ABSTRACT
No previous investigation has summarized findings from prospective cohort studies on the association between dietary intake of fiber, fruit, and vegetables and risk of inflammatory bowel disease (IBD). Dietary fiber and its major sources can influence the risk of IBD by modulation of the gut microbiota. This study summarizes findings from published cohort studies on the association between dietary fiber, fruit, and vegetable consumption and risk of IBD. Relevant articles published up to January 2019 were searched via PubMed, MEDLINE, Scopus, Embase, Cochrane Library, and Google Scholar. All prospective cohort studies investigating the association between dietary fiber, fruit, and vegetable intake and risk of IBD were included. Combining 7 effect sizes from 6 studies, no significant association was found between dietary intake of fiber and risk of ulcerative colitis (UC) (RR: 1.09; 95% CI: 0.88, 1.34). However, a significant inverse association was found between dietary fiber intake and risk of Crohn disease (CD) (RR: 0.59; 95% CI: 0.46, 0.74), based on 5 studies with 6 effect sizes. Pooling information from 4 studies, we found a significant protective association between dietary intake of fruit and risk of UC (RR: 0.69; 95% CI: 0.55, 0.86) and CD (RR: 0.47; 95% CI: 0.38, 0.58). We also found a significant inverse association between vegetable consumption and risk of UC (RR: 0.56; 95% CI: 0.48, 0.66) and CD (RR: 0.52; 95% CI: 0.46, 0.59). In conclusion, dietary intake of fruit and vegetables was inversely associated with risk of IBD and its subtypes. Dietary fiber intake was also inversely associated with incidence of IBD and CD, but not with UC. Further studies are warranted to examine the association of other fiber-rich foods with IBD.
Keywords: fiber, fruit, inflammatory bowel disease, intake, vegetable
Introduction
Inflammatory bowel disease (IBD) is a chronic, relapsing intestinal inflammatory disorder that occurs in 2 forms, Crohn disease (CD) and ulcerative colitis (UC) (1). Whereas UC is limited to the colon, CD can occur anywhere between the mouth and the anus (2). The prevalence of IBD is increasing worldwide (3). It is estimated that ∼3 million people in European countries and ∼1.5 million people in the United States are affected (1, 3). Similar to other gastrointestinal (GI) disorders, IBD is a costly condition, which imposes a huge economic burden on society and negatively influences the quality of life (4).
Alterations in the gut microbiota, taking oral contraceptives, living in urban areas, and stressful lifestyle have been reported to play a role in IBD development; however, limited information is available about the contribution of dietary factors in IBD pathogenesis (5). Overall, the role of dietary fiber intake and high-fiber foods has long been at the center of several studies focused on GI disorders (6). In a meta-analysis in 2015, it was concluded that high dietary fiber intake was significantly associated with a reduced risk of IBD (7). However, a prospective study in children revealed no significant difference in consumption of dietary fiber between individuals with and without IBD (8). In addition, findings from the Nurses' Health Study demonstrated that neither long-term total fiber intake nor fiber intake from specific sources was associated with the risk of UC (9). Besides dietary fiber, fruit and vegetable consumption has also been extensively examined in relation to risk of developing chronic diseases (10, 11), but no conclusive evidence is available about its role in IBD patients.
Fruit and vegetables, which are rich sources of dietary fiber, micronutrients, and phytochemicals (10), might influence the risk of IBD by their effects on the gut microbiota. Although some previous studies demonstrated a significant inverse association between fruit or vegetable consumption and the incidence of UC and CD (12), others failed to find such association. In a study in Japan, no significant association was observed between consumption of fruit or vegetables and risk of UC (13). However, a meta-analysis in 2015 indicated that consumption of fruit and vegetables was inversely associated with the risk of UC (12). One possible explanation for the inconsistent findings in previous studies might be the different pathophysiological mechanisms of UC and CD development. Previous meta-analyses on the association between dietary fiber, fruit, and vegetable consumption and risk of IBD have mainly focused on findings from case-control studies (7, 12). Therefore, their conclusions might be misleading due to the inherent limitations in such a study design. Although several prospective studies have been conducted on this topic, there is no meta-analysis summarizing these publications. Therefore, we conducted this meta-analysis to summarize findings from earlier prospective cohort studies on the association between dietary fiber, fruit, and vegetable consumption and risk of IBD.
Methods
Search strategy
We searched for relevant articles published up to January 2019 in PubMed, MEDLINE, Scopus, Embase, Cochrane Library, and Google Scholar using MESH and non-MESH keywords: ((“Dietary fiber”[tiab] OR “fiber*”[tiab] OR “dietary fiber”[MESH] OR “fibre*”[tiab] OR “fruit” OR “vegetable” OR “polysaccharides*”[tiab] OR “psyllium*”[tiab] OR “Metamucil*”[tiab] OR “polymers*”[tiab] OR “carbohydrate*”[tiab] OR “dietary carbohydrate*”[tiab] OR “fermentable” [tiab] OR “fructan*”[tiab] OR “asteraceae”[tiab] OR “fructooligosaccharide*”[tiab] OR “oligofructose*”[tiab] OR “inulin”[tiab] OR “lactulose”[tiab] OR “whole grain*”[tiab] OR “wholegrain”[tiab] OR “whole grains”[MESH] OR “whole meal”[tiab] OR “whole wheat”[tiab] OR “edible grain”[MESH] OR “wheat”[tiab] OR “rice”[tiab] OR “brown rice”[tiab] OR “maize”[tiab] OR “oat”[tiab] OR “barley”[tiab] OR “corn”[tiab] OR “rye”[tiab] OR “millet”[tiab] OR “sorghum”[tiab] OR “cereals”[tiab] OR “bread”[tiab] OR “sweets”[tiab] OR “desserts”[tiab] OR “pasta”[tiab] OR “muffin”[tiab] OR “biscuit”[tiab] OR “pancake”[tiab] OR “waffle”[tiab]) AND (“Inflammatory bowel disease”[tiab] OR “Inflammatory bowel diseases”[MESH] OR “Crohn disease”[tiab] OR “Crohn disease”[MESH] OR “colitis, ulcerative”[MESH] OR “ulcerative colitis”[tiab] OR “IBD”[tiab] OR “Crohn's disease”[tiab])).
