ABSTRACT
This systematic review aimed to evaluate the effectiveness and safety of probiotics for glycemic control in adults with impaired glucose control, including prediabetes and type 2 diabetes mellitus (T2DM). We searched PubMed, Embase, and Cochrane databases, and trial registries up to February 2019. We included randomized controlled trials (RCTs) of participants with prediabetes or T2DM. Eligible trials compared probiotics versus either placebo, no intervention, or comparison probiotics, or compared synbiotics versus prebiotics. Primary outcomes were mean change in fasting blood glucose (FBG) and glycated hemoglobin (HbA1c) from baseline to short term (<12 wk) and long term (≥12 wk). We performed meta-analyses using the random-effects model. We included 28 RCTs (1947 participants). Overall, probiotics reduced FBG more than the placebo/no intervention group with a mean difference (MD) of –12.99 mg/dL (95% CI: –23.55, –2.42; P value: 0.016) over the short term; and –2.99 mg/dL (95% CI: –5.84, –0.13; P value: 0.040) over the long term. There was also some evidence for reduced HbA1c in the probiotics group at both short term (MD: –0.17; 95% CI: –0.37, 0.02; P value: 0.084) and long term (MD: –0.14; 95% CI: –0.34, 0.06; P value: 0.172), however, these did not reach statistical significance possibly because only a few trials reported HbA1c as an outcome. Subgroup analyses showed a greater reduction in HbA1c in participants not receiving insulin therapy than those receiving insulin therapy. Furthermore, the effect of probiotics on the reduction of FBG was more pronounced in participants with FBG >130 mg/dL and those not receiving insulin therapy than their counterparts. Probiotics were also effective in lowering serum cholesterol over the short and long term. In conclusion, we found that probiotics may have a glucose-lowering effect in T2DM participants. The effect appeared to be stronger in participants with poorly controlled diabetes and those not on insulin therapy. Systematic review registration: CRD42019121682.
Keywords: probiotics, type 2 diabetes mellitus, glycemic control, systematic review, meta-analysis
Meta-analysis of 1947 participants in 28 RCTs suggested a glucose-lowering effect of probiotics in T2DM participants, especially those with poorly controlled diabetes and those not on insulin therapy.
Introduction
Probiotics—live microbial communities (microbiota) that may benefit host health (1, 2)—are 1 of the most commonly used nutritional supplements worldwide (3). The gut microbiota has been shown to play a role in diabetes—a disease estimated to impact 451 million people in 2017 and projected to impact 693 million by 2045 (3, 4). Several randomized controlled trials (RCTs) have tested whether probiotics can improve glycemic control in adults with type 2 diabetes mellitus (T2DM). Although some RCTs have found that probiotics lower blood sugar (5, 4, 6, 7, 8, 9), overall the evidence is inconsistent (10, 11, 12, 13). Previous systematic reviews and meta-analyses have concluded an overall beneficial effect of probiotics in adults with T2DM. However, the literature searches in these systematic reviews were not comprehensive and the trials included had a short treatment duration and follow-up period (14, 15, 16, 17, 18, 19, 20). Since the publication of these reviews, ≥2 RCTs with a longer treatment duration have been published (6, 9).
The purpose of this systematic review was to assess the effectiveness and safety of probiotics for glycemic control (fasting glucose and glycated hemoglobin [HbA1c]) over the short term and long term in adults with impaired glucose control, including prediabetes and T2DM. In addition, we examined plasma insulin (μU/mL), triglyceride, cholesterol, LDL cholesterol, HDL cholesterol (mg/dL), and health service outcomes. Finally, we determined whether treatment effects differed by the risk of bias, funding, diabetes severity and treatment, and probiotic strains.
Methods
We registered the systematic review with International Prospective Register of Systematic Reviews (PROSPERO) (registration number: CRD42019121682). We followed a pre-established protocol in conducting the review, which was previously published (21). Briefly, we searched PubMed, Embase, and Cochrane databases, and trial registries up to February 2019. We included RCTs of participants with prediabetes or T2DM. Eligible trials either compared probiotics with placebo, comparison probiotics, or no intervention, or they compared synbiotics (probiotics + prebiotics) with prebiotics. Two reviewers (TR, KJ) independently screened titles and abstracts, reviewed full texts, extracted information, and assessed the risk of bias using Cochrane Risk of Bias 2 (22, 23). The tool is structured into 5 domains through which bias might be introduced into a result: bias arising from the randomization process, bias due to deviations from intended interventions, bias due to missing outcome data, bias in measurement of the outcome, and bias in selection of the reported results. We rated each domain as either “low risk of bias,” “high risk of bias,” or “some concerns” following a series of signaling questions. The overall risk of bias for the result is the least favorable assessment across the domains of bias.
We assessed publication bias and reporting bias by comparing information in the trial protocols and/or trial registrations with the publications of trials when they were available. Publication bias is suspected when a trial is completed but there are no publications available. Reporting bias is suspected when outcomes registered or described in the protocols are not reported in the publications.
We examined each outcome, described below, over the short term (<12 wk) and the long term (≥12 wk). Within each time frame, we chose the outcome measurement at the longest follow-up time point. The primary outcomes were mean change in fasting blood glucose (FBG; mg/dL) and mean change in HbA1c (%) from the baseline. For secondary outcomes, we focused on mean change in plasma insulin (μU/mL), triglyceride, cholesterol, LDL cholesterol, and HDL cholesterol (mg/dL) from the baseline. For adverse outcomes, we focused on the proportion of participants that experienced abdominal cramping, abdominal pain, nausea, taste disturbance, soft stools, diarrhea, flatulence, bloating, and systemic infection such as septicemia and endocarditis (24). For health service outcomes, we looked for costs associated with the intervention and mean number of hospital or health professional visits.
For statistical analysis, we used mean difference (MD) for continuous outcomes and risk ratio for binary outcomes. In cases where the MD was not reported, we calculated the MD as the mean (or mean change from baseline) in the intervention group minus the mean (or mean change from baseline) in the comparison group. We calculated SD from the SE or 95% CI whenever possible but did not impute the variability for the MD when they were not reported. We performed meta-analyses using random-effects models. The sources of heterogeneity were qualitatively investigated in the analyses that showed substantial statistical heterogeneity (I2 was 50–90%). We conducted subgroup analyses by the risk of bias of trials (high risk of bias versus low risk of bias or some concern), funding (funded by food industry versus others), stage of disease (prediabetes versus T2DM), participants’ baseline FBG (≤130 mg/dL versus >130 mg/dL), whether participants received insulin therapy at the baseline, type of vehicles for probiotics (foods versus capsules), and whether the probiotics contained the Bifidobacterium genus.
Results
Description of studies
Results of the search
The electronic search yielded 4189 records, of which 66 records of 28 trials were included in our systematic review and 26 trials were included in meta-analyses (2 trials did not provide sufficient data for meta-analysis). We identified 22 ongoing studies and 17 studies that are awaiting classification (Figure 1).
FIGURE 1.
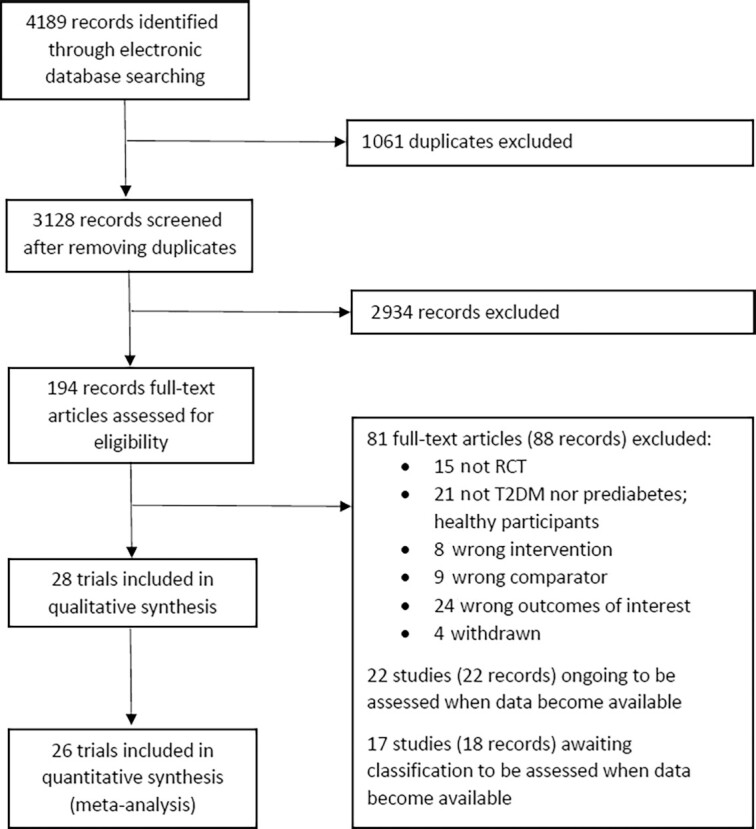
Study flow diagram. RCT, randomized controlled trial; T2DM, type 2 diabetes mellitus.
Included studies
We included 28 RCTs published between 2011 and 2019. Most RCTs (26, 90%) were single-center trials. The maximal planned length of follow-up ranged from 6 wk to 9 mo (median: 12; IQR: 8–12 wk). Of the 27 trials that reported receiving financial and nonfinancial support, 11 (39%) received funding from the food industry (Table 1).
TABLE 1.
