ABSTRACT
Despite increasing evidence for the association of food-based dietary patterns with breast cancer risk, knowledge about the shape of the relationship and the quality of meta-evidence are insufficient. We aimed to summarize the associations between food groups and risks of breast cancer. We performed a systematic literature search of the PubMed and Embase databases up to March 2020. We included cohort, case-cohort, nested case-control studies, and follow-up studies of randomized controlled trials that investigated the relationship between breast cancer risk and at least 1 of the following food groups: red meat, processed meat, fish, poultry, egg, vegetables, fruit, dairy product (overall, milk, yogurt, and cheese), grains/cereals, nuts, legumes, soy, and sugar-sweetened beverages. Summary risk ratios (RRs) and 95% CIs were estimated using a random-effects model for linear and nonlinear relationships. Inverse linear associations were observed for vegetables (RR per 100 g/d, 0.97; 95% CI, 0.95–0.99), fruit (RR per 100 g/d, 0.97; 95% CI, 0.95–0.99), cheese (RR per 30 g/d, 0.95; 95% CI, 0.91–1.00), and soy (RR per 30 g/d, 0.96; 95% CI, 0.94–0.99), while positive associations were observed for red (RR per 100 g/d, 1.10; 95% CI, 1.03–1.18) and processed meat (RR per 50 g/d, 1.18; 95% CI, 1.04–1.33). None of the other food groups were significantly associated with breast cancer risk. A nonlinear association was observed only for milk, such that the intake of >450 g/d increased the risk, while no association was observed for lower intake amounts. High intakes of vegetables, fruit, cheese, and soy products and low intakes of red and processed meat were associated with lower risks of breast cancer. However, causality cannot be inferred from these statistical correlations.
Keywords: breast cancer, hormone receptor-positive, ER/PR-positive, refined grain, whole grain
Introduction
Breast cancer is the most commonly diagnosed cancer among females and the leading cause of cancer-related death in women. In 2018, 2.1 million new breast cancer cases were estimated, accounting for approximately 11.6% of all cancers in the world (1), whilst in the same time-period, an estimated 600 000 deaths occurred worldwide in 2018, accounting for 6.6% of deaths from all cancer types (2).
Knowledge of the etiology of breast cancer is still limited (2), but a variety of modifiable and nonmodifiable risk factors have been identified. Indeed, race, ethnicity, family history of cancer, and genetic traits have been identified as important nonmodifiable risk factors in epidemiologic studies. However, modifiable risk factors have also been identified, such as increased alcohol consumption, physical inactivity, exogenous hormone uses, and certain female reproductive factors, such as pregnancy and age at first birth (3). Importantly, the potential role of diet on the risk of breast cancer has been examined in a large volume of epidemiologic studies; however, the specific associations between numerous specific food groups and breast cancer risks are relatively unclear.
Indeed, multiple systematic reviews and meta-analyses have evaluated the association of single food groups with breast cancer risks, and most of the prior meta-analyses have only compared breast cancer risks in the highest versus lowest intakes of selected food groups. Moreover, multiple systematic reviews have examined dietary patterns (i.e., multiple food groups in combination) and breast cancer risks, finding moderate evidence to indicate that dietary patterns rich in vegetables, fruits, and whole grains and lower in animal-source foods and refined carbohydrates are correlated with decreased risks of postmenopausal breast cancer. The data pertaining to these dietary patterns and premenopausal breast cancer risks follow the same direction, but the evidence remains insufficient since few studies include premenopausal breast cancer (4, 5). However, the present study seeks to strengthen the field by taking a novel approach to examining individual foods/food groups. Thus, the objective of our comprehensive meta-analysis was to assess the shape of the diet/breast cancer relationship by performing linear and nonlinear does-response analyses. We estimated the summary associations between intake of 13 food groups [as defined by the Schwingshackl et al. methodology (6)] and breast cancer risks.
Methods
The protocol of this meta-analysis has been registered in the International Prospective Register of Systematic Reviews (PROSPERO; www.crd.york.ac.uk/prospero/index.asp; identifier CRD42019144956). This systematic review was developed based on the standards of the Meta-Analysis of Observational Studies in Epidemiology guidelines (7).
Study selection
To be eligible for inclusion, studies were required to: 1) be of cohort, case-cohort, or nested case-control design, including follow-up studies of randomized controlled trials (RCTs); 2) provide data on the association between the risk of breast cancer and at least 1 of the following 13 food groups: grains/cereals, vegetables, fruit, eggs, dairy products (overall or milk, yogurt, and cheese), fish, poultry, red meat, processed meat, nuts, legumes, soy product, sugar-sweetened beverages (SSB); 3) include participants aged ≥18 y; and 4) assess dietary intake at the beginning of the study. When dietary intake was assessed during adolescence or early adulthood, the study was not included in our meta-analysis. If ≥2 studies were published on the same exposure-outcome pair, we included only the most recent study with the longest follow-up, and thus the greatest number of events. Moreover, studies that only investigated the highest versus lowest categories were excluded. We also excluded studies conducted on micro- and macronutrients (i.e., soy fiber or phytoestrogen), and focused our evaluation on dietary groups. Studies that only assessed cancer recurrence or survivorship as the outcome were excluded, and studies with case-control and cross-sectional designs and RCTs and non-RCTs were excluded. We imposed no limitation or restriction on the geographical location and health status of participants.
Search strategy
Articles published through March 2020 and indexed in PubMed and Embase were searched for prospective studies, based on the above inclusion criteria, with no language restriction. The search terms used as keywords in the search strategy are listed in Supplemental Table 1. In addition, the bibliographies of all relevant prior reviews and primary studies identified by the electronic search strategy were scanned for relevant papers.
Data extraction
Our 2 reviewers independently extracted the following information: name of first author, year of publication, country, cohort name, age at entry, menopause status, sample size, total cases, dietary assessment, outcome, outcome assessment, type and quantity of food group, adjustment factors, duration of study, and risk estimate [risk ratios (RRs), HRs, or ORs with their corresponding 95% CIs]. Results for the fully adjusted model were extracted as the preferential data for our analyses. When a study did not report sufficient information for data extraction, we contacted the corresponding author by e-mail at least 2 times, 1 week apart; accordingly, we attained additional data for 2 papers using this method (8, 9). For the linear dose-response relationship, no studies were excluded because of incomplete data. But for the nonlinear analysis, 9 studies did not report the number of cases in each category and 1 study did not provide data on the amount of dietary intake in each category. Since we could not obtain required data after contacting the corresponding authors, we excluded these studies from the nonlinear analysis.
