ABSTRACT
There is growing awareness that intestinal dysfunction determines the clinical outcomes of situations as diverse as undernourished children in urban tropical slums and undernourished surgical patients in intensive care units. As experimental starvation in humans has only rarely been studied, and largely not using current biomedical research tools, we must draw inference from disparate clinical and experimental observations as to the derangements present in the starved gut. There is good evidence of intestinal atrophy and achlorhydria in starvation and severe undernutrition. Historical reports from concentration camps and conflict settings consistently reported a noncontagious phenomenon called “hunger diarrhea,” but in settings where starved individuals are isolated from others (prisoners on hunger strike, anorexia nervosa) diarrhea is not a feature. Changes in intestinal permeability and absorption have been infrequently studied in experimental starvation; available data suggest that short-term starvation reduces sugar absorption but not permeability. Severe acute malnutrition in children is associated with severe changes in the intestinal mucosa. Experimental animal models may help explain some observations in humans. Starved rats develop a hypersecretory state and intestinal barrier defects. Starved pigs demonstrate prolongation of rotavirus diarrhea and reproduce some of the absorptive and barrier defects observed in malnourished children. However, there remains much to be learned about the effects of starvation on the gut. Given the high prevalence of undernutrition in hospitals and disadvantaged communities, the lack of attention to the interaction between undernutrition and gastrointestinal damage is surprising and needs to be corrected. Current sophisticated cellular and molecular techniques now provide the opportunity to create fresh understanding of gastrointestinal changes in pure undernutrition, using volunteer studies and samples from anorexia nervosa.
Keywords: starvation, malnutrition, enteropathy, malabsorption, diarrhea, anorexia nervosa
Introduction
Malnutrition is an ancient scourge of humans. Famines have been part of the human experience since time immemorial (1–3). Malnutrition, a term which recently has come to include obesity, is still common in the form of undernutrition in hospitals and in disadvantaged communities around the world (1). Largely because the intestine was difficult to study until the advent of biopsy capsules and endoscopes, and because it autolyses postmortem, our understanding of the effects of malnutrition on the intestine is limited. The purpose of this review is to survey what we know about the effects on the gut of starvation and semi-starvation (severe restriction of nutrient intake), separated as far as possible from the effects of infectious and inflammatory disorders. I will draw on historical accounts, studies of anorexia nervosa, some relevant experimental and volunteer studies in humans, recent work on acute and chronic malnutrition in children living in low- and middle-income countries, and then selected reports of animal experimentation, which may help explain these clinical and experimental observations. The cardinal features and etiologies of the different malnutrition disorders included are summarized in Table 1.
TABLE 1.
Summary of cardinal features and etiologies of different human malnutrition disorders reviewed1
| Concentration camps and the Warsaw ghetto | Anorexia nervosa | Hunger strikes | Experimental starvation or semi-starvation | SAM | |
|---|---|---|---|---|---|
| Age group | Adults | Adolescents and adults | Adults | 20–33 y | Under 5 y |
| Sex | Both | Mostly women | Mostly men | Men only | Both |
| Setting | Starvation and violence during genocide | Homes and hospitals in affluent countries | Prison hospitals | Research setting | Hospitals in tropical countries |
| Nutrients | Extremely reduced | Extremely reduced | Usually no macronutrients at all | Limited to 1570 kcal/d (Minnesota) or nil (Blaine) | Reduced diversity and calories but variable |
| Intestinal infection | Not proved and not contagious, but likely to have been present | No | No | No | Yes |
| Diarrhea: a major clinical feature or not? | Yes, often a terminal event in extreme wasting | No | No | No | Frequent |
SAM, severe acute malnutrition.
Historical Accounts of Starvation and Diarrhea during Wartime
During the Second World War, millions of victims of the Nazi regime were starved, tortured, and then executed in ghettos and concentration camps. Despite the horrific conditions, a number of courageous physicians attempted to learn what they could about undernutrition from the conditions in which they found themselves, and to which many of them also eventually succumbed. The most remarkable of these witness accounts were the records kept by physicians from several medical disciplines, including pathology, in the Warsaw ghetto. These accounts were later published by people who were able to retrieve hidden fragments of the accounts written by physicians who perished in the ghetto or later in concentration camps (4). Diarrhea was reported as a very common consequence of starvation in both adults and children. In 15 children in whom it could be measured, the gastric aspirate was devoid of acid. Reports from the ghetto physicians included postmortem examinations. Intestinal atrophy and edema were recorded in 27% of cases, which they described as “pseudodysentery.” Petechiae were sometimes observed in the postmortem intestine.
The liberation of Belsen in 1945 provided army physicians with first-hand evidence of the high prevalence of “nutritional diarrhea” in prisoners who had been subjected to extreme nutrient deprivation (5, 6). Photographs taken at the time illustrate the extreme wasting of many tissues, including abdominal viscera (Figure 1). The liberating soldiers also discovered the importance of the re-feeding syndrome: “With long-standing starvation the body had become adapted to low intake of food and water, and the digestive system did not tolerate rapid increase of diet, especially if it contained much fat. Liberal feeding with rich foods, such as were supplied with well-meant but mistaken kindness by fighting units when the camp was first uncovered, or even by injudicious dieting in hospital, caused abdominal pain and vomiting, and precipitated or exacerbated diarrhea, sometimes with fatal results.” This “alimentary diarrhea,” precipitated by re-feeding, was also noted in the Danish report (see below). Intravenous nutrition was attempted but noted to carry a risk of volume overload.