Study design of included trials that evaluated the effectiveness and safety of probiotics for glycemic control in patients with type 2 diabetes mellitus1
| Study | Interventions compared | Allowed antidiabetic standard therapy during the trial | Allowed pretreatment with antibiotics | Multi/single center trial (n, recruiting centers) | Country(ies) in which participants were recruited | Maximal planned length of follow-up, wk | Number of participants randomized |
|---|---|---|---|---|---|---|---|
| Ejtahed et al., 2011 (25) | Probiotics; control probiotics | OGM | NR | Single | Iran | 6 | 64 |
| Ejtahed et al., 2012 (4) | Probiotics; control probiotics | OGM | NR | Single | Iran | 6 | 64 |
| Tripolt et al., 2013 (26) | Probiotics; no intervention | NR | No | Single | Austria | 12 | 30 |
| Mazloom et al., 2013 (13) | Probiotics; placebo | NR | NR | Single | Iran | 6 | NR |
| Asemi et al., 2013 (5) | Synbiotics; prebiotics | OGM | NR | Single | Iran | 8 | 60 |
| Barreto et al., 2014 (27) | Probiotics; placebo | NR | NR | Single | Brazil | 12 | 24 |
| Shakeri et al., 2014 (28) | Probiotics; placebo | OGM | NR | Single | Iran | 8 | 78 |
| Mohamadshahi et al., 2014 (7) | Probiotics; control probiotics | OGM | NR | Single | Iran | 8 | 44 |
| Jung et al., 2014 (29) | Probiotics; no intervention | LM, OGM | NR | Single | Korea | 12 | 48 |
| Tajadadi-Ebrahimi et al., 2014 (30) | Probiotics; placebo | OGM | NR | Single | Iran | 8 | 81 |
| Ostadrahimi et al., 2015 (31) | Probiotics; control probiotics | OGM | NR | Single | Iran | 8 | 68 |
| Bayat et al., 2016 (32) | Probiotics; no intervention | OGM | NR | Single | Iran | 8 | 80 |
| Bernini et al., 2016 (33) | Probiotics; no intervention | NR | No | Single | Brazil | 45 days | 51 |
| Sato et al., 2017 (34) | Probiotics; placebo | LM, OGM, insulin therapy | NR | Single | Japan | 16 | 70 |
| Mobini et al., 2017 (35) | Probiotics; placebo | LM, OGM, insulin therapy | No | Single | Sweden | 12 | 46 |
| Firouzi et al., 2017 (36) | Probiotics; placebo | LM, OGM | No | Single | Malaysia | 12 | 136 |
| Tonucci et al., 2017 (37) | Probiotics; control probiotics | OGM | No | Unclear2 | Brazil | 6 | 50 |
| Feizollahzadeh et al., 2017 (38) | Probiotics; placebo | OGM, insulin therapy | No | Single | Iran | 8 | 48 |
| Yuan et al., 2017 (39) | Probiotics; placebo | LM, OGM, insulin therapy | No | Multi (7) | China | 12 | 234 |
| Raygan et al., 2018 (40) | Probiotics; placebo | OGM, insulin therapy | No | Single | Iran | 12 | 60 |
| Kassaian et al., 2018 (41) | Probiotics; placebo | NR | No | Single | Iran | 24 | 120 |
| Sabico et al., 2018 (9) | Probiotics; placebo | NR | No | Single | Saudi Arabia | 6 months | 96 |
| Kobyliak et al., 2018 (12) | Probiotics; placebo | OGM, insulin therapy | No | Single | Ukraine | 8 | 53 |
| Hsieh et al., 2018 (6) | Probiotics; placebo | OGM, insulin therapy | No | Single | Taiwan | 9 months | 74 |
| Mazruei et al., 2019 (42) | Probiotics; placebo | OGM, insulin therapy | No | Single | Iran | 12 | 60 |
| Naito et al., 2018 (43) | Probiotics; placebo | NR | No | Single | Japan | 12 | 100 |
| Razmpoosh et al., 2019 (44) | Synbiotics; prebiotics | OGM | No | Single | Iran | 6 | 68 |
| Khalili et al., 2019 (45) | Probiotics; placebo | OGM | No | Single | Iran | 8 | 40 |
LM, lifestyle modification; NR, not reported; OGM, oral glucose-lowering medication.
Participants were recruited from 2 clinics in the same city.
Participants
A total of 1947 participants were included. The number of participants per trial ranged from 24 to 234 (median: 64; IQR: 49–79). Half of participants (1031, 53%) were recruited from the Middle East (Iran: 935, 48%; Saudi Arabia: 96, 5%). Other participants were recruited from Austria (30, 1.5%), Brazil (125, 6%), China (234, 12%), Japan (170, 9%), Korea (48, 2.5%), Malaysia (136, 7%), Sweden (46, 2%), Taiwan (74, 4%), and the Ukraine (53, 3%). Participants were both men and women with ages ranging from 35 to 76 y. All participants had been diagnosed either with prediabetes or T2DM for 1–26 y and all were overweight. Most T2DM participants were fair to well-controlled in terms of FBG and HbA1c. All T2DM participants received an oral glucose-lowering medication(s) and one-third (33%; 645) received the additional insulin therapy at baseline. No participants with prediabetes were taking type 2 diabetes medication (Table 2).
TABLE 2.
Baseline participant characteristics of intervention versus comparison of included trials that evaluated the effectiveness and safety of probiotics for glycemic control in patients with type 2 diabetes mellitus1
| Age, y | Participants, n (% female) | Duration of diabetes, y | BMI, kg/m2 | 2-h OGTT, mg/dL | HbA1c, % | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study | Intervention | Comparison | Intervention | Comparison | Intervention | Comparison | Intervention | Comparison | Intervention | Comparison | Intervention | Comparison |
| Ejtahed et al., 2011 (25) | 50.87 ± 1.40 | 51.00 ± 1.34 | 19 (63) | 18 (60) | 5.82 ± 0.90 | 4.08 ± 0.78 | 28.95 ± 0.67 | 29.14 ± 0.78 | NR | NR | NR | NR |
| Ejtahed et al., 2012 (4) | 50.87 ± 7.68 | 51.00 ± 7.32 | 19 (63) | 18 (60) | 5.82 ± 4.95 | 4.08 ± 4.28 | 28.95 ± 3.65 | 29.14 ± 4.30 | NR | NR | 7.29 ± 1.21 | 6.87 ± 0.81 |
| Tripolt et al., 2013 (26) | 51.00 ± 11.00 | 55.00 ± 9.00 | 4 (31) | 6 (40) | NR | NR | 34.9 ± 5.3 | 31.4 ± 3.7 | 7.90 ± 2.30 | 8.2 ± 2.90 | NR | NR |
| Mazloom et al., 2013 (13) | 55.40 ± 8.00 | 51.80 ± 10.20 | 26 (76) | NR | NR | 27.97 ± 3.81 | 27.24 ± 2.73 | NR | NR | NR | NR | NR |
| Asemi et al., 2013 (5) | 50.51 ± 9.82 | 52.59 ± 7.14 | 42 (70) | NR | NR | 31.16 ± 6.36 | 30.17 ± 4.23 | NR | NR | NR | 7.71 ± 0.373 | 6.35 ± 0.303 |
| Barreto et al., 2014 (27) | 62 (58.3–67)2 | 63 (60.5–75.7)2 | NR | NR | NA | NA | 27.5 (26.0–31.3)2 | 27.5 (24.3–30.0)2 | NR | NR | NR | NR |
| Shakeri et al., 2014 (28) | 52.30 ± 8.20 | 53.10 ± 7.50 | NR | NR | NR | NR | 29.50 ± 5.70 | 30.6 ± 4.10 | NR | NR | NR | NR |
| Mohamadshahi et al., 2014 (7) | 53.00 ± 5.90 | 49.00 ± 7.08 | NR | NR | NR | NR | 28.36 ± 4.14 | 29.22 ± 3.20 | NR | NR | 8.24 ± 1.68 | 8.33 ± 1.46 |
| Jung et al., 2014 (29) | 63.30 ± 2.00 | 60.20 ± 1.90 | 9 (43) | 10 (50) | NR | NR | 25.90 ± 0.90 | 25.60 ± 0.70 | NR | NR | 6.77 ± 0.203 | 6.77 ± 0.23 |
| Tajadadi-Ebrahimi et al., 2014 (30) | 52.00 ± 7.20 | 53.40 ± 7.50 | NR | NR | NR | NR | 29.80 ± 5.70 | 30.50 ± 4.10 | NR | NR | NR | NR |
| Ostadrahimi et al., 2015 (31) | NR | NR | 12 (40) | 14 (47) | 6.47 ± 0.90 | 7.36 ± 0.84 | 28.89 ± 4.77 | 27.47 ± 3.55 | NR | NR | 7.61 ± 1.22 | 6.98 ± 1.63 |
| Bayat et al., 2016 (32) | 54.10 ± 9.54 | 46.95 ± 9.34 | 17 (85) | 11 (55) | NR | NR | 28.77 ± 4.59 | 29.75 ± 4.66 | NR | NR | 7.06 ± 1.58 | 7.54 ± 2.03 |
| Bernini et al., 2016 (33) | NR | NR | NR | NR | NR | NR | 30.8 (27.2–33.7)2 | 35.8 (33.4–44.5)2 | NR | NR | NR | NR |
| Sato et al., 2017 (34) | 64.00 ± 9.20 | 65.00 ± 8.3 | 5 (15) | 14 (41) | NR | NR | 24.20 ± 2.60 | 24.60 ± 2.60 | NR | NR | NR | NR |
| Mobini et al., 2017 (35) | 64.00 ± 6.00 | 65.00 ± 5.00 | 3 (21) | 4 (27) | 14.40 ± 9.60 | 18.30 ± 7.30 | 32.30 ± 3.40 | 30.70 ± 4.00 | NR | NR | 8.10 ± 0.70 | 7.70 ± 0.50 |
| Firouzi et al., 2017 (36) | 52.90 ± 9.20 | 54.2 ± 8.30 | 65 (48) | NR | NR | 29.20 ± 5.60 | 29.30 ± 5.30 | NR | NR | NR | 7.46 ± 1.20 | 7.29 ± 1.60 |