Risk of bias assessment and quality of evidence
We used the Newcastle-Ottawa Scale to assess the methodological quality of included studies (10). We examined 3 main domains—selection, comparability, and outcome—to rate the quality of studies. In the selection domain, 4 items were assessed: representativeness of the exposed cohort, selection of the nonexposed cohort, ascertainment of exposure, and demonstration that the outcomes were not present at the start of the study. In the comparability domain, the control of confounders in the design or analysis of the studies was checked. Finally, in the outcome domain, the outcomes ascertainment, duration of follow-up, and adequacy of follow-up of cohorts were considered. If a study received 3–4 stars in the selection domain, 1–2 stars in the comparability domain, and 2–3 stars in the outcome domain, the quality was rated as good. If a study received 2 stars in the selection domain, 1–2 stars in the comparability domain, and 2–3 stars in the outcome domain, the quality was rated as fair. If a study received 0–1 star in the selection domain, 0 stars in the comparability domain, or 0–1 star in the outcome domain, the quality was rated as poor.
The overall quality of the studies included in this meta-analysis was also evaluated by the use of the NutriGrade scoring system (11), which comprises the following items: 1) risk of bias, study quality, and study limitations (0–2 points); 2) precision (0–1 point); 3) heterogeneity (0–1 point); 4) directness (0–1 point); 5) publication bias (0–1 point); 6) funding bias (0–1 point); 7) effect size (+ 2 points); and 8) dose response (+1 point). This scoring system recommends 4 categories to define the meta-evidence as high (≥ 8 points), moderate (6–7.99 points), low (4–5.99 points), or very low (0–3.99 points).
Statistical analysis
We used HRs and 95% CIs as the effect sizes for all analyses. The reported RRs or ORs in the primary studies were considered to be equal to HRs. The dose-response meta-analysis was performed using the method proposed by Greenland and Longnecker (12) and Orsini et al. (13) and consists of 2 parts: linear analysis and nonlinear analysis. Using a random-effects model, we performed a linear dose-response meta-analysis by pooling the HRs for each increment of 100 grams of meat, poultry, fish, fruit, and vegetable intake; 50 grams of processed meat, egg, fruit juice, and legume intake; 200 grams of dairy (as a whole), milk, and yogurt intake; 30 grams of cheese and soy intake; 20 grams of cereals intake; and 28 grams of nut intake.
To assess the nonlinear dose-response relationship, a 2-stage hierarchical regression model was used, in which the difference between category-specific and reference-specific doses, expressed in quadratic terms, was calculated. Then, the dose-response association, considering within- and between-study variances, was estimated through the use of spline transformations. This method requires the distribution of cases and noncases across >3 categories of food groups, using the median value and the adjusted RRs with their 95% CIs for each category of exposure. For the estimation quantity of food consumption, the median intake of each food group was used. If a study reported both the mean and median of the group, we used the median. Only mean intakes were reported in 11 papers, so for these studies the mean intake was used. In instances where the amount of food intake in each category was reported in the closed interval, consumption was considered as the midpoint of the interval. For the open-ended exposure categories, we considered the length of the open-ended interval to be the same as that of the adjacent interval. We set 2-sided statistical significance a priori at P < 0.05.
The Q test and the I2 statistic (with a value of I2 >50% considered to represent potentially important statistical heterogeneity) was used to explore heterogeneity between studies. To discern the source of heterogeneity, we performed subgroup analyses of potential influencing factors, including menopause status, presence of estrogen receptor, follow-up duration, geographical location, number of cases, and characteristics of the food items (e.g., high- vs. low-fat content or whole vs. refined grain). However, it was not possible to perform subgroup analyses by all of these factors for all of food groups, because in some cases fewer than 2 studies were in a subgroup or the primary studies did not report the results appropriately; for example, for milk, some primary studies reported data separately according to the fat content (low- vs. high-fat intake) for dairy, yogurt, cheese, and meat, while some studies did not report results according to the fat content.
If at least 10 studies were available, we explored potential small-study effects, such as publication bias, by using Egger's test and funnel plots. Stata version 13 software was used to conduct all statistical analyses.
Results
As detailed in Figure 1, 7635 records were obtained following the literature search. Of these, 210 articles were potentially relevant for inclusion in the meta-analysis because they reported ≥1 of the 13 food groups and breast cancer risk in the title or abstract. Finally, the number of studies included in the meta-analysis for each food group were as follows: total meat: 13; red meat: 20; processed meat: 17; poultry: 13; fish: 17; egg: 11; fruit: 15; vegetable: 14; dairy: 10; milk: 13; yogurt: 6; cheese: 10; total cereals (both whole and refined): 14; soy and soy products: 7; nuts: 6; and legumes: 4. The number of studies on SSB was not adequate. The included studies were performed in Asia, Europe, North America, and Australia (1 study), and characteristics of all studies are presented in Table 1.
FIGURE 1.