FIGURE 1.
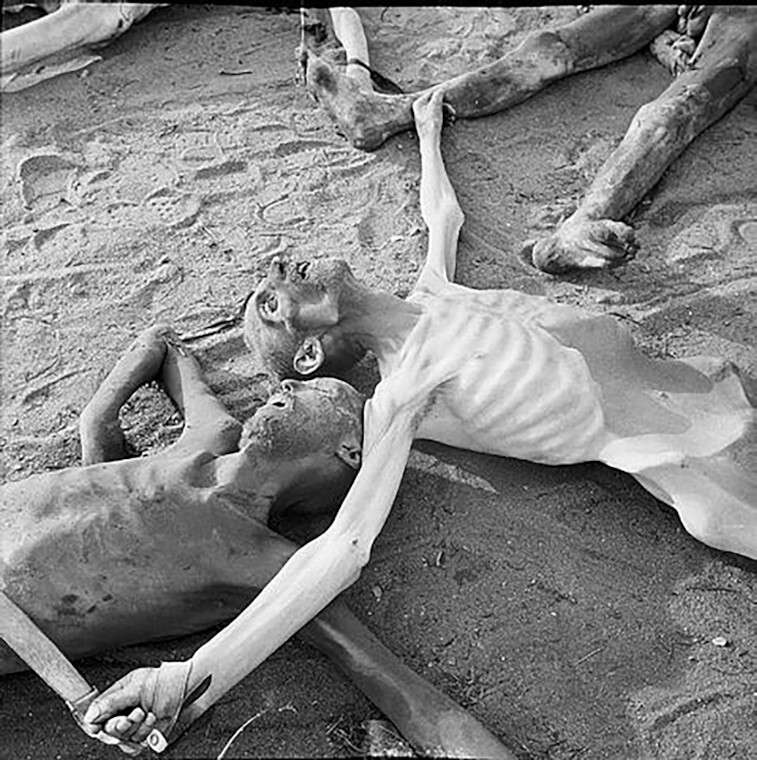
Image of unidentified victims of Nazi brutality in Belsen concentration camp, April 1945. The extreme severity of atrophy of the abdominal viscera is obvious. Reproduced with permission from Imperial War Museum.
Following the war's end, several physicians, themselves survivors of the concentration camps, recounted their experiences and observations. Dr. Adelsberger described her own experiences in Auschwitz (7). She described diarrhea as the most feared illness, as it was obviously associated with a fatal outcome. Surprisingly, given the deliberately degrading conditions and meager resources, some bacteriology was possible and dysenteric organisms were found to be rare. She commented that many cases responded to sulfonamides, and that some cases responded to thiamin. Dr. Wolff-Eisner wrote, in German, about life and death in Theresienstadt concentration camp, and this was helpfully summarized in English in The Lancet in 1948 (8). Again, the narrative describes diarrhea as the most feared disease, and notes that once it started it would not remit. The author clearly attributed it to ‘“famine diarrhea,’ with which workers at Belsen and in the Bengal famine became so familiar.”
Danish physicians reported on their experience of the concentration camps in a large report (9), which includes an entire chapter on “hunger diarrhea.” The authors specifically noted that the diarrhea was a late manifestation of starvation and did not behave as an infectious disease would behave in unsanitary and overcrowded conditions (i.e., in an epidemic manner). They were also able to distinguish it from “alimentary diarrhea” due to re-feeding, which was a response to the sudden introduction of nutrients into a starved gut. The chapter begins with a review of the evidence for the existence of “hunger diarrhea” in previous famines: Ireland in 1847, Finland in 1868, Paris in 1871, Madras in 1877, Kut (Iraq) in 1916, Dresden in 1917, Russia in 1917–1922, Finland in 1918, Tanzania in 1940, Leningrad in 1941–1942, Budapest in 1944, and Holland in 1945. In Ireland, Iraq, and Dresden the authors specifically provided evidence to refute the hypothesis that the diarrhea was infectious, and in Leningrad the onset of diarrhea often coincided with the onset of famine edema. In the prison camps for Danish prisoners hunger diarrhea only afflicted prisoners who had become markedly malnourished, was not transmissible to other prisoners even in the next bed, was not accompanied by fever or vomiting, and displayed no seasonal pattern. When postmortem examinations were carried out the intestine was found to be severely atrophic, even transparent. Hunger diarrhea was reported to have afflicted 26% of the survivors, but it was frequently fatal. Barium tests suggested slow intestinal transit, and oral-glucose-tolerance tests frequently showed “flat” curves.
In the years after the war, the restoration of agriculture and food distribution took time, and following on from the severe dislocation of the years of conflict, the German population suffered from severe malnutrition. The British Medical Research Council commissioned a series of studies in Wuppertal, a Ruhr town in the grip of a severe food shortage in 1946 (10). Once more, the authors noted that diarrhea was a common accompaniment of hunger, although there was no comment about it being noninfectious. The small intestine was examined radiologically in 78 adults; the principal findings were of dilatation of the small intestine with slow transit. Fat malabsorption was not noted. This report also includes a remarkable, comprehensive review of evidence for “hunger edema,” a phenomenon associated with famines as far back as Biblical times. Edematous malnutrition is still seen in malnourished adults and children and often referred to as “kwashiorkor” in children (11–13).
