ABSTRACT
Dysglycemia is a common complication of severe acute malnutrition (SAM) in children. Its prevalence and impact on short- and long-term outcomes are not well described. This systematic review was undertaken to review the available evidence on dysglycemia (either hypo- or hyperglycemia) in hospitalized children with SAM. The 2 primary objectives of this systematic review were to understand the prevalence of hypoglycemia and hyperglycemia in children with SAM. A secondary objective was to understand the relation between dysglycemia and clinical outcomes like mortality in children with SAM. MEDLINE was searched with terms related to children, SAM, and dysglycemia. A meta-analysis of proportions was completed to determine the hypoglycemia prevalence and a standard meta-analysis was done to determine the relation between hypoglycemia and mortality. The certainty of the evidence was evaluated using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach. A total of 2148 articles were identified in the database search of which 16 met the inclusion criteria for the systematic review based on screening done by multiple reviewers. The overall prevalence of hypoglycemia in SAM across studies based on the meta-analysis of proportions was 9% (95% CI: 7%, 12%; I2 = 92%). Meta-analysis results showed that hypoglycemia was associated with a higher chance of mortality during hospitalization in children with SAM (OR: 4.29; 95% CI: 3.04, 6.05; I2 = 0%). According to the GRADE evaluation, the certainty of the evidence for the prevalence of hypoglycemia was low and for hyperglycemia was very low. For the relation between hypoglycemia and mortality, the certainty of the evidence was moderate. A meta-analysis was not carried out for the prevalence of hyperglycemia due to the wide range of definitions used for across studies, but the prevalence ranged from 2% to 38% in the literature. This systematic review highlights the need for further work in this area to include serial glucose measurements to understand the clinical importance of dysglycemia during hospitalization in children with SAM.
Keywords: severe acute malnutrition, hypoglycemia, hyperglycemia, pediatric, dysglycemia
Introduction
Malnutrition is a major global health problem and contributes to at least a third of all child deaths globally (1). In 2018, over 49 million children under the age of 5 y were wasted, of whom 17 million were severely wasted (2). Children with malnutrition are more likely to die when hospitalized for an intercurrent illness such as gastroenteritis, malaria, lower respiratory tract infections, or measles (3).
Hypoglycemia is the state of abnormally low blood glucose concentrations. The definition of hypoglycemia varies within the scientific literature and across clinical practice, but is defined by the WHO as a glycemia concentration <2.5 mmol/L (45 mg/dL) in an adequately nourished child, or <3.0 mmol/L (54 mg/dL) in a severely malnourished child (4). Hyperglycemia is an adaptive stress response often seen in critically ill patients and is most commonly defined as a glucose concentration >7.0 mmol/L (126 mg/dL) (5) although currently no agreed definition exists in nondiabetic patients.
There is strong evidence that dysglycemia (either hypoglycemia or hyperglycemia) is associated with higher mortality in children hospitalized with a severe illness (6, 7). Hypoglycemia has been found to have proinflammatory and prothrombotic effects and is associated with an increased risk of cardiovascular events (8). It can also have severe neurological consequences such as seizures and coma (9). The hippocampus is particularly sensitive to hypoglycemia and can lead to deficits in cognitive development particularly with regard to working memory (10, 11).
Severe acute malnutrition (SAM) is thought to predispose children to develop either hypo- or hyperglycemia. Low glycogen stores and wasting with reduced lean mass and adipose tissue reserves have been linked to hypoglycemia in SAM (12). In addition, hepatic glucose production has been found to be lower, especially in children with edematous SAM (13). Hormonal changes with impaired insulin responses as well as impaired glucose clearance potentially increase the risk of hyperglycemia in SAM (14, 15).
The WHO guidelines on the inpatient treatment of SAM at nutritional rehabilitation units recommend small 2–3 hourly feeds in the early stages of treatment with an aim to prevent episodes of hypoglycemia (16). They provide clear guidance on the screening, recognition, and treatment of hypoglycemia in emergency situations (4). Despite this, mortality rates in ill, severely malnourished children who are hospitalized often continue to be higher than the WHO target of 5–10% (17, 18). Hemodynamic instability and metabolic disturbances are thought to be important risk factors for death during hospital admission (14, 17). It is, however, not clear from the WHO guidelines what the prevalence of dysglycemic events is in children with SAM, nor to what extent dysglycemia affects the risk of morbidity and mortality in this vulnerable population.
The main objectives of this systematic review were to understand the prevalence of hypoglycemia and hyperglycemia in children admitted for inpatient treatment of SAM. A secondary objective was to determine the relation between dysglycemia and clinical outcomes like mortality in children with SAM. The review also explores current knowledge gaps in the literature around dysglycemia in children with SAM.
Methods
A systematic review protocol was submitted in PROSPERO (International Prospective Register of Systematic Reviews) (CRD42020184163). The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines were followed throughout the review.
Inclusion and exclusion criteria
The population of interest for this review was children aged 6–60 mo admitted for inpatient treatment of SAM. The current WHO definition of SAM includes: severe wasting (marasmus) defined by a weight-for-height z-score (WHZ) below –3 SDs or mid-upper arm circumference of <11.5 cm; the presence of nutritional edema (kwashiorkor); or a combination of wasting and nutritional edema (marasmic-kwashiorkor) (19). To be eligible, studies had to involve glucose measurements during inpatient treatment. The primary outcomes were the prevalence of hypoglycemia and hyperglycemia in this population with a secondary outcome being the relation between dysglycemia and clinical outcomes. Studies that examined either hypoglycemia or hyperglycemia were eligible for inclusion. Peer-reviewed observational and experimental studies were eligible, but systematic or narrative reviews, conference proceedings, animal studies, and case reports were excluded. Only articles in English and those published after 1990 were considered for inclusion due to the change in terminology and definition of SAM that have occurred over time.