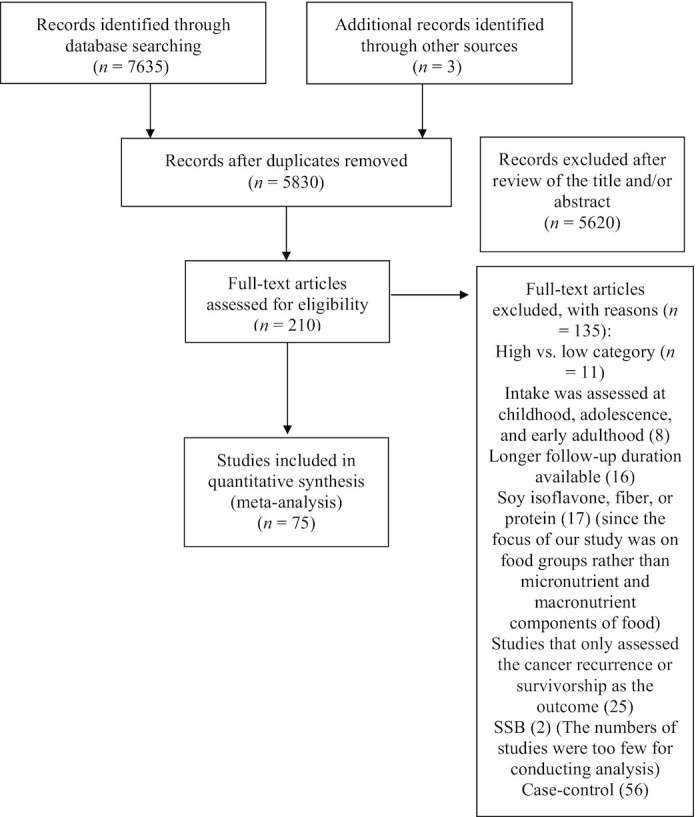
Flowchart of study selection.
TABLE 1.
General study characteristics of the included studies investigating the association between various food groups and risk of breast cancer
| Author, year | Study design (n follow-ups) | Cohort name | Participants, n | Age, y1 | Follow-up rate, %2 | Cases, n | Interested exposure | Exposure assessment (n items) | Outcome assessment | Adjusted covariate |
|---|---|---|---|---|---|---|---|---|---|---|
| Adebamowo et al., 2005 (47) | Prospective cohort (8) | Nurses' Health Study (NHS) II, USA | 90 638 | 57.8 | 90 | 710 | Bean and lentil | Validated FFQ (130) | Cancer registration system | Age, study area, family history of breast cancer, age at menopause, age at first birth, parity, use of exogenous female hormone, smoking, consumption of green leafy vegetables, walking time, BMI, and total EI |
| Anderson et al., 2018 (34) | Prospective cohort (7) | UK Biobank | 258 922 | 40–69 | NM | 9701 | Red meat, processed meat | Not validated FFQ (NM) | Linkage to 3 routine administrative databases | Age, deprivation and ethnic group, smoking, alcohol, BMI and physical activity, consumption of cooked and raw vegetables, and type of bread |
| Boggs et al., 2010 (48) | Prospective cohort (12) | Black Women's Health Study (BWHS), USA | 51 928 | 38.9 | 85 | 1597 | Total fruit and vegetable, yellow orange | Validated FFQ (85) | Medical record or cancer registry data | Age, energy intake, age at menarche, BMI at age 18 y, family history of breast cancer, education, geographic region, parity, age at first birth, OCP use, menopausal status, age at menopause, menopausal hormone use, vigorous activity, smoking status, alcohol intake, and multivitamin use |
| Butler et al., 2010 (49) | Prospective cohort (10.7) | Singapore Chinese Health Study (SCHS), Singapore | 34 028 Post | 55 | NM | 629 | Vegetable, soy food | Validated FFQ (165) | Cancer registry | Age at interview, dialect group, interview year, education, parity, BMI, first-degree relative with diagnosis of breast cancer, EI |
| Cho et al., 2006 (29) | Prospective cohort (12) | NHS II, USA | 349 573 Pre | 36 | >90 | 10 722 | Red meat | Validated FFQ (130) | Self-reported | Age at start of follow-up and calendar year of the current questionnaire cycle and was simultaneously adjusted for smoking, height, parity and age at first birth, BMI, age at menarche, family history of breast cancer, history of benign breast disease, OCP use, alcohol intake, EI |
| Couto et al., 2013 (35) | Prospective cohort (16) | Women's Lifestyle and Health (WLH),Sweden | 44 840 (40 031 Pre and 27 509 Post) | 30–49 | 93 | 1278 | Cereals, fruits and nuts, vegetable, dairy products, red meat, fish | Validated FFQ (80) | National cancer register | History of breast cancer in mother and/or sister(s), personal history of benign breast disease, smoking status, BMI, height, age at first birth and number of children, educational level, age at menarche, total energy intake, and consumption of beverages, potatoes, sweets, and eggs |
| Daniel et al., 2011 (46) | Prospective cohort (9) | National Institute of Health-American Association of Retired Persons (NIH-AARP), USA | 184 488 Post | >18 | 90 | 7181 | Fish | Validated FFQ (124) | Linking cohort members to state cancer registries and to the US National Death Index | EI, age at entry, BMI, age at first menstrual period, age at first live birth, family history of breast cancer, HRT, education, race, saturated fat, alcohol intake, physical activity, smoking, age at menopause, number of breast biopsies, height |
| Dunneram et al., 2019 (15) | Prospective cohort (18) | UK Women's Cohort | 29 183 | 53.2 | NM | 1625 | Red meat, processed meat, fish, poultry, egg | Validated FFQ (217) | National Health Service Central Register | Age, ethanol intake, duration of breastfeeding, physical activity, smoking, social class, menopausal status |
| Diallo et al., 2018 (50) | Prospective cohort (4.1) | NutriNet-Sante, France | 590 742 | 51.7 | 87 | 13 010 | Red meat, processed meat | Validated web based 24-hr dietary records | Medical records | Age, energy intake without alcohol, number of 24-hour dietary records, smoking, educational level, physical activity, height, BMI, alcohol intake, family history of cancers, lipids intake, fruits, vegetables, hormone replacement therapy, number of children, contraception, red meat intake, and processed meat intake |
| Egeberg et al., 2008 (16) | Nested case-control (4.2) | Diet, Cancer and Health (DCH), Denmark | 1134 Post | 57 | NM | 378 | Total meat, red meat, poultry, processed meat, fish | Validated FFQ (192) | Danish Cancer Registry | Parity, age at first birth, education, duration of HRT use, intake of alcohol, and BMI |
| Egeberg et al., 2009 (51) | Prospective cohort (9.6) | DCH, Denmark | 25 278 Post | 56 | 100 | 978 | Whole-grain product, | Validated FFQ (192) | Danish Cancer Registry | Parity, age at first birth, education, duration of HRT use, use of HRT, intake of alcohol, and BMI |
| Emaus et al., 2016 (52) | Prospective cohort (11.5) | European Prospective Investigation into Cancer and Nutrition (EPIC), European countries | 335 054 | 50.8 | 100 | 10 197 | Fruit, vegetable | Validated FFQ (260), UK and Sweden (FFQ and a 7-day record) | Record linkage and cancer registries | Stratified by age and center and adjusted for energy intake divided into energy from fat and energy from nonfat sources, saturated fat intake, age at menarche, oral contraceptive use, age at first full-term pregnancy, menopausal status, hormone replacement therapy use, BMI, BMI at menopause, physical activity, smoking status and intensity, alcohol user (yes or no), alcohol consumption, educational level |