Prisoners in Japanese prisoner-of-war camps were also starved and overworked, but in tropical conditions cholera, amoebic dysentery, and strongyloidiasis dominated the clinical burden of disease (14), and the clinical reports differ considerably from those from northern Europe.
Over the last century there have been several large, often catastrophic famines, but few of these have been reported in detail, and this is a notable gap in the literature. A report from the Biafran Civil War (1967–1969) by Hughes (15) again attested to the heavy burden of diarrhea but did not give details of prevalence, response, or mortality. An interesting report from Sudan during a famine (16) concluded that malnourished children had less-severe cholera than nonmalnourished children, but no data were provided. Data from Somalia during the civil war (17) provided evidence of astonishingly high mortality among children, but no attribution to mortality was possible. Many other recent studies of famine survivors deal mainly with noncommunicable disease risk in later life, and have little to say on diarrhea or intestinal dysfunction during the famine itself. This is not surprising, given the circumstances in which they usually occur (18).
Anorexia Nervosa
A contrary situation exists in patients with anorexia nervosa: they rarely experience diarrhea and constipation is the greater problem. A study of 65 patients with severe malnutrition [mean BMI (in kg/m2): 12.3] did not report diarrhea as a major problem, and in fact, high-calorie enteral tube feeding (3000 kcal/d) was practiced without significant complications (19). High-calorie refeeding was also not found to be problematic in a randomized controlled trial (20). In a systematic review of mortality in 6000 patients, gastrointestinal complications were not a major contributor to death (n = 6, compared to 84 suicides and 29 cancers) (21). The same was found in a German series (22). There seems to be little evidence of increased susceptibility to diarrhea in anorexia nervosa. Given that some patients with anorexia nervosa develop extreme undernutrition (23) there must be intestinal atrophy, and bowel infarction has been reported (24). One study reported that intestinal permeability was reduced in anorexia nervosa (25). To my knowledge, no studies on anorexia nervosa have included intestinal biopsy.
Hunger Strikes
The majority of hunger strikes are carried out in times of political and civic strife, and it is rare for detailed assessments to be permitted. Postmortem reports of 3 Turkish hunger strikers used very similar language to reports from concentration camps, with “transparent intestines” (26). Israeli hunger strikers, who had lost 18.6% of body weight, none of whom died, were not reported as experiencing diarrhea (27). A South African report identified diarrhea on admission in 4 (13%) of 31 prisoners with weight loss of 6–20% (28).
Experimental and Clinical Studies in Humans
The prolonged and very public 44-d fast of David Blaine the entertainer, however, in London in 2007 allowed very detailed assessment of his clinical condition, showing substantial weight loss and hypophosphatemia during re-feeding (29). No tendency to diarrhea was reported, but his fast was conducted in seclusion, with clean water available ad libitum.
The most extensive experiment on human semi-starvation was conducted in Minnesota, United States, from 1944 to 1946 (3). Over 1 y, 32 healthy young men were monitored carefully through a 12-wk control period, 26 wk of semi-starvation (1570 kcal/d, with 50 g protein and 30 g fat), then a further 58 wk of re-feeding and further follow-up. The mean weight loss during semi-starvation was 24%, not as severe as that recorded in concentration camps (which was typically 50%) but perhaps comparable to the levels of malnutrition seen in refugees and civilian survivors of many war famines. In the background to the chapter on the gastrointestinal system, Keys summarized recent experience in areas affected by famine to the effects that “diarrhea, colic, flatulence, and a protruding abdomen are universally recognized symptoms of caloric undernutrition,” and backed up this statement with a comprehensive review of historical evidence from multiple famines. Volunteers in the Minnesota study did not experience diarrhea (30). In reports from concentration camps it was frequently noted that the development of hunger edema followed the onset of hunger diarrhea, but in Minnesota, volunteers’ hunger edema developed without antecedent diarrhea.
In British volunteers who fasted for just 5 d, mannitol absorption was dramatically (47%) reduced, but intestinal permeability (measured by 51 Cr-EDTA and lactulose) was unaffected (31). There was no effect on transit time. Other studies of malnutrition (32, 33) generally included patients with neoplastic and inflammatory conditions and are harder to interpret. One of these papers (33) did include data from volunteers who were starved for 36 h and no change in permeability was observed. Available, although limited, data therefore suggest that short-term starvation does not increase permeability but that increased permeability may be observed when associated with inflammation or neoplasia. Only superficial data are available on sugar absorption (as distinct from permeation), which current technologies could quantify much more precisely.
Children with Severe Acute Malnutrition
Children with severe acute malnutrition (SAM) have usually endured periods of markedly reduced energy intake accompanied by deficiencies of protein, micronutrients, and essential fatty acids. Children with SAM, however, may have multiple infectious complications (so-called “complicated SAM”) and it is these children who require admission to the hospital. Children with SAM are very susceptible to diarrhea, and it is a major contributor to high mortality rates in this disorder (34, 35), so the effects on the gut are of great interest. There have been several studies of children with complicated SAM in which intestinal biopsies have been assessed and villus height and crypt depth measured directly (Table 2). These biopsies are characterized by villus atrophy, with or without crypt hyperplasia (Table 2). Data are scanty, but extant data support evidence from animal models and in vitro work that suggest that epithelial surface area and enterocyte cell mass are probably substantially reduced in malnutrition.