We did not perform language or time restrictions. Duplicate citations were removed. In addition, we reviewed the reference list of available original and review studies to avoid missing any relevant publication. Congress abstracts, dissertations, and patents were not included in the current meta-analysis.
Inclusion criteria
All prospective cohort or nested case-control studies that reported HRs or RRs and 95% CIs for IBD across categories of dietary fiber, fruit, or vegetable consumption were included. If several reports were published from the same dataset, we included the most comprehensive one. In addition, if a study had reported data for specific subgroups, findings for the whole population were included.
Exclusion criteria
Studies were excluded if they: 1) were done on animals, pregnant women, children, or elderly people; 2) had cross-sectional or case-control designs or were clinical trials; or 3) had reported data for dietary sources of fiber or reported the association for dietary fiber intake from specific sources.
Data extraction
Two independent reviewers extracted the following data from included studies: first author's name, publication year, cohort name, study location, mean age of study participants, subjects’ gender, study sample size, number of participants with IBD, follow-up duration, person-years, type of exposure, methods used to assess exposure, methods used to examine outcomes, HRs or RRs with 95% CIs for IBD or its subtypes, and list of confounders controlled for in statistical analysis.
Assessment of study quality
The methodological quality of the included publications was examined by 2 independent reviewers using the Newcastle-Ottawa Scales adapted for cohort studies (14). This scale assesses the selection of study groups (0–4 stars) and adequacy of adjustment for confounders (0–2 stars) as well as ascertainment of outcome of interest (0–3 stars).
Statistical analysis
The overall effect size was calculated using a random-effects model. When between-studies heterogeneity was low, we used a fixed-effects model rather than a random-effects model. To compute the overall effect size, log HRs or RRs and its SE were calculated based on reported HRs, RRs, and their 95% CIs. Between-study heterogeneity was examined using the Cochran Q test and I2 statistic. Subgroup analyses were used to find probable sources of heterogeneity using a fixed-effects model. Dose-dependent nonlinear association between dietary fiber, fruit, and vegetable consumption and risk of UC and CD was examined using the method proposed by Greenland and Longnecker (15) and Orsini et al. (16). Studies that reported RRs across categories of fiber, fruit, and vegetable consumption (≥3 categories) and those that reported total population as well as number of patients with UC or CD in each category met the criteria for dose–response meta-analysis. The midpoint of consumption category was considered as the corresponding HR/RR estimate, whereas the open-ended categories were considered as the same width as the neighboring categories. The 2-stage random-effects dose–response meta-analysis was used to explore nonlinear relations between dietary fiber, fruit, and vegetable consumption and risk of UC or CD. In this analysis, dietary intake of each item was modeled using restricted cubic splines with 3 knots at fixed percentiles of 10%, 50%, and 90% of the distribution (17), which were calculated using generalized least squares regression, taking into account the correlation within each set of the HRs/RRs (18). Subsequently, the study-specific estimates were combined using the restricted maximum likelihood method in a multivariate random-effects meta-analysis (19). We examined the null hypothesis that considered the coefficient of the second spline equal to 0. Moreover, a linear dose–response relation of 10 g/d increment in fiber intake and 1 serving/d increase in fruit or vegetable consumption with risk of UC or CD was estimated using the 2-stage generalized least-squares trend estimation (15, 18, 20). The overall average slope was calculated using the estimated study-specific slope lines combined with studies that directly reported the slopes (20). All statistical analyses were conducted using STATA version 14.0 software (Stata Corp LLC). P values <0.05 were considered statistically significant.
Results
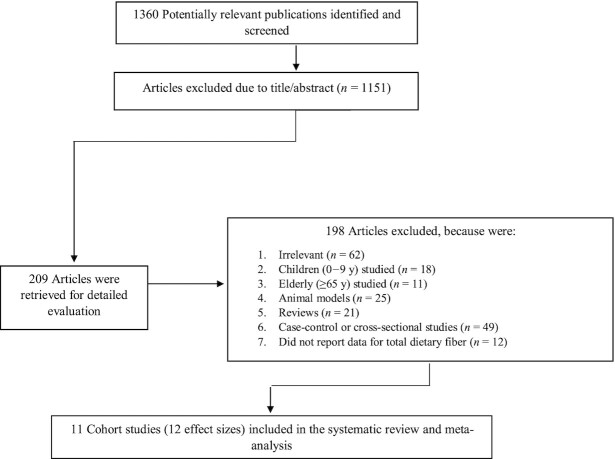
Of 1360 publications found in our initial search, 209 articles remained for further investigation after the first stage assessment based on title and abstract. Evaluation of full texts in the second stage resulted in 11 cohort or nested case-control studies. Therefore, these 11 studies, which provided 12 effect sizes, were included in the current systematic review and meta-analysis (9, 21–30). A flow diagram of study selection is shown in Figure 1.
FIGURE 1.
Flow diagram of study selection.
Study characteristics
Characteristics of included studies are presented in Table 1. These studies were published between 1992 and 2018. Six studies were prospective cohorts (9, 21, 23–25, 27) and the 5 remaining studies were nested case-control studies (22, 26, 28–30). The studies were conducted in Sweden (25, 30), the United States (9, 21, 23, 24), Australia (29), and Denmark (25, 26). Moreover, 3 studies were done in several neighboring countries (22, 27, 28). Participants were aged between 10 and 80 y. Most studies enrolled both genders (22–30), whereas 2 studies were confined to females (9, 21). Duration of follow-up in prospective studies varied from 6 mo to 26 y. A total of 478,604 participants were enrolled in the included studies.
TABLE 1.
