38, 41, 43, 45, 49), 1 study showed a positive association (48), and others illustrated no significant association. For tree nut intake and cancer risk, 2 studies reported an inverse association (21, 28), whereas others indicated no significant association. For peanut intake, none of the articles revealed a significant association between peanut intake and cancer incidence. Regarding peanut butter, 1 study showed an inverse association between peanut butter intake and cancer risk (13). In the case of cancer mortality, 6 studies showed a protective association between total nut intake and cancer mortality (52–54, 57–59). Such an inverse association was also seen for peanut intake in 1 study (59) and tree nut intake in another study (53), whereas others did not find any significant association.
Findings from the meta-analysis on total nut intake and risk of cancer
Thirty-eight studies with a total of 1,436,744 participants and 63,844 cancer cases were included in this association (9–30, 32, 33, 35, 36, 38–43, 45, 47–50). The summary ES for the risk of overall cancer, comparing the highest with the lowest intake of total nuts, was 0.86 (95% CI: 0.81–0.92, P < 0.001), indicating a significant inverse association (Table 1). However, there was evidence of significant heterogeneity between studies (I2 = 58.1%; P < 0.01). Findings from subgroup analyses revealed that study design, gender, sample size, follow-up duration, methods used for the diagnosis of cancer and the assessment of nut intake, type of ES, and cancer type explained the between-study heterogeneity. These analyses showed a significant inverse association between total nut intake and risk of overall cancer in most subgroups of studies, particularly prospective cohort studies, those that controlled their analysis for energy intake and BMI, and studies that reported HR as an ES.
TABLE 1.
Summary risk estimates for the association between total nut intake and risk of cancer in adults aged ≥18 y1
| n 2 | Pooled ES (95% CI)3 | I 2 (%)4 | Q-statistic5 | |
|---|---|---|---|---|
| The highest vs. lowest comparison of total nut intake6 | ||||
| Overall cancer | 48 | 0.86 (0.81, 0.92)* | 58.1 | 112.0* |
| Study design | ||||
| Prospective | 33 | 0.90 (0.86, 0.94)* | 10.9 | 35.9 |
| Case-control | 15 | 0.77 (0.60, 0.97)* | 81.3 | 74.7* |
| Gender | ||||
| Male | 9 | 0.93 (0.82, 1.05) | 47.3 | 15.1 |
| Female | 16 | 0.91 (0.85, 0.98)* | 9.4 | 16.5 |
| Both | 23 | 0.86 (0.81, 0.92)* | 68.5 | 69.8* |
| Geographical region | ||||
| USA | 17 | 0.86 (0.79, 0.92)* | 47.9 | 30.6* |
| Non-USA countries | 31 | 0.87 (0.79, 0.96)* | 62.8 | 80.6* |
| Sample size, individuals | ||||
| <10,000 | 28 | 0.83 (0.73, 0.94)* | 71 | 93.2 |
| ≥10,000 | 20 | 0.89 (0.85, 0.92)* | 0 | 18.7 |
| Follow-up, year | ||||
| <15 | 9 | 0.84 (0.80, 0.99)* | 2.6 | 8.2 |
| ≥15 | 24 | 0.90 (0.85, 0.94)* | 16.9 | 27.6 |
| Nut intake assessment | ||||
| Only FFQ | 45 | 0.86 (0.80, 0.92)* | 60.0 | 109.8* |
| FFQ and dietary recall | 3 | 0.94 (0.81, 1.09) | 0 | 1.6 |
| Cancer assessment | ||||
| Medical records | 33 | 0.87 (0.81, 0.93)* | 57.2 | 74.8* |
| Pathological or histological | 15 | 0.85 (0.71, 1.02) | 61.9 | 36.7* |
| Adjustment for energy | ||||
| Yes | 41 | 0.86 (0.81, 0.92)* | 56.9 | 92.1* |
| No | 7 | 0.92 (0.68, 1.24) | 68.6 | 19.1* |
| Adjustment for BMI | ||||
| Yes | 39 | 0.86 (0.81, 0.92)* | 58.4 | 91.4* |
| No | 9 | 0.90 (0.67, 1.23) | 60.3 | 20.1* |
| Effect size type | ||||
| HR | 28 | 0.89 (0.85, 0.94)* | 18.5 | 33.1 |
| RR | 7 | 0.92 (0.73, 1.16) | 60.1 | 15.0* |
| OR | 13 | 0.73 (0.57, 0.92)* | 79.9 | 59.6* |
| Specific cancers | ||||
| Prostate | 6 | 0.96 (0.85, 1.10) | 43.2 | 8.8 |
| Colorectal | 3 | 0.64 (0.39, 1.05) | 92 | 24.8* |
| Colon | 5 | 0.61 (0.43, 0.85)* | 75.9 | 16.6* |
| Rectal | 3 | 0.68 (0.34, 1.37) | 89.2 | 18.5* |
| Lung | 5 | 0.85 (0.81, 0.90)* | 0 | 1.7 |
| Esophageal | 5 | 0.84 (0.65, 1.09) | 51 | 8.1 |
| Gastric | 7 | 0.88 (0.68, 1.13) | 64.6 | 16.9* |
| Pancreatic | 5 | 0.83 (0.72, 0.97)* | 0 | 3.8 |
| Liver | 1 | 0.84 (0.56, 1.26) | — | — |
| Breast | 5 | 0.92 (0.84, 1.02) | 14.2 | 4.6 |
| Ovarian | 3 | 0.97 (0.78, 1.21) | 0 | 1.5 |
| Leukemia | 3 | 0.69 (0.32, 1.52) | 87.3 | 15.7* |
| Endometrial | 2 | 0.77 (0.30, 2.02) | 85.2 | 6.7* |
| Glioma | 2 | 1.15 (0.75, 1.75) | 0 | 0.8 |
| Linear dose-response association (per 5-g/d increase in total nut intake)6 | ||||
| Overall cancer | 32 | 0.97 (0.95, 0.98)* | 61.7 | 80.8* |
| Study design | ||||
| Prospective | 29 | 0.98 (0.96, 0.99)* | 31.4 | 40.8 |
| Case-control | 3 | 0.78 (0.61, 1.01) | 94.9 | 39.4* |
| Specific cancers | ||||
| Colorectal | 3 | 0.78 (0.59, 1.03) | 93.5 | 30.9* |
| Colon | 4 | 0.75 (0.60, 0.94)* | 84.8 | 19.6* |
| Rectal | 3 | 0.80 (0.53, 1.20) | 90 | 21.9* |
| Lung | 4 | 0.97 (0.95, 0.98)* | 0 | 1.9 |
| Esophageal | 5 | 0.91 (0.80, 1.04) | 59 | 9.7* |
| Gastric | 4 | 0.93 (0.83, 1.04) | 61.4 | 7.7* |
| Pancreatic | 5 | 0.94 (0.89, 0.99)* | 16.9 | 4.8 |
| Breast | 4 | 0.98 (0.96, 0.99)* | 0 | 0 |
ES, effect size; RR, relative risk.
Number of effect sizes.
Obtained from the random-effects model.
Inconsistency – the percentage of variation across studies due to heterogeneity.
Obtained from the Q-test.
Total nut intake consisted of tree nut and peanut consumption.
P value < 0.05.