TABLE 2.
Summary of available published morphometric measurements of villi and crypts in children with malnutrition compared to healthy controls1
| Study (reference) | Country | Condition | n | VH, μm | CD, μm |
|---|---|---|---|---|---|
| Penna et al. (36) | UK | Healthy | 24 | 332 (45) | 169 (28) |
| Campbell et al. (37) | UK | Healthy | 19 | 355 (35) | 170 (20) |
| Cook and Lee (38) | Uganda | Previous kwashiorkor, recovered for 4 years | 20 | 321 [271–359] | |
| Gendrel et al. (39) | Gabon | Malnutrition | 13 | 218 (43) | 154 (17) |
| Campbell et al. (37) | Gambia | SAM | 38 | 243 (68) | 278 (69) |
| Amadi et al. (40) | Zambia | SAM | 22 | 200 (56) | 165 (30) |
Values are means (SDs) or medians [ranges]. CD, crypt depth; SAM, severe acute malnutrition; VH, villus height.
There have been very few longitudinal studies. In children with SAM in The Gambia, epithelial surface area in biopsies did not change significantly from admission to recovery (41). A morphometric study from Panama did not independently measure villi and crypts, and so the data are not presented in Table 1, but did measure total mucosal thickness, epithelial cell height, and brush border height. All 3 of these parameters increased during recovery from malnutrition (42).
Our work in Zambia in the enteropathy of SAM shows severe epithelial damage with micro-erosions (40). This may be the origin of the increased permeability to lactulose observed in many studies of malnourished children (43) [note that mannitol may also permeate paracellularly (44)]. Transcriptomic analysis reveals that severity of enteropathy correlates with expression of genes for mucins and mucus integrity, antimicrobial defense, nutrient absorption, C-X-C chemokines, proteases, and anti-proteases. Amino acid transporters and ZIPzinc transporters were increased in severe enteropathy, but transcripts for xenobiotic metabolizing enzymes were reduced (45). The therapeutic implications of these findings remain to be determined, but it is not self-evident that these changes could be entirely due to reduced nutrient intake in view of the infectious burden these children carry.
This infectious burden has been studied in detail recently. In the Global Enteric Multicenter Study (GEMS), a large multicountry analysis of diarrhea and malnutrition, the odds of diarrhea and death were studied in malnourished children and nonmalnourished children (46). Two pathogens, typical enteropathogenic Escherichia coli (EPEC) and ST-enterotoxigenic Escherichia coli (ST-ETEC), were more pathogenic in malnourished children than in nonmalnourished children. Conversely, norovirus and Shigella spp. were less pathogenic in malnourished children. Five pathogens demonstrated malnutrition-associated increases in case fatality: Entamoeba histolytica, typical EPEC, Shigella spp., atypical EPEC, and ST-ETEC. These data suggest that, in malnutrition, the observed mucosal changes may allow enhanced expression of pathogenicity by some, but not all, pathogens. Whether this is attributable to changes in pathogen or host is not known.
Experimental Studies in Animals
Starvation or semi-starvation of experimental animals results in well-described changes in epithelial cell mass, fluid and electrolyte fluxes, and absorptive capacity (47, 48). In a series of highly informative experiments 3 decades ago, Young and Levin (49, 50) showed that the intestine of starved rats develops a hypersecretory state. They also demonstrated that the jejunal secretion was greatly enhanced in the starved animals, which were treated with bethanechol, a cholinergic agonist, prostaglandin E2, or the stable toxin of E. coli (51). These findings may help explain the hunger diarrhea described above, and the occurrence of diarrhea in the re-feeding syndrome which is not now considered part of it.
An extensive literature exists on the effects of calorie restriction or protein restriction on the intestinal barrier in experimental models. Starvation of experimental animals induces marked loss of intestinal weight, villus surface area, and enterocyte mass (47, 48). Starvation increases intestinal permeability (48, 52), although recent evidence suggests that this may be complex as starvation can also reduce expression of claudin-2 (which increases paracellular ion flux) through autophagy (53). A neglected facet of intestinal barrier function is that posed by the secretion of antimicrobial peptides into the mucus layer of the intestine. Mucin secretion is reduced in experimental starvation (54). Starvation and parenteral nutrition compromise the Paneth cell (antimicrobial peptide) contribution to barrier function (55, 56).
Nutritional restriction in pigs helps elucidate some of the observations made in humans. An American study demonstrated that malnutrition led to prolongation of diarrhea due to rotavirus, accompanied by villus blunting and an impaired crypt hypertrophic response (57). Insulin and insulin-like growth factor binding protein 3 (IGFBP3) were also reduced. A Danish study found villus atrophy in animals fed a protein-restricted diet, accompanied by reduced expression of lactase and aminopeptidase N (58). A study performed in China confirmed intestinal and villus atrophy in undernourished pigs but found increased expression of transporters of glucose and dipeptides (59). Undernutrition was associated with epithelial fragility at the villus tips, strikingly consistent with observations in malnourished children in Zambia (40). A second Chinese study found that energy restriction of pregnant pigs was associated with reduced brush border enzyme expression in their offspring, accompanied by reduced expression of ZO-1, a tight junction protein (60). These experimental results may help explain the overall lack of crypt hypertrophic response in the presence of undernutrition, the epithelial fragility observed in severely malnourished children, and some of the changes in solute transporter expression observed.