General characteristics of included studies on the association of dietary fiber, fruit, or vegetable consumption with risk of inflammatory bowel disease1
| First author (year) | Country | Age2 y | Sex | Sample size | Study design | Follow-up, y | Exposure | Exposure assessment method | Compared categories | Outcome assessment method | OR or RR or HR (95% CI) | Adjustments3 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Persson et al. (1992) (30) | Sweden | 15–792 | Both |
755 Case: 365 Control: 390 |
Case-control from cohort | 5 | Vegetables, fibers | FFQ3 |
For fibers: ≥15 g/d vs. ≤11 g/d For vegetables: daily vs. less frequency |
Not reported |
Fibers: Men: UC (RR): 1.2 (0.5, 2.6) CD: 0.7 (0.3, 1.6) Women: UC: 1.9 (0.8, 4.2) CD: 0.4 (0.2, 1.0) Vegetables: 0.7 (0.4, 1.0) |
1, 2, 3 |
| Halfvarson et al. (2006) (25) | Sweden, Denmark | 5–79 | Both | 227 | Cohort | Not reported | Fruits | A questionnaire | Less frequently vs. daily | Self-report, confirms |
Fruits: UC (OR): 2.90 (0.90, 9.40) CD: 0.20 (0.10, 0.90) |
4 |
| Hart et al. (2008) (27) | 5 European countries | 20–80 | Both | 260,686 | Cohort | 3.8 | Fiber | FFQ | Q4 (23.3–47.1 g/d) vs. Q1 (6.2–14.8 g/d) and continuous | Registries |
Fibers: UC (OR): 1.12 (0.59, 2.11) For continuous: 1.03 (0.84, 1.25) |
— |
| Hansen et al. (2011) (26) | Denmark | Case: 37.5Control: 39 | Both |
534 Case: 267 Control: 267 |
Case-control from cohort | Not reported | Fruits, vegetables | A questionnaire | Case vs. controls | Not reported |
Fruits: UC (OR): 0.56 (0.33, 0.95) CD: 0.39 (0.22, 0.70) Vegetables: UC: 0.51 (0.31, 0.84) CD: 0.41 (0.24, 0.71) |
5, 6, 7, 8, 9, 10, 11, 12 |
| Ananthakrishnan et al. (2013) (9) | USA | 30–55 | Female | 170,311 | Cohort | 26 | Fiber | FFQ | Q5 (22.8–26.8 g/d) vs. Q1 (11.4–13.6 g/d) | Self-reports and medical records confirmed by physician |
UC (HR): 0.82 (0.58, 1.17) CD: 0.59 (0.39, 0.90) |
1, 5, 8, 13, 14, 15, 16 |
| Ananthakrishnan et al. (2013) (9) | USA | 30–55 | Female | 170,311 | Cohort | 26 | Fruits, vegetables | FFQ | Q5 vs. Q1 | Self-reports and medical records confirmed by physician |
Fruits: UC (HR): 0.78 (0.54, 1.12) CD: 0.57 (0.38, 0.85) Vegetables: UC: 0.82 (0.58, 1.17) CD: 0.88 (0.61, 1.25) |
1, 5, 8, 13, 14, 15, 16 |
| Cohen et al. (2013) (24) | USA | 42–49 | Both | 1718 | Cohort | Not reported | Fruits, vegetables | FFQ | Q4 vs. Q1 | Self-report |
Fruits: UC (OR): 0.39 (0.27, 0.55) CD: 0.43 (0.33, 0.57) Leafy vegetables: UC: 0.40 (0.30, 0.53) CD: 0.44 (0.36, 0.54) Nonleafy vegetables: UC: 0.59 (0.45, 0.77) CD: 0.54 (0.44, 0.65) |
1, 2, 17 |
| Ananthakrishnan et al. (2015) (21) | USA | 25–4y | Female | 39,511 | Cohort | 19.31 | Fiber, fruits, vegetables | FFQ | Q4 vs. Q1 | Self-reports and medical records confirmed by physician |
Fiber: UC (HR): 1.06 (0.59, 1.89) CD: 0.55 (0.27, 1.12) Fruits: UC: 1.47 (0.84, 2.57) CD: 0.65 (0.32, 1.32) Vegetables: UC: 0.74 (0.40, 1.37) CD: 0.50 (0.25, 1.03) |
5, 8, 9, 13, 14, 15, 18, 19, 20 |
| Ng et al. (2015) (28) | Nine countries/regions in Asia-Pacific | 25–50 | Both |
1382 Case: 442 Control: 940 |
Case-control from cohort | — | Fruits, vegetables | A questionnaire | Continuous | On basis of clinical symptoms, endoscopy, histology, and radiology |
Fruits: UC (OR): 0.95 (0.70, 1.28) CD: 0.92 (0.64, 1.31) Vegetables: UC: 1.02 (0.68, 1.54) CD: 0.81 (0.51, 1.28) |
1, 2, 21 |
| Brotherton et al. (2016) (23) | USA | >18 | Both | 1619 | Cohort | 0.5 | Fiber | A validated questionnaire | Q4 (23.7–24.5 g/d) vs. Q1 (10.4–10.8 g/d) | Not reported |
UC (OR): 1.59 (0.83, 3.05) CD: 0.57 (0.37, 0.87) |
1, 2, 17, 22, 23 |
| Niewiadomski et al. (2016) (29) | Australia | 24–70 | Both |
236 Case: 132 Control: 104 |
Case-control from cohort | — | Fruits | A questionnaire | Continuous | Local specialists, hospitals, pharmacies and the pathology centers |
Fruits: UC (OR): 0.59 (0.40, 0.88) |
— |
| Andersen et al. (2018) (22) | 8 European centers | 20–80 | Both |
1625 Case: 325 Control: 1300 |
Case-control from a cohort | Not reported | Fiber | FFQ | 27.1–75.2 g/d vs. 6.4 to <17.3 g/d | Registry linkage, questionnaires |
UC (OR): 1.22 (0.71, 2.08) CD: 0.83 (0.38, 1.81) |
3, 5 |
CD, Crohn disease; Q, quartile; UC, ulcerative colitis.