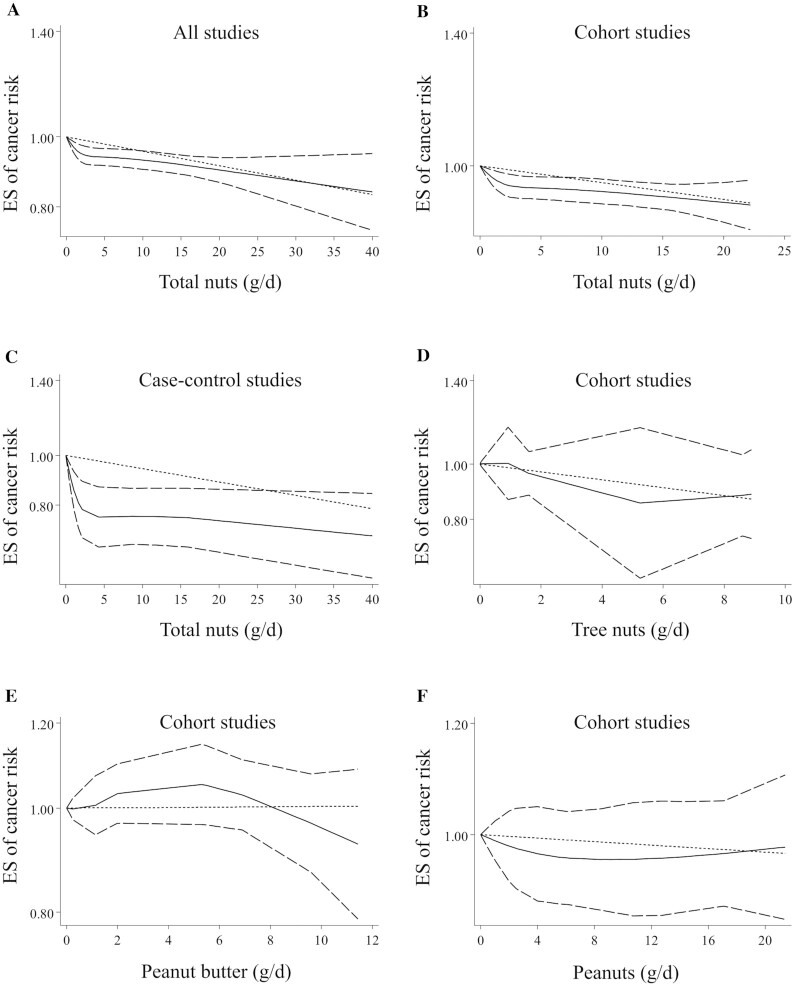
Twenty-two articles with sufficient data for inclusion in the dose-response analysis were identified (9–14, 16, 18, 20–25, 27, 28, 30–33, 38, 41). We found that a 5-g/d increase in total nut intake was associated with a 3% lower risk of overall cancer (pooled ES: 0.97, 95% CI: 0.95–0.98, P < 0.01) (Table 1). Excluding 3 case-control studies (18, 38, 41) and retaining only prospective studies, the linear association remained significant (pooled ES: 0.98, 95% CI: 0.96–0.99, P < 0.01). Findings from the nonlinear dose-response meta-analysis showed a nonlinear association between total nut intake and risk of overall cancer (P-nonlinearity < 0.001) (Figure 2A), in which the risk decreased continuously with increasing total nut intake from zero to higher amounts. However, a steeper decrease in the risk was seen from 0 to 3 g/d. Such a nonlinear association was observed when the analysis was confined to prospective studies (P-nonlinearity < 0.001) (Figure 2B). However, it was not seen among case-control studies (P-nonlinearity = 0.09) (Figure 2C).
FIGURE 2.
Nonlinear dose-response associations between total nut (A, B, C), tree nut (D), peanut butter (E), and peanut (F) intake and risk of overall cancer in adults aged ≥18 y among all (A), cohort (B, D, E, F), and case-control studies (C). Dietary intake of nuts was modeled in a random-effects model using restricted cubic splines with knots fixed at the 5th, 35th, 65th, and 95th percentiles of the distribution. The dotted line indicates the linear model. The solid line represents point estimates of the association between nut intake and ESs. The dashed line presents the 95% CI. ES, effect size.
Findings from the meta-analysis on different types of nuts and risk of cancer
Tree nut intake in relation to cancer risk was examined in 7 articles with a prospective design that included 168,022 participants and 11,641 cases of cancer (9, 20–23, 28, 30). Comparing the highest with the lowest intake of tree nuts, a significant inverse association was seen between tree nut intake and risk of overall cancer (pooled ES: 0.87, 95% CI: 0.78–0.96, P < 0.01), with no significant heterogeneity among the studies (I2 = 15.8%; P = 0.28) (Table 2). Based on the dose-response analysis on 5 articles with complete data (9, 22, 23, 28, 30), tree nut intake was not dose-dependently associated with overall cancer risk (Table 2 and Figure 2D). Also, there was no significant association between tree nut intake and specific types of cancer (Table 2). The association between peanut butter intake and cancer risk was assessed in 8 prospective studies with a total sample size of 655,099 individuals and 14,305 cancer cases (9, 13, 20–23, 28, 30) and with complete data for the dose-response analysis. No significant association was found either in the highest versus lowest comparison (pooled ES: 0.97, 95% CI: 0.88–1.07, P = 0.49) or in the dose-response analyses (Table 2 and Figure 2E). Between-study heterogeneity was not significant in this regard (I2 = 33.9%; P = 0.10). This association remained nonsignificant for specific types of cancer (Table 2).
TABLE 2.
Summary risk estimates for the association between specific types of nuts and risk of cancer in adults aged ≥18 y1
| n 2 | Pooled ES (95% CI)3 | I 2 (%)4 | Q-statistic5 | |
|---|---|---|---|---|
| The highest vs. lowest comparison of nut intake | ||||
| Tree nuts6 (all prospective) | ||||
| Overall cancer | 13 | 0.87 (0.78–0.96)* | 15.8 | 14.2 |
| Specific cancers | ||||
| Lung | 2 | 0.90 (0.77–1.06) | 0 | 0.6 |
| Esophageal | 2 | 0.79 (0.42–1.49) | 79.4 | 4.8* |
| Gastric | 2 | 0.85 (0.71–1.02) | 0 | 0.4 |
| Pancreatic | 2 | 0.65 (0.40–1.03) | 0 | 0.01 |
| Peanut butter (all prospective) | ||||
| Overall cancer | 17 | 0.97 (0.88–1.07) | 33.9 | 24.2 |
| Specific cancers | ||||
| Lung | 2 | 0.95 (0.75–1.19) | 0 | 0.1 |
| Esophageal | 4 | 1.09 (0.83–1.42) | 53.4 | 6.4 |
| Gastric | 4 | 0.88 (0.72–1.08) | 45.5 | 5.5 |
| Pancreatic | 2 | 0.75 (0.54–1.06) | 0 | 0.58 |
| Peanuts7 | ||||
| Overall cancer | 21 | 0.92 (0.81–1.05) | 52.4 | 41.9* |
| Study design | ||||
| Prospective | 16 | 0.94 (0.86–1.02) | 4.8 | 15.7 |
| Case-control | 5 | 1.19 (0.50–2.82) | 84.6 | 25.9* |
| Gender | ||||
| Male | 5 | 0.98 (0.82–1.19) | 49.5 | 7.9 |
| Female | 7 | 0.87 (0.70–1.09) | 48 | 11.5 |
| Both | 9 | 0.94 (0.70–1.26) | 62.7 | 21.1* |
| Geographical region | ||||
| USA | 3 | 0.96 (0.75–1.22) | 0 | 1.1 |
| Non-USA countries | 18 | 0.91 (0.78–1.06) | 58.4 | 40.8* |
| Sample size, individuals | ||||
| <10,000 | 17 | 0.97 (0.84–1.12) | 53.2 | 34.1* |
| ≥10,000 | 4 | 0.74 (0.58–0.94)* | 3.3 | 3.1 |
| Follow-up, year | ||||
| <15 | 3 | 0.68 (0.48–0.95)* | 0 | 8.6 |
| ≥15 | 13 | 0.97 (0.89–1.05) | 18.1 | 2.4 |
| Nut intake assessment | ||||
| FFQ | 15 | 0.94 (0.86–1.03) | 5.1 | 14.7 |
| Other instruments | 6 | 1.05 (0.53–2.07) | 81.6 | 27.2* |
| Cancer assessment | ||||
| Medical records | 17 | 0.94 (0.82–1.07) | 49.1 | 31.4* |
| Pathological or histological | 4 | 0.80 (0.50–1.30) | 70.3 | 10.0* |
| Adjustment for energy | ||||
| Yes | 17 | 0.91 (0.82–1.01) | 23.3 | 20.8 |
| No | 4 | 1.57 (0.56–4.40) | 82.3 | 16.9* |
| Adjustment for BMI | ||||
| Yes | 17 | 0.91 (0.82–1.01) | 23.3 | 20.8 |
| No | 4 | 1.57 (0.56–4.40) | 82.3 | 16.9* |
| Effect size type | ||||
| HR | 14 | 0.96 (0.88–1.05) | 0 | 9.3 |
| RR | 2 | 0.58 (0.34–1.00) | 37.3 | 1.5 |
| OR | 5 | 1.19 (0.50–2.82) | 84.6 | 25.9* |
| Specific cancers | ||||
| Colorectal | 2 | 0.58 (0.34–0.99)* | 37.3 | 1.5 |
| Lung | 2 | 0.92 (0.77–1.10) | 0 | 0.5 |
| Esophageal | 3 | 0.87 (0.61–1.25) | 35.7 | 3.1 |
| Gastric | 2 | 0.88 (0.69–1.13) | 0 | 0.3 |
| Pancreatic | 2 | 0.86 (0.64–1.16) | 0 | 0.1 |
| Liver | 5 | 1.30 (0.65–2.57) | 80.2 | 20.1* |
| Endometrial | 2 | 0.76 (0.32–1.80) | 81.6 | 5.4* |
| Linear dose-response association (per 5-g/d increase) | ||||
| Tree nuts6 (all prospective) | ||||
| Overall cancer | 7 | 0.90 (0.80–1.01) | 27.4 | 8.2 |
| Peanut butter (all prospective) | ||||
| Overall cancer | 14 | 0.99 (0.96–1.03) | 0 | 11.6 |
| Peanuts7 (all prospective) | ||||
| Overall cancer | 14 | 0.99 (0.97–1.01) | 0 | 8.1 |
ES, effect size; RR, relative risk.