| Engeset et al., 2006 (53) | Prospective cohort (6.4) | EPIC, European countries | 1 932 107 | >18 | NM | 4776 | Fish | Validated FFQ (260), UK and Sweden (FFQ and a 7-day record) | Health insurance and cancer and pathology registries | Stratified by center, adjusted for time of follow-up, energy intake from fat, EI from CHO and protein, alcohol intake, height, weight, age at menarche, number of full-term pregnancies and age at first FTP, current use of hormone replacement therapy, current use of OCP, and menopausal status |
| Farvid et al., 2014 (36) | Prospective cohort (20) | NHS II, USA | 88 803 | 36.4 | 95 | 2826 | Egg, total red meat (unprocessed and processed), poultry (chicken and turkey), fish, nut | Validated FFQ (130) | Self-reported + review of pathology reports | Age at start of follow-up and calendar year of current questionnaire cycle and was simultaneously adjusted for race, family history of breast cancer in mother or sisters, history of benign breast disease, smoking, BMI at menarche, parity and age at first birth, OCP use, alcohol intake, and energy. In Post women, we additionally adjusted for postmenopausal hormone use and age at menopause |
| Farvid et al., 2016 (54) | Prospective cohort (11.5) | NHS II, USA | 90 476 | 27–44 | 98 | 6459 | Fruit, vegetable, whole-grain, total refined-grain food intake | Validated FFQ (130) | Self-report | Race, family history of breast cancer in mother or sisters, history of benign breast disease, smoking, height, BMI at age 18, weight change since age 18, age at menarche, parity and age at first birth, OCP use, alcohol intake, and energy. In postmenopausal women, additionally adjusted for hormone use, age at menopause, hormone use and menopausal status, and age at menopause |
| Farvid et al., 2018 (55) | Prospective cohort (22) | NHS II, USA | 90 503 (56 231 Pre and 34 272 Post) | 36.5 | 96 | 3191 (1706 Pre- and 1134 Postmenopausal) | Dairy | Validated FFQ (130) | Self-report | Smoking, race, parity and age at first birth, height, BMI at age 18 y, weight change since age 18, age at menarche, family history of breast cancer, history of benign breast disease, OCP use, adolescent alcohol intake, adult alcohol intake, physical activity, adolescent energy intake. In postmenopausal women, additional adjustment for hormone use and age at menopause. Among all women, additionally adjusted for hormone use and menopausal status and age at menopause |
| Farvid et al., 2019 (56) | Prospective cohort (23.7) | NHS I and NHS II, USA | 93 844 | 36 | 96 | 3300 | Fruits, fruit juice, vegetable | NHS II: Validated FFQ (130), NHS I: Validated FFQ (61) | Hospital records and pathology reports | Family history of breast cancer, history of benign breast disease, height, BMI at age 18 y, weight change since age 18, smoking, physical activity, OCP, alcohol intake, total EI, age at menarche, parity and age at first birth, and menopausal status, age at menopause, and MHT |
| Ferrucci et al., 2009 (37) | Prospective cohort (5.5) | Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial (PLCO), USA | 52 158 | 65.2 | NM | 6464 | Red meat, white meat, poultry, processed meat | Validated FFQ (124) | Self-report and physician reports | Age, race, education, study center, randomization group, family history of breast cancer, age at menarche and menopause, age at first birth and number of live births, history of benign breast disease, number of mammograms during past 3 y, menopausal hormone therapy, BMI, alcohol, total fat, and EI |
| Folsom and Demissie, 2004 (57) | Prospective cohort (14) | Iowa Women's Health Study (IWHS), USA | 3 377 526 Post | 55–69 | NM | 10 147 | Fish | Validated FFQ (127) | Linkage of cohort identifiers to Iowa State–wide cancer incidence and death records | Age, EI, educational level, physical activity level, alcohol consumption, smoking, pack-years of cigarette smoking, age at first live birth, estrogen use, vitamin use, BMI, waist/hip ratio, diabetes, hypertension, and intake of whole grains, fruit and vegetables, red meat, cholesterol, and saturated fat |
| Fung et al., 2011 (58) | Prospective cohort (26) | NHS I, USA | 75 929 | 38–63 | >90 | 792 | Total fruit and vegetable, fruit juice, whole grain, nut | Validated FFQ (61) | Self-report | Age, energy intake, smoking, alcohol, weight change since age 18, height, postmenopausal hormone use, physical activity, BMI at age 18, family history of benign breast disease, modified Alternate Mediterranean Diet score |
| Gaard et al., 1995 (17) | Prospective cohort (6) | Norwegian Women Study, Norway | 24 897 | 43 | 100 | 248 | Total meat, egg, milk | Validated FFQ (50) | Linkage to cancer registry | Age |
| Genkinger et al., 2013 (18) | Prospective cohort (12) | BWHS, USA | 52 062 | 38.8 | 85 | 1516 | Total meat, red meat, white meat, processed meat, fish, milk, cheese | Validated FFQ (85) | Medical record or cancer registry data | Energy intake, age at menarche, BMI, family history of breast cancer, education, parity and age at first live birth, OCP use, menopausal status, age at menopause, menopausal hormone use, vigorous physical activity, smoking status, and alcohol intake |
| George et al., 2009 (59) | Prospective cohort (8) | NIH-AARP Diet and Health STUDY | 195 229 Post | 61.5 | 90 | 5815 | Fruit and vegetable | Validated FFQ, 124 food items | Cancer registry | Age, smoking, energy intake, BMI, alcohol, physical activity, education, race, marital status, family history of cancer, menopausal HRT, mutual adjustment between fruit and vegetables |
| Giles et al., 2006 (60) | Prospective cohort (9.1) | Melbourne Collaborative Cohort Study (MCCS), Australia | 12 273 Post | 40–69 | 100 | 245 | All cereal products, rice | Validated FFQ (122) | Cancer registry and medical records | Age at attendance, country of birth, total energy intake, and HRT |