Age is mean or range.
Adjustments: 1: age; 2: gender; 3: total energy; 4: multiple testing; 5: smoking; 6: appendectomy; 7: tonsillectomy; 8: oral contraceptives; 9: fiber; 10: sugar; 11: coffee; 12: eggs; 13: BMI; 14: postmenopausal hormone therapy; 15: nonsteroidal anti-inflammatory drugs; 16: aspirin; 17: surgery; 18: menopausal status; 19: physical activity; 20: vitamin D; 21: country income; 22: hospitalization; 23: duration of disease.
Dietary fiber (9, 21–23, 27, 30), fruit (21, 24–26, 28, 29), and vegetables (21, 24, 26, 28, 30) were considered as the exposure in the included studies. Assessment of exposure was performed using FFQs in some studies (9, 21, 22, 24, 27, 30) and specifically designed questionnaires in others (23, 25, 26, 28, 29). Detailed information about exposure assessment tools as well as dietary intake of total energy and major contributing macronutrients to energy is presented in Supplemental Table 1.
The exposures of interest were investigated in relation to the risk of UC (9, 21–30), CD (9, 21–26, 28, 30), and IBD (both UC and CD) (30) in the included studies. Study outcomes were assessed through participants’ self-reports or medical records confirmed by a physician (9, 21, 25), registry linkage (22, 27), only self-reports (24), or clinical examinations (28, 29). Moreover, 3 studies did not report the method of outcome assessment (23, 26, 30). Adjustment for dietary intake was performed only in 4 studies (21, 22, 26, 30). Quality assessment by the Newcastle-Ottawa scale showed that all included studies had a quality score ≥3 (Supplemental Table 2).
Findings for the association between dietary fiber intake and risk of IBD
When we combined 7 effect sizes from 6 prospective cohort studies, no significant association was found between dietary fiber intake and risk of UC (RR: 1.09; 95% CI: 0.88, 1.34; I2 = 0.0%) (Supplemental Figure 1). This finding remained unchanged in all subgroup analyses (Table 2). In addition, there was no significant nonlinear (Supplemental Figure 2) or linear (Supplemental Figure 3) association between dietary fiber intake and risk of UC (Pnonlinearity = 0.52; Plinearity = 0.09).
TABLE 2.
Subgroup analyses for the association of dietary fiber intake with risk of UC and CD1
| Variables | Subgroups | Number of effect sizes | Pooled RR (95% CI) | I 2, % | |
|---|---|---|---|---|---|
| Risk of UC | Age | Adult2 | 3 | 0.97 (0.74, 1.28) | 37.2 |
| Adult + elderly3 | 4 | 1.28 (0.91, 1.79) | 0.0 | ||
| Sex | Female | 2 | 0.88 (0.65, 1.19) | 0.0 | |
| Male + female | 5 | 1.34 (0.99, 1.80) | 0.0 | ||
| Study location | USA | 3 | 0.97 (0.74, 1.28) | 37.2 | |
| Other | 4 | 1.28 (0.91, 1.79) | 0.0 | ||
| Sample size | n <10,000 | 4 | 1.41 (1.00, 1.97) | 0.0 | |
| n ≥10,000 | 3 | 0.92 (0.70, 1.20) | 0.0 | ||
| Study type | Cohort | 4 | 1.00 (0.77, 1.28) | 10.1 | |
| Nested case-control | 3 | 1.34 (0.91, 2.00) | 0.0 | ||
| Age | Adult | 3 | 0.58 (0.44, 0.76) | 0.0 | |
| Adult + elderly | 3 | 0.62 (0.39, 0.98) | 0.0 | ||
| Risk of CD | Sex | Female | 2 | 0.58 (0.40, 0.83) | 0.0 |
| Male + female | 4 | 0.59 (0.43, 0.81) | 0.0 | ||
| Study location | USA | 3 | 0.58 (0.44, 0.76) | 0.0 | |
| Other | 3 | 0.62 (0.39, 0.98) | 0.0 | ||
| Sample size | n <10,000 | 4 | 0.59 (0.43, 0.81) | 0.0 | |
| n ≥10,000 | 2 | 0.58 (0.40, 0.83) | 0.0 | ||
| Study type | Cohort | 3 | 0.58 (0.44, 0.76) | 0.0 | |
| Nested case-control | 3 | 0.62 (0.39, 0.98) | 0.0 | ||
CD, Crohn disease; UC, ulcerative colitis.
Studies done on people aged 20–65 y.
Studies done on adults and elderly participants (≥20 y).
However, pooling 6 effect sizes from 5 studies revealed a significant inverse association between dietary fiber intake and risk of CD (RR: 0.59; 95% CI: 0.46, 0.74; I2 = 0.0%) (Supplemental Figure 4). This was also the case in all subgroups (Table 3). Moreover, a significant nonlinear association was found between dietary fiber intake and risk of CD (Pnonlinearity < 0.001) such that the highest risk reduction was seen for fiber intake >22 g/d (Supplemental Figure 5). In the linear association, we found that an additional 10 g/d of fiber intake was associated with a 14% reduction in CD risk (Plinearity < 0.001) (Supplemental Figure 6).
TABLE 3.
Subgroup analyses for the association of dietary fruit intake with risk of UC and CD1
| Variables | Subgroups | Number of effect sizes | Pooled RR (95% CI) | I 2, % | |
|---|---|---|---|---|---|
| Risk of UC | Sex | Female | 2 | 0.94 (0.69, 1.28) | 71.1 |
| Male + female | 2 | 0.46 (0.33, 0.65) | 90.3 | ||
| Sample size | n <10,000 | 2 | 0.46 (0.33, 0.65) | 90.3 | |
| n ≥10,000 | 2 | 0.94 (0.69, 1.28) | 71.1 | ||
| Risk of CD | Sex | Female | 2 | 0.59 (0.41, 0.84) | 0.0 |
| Male + female | 2 | 0.41 (0.32, 0.54) | 43.1 | ||
| Sample size | n <10,000 | 2 | 0.41 (0.32, 0.54) | 43.1 | |
| n ≥10,000 | 2 | 0.59 (0.41, 0.84) | 0.0 | ||
CD, Crohn disease; UC, ulcerative colitis.