Number of effect sizes.
Obtained from the random-effects model.
Inconsistency – the percentage of variation across studies due to heterogeneity.
Obtained from the Q-test.
Including walnuts, pistachios, macadamia nuts, pecans, cashews, almonds, hazelnuts, and Brazil nuts.
Including peanuts without considering peanut butter.
P value < 0.05.
Regarding peanut intake, a total of 14 articles (9, 14, 20–23, 28, 30, 34, 37, 43, 44, 46, 51) with prospective (n = 9) or case-control (n = 5) designs were included in the highest versus lowest comparison. These studies enrolled 239,332 participants and 13,119 cancer cases. Comparing the highest versus lowest intake of peanuts, there was no significant association with the risk of overall cancer (pooled ES: 0.92, 95% CI: 0.81–1.05, P = 0.21) (Table 2). The heterogeneity between studies was significant; therefore, we performed subgroup analysis for this association. Based on this analysis, the heterogeneity was reduced after stratifying studies by study design, geographical region, sample size, methods used for the assessment of nut intake, follow-up duration, cancer type, type of ES, and adjustment for energy intake and BMI. In addition, a significant inverse association was seen between peanut intake and risk of overall cancer in studies with a sample size of ≥10,000 participants, and those with a follow-up duration of <15 y. Dose-response meta-analysis on the 8 cohort studies with sufficient data (9, 14, 20–23, 28, 30) showed that peanut intake was not dose-dependently associated with the risk of overall cancer (Table 2 and Figure 2F). Considering the type of cancer, a significant inverse association was seen between peanut intake and risk of colorectal cancer (Table 2).
Findings from the meta-analysis on total nut intake and risk of specific cancer
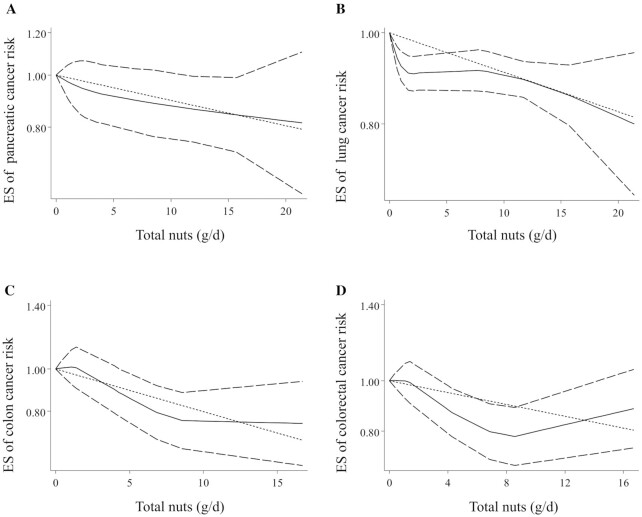
Combining 5 ESs from 4 studies (10, 12, 20, 24), including 604,266 participants and 2386 cases of pancreatic cancer, a significant inverse association was found between total nut intake and risk of pancreatic cancer (pooled ES: 0.83, 95% CI: 0.72–0.97, P < 0.05) with no evidence of significant heterogeneity between studies (I2 = 0; P = 0.43) (Table 1). This was also the case for the dose-response analysis, in which an increase of 5 g/d from total nut intake was associated with a 6% lower risk of pancreatic cancer (pooled ES: 0.94, 95% CI: 0.89–0.99, P < 0.05) (Table 1). This association was nonlinear according to the nonlinear dose-response analysis (P-nonlinearity < 0.05) (Figure 3A); such that, the risk of pancreatic cancer decreased from zero to 20 g/d total nut intake; however, the risk reduction slowed down at the dosage of 5 g/d. A similar association was seen for lung cancer when comparing the highest and lowest categories of total nut intake (pooled ES: 0.85, 95% CI: 0.81–0.90, P < 0.001, I2 = 0%; P = 0.78) (Table 1), and also in the linear dose-response analysis (pooled ES: 0.97, 95% CI: 0.95–0.98, P < 0.001) (Table 1). No nonlinear association was found for this association (P-nonlinearity = 0.60) (Figure 3B).
FIGURE 3.
Nonlinear dose-response associations between total nut intake and risk of pancreatic (A), lung (B), colon (C), and colorectal (D) cancers in adults aged ≥18 y. Dietary intake of total nuts was modeled in a random-effects model using restricted cubic splines with knots fixed at the 5th, 35th, 65th, and 95th percentiles of the distribution. The dotted line indicates the linear model. The solid line represents point estimates of the association between total nut intake and ESs. The dashed line presents the 95% CI. ES, effect size.
Considering 5 ESs from 4 studies (16, 26, 33, 38) that included a total of 588,540 participants and 2604 cases of colon cancer, the overall ES for risk of colon cancer, comparing the highest and lowest categories of total nut intake, was 0.61 (95% CI: 0.43–0.85, P < 0.001), indicating a significant inverse association (Table 1). Between-study heterogeneity was significant in this regard (I2 = 75.9%; P < 0.01). Three studies required data for the dose-response analysis on the link between total nut intake and colon cancer risk (16, 33, 38). Based on this analysis, a 5-g/d increase in total nut intake was associated with a 25% lower risk of colon cancer (pooled ES: 0.75, 95% CI: 0.60–0.94, P < 0.01) (Table 1). Furthermore, a nonlinear association was found in this regard in which the risk of colon cancer decreased continuously from 2 g/d to higher amounts of total nuts; however, this reduction slowed down at the dosage of 9 g/d (Figure 3C).
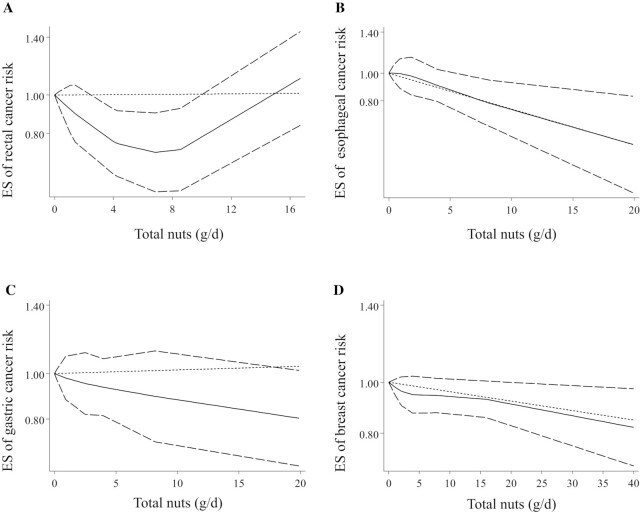
For other types of cancer including colorectal (16, 33, 38), rectal (16, 33, 38), prostate (9, 19, 32, 39, 42, 45), esophageal (13, 14, 21), gastric (13, 21, 36, 47, 48), liver (28), ovarian (15, 23, 50), endometrial (23, 43), and breast (11, 27, 30, 35, 41) cancers as well as glioma (40), there was no significant association with total nut intake after comparing the highest with the lowest intake (Table 1). The same results were also observed in the dose-response analysis (Table 1, Figure 3D, and Figure 4A–C). However, an inverse dose-dependent association was observed for breast cancer, by considering 4 articles with complete data (11, 27, 30, 41), indicating a 2% risk reduction for a 5-g/d increase in total nut intake (pooled ES: 0.98, 95% CI: 0.96–0.99, P < 0.05) (Table 1). This association was nonlinear; such that, the risk of breast cancer decreased continuously from zero to 40 g/d total nuts with a slow reduction between 5 and 15 g/d (P-nonlinearity = 0.01) (Figure 4D). Of note, due to a limited number of studies, we were unable to perform dose-response analysis for glioma and leukemia as well as for prostate, ovarian, and endometrial cancers.
FIGURE 4.
Nonlinear dose-response associations between total nut intake and risk of rectal (A), esophageal (B), gastric (C), and breast (D) cancers in adults aged ≥18 y. Dietary intake of total nuts was modeled in a random-effects model using restricted cubic splines with knots fixed at the 5th, 35th, 65th, and 95th percentiles of the distribution. The dotted line indicates the linear model. The solid line represents point estimates of the association between total nut intake and ESs. The dashed line presents the 95% CI. ES, effect size.