Search strategy and study selection
MEDLINE (epub ahead of print, in-process, and other nonindexed citations, Ovid MEDLINE® Daily and Ovid MEDLINE® 1946-present) was searched on 30 April, 2020 using the following terms: (hypoglyc* OR hyperglyc* OR gluc*) AND (malnutr* OR undernutr* OR thin* OR marasmus OR kwashiorkor) AND (child* OR pediatr* OR paediatr*). Based on reviewer feedback, the Cochrane Library and Web of Science were searched using the same search terms on 15 July, 2020. Titles and abstracts were then screened in duplicate by multiple authors (EL, PH, and AD) and full texts were subsequently reviewed in duplicate by the same authors using Covidence software. Reference lists of included studies were examined to identify additional articles and additional informal online searches were done.
Synthesis of results
Data were extracted in duplicate by 2 authors (EL and AD), including country, methodology, dysglycemia definition, time point glucose was measured, and prevalence of dysglycemia. Individual studies were summarized in a table and described qualitatively in the text. Quantitative data were analyzed in Stata version 16 (StataCorp LP). Random-effects meta-analysis of proportions was completed using metaprop to understand the prevalence of hypoglycemia. Only studies that defined hypoglycemia by blood glucose <3.0 mmol/L were pooled in this analysis. Effect sizes representing proportions and 95% CIs were displayed. A meta-analysis was then done to examine the relation between hypoglycemia and mortality. ORs and 95% CIs were presented for this meta-analysis. Effect sizes were considered significant at P values <0.05. Statistical heterogeneity was evaluated using the I2 value. Results were not pooled for hyperglycemia because of a lack of established cut-off values for glucose concentrations and the variability in individual study methods for determining hyperglycemia.
Certainty of evidence
The Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach was carried out by 2 authors to evaluate the certainty of evidence of the prevalence of hypoglycemia and hyperglycemia, as well as the relation between hypoglycemia and mortality, across studies. This involved examining reasons for downgrading the certainty of the evidence, including inconsistency, indirectness of effect, imprecision, and publication bias in addition to individual study risk of bias.
Results
The search identified 2145 titles and abstracts and an additional 3 articles were identified from hand searches of reference lists of included articles and additional informal online searches (Figure 1). In total, 2110 articles were excluded and 38 were reviewed in full. Sixteen articles met the inclusion criteria for this systematic review, with 15 assessing hypoglycemia and 5 assessing hyperglycemia.
FIGURE 1.
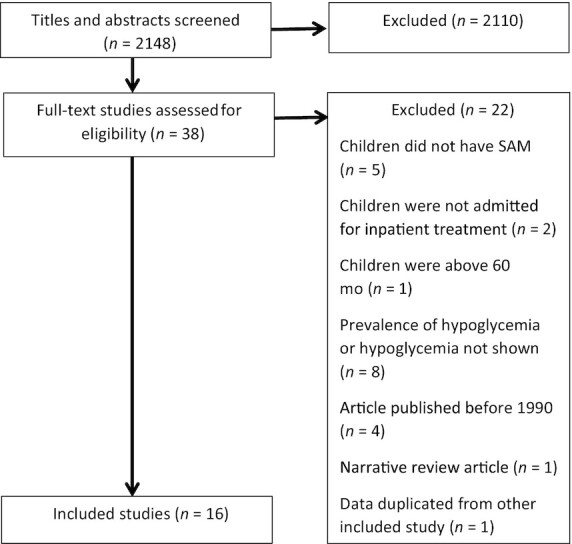
Literature search methodology. SAM, severe acute malnutrition.
Prevalence of hypoglycemia in SAM
Table 1 summarizes 15 studies we identified that reported hypoglycemia prevalence. Six of these were retrospective studies, 5 were prospective observational studies, 2 cross-sectional studies, 1 randomized controlled trial (RCT), and 1 case-control study. Studies were performed in countries in Asia and sub-Saharan Africa. Sample sizes for the studies ranged from 16 to 6136 children with SAM. Based on the results of the meta-analysis of proportions, the prevalence of hypoglycemia defined by blood glucose <3.0 mmol/L was 9% (95% CI: 7%; 12%; I2 = 92%) as shown in Figure 2.
TABLE 1.
Prevalence of hypoglycemia in hospitalized children with SAM
| Author/year | Country | Methodology | Hypoglycemia cut-off used (blood glucose concentration) | Time point for glucose measurement | Prevalence of hypoglycemia |
|---|---|---|---|---|---|
| Ahmed et al., 1999 (22) | Bangladesh | • Cross-sectional study• SAM children aged 0–5 y with diarrhea• Standardized “protocol treatment” (n = 334) nonprotocol treatment (n = 293) | <3 mmol/L | Admission and during hospitalization | Admission: 7.1% protocol, 8.1% nonprotocolDuring hospitalization: 2.7% protocol, 5.8% nonprotocol |
| Osier et al., 2003 (25) | Kenya | • Prospective observational cohort | <2.2 mmol/L | Admission | 9.4% |
| • SAM children (n = 522) | |||||
| Elusiyan et al., 2006 (26) | Nigeria | • Prospective cohort study | <2.5 mmol/L | Admission | 12.5% |
| • Children aged 1–14 y (n = 392) | |||||
| • SAM children (n = 16) | |||||
| Maitland et al., 2006 (17) | Kenya | • Retrospective study | <3 mmol/L | Admission | 13.6% |
| • SAM children aged >3 mo (n = 742 with glucose measurements) | |||||
| Alam et al., 2009 (27) | Bangladesh | • RCT of cholera treatment | <3 mmol/L | Admission | 8% |
| • SAM children aged 6 mo to 5 y with <48 h history of diarrhea (n = 175) | |||||
| Chisti et al., 2010 (28) | Bangladesh | • Retrospective study | <3 mmol/L | Admission | 16.4% |
| • Infants with SAM and diarrhea (n = 61) | |||||
| Roy et al., 2011 (24) | Bangladesh | • Case-control study• SAM children <3 y• Cases (n = 98), children died from acute diarrheaControls (n = 81), children who survived. | <3 mmol/L | Admission | 31% in cases (children who died), 11% in controls (children who survived) |
| Nhampossa et al., 2013 (20) | Mozambique | • Retrospective study | <2.2 mmol/L severe | Admission | 6% |
| • SAM children aged under 5 y (n = 2522) | 2.3–3.0 mmol/L moderate | ||||
| Shah and Javdekar, 2014 (29) | India | • Prospective observational cohort | Not provided | Admission | 5% |
| • SAM children <5 y (n = 60) | |||||
| Kariyappa and Shepur, 2015 (30) | India | • Prospective observational study | <3 mmol/L | Admission | 28.5% |
| • SAM children <5 y with either no signs or some signs of dehydration (n = 21) | |||||
| Madrid et al., 2016 (21) | Mozambique | • Retrospective study | <3 mmol/L | Admission | 4.1% |
| • SAM children aged <15 y (n = 6136) | |||||
| Girum et al., 2017 (31) | Ethiopia | • Retrospective study | Not provided | Admission | 8.8% |
| • SAM children <5 y (n = 545) | |||||
| Tumwebaze et al., 2018 (32) | Uganda | • Cross-sectional study | <3.3 mmol/L | Within 3 d of admission and 2 h after last feed | 1.3% |
| • SAM children between 1 and 60 mo (n = 235) | |||||
| Abhinay et al., 2019 (33) | India | • Cross-sectional | <3 mmol/L | Admission | 21.3% |
| • SAM children 6–60 mo (n = 108) | |||||
| Vonasek et al., 2020 (34) | Malawi | • Retrospective review of electronic medical records database | <3 mmol/L | Within 24 h after admission | 4% |
| • SAM children aged 6 mo to 3 y (n = 176) |
RCT, randomized controlled trial; SAM, severe acute malnutrition.