When we combined the studies on UC and CD, a significant inverse association was found between dietary fiber intake and risk of IBD (RR: 0.83; 95% CI: 0.70, 0.97; I2 = 45.6%) (Supplemental Figure 7). Excluding studies that did not report the outcome assessment method did not change our findings (for UC, RR: 0.97; 95% CI: 0.76, 1.24; and for CD, RR: 0.62; 95% CI: 0.44, 0.86).
Findings for the association between fruit consumption and risk of IBD
Combining data from 4 studies, we found a significant inverse association between fruit consumption and risk of UC; such that those in the highest category of fruit intake had a 31% lower risk of UC compared with those in the lowest category (RR: 0.69; 95% CI: 0.55, 0.86; I2 = 87.0%) (Supplemental Figure 8). When we did subgroup analysis to find a possible source of heterogeneity, the negative association disappeared in studies conducted exclusively in females and in those with a large sample size (for both, RR: 0.94; 95% CI: 0.69, 1.28; I2 = 71.1%) (Table 2). Although an inverted U-shaped nonlinear association was found between fruit intake and risk of UC, the association was statistically nonsignificant (Pnonlinearity = 0.68) (Supplemental Figure 9). In this analysis, RRs >1.00 were seen for fruit intake of 1–3 servings/d. No significant linear association was found between fruit intake and risk of UC (Plinearity = 0.26) (Supplemental Figure 10).
Pooling effect sizes from 4 studies on CD, we observed that individuals with the highest fruit consumption had a lower risk of CD development compared with those with the lowest intake (RR: 0.47; 95% CI: 0.38, 0.58; I2 = 32.1%) (Supplemental Figure 11). This association was also significant in all subgroups (Table 2). Moreover, a significant nonlinear association was seen between fruit consumption and risk of CD (Pnonlinearity < 0.001) (Supplemental Figure 12). Furthermore, an additional 1 serving/d of fruit was associated with a 19% lower risk of CD (Plinearity < 0.001) (Supplemental Figure 13).
A significant inverse association was also found between fruit consumption and risk of IBD based on 4 effect sizes (RR: 0.56; 95% CI: 0.48, 0.65; I2 = 79.0%) (Supplemental Figure 14).
Findings for the association between vegetable consumption and risk of IBD
Pooling data from 3 studies, we found a 44% lower risk of UC in individuals in the top category of vegetable consumption compared with those in the bottom category (RR: 0.56; 95% CI: 0.48, 0.66; I2 = 72.0%) (Supplemental Figure 15). In addition, a significant inverse association was seen between vegetable consumption and risk of CD based on data from 3 studies (RR: 0.52; 95% CI: 0.46, 0.59; I2 = 78.9%) (Supplemental Figure 16). Moreover, combining studies in UC and CD, we found a significant inverse association between consumption of vegetables and risk of IBD so that those with the highest intake of vegetables had a 46% lower risk of IBD development compared with those with the lowest intake (RR: 0.54; 95% CI: 0.49, 0.60; I2 = 67.3%) (Supplemental Figure 17). Due to the limited number of included studies, we were not able to examine the nonlinear association between vegetable consumption and the risk of UC or CD. Based on a linear association, an additional 1 serving/d of vegetables was associated with an 11% reduced risk of CD (Plinearity < 0.001) (Supplemental Figure 18), but not UC (Plinearity = 0.11) (Supplemental Figure 19).
Discussion
Our meta-analysis found a significant inverse association between fruit and vegetable consumption and risk of UC, CD, and IBD. In addition, a significant inverse association was found between dietary fiber intake and risk of CD and IBD. However, there was no significant association between dietary fiber intake and risk of UC in the current meta-analysis.
Our findings revealed a significant inverse association between fruit and vegetable consumption and risk of UC and CD. These findings were in line with an earlier meta-analysis of case-control studies in 2015, in which high intake of fruit was inversely associated with the risk of UC and CD. Although such a significant association was also found between vegetable consumption and risk of UC, it was nonsignificant for CD (12). Subgroup analysis in that study revealed a significant inverse association between vegetable intake and risk of CD for studies done in European countries. It seems that the types of vegetable consumed are an important factor, because these differ greatly between countries (31). A systematic review in 2011 showed a significant inverse association between fruit and vegetable consumption and risk of UC and CD (32). Only 2 cohort studies included in the current meta-analysis reported a significant association between fruit intake and risk of CD (9, 21). It must be kept in mind that these 2 studies were done by the same team and that they recruited only women in their studies. Therefore, further investigations, particularly on men and combined genders, are required to shed light on this issue.
We also found a significant inverse association between dietary fiber intake and risk of CD, but not UC. A meta-analysis of observational studies in 2015 indicated that dietary fiber intake was significantly associated with a reduced risk of CD and UC (7). However, observational studies with different study designs were combined in that meta-analysis. In another meta-analysis of observational studies on the association between dietary fiber intake and risk of UC, no significant finding was reported (33). Most included studies in that meta-analysis had a case-control design. In addition, findings from a systematic review and meta-analysis showed a lower risk of CD with high dietary fiber intake (34). It should be noted that stratification by the study design and adjustment for smoking in that study influenced the overall findings. Therefore, further long-term and preferably prospective cohort studies are needed to confirm our findings. Although subgroup analyses influenced our overall findings in some cases, the included number of studies in each subgroup was relatively low to reach firm conclusions. Furthermore, it should be noted that UC is limited to the colon, whereas CD can occur anywhere throughout the GI tract (2). Several effects of dietary fiber on digestion can also occur in the upper digestive tract, whereas some dietary fibers are fermented in the distal colon (35). Therefore, more attention needs to be paid to the impacts of dietary fiber on the gut environment other than its influence on the gut microbiota. Moreover, dietary fiber is usually derived from legumes, whole grains, fruit, or vegetables in a daily diet (10). Therefore, the other beneficial components of these foods, which might influence IBD and its subtypes, must also be taken into consideration.