Findings from the meta-analysis on nut intake and cancer mortality
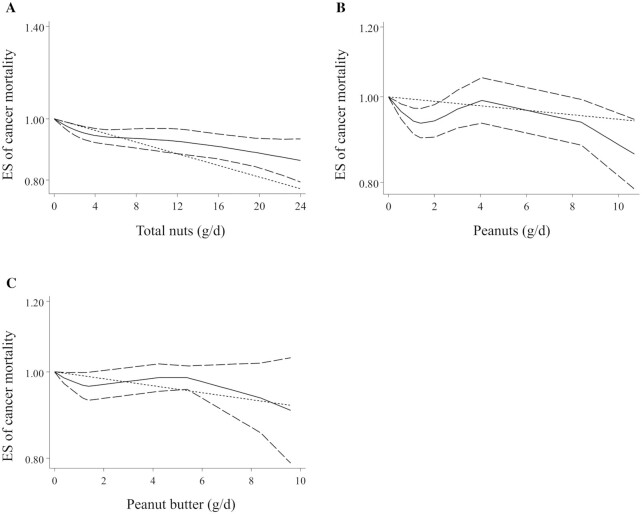
The association between total nut intake and risk of cancer mortality was examined in 9 articles (52–60), which enrolled 819,851 participants with 48,038 cases of cancer death. Combining 10 ESs for comparing the highest versus lowest intake of total nuts, we found a significant inverse association between total nut intake and risk of cancer mortality (pooled ES: 0.87, 95% CI: 0.82–0.91, P < 0.001), with no evidence of significant heterogeneity among the studies (I2 = 23%; P = 0.21) (Table 3). Considering the 9 articles in the linear dose-response analysis (52–60), a similar result was reached, in which a 5-g/d increase in total nut intake was associated with a 4% lower risk of cancer mortality (pooled ES: 0.96, 95% CI: 0.95–0.98, P < 0.001) (Table 3). This association was found to be nonsignificant in the nonlinear dose-response analysis (P-nonlinearity = 0.29) (Figure 5A). Moreover, the summary ESs for cancer mortality risk when comparing the highest versus lowest intakes of tree nuts and peanuts were 0.82 (95% CI: 0.76–0.90) and 0.92 (95% CI: 0.86–0.99, P < 0.05), respectively, indicating an inverse association (Table 3). However, the dose-response meta-analysis showed that peanut intake was not dose-dependently associated with the risk of overall cancer (Table 3 and Figure 5B). Due to the limited number of studies, we could not perform dose-response analysis for tree nut intake. In terms of peanut butter intake, no significant association was seen with cancer mortality either in the highest versus lowest comparison or in the dose-response meta-analysis (Table 3 and Figure 5C).
TABLE 3.
Summary risk estimates for the association of total and specific nuts with risk of cancer mortality in adults aged ≥18 y by considering prospective cohort studies1
| n 2 | Pooled ES (95% CI)3 | I 2 (%)4 | Q-statistic5 | |
|---|---|---|---|---|
| The highest vs. lowest comparison of nut intake | ||||
| Total nuts6 | 10 | 0.87 (0.82, 0.91)* | 23 | 14.2 |
| Tree nuts7 | 3 | 0.82 (0.76, 0.90)* | 0 | 0.03 |
| Peanut butter | 4 | 0.92 (0.82, 1.04) | 54.1 | 6.5 |
| Peanuts8 | 4 | 0.92 (0.86, 0.99)* | 33.5 | 4.5 |
| Linear dose-response association (per 5-g/d increase) | ||||
| Total nuts6 | 10 | 0.96 (0.95, 0.98)* | 30.1 | 12.8 |
| Peanut butter | 4 | 0.97 (0.91, 1.02) | 46.1 | 5.5 |
| Peanuts8 | 4 | 0.97 (0.92, 1.02) | 48.1 | 5.7 |
ES, effect size.
Number of effect sizes.
Obtained from the random-effects model.
Inconsistency – the percentage of variation across studies due to heterogeneity.
Obtained from the Q-test.
Including tree nuts and peanuts.
Including walnuts, pistachios, macadamia nuts, pecans, cashews, almonds, hazelnuts, and Brazil nuts.
Including peanuts without considering peanut butter.
P value <0.05.
FIGURE 5.
Nonlinear dose-response association between total nut (A), peanut (B), and peanut butter (C) intake and risk of cancer mortality in adults aged ≥18 y. All included studies were prospective cohorts. Dietary intake of nuts was modeled in a random-effects model using restricted cubic splines with knots fixed at the 5th, 35th, 65th, and 95th percentiles of the distribution. The dotted line indicates the linear model. The solid line represents point estimates of the association between nut intake and ESs. The dashed line presents the 95% CI. ES, effect size.
Sensitivity analyses and publication bias
Sensitivity analyses based on a fixed-effects model showed that excluding any single study from the analysis did not significantly alter the pooled ESs. Based on the Egger's test, we found no substantial publication bias for the associations examined in the current meta-analysis, except for the association between total nut intake and risk of overall cancer (P < 0.001). However, the application of the trim-and-fill method did not alter the pooled ES, indicating the results were not affected by the publication bias.
Discussion
We found 14% and 13% risk reductions in overall cancer with higher intakes of total nuts and tree nuts, respectively. These risk reductions were also seen for specific cancers including colon, pancreatic, and lung cancers in relation to total nut intake. In the dose-response analysis, each 5-g/d increase of total nut intake was associated with a 3% lower risk of overall cancer. There was evidence of a nonlinear association in which the overall cancer risk decreased when the consumption of total nuts increased from very low levels to higher amounts with a steeper decrease from 0 to 3 g/d. The meta-analysis of cancer mortality risk and consumption of total nuts and tree nuts revealed, respectively, 13% and 8% risk reductions. To the best of our knowledge, this study is among the first comprehensive meta-analyses to summarize prior publications on the association of total and individual nut intake with overall and specific cancer risk and also cancer mortality. Moreover, 3 earlier meta-analyses of cancer risk (61, 62, 66) had several limitations that make their findings misleading; and 3 meta-analyses of cancer mortality (59, 67, 83) needed to be updated.
Total and specific types of nuts and cancer risk
Our study showed a significant risk reduction for overall cancer with the higher consumption of total nuts. In a recent meta-analysis on 28 prospective cohorts and 5 case-cohort studies, which enrolled 50,879 cancer cases, total nut intake was associated with a 10% reduction in overall cancer risk (61). Some methodological limitations may compromise the validity of their underestimated ES. The authors included an ineligible study in which the intake of fatty acids (from nuts) was investigated in relation to cancer incidence (63). This inclusion is unreasonable because nuts may affect the risk of cancer through their fiber, selenium, and MUFA and PUFA content, rather than only their fatty acids. They also did not include an eligible study despite the inclusion criteria (31), which resulted in fewer cases of cancer being included in that meta-analysis. Two other meta-analyses, which were published before 2016, found a significant reduction of 5–8% in overall cancer risk; however, they included studies in which a combination of fruit, nut, seed, and legume intake, rather than nut intake alone, was considered as the exposure variable (64, 77, 78, 65). Moreover, none of these meta-analyses examined the dose-response association between various types of nuts and the risk of specific types of cancer.
It should be noted that we found a substantial publication bias for the association between total nut intake and risk of overall cancer (P < 0.001). To handle this issue, we applied the trim-and-fill method to calculate the bias-corrected overall estimate (84). Based on this method, we first trimmed the studies that caused asymmetry of the funnel plot so that the overall effect estimate produced by the remaining studies could be considered minimally impacted by publication bias. We then filled imputed missing studies in the funnel plot based on the bias-corrected overall estimate. Correcting the potential bias by the “trim-and-fill” method provided the same results for the association between total nut intake and cancer risk, indicating the results were not affected by the publication bias.
We observed an inverse association for tree nut intake, but not for peanut and peanut butter. Consistently, previous meta-analyses found a reduced risk of cancer in relation to tree nut intake (24, 61). Different types of nuts contain different compositions of nutrients; such that peanuts contain less total fat compared with walnuts, hazelnuts, and almonds, but a higher amount of carbohydrates, proteins, folate, and phytosterols; and the amount of fiber, magnesium, and calcium in almonds are much higher than those in peanuts (85). Although peanut butter contains the beneficial components of peanut, some additives such as sugar or salt may reduce the beneficial effects of plain peanuts (86).
In the subgroup analyses, the association between total nut intake and cancer risk was not significant in studies that did not control their analysis for energy intake and BMI. To get the most benefit of nuts, it is recommended to incorporate 4–5 servings/wk nuts (14 g), seeds (14 g), and cooked dry beans (90 g) into a heart-healthy eating pattern containing 2000 kcal/d (87). Since nuts are among the calorie-dense foods (5.6–7 kcal/g), they raise a concern regarding weight gain, a known risk factor for cancer. However, if they are substituted for other food choices rather than being added to an existing diet, weight gain may not occur. We also could not find a significant association among studies that recruited only men. Such data may come from the observations that male subcohort members consumed a lesser amount of nuts (22) and more alcohol and processed meat than females (21).