In addition, further studies in pigs help distinguish between loss of body tissue and withdrawal of luminal nutrients (61). By using total parenteral nutrition (TPN) to provide enough nutrients to permit healthy weight gain, collaborators in the United States and Denmark have shown that withdrawal of luminal nutrients can induce degrees of mucosal atrophy and dysfunction that are comparable to starved animals (62). Mucosal atrophy was accompanied by dramatically reduced blood splanchnic blood flow. However, TPN did not induce microbial translocation (63). Luminal nutrients have also been shown to be important for stimulation of glucagon-like peptide 2, epidermal growth factor, insulin-like growth factor 1, and other trophic factors from the gut during intestinal adaptation to resection (64, 65).
Effects of Reduced Intake on the Microbiota
In general, studies on the microbiota are in their infancy, but there is no question that the microbiota has key roles in regulation of appetite, energy balance, and gastrointestinal disease susceptibility. Patients with anorexia nervosa have altered composition of the microbiota (66), and rapid weight loss after bariatric surgery for obesity also alters it (67). Intriguingly, starvation can reduce the virulence gene expression of Salmonella enterica serovar typhimurium (68), which prompts speculation that such changes may complicate our understanding of altered susceptibility to diarrhea during starvation. Further work is clearly needed.
Conclusions
Historical data, although lacking the sophistication of modern studies, were collected with sufficient care to enable us to draw some interesting conclusions about “hunger diarrhea.” The remarkable consistency in the various accounts from authors from different parts of Europe suggests to me that hunger diarrhea is a discrete nosological entity. Given the grossly unsanitary conditions prevailing, intestinal infection is likely to have contributed to the diarrhea, but the pathogens implicated were not responsible for clinically apparent outbreaks. Starvation in settings where there is seclusion and minimal exposure to intestinal pathogens (Table 1) is generally not associated with severe, life-threatening diarrhea. Physiological studies in starved experimental animals may provide a plausible explanation for the high incidence of pre-terminal diarrhea, through intestinal atrophy, reduced mucosal barrier and antimicrobial function, and changes in chloride secretion in response to secretagogues and bacterial toxins. These changes may also contribute to the recently observed enhanced enteropathogen virulence in SAM. In 1968, Scrimshaw et al. (69) developed the theory of the infection–malnutrition cycle, an interaction in which undernutrition, impaired host defense, and infection exacerbate each other, leading to accelerating mortality in children in disadvantaged communities in much of the tropical world. The state of the world's children is improving, but slowly, and there is an urgent need for sophisticated studies that dissect how the infection–malnutrition cycle in the stomach and intestine might be interrupted. These studies should include changes in cell biology and immunology, and studies in settings where intestinal infection is not a feature, such as volunteer studies and anorexia nervosa, may yield important information about the starved gut.
Acknowledgments
I am grateful to Professor Jeremy Powell-Tuck for helpful discussion of the manuscript. The photograph in Figure 1 was taken by Lieutenant MH Wilson of No. 5 Army Film and Photo Section, Army Film and Photographic Unit, British Armed Forces, sometime between 18 and 28 April 1945, during the liberation of Bergen-Belsen concentration camp. The photograph is BU3760 in the photographic archive of the Imperial War Museum, London, and is available on the Imperial War Museum website (https://www.iwm.org.uk/collections/search?query=Belsen&pageSize=30&media-records=records-with-media&style=list&page=2). The sole author conceived and wrote the manuscript.
Notes
The author reported no funding received for this work.
Author disclosures: The author reports no conflicts of interest.
Abbreviations used: EPEC, enteropathogenic Escherichia coli; ETEC, enterotoxigenic Escherichia coli; SAM, severe acute malnutrition; TPN, total parenteral nutrition.