Although the mechanisms through which dietary fiber, fruit, and vegetable consumption influence the risk of IBD are not completely understood, several suggestions have been made. Dietary fiber and its main sources, including fruit and vegetables, influence the composition and function of the gut microbiota to affect immune responses and immunological homeostasis (36). Insoluble fiber is an effective laxative, and soluble fiber modulates gut inflammation (37). Fermentation of specific types of dietary fiber, such as prebiotics, by the gut microbiota produces SCFAs, which have several anti-inflammatory properties (38, 39). Furthermore, fermentable oligosaccharides, disaccharides, monosaccharides, and polyols in fruit and vegetables are also fermented to produce SCFAs (40, 41). Butyrate, one of the end-products of intestinal fermentation of dietary fiber, has several anti-inflammatory properties (42). Butyrate is also thought to reduce colonic permeability through enhancement of peroxisome proliferator-activated receptor C activation (36, 43). Intestinal permeability is proposed to be an important contributing factor in the pathogenesis of IBD (44). Butyrate is the preferred energy source of colonocytes, promoting growth and healthy turnover of colonic epithelium (42). In addition, the anti-inflammatory effect of fruit and vegetables has been reported previously (10, 45). Fruit and vegetables are rich in micronutrients and phytochemicals (46). The influence of some micronutrients on GI health and the role of flavonoids in the maintenance of intercellular tight junctions have been reported in earlier studies (47, 48).
To our knowledge, this is the first systematic review and meta-analysis on the association between dietary fiber, fruit, and vegetable consumption and risk of IBD and it subtypes. We confined the analysis to prospective cohort studies because findings from case-control and cross-sectional studies are subject to bias. However, the findings should be interpreted in light of some limitations of the current study. Available studies did not consider different sources of dietary fiber and different types of fruit or vegetables. When we did subgroup analyses, the number of included studies in each subgroup was not adequate to reach a firm conclusion. Although total energy intake and major contributing macronutrients to energy intake are important, most studies did not report sufficient data on this issue. Given the use of FFQs for dietary assessment in most studies, misclassification of participants in terms of dietary intake should also be taken into account. Moreover, some studies considered baseline dietary intake of participants as the main study parameter instead of the average of repeated assessments of diet intake. Furthermore, dietary intake by patients with IBD can change by the disease stage. Included studies did not provide sufficient data about disease severity. In addition, some studies did not adequately report their outcome assessment method. Finally, high between-study heterogeneity was another concern.
In conclusion, in summarizing earlier studies we found a significant inverse association between fruit and vegetable consumption and risk of IBD and its subtypes. Dietary fiber intake was also associated with a reduced risk of IBD and CD, but not with the risk of UC. Future well-powered prospective studies and clinical trials are needed to expand our knowledge in this regard.
Supplementary Material
Acknowledgments
The authors’ contributions were as follows—AM and AE: designed the study; AM, BL, and NE-D: collected data; AM and AE: analyzed data; AM, AE, and LAD: wrote the manuscript; and all authors: read and approved final manuscript.
Notes
The authors reported no funding received for this study.
Author disclosures: The authors report no conflicts of interest.
Supplemental Figures 1–19 and Supplemental Tables 1 and 2 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/advances
Abbreviations: CD, Crohn disease; GI, gastrointestinal; IBD, inflammatory bowel disease; UC, ulcerative colitis.
Contributor Information
Alireza Milajerdi, Department of Community Nutrition, School of Nutritional Sciences and Dietetics, Tehran University of Medical Sciences, Tehran, Iran; Research Center for Biochemistry and Nutrition in Metabolic Diseases, Kashan University of Medical Sciences, Kashan, Islamic Republic of Iran; Department of Health, Aja University of Medical Sciences, Tehran, Iran.
Nasser Ebrahimi-Daryani, Department of Gastroenterology and Hepatology, Tehran University of Medical Sciences, Tehran, Iran.
Levinus A Dieleman, Division of Gastroenterology, University of Alberta, Edmonton, Alberta, Canada.
Bagher Larijani, Endocrinology and Metabolism Research Center, Endocrinology and Metabolism Clinical Sciences Institute, Tehran University of Medical Sciences, Tehran, Iran.
Ahmad Esmaillzadeh, Department of Community Nutrition, School of Nutritional Sciences and Dietetics, Tehran University of Medical Sciences, Tehran, Iran; Obesity and Eating Habits Research Center, Endocrinology and Metabolism Molecular-Cellular Sciences Institute, Tehran University of Medical Sciences, Tehran, Iran; Food Security Research Center, Department of Community Nutrition, School of Nutrition and Food Science, Isfahan University of Medical Sciences, Isfahan, Iran.