FIGURE 2.

Meta-analysis of proportions for hypoglycemia in hospitalized children with SAM. CI, confidence intervals; ES, effect size; SAM, severe acute malnutrition.
A large 10-y retrospective study of hospital records of 274,813 children in Mozambique found only 6% of children with SAM had a recorded episode of hypoglycemia (20). Another 13-y retrospective study of 45,000 children in Mozambique, where blood glucose was measured on admission (21), found a prevalence of hypoglycemia of 4.1% in the 6136 children with SAM. The authors noted that a significant number (14–18%) of children had an incorrect registration of height so predominantly weight-for-age was used to diagnose malnutrition, which could have led to an incorrect diagnosis of SAM. These retrospective studies had different types of bias. For example, glucose concentrations may have not been measured in asymptomatic patients.
Ahmed et al. compared outcomes in SAM children admitted with diarrhea treated by a standardized “protocol treatment” developed by the study investigators (n = 334) to those who had nonprotocol treatment (n = 293) (22). The “protocol treatment” comprised of slow rehydration (slower than the WHO guideline of the time) (23). Children who were treated following this protocol were compared to historical controls of children that had received the current nonprotocol treatment where feeds were delayed until after dehydration had been treated (dehydration and malnutrition being treated entirely separately with separate WHO guidelines of the time) (23). Prevalence of hypoglycemia (<3 mmol/L) on admission was 7.1% and 8.1% for those treated according to the protocol and those treated using the current nonprotocol treatment, respectively.
A case-control study in Bangladesh investigated risk factors of mortality in children with SAM who had diarrhea (24). Cases were children who died from acute diarrhea whereas controls were those who survived. This study found a high prevalence of hypoglycemia of 31% among cases (n = 98) and 11% among controls (n = 81). All patients had their glucose measured on admission.
A group in Kenya examined the utility of the WHO protocol to identify children with SAM at greatest risk of death (17) using a retrospective case note review of 920 children from Kilifi. Out of 742 children with SAM they found a prevalence of hypoglycemia of 13.6%. This was a retrospective analysis of case notes, relying on hypoglycemia being recognized and tested for; the prevalence of hypoglycemia was therefore likely underestimated.
An observational study of 3742 Kenyan children at admission to a pediatric ward reported a hypoglycemia prevalence (<2.2 mmol/L) of 7.3% across all diagnoses (25). For children with SAM, they found a prevalence of 9.4% amongst the 512 patients whose glucose levels were tested on admission due to protocol and clinical indicators (25).
A retrospective study in Ethiopia (31) in 545 children with SAM found hypoglycemia was the third most common comorbidity and complication on admission (after pneumonia and anemia) and was identified in 8.8% of children admitted. The major limitation was the retrospective nature of the study and its reliance on both clinician discretion to test glucose and subsequent recording in the clinical notes of the result.
Tumwebaze et al. found 1.3% of children admitted with SAM (n = 235) to a nutritional rehabilitation unit in Uganda were hypoglycemic (32). Glucose was measured the morning after admission and ≥2 h after feeds.
An RCT carried out in Bangladesh to assess the safety of rapid intravenous rehydration in children with acute dehydrating cholera (<48 h of diarrhea) found a prevalence of hypoglycemia of 8% in 175 children with SAM and cholera (27).
In an article published this year from Malawi, 7 out of 176 (4%) SAM patients were recorded to have hypoglycemia in the first 24 h of admission based on an electronic medical records database. Again, a limitation was that there was retrospective bias and only 27% of patients had a blood glucose documented in this system within 4 h of admission (WHO standards indicate that all patients with SAM should have blood glucose measured and documented) (34).
Finally, 5 other studies with small subject numbers found prevalence rates of hypoglycemia of 28.5% (n = 21) (30), 5% (n = 60) (29), 16.4% (28), 21.3% (33), and 12.5% (n = 16) (26).
In summary, the studies show varying prevalence of hypoglycemia in children with SAM ranging from 1.3% to 31%. All but 2 (20, 24) of these studies were based on a 1-time glucose measurement systematically done on admission or a day after. In 1 study, authors did not clarify whether the measurements during hospital stay were on clinical indication or not (22). Common limitations of these articles were that there was no mention of the timing of the glucose measurement in relation to feeds or time of day and limited discussion of the severity of children's illnesses.