| Haraldsdottir et al., 2017 (61) | Prospective cohort (27.3) | Reykjavik Study, Iceland | 9340 | 40–50 | 100 | 744 | Fish | Validated FFQ (NM) | Cancer registry | Age, education, family history of breast cancer, BMI in midlife, age at first child, age at menarche, and intake of milk, rye, meat, fish liver oil, fish, salted/smoked fish, alcohol |
| Harris et al., 2015 (30) | Prospective cohort (15) | Swedish Mammography Cohort (SMC), Sweden | 37 004 | 61.8 | 100 | 1603 | Red meat | Validated FFQ (96) | Linkage of the study cohort with Swedish cancer register | Age, energy intake, height, BMI, education, OCP use, HRT, age at menarche, age at menopause, family history of breast cancer, history of benign breast disease, smoking status, physical activity, and alcohol intake |
| Hirvonen et al., 2006 (62) | Prospective cohort (6.6) | Supplementation in Vitamins and Mineral Antioxidants (SUVIMAX), France | 91 016 | 49.9 | NM | 5294 | Fruit juice | 24-hour dietary record every 2 months | Self-report | Age, smoking, number of children, use of OCP, family history of breast cancer, and menopausal status |
| Hjartåker et al., 2010 (63) | Prospective cohort (8.6) | Norwegian Women and Cancer Study (NOWAC), Norway | 64 903 (36 605 Pre and 28 298 Post) | 51 | 100 | 947 (151 Pre and 796 Post) | Dairy, milk, yogurt, cheese | Validated FFQ (NM) | Cancer registry | Age, energy intake, alcohol intake, height, weight, increase since age 18, level of physical activity, years of education, maternal history of breast cancer, mammography practice, and use of OCP |
| Holmes et al., 2003 (19) | Prospective cohort (18) | NHS I, USA | 88 647 | 46.7 | 98 | 4107 | Total meat, red meat, white meat, processed meat, egg, fish | Validated FFQ (61) | Self-report | Age, 2-y time period; total EI; alcohol intake; parity and age at first birth categories; BMI at age 18 in kg/m2; weight change since age 18 in kg; height in inches; family history of breast cancer; history of benign breast disease; age at menarche in years, menopausal status, age at menopause, HRT use categories, and duration of menopause |
| Inoue-Choi et al., 2016 (20) | Prospective cohort (9.4) | NIH-AARP, USA | 38 748 Post | 62 | 90 | 15 230 | Total meat, red meat, processed meat | Validated FFQ (124) | Linkage to cancer registries | Age, race, BMI, height, education level, alcohol intake, physical activity, familial history of breast cancer, age at menarche, age at menopause, age at first live birth, number of live births, hormone use, OCP use, numbers of previous breast biopsy, total calorie intake, total fat intake, fiber intake, and intake of other types of meat (white, red, processed, processed red, processed white, unprocessed) |
| Kabat et al., 2009 (14) | Prospective cohort (8) | NIH-AARP, USA | 120 755 Post | 62 | 90 | 38 748 | Total meat, red meat, white meat, processed meat | Validated FFQ (124) | Linking to state cancer registries and to the US National Death Index | Energy intake, age at entry, BMI, age at first menstrual period, age at first live birth, family history of breast cancer, HRT, education, race, total EI, saturated fat, alcohol intake, physical activity, smoking, age at menopause, number of breast biopsies, height |
| Kesse-Guyot et al., 2007 (64) | Prospective cohort (7.7) | SUVIMAX, France | 3627 | 49 | >99 | 82 | Dairy, milk, yogurt, cheese | 24-hour record every 2 months | Self-report and validated pathological report | Educational level, parity, group of treatment, smoking status, overall physical activity, marital status, energy from fat, energy from other sources, alcohol intake, BMI, family history of breast cancer in first degree, menopausal status, and HRT use at baseline for the whole population, HRT use for menopausal women, dietary energy–adjusted calcium intake |
| Key et al., 1999 (44) | Prospective cohort (12) | Life Span Study (LSS), Japan | 11 067 | >18 | NM | 400 | Processed meat, egg, fish, fruit, rice, low fiber bread, soy product (miso paste, tofu) | Food and drink questionnaire (19) | Cancer registries | None |
| Key et al., 2018 (21) | Prospective cohort (11.9) | Million Women Study (MWS), UK | 691 571 | 59.9 | 90 | 27 863 | Fruits, vegetable, milk, yogurt, egg, total meat, processed meat, cheese | Validated FFQ (130) | Record linkage to the UK NHS databases | Socioeconomic status, BMI, height, smoking, current use of MHT, dietary EI, and alcohol consumption |
| Kiyabu et al., 2015 (65) | Prospective cohort (14.1) | Japan Public Health Center-based Prospective Study (JPHCPS), Japan | 38 234 | 57.3 | 100 | 556 | Fish | Validated FFQ (138) | Cancer registry | Age, BMI, age at menarche, age at first birth, parity, menopausal age, menopausal status at baseline, use of exogenous female hormones that |
| include OCP and MHT, leisure-time physical activity, smoking status, alcohol intake, and total energy-adjusted intake of isoflavones | ||||||||||
| Larsson et al., 2009 (38) | Prospective cohort (17.4) | SMC, Sweden | 61 433 | 60 | 100 | 2952 | Red meat | Validated FFQ (96) | National and regional Swedish cancer registers | Stratified by age in months at the start of each follow-up period and calendar year of the questionnaire cycle education, BMI, height, parity and age at first birth, age at menarche, age at menopause, use of OCP, use of postmenopausal hormones, family history of breast cancer, and intakes of total energy and alcohol |
| Lin et al., 2007 (66) | Prospective cohort (10) | Women's Health Study (WHS), USA | 31 487 (10 578 pre and 20 909 Post) | 55.18 | NM | 1019 (276 Pre and 743 Post) | Dairy | Validated FFQ (131) | Either self-report or vital record | Age, BMI, physical activity, family history of breast cancer in a first-degree relative, history of benign breast disease, age at menarche, parity, age at first birth, multivitamin, smoking status, alcohol consumption, total EI, age at menopause, and baseline MHT |