| Tonucci et al., 2017 (37) | 51.83 ± 6.64 | 50.95 ± 7.20 | 11 (47) | 8 (37) | 6.0 (2–17)2 | 4.5 (2–15)2 | 27.49 ± 3.97 | 29.20 ± 5.60 | NR | NR | 6.07 (5.4–7.0)2 | 5.35 (4.9–6.1)2 |
| Feizollahzadeh et al., 2017 (38) | 56.90 ± 1.813 | 53.60 ± 1.603 | 11 (55) | 10 (50) | 8.70 ± 2.10 | 6.90 ± 4.90 | 26.68 ± 0.71 | 26.58 ± 0.73 | NR | NR | NR | NR |
| Yuan et al., 2017 (39) | 57.43 ± 9.50 | 57.71 (8.20) | 61 | 56 | 9.49 ± 6.43 | 9.20 ± 6.20 | 25.53 ± 4.26 | 24.91 ± 2.81 | NR | NR | 8.00 ± 1.08 | 7.99 ± 1.03 |
| Raygan et al., 2018 (40) | 60.70 ± 9.40 | 61.8 (9.8) | NR | NR | 6.60 ± 1.90 | 6.80 ± 2.20 | 30.30 ± 5.20 | 29.30 ± 4.10 | NR | NR | NR | NR |
| Kassaian et al., 2018 (41) | 52.90 ± 6.30 | 52.97 ± 5.90 | 14 (52) | 16 (57) | NR | NR | 29.60 ± 3.50 | 30.40 ± 3.20 | NR | NR | 5.68 ± 0.40 | 5.70 ± 0.40 |
| Sabico et al., 2018 (9) | 48.00 ± 8.30 | 46.60 ± 5.9 | 20 (51) | 18 (46) | NR | NR | 29.40 ± 5.20 | 30.10 ± 5.00 | NR | NR | NR | NR |
| Kobyliak et al., 2018 (12) | 52.23 ± 1.74 | 57.18 ± 2.06 | NR | NR | 6.16 ± 0.92 | 5.91 ± 0.87 | 34.70 ± 1.29 | 35.65 ± 1.57 | NR | NR | 8.40 ± 0.22 | 8.31 ± 0.29 |
| Hsieh et al., 2018 (6) | 52.32 ± 10.20 | 55.77 ± 8.55 | 10 (46) | 9 (41) | NR | NR | 28.04 ± 4.29 | 27.53 ± 3.15 | NR | NR | 7.91 ± 0.68 | 7.91 ± 0.62 |
| Mazruei et al., 2019 (42) | 62.70 ± 9.10 | 60.30 ± 8.50 | NR | NR | NR | NR | 30.30 ± 5.60 | 31.10 ± 4.60 | NR | NR | NR | NR |
| Naito et al., 2018 (43) | 46.60 ± 1.103 | 47.40 ± 1.003 | 0 | 0 | NA | NA | 29.5 ± 0.403 | 29.0 ± 0.403 | 161.50 ± 3.503 | 165.80 ± 4.603 | 5.74 ± 0.043 | 5.79 ± 0.043 |
| Razmpoosh et al., 2019 (44) | 58.60 ± 6.50 | 61.30 ± 5.20 | 13 (43) | 14 (47) | 6.20 ± 3.10 | 5.90 ± 2.90 | 27.70 ± 4.20 | 27.20 ± 4.20 | NR | NR | NR | NR |
| Khalili et al., 2019 (45) | 43.95 ± 8.14 | 45.00 ± 5.37 | 13 (65) | 13 (65) | 4.00 ± 3.81 | 3.67 ± 4.00 | 29.50 ± 3.34 | 31.94 ± 5.76 | NR | NR | 7.30 ± 0.65 | 6.83 ± 0.95 |
Values are means ± SDs or n (%) unless otherwise indicated; HbA1c, glycated hemoglobin; NA, not applicable; NR, not reported; 2-h OGTT = 2-h oral-glucose-tolerance test.
Data reported as median (range).
Data reported as mean ± SE.
Type of interventions
We focused on 3 comparisons: (2) probiotics versus placebo or no intervention (21 RCTs), (1) probiotics versus comparison probiotics (5 RCTs), and (3) synbiotics versus prebiotics (2 RCTs). Most of the trials (22, 76%) allowed participants to continue their diabetic standard therapy and remain on their usual diet during the trial. Half (16, 55%) of the trials mentioned that they did not allow pretreatment with antibiotics before and during the trial (Table 3).
TABLE 3.
Intervention and comparison of included trials that evaluated the effectiveness and safety of probiotics for glycemic control in patients with type 2 diabetes mellitus1
| Type of vehicles for probiotics/synbiotics | Microbial composition and content (CFU) per dose | Dose | Frequency of usage | Duration of treatment, week | |||||
|---|---|---|---|---|---|---|---|---|---|
| Study | Intervention | Comparison | Intervention | Comparison | Intervention | Comparison | Intervention | Comparison | |
| Ejtahed et al., 2011 (25) | Yogurt | Yogurt | Lactobacillus acidophilus La5 (2.2 × 109 CFU); Bifidobacterium lactis Bb12 (1.8 × 109 CFU); Lactobacillus bulgaricus (NR); Streptococcus thermophilus (NR) | Lactobacillus bulgaricus (NR); Streptococcus thermophilus (NR) | 300 g | 300 g | once a day | once a day | 6 |
| Ejtahed et al., 2012 (4) | Yogurt | Yogurt | Lactobacillus acidophilus La5 (2.2 × 109 CFU); Bifidobacterium lactis Bb12 (1.8 × 109 CFU); Lactobacillus bulgaricus (NR); Streptococcus thermophilus (NR) | Lactobacillus bulgaricus (NR); Streptococcus thermophilus (NR) | 300 g | 300 g | once a day | once a day | 6 |
| Tripolt et al., 2013 (26) | Milk2 | NA | Lactobacillus casei Shirota (6.5 × 109 CFU) | NA | 65 mL | NA | thrice a day | NA | 12 |
| Mazloom et al., 2013 (13) | Capsules | Capsules | Lactobacillus acidophilus (NR); Lactobacillus bulgaricus (NR); Lactobacillus bifidum (NR); Lactobacillus casei (NR) | NA | 1.5 g | 1.5 g | twice a day | twice a day | 6 |
| Lactobacillus bifidum (NR); Lactobacillus casei (NR) | Capsules | Capsules | Bifidobacterium breve (2 ×1010 CFU); Lactobacillus casei (7 × 109 CFU); Bifidobacterium longum (7 × 109 CFU); Lactobacillus acidophilus (2 × 109 CFU); Lactobacillus rhamnosus (1.5 × 109 CFU); Streptococcus thermophilus (1.5 × 109 CFU); Lactobacillus bulgaricus (2 × 108 CFU) | NA | NR | NR | once a day | once a day | 8 |
| Asemi et al., 2013 (5) | Milk2 | NA | Lactobacillus plantarum LP 115 (1 × 109 CFU) | NA | 80 mL | 80 mL | once a day | once a day | 12 |
| Lactobacillus bulgaricus (2 × 108 CFU) | Bread | Bread | Lactobacillus sporogenes (4 × 109 CFU) | NA | 40 g | 40 g | thrice a day | thrice a day | 8 |
| Barreto et al., 2014 (27) | Yogurt | Yogurt | Lactobacillus acidophilus La5 (5.55 × 108 CFU); Bifidobacterium lactis Bb12 (5.55 × 108 CFU); Lactobacillus delbrueckii (NR); Streptococcus thermophilus (NR) | Lactobacillus delbrueckii (NR); Streptococcus thermophilus (NR) | 150 g | 150 g | twice a day | twice a day | 8 |
| Shakeri et al., 2014 (28) | Kimchi4 | NA | NR | NA | NR | NA | thrice a day | NA | 12 |
| Mohamadshahi et al., 2014 (7) | Bread | Bread | Lactobacillus sporogenes (4 × 109 CFU) | NA | 40 g | 40 g | thrice a day | thrice a day | 8 |
| Jung et al., 2014 (29) | Milk2 | Milk2 | Lactobacillus acidophilus (1.5 × 1010 CFU); Lactobacillus casei (9 × 109 CFU); Bifidobacterium lactis (4.8 × 109 CFU); Streptococcus thermophilus (NR) | Streptococcus thermophilus (NR); Lactobacillus bulgaricus (NR) | 600 mL | 600 mL | twice a day | twice a day | 8 |
| Tajadadi-Ebrahimi et al., 2014 (30) | Yogurt | NA | NR | NA | 150 g | NA | once a day | NA | 8 |
| Ostadrahimi et al., 2015 (31) | Milk2 | NA | Bifidobacterium animalis HN019 (2.72 × 1010 CFU) | NA | 80 mL | NA | once a day | NA | 45 days |
| Bayat et al., 2016 (32) | Milk2 | Milk3 | Lactobacillus casei Shirota (4 × 1010 CFU) | NA | 80 mL | 80 mL | once a day | once a day | 16 |
| Bernini et al., 2016 (33) | Powders | Powders | Lactobacillus reuteri DSM 17,938 (1010 CFU) | NA | 1010 CFU | NR | once a day | once a day | 12 |
| Sato et al., 2017 (34) | Powders | Powders | Lactobacillus acidophilus (105 CFU); Lactobacillus casei (105 CFU); Lactobacillus lactis (105 CFU); Bifidobacterium bifidum (105 CFU); Bifidobacterium longum (105 CFU); Bifidobacterium infantis (105 CFU) | NA | 3 × 1010 CFU | NR | twice a day | twice a day | 12 |
| Mobini et al., 2017 (35) | Milk2 | Milk2 | Lactobacillus acidophilus La-5 (109 CFU); Bifidobacterium animalis BB-12 (109 CFU) | Streptococcus thermophilus TA-40 (NR) | 120 g | 120 g | once a day | once a day | 6 |
| Firouzi et al., 2017 (36) | Soymilk | Soymilk | Lactobacillus plantarum A7 (2 × 107 CFU) | NA | 200 mL | 200 L | once a day | once a day | 8 |
| Tonucci et al., 2017 (37) | Tablets | Tablets | Bacillus cereus (>0.5 × 106 CFU); Bifidobacterium infantis (>0.5 × 106 CFU); Enterococcus faecalis (>0.5 × 106 CFU); Lactobacillus acidophilus (>0.5 × 106 CFU) | NA | 1.5 g | 1.5 g | thrice a day | thrice a day | 8 |
| Feizollahzadeh et al., 2017 (38) | Capsules | Capsules | Bifidobacterium bifidum (2 × 109 CFU); Lactobacillus casei (2 × 109 CFU); Lactobacillus acidophilus (2 × 109 CFU) | NA | NR | NR | once a day | NR | 12 |