Prevalence of hyperglycemia in SAM
Currently, no guidelines exist on the management of nondiabetic hyperglycemic ill children with SAM. We identified 5 studies that explored the prevalence of hyperglycemia in SAM as shown in Table 2.
TABLE 2.
Prevalence of hyperglycemia in hospitalized children with SAM
| Author | Country | Methodology | Hyperglycemia cut-off used (blood glucose concentration) | Time point for glucose measurement | Prevalence of hyperglycemia |
|---|---|---|---|---|---|
| Seth and Aneja, 1995 (35) | India | • Prospective observational cohort | Not provided | Admission | 10% |
| • SAM children aged 1–4 y with mild or moderate dehydration (n = 50) | |||||
| Osier et al., 2003 (25 ) | Kenya | • Prospective observational cohort | >10 mmol/L | Admission | 2% |
| • SAM children age >1 mo, max age admitted to pediatric ward (not stated) (n = 522) | |||||
| Roy et al., 2011 (24) | Bangladesh | • Case-control study• SAM children <3 y• Cases (n = 98)—children died from acute diarrheaControls (n = 81)—children who survived | >6.5 mmol/L | Admission | 38% cases (children who died), 28% controls (children who survived) |
| Kariyappa and Shepur, 2015 (30) | India | • Prospective observational study | Not provided | Admission | 14% |
| <5 y SAM children with some signs or no signs of dehydration (n = 21) | |||||
| Tumwebaze et al., 2018 (32) | Uganda | • Cross-sectional study | 8.3 mmol/L | Within 3 d of admission | 16.6% |
| • SAM children aged 1–60 mo (n = 235) |
SAM, severe acute malnutrition.
Osier et al., in a large observational study, found a hyperglycemia (>10 mmol/L) prevalence of 2% in the 512 patients with SAM admitted to a rural district hospital in Kenya who had blood glucose tested on admission (25).
Tumwebaze et al. found 16.6% of children admitted with SAM (n = 235) in a cross-sectional study of a nutritional rehabilitation unit in Uganda (32) were hyperglycemic (defined as >8.3 mmol/L). Glucose was measured the morning after admission and ≥2 h after feeds. Children with oral sores were found to be almost 3 times more likely to have hyperglycemia.
A case-control study in Bangladesh investigating risk factors of mortality in children with SAM with diarrhea found a high prevalence of 38% among cases (n = 98) and 28% among controls (n = 81), with hyperglycemia defined as >6.5 mmol/L (24). Cases were children who died from acute diarrhea whereas controls were those who survived.
Finally, 2 small studies in India, Seth and Aneja (n = 55) and Kariyappa and Shepur (n = 21), found hyperglycemia prevalence of 9% and 14.2%, respectively (30, 35), in children with SAM presenting with dehydrating gastroenteritis. In both studies, all children with hyperglycemia only received an oral rehydration solution for dehydration. The studies did not state the definition used for hyperglycemia.
In summary, data for all studies were based on a 1-time glucose measurement on admission and the prevalence ranged between 9% and 38%. The definition of hyperglycemia varied in the different studies which likely contributed to the observed differences in the prevalence.
Relation between dysglycemia and clinical outcomes
Twelve studies discussed the relation between dysglycemia and clinical outcomes for children with SAM, describing increases in mortality rates, and the use of dysglycemia as a predictor of poor outcomes in this population (Table 3). Results from the meta-analysis showed that hypoglycemia on admission is associated with higher odds of mortality in children with SAM (OR: 4.29; 95% CI: 3.04, 6.05; I2 = 0%) (Figure 3).
TABLE 3.
Clinical outcomes in hospitalized children with SAM and dysglycemia
| Author | Country | Methodology | Time point for glucose measurement | Results |
|---|---|---|---|---|
| Ahmed et al., 1999 (22) | Bangladesh | • Cross-sectional study• Children aged 0–5 y with diarrea• Standardized protocol (n = 334) nonprotocol treatment (n = 293) | Admission and during hospitalization | Risk factors for mortality in standardized protocol included increased frequency of hypoglycemia |
| Osier et al., 2003 (25) | Kenya | • An observational study• All children included (n = 3742) | Admission | Hypoglycemic patients who died, 32% were children “severely ill” or malnourished |
| Maitland et al., 2006 (17) | Kenya | • Retrospective study | Admission | 1) Overall mortality 19% |
| • SAM children aged >3 mo (n = 920)• 19% (178 of 920) did not have a glucose reading | 2) The likelihood ratio for hypoglycemia identifying early deaths (within 48 h of admission) was 4.0 (95% CI: 2.5, 6.3) and for late deaths (after 48 h) was 2.2 (95% CI: 1.4, 3.6) | |||
| Alam et al., 2009 (27) | Bangladesh | • RCT of cholera treatment• SAM children aged 6 mo to 5 y with <48 h history of diarrhea (n = 175) | Admission | Of 14 hypoglycemic SAM patients there were 0 deaths |
| Chisti et al., 2010 (28) | Bangladesh | • Retrospective study• Infants with SAM and diarrhea (n = 61) | Admission | Death significantly associated with hypoglycemia40% cases vs. 12% controls hypoglycemic |
| • Cases (n = 10)—deaths | ||||
| • Controls (n= 51)—survivors | ||||
| Roy et al., 2011 (24) | Bangladesh | • Case-control study• SAM children <3 y | Admission | Hypoglycemia associated with 3.8 times the odds of death |
| • Cases (n = 98)—children died from acute diarrhea | ||||
| Controls (n = 81)—children who survived | ||||
| Nhampossa et al., 2013 (20) | Mozambique | • Retrospective study• SAM children aged under 5 y (n = 2522) | Admission | Hypoglycemia—an independent risk factor for mortality |
| Shah and Javdekar, 2014 (29) | India | • Prospective cohort | Admission | 1/3 hypoglycaemic SAM patients died |
| • SAM children <5 y (n = 60) | ||||
| Madrid et al., 2016 (21) | Mozambique | • Retrospective study• All children aged under 15 y (n = 45,573) | Admission | 1) Malnutrition an independent risk factor of mortality in hypoglycemic children |
| 2) 58.4% of hypoglycemia related deaths were in children with malnutrition (AOR: 1.21; 95% CI: 1.03, 1.42) | ||||
| Girum et al., 2017 (31) | Ethiopia | • Retrospectively study | Admission | 1) Overall mortality 9.3% |
| • <5 y SAM children (n = 545) | 2) Hypoglycemia an independent risk factor for mortality—adjusted HR of 2.74 (95% CI: 1.279, 5.87) | |||
| Tumwebaze et al., 2018 (32) | Uganda | • Cross-sectional study | Within 3 d of admission | 1) Overall mortality 20.2% |
| • SAM children aged 1–60 mo (n = 235) | 2) Hyperglycemic children mortality = 56.4% | |||
| 3) Hyperglycemic children 8 times more likely to die | ||||
| Abhinay et al., 2019 (33) | India | • Prospective cross-sectional study• SAM children 6–60 mo (n = 108) | Admission | Deaths 3.6 times more likely to be hypoglycemic |
| Vonasek et al., 2020 (34) | Malawi | • Retrospective review of electronic medical records database | Within 24 h after admission | 5 of 7 of hypoglycemic SAM patients died |
| • SAM children aged 6 mo to 3 y (n = 176) |
AOR, adjusted odds ratio; CI, confidence intervals; HR, hazard ratio; RCT, randomized control trial; SAM, severe acute malnutrition.