| Lo et al., 2019 (22) | Prospective cohort (7.6) | Sister Study, USA | 275 921 | 55.3 | NM | 1536 | Red meat, processed meat, poultry | Validated FFQ (110 items) | Medical records | Age; ethnicity; household income; education; baseline menopausal status; BMI; interaction term between baseline menopausal status and BMI; waist-to-hip ratio; total EI; consumption of vegetables, fruit, dairy, and other meat categories, including organ meat; percent calories from fat; number of relatives diagnosed with breast cancer before the age of 50; lifetime duration of breastfeeding; hormone therapy; parity; use of OCP; alcohol; physical activity; smoking |
| Marcondes et al., 2019 (39) | Prospective cohort (17) | Rotterdam Study (RS), Netherlands | 3209 Post | 67 | 100 | 199 | Red meat, processed meat, egg, fish, poultry, dairy, milk, cheese, yogurt | Validated FFQ (170) | Medical records and histopathological data | Age, physical activity, smoking status, history of breast cancer in first-degree relatives, MHT, parity, breast-feeding history and age of menopause, education, alcohol intake, iron intake did not alter the HR with more than 10% |
| Masala et al., 2012 (67) | Prospective cohort (11.25) | EPIC, Italy | 31 510 | 50 | NM | 1072 | Fruit juice, citrus fruit, nut and seeds | Validated FFQ (188) | Linkages with hospital discharge system | Weight, height, education, number of children, age at menarche, menopausal status, EI (except alcohol), alcohol intake, current use of HR, smoking status, physical activity |
| McCullough et al., 2005 (68) | Prospective cohort (16) | Cancer Prevention Study (CPS) II , USA | 140 725 Post | 62.4 | 91 | 3976 | Dairy, milk | Validated FFQ (68) | Medical record reports and cancer registries | Age, energy, history of breast cyst, family history of breast cancer, height, weight gain since age 18, alcohol use, race, age at menopause, age at first birth and number of live births, education, mammography history, and HRT use |
| Mills et al., 1989 (23) | Prospective cohort (6) | Seventh Day Adventist, USA | 20 341 | 55.4 | 99 | 199 | Total meat, processed meat, egg, fish, poultry, milk, cheese | Validated FFQ with five 24-hour dietary recalls | Self-report and diagnosis by senior medical personnel | Age at entry, age at first live birth, age at menarche, menopausal status, history of benign breast disease, maternal history of breast cancer, educational attainment, and BMI |
| Narita et al., 2017 (69) | Prospective cohort (14) | JPHCPS, Japan | 44 444 | 58 | 100 | 681 | Rice, soy | Validated FFQ (138) | Cancer registry | Age, area, BMI at 5-y follow-up, age at menarche, age at first birth, parity, age at menopause, use of exogenous female hormones, smoking status, leisure-time physical activity, alcohol intake, and total energy-adjusted intakes of fat, isoflavones, vitamin C, and carbohydrate |
| Nicodemus et al., 2001 (70) | Prospective cohort (9) | IWHS, USA | 29 119 Post | 61.3 | NM | 977 | Whole grain, refined grain | Validated FFQ (NM) | National Cancer Institute's surveillance | Age, energy intake, estrogen use, personal history of benign breast disease, family history of breast cancer, mammography, status, age at first live birth, number of live births, current weight, waist-to-hip ratio, vitamin use, educational attainment, and vitamin A and refined grain intake |
| Nilsson et al., 2020 (8) | Prospective cohort (11.2) | Northern Sweden Diet Database (NSDD), Sweden | 185 987 | >18 | NM | 5907 | Milk, yogurt, cheese | Validated FFQ (84) | Linkage to the Swedish cancer register | Age, screening year, dairy product category, BMI, civil status, education level, physical activity in leisure time, smoking status, recruitment cohort, and quintiles of fruit and vegetables, alcohol, and EI |
| Nishio et al., 2007 (71) | Prospective cohort (8) | JACC, Japan | 20 129 | 57.7 | NM | 237 | Soy product (miso paste, tofu) | Validated FFQ (NM | Cancer registration system | Age, study area, family history of breast cancer, age at menopause, age at first birth, parity, use of HRT, smoking, consumption of green leafy vegetables, walking time, BMI, and total EI |
| Olsen et al., 2003 (72) | Prospective cohort (4.7) | DCH, Denmark | 23 798 Post | 57 | 100 | 819 | Fruit, vegetable | Validated FFQ (192) | Danish cancer registry | Age, duration, baseline values of parity, previous benign breast tumor surgery, education, use of HRT, duration of HRT, intake of alcohol, and BMI |
| Pala et al., 2009 (40) | Prospective cohort (8.6) | EPIC, European countries | 319 826 | 51 | NM | 7119 | Milk, egg, red meat, poultry, processed meat, milk, cheese | Validated FFQ (260), UK and Sweden (FFQ and a 7-day record) | Cancer registry | Age, EI, alcohol intake, height, weight, increase since age 18, level of physical activity, years of education, maternal history of breast cancer, mammography practice, and use of OCP |
| Park et al., 2009 (73) | Prospective cohort (7) | NIH-AARP, USA | 198 903 | 50–71 | 90 | 5856 | Dairy | Validated FFQ (124) | Linking cohort members to state cancer registries and to the US National Death Index | Race/ethnicity, education; marital status; BMI; family history of cancer; vigorous physical activity; MHT; alcohol consumption; intakes of red meat and total energy; smoking; combined age at first birth and number of children,age at menopause; and intake of fat |