| Yuan et al., 2017 (39) | Powders | Powders | Lactobacillus acidophilus (1 × 109 CFU); Bifidobacterium lactis (1 ×109 CFU); Bifidobacterium bifidum (1×109 CFU); Bifidobacterium longum (1 × 109 CFU) | NA | 6 g | NR | once a day | once a day | 24 |
| Raygan et al., 2018 (40) | Powders | Powders | Bifidobacterium bifidum W23 (NR); Bifidobacterium lactis W52 (NR); Lactobacillus acidophilus W37 (NR); Lactobacillus brevis W63 (NR); Lactobacillus casei W56 (NR); Lactobacillus salivarius W24 (NR); Lactobacillus lactis W19 (NR); Lactobacillus lactis W58 (NR) | NA | 5 × 109 CFU | 2 g | twice a day | twice a day | 6 months |
| Kassaian et al., 2018 (41) | Powders | Powders | Lactobacillus + Lactococcus (6 × 1011 CFU); Propionibacterium (3 × 1011 CFU); Bifidobacterium (1 × 1011 CFU); Acetobacter (1 × 107 CFU) | NA | 10 g | 10 g | once a day | once a day | 8 |
| Sabico et al., 2018 (9) | Capsules | Capsules | Lactobacillus reuteri GMNL-89 (2 × 109 CFU) | NA | 4 × 109 CFU | NR | once a day | once a day | 6 months |
| Kobyliak et al., 2018 (12) | Honey | Honey | Bacillus coagulans T4 (2.5 × 109 CFU) | NA | 25 g | 25 g | once a day | once a day | 12 |
| Hsieh et al., 2018 (6) | Milk2 | Milk3 | Lactobacillus casei Shirota YIT 9029 (>1.0 × 1011 CFU) | NA | 100 mL | 100 mL | once a day | once a day | 8 |
| Mazruei et al., 2019 (42) | Capsules | Capsules | Bifidobacterium breve (3 × 1010 CFU); Lactobacillus casei (7 × 109 CFU); Bifidobacterium longum (7 × 109 CFU); Lactobacillus acidophilus (2 × 109 CFU); Lactobacillus rhamnosus (1.5 × 109 CFU); Streptococcus thermophilus (1.5 × 109 CFU); Lactobacillus bulgaricus (2 × 108 CFU) | NA | NR | NA | twice a day | twice a day | 6 |
| Naito et al., 2018 (43) | Capsules | Capsules | Lactobacillus casei (108 CFU) | NA | 108 CFU | NR | once a day | once a day | 8 |
NA, not applicable; NR, not reported.
Fermented milk.
Nonfermented milk.
Kimchi and fermented soy-based condiments.
Probiotics versus placebo or no intervention
Of the 21 RCTs that compared probiotics to placebo or no intervention, 7 (33%) evaluated multistrain probiotics versus placebo or no intervention. The number of strains ranged from 3 to 14 (median: 4; IQR: 4–7). Eleven RCTs (53%) compared single-strain probiotics versus placebo or no intervention. The remaining 3 RCTs (14%) did not report the number of strains. The type of vehicles for probiotics varied including fermented foods (i.e. yogurt, fermented milk, kimchi), functional foods (i.e. honey, bread), and dietary supplements (i.e. probiotic capsules or tablets). The microbial compositions were similar in terms of the genera, which were mainly Lactobacillus and Bifidobacterium; however, the species and strains differed, and the daily dose ranged from 106 to 1019 CFU across the trials. The treatment duration also varied across these 21 RCTs: 8 (38%) evaluated short-term treatment duration that ranged from 6 to 8 wk (median: 8; IQR: 7.6–8); 13 (62%) evaluated long-term treatment duration ranging from 12 to 36 wk (median: 12; IQR: 12–16) (Table 3).
Probiotics versus comparison probiotics
Of the 5 RCTs that compared probiotics to comparison probiotics, 4 (80%) evaluated 4-strain probiotics versus 2-strain control probiotics; and 1 (20%) evaluated 2-strain probiotics versus single-strain control probiotics. In these comparisons, all probiotics were fermented foods (i.e. yogurt and fermented milk). The microbial composition of probiotics was similar in terms of the genera, which were Lactobacillus, Bifidobacterium, and Streptococcus; however, the species and strains varied among trials. The daily dose ranged from 109 to 1010 CFU. The treatment was short term in all 5 RCTs, ranging from 6 to 8 wk (Table 3).
Synbiotics versus prebiotics
Two RCTs compared prebiotics versus 7-strain synbiotics in dietary supplements. Both trials were similar concerning the microbial composition; however, the daily dose differed between trials. Treatment duration was 6 and 8 wk in these trials (Table 3).
Type of outcomes
Apart from Ejtahed (2011) (25), which measured only mean change in triglyceride, cholesterol, LDL cholesterol, and HDL cholesterol (mg/dL) from the baseline, all included trials measured ≥1 of our primary outcomes of interest. For our secondary outcomes of interest, the number of trials reporting each outcome ranged from 19 to 21. None of the studies reported health service utilization outcomes and half of the studies (15, 52%) reported adverse events (Table 4).
TABLE 4.
Outcome measures of included trials that evaluated the effectiveness and safety of probiotics for glycemic control in patients with type 2 diabetes mellitus1
| Study | FBG | Insulin | HbA1c | Cholesterol | Triglyceride | HDL-C | LDL-C |
|---|---|---|---|---|---|---|---|
| Ejtahed et al., 2011 (25) | — | — | — | ✓ | ✓ | ✓ | ✓ |
| Ejtahed et al., 2012 (4) | ✓ | ✓ | ✓ | — | — | — | — |
| Tripolt et al., 2013 (26) | ✓ | ✓ | — | — | — | — | — |
| Mazloom et al., 2013 (13) | ✓ | ✓ | — | ✓ | ✓ | ✓ | ✓ |
| Asemi et al., 2013 (5) | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Barreto et al., 2014 (27) | ✓ | ✓ | — | ✓ | ✓ | ✓ | ✓ |
| Shakeri et al., 2014 (28) | ✓ | — | — | ✓ | ✓ | ✓ | ✓ |
| Mohamadshahi et al., 2014 (7) | ✓ | — | ✓ | ✓ | ✓ | ✓ | ✓ |
| Jung et al., 2014 (29) | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Tajadadi-Ebrahimi et al., 2014 (30) | ✓ | ✓ | — | — | — | — | — |
| Ostadrahimi et al., 2015 (31) | ✓ | — | ✓ | ✓ | ✓ | ✓ | ✓ |
| Bayat et al., 2016 (32) | ✓ | — | ✓ | ✓ | ✓ | ✓ | ✓ |
| Bernini et al., 2016 (33) | ✓ | ✓ | — | ✓ | ✓ | ✓ | ✓ |
| Sato et al., 2017 (34) | ✓ | — | ✓ | ✓ | ✓ | ✓ | |
| Mobini et al., 2017 (35) | ✓ | — | ✓ | ✓ | ✓ | ✓ | ✓ |
| Firouzi et al., 2017 (36) | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Tonucci et al., 2017 (37) | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Feizollahzadeh et al., 2017 (38) | ✓ | — | — | — | ✓ | ✓ | ✓ |
| Yuan et al., 2017 (39) | ✓ | — | ✓ | — | — | — | — |
| Raygan et al., 2018 (40) | ✓ | ✓ | — | ✓ | ✓ | ✓ | ✓ |
| Kassaian et al., 2018 (41) | ✓ | ✓ | ✓ | — | — | — | — |
| Sabico et al., 2019 (9) | ✓ | ✓ | — | ✓ | ✓ | ✓ | ✓ |
| Kobyliak et al., 2018 (12) | ✓ | ✓ | ✓ | — | — | — | — |
| Hsieh et al., 2018 (6) | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Mazruei et al., 2019 (42) | ✓ | ✓ | — | ✓ | ✓ | ✓ | ✓ |
| Naito et al., 2018 (43) | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Razmpoosh et al., 2019 (44) | ✓ | ✓ | — | ✓ | ✓ | ✓ | ✓ |
| Khalili et al., 2019 (45) | ✓ | ✓ | ✓ | — | — | — | — |
FBG, fasting blood glucose; HbA1c, glycated hemoglobin.
Risk of bias in included studies
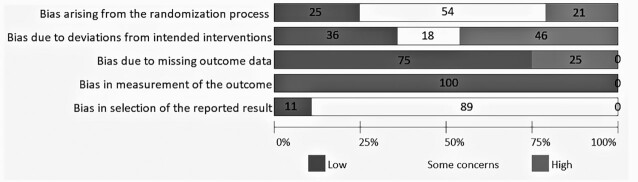
The overall risk of bias of the included RCTs was either some concern or high (Figure 2 and Supplementary Table 1). Six trials (21%) were rated at a high risk of bias arising from the randomization process. Thirteen trials (46%) were rated at a high risk of bias due to deviation from intended interventions. Seven trials (25%) were rated at a high risk of bias due to missing outcome data. None of the trials was rated at a high risk of bias in the measurement of the outcome and selection of the reported results. We did not find evidence for publication or reporting bias.