FIGURE 3.
Meta-analysis of hypoglycemia and mortality in hospitalized children with SAM. CI, confidence intervals; OR, odds ratio; REML, restricted maximum likelihood; SAM, severe acute malnutrition.
A large 10-y retrospective study of hospital records of 274,813 children in Mozambique found a case fatality rate (CFR) of 7% in children with SAM (20). Multivariable analysis showed that there was an associated risk of death with hypoglycemia in SAM. The authors recommended early screening in SAM to detect and treat hypoglycemia.
In another large retrospective study from Mozambique (21), 3.2% of all admissions were hypoglycemic (blood glucose concentration <3.0 mmol/L) with a CFR associated with hypoglycemia of 19.3% versus 3.3% in those who were not hypoglycemic. There were 3 main groups independently associated with both risk of hypoglycemia and higher odds of death among patients with hypoglycemia; 1) unable to feed/unconscious, 2) malnutrition, and 3) concomitant infections. SAM was an independent risk factor of hypoglycemia [adjusted odds ratio (AOR): 1.12; 95% CI: 1.07, 1.18]. Fifty-eight percent of hypoglycemia-related deaths were in children with SAM (AOR: 1.21; 95% CI: 1.03, 1.42). There was also a significantly higher CFR of 12.1% with hyperglycemia compared with normoglycemia. This study also drew attention to the fact that, like many of the previously discussed studies in this review, glucose concentrations were only assessed at 1 fixed point on admission. This likely underestimated the prevalence of hypoglycemia in this population.
A retrospective cohort study done in Ethiopia (31) explored predictors of mortality among children admitted with SAM. Overall mortality in children with SAM was 9.3%. Children with hypoglycemia had a significantly higher risk of death compared with normoglycemic children with an adjusted hazard ratio of 2.74 (95% CI: 1.279, 5.87).
Tumwebaze et al. (32) investigated the prevalence and outcomes of hyperglycemia (>8.3 mmol/L) among severely malnourished children (n = 235) in Uganda. Glucose concentrations were measured once after admission and ≥2 h after feeds. Those with hyperglycemia had a mortality rate of 56.4% and were more likely to die compared with those without hyperglycemia (OR: 8.7; 95% CI: 4.1, 18.7).
A Kenyan study using the WHO protocol of the time to identify children with SAM at greatest risk of death (17) found 19% of these children died, with a third of deaths occurring in the first 48 h of admission. The likelihood ratio for predicting deaths in the first 48 h of admission in patients with hypoglycemia (defined as glucose <3 mmol/L) was 4.0 (95% CI: 2.5, 6.3) and for deaths after 48 h was 2.2 (95% CI: 1.4, 3.6).
Another study from Kenya (n = 3742) reported a higher mortality in children (Weight for Height Z score < −3) with hypoglycemia compared to children with normoglycemia (20% versus 3.8%) (25). A key finding of this study was that despite an established protocol for the recognition and treatment of hypoglycemia, almost a third of children with hypoglycemia, and either a severe illness or malnutrition, died.
Three studies in Bangladesh looked at risk factors and clinical outcomes of severely malnourished children with diarrhea (22, 24, 28). In the study comparing “protocol” treatment of SAM children developed by the study investigators with diarrhea with retrospective nonprotocol treatment, mortality in the protocol group was 9% and significantly lower than the 17% mortality in the nonprotocol group (22). Most of the deaths in children treated according to the standardized protocol occurred within 48 h of admission and the risk factors for mortality were: younger age, poorer nutritional status, increased frequency of hypoglycemia and septicemia on admission, bacteremia, and greater volume of intravenous fluids infused. It is difficult to infer which aspects of management were related to mortality. Furthermore, the use of historical controls was a limitation of this study.
Roy et al. (24) compared risk factors of mortality in SAM children who died from diarrhea (cases) to those who survived (controls). The presence of hypoglycemia on admission had 3.8 times (95% CI: 1.5, 9.8) the odds of death compared to those with normal blood glucose concentrations. Other significant risk factors for mortality included: septicemia (AOR: 8.8; 95% CI: 3.7, 21.1), hypothermia (AOR: 3.5; 95% CI: 1.3, 9.4), and bronchopneumonia (AOR: 3.0; 95% CI: 1.2, 7.3).