References
- 1. Park SJ, Kim WH, Cheon JH. Clinical characteristics and treatment of inflammatory bowel disease: a comparison of Eastern and Western perspectives. World J Gastroenterol. 2014;20(33):11525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wolf DC. Biosimilars in Crohn's disease and ulcerative colitis. Inflamm Bowel Dis. 2016;22(4):994–7. [DOI] [PubMed] [Google Scholar]
- 3. Burisch J, Munkholm P. Inflammatory bowel disease epidemiology. Curr Opin Gastroenterol. 2013;29(4):357–62. [DOI] [PubMed] [Google Scholar]
- 4. Sack C, Phan V, Grafton R, Holtmann G, Van Langenberg D, Brett K, Clark M, Andrews J. A chronic care model significantly decreases costs and healthcare utilisation in patients with inflammatory bowel disease. J Crohns Colitis. 2012;6(3):302–10. [DOI] [PubMed] [Google Scholar]
- 5. Rampton DS. The influence of stress on the development and severity of immune-mediated diseases. J Rheumatol Suppl. 2011;88:43–7. [DOI] [PubMed] [Google Scholar]
- 6. Holscher HD. Dietary fiber and prebiotics and the gastrointestinal microbiota. Gut Microbes. 2017;8(2):172–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Liu X, Wu Y, Li F, Zhang D. Dietary fiber intake reduces risk of inflammatory bowel disease: result from a meta-analysis. Nutr Res. 2015;35(9):753–8. [DOI] [PubMed] [Google Scholar]
- 8. Pituch-Zdanowska A, Albrecht P, Banasiuk M, Banaszkiewicz A. Dietary fiber intake in children with inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2018;66(4):624–9. [DOI] [PubMed] [Google Scholar]
- 9. Ananthakrishnan AN, Khalili H, Konijeti GG, Higuchi LM, de Silva P, Korzenik JR, Fuchs CS, Willett WC, Richter JM, Chan AT. A prospective study of long-term intake of dietary fiber and risk of Crohn's disease and ulcerative colitis. Gastroenterology. 2013;145(5):970–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Saghafian F, Malmir H, Saneei P, Milajerdi A, Larijani B, Esmaillzadeh A. Fruit and vegetable consumption and risk of depression: accumulative evidence from an updated systematic review and meta-analysis of epidemiological studies. Br J Nutr. 2018;119(10):1087–101. [DOI] [PubMed] [Google Scholar]
- 11. Zamani B, Milajerdi A, Tehrani H, Bellissimo N, Brett NR, Azadbakht L. Association of a plant‐based dietary pattern in relation to gestational diabetes mellitus. Nutr Diet. 2019;76:589–96. [DOI] [PubMed] [Google Scholar]
- 12. Li F, Liu X, Wang W, Zhang D. Consumption of vegetables and fruit and the risk of inflammatory bowel disease: a meta-analysis. Eur J Gastroenterol Hepatol. 2015;27(6):623–30. [DOI] [PubMed] [Google Scholar]
- 13. Kurata JH. Dietary and other risk factors of ulcerative colitis: a case-control study in Japan. J Clin Gastroenterol. 1994;19(2):166–71. [PubMed] [Google Scholar]
- 14. Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. 2011.
- 15. Greenland S, Longnecker MP. Methods for trend estimation from summarized dose-response data, with applications to meta-analysis. Am J Epidemiol. 1992;135(11):1301–9. [DOI] [PubMed] [Google Scholar]
- 16. Orsini N, Bellocco R, Greenland S. Generalized least squares for trend estimation of summarized dose–response data. Stata Journal. 2006;6(1):40–57. [Google Scholar]
- 17. Harre FE Jr., Lee KL, Pollock BG. Regression models in clinical studies: determining relationships between predictors and response. J Natl Cancer Inst. 1988;80(15):1198–202. [DOI] [PubMed] [Google Scholar]
- 18. Orsini N, Bellocco R, Greenland S. Generalized least squares for trend estimation of summarized dose-response data. Stata Journal. 2006;6(1):40. [Google Scholar]
- 19. Jackson D, White IR, Thompson SG. Extending DerSimonian and Laird's methodology to perform multivariate random effects meta‐analyses. Statist Med. 2010;29(12):1282–97. [DOI] [PubMed] [Google Scholar]
- 20. Berlin JA, Longnecker MP, Greenland S. Meta-analysis of epidemiologic dose-response data. Epidemiology. 1993;4:218–28. [DOI] [PubMed] [Google Scholar]
- 21. Ananthakrishnan AN, Khalili H, Song M, Higuchi LM, Richter JM, Nimptsch K, Wu K, Chan AT. High school diet and risk of Crohn's disease and ulcerative colitis. Inflamm Bowel Dis. 2015;21(10):2311–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Andersen V, Chan S, Luben R, Khaw KT, Olsen A, Tjonneland A, Kaaks R, Grip O, Bergmann MM, Boeing Het al. Fibre intake and the development of inflammatory bowel disease: a European prospective multi-centre cohort study (EPIC-IBD). J Crohns Colitis. 2018;12(2):129–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Brotherton CS, Martin CA, Long MD, Kappelman MD, Sandler RS. Avoidance of fiber is associated with greater risk of Crohn's disease flare in a 6-month period. Clin Gastroenterol Hepatol. 2016;14(8):1130–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cohen AB, Lee D, Long MD, Kappelman MD, Martin CF, Sandler RS, Lewis JD. Dietary patterns and self-reported associations of diet with symptoms of inflammatory bowel disease. Dig Dis Sci. 2013;58(5):1322–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Halfvarson J, Jess T, Magnuson A, Montgomery SM, Orholm M, Tysk C, Binder V, Järnerot G. Environmental factors in inflammatory bowel disease: a co-twin control study of a Swedish-Danish twin population. Inflamm Bowel Dis. 2006;12(10):925–33. [DOI] [PubMed] [Google Scholar]
- 26. Hansen TS, Jess T, Vind I, Elkjaer M, Nielsen MF, Gamborg M, Munkholm P. Environmental factors in inflammatory bowel disease: a case-control study based on a Danish inception cohort. J Crohns Colitis. 2011;5(6):577–84. [DOI] [PubMed] [Google Scholar]