We observed a linear dose-dependent association between total nut intake and the risk of overall cancer and pancreatic and colon cancers, in which there were, respectively, 3%, 6%, and 25% risk reductions for a 5-g/d per day increase in total nut intake. Also, in the nonlinear analysis, we found that the risk of overall and colon cancers decreased more slowly when the intake exceeded from 3 g/d to 9 g/d, respectively. Dose-response associations between total nut intake and overall cancer risk have also been reported in previous studies. In a recent meta-analysis (61), a 20-g/d increase in total nut intake was associated with a 10% decrease in overall cancer risk. In the study by Aune et al. (66), there was a 15% risk reduction for 1 serving/d (28 g/d) increment in total nut consumption. However, dose-response associations for specific types of cancers were not examined in the mentioned meta-analyses. Moreover, in the European Prospective Investigation into Cancer and Nutrition (EPIC) study, the highest quintile of total nut intake (>6.2 g/d) was associated with a 31% reduction in colorectal cancer, compared with the lowest quintile (nonconsumers) (16).
Among the 14 different cancer types reported in the included studies, the inverse association was observed only for colon, pancreatic, and breast cancers. The rationale behind this differential effect of total nut intake on specific cancers is not well understood. However, individual anticancer components of nuts may play a role. For example, dietary fiber in nuts can increase fecal bulking and viscosity, reduce the time for proteolytic fermentation that results in harmful substances, and shorten the contact time between potential carcinogens and mucosal cells (88). Also, dietary fiber can bind and excrete potential gut carcinogens (e.g. secondary bile acids), reduce fecal pH in the colon, and finally provide a healthy intestinal environment (88, 89). Moreover, ellagic acid in nuts seems to inhibit the activation, proliferation, and migration of pancreatic stellate cells (90), and the resveratrol content of nuts can increase the expression of p53 target genes and induce p53-dependent apoptosis in breast cancer cells (91).
It should be noted that in some food cultures, salt is used for nut processing and preparation. A high consumption of salt is associated with an increased risk of gastric and colorectal cancers and even all-cause mortality (92, 93). Also, aflatoxin concentrations may increase under improper storage conditions in different types of nuts such as peanuts, pistachios, almonds, and walnuts (94). Aflatoxin is a mycotoxin with carcinogenic properties produced by Aspergillus flavus and Aspergillus parasiticus spp. (94). Therefore, the beneficial effects of nuts might be reduced by improper processing methods and storage conditions.
Total and specific types of nuts and cancer mortality
We found 13%, 18%, and 8% risk reductions in the risk of cancer mortality with the higher intake of total nuts, tree nuts, and peanuts, respectively, but no significant association was found for peanut butter consumption. A similar result was reached in the linear dose-response analysis, in which a 5-g/d increase in total nut intake was associated with a 4% lower risk of cancer mortality. Two earlier meta-analyses on 3 and 5 eligible studies, conducted before 2016, showed 14–15% risk reduction in cancer mortality with higher total nut intake (59, 83). A recent meta-analysis of 8 prospective studies, involving 21,353 cancer deaths, revealed significant 13%, 16%, and 7% reductions in the risk of cancer mortality by comparing the highest versus lowest categories of total nut, tree nut, and peanut intake, respectively (67). They also found a linear dose-response association in which a 3% lower risk of cancer mortality was observed by a 1 serving (28 g) per week increment of total nut intake (67). The protective effect of total nut intake on the prevention of cancer mortality may be attributed to its antioxidant and anti-inflammatory properties, for example, by reducing lipid peroxidation or oxidative DNA damage (95). The preventive effect of nut consumption may occur due to the presence of fiber, which decreases the intestinal mucosa's exposure to carcinogens, as well as folate, which is necessary for normal cellular function, DNA synthesis, and metabolism (96, 97). Possible reasons for the different associations of peanut and peanut butter intakes with cancer mortality would be the addition of partially hydrogenated vegetable fats (containing trans fats) to peanut butter (98). Trans fats can reduce the beneficial effects of plain peanuts.
Strengths and limitations
We conducted the most comprehensive systematic review, meta-analysis, and dose-response analysis on a large number of prospective cohort and case-control studies that investigated the association between nut intake and risk of cancer and its mortality to overcome earlier methodological defects and to update the previous meta-analyses in this regard. We also performed stratified analysis to find the association of various types of nuts with the risk of specific types of cancer and cancer mortality. However, potential limitations must be considered when interpreting our findings. First, residual or unmeasured confounding factors may have affected the magnitude of the association between nut intake and risk of cancer. Although most studies had controlled for potential confounders, some did not control the analyses for dietary intake of other food groups (e.g. fruits and vegetables), and some others did not consider total energy intake and BMI as covariates. Lack of controlling for such potential confounders might affect the independent association between nut intake and cancer risk. Second, participants with a higher intake of nuts may have a healthier lifestyle; and therefore, it is unclear whether the observed protective effect is mediated by nut consumption or through the clustering of healthy food preferences. Third, although we tried to assess different types of nuts in the current meta-analysis, data on some other types such as walnut, pistachio, and hazelnut were not sufficient for a meta-analysis. Fourth, using different methods with different accuracy for dietary assessment including FFQ, dietary recall, and researcher-made questionnaires is another limitation of our meta-analysis. Last, but not least, most of the included studies performed a single measurement for baseline dietary nut intake, which may not provide accurate information for long-term nut exposure.
Conclusions
In summary, our results provide further evidence that the higher intake of total nuts and tree nuts is associated with a lower risk of cancer and its mortality. Among different types of nuts, tree nuts showed the most protective effects. The consumption of 5-g/d total nuts is associated with a 3% reduced risk of cancer and a 4% reduction in cancer mortality. We further understand that peanut intake may have a weaker association in relation to cancer mortality, but the results were not significant for peanut butter. These findings support recommendations to increase the consumption of total nuts and tree nuts in the general population. However, caution should be considered due to the possibility of aflatoxin contamination as well as the allergic potential of nuts (99, 100). Our results need to be confirmed by interventional studies. Future prospective studies should focus on other unclear aspects of the association such as the effect of preparation methods and the differential association for individual tree nut intake.
Supplementary Material
Acknowledgments
We appreciate the Abadan Faculty of Medical Sciences, Abadan, Iran for its financial support. This study was approved by the ethic committe of the Abadan Faculty of Medical Sciences, Abadan, Iran (IR.ABADANUMS.REC.1398.089).
The authors’ contributions were as follows—SN, SM, MA, and OS: contributed to the conception, design, and literature search; SN and OS: contributed to the data extraction and data analysis; OS, MN, and MS: contributed to the interpretation of the data and drafted the manuscript; SM and MA: contributed to manuscript editing; and all authors: read and approved the final manuscript.
Notes
This study was supported by the Abadan Faculty of Medical Sciences, Abadan, Iran (code: 695).
Author disclosure: The authors report no conflicts of interest.
Supplemental Tables 1–5 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at http://academic.oup.com/advances/.
MA and OS contributed equally to this work.
Abbreviations used: ES, effect size; NOS, Newcastle Ottawa Scale.
Contributor Information
Sina Naghshi, Students' Scientific Research Center, Tehran University of Medical Sciences, Tehran, Iran; Department of Clinical Nutrition, School of Nutritional Sciences and Dietetics, Tehran University of Medical Sciences, Tehran, Iran.
Mehdi Sadeghian, Student Research Committee, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran.
Morteza Nasiri, Student Research Committee, Shiraz University of Medical Sciences, Shiraz, Iran; Department of Operating Room Nursing, School of Nursing and Midwifery, Shiraz University of Medical Sciences, Shiraz, Iran.
Sara Mobarak, Department of Infectious and Tropical Diseases, Abadan Faculty of Medical Sciences, Abadan, Iran.
Masoomeh Asadi, Department of Operating Room Nursing, Abadan Faculty of Medical Sciences, Abadan, Iran.
Omid Sadeghi, Students' Scientific Research Center, Tehran University of Medical Sciences, Tehran, Iran; Department of Community Nutrition, School of Nutritional Sciences and Dietetics, Tehran University of Medical Sciences, Tehran, Iran.