The third study from Bangladesh, including children (age not given) with SAM and diarrhea, compared 10 children who died with 51 survivors (28). They found deaths were significantly associated with hypoglycemia (OR 5.0; 95% CI: 1.1, 23.0) and the sensitivity and specificity of hypoglycemia to predict death were 40% and 88%, respectively.
In the retrospective study of electronic medical records in Malawi (34), of the 7 SAM patients with documented hypoglycemia, 5 died and 2 survived. In another study done in India with a very small sample size, 2 hypoglycemic SAM patients survived and 1 died (29).
A prospective study in India looking at clinical outcomes in 108 children with SAM using the WHO triage framework (4) found that those that died were 2.07 times more likely to have hypoglycemia on admission after being identified as having priority signs on triage (33).
In summary, there is an established associated relation between increased mortality risk and dysglycemia in children with SAM, but the strength of that relation varies strongly between studies. It is also worth noting that in all these studies, glucose concentrations were measured mainly on admission, and therefore, the risk of dysglycemia for adverse clinical outcomes during hospitalization and its relative contribution has not been established for children with SAM.
Certainty of evidence
Based on the GRADE assessment, there was very serious inconsistency for hypoglycemia prevalence based on an I2 value of 92%. There were also concerns of indirectness of effect as there was variability in when glucose was assessed upon hospitalization, which was the case for hypoglycemia and hyperglycemia. There was also imprecision for hypoglycemia as the sample size was small within and across studies. The certainty of the evidence around hypoglycemia prevalence was therefore low and for hyperglycemia was very low. Lastly, the certainty of the evidence for mortality was moderate, with imprecision as the reason for downgrading because of the small sample size within and across studies.
Discussion
This review has provided evidence that hypoglycemia is relatively common in children with SAM with prevalence described in the range of 1.3–28.5%, and up to 31% in children that died (17, 20–22, 24–34). There were few studies that reported on the prevalence of hyperglycemia in SAM but limited data do suggest that it is common in children with SAM, with prevalence described as 2–16.6%, and up to 38% in children who died (24, 25, 30, 32, 35). There are consistent data reporting an association between dysglycemia on admission to hospital and mortality in children with SAM (17, 20–22, 24, 25, 28, 29, 31–34).
A common limitation for many of these studies was the reliance on a single time point for the measurement of blood glucose, often only on admission. The associations between blood glucose and feeds, and time of day, have not been described and the immediate and short-term effects of treatment of states of hypoglycemia have not been reported in children with SAM.
There are scarce data on whether specific age groups, such as young infants, are at increased risk of dysglycemia, with 1 study including only 21 children with SAM and diarrhea reporting an increased risk of dysglycemia in those aged <2 y compared with older children (30). There is also a limited understanding of how patterns of glucose variability may relate to long-term developmental outcomes in survivors of childhood malnutrition (36, 37) and this warrants further study.
There was variation in the definition of hypoglycemia (<2.2 mmol/L to <3.3 mmol/L) amongst the studies and even wider variation in the definition of hyperglycemia (>6.5 mmol/L to 10 mmol/L) with some articles not specifying the definition used. Furthermore, many studies did not specify if they assessed fasting blood glucose, but this may not have been possible in certain circumstances such as samples taken upon admission. This made comparison of prevalence data difficult. In many studies, glucose concentrations were measured based on clinician-driven decisions, which could underestimate its true prevalence or could alternatively introduce bias as children with symptoms consistent with hypoglycemia might have a higher chance of glucose concentration being measured. Several studies relied on retrospective analysis of medical notes, which depended on the blood glucose result being recorded accurately. In practice, this task is easily forgotten on a busy medical ward and may also lead to an inaccurate estimate of prevalence.
Furthermore, it is important to note that the certainty of evidence was low for the prevalence of hypoglycemia, very low for the prevalence of hyperglycemia, and moderate for estimates of mortality in children with SAM who had hypoglycemia. One of the limitations of this systematic review was that it was not possible to assess the risk of bias of individual studies since this review examined predominantly observational studies, many of which had retrospective study designs.
The exact patterns of glycemia in SAM remain largely unknown (37), and in high-resource contexts it is reported that glucose variability is associated with increased mortality in sick children (36). A way of investigating the patterns of blood glucose concentrations and identifying any episodes of asymptomatic hypoglycemia is using a continuous glucose monitoring system.These have been used in very low birth weight infants (38) and children with malaria where over 15% of children with hypoglycemia were asymptomatic (39). Continuous glucose monitoring systems have not been used in children with SAM before, and could provide valuable insights into the occurrence, risk factors, and clinical impact of dysglycemia in these children. Further information on glucose patterns could impact clinical management guidelines, including refeeding formulations. A potential focus could be to assess glucose concentrations overnight where much of the mortality in this population occurs (18, 26).
In conclusion, this review has demonstrated that children with SAM are at risk of hypo- or hyperglycemia, and hypoglycemia relates to clinical outcomes like mortality. Future research should focus on generating more in-depth data on the prevalence and patterns of dysglycemia during hospitalization in relation to short-term survival and long-term health outcomes.
Acknowledgments
The authors’ contributions were as follows—EL and PH: undertook the initial search and first draft of the manuscript; AD, TC, AP, and RB: edited and provided technical advice on the subject; AD: analyzed data for the meta-analysis; EL: wrote the subsequent final version of the manuscript; and all authors: read and approved the final manuscript.
Notes
TC is a Medical Research Council Clinical Research Training Fellow and RB has funding from the Bill and Melinda Gates Foundation. Neither funding body had any input into this manuscript.
Author disclosures: The authors report no conflicts of interest.
Abbreviations used: AOR, adjusted odds ratio; CFR, case fatality rate; CI, confidence intervals; GRADE, Grading of Recommendations Assessment, Development and Evaluation; NA, not applicable/provided; OR, odds ratio; RCT, randomized controlled trial; SAM, severe acute malnutrition.
Contributor Information
Elizabeth Ledger, Medical Research Council The Gambia at the London School of Hygiene & Tropical Medicine, Keppel Street, London, UK.