- 27. Hart AR, Luben R, Olsen A, Tjonneland A, Linseisen J, Nagel G, Berglund G, Lindgren S, Grip O, Key T. Diet in the aetiology of ulcerative colitis: a European prospective cohort study. Digestion. 2008;77(1):57–64. [DOI] [PubMed] [Google Scholar]
- 28. Ng SC, Tang W, Leong RW, Chen M, Ko Y, Studd C, Niewiadomski O, Bell S, Kamm MA, De Silva H. Environmental risk factors in inflammatory bowel disease: a population-based case-control study in Asia-Pacific. Gut. 2015;64(7):1063–71. [DOI] [PubMed] [Google Scholar]
- 29. Niewiadomski O, Studd C, Wilson J, Williams J, Hair C, Knight R, Prewett E, Dabkowski P, Alexander S, Allen B. Influence of food and lifestyle on the risk of developing inflammatory bowel disease. Intern Med J. 2016;46(6):669–76. [DOI] [PubMed] [Google Scholar]
- 30. Persson PG, Ahlbom A, Hellers G. Diet and inflammatory bowel disease: a case-control study. Epidemiology. 1992;3(1):47–52. [DOI] [PubMed] [Google Scholar]
- 31. Del Gobbo LC, Khatibzadeh S, Imamura F, Micha R, Shi P, Smith M, Myers SS, Mozaffarian D. Assessing global dietary habits: a comparison of national estimates from the FAO and the Global Dietary Database. Am J Clin Nutr. 2015;101(5):1038–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hou JK, Abraham B, El-Serag H. Dietary intake and risk of developing inflammatory bowel disease: a systematic review of the literature. Am J Gastroenterol. 2011;106(4):563. [DOI] [PubMed] [Google Scholar]
- 33. Wang F, Feng J, Gao Q, Ma M, Lin X, Liu J, Li J, Zhao Q. Carbohydrate and protein intake and risk of ulcerative colitis: systematic review and dose-response meta-analysis of epidemiological studies. Clin Nutr. 2017;36(5):1259–65. [DOI] [PubMed] [Google Scholar]
- 34. Zeng L, Hu S, Chen P, Wei W, Tan Y. Macronutrient intake and risk of Crohn's disease: systematic review and dose–response meta-analysis of epidemiological studies. Nutrients. 2017;9(5):500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rose DJ, DeMeo MT, Keshavarzian A, Hamaker BR. Influence of dietary fiber on inflammatory bowel disease and colon cancer: importance of fermentation pattern. Nutr Rev. 2008;65(2):51–62. [DOI] [PubMed] [Google Scholar]
- 36. Issa M, Saeian K. Diet in inflammatory bowel disease. Nutr Clin Pract. 2011;26(2):151–4. [DOI] [PubMed] [Google Scholar]
- 37. Kumar V, Sinha AK, Makkar HP, De Boeck G, Becker K. Dietary roles of non-starch polysachharides in human nutrition: a review. Crit Rev Food Sci Nutr. 2012;52(10):899–935. [DOI] [PubMed] [Google Scholar]
- 38. Milajerdi A, Mousavi SM, Sadeghi A, Salari-Moghaddam A, Parohan M, Larijani B, Esmaillzadeh A. The effect of probiotics on inflammatory biomarkers: a meta-analysis of randomized clinical trials. Eur J Nutr. 2019;59:633–49. [DOI] [PubMed] [Google Scholar]
- 39. Venegas DP, Marjorie K, Landskron G, González MJ, Quera R, Dijkstra G, Harmsen HJ, Faber KN, Hermoso MA. Short chain fatty acids (SCFAs)-mediated gut epithelial and immune regulation and its relevance for inflammatory bowel diseases. Front Immunol. [Internet]2019;10:277. doi:10.3389/fimmu.2019.00277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Milajerdi A, Sadeghi O, Siadat SD, Keshavarz SA, Sima A, Vahedi H, Adibi P, Esmaillzadeh A. A randomized controlled trial investigating the effect of a diet low in fermentable oligosaccharides, disaccharides, monosaccharides, and polyols on the intestinal microbiome and inflammation in patients with ulcerative colitis: study protocol for a randomized controlled trial. Trials. [Internet]2020;21:201. doi:10.1186/s13063-020-4108-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Halajzadeh J, Milajerdi A, Reiner Ž, Amirani E, Kolahdooz F, Barekat M, Mirzaei H, Mirhashemi SM, Asemi Z. Effects of resistant starch on glycemic control, serum lipoproteins and systemic inflammation in patients with metabolic syndrome and related disorders: a systematic review and meta-analysis of randomized controlled clinical trials. Crit Rev Food Sci Nutr. 2020;60:3172–84. [DOI] [PubMed] [Google Scholar]
- 42. Liu J, Wang F, Luo H, Liu A, Li K, Li C, Jiang Y. Protective effect of butyrate against ethanol-induced gastric ulcers in mice by promoting the anti-inflammatory, anti-oxidant and mucosal defense mechanisms. Int Immunopharmacol. 2016;30:179–87. [DOI] [PubMed] [Google Scholar]
- 43. Venkatraman A, Ramakrishna B, Shaji R, Kumar NN, Pulimood A, Patra S. Amelioration of dextran sulfate colitis by butyrate: role of heat shock protein 70 and NF-κB. Am J Physiol Gastrointest Liver Physiol. 2003;285(1):G177–G84. [DOI] [PubMed] [Google Scholar]
- 44. Fiocchi C. IBD: advances in pathogenesis, complications, diagnosis, and therapy. Curr Opin Gastroenterol. 2012;28(4):297–300. [DOI] [PubMed] [Google Scholar]
- 45. Zhu F, Du B, Xu B. Anti-inflammatory effects of phytochemicals from fruits, vegetables, and food legumes: a review. Crit Rev Food Sci Nutr. 2018;58(8):1260–70. [DOI] [PubMed] [Google Scholar]
- 46. Slavin JL, Lloyd B. Health benefits of fruits and vegetables. Adv Nutr. 2012;3(4):506–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Mora J, Von Andrian U. Differentiation and homing of IgA-secreting cells. Mucosal Immunol. 2008;1(2):96. [DOI] [PubMed] [Google Scholar]
- 48. Suzuki T, Hara H. Role of flavonoids in intestinal tight junction regulation. J Nutr Biochem. 2011;22(5):401–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.