References
- 1. Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, Rosso S, Coebergh JW, Comber H, Forman D, Bray F. Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. Eur J Cancer. 2013;49(6):1374–403. [DOI] [PubMed] [Google Scholar]
- 2. Fitzmaurice C, Allen C, Barber RM, Barregard L, Bhutta ZA, Brenner H, Dicker DJ, Chimed-Orchir O, Dandona R, Dandona Let al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990 to 2015: a systematic analysis for the global burden of disease study. JAMA Oncol. 2017;3(4):524–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lortet-Tieulent J, Soerjomataram I, Lin CC, Coebergh JWW, Jemal A. U.S. burden of cancer by race and ethnicity according to disability-adjusted life years. Am J Prev Med. 2016;51(5):673–81. [DOI] [PubMed] [Google Scholar]
- 4. Parohan M, Sadeghi A, Khatibi SR, Nasiri M, Milajerdi A, Khodadost M, Sadeghi O. Dietary total antioxidant capacity and risk of cancer: a systematic review and meta-analysis on observational studies. Crit Rev Oncol Hematol. 2019;138:70–86. [DOI] [PubMed] [Google Scholar]
- 5. Nachvak SM, Moradi S, Anjom-Shoae J, Rahmani J, Nasiri M, Maleki V, Sadeghi O. Soy, soy isoflavones, and protein intake in relation to mortality from all causes, cancers, and cardiovascular diseases: a systematic review and dose-response meta-analysis of prospective cohort studies. J Acad Nutr Diet. 2019;119(9):1483–500.e17. [DOI] [PubMed] [Google Scholar]
- 6. Sadeghi A, Sadeghian M, Nasiri M, Rahmani J, Khodadost M, Pirouzi A, Maleki V, Sadeghi O. Carbohydrate quantity and quality affect the risk of endometrial cancer: a systematic review and dose-response meta-analysis. Clin Nutr. 2020;39(6):1681–91. [DOI] [PubMed] [Google Scholar]
- 7. Alasalvar C, Bolling BW. Review of nut phytochemicals, fat-soluble bioactives, antioxidant components and health effects. Br J Nutr. 2015;113:(Suppl 2):S68–78. [DOI] [PubMed] [Google Scholar]
- 8. Garcia CP, Lamarque AL, Comba A, Berra MA, Silva RA, Labuckas DO, Das UN, Eynard AR, Pasqualini ME. Synergistic anti-tumor effects of melatonin and PUFAs from walnuts in a murine mammary adenocarcinoma model. Nutrition. 2015;31(4):570–7. [DOI] [PubMed] [Google Scholar]
- 9. Boudewijns EA, Nieuwenhuis L, Geybels MS, van den Brandt PA. Total nut, tree nut, peanut, and peanut butter intake and the risk of prostate cancer in the Netherlands Cohort Study. Prostate Cancer Prostatic Dis. 2019;22(3):467–74. [DOI] [PubMed] [Google Scholar]
- 10. Bao Y, Hu FB, Giovannucci EL, Wolpin BM, Stampfer MJ, Willett WC, Fuchs CS. Nut consumption and risk of pancreatic cancer in women. Br J Cancer. 2013;109(11):2911–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Farvid MS, Cho E, Chen WY, Eliassen AH, Willett WC. Dietary protein sources in early adulthood and breast cancer incidence: prospective cohort study. BMJ. 2014;348:g3437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ghorbani Z, Pourshams A, Fazeltabar Malekshah A, Sharafkhah M, Poustchi H, Hekmatdoost A. Major dietary protein sources in relation to pancreatic cancer: a large prospective study. Arch Iran Med. 2016;19(4):248–56. [PubMed] [Google Scholar]
- 13. Hashemian M, Murphy G, Etemadi A, Dawsey SM, Liao LM, Abnet CC. Nut and peanut butter consumption and the risk of esophageal and gastric cancer subtypes. Am J Clin Nutr. 2017;106(3):858–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hashemian M, Murphy G, Etemadi A, Poustchi H, Sharafkhah M, Kamangar F, Pourshams A, Malekshah AF, Khoshnia M, Gharavi Aet al. Nut consumption and the risk of oesophageal squamous cell carcinoma in the Golestan Cohort Study. Br J Cancer. 2018;119(2):176–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hedelin M, Lof M, Andersson TM, Adlercreutz H, Weiderpass E. Dietary phytoestrogens and the risk of ovarian cancer in the women's lifestyle and health cohort study. Cancer Epidemiol Biomarkers Prev. 2011;20(2):308–17. [DOI] [PubMed] [Google Scholar]
- 16. Jenab M, Ferrari P, Slimani N, Norat T, Casagrande C, Overad K, Olsen A, Stripp C, Tjonneland A, Boutron-Ruault MCet al. Association of nut and seed intake with colorectal cancer risk in the European Prospective Investigation into Cancer and Nutrition. Cancer Epidemiol Biomarkers Prev. 2004;13(10):1595–603. [PubMed] [Google Scholar]
- 17. Gnagnarella P, Maisonneuve P, Bellomi M, Rampinelli C, Bertolotti R, Spaggiari L, Palli D, Veronesi G. Red meat, Mediterranean diet and lung cancer risk among heavy smokers in the COSMOS screening study. Ann Oncol. 2013;24(10):2606–11. [DOI] [PubMed] [Google Scholar]
- 18. Lee JT, Lai GY, Liao LM, Subar AF, Bertazzi PA, Pesatori AC, Freedman ND, Landi MT, Lam TK. Nut consumption and lung cancer risk: results from two large observational studies. Cancer Epidemiol Biomarkers Prev. 2017;26(6):826–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mills PK, Beeson WL, Phillips RL, Fraser GE. Cohort study of diet, lifestyle, and prostate cancer in Adventist men. Cancer. 1989;64(3):598–604. [DOI] [PubMed] [Google Scholar]
- 20. Nieuwenhuis L, van den Brandt PA. Total nut, tree nut, peanut, and peanut butter consumption and the risk of pancreatic cancer in the Netherlands Cohort Study. Cancer Epidemiol Biomarkers Prev. 2018;27(3):274–84. [DOI] [PubMed] [Google Scholar]
- 21. Nieuwenhuis L, van den Brandt PA. Tree nut, peanut, and peanut butter consumption and the risk of gastric and esophageal cancer subtypes: the Netherlands Cohort Study. Gastric Cancer. 2018;21(6):900–12. [DOI] [PubMed] [Google Scholar]
- 22. Nieuwenhuis L, van den Brandt PA. Nut and peanut butter consumption and the risk of lung cancer and its subtypes: a prospective cohort study. Lung Cancer. 2019;128:57–66. [DOI] [PubMed] [Google Scholar]
- 23. Nieuwenhuis L, van den Brandt PA. Nut and peanut butter intake are not directly associated with the risk of endometrial or ovarian cancer: results from a Dutch prospective cohort study. Clin Nutr. 2020;39(7):2202–10. [DOI] [PubMed] [Google Scholar]
- 24. Obon-Santacana M, Lujan-Barroso L, Freisling H, Naudin S, Boutron-Ruault MC, Mancini FR, Rebours V, Kuhn T, Katzke V, Boeing Het al. Consumption of nuts and seeds and pancreatic ductal adenocarcinoma risk in the European Prospective Investigation into Cancer and Nutrition. Int J Cancer. 2020;146(1):76–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Saberi Hosnijeh F, Peeters P, Romieu I, Kelly R, Riboli E, Olsen A, Tjonneland A, Fagherazzi G, Clavel-Chapelon F, Dossus Let al. Dietary intakes and risk of lymphoid and myeloid leukemia in the European Prospective Investigation into Cancer and Nutrition (EPIC). Nutr Cancer. 2014;66(1):14–28. [DOI] [PubMed] [Google Scholar]
- 26. Singh PN, Fraser GE. Dietary risk factors for colon cancer in a low-risk population. Am J Epidemiol. 1998;148(8):761–74. [DOI] [PubMed] [Google Scholar]
- 27. Sonestedt E, Borgquist S, Ericson U, Gullberg B, Landberg G, Olsson H, Wirfält E. Plant foods and oestrogen receptor α- and β-defined breast cancer: observations from the Malmö Diet and Cancer cohort. Carcinogenesis. 2008;29(11):2203–9. [DOI] [PubMed] [Google Scholar]
- 28. Sui J, Yang W, Ma Y, Li TY, Simon TG, Meyerhardt JA, Liang G, Giovannucci EL, Chan AT, Zhang X. A prospective study of nut consumption and risk of primary hepatocellular carcinoma in the U.S. women and men. Cancer Prev Res. 2019;12(6):367–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Thompson CA, Habermann TM, Wang AH, Vierkant RA, Folsom AR, Ross JA, Cerhan JR. Antioxidant intake from fruits, vegetables and other sources and risk of non-Hodgkin's lymphoma: the Iowa Women's Health Study. Int J Cancer. 2010;126(4):992–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. van den Brandt PA, Nieuwenhuis L. Tree nut, peanut, and peanut butter intake and risk of postmenopausal breast cancer: the Netherlands Cohort Study. Cancer Causes Control. 2018;29(1):63–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. von Ruesten A, Feller S, Bergmann MM, Boeing H. Diet and risk of chronic diseases: results from the first 8 years of follow-up in the EPIC-Potsdam study. Eur J Clin Nutr. 2013;67(4):412–9. [DOI] [PubMed] [Google Scholar]