References
- 1. Elia M. Hunger disease. Clin Nutr. 2000;19:379–86. [DOI] [PubMed] [Google Scholar]
- 2. Wells JCK. The evolutionary biology of human body fatness. Cambridge (UK): Cambridge University Press; 2010. [Google Scholar]
- 3. Keys A, Brozek J, Henschel A, Mickelsen O, Taylor HL. The biology of human starvation. St. Paul (MN): University of Minnesota Press; 1950. [Google Scholar]
- 4. Winick M. Hunger disease. New York: Wiley; 1979. [Google Scholar]
- 5. Lipscomb FM. Medical aspects of Belsen concentration camp. Lancet North Am Ed. 1945;246:313–15. [Google Scholar]
- 6. Mollison PL. Observations on cases of starvation at Belsen. BMJ. 1946;1(4435):4–8. [PMC free article] [PubMed] [Google Scholar]
- 7. Adelsberger L. Medical observations in Auschwitz concentration camp. Lancet North Am Ed. 1946;247:317–19. [DOI] [PubMed] [Google Scholar]
- 8. Anonymous. Life and death in a concentration camp. Lancet. 1948;ii:228–9. [Google Scholar]
- 9. Helwig-Larsen P, Hoffmeyer H, Kieler J, Thaysen EH, Thaysen JH, Thygesen P, Wulff MH. Famine disease in German concentration camps: complications and sequels. Acta Med Scand. 1952;Suppl 274:124–60. [PubMed] [Google Scholar]
- 10. Medical Research Council . Studies of undernutrition in Wuppertal 1946–1949. Special report no. 275. London: HMSO; 1951. [Google Scholar]
- 11. Williams CD. Kwashiorkor: a nutritional disease of children associated with a maize diet. Lancet North Am Ed. 1935;226:1151–2. [Google Scholar]
- 12. Bhutta ZA, Berkley JA, Bandsma RHJ, Kerac M, Trehan I, Briend A. Severe childhood malnutrition. Nat Rev Dis Primers. 2017;3:17067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Trehan I, Manary MJ. Management of severe acute malnutrition in low-income and middle-income countries. Arch Dis Child. 2015;100:283–7. [DOI] [PubMed] [Google Scholar]
- 14. Dunlop EE. Medical experiences in Japanese captivity. BMJ. 1946;2(4474):481–6. [PMC free article] [PubMed] [Google Scholar]
- 15. Hughes SPF. Malnutrition in the field, Nigerian Civil War 1968–9. BMJ. 1969;2:436–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Malholland K. Cholera in Sudan: an account of an epidemic in a refugee camp in eastern Sudan, May-June 1985. Disasters. 1985;9(4):247–58. [DOI] [PubMed] [Google Scholar]
- 17. Moore PS, Marfin AA, Quenemoen LE, Gessner BD, Ayub YS, Miller DS, Sullivan KM, Toole MJ. Mortality rates in displaced and resident populations of central Somalia during 1992 famine. Lancet North Am Ed. 1993;341(8850):935–8. [DOI] [PubMed] [Google Scholar]
- 18. Collins S. The limit of human adaptation to starvation. Nat Med. 1995;1:810–14. [DOI] [PubMed] [Google Scholar]
- 19. Born C, de la Fontaine L, Winter B, Müller N, Schaub A, Früstück C, Schüle C, Voderholzer U, Cuntz U, Falkai Pet al. First results of a refeeding program in a psychiatric intensive care unit for patients with extreme anorexia nervosa. BMC Psychiatry. 2015;15:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. O'Connor G, Nicholls D, Hudson L, Singhal A. Refeeding low weight hospitalized adolescents with anorexia nervosa: a multicentre randomized controlled trial. Nutr Clin Pract. 2016;31:681–9. [DOI] [PubMed] [Google Scholar]
- 21. Papadopoulos FC, Ekbom A, Brandt L, Ekselius L. Excess mortality, causes of death and prognostic factors in anorexia nervosa. Br J Psychiatry. 2009;194(1):10–17. [DOI] [PubMed] [Google Scholar]
- 22. Fichter MM. Mortality in eating disorders—results of a large prospective clinical longitudinal study. Int J Eating Dis. 2016;49:391–401. [DOI] [PubMed] [Google Scholar]
- 23. Frolich J, Palm CVB, Stoving RK. To the limit of extreme malnutrition. Nutrition. 2016;32:146–8. [DOI] [PubMed] [Google Scholar]
- 24. Neycheva V, Borruso J. Bowel ischemia and necrosis in anorexia nervosa: a case report and review of the literature. Int J Surg Case Rep. 2015;8:141–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Monteleone P, Carratù R, Cartenì M, Generoso M, Lamberti M, De Magistris L, Brambilla F, Colurcio B, Secondulfo M, Maj M. Intestinal permeability is decreased in anorexia nervosa. Mol Psychiatry. 2004;9:76–80. [DOI] [PubMed] [Google Scholar]
- 26. Altun G, Akansu B, Altun BU, Azmak D, Yilmaz A. Deaths due to hunger strike: post-mortem findings. Forensic Sci Int. 2004;146(1):35–8. [DOI] [PubMed] [Google Scholar]
- 27. Gordon D, Drescher M, Shiber S. Security hunger-strike prisoners in the emergency department: physiological and laboratory findings. J Emerg Med. 2018;55:185–91. [DOI] [PubMed] [Google Scholar]
- 28. Kalk WJ, Felix M, Snoey ER, Veriawa Y. Voluntary total fasting in political prisoners—clinical and biochemical observations. S Afr Med J. 1993;83(6):391–4. [PubMed] [Google Scholar]
- 29. Jackson JM, Blaine D, Powell-Tuck J, Korbonits M, Carey A, Elia M. Macro- and micronutrient losses and nutritional status resulting from 44 days of total fasting in a non-obese man. Nutrition. 2006;22:889–97. [DOI] [PubMed] [Google Scholar]