FIGURE 2.
Risk of bias.
Effects of interventions
Comparison I: probiotics versus placebo or no intervention (Table 5)
TABLE 5.
Summary estimates for primary and secondary outcomes at short term and long term derived from meta-analyses on 3 comparisons from 26 trials1
| Intervention | Comparison | Outcomes | Studies, n | Participants, n | MD | 95% CI | P value | In favor of | Tau-squared | I-squared |
|---|---|---|---|---|---|---|---|---|---|---|
| Probiotics | Placebo/no intervention | Short term | ||||||||
| Primary Outcomes | ||||||||||
| Fasting blood glucose | 8 | 428 | –12.99 | (–23.55, –2.42) | 0.016 | Probiotics | 0.005 | 65.7 | ||
| HbA1c | 4 | 231 | –0.17 | (–0.37, 0.02) | 0.084 | — | 0.194 | 36.3 | ||
| Secondary Outcomes | ||||||||||
| Serum insulin | 5 | 296 | –0.98 | (–2.38, 0.42) | 0.170 | — | 0.122 | 45.0 | ||
| Triglyceride | 5 | 281 | –20.98 | (–63.96, 22.01) | 0.339 | — | 0.021 | 65.3 | ||
| Cholesterol | 4 | 241 | –10.69 | (–19.53, –1.85) | 0.018 | Probiotics | 0.680 | 0 | ||
| LDL-C | 5 | 281 | –9.55 | (–17.13, –1.98) | 0.013 | Probiotics | 0.942 | 0 | ||
| HDL-C | 5 | 281 | 3.25 | (–0.05, 6.54) | 0.054 | — | 0.176 | 36.8 | ||
| Long term | ||||||||||
| Primary outcomes | ||||||||||
| Fasting blood glucose | 12 | 805 | –2.99 | (–5.84, –0.13) | 0.040 | Probiotics | 0.774 | 0 | ||
| HbA1c | 7 | 572 | –0.14 | (–0.34, 0.06) | 0.172 | — | 0.001 | 72.1 | ||
| Secondary outcomes | ||||||||||
| Serum insulin | 9 | 474 | –1.79 | (–3.33, –0.24) | 0.023 | Probiotics | 0.011 | 59.4 | ||
| Triglyceride | 10 | 516 | –12.39 | (–23.78, –0.99) | 0.033 | Probiotics | 0.195 | 27.0 | ||
| Cholesterol | 10 | 516 | –4.77 | (–9.20, 0.33) | 0.035 | Probiotics | 0.981 | 0 | ||
| LDL-C | 8 | 420 | –2.67 | (–7.48, 2.14) | 0.277 | — | 0.886 | 0 | ||
| HDL-C | 9 | 488 | 1.49 | (–0.47, 3.46) | 0.136 | — | 0.030 | 53.0 | ||
| Synbiotics | Prebiotics | Short term | ||||||||
| Primary outcomes | ||||||||||
| Fasting blood glucose | 2 | 114 | –19.52 | (–32.42, –6.62) | 0.003 | Synbiotics | 0.341 | 0 | ||
| HbA1c | 1 | 54 | –0.48 | (–1.43, 0.47) | 0.320 | — | . | . | ||
| Secondary outcomes | ||||||||||
| Serum insulin | 2 | 114 | –1.66 | (–3.72, 0.40) | 0.115 | — | 0.510 | 0 | ||
| Triglyceride | 2 | 114 | –8.71 | (–28.79, 11.37) | 0.395 | — | 0.568 | 0 | ||
| Cholesterol | 2 | 114 | –4.92 | (–19.17, 9.33) | 0.499 | — | 0.694 | 0 | ||
| LDL-C | 2 | 114 | –3.59 | (–13.60, 6.43) | 0.483 | — | 0.870 | 0 | ||
| HDL-C | 2 | 114 | 0.99 | (–2.70, 4.69) | 0.598 | — | 0.560 | 0 | ||
| Probiotics | Control probiotics | Long term | ||||||||
| Primary outcomes | ||||||||||
| Fasting blood glucose | 3 | 165 | –8.56 | (–37.19, 20.08) | 0.558 | — | 0.017 | 75.5 | ||
| HbA1c | 3 | 165 | –0.15 | (–0.55, 0.25) | 0.452 | — | 0.547 | 0 | ||
| Secondary outcomes | ||||||||||
| Serum insulin | 2 | 115 | 0.35 | (–1.28, 1.98) | 0.672 | — | 0.853 | 0 | ||
| Triglyceride | 3 | 165 | –2.39 | (–29.69, 24.91) | 0.864 | — | 0.264 | 24.9 | ||
| Cholesterol | 3 | 165 | –9.95 | (–31.42, 11.53) | 0.364 | — | 0.061 | 64.2 | ||
| LDL-C | 3 | 165 | –2.08 | (–14.46, 10.31) | 0.742 | — | 0.208 | 36.3 | ||
| HDL-C | 3 | 165 | 0.29 | (–3.30, 3.88) | 0.873 | — | 0.996 | 0 |
There are 20 trials in probiotics versus placebo or no intervention comparison, 2 trials in synbiotics versus prebiotics, and 4 trials in probiotics versus control probiotics (total 26 trials) that reported sufficient data to permit meta-analysis; HbA1c, glycated hemoglobin; MD, mean difference; short term is defined as <12 wk; long term is defined as ≥12 wk.
FBG
Twenty-one RCTs (1529 participants) were included for comparison I. Nine RCTs (428 participants) evaluated FBG in the short term. Eight RCTs reported sufficient data to permit meta-analysis and the average effect from these RCTs was in favor of probiotics (MD: –12.99; 95% CI: –23.55, –2.42; P value: 0.016; I2 = 65.7%). Twelve RCTs (805 participants) evaluated FBG in the long term. All reported sufficient data to permit meta-analysis and the average effect from these RCTs was in favor of probiotics (MD: –2.99; 95% CI: –5.84, –0.13; P value: 0.040; I2 = 0%).
HbA1c
Four RCTs (231 participants) evaluated HbA1c in the short term. All RCTs reported sufficient data to permit meta-analysis. Although the average effect appears to favor probiotics, it did not meet the threshold for statistical significance (MD: –0.17; 95% CI: –0.37, 0.02; P value: 0.084; I2 = 36.3%). Seven RCTs (572 participants) evaluated HbA1c in the long term. All reported sufficient data to permit meta-analysis. Although the average effect favored probiotics, the difference was not statistically significant (MD: –0.14; 95% CI: –0.34, 0.06; P value: 0.172; I2 = 72.1%).
Secondary outcomes
We found statistically significant differences in mean change in serum cholesterol and LDL cholesterol from baseline in favor of probiotics in the short term and statistically significant differences in mean change in serum insulin, triglyceride, and serum cholesterol from baseline in favor of probiotics in the long term. The estimates and 95% CIs are available in Table 5.
Health services outcomes
No trials reported costs associated with the intervention or mean number of hospital or health professional visits.
Sources of heterogeneity
We concluded that the statistical heterogeneity could be due to differences in participants’ ethnicity, blood sugar control, bacterial strains, and dose of probiotics.
Subgroup analysis for comparison I: probiotics versus placebo or no intervention (Table 6)
TABLE 6.