Philliness Prisca Harawa, Department of Biomedical Sciences, College of Medicine, University of Malawi, Blantyre, Malawi; Malawi-Liverpool Wellcome Trust Clinical Research Programme, Blantyre, Malawi.
Allison I Daniel, Centre for Global Child Health, Hospital for Sick Children, Toronto, Canada; Department of Nutritional Sciences, Faculty of Medicine, University of Toronto, Toronto, Canada.
Toby Candler, Medical Research Council The Gambia at the London School of Hygiene & Tropical Medicine, Keppel Street, London, UK.
Andrew M Prentice, Medical Research Council The Gambia at the London School of Hygiene & Tropical Medicine, Keppel Street, London, UK.
Robert H J Bandsma, Department of Biomedical Sciences, College of Medicine, University of Malawi, Blantyre, Malawi; Centre for Global Child Health, Hospital for Sick Children, Toronto, Canada; Department of Nutritional Sciences, Faculty of Medicine, University of Toronto, Toronto, Canada; The Childhood Acute Illness and Nutrition Network (CHAIN), Nairobi, Kenya.
References
- 1. Black RE, Victora CG, Walker SP, Bhutta ZA, Christian P, De Onis M, Ezzati M, Grantham-Mcgregor S, Katz J, Martorell Ret al. Maternal and child undernutrition and overweight in low-income and middle-income countries. Lancet North Am Ed. 2013;382:427–51. [DOI] [PubMed] [Google Scholar]
- 2. UNICEF/WHO/World Bank . Levels and Trends in Child Malnutrition – UNICEF WHO The World Bank Joint Child Malnutrition Estimates, key findings of the 2019 edition. UNICEF. 2019. [Google Scholar]
- 3. Caulfield LE, de Onis M, Blössner M, Black RE. Undernutrition as an underlying cause of child deaths associated with diarrhea, pneumonia, malaria, and measles. Am J Clin Nutr. 2004;80:193–8. [DOI] [PubMed] [Google Scholar]
- 4. World Health Organization . Guideline: Updates on Paediatric Emergency Triage, Assessment and Treatment: Care of Critically-Ill Children. World Health Organization, Geneva. 2016. [PubMed] [Google Scholar]
- 5. Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998;15:539–53. [DOI] [PubMed] [Google Scholar]
- 6. Wintergerst KA, Buckingham B, Gandrud L, Wong BJ, Kache S, Wilson DM. Association of hypoglycemia, hyperglycemia, and glucose variability with morbidity and death in the pediatric intensive care unit. Pediatrics. 2006;118:173–9. [DOI] [PubMed] [Google Scholar]
- 7. Faustino EVS, Hirshberg EL, Asaro LA, Biagas KV, Pinto N, Srinivasan V, Bagdure DN, Steil GM, Coughlin-Wells K, Wypij Det al. Short-term adverse outcomes associated with hypoglycemia in critically ill children. Crit Care Med. 2019;47:706–14. [DOI] [PubMed] [Google Scholar]
- 8. Ratter JM, Rooijackers HMM, Tack CJ, Hijmans AGM, Netea MG, de Galan BE, Stienstra R. Proinflammatory effects of hypoglycemia in humans with or without diabetes. Diabetes. 2017;66:1052–61. [DOI] [PubMed] [Google Scholar]
- 9. Mahajan G, Mukhopadhyay K, Attri S, Kumar P. Neurodevelopmental outcome of asymptomatic hypoglycemia compared with symptomatic hypoglycemia and euglycemia in high-risk neonates. Pediatr Neurol. 2017;74:74–9. [DOI] [PubMed] [Google Scholar]
- 10. Holemans X, Dupuis M, Misson N, Vanderijst JF. Reversible amnesia in a type 1 diabetic patient and bilateral hippocampal lesions on magnetic resonance imaging (MRI). Diabet Med. 2001;18:761–3. [DOI] [PubMed] [Google Scholar]
- 11. Deary IJ, Sommerfield AJ, McAulay V, Frier BM. Moderate hypoglycaemia obliterates working memory in humans with and without insulin treated diabetes. J Neurol Neurosurg Psychiatry. 2003;74::278–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ashworth A. Treatment of severe malnutrition. J Pediatr Gastroenterol Nutr. 2001;32:516–8. [DOI] [PubMed] [Google Scholar]
- 13. Bandsma RH, Mendel M, Spoelstra MN, Reijngoud DJ, Boer T, Stellaard F, Brabin B, Schellekens R, Senga E, Heikens GT. Mechanisms behind decreased endogenous glucose production in malnourished children. Pediatr Res. 2010;68:(5):423–8. [DOI] [PubMed] [Google Scholar]
- 14. Bhutta ZA, Berkley JA, Bandsma RHJ, Kerac M, Trehan I, Briend A. Severe childhood malnutrition. Nat Rev Dis Primers. 2017;3:17067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Spoelstra MN, Mari A, Mendel M, Senga E, van Rheenen P, van Dijk TH, Reijngoud D-J, Zegers RGT, Heikens GT, Bandsma RHJ. Kwashiorkor and marasmus are both associated with impaired glucose clearance related to pancreatic β-cell dysfunction. Metabolism. 2012;61:1224–30. [DOI] [PubMed] [Google Scholar]
- 16. Roberfroid D, Verstraeten R. Management of oedematous malnutrition in infants and children aged >6 months: a systematic review of the evidence. World Health Organization. 2013. [Google Scholar]
- 17. Maitland K, Berkley JA, Shebbe M, Peshu N, English M, Newton C. Children with severe malnutrition: can those at highest risk of death be identified with the WHO protocol?. PLoS Med. 2006;3:e500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Karaolis N, Jackson D, Ashworth A, Sanders D, Sogaula N, McCoy D, Chopra M, Schofield C. WHO guidelines for severe malnutrition: are they feasible in rural African hospitals?. Arch Dis Child. 2007;92:198–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. World Health Organization . WHO Child Growth Standards and the identification of severe acute malnutrition in infants and children. [Internet]. WHO Library. Geneva; 2009. Available from: https://apps.who.int/iris/bitstream/handle/10665/44129/9789241598163_eng.pdf. [PubMed] [Google Scholar]