- 30. Keys A, Brozek J, Henschel A, Mickelsen O, Taylor HL. The biology of human starvation. Vol. 2. St. Paul (MN): University of Minnesota Press; 1950. [Google Scholar]
- 31. Elia M, Goren A, Behrens R, Barber RW, Neale G. Effect of total starvation and very low calorie diets on intestinal permeability in man. Clin Sci (Lond). 1987;73(2):205–10. [DOI] [PubMed] [Google Scholar]
- 32. Welsh FK, Farmery SM, MacLennan K, Sheridan MB, Barclay GR, Guillou PJ, Reynolds JV. Gut barrier function in malnourished patients. Gut. 1998;42:396–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Maxton DG, Menzies IS, Slavin B, Thompson RP. Small-intestinal function during enteral feeding and starvation in man. Clin Sci. 1989;77:401–6. [DOI] [PubMed] [Google Scholar]
- 34. Attia S, Versloot CJ, Voskuijl W, van Vliet SJ, Di Giovanni V, Zhang L, Richardson S, Bourdon C, Netea MG, Berkley JAet al. Mortality in children with complicated severe acute malnutrition is related to intestinal and systemic inflammation: an observational cohort study. Am J Clin Nutr. 2016;104(5):1441–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Nabukeera-Barungi N, Grenov B, Lanyero B, Namusoke H, Mupere E, Christensen VB, Michaelsen KF, Mølgaard C, Rytter MJ, Friis H. Predictors of mortality among hospitalized children with severe acute malnutrition: a prospective study from Uganda. Pediatr Res. 2018;84:92–8. [DOI] [PubMed] [Google Scholar]
- 36. Penna FJ, Hill ID, Kingston D, Robertson K, Slavin G, Shiner M. Jejunal mucosal morphometry in children with and without gut symptoms and in normal adults. J Clin Pathol. 1981;34:386–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Campbell DI, Murch SH, Elia M, Sullivan PB, Sanyang MS, Jobarteh B, Lunn PG. Chronic T cell-mediated enteropathy in rural west African children: relationship with nutritional status and small bowel function. Pediatr Res. 2003;54:306–11. [DOI] [PubMed] [Google Scholar]
- 38. Cook GC, Lee FD. The jejunum after kwashiorkor. Lancet North Am Ed. 1966;288:1263–7. [DOI] [PubMed] [Google Scholar]
- 39. Gendrel D, Gahouma D, Ngou-Milama E, Nardou M, Chamlian A, Philippe E. Anomalies de la muqueuse jejunale et malnutrition protein-calorique chez le nourrisson en Afrique equatorial. Ann Pediatr. 1984;31:871–6. [PubMed] [Google Scholar]
- 40. Amadi B, Besa E, Zyambo K, Kaonga P, Louis-Auguste J, Chandwe K, Tarr PI, Denno DM, Nataro JP, Faubion Wet al. Impaired barrier function and autoantibody generation in malnutrition enteropathy in Zambia. EBioMedicine. 2017;22:191–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sullivan P, Lunn PG, Northrop-Clewes C, Crowe PT, Marsh MN, Neale G. Persistent diarrhea and malnutrition—the impact of treatment on small bowel structure and permeability. JPGN. 1992;14:205–15. [DOI] [PubMed] [Google Scholar]
- 42. Schneider RE, Viteri FE. Morphological aspects of the duodenal mucosa in protein-calorie malnourished children and during recovery. Am J Clin Nutr. 1972;25:1092–102. [DOI] [PubMed] [Google Scholar]
- 43. Denno DM, VanBuskirk K, Nelson ZC, Musser CA, Hay Burgess DC, Tarr PI. Use of the lactulose to mannitol ratio to evaluate childhood environmental enteric dysfunction: a systematic review. Clin Infect Dis. 2014;59(Suppl 4):S213–19. [DOI] [PubMed] [Google Scholar]
- 44. Ordiz MI, Davitt C, Stephenson K, Agapova S, Divala O, Shaikh N, Manary MJ. EB 2017 article: interpretation of the lactulose:mannitol test in rural Malawian children at risk for perturbations in intestinal permeability. Exp Biol Med (Maywood). 2018 May;243(8):677–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Chama M, Amadi B, Chandwe K, Besa E, Zyambo K, Shaikh N, Ndao M, Tarr P, Storer C, Head Ret al. Transcriptomic analysis of enteropathy in Zambian children with severe acute malnutrition. EBioMedicine. 2019;45:456–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Tickell KD, Sharmin R, Deichsel EL, Lamberti LM, Walson JL, Faruque ASG, Pavlinac PB, Kotloff KL, Chisti MJ. The effect of acute malnutrition on enteric pathogens, moderate-to-severe diarrhoea, and associated mortality in the Global Enteric Multicenter Study cohort: a post-hoc analysis. Lancet Glob Health. 2020;8(2):e215–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ferraris RP, Carey HP. Intestinal transport during fasting and malnutrition. Annu Rev Nutr. 2000;20:195–219. [DOI] [PubMed] [Google Scholar]
- 48. Genton L, Cani PD, Schrenzel J. Alterations of gut barrier and gut microbiota in food restriction, food deprivation and protein-energy wasting. Clin Nutr. 2015;34:341–9. [DOI] [PubMed] [Google Scholar]
- 49. Young A, Levin RJ. Diarrhea of famine and malnutrition: investigations using a rat model. 1. Jejunal hypersecretion induced by starvation. Gut. 1990;31:43–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Young A, Levin RJ. Diarrhea of famine and malnutrition: investigations using a rat model. 2. Ileal hypersecretion induced by starvation. Gut. 1990;31:162–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Young A, Levin RJ. Intestinal hypersecretion of the refed starved rat: a model for alimentary diarrhea. Gut. 1992;33:1050–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Tsujikawa T, Itoh A, Yasuoka T, Fukunaga T, Satoh J, Uda K, Ihara T, Sasaki M, Fujiyama Y. Mucosal permeability regulates receptor binding of luminal epidermal growth factor in the adult rat intestine. Int J Mol Med. 2003;11(3):349–52. [PubMed] [Google Scholar]