Summary estimates on fasting blood glucose and glycated hemoglobin from subgroup analysis and additional analysis on probiotics versus placebo or no intervention comparison1
| n, studies | n, participants | MD | (95% CI) | P value | In favor of | Tau-squared | I-squared | P value | |
|---|---|---|---|---|---|---|---|---|---|
| Fasting blood glucose | |||||||||
| High risk of bias | 10 | 522 | −2.68 | (−5.75, 0.39) | 0.087 | — | 0.987 | 0.0 | 0.396 |
| Not high risk of bias | 10 | 711 | −10.26 | (−18.23, −2.30) | 0.012 | Probiotics | 0.003 | 64.3 | |
| Funded by industry | 11 | 615 | −2.99 | (−5.84, −0.14) | 0.040 | Probiotics | 0.713 | 0.0 | 0.501 |
| Not funded by industry | 9 | 618 | −8.12 | (−15.76, −0.48) | 0.037 | Probiotics | 0.008 | 61.2 | |
| T2DM | 15 | 977 | −9.14 | (−15.12, −3.17) | 0.003 | Probiotics | 0.062 | 38.9 | 0.081 |
| Prediabetes | 2 | 153 | −2.54 | −5.45, 0.37) | 0.087 | — | 0.794 | 0.0 | |
| Metabolic syndrome | 3 | 103 | −0.30 | (−6.14, 5.53) | 0.919 | — | 0.928 | 0.0 | |
| FBG ≤ 130 mg/dL | 7 | 401 | −2.58 | (−4.99, −0.16) | 0.036 | Probiotics | 0.953 | 0.0 | 0.000 |
| FBG > 130 mg/dL | 10 | 530 | −16.15 | (−24.62, −7.68) | 0.000 | Probiotics | 0.211 | 25.2 | |
| Foods | 11 | 556 | −4.55 | (−8.97, −0.12) | 0.044 | Probiotics | 0.140 | 32.4 | 0.663 |
| Capsules | 9 | 677 | −6.20 | (−12.64, 0.24) | 0.059 | — | 0.105 | 39.4 | |
| Contained Bifidobacterium | 4 | 268 | −3.53 | (−7.42, 0.36) | 0.075 | — | 0.745 | 0.0 | 0.828 |
| Do not contain Bifidobacterium | 6 | 296 | −7.12 | (−15.39, 1.15) | 0.091 | — | 0.024 | 61.3 | |
| Received insulin therapy | 8 | 588 | −3.52 | (−8.50, 1.45) | 0.165 | — | 0.660 | 0.0 | 0.006 |
| Did not received insulin therapy | 6 | 328 | −18.40 | (−30.20, −6.60) | 0.000 | Probiotics | 0.073 | 50.4 | |
| Additional analysis: short- and long-term combination | 20 | 1233 | −4.95 | (−8.36, −1.54) | 0.004 | Probiotics | 0.080 | 32.6 | |
| Glycated hemoglobin | |||||||||
| High risk of bias | 5 | 318 | −0.16 | (−0.35, 0.03) | 0.098 | — | 0.009 | 70.2 | 0.923 |
| Not high risk of bias | 6 | 485 | −0.17 | (−0.43, 0.08) | 0.182 | — | 0.025 | 61.0 | |
| Funded by industry | 4 | 296 | −0.01 | (−0.17, 0.16) | 0.946 | — | 0.070 | 57.5 | 0.002 |
| Not funded by industry | 7 | 507 | −0.29 | (−0.45, −0.12) | 0.001 | Probiotics | 0.148 | 36.8 | |
| T2DM | 9 | 650 | −0.18 | (−0.38, 0.02) | 0.078 | — | 0.001 | 68.3 | 0.996 |
| Prediabetes | 2 | 153 | −0.11 | (−0.23, −0.002) | 0.046 | Probiotics | 0.310 | 3.1 | |
| FBG ≤ 130 mg/dL | 4 | 262 | −0.14 | (−0.34, 0.06) | 0.161 | — | 0.004 | 77.4 | 0.477 |
| FBG > 130 mg/dL | 5 | 263 | −0.16 | (−0.42, 0.11) | 0.248 | — | 0.082 | 51.6 | |
| Foods | 4 | 247 | −0.19 | (−0.46, 0.09) | 0.180 | — | 0.001 | 80.7 | 0.235 |
| Capsules | 7 | 556 | −0.16 | (−0.32, −0.01) | 0.038 | Probiotics | 0.158 | 35.4 | |
| Contained Bifidobacterium | 2 | 156 | −0.19 | (−0.35, −0.03) | 0.021 | Probiotics | 0.757 | 0.0 | 0.401 |
| Did not contain Bifidobacterium | 2 | 138 | −0.12 | (−0.30, 0.06) | 0.180 | — | 0.264 | 19.9 | |
| Received insulin therapy | 5 | 428 | −0.04 | (−0.28, 0.21) | 0.759 | — | 0.031 | 62.3 | 0.002 |
| Not received insulin therapy | 4 | 222 | −0.34 | (−0.58, −0.11) | 0.004 | Probiotics | 0.170 | 40.3 | |
| Additional analysis: short- and long-term combination | 11 | 803 | −0.16 | (−0.30, −0.02) | 0.023 | Probiotics | 0.003 | 61.9 |
FBG, fasting blood glucose; MD, mean difference; T2DM, type 2 diabetes mellitus.
FBG
In the subgroups of trials without the high risk of bias, trials not funded by the food industry, trials of participants with T2DM, trials testing food-type probiotics, and trials among participants that did not receive insulin therapy, there was a statistically significantly difference in mean change in FBG from baseline, in favor of probiotics, between the probiotics and placebo or no intervention comparison. The magnitude of reduction was significantly greater in the subgroup of participants with baseline FBG >130 mg/dL than in participants with baseline FBG ≤130 mg/dL (P < 0.001); and significantly greater in the subgroup of participants not receiving insulin therapy at baseline (P = 0.006).
HbA1c
In subgroups of trials not funded by the food industry, probiotics that contained Bifidobacterium, capsule-type probiotics, trials among prediabetic participants, and trials among participants not receiving insulin therapy, there was a statistically significant difference in mean change in HbA1c from baseline, in favor of probiotics, between the probiotics and placebo or no intervention comparison. The magnitude of reduction was significantly greater in the subgroups that were not funded by the food industry (P = 0.002) and in participants not receiving insulin therapy (P = 0.002).
Additional analysis
When all trials were combined regardless of the duration of treatment, we found significant differences in mean change in FBG (MD: –4.95; 95% CI: –8.36, –1.54; P value: 0.004; I2 = 32.6%) and HbA1c from baseline (MD: –0.16; 95% CI: –0.30, –0.02; P value: 0.023; I2 = 61.9%) in favor of probiotics.
Comparison II: synbiotics (probiotics with added prebiotics) versus prebiotics (Table 5)
Two RCTs (114 participants) made this comparison. We found a statistically significant difference in mean change in FBG in favor of synbiotics (MD: –19.52; 95% CI: –32.42, –6.62; P value: 0.003; I2 = 0%) in the short-term trials. We found either no data or no evidence of a difference for other outcomes analyzed (Table 5).
Comparison III: probiotics versus other probiotics (Table 5)
Five RCTs (225 participants) made this comparison. Of these 5 RCTs, 3 reported FBG, 3 reported HbA1c, 4 reported serum insulin, 3 reported triglyceride, 3 reported LDL cholesterol, and 3 reported HDL cholesterol. One RCT did not contribute data to any meta-analysis (7). We found either no data or no evidence of a difference for all outcomes analyzed.
Adverse events
Of 15 trials (53.6%) that reported adverse events, none reported serious adverse events. Three trials reported minor adverse events observed in the probiotics group which were abdominal cramping, dyspepsia, or diarrhea or soft stools. However, the number of participants reporting minor adverse events was <5% in each trial. We did not have enough data to calculate a between-group difference.
Discussion
We conducted a systematic review to evaluate the effectiveness and safety of probiotics for improving glucose control in adults with impaired blood control, including prediabetes and T2DM. Probiotics were more effective than placebo in reducing FBG from baseline, both over the short term and long term. However, the effect of probiotics over the long term seems to have a less meaningful effect compared with the effect over the short term. Synbiotics were also effective. Subgroup analyses suggested that probiotics might be more effective in adults not on insulin therapy or with poorly controlled T2DM. In addition, probiotics were more effective than placebo in reducing serum cholesterol and LDL cholesterol from baseline in the short term and in reducing triglyceride and serum cholesterol from baseline in the long term.
Previous meta-analyses reported either inconclusive results or modest probiotic effects on glycemic control (14, 15, 16, 17, 18, 19, 20). We found a statistically significant difference in reducing FBG and some effect of reducing HbA1c in type 2 diabetic patients. Previous systematic reviews included between 6 and 12 trials, whereas we included 28 trials, 15 of which were published between 2017 and 2019. Unlike previous systematic reviews, we performed meta-analyses of the effect of probiotics both in the short term and long term to further understand how the effects may vary over time (14, 15, 16, 17, 18, 19, 20). Furthermore, we performed subgroup analyses based on various trial characteristics (e.g. industry funding compared with not) and participant characteristics (e.g. participants receiving insulin therapy compared with not) that have the potential to influence the effect of probiotics on glycemic control.
Mechanisms through which probiotics improve glucose homeostasis likely stem from changing the composition of the host gut microbiota. Altering the gut microbiota can improve intestinal barrier integrity to reduce circulating bacterial endotoxin, and ultimately, reduce systemic inflammation (46, 47, 48). The gut microbiota may also modulate glucagon-like peptide-1 (GLP-1), 1 of the enteroendocrine peptides produced by L-cells in the gut, and alter the secretion of GLP-1 which results in a reduction of gastric emptying time and food intake, and an increase in insulin secretion (49, 50). Also, probiotics may alter microbiota-derived metabolites, such as butyrate and acetate, which have been associated with changes in glucose and lipid metabolism as well as appetite signaling (51).
In the subgroup analyses, we found that the magnitude of the probiotic effect on glycemic outcomes appears to be stronger in participants with poorly controlled diabetes (FBG >130 mg/dL). In addition, the magnitude of reduction in FBG was more pronounced in those not receiving insulin therapy compared with those receiving insulin therapy. T2DM patients who require additional insulin therapy may have compromised β-cell function (52). Probiotics may exert glycemic effects via improved insulin sensitivity and therefore be less likely to have a significant impact on reducing blood glucose in diabetics on insulin.
It should be noted that differences in probiotic strains, host conditions, as well as dietary patterns can affect the composition of gut microbiota which may result in an interindividual difference in response to probiotic treatment (53, 54, 55, 56). Our systematic review highlights the need for future studies to (1) explicitly report specific strains and dosages of each specific bacteria contained in probiotic supplements, (2) carefully monitor participants’ dietary intake and antibiotic use to minimize bias and to determine whether there is heterogeneity between interventions, (3) be adequately powered and stratified by severity of T2DM, and (4) use appropriate randomization and allocation concealment as well as blinding of participants, study personnel, and outcome assessors. Moreover, to determine the benefit of probiotics on reducing morbidity and mortality of prediabetes and T2DM, future studies should measure long-term, patient-centered outcomes and long-term micro- and macrovascular complications, mortality, health services outcomes, and adverse events in addition to lab measures.
There were several limitations of the trials included in our meta-analysis. First, most included trials did not report specific probiotic strain composition, thus our meta-analysis grouped unrelated microorganisms. Also, many of the organisms in the trials reviewed have yet to meet the definition of probiotic and should thus be considered only as putative probiotic organisms. Another limitation of the reviewed studies is that certain racial or ethnic groups were underrepresented. Half of the included trials were conducted in the Middle East, in which dietary, genetic, and gut microbiome profiles of the population may differ from those in other regions. Since individuals may respond to probiotics differently based on the strain of probiotics as well as an individual's genetic, diet, and gut microbiome profile, different responses to probiotics among the studies can be expected (57). Finally, all of the included trials were rated as having some concern or high risk of bias, which reduced the certainty of evidence.
In conclusion, we found that probiotics have a beneficial effect on fasting glucose in adults with T2DM, and the effect was stronger in participants with poorly controlled diabetes and those not on insulin therapy. Probiotics also may have a beneficial glycemic effect in adults with prediabetes, although the number of included trials was too small to demonstrate statistical significance. There was also suggestive evidence that probiotics lower HbA1c, however, these differences did not meet the threshold for statistical significance likely due to the smaller number of trials contributing to the HbA1c analyses. If probiotics are selected to be supplementary therapy for prediabetes and T2DM patients, all factors including its effectiveness, cost, safety, and patients’ preference should be considered.