| Pouchieu et al., 2014 (41) | Prospective cohort (11.3) | SUVIMAX, France | 2367 | 47 | 84 | 102 | Red meat, processed meat | 24-h dietary records | Medical data and validated pathological reports | Age, intervention group, number of dietary records, smoking status, educational level, physical activity, height, BMI, family history of breast cancer, menopausal status at baseline, MHT at baseline, number of live births, without-alcohol EI, alcohol intake, total lipid intake. In addition, the red meat model is adjusted for processed meat intake, and the processed meat intake model is adjusted for red meat (mutual adjustment) |
| Rohan et al., 1993 (74) | Nested case-control (5) | Canadian National Breast Screening Study (CNBSS), Canada | 1700 | 40–59 | NM | 518 | Cereals, bread | Dietary history questionnaire | Medical records | Age, age at menarche, surgical menopause, age at first live birth, years of education, family history of breast cancer, history of benign breast disease, and other contributors to total food intake |
| Shannon et al., 2005 (24) | Nested case control (10) | Breast self-exam trial, China | 1070 | – | 100 | 378 | Total meat, red meat, processed meat, poultry, fish, egg, fruit, vegetable, dairy, soy, cereals | Validated FFQ (117) | Evaluated by medical workers | Age and total energy intake |
| Shibata et al., 1992 (75) | Prospective cohort (8) | Leisure World Cohort study, USA | 7200 post | 73.8 | 100 | 215 | Fruit and vegetable | FFQ (59) | Local hospitals records | Age, smoking |
| Shin et al., 2002 (76) | Prospective cohort (16) | NHS I, USA | 67 956 (42 990 pre and 24 966 Post) | 46.7 | >90 | 3172 (827 Pre and 2345 Post) | Dairy | Validated FFQ (61) | Either self-report or vital record | Age, time period, physical activity, history of benign breast disease, family history of breast cancer, height, weight change since age 18, BMI at age 18, age at menarche, parity, age at first birth, alcohol intake, total energy intake, total fat intake, glycemic index, B-carotene intake, vitamin E and D intake; for postmenopausal women, age at menopause and MHT were added |
| Shin et al., 2019 (77) | Prospective cohort (6.3) | Health Examinees-Gem, South Korea | 78 320 | 40–69 | NM | 359 | Milk | Validated FFQ (106) | Cancer Registry | BMI, total EI, educational level, parity, age at first birth, age at menarche, OCP use, regular exercise, alcohol consumption, and the presence of a family history of breast cancer |
| Sonestedt et al., 2008 (78) | Prospective cohort (10.3) | Malmö Diet and Cancer (MDC), Sweden | 15 773 | >18 | NM | 544 | Total fruit and vegetable, fruit juice, cereals, low-fiber bread, nut | Validated FFQ (168) | Cancer registries | Season of data collection, diet interviewer, method version, age, total energy, weight, height, educational status, smoking habits, leisure time physical activity, hours of household activities, alcohol consumption, age at menopause, parity, and current use of MHT |
| Stolzenberg-Solomon et al., 2006 (79) | Prospective cohort (10) | PLCO, USA | 25 400 Post | 62.9 | NM | 91 | Orange, grapefruit juice, or both | Validated FFQ (137) | Cancer registries, death certificates, physician reports | Energy, education, use of HRT, birth control pill use, mammography screening history, history of benign breast disease, family history of breast cancer, age at menarche, age at menopause, age at first birth, and number of live births |
| Stripp et al., 2003 (80) | Prospective cohort (4.8) | DCH, Denmark | 23 693 Post | 57 | 90 | 394 | Fish | Validated FFQ (192) | Record linkage to cancer register | Baseline values of parity, previous benign breast tumor surgery, school education, use of HRT, duration of HRT use, intake of alcohol, and BMI |
| Suzuki et al., 2013 (81) | Prospective cohort (10.2) | JPHCPS, Japan | 47 289 | 56 | 77 | 22 397 | Total fruit and vegetable | Validated FFQ (138) | Linkage to population–based registries | Age time-scales, area, height, recent BMI, BMI at age 20 y, age at menarche, age at first birth, parity, menopausal status, use of exogenous female hormones, smoking status, leisure-time physical activity, alcohol intake, total energy-adjusted intake of isoflavones, vitamin C supplement and combination variables, recent BMI, and menopausal status |
| Taylor et al., 2007 (25) | Prospective cohort (8) | UK Women's Cohort Study (UKWCS), UK | 34 403 | 52 | NM | 678 | Total meat, red meat, processed meat, poultry | Validated FFQ (217) | Central register | Age, energy intake, menopausal status, BMI, physical activity, smoking status, HRT use, OCP use, parity, and total fruit and vegetable intake |
| Toniolo et al., 1994 (26) | Nested case-control (6) | New York University Women's Health Study, USA | 829 | 52.2 | NM | 180 | Total meat, fish, poultry, dairy | Validated FFQ (71) | Self-report | Height, BMI, age at menarche, age at first full-term pregnancy, number of full-term pregnancies, first-degree family history of breast cancer, history of benign breast conditions, race, religion, EI |
| Trichopoulou et al., 2010 (31) | Prospective cohort (9.8) | EPIC, Greece | 14 807 | >18 | 91.5 | 240 | Cereals, fruit, vegetable, dairy, meat, and meat product | Validated FFQ (150) | Medical records and death certificates | Age, smoking, education, BMI, EI, age of menarche, age at first delivery, menopausal status, age at menopause, HRT, metabolic equivalents of task |
| van den Brandt et al., 2018 (82) | Case-cohort (20.3) | Netherlands Cohort Study (NLCS), Netherlands | 62 573 Post | 62 | 100 | 2321 | Nut | Validated FFQ (150) | Medical records and pathology reports | Age at baseline, cigarette use duration, BMI, nonoccupational physical activity, education, family history of breast cancer, history of benign breast disease, age at menarche, parity, age at first birth, age at menopause, OCP use, postmenopausal HRT, EI, alcohol, and alternate Mediterranean Diet Score, excluding alcohol and nuts |
| Van Der Hel et al., 2004 (27) | Nested case-control (10) | Dutch prospective cohort, Netherlands | 261 | 47.5 | 100 | 228 | Total meat, processed meat | Short validated FFQ (90) | Linkage to cancer registry | Age, menopausal status, town, EI, smoking, alcohol, age at menarche, and BMI |