- 53. Nighot PK, Hu CA, Ma TY. Autophagy enhances intestinal epithelial tight junction barrier function by targeting claudin-2 protein degradation. J Biol Chem. 2015;290(11):7234–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Sherman P, Forstner J, Roomi N, Khatri I, Forstner G. Mucin depletion in the intestine of malnourished rats. Am J Physiol. 1985;248:G418, e23. [DOI] [PubMed] [Google Scholar]
- 55. Hodin CM, Lenaerts K, Grootjans J, de Haan JJ, Hadfoune M, Verheyen FK, Kiyama H, Heineman E, Buurman WA. Starvation compromises Paneth cells. Am J Pathol. 2011;179(6):2885–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Hodin CM, Visschers RG, Rensen SS, Boonen B, Olde Damink SW, Lenaerts K, Buurman WA. Total parenteral nutrition induces a shift in the Firmicutes to Bacteroidetes ratio in association with Paneth cell activation in rats. J Nutr. 2012;142(12):2141–7. [DOI] [PubMed] [Google Scholar]
- 57. Zijlstra RT, Donovan SM, Odle J, Gelberg HB, Petschow BW, Gaskins HR. Protein-energy malnutrition delays small-intestinal recovery in neonatal pigs infected with rotavirus. J Nutr. 1997;127:1118–27. [DOI] [PubMed] [Google Scholar]
- 58. Lykke M, Hother AL, Hansen CF, Friis H, Mølgaard C, Michaelsen KF, Briend A, Larsen T, Sangild PT, Thymann T. Malnutrition induces gut atrophy and increases hepatic fat infiltration: studies in a pig model of childhood malnutrition. Am J Transl Res. 2013;5(5):543–54. [PMC free article] [PubMed] [Google Scholar]
- 59. Cao M, Che L, Wang J, Yang M, Su G, Fang Z, Lin Y, Xu S, Wu D. Effects of maternal over- and undernutrition on intestinal morphology, enzyme activity, and gene expression of nutrient transporters in newborn and weaned pigs. Nutrition. 2014;30(11–12):1442–7. [DOI] [PubMed] [Google Scholar]
- 60. Chen Y, Mou D, Hu L, Zhen J, Che L, Fang Z, Xu S, Lin Y, Feng B, Li Jet al. Effects of maternal low-energy diet during gestation on intestinal morphology, disaccharidase activity, and immune response to lipopolysaccharide challenge in pig offspring. Nutrients. 2017;9(10):1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Sangild PT, Ney DM, Sigalet DL, Vegge A, Burrin D. Animal models of infant short bowel syndrome: translational relevance and challenges. Am J Physiol Gastrointest Liver Physiol. 2014;307:G1147–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Burrin DG, Stoll B, Chang X, Van Goudoever JB, Fujii H, Hutson SM, Reeds PJ. Parenteral nutrition results in impaired lactose digestion and hexose absorption when enteral feeding is initiated in infant pigs. Am J Clin Nutr. 2003;78:461–70. [DOI] [PubMed] [Google Scholar]
- 63. Niinikoski H, Stoll B, Guan X, Kansagra K, Lambert BD, Stephens J, Hartmann B, Holst JJ, Burrin DG. Onset of small intestinal atrophy is associated with reduced intestinal blood flow in TPN-fed neonatal piglets. J Nutr. 2004;134(6):1467–74. [DOI] [PubMed] [Google Scholar]
- 64. Burrin DG, Sangild PT, Stoll B, Thymann T, Buddington R, Marini J, Oluytoye O, Shulman RJ. Translational advances in pediatric nutrition and gastroenterology: new insights from pig models. Annu Rev Anim Biosci. 2020;8:321–54. [DOI] [PubMed] [Google Scholar]
- 65. Burrin DG, Stoll B, Jiang R, Chang X, Hartmann B, Holst JJ, Greeley GH Jr, Reeds PJ. Minimal enteral nutrient requirements for intestinal growth in neonatal pigs: how much is enough?. Am J Clin Nutr. 2000;71:1603–10. [DOI] [PubMed] [Google Scholar]
- 66. Mack I, Penders J, Cook J, Dugmore J, Mazurak N, Enck P. Is the impact of starvation on the gut microbiota specific or unspecific to anorexia nervosa? A narrative review based on a systematic literature search. Curr Neuropharmacol. 2018;16: 1131–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Furet JP, Kong LC, Tap J, Poitou C, Basdevant A, Bouillot JL, Mariat D, Corthier G, Doré J, Henegar Cet al. Differential adaptation of human gut microbiota to bariatric surgery-induced weight loss: links with metabolic and low-grade inflammation markers. Diabetes. 2010;59:3049–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Yurist-Doutsch S, Arrieta MC, Tupin A, Valdez Y, Antunes LC, Yen R, Finlay BB. Nutrient deprivation affects salmonella invasion and its interaction with the gastrointestinal microbiota. PLoS One. 2016;11:e0159676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Scrimshaw NS, Taylor CE, Gordon JE; World Health Organization . Interactions of nutrition and infection. Geneva (Switzerland): World Health Organization; 1968; [Internet]. Available from: https://apps.who.int/iris/handle/10665/41782. [PubMed] [Google Scholar]