Supplementary Material
Acknowledgments
The authors’ contributions were as follows—TR and TL: designed the research; TR and KJ: conducted the research; TR, KP, and TL: provided essential reagents or materials; TR: performed the statistical analysis; TR, KP, NTM, and TL: analyzed data; TR: wrote the manuscript; TR, KP, NTM, and TL: had primary responsibility for final content; and all authors: read and approved the final manuscript.
Notes
TR was a visiting scholar at Johns Hopkins Bloomberg School of Public Health when the work was performed. Her scholarship was funded by the Prince Mahidol Award Foundation under the Royal Patronage. The project received partial funding support from the Thailand Research Fund (RDG6150124). The sponsors were not involved in the data analysis, data interpretation, or manuscript preparation.
Author disclosures: The authors report no conflicts of interest.
Supplementary Table 1 is available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/advances.
Abbreviations used: FBG, fasting blood glucose; GLP-1, glucagon-like peptide-1; HbA1c, glycated hemoglobin; MD, mean difference; RCT, randomized controlled trial; T2DM, type 2 diabetes mellitus.
Contributor Information
Thanitsara Rittiphairoj, Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, USA; Department of Preventive and Social Medicine, Faculty of Medicine, Chulalongkorn University, Bangkok, Thailand.
Krit Pongpirul, Department of Preventive and Social Medicine, Faculty of Medicine, Chulalongkorn University, Bangkok, Thailand; Department of International Health, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, USA.
Kantima Janchot, Panacee Group Co., Ltd, Bangkok, Thailand.
Noel T Mueller, Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, USA; Welch Center for Prevention, Epidemiology and Clinical Research, Baltimore, MD, USA.
Tianjing Li, Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, USA; Department of Ophthalmology, School of Medicine, University of Colorado Anschutz Medical Campus, Aurora, CO, USA.
Reference
- 1. Reid G, Jass J, Sebulsky MT, McCormick JK. Potential uses of probiotics in clinical practice. Clin Microbiol Rev. 2003;16(4):658–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Senok AC, Ismaeel AY, Botta GA. Probiotics: facts and myths. Clin Microbiol Infect. 2005;11(12):958–66. [DOI] [PubMed] [Google Scholar]
- 3. Clarke TC, Black LI, Stussman BJ, Barnes PM, Nahin RL. Trends in the use of complementary health approaches among adults: United States, 2002–2012. Natl Health Stat Report. 2015(79):1–16. [PMC free article] [PubMed] [Google Scholar]
- 4. Ejtahed HS, Mohtadi-Nia J, Homayouni-Rad A, Niafar M, Asghari-Jafarabadi M, Mofid V. Probiotic yogurt improves antioxidant status in type 2 diabetic patients. Nutrition. 2012;28(5):539–43. [DOI] [PubMed] [Google Scholar]
- 5. Asemi Z, Zare Z, Shakeri H, Sabihi SS, Esmaillzadeh A. Effect of multispecies probiotic supplements on metabolic profiles, hs-CRP, and oxidative stress in patients with type 2 diabetes. Ann Nutr Metab. 2013;63(1–2):1–9. [DOI] [PubMed] [Google Scholar]
- 6. Hsieh MC, Tsai WH, Jheng YP, Su SL, Wang SY, Lin CC, Chen YH, Chang WW. The beneficial effects of Lactobacillus reuteri ADR-1 or ADR-3 consumption on type 2 diabetes mellitus: a randomized, double-blinded, placebo-controlled trial. Sci Rep. 2018;8(1):16791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mohamadshahi M, Veissi M, Haidari F, Shahbazian H, Kaydani GA, Mohammadi F. Effects of probiotic yogurt consumption on inflammatory biomarkers in patients with type 2 diabetes. Bioimpacts. 2014;4(2):83–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rajkumar H, Kumar M, Das N, Kumar SN, Challa HR, Nagpal R. Effect of probiotic Lactobacillus salivarius UBL S22 and prebiotic fructo-oligosaccharide on serum lipids, inflammatory markers, insulin sensitivity, and gut bacteria in healthy young volunteers: a randomized controlled single-blind pilot study. J Cardiovasc Pharmacol Ther. 2015;20(3):289–98. [DOI] [PubMed] [Google Scholar]
- 9. Sabico S, Al-Mashharawi A, Al-Daghri NM, Wani K, Amer OE, Hussain DS, Ahmed Ansari MG, Masoud MS, Alokail MS, McTernan PG. Effects of a 6-month multi-strain probiotics supplementation in endotoxemic, inflammatory and cardiometabolic status of T2DM patients: a randomized, double-blind, placebo-controlled trial. Clin Nutr. 2019;38(4):1561–9. [DOI] [PubMed] [Google Scholar]
- 10. Ivey KL, Hodgson JM, Kerr DA, Lewis JR, Thompson PL, Prince RL. The effects of probiotic bacteria on glycaemic control in overweight men and women: a randomised controlled trial. Eur J Clin Nutr. 2014;68(4):447–52. [DOI] [PubMed] [Google Scholar]
- 11. Ivey KL, Hodgson JM, Kerr DA, Thompson PL, Stojceski B, Prince RL. The effect of yoghurt and its probiotics on blood pressure and serum lipid profile; a randomised controlled trial. Nutr Metab Cardiovasc Dis. 2015;25(1):46–51. [DOI] [PubMed] [Google Scholar]
- 12. Kobyliak N, Falalyeyeva T, Mykhalchyshyn G, Kyriienko D, Komissarenko I. Effect of alive probiotic on insulin resistance in type 2 diabetes patients: randomized clinical trial. Diabetes Metab Syndr. 2018;12(5):617–24. [DOI] [PubMed] [Google Scholar]
- 13. Mazloom Z, Yousefinejad A, Dabbaghmanesh MH. Effect of probiotics on lipid profile, glycemic control, insulin action, oxidative stress, and inflammatory markers in patients with type 2 diabetes: a clinical trial. Iran J Med Sci. 2013;38(1):38–43. [PMC free article] [PubMed] [Google Scholar]
- 14. He J, Zhang F, Han Y. Effect of probiotics on lipid profiles and blood pressure in patients with type 2 diabetes: a meta-analysis of RCTs. Medicine (Baltimore). 2017;96(51):e9166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kasinska MA, Drzewoski J. Effectiveness of probiotics in type 2 diabetes: a meta-analysis. Pol Arch Med Wewn. 2015;125(11):803–13. [DOI] [PubMed] [Google Scholar]
- 16. Li C, Li X, Han H, Cui H, Peng M, Wang G, Wang Z. Effect of probiotics on metabolic profiles in type 2 diabetes mellitus: a meta-analysis of randomized, controlled trials. Medicine (Baltimore). 2016;95(26):e4088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Samah S, Ramasamy K, Lim SM, Neoh CF. Probiotics for the management of type 2 diabetes mellitus: a systematic review and meta-analysis. Diabetes Res Clin Pract. 2016;118:172–82. [DOI] [PubMed] [Google Scholar]
- 18. Tao YW, Gu YL, Mao XQ, Zhang L, Pei YF. Effects of probiotics on type II diabetes mellitus: a meta-analysis. J Transl Med. 2020;18(1):30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yao K, Zeng L, He Q, Wang W, Lei J, Zou X. Effect of probiotics on glucose and lipid metabolism in type 2 diabetes mellitus: a meta-analysis of 12 randomized controlled trials. Med Sci Monit. 2017;23:3044–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhang Q, Wu Y, Fei X. Effect of probiotics on glucose metabolism in patients with type 2 diabetes mellitus: a meta-analysis of randomized controlled trials. Medicina (B Aires). 2016;52(1):28–34. [DOI] [PubMed] [Google Scholar]
- 21. Rittiphairoj T, Pongpirul K, Mueller NT, Li T. Probiotics for glycemic control in patients with type 2 diabetes mellitus: protocol for a systematic review. Syst Rev. 2019;8(1):227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Higgins JPT, Savović J, Page MJ, Elbers RG, Sterne JAC. Chapter 8: Assessing risk of bias in a randomized trialIn: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane; 2019. [Google Scholar]
- 23. Sterne JAC, Savovic J, Page MJ, Elbers RG, Blencowe NS, Boutron I, Cates CJ, Cheng HY, Corbett MS, Eldridge SMet al. . RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. [DOI] [PubMed] [Google Scholar]
- 24. Doron S, Snydman DR. Risk and safety of probiotics. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2015;60(Suppl 2):S129–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ejtahed HS, Mohtadi-Nia J, Homayouni-Rad A, Niafar M, Asghari-Jafarabadi M, Mofid V, Akbarian-Moghari A. Effect of probiotic yogurt containing Lactobacillus acidophilus and Bifidobacterium lactis on lipid profile in individuals with type 2 diabetes mellitus. J Dairy Sci. 2011;94(7):3288–94. [DOI] [PubMed] [Google Scholar]
- 26. Tripolt NJ, Leber B, Blattl D, Eder M, Wonisch W, Scharnagl H, Stojakovic T, Obermayer-Pietsch B, Wascher TC, Pieber TRet al. . Short communication: effect of supplementation with Lactobacillus casei Shirota on insulin sensitivity, beta-cell function, and markers of endothelial function and inflammation in subjects with metabolic syndrome—a pilot study. J Dairy Sci. 2013;96(1):89–95. [DOI] [PubMed] [Google Scholar]