- 20. Nhampossa T, Sigaúque B, Machevo S, Macete E, Alonso P, Bassat Q, Menéndez C, Fumadó V. Severe malnutrition among children under the age of 5 years admitted to a rural district hospital in southern Mozambique. Public Health Nutr. 2013;16:1565–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Madrid L, Acacio S, Nhampossa T, Lanaspa M, Sitoe A, Maculuve SA, Mucavele H, Quintó L, Sigaúque B, Bassat Q. Hypoglycemia and risk factors for death in 13 years of pediatric admissions in Mozambique. Am J Trop Med Hyg. 2016;94:218–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ahmed T, Ali M, Ullah MM, Choudhury IA, Haque ME, Salam MA, Rabbani GH, Suskind RM, Fuchs GJ. Mortality in severely malnourished children with diarrhoea and use of a standardised management protocol. Lancet North Am Ed. 1999;353:1919–22. [DOI] [PubMed] [Google Scholar]
- 23. World Health Organization . Management of severe malnutrition: a manual for physicians and other senior health workers. [Internet]. Geneva; 1999. Available from: https://www.who.int/nutrition/publications/en/manage_severe_malnutrition_eng.pdf. [Google Scholar]
- 24. Roy SK, Buis M, Weersma R, Khatun W, Chowdhury S, Begum A, Sarker D, Thakur SK, Khanam M. Risk factors of mortality in severely-malnourished children hospitalized with diarrhoea. J Health Popul Nutr. 2011;29:229–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Osier FHA, Berkley JA, Ross A, Sanderson F, Mohammed S, Newton C. Abnormal blood glucose concentrations on admission to a rural Kenyan district hospital: prevalence and outcome. Arch Dis Child. 2003;88:621–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Elusiyan JBE, Adejuyigbe EA, Adeodu OO. Hypoglycaemia in a Nigerian paediatric emergency ward. J Trop Pediatr. 2006;52:96–102. [DOI] [PubMed] [Google Scholar]
- 27. Alam NH, Islam S, Sattar S, Monira S, Desjeux J-F. Safety of rapid intravenous rehydration and comparative efficacy of 3 oral rehydration solutions in the treatment of severely malnourished children with dehydrating cholera. J Pediatr Gastroenterol Nutr. 2009;48:318–27. [DOI] [PubMed] [Google Scholar]
- 28. Chisti MJ, Ahmed T, Bardhan PK, Salam MA. Evaluation of simple laboratory investigations to predict fatal outcome in infants with severe malnutrition presenting in an urban diarrhoea treatment centre in Bangladesh. Trop Med Int Heal. 2010;15:1322–5. [DOI] [PubMed] [Google Scholar]
- 29. Shah R, Javdekar B. Management of children with severe acute malnutrition: experience of nutrition rehabilitation centre at Baroda, Gujarat. Int J Contemp Pediatr. 2014;1:3. [Google Scholar]
- 30. Kariyappa M, Shepur TA. Hyperglycemia in grade III and grade IV malnutrition with dehydrating gastroenteritis. JEBMH. 2015;2:4623–9. [Google Scholar]
- 31. Girum T, Kote M, Tariku B, Bekele H. Survival status and predictors of mortality among severely acute malnourished children <5 years of age admitted to stabilization centers in Gedeo Zone: a retrospective cohort study. Ther Clin Risk Manag. 2017;; 13:101–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tumwebaze A, Kiboneka E, Mugalu J, Kikabi EM, Tumwine JK. Prevalence and outcome of stress hyperglycaemia among severely malnourished children admitted to Mulago referral and teaching hospital in Kampala, Uganda. BMC Nutr. 2018;4:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Abhinay A, Kumar D, Rao SK. Triage of children with severe acute malnutrition and its outcome: single centre cross-sectional study. JCDR. 2019;13:4–6. [Google Scholar]
- 34. Vonasek BJ, Chiume M, Crouse HL, Mhango S, Kondwani A, Ciccone EJ, Kazembe PN, Gaven W, Fitzgerald E. Risk factors for mortality and management of children with complicated severe acute malnutrition at a tertiary referral hospital in Malawi. Paediatr Int Child Health. 2020;40(3):148–57. [DOI] [PubMed] [Google Scholar]
- 35. Seth A, Aneja S. Hyperglycemia in malnourished children with dehydrating gastroenteritis. Indian J Pediatr. 1995;62:353–5. [DOI] [PubMed] [Google Scholar]
- 36. Leite HP, de Lima LFP, de Oliveira Iglesias SB, Pacheco JC, de Carvalho WB. Malnutrition may worsen the prognosis of critically ill children with hyperglycemia and hypoglycemia. JPEN J Parenter Enteral Nutr. 2013;37:335–41. [DOI] [PubMed] [Google Scholar]
- 37. Bila R, Varo R, Madrid L, Sitoe A, Bassat Q. Continuous glucose monitoring in resource-constrained settings for hypoglycaemia detection: looking at the problem from the other side of the coin. Biosensors. 2018;8((2):):43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Beardsall K, Vanhaesebrouck S, Ogilvy-Stuart AL, Vanhole C, Palmer CR, Ong K, Van Weissenbruch M, Midgley P, Thompson M, Thio Met al. Prevalence and determinants of hyperglycemia in very low birth weight infants: cohort analyses of the NIRTURE Study. J Pediatr. 2010;157:715–9.e3. [DOI] [PubMed] [Google Scholar]
- 39. Madrid L, Sitoe A, Varo R, Nhampossa T, Lanaspa M, Nhama A, Acácio S, Riaño I, Casellas A, Bassat Q. Continuous determination of blood glucose in children admitted with malaria in a rural hospital in Mozambique. Malar J. 2017;16(1):184. [DOI] [PMC free article] [PubMed] [Google Scholar]