- 32. Wang W, Yang M, Kenfield SA, Hu FB, Stampfer MJ, Willett WC, Fuchs CS, Giovannucci EL, Bao Y. Nut consumption and prostate cancer risk and mortality. Br J Cancer. 2016;115(3):371–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yang M, Hu FB, Giovannucci EL, Stampfer MJ, Willett WC, Fuchs CS, Wu K, Bao Y. Nut consumption and risk of colorectal cancer in women. Eur J Clin Nutr. 2016;70(3):333–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yeh CC, You SL, Chen CJ, Sung FC. Peanut consumption and reduced risk of colorectal cancer in women: a prospective study in Taiwan. World J Gastroenterol. 2006;12(2):222–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jacobs I, Taljaard-Krugell C, Ricci C, Vorster H, Rinaldi S, Cubasch H, Laubscher R, Joffe M, van Zyl T, Norris SAet al. Dietary intake and breast cancer risk in black South African women: the South African Breast Cancer study. Br J Nutr. 2019;121(5):591–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hoshiyama Y, Sasaba T. A case-control study of stomach cancer and its relation to diet, cigarettes, and alcohol consumption in Saitama Prefecture, Japan. Cancer Causes Control. 1992;3(5):441–8. [DOI] [PubMed] [Google Scholar]
- 37. Chen CJ, Liang KY, Chang AS, Chang YC, Lu SN, Liaw YF, Chang WY, Sheen MC, Lin TM. Effects of hepatitis B virus, alcohol drinking, cigarette smoking and familial tendency on hepatocellular carcinoma. Hepatology. 1991;13(3):398–406. [PubMed] [Google Scholar]
- 38. Lee J, Shin A, Oh JH, Kim J. The relationship between nut intake and risk of colorectal cancer: a case control study. Nutr J. 2018;17(1):37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Moller E, Galeone C, Andersson TM, Bellocco R, Adami HO, Andren O, Gronberg H, La Vecchia C, Mucci LA, Balter K. Mediterranean Diet Score and prostate cancer risk in a Swedish population-based case-control study. J Nutr Sci. 2013;2:e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Giles GG, McNeil JJ, Donnan G, Webley C, Staples MP, Ireland PD, Hurley SF, Salzberg M. Dietary factors and the risk of glioma in adults: results of a case-control study in Melbourne, Australia. Int J Cancer. 1994;59(3):357–62. [DOI] [PubMed] [Google Scholar]
- 41. Liu Y, Colditz GA, Cotterchio M, Boucher BA, Kreiger N. Adolescent dietary fiber, vegetable fat, vegetable protein, and nut intakes and breast cancer risk. Breast Cancer Res Treat. 2014;145(2):461–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Jackson M, Tulloch-Reid M, Walker S, McFarlane-Anderson N, Bennett F, Francis D, Coard K. Dietary patterns as predictors of prostate cancer in Jamaican men. Nutr Cancer. 2013;65(3):367–74. [DOI] [PubMed] [Google Scholar]
- 43. Takayama S, Monma Y, Tsubota-Utsugi M, Nagase S, Tsubono Y, Numata T, Toyoshima M, Utsunomiya H, Sugawara J, Yaegashi N. Food intake and the risk of endometrial endometrioid adenocarcinoma in Japanese women. Nutr Cancer. 2013;65(7):954–60. [DOI] [PubMed] [Google Scholar]
- 44. Zhang JY, Dai M, Wang X, Lu WQ, Li DS, Zhang MX, Wang KJ, Dai LP, Han SG, Zhou YFet al. A case-control study of hepatitis B and C virus infection as risk factors for hepatocellular carcinoma in Henan, China. Int J Epidemiol. 1998;27(4):574–8. [DOI] [PubMed] [Google Scholar]
- 45. Raimondi S, Mabrouk JB, Shatenstein B, Maisonneuve P, Ghadirian P. Diet and prostate cancer risk with specific focus on dairy products and dietary calcium: a case-control study. Prostate. 2010;70(10):1054–65. [DOI] [PubMed] [Google Scholar]
- 46. Soliman AS, Hung CW, Tsodikov A, Seifeldin IA, Ramadan M, Al-Gamal D, Schiefelbein EL, Thummalapally P, Dey S, Ismail K. Epidemiologic risk factors of hepatocellular carcinoma in a rural region of Egypt. Hepatol Int. 2010;4(4):681–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wang XQ, Yan H, Terry PD, Wang JS, Cheng L, Wu WA, Hu SK. Interaction between dietary factors and Helicobacter pylori infection in noncardia gastric cancer: a population-based case-control study in China. J Am Coll Nutr. 2012;31(5):375–84. [DOI] [PubMed] [Google Scholar]
- 48. Trichopoulos D, Ouranos G, Day NE, Tzonou A, Manousos O, Papadimitriou C, Trichopoulos A. Diet and cancer of the stomach: a case-control study in Greece. Int J Cancer. 1985;36(3):291–7. [PubMed] [Google Scholar]
- 49. Yamamura Y, Oum R, Gbito KY, Garcia-Manero G, Strom SS. Dietary intake of vegetables, fruits, and meats/beans as potential risk factors of acute myeloid leukemia: a Texas case-control study. Nutr Cancer. 2013;65(8):1132–40. [DOI] [PubMed] [Google Scholar]
- 50. Pan SY, Ugnat AM, Mao Y, Wen SW, Johnson KC. A case-control study of diet and the risk of ovarian cancer. Cancer Epidemiol Biomarkers Prev. 2004;13(9):1521–7. [PubMed] [Google Scholar]
- 51. Yu SZ, Huang XE, Koide T, Cheng G, Chen GC, Harada K, Ueno Y, Sueoka E, Oda H, Tashiro Fet al. Hepatitis B and C viruses infection, lifestyle and genetic polymorphisms as risk factors for hepatocellular carcinoma in Haimen, China. Jpn J Cancer Res. 2002;93(12):1287–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Guasch-Ferre M, Bullo M, Martinez-Gonzalez MA, Ros E, Corella D, Estruch R, Fito M, Aros F, Warnberg J, Fiol Met al. Frequency of nut consumption and mortality risk in the PREDIMED nutrition intervention trial. BMC Med. 2013;11:164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Bao Y, Han J, Hu FB, Giovannucci EL, Stampfer MJ, Willett WC, Fuchs CS. Association of nut consumption with total and cause-specific mortality. N Engl J Med. 2013;369(21):2001–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Bonaccio M, Di Castelnuovo A, De Curtis A, Costanzo S, Bracone F, Persichillo M, Donati MB, de Gaetano G, Iacoviello L. Nut consumption is inversely associated with both cancer and total mortality in a Mediterranean population: prospective results from the Moli-sani study. Br J Nutr. 2015;114(5):804–11. [DOI] [PubMed] [Google Scholar]
- 55. Eslamparast T, Sharafkhah M, Poustchi H, Hashemian M, Dawsey SM, Freedman ND, Boffetta P, Abnet CC, Etemadi A, Pourshams Aet al. Nut consumption and total and cause-specific mortality: results from the Golestan Cohort Study. Int J Epidemiol. 2017;46(1):75–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Hshieh TT, Petrone AB, Gaziano JM, Djousse L. Nut consumption and risk of mortality in the Physicians’ Health Study. Am J Clin Nutr. 2015;101(2):407–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Luu HN, Blot WJ, Xiang YB, Cai H, Hargreaves MK, Li H, Yang G, Signorello L, Gao YT, Zheng Wet al. Prospective evaluation of the association of nut/peanut consumption with total and cause-specific mortality. JAMA Intern Med. 2015;175(5):755–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Amba V, Murphy G, Etemadi A, Wang S, Abnet CC, Hashemian M. Nut and peanut butter consumption and mortality in the National Institutes of Health-AARP Diet and Health Study. Nutrients. 2019;11(7):1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. van den Brandt PA, Schouten LJ. Relationship of tree nut, peanut and peanut butter intake with total and cause-specific mortality: a cohort study and meta-analysis. Int J Epidemiol. 2015;44(3):1038–49. [DOI] [PubMed] [Google Scholar]
- 60. Fernandez-Montero A, Bes-Rastrollo M, Barrio-Lopez MT, Fuente-Arrillaga Cde L, Salas-Salvado J, Moreno-Galarraga L, Martinez-Gonzalez MA. Nut consumption and 5-y all-cause mortality in a Mediterranean cohort: the SUN project. Nutrition. 2014;30(9):1022–7. [DOI] [PubMed] [Google Scholar]
- 61. Long J, Ji Z, Yuan P, Long T, Liu K, Li J, Cheng L. Nut consumption and risk of cancer: a meta-analysis of prospective studies. Cancer Epidemiol Biomarkers Prev. 2020;29(3):565–73. [DOI] [PubMed] [Google Scholar]
- 62. Wu L, Wang Z, Zhu J, Murad AL, Prokop LJ, Murad MH. Nut consumption and risk of cancer and type 2 diabetes: a systematic review and meta-analysis. Nutr Rev. 2015;73(7):409–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Thiebaut AC, Chajes V, Gerber M, Boutron-Ruault MC, Joulin V, Lenoir G, Berrino F, Riboli E, Benichou J, Clavel-Chapelon F. Dietary intakes of omega-6 and omega-3 polyunsaturated fatty acids and the risk of breast cancer. Int J Cancer. 2009;124(4):924–31. [DOI] [PubMed] [Google Scholar]