- 27. Barreto FM, Colado Simao AN, Morimoto HK, Batisti Lozovoy MA, Dichi I, Helena da Silva Miglioranza L. Beneficial effects of Lactobacillus plantarum on glycemia and homocysteine levels in postmenopausal women with metabolic syndrome. Nutrition. 2014;30(7–8):939–42. [DOI] [PubMed] [Google Scholar]
- 28. Shakeri H, Hadaegh H, Abedi F, Tajabadi-Ebrahimi M, Mazroii N, Ghandi Y, Asemi Z. Consumption of synbiotic bread decreases triacylglycerol and VLDL levels while increasing HDL levels in serum from patients with type-2 diabetes. Lipids. 2014;49(7):695–701. [DOI] [PubMed] [Google Scholar]
- 29. Jung SJ, Park SH, Choi EK, Cha YS, Cho BH, Kim YG, Kim MG, Song WO, Park TS, Ko JKet al. . Beneficial effects of Korean traditional diets in hypertensive and type 2 diabetic patients. J Med Food. 2014;17(1):161–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tajadadi-Ebrahimi M, Bahmani F, Shakeri H, Hadaegh H, Hijijafari M, Abedi F, Asemi Z. Effects of daily consumption of synbiotic bread on insulin metabolism and serum high-sensitivity C-reactive protein among diabetic patients: a double-blind, randomized, controlled clinical trial. Ann Nutr Metab. 2014;65(1):34–41. [DOI] [PubMed] [Google Scholar]
- 31. Ostadrahimi A, Taghizadeh A, Mobasseri M, Farrin N, Payahoo L, Beyramalipoor Gheshlaghi Z, Vahedjabbari M. Effect of probiotic fermented milk (kefir) on glycemic control and lipid profile in type 2 diabetic patients: a randomized double-blind placebo-controlled clinical trial. Iran J Public Health. 2015;44(2):228–37. [PMC free article] [PubMed] [Google Scholar]
- 32. Bayat A, Azizi-Soleiman F, Heidari-Beni M, Feizi A, Iraj B, Ghiasvand R, Askari G. Effect of Cucurbita ficifolia and probiotic yogurt consumption on blood glucose, lipid profile, and inflammatory marker in type 2 diabetes. Int J Prev Med. 2016;7:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bernini LJ, Simao AN, Alfieri DF, Lozovoy MA, Mari NL, de Souza CH, Dichi I, Costa GN. Beneficial effects of Bifidobacterium lactis on lipid profile and cytokines in patients with metabolic syndrome: a randomized trial. Effects of probiotics on metabolic syndrome. Nutrition. 2016;32(6):716–9. [DOI] [PubMed] [Google Scholar]
- 34. Sato J, Kanazawa A, Azuma K, Ikeda F, Goto H, Komiya K, Kanno R, Tamura Y, Asahara T, Takahashi Tet al. . Probiotic reduces bacterial translocation in type 2 diabetes mellitus: a randomised controlled study. Sci Rep. 2017;7(1):12115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mobini R, Tremaroli V, Stahlman M, Karlsson F, Levin M, Ljungberg M, Sohlin M, Berteus Forslund H, Perkins R, Backhed Fet al. . Metabolic effects of Lactobacillus reuteri DSM 17938 in people with type 2 diabetes: a randomized controlled trial. Diabetes Obes Metab. 2017;19(4):579–89. [DOI] [PubMed] [Google Scholar]
- 36. Firouzi S, Majid HA, Ismail A, Kamaruddin NA, Barakatun-Nisak MY. Effect of multi-strain probiotics (multi-strain microbial cell preparation) on glycemic control and other diabetes-related outcomes in people with type 2 diabetes: a randomized controlled trial. Eur J Nutr. 2017;56(4):1535–50. [DOI] [PubMed] [Google Scholar]
- 37. Tonucci LB, Olbrich Dos Santos KM, Licursi de Oliveira L, Rocha Ribeiro SM, Duarte Martino HS. Clinical application of probiotics in type 2 diabetes mellitus: a randomized, double-blind, placebo-controlled study. Clin Nutr. 2017;36(1):85–92. [DOI] [PubMed] [Google Scholar]
- 38. Feizollahzadeh S, Ghiasvand R, Rezaei A, Khanahmad H, Sadeghi A, Hariri M. Effect of probiotic soy milk on serum levels of adiponectin, inflammatory mediators, lipid profile, and fasting blood glucose among patients with type II diabetes mellitus. Probiotics Antimicro Prot. 2017;9(1):41–7. [DOI] [PubMed] [Google Scholar]
- 39. Yuan T, Zhao W, Cao Y, Li Q, Yao M, Hao X, Yu H, Jiang C, Wang H, Wang Set al. . Effect of Bifidobacterium tetragenous viable bacteria tablets on blood glucose level in patients with type 2 diabetes mellitus. Chinese J Clin Nutr. 2017;25(4):205–13. [DOI] [PubMed] [Google Scholar]
- 40. Raygan F, Rezavandi Z, Bahmani F, Ostadmohammadi V, Mansournia MA, Tajabadi-Ebrahimi M, Borzabadi S, Asemi Z. The effects of probiotic supplementation on metabolic status in type 2 diabetic patients with coronary heart disease. Diabetol Metab Syndr. 2018;10:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kassaian N, Feizi A, Aminorroaya A, Jafari P, Ebrahimi MT, Amini M. The effects of probiotics and synbiotic supplementation on glucose and insulin metabolism in adults with prediabetes: a double-blind randomized clinical trial. Acta Diabetol. 2018;55(10):1019–28. [DOI] [PubMed] [Google Scholar]
- 42. Mazruei Arani N, Emam-Djomeh Z, Tavakolipour H, Sharafati-Chaleshtori R, Soleimani A, Asemi Z. The effects of probiotic honey consumption on metabolic status in patients with diabetic nephropathy: a randomized, double-blind, controlled trial. Probiotics Antimicro Prot. 2019;11(4):1195–201. [DOI] [PubMed] [Google Scholar]
- 43. Naito E, Yoshida Y, Kunihiro S, Makino K, Kasahara K, Kounoshi Y, Aida M, Hoshi R, Watanabe O, Igarashi Tet al. . Effect of Lactobacillus casei strain Shirota-fermented milk on metabolic abnormalities in obese prediabetic Japanese men: a randomised, double-blind, placebo-controlled trial. Biosci Microbiota Food Health. 2018;37(1):9–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Razmpoosh E, Javadi A, Ejtahed HS, Mirmiran P, Javadi M, Yousefinejad A. The effect of probiotic supplementation on glycemic control and lipid profile in patients with type 2 diabetes: a randomized placebo controlled trial. Diabetes Metab Syndr. 2019;13(1):175–82. [DOI] [PubMed] [Google Scholar]
- 45. Khalili L, Alipour B, Asghari Jafar-Abadi M, Faraji I, Hassanalilou T, Mesgari Abbasi M, Vaghef-Mehrabany E, Alizadeh Sani M. The effects of Lactobacillus casei on glycemic response, serum sirtuin1 and fetuin-a levels in patients with type 2 diabetes mellitus: a randomized controlled trial. Iran Biomed J. 2019;23(1):68–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Cani PD, Bibiloni R, Knauf C, Waget A, Neyrinck AM, Delzenne NM, Burcelin R. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes. 2008;57(6):1470–81. [DOI] [PubMed] [Google Scholar]
- 47. Harkins CP, Kong HH, Segre JA. Manipulating the human microbiome to manage disease. JAMA. 2020;323(4):303–4. [DOI] [PubMed] [Google Scholar]
- 48. Noble EE, Hsu TM, Kanoski SE. Gut to brain dysbiosis: mechanisms linking Western diet consumption, the microbiome, and cognitive impairment. Front Behav Neurosci. 2017;11:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Cani PD, Everard A, Duparc T. Gut microbiota, enteroendocrine functions and metabolism. Curr Opin Pharmacol. 2013;13(6):935–40. [DOI] [PubMed] [Google Scholar]
- 50. Tolhurst G, Heffron H, Lam YS, Parker HE, Habib AM, Diakogiannaki E, Cameron J, Grosse J, Reimann F, Gribble FM. Short-chain fatty acids stimulate glucagon-like peptide-1 secretion via the G-protein-coupled receptor FFAR2. Diabetes. 2012;61(2):364–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Mueller NT, Zhang M, Juraschek SP, Miller ER, Appel LJ. Effects of high-fiber diets enriched with carbohydrate, protein, or unsaturated fat on circulating short chain fatty acids: results from the OmniHeart randomized trial. Am J Clin Nutr. 2020. doi: 10.1093/ajcn/nqz322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Cernea S, Dobreanu M. Diabetes and beta cell function: from mechanisms to evaluation and clinical implications. Biochem Med. 2013;23(3):266–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kankaanpaa PE, Salminen SJ, Isolauri E, Lee YK. The influence of polyunsaturated fatty acids on probiotic growth and adhesion. FEMS Microbiol Lett. 2001;194(2):149–53. [DOI] [PubMed] [Google Scholar]
- 54. Pelto L, Isolauri E, Lilius EM, Nuutila J, Salminen S. Probiotic bacteria down-regulate the milk-induced inflammatory response in milk-hypersensitive subjects but have an immunostimulatory effect in healthy subjects. Clin Exp Allergy. 1998;28(12):1474–9. [DOI] [PubMed] [Google Scholar]
- 55. Roessler A, Friedrich U, Vogelsang H, Bauer A, Kaatz M, Hipler UC, Schmidt I, Jahreis G. The immune system in healthy adults and patients with atopic dermatitis seems to be affected differently by a probiotic intervention. Clin Exp Allergy. 2008;38(1):93–102. [DOI] [PubMed] [Google Scholar]
- 56. Suwal S, Wu Q, Liu W, Liu Q, Sun H, Liang M, Gao J, Zhang B, Kou Y, Liu Zet al. . The probiotic effectiveness in preventing experimental colitis is correlated with host gut microbiota. Front Microbiol. 2018;9:2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Suez J, Zmora N, Segal E, Elinav E. The pros, cons, and many unknowns of probiotics. Nat Med. 2019;25(5):716–29. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.