- 64. Jain MG, Hislop GT, Howe GR, Ghadirian P. Plant foods, antioxidants, and prostate cancer risk: findings from case-control studies in Canada. Nutr Cancer. 1999;34(2):173–84. [DOI] [PubMed] [Google Scholar]
- 65. Petridou E, Kedikoglou S, Koukoulomatis P, Dessypris N, Trichopoulos D. Diet in relation to endometrial cancer risk: a case-control study in Greece. Nutr Cancer. 2002;44(1):16–22. [DOI] [PubMed] [Google Scholar]
- 66. Aune D, Keum N, Giovannucci E, Fadnes LT, Boffetta P, Greenwood DC, Tonstad S, Vatten LJ, Riboli E, Norat T. Nut consumption and risk of cardiovascular disease, total cancer, all-cause and cause-specific mortality: a systematic review and dose-response meta-analysis of prospective studies. BMC Med. 2016;14(1):207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Chen GC, Zhang R, Martinez-Gonzalez MA, Zhang ZL, Bonaccio M, van Dam RM, Qin LQ. Nut consumption in relation to all-cause and cause-specific mortality: a meta-analysis 18 prospective studies. Food Funct. 2017;8(11):3893–905. [DOI] [PubMed] [Google Scholar]
- 68. Page MJ, Moher D. Evaluations of the uptake and impact of the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) statement and extensions: a scoping review. Syst Rev. 2017;6(1):263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–5. [DOI] [PubMed] [Google Scholar]
- 70. DerSimonian R, Laird N. Meta-analysis in clinical trials revisited. Contemp Clin Trials. 2015;45(Pt A):139–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Higgins JP, Green S. Cochrane handbook for systematic reviews of interventions. Chichester (England); Hoboken (NJ): Wiley-Blackwell; 2008. [Google Scholar]
- 72. van Enst WA, Ochodo E, Scholten RJ, Hooft L, Leeflang MM. Investigation of publication bias in meta-analyses of diagnostic test accuracy: a meta-epidemiological study. BMC Med Res Methodol. 2014;14:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Lin L. Hybrid test for publication bias in meta-analysis. Stat Methods Med Res. 2020:29(10):2881–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Greenland S, Longnecker MP. Methods for trend estimation from summarized dose-response data, with applications to meta-analysis. Am J Epidemiol. 1992;135(11):1301–9. [DOI] [PubMed] [Google Scholar]
- 75. Orsini N, Bellocco R, Greenland S. Generalized least squares for trend estimation of summarized dose-response data. The Stata Journal. 2006;6(1):40–57. [Google Scholar]
- 76. Jackson D, White IR, Thompson SG. Extending DerSimonian and Laird's methodology to perform multivariate random effects meta-analyses. Statist Med. 2010;29(12):1282–97. [DOI] [PubMed] [Google Scholar]
- 77. Samoli E, Lagiou A, Nikolopoulos E, Lagogiannis G, Barbouni A, Lefantzis D, Trichopoulos D, Brennan P, Lagiou P. Mediterranean diet and upper aerodigestive tract cancer: the Greek segment of the Alcohol-Related Cancers and Genetic Susceptibility in Europe study. Br J Nutr. 2010;104(9):1369–74. [DOI] [PubMed] [Google Scholar]
- 78. Kune S, Kune GA, Watson LF. Case-control study of dietary etiological factors: the Melbourne Colorectal Cancer Study. Nutr Cancer. 1987;9(1):21–42. [DOI] [PubMed] [Google Scholar]
- 79. Ibiebele TI, Nagle CM, Bain CJ, Webb PM. Intake of omega-3 and omega-6 fatty acids and risk of ovarian cancer. Cancer Causes Control. 2012;23(11):1775–83. [DOI] [PubMed] [Google Scholar]
- 80. Berkey CS, Willett WC, Tamimi RM, Rosner B, Frazier AL, Colditz GA. Vegetable protein and vegetable fat intakes in pre-adolescent and adolescent girls, and risk for benign breast disease in young women. Breast Cancer Res Treat. 2013;141(2):299–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Farvid MS, Cho E, Chen WY, Eliassen AH, Willett WC. Adolescent meat intake and breast cancer risk. Int J Cancer. 2015;136(8):1909–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. van den Brandt PA. Red meat, processed meat, and other dietary protein sources and risk of overall and cause-specific mortality in the Netherlands Cohort Study. Eur J Epidemiol. 2019;34(4):351–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Grosso G, Yang J, Marventano S, Micek A, Galvano F, Kales SN. Nut consumption on all-cause, cardiovascular, and cancer mortality risk: a systematic review and meta-analysis of epidemiologic studies. Am J Clin Nutr. 2015;101(4):783–93. [DOI] [PubMed] [Google Scholar]
- 84. Shi L, Lin L. The trim-and-fill method for publication bias: practical guidelines and recommendations based on a large database of meta-analyses. Medicine (Baltimore). 2019;98(23):e15987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Ros E. Health benefits of nut consumption. Nutrients. 2010;2(7):652–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Stichting Nederlands Voedingsstoffenbestand . NEVO-table. Dutch Food Composition Table 1986–1987 NvTH. The Netherlands: Voorlichtingsbureau voor de Voeding; 1986. [Google Scholar]
- 87. Karanja N, Erlinger TP, Pao-Hwa L, Miller ER 3rd, B GA. The DASH diet for high blood pressure: from clinical trial to dinner table. Cleve Clin J Med. 2004;71(9):745–53. [DOI] [PubMed] [Google Scholar]
- 88. Zeng H, Lazarova DL, Bordonaro M. Mechanisms linking dietary fiber, gut microbiota and colon cancer prevention. World J Gastrointest Oncol. 2014;6(2):41–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Gonzalez CA, Salas-Salvado J. The potential of nuts in the prevention of cancer. Br J Nutr. 2006;96(Suppl 2):S87–94. [DOI] [PubMed] [Google Scholar]
- 90. Edderkaoui M, Lugea A, Hui H, Eibl G, Lu QY, Moro A, Lu X, Li G, Go VL, Pandol SJ. Ellagic acid and embelin affect key cellular components of pancreatic adenocarcinoma, cancer, and stellate cells. Nutr Cancer. 2013;65(8):1232–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Chin YT, Hsieh MT, Yang SH, Tsai PW, Wang SH, Wang CC, Lee YS, Cheng GY, HuangFu WC, London Det al. Anti-proliferative and gene expression actions of resveratrol in breast cancer cells in vitro. Oncotarget. 2014;5(24):12891–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Yakoob MY, Baig-ansari N. Dietary sodium (salt) intake and risk of colorectal cancer: a systematic review (P05-039-19). Current Developments in Nutrition. 2019;3(Supplement_1):nzz030. P05–9-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. D'Elia L, Rossi G, Ippolito R, Cappuccio FP, Strazzullo P. Habitual salt intake and risk of gastric cancer: a meta-analysis of prospective studies. Clin Nutr. 2012;31(4):489–98. [DOI] [PubMed] [Google Scholar]
- 94. Eneroth H, Wallin S, Leander K, Nilsson Sommar J, Akesson A. Risks and benefits of increased nut consumption: cardiovascular health benefits outweigh the burden of carcinogenic effects attributed to aflatoxin B(1) exposure. Nutrients. 2017;9(12):1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Falasca M, Casari I. Cancer chemoprevention by nuts: evidence and promises. Front Biosci. 2012;S4:109–20. [DOI] [PubMed] [Google Scholar]
- 96. Salas-Salvado J, Bullo M, Perez-Heras A, Ros E. Dietary fibre, nuts and cardiovascular diseases. Br J Nutr. 2006;96:(Suppl 2):S46–51. [DOI] [PubMed] [Google Scholar]
- 97. Segura R, Javierre C, Lizarraga MA, Ros E. Other relevant components of nuts: phytosterols, folate and minerals. Br J Nutr. 2006;96:(Suppl 2):S36–44. [DOI] [PubMed] [Google Scholar]
- 98. Mozaffarian D, Katan MB, Ascherio A, Stampfer MJ, Willett WC. Trans fatty acids and cardiovascular disease. N Engl J Med. 2006;354(15):1601–13. [DOI] [PubMed] [Google Scholar]
- 99. Bosetti C, Turati F, La Vecchia C. Hepatocellular carcinoma epidemiology. Best Pract Res Clin Gastroenterol. 2014;28(5):753–70. [DOI] [PubMed] [Google Scholar]
- 100. Willison LN, Sathe SK, Roux KH. Production and analysis of recombinant tree nut allergens. Methods. 2014;66(1):34–43. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.