| Vatten et al., 1990 (28) | Prospective cohort (12) | Norwegian Women Study, Norway | 14 500 | 35–51 | 95 | 152 | Total meat | Validated FFQ (60) | Cancer registry | Age |
| Verhoeven et al., 1997 (83) | Prospective cohort (4.3) | NLCS, Netherlands | 1812 post | 55–69 | 96 | 519 | Fruit and vegetable | Validated FFQ, (150) | Linkage to cancer registries and national database of pathology reports | Age, energy intake, alcohol intake, benign breast disease, maternal breast cancer, breast cancer in sister(s), age at menarche, age at menopause, age at first birth, parity |
| Voorrips et al., 2002 (45) | Prospective cohort (6.3) | NLCS, Netherlands | 62 573 Post | 55–69 | 96 | 2368 | Processed meat, milk and milk product, cheese | Validated FFQ (150) | Linkage to regional cancer registries and national database of pathology reports | Age, history of benign breast disease, maternal breast cancer, breast cancer in 1 or more sisters, age at menarche and menopause, OCP use, parity, age at first childbirth, BMI, education, alcohol use, cigarette smoking, and energy intake |
| Wada et al., 2013 (84) | Prospective cohort (15) | Takayama study, Japan | 15 607 | 52.2 | NM | 172 | Soy | Validated FFQ (169) | Cancer registries | Area, age, age at menarche, number of pregnancies, menopausal status, age at first pregnancy, smoking, alcohol consumption, leisure-time physical activity, educational level, total energy, and meat, fish, vegetable, and fruit consumption |
| Wilson et al., 2009 (85) | Prospective cohort (14) | NHS II, USA | 90 628 Pre | 36 | >90 | 1697 | Bread/ starches | Validated FFQ (130) | Self-report | Age in months and calendar year and adjusted for the following: BMI, height, OCP use, parity and age at first birth, age at menarche, family history of breast cancer, history of benign breast disease, smoking, physical activity, animal fat, glycemic load, alcohol intake, and total energy intake |
| Wirfalt et al., 2011 (42) | Prospective cohort (10.3) | MDC, Sweden | 15 773 | 45–73 | 100 | 544 | Milk, yogurt, egg, red meat, fish, cheese | Modified diet history, 7 days | Patients’ medical records and an immunohistochemistry methodology | MHT, height, weight, alcohol habits, household activity, leisure-time physical activity, work activity |
| Wu et al., 2019 (86) | Case-cohort (5.3) | DCH, Denmark | 1347 | 61 | NM | 414 | Whole-grain rye and whole-grain wheat | Validated FFQ (192) | Linkage to the Danish Cancer Registry | BMI, education, parity, HRT, age at first birth, age at menopause, age at first period, exercise, smoking status, waist-hip ratio, menopause status, and alcohol intake |
| Zhang et al., 2016 (9) | Prospective cohort (22) | NHS I and NHS II, USA | 143 206 | 50.4 | >90 | 5714 | Total rice intake (white and brown) | NHS II: Validated FFQ (130), NHS I: Validated FFQ (61) | Medical records and pathological reports | Age, ethnicity, BMI, smoking, physical activity, family history of cancer, multivitamin supplementation, total EI, MHT, and intake of alcohol, fruit, vegetables, red meat, fish, nuts, whole grains, and sugar-sweetened beverage |
CHO, carbohydrate; EI, energy intake; FTP, full-term pregnancy; HRT, hormone replacement therapy; JACC, Japan Collaborative Cohort; MHT, menopausal hormone therapy; NM, not mentioned; OCP, oral contraceptive pill.
Values are means or ranges.
The percentage of participants who completed the study.
Total meat
From 14 studies, we investigated the association of total meat consumption with breast cancer, where 1 study was excluded (14) due to an identical publication with a longer duration being available. Therefore, 13 studies, with 48 590 breast cancer cases, were included in the linear dose-response meta-analysis (15–28). Each additional 100-g/d increase of total meat was associated with a small increase in the risk of breast cancer (RR, 1.07; 95% CI, 1.01–1.13; I2, 75.5%; P-heterogeneity < 0.001; Supplemental Figure 1). A subgroup analysis by duration, number of cases, and location indicated that this association persisted only in studies with a duration of <10 y, case numbers of ≥1000, and studies conducted in Europe; while in studies with a duration of ≥10 y, case numbers of <1000, and studies conducted in the United States, no association was observed (Table 2). Moreover, the difference between premenopausal and postmenopausal status was nonsignificant (Table 2).
TABLE 2.
Subgroup analysis of studies investigated the association of various food groups with risk of breast cancer
| Subgroup factors | n of studies | RR (95% CI) | I², % |
|---|---|---|---|
| Red meat, 100 grams/day | |||
| Menopause status | |||
| Premenopause | 9 | 1.02 (0.94–1.12) | 17.1 |
| Postmenopause | 11 | 1.09 (0.99–1.20) | 63.0 |
| P between-group heterogeneity | 0.34 | ||
| Estrogen-progesterone receptor | |||
| Er+Pr+ | 4 | 1.21 (0.93–1.58) | 75.6 |
| Er+Pr− | 2 | 0.97 (0.81–1.15) | 0.0 |
| Er−Pr− | 3 | 1.22 (0.91–1.64) | 30.3 |
| P between-group heterogeneity | 0.17 | ||
| Follow-up | |||
| <10 y | 8 | 1.22 (1.09–1.37) | 44.1 |
| ≥10 y | 12 | 1.03 (0.96–1.11) | 47.4 |
| P between-group heterogeneity | <0.001 | ||
| Geographic location | |||
| Europe | 12 | 1.12 (1.02–1.22) | 51 |
| North America | 7 | 1.09 (0.98–1.21) | 71.6 |
| P between-group heterogeneity | 0.12 | ||
| Number of cases | |||
| <1000 | 6 | 1.14 (0.95–1.36) | 44.4 |
| ≥1000 | 14 | 1.09 (1.02–1.17) | 63.1 |
| P between-group heterogeneity | 0.06 | ||
| Poultry, 100 grams/day | |||
| Menopause status | |||
| Premenopause | 5 | 1.01 (0.98–1.05) | 27.6 |
| Postmenopause | 8 | 0.99 (0.96–1.02) | 59.3 |
| P between-group heterogeneity | 0.27 | ||
| Follow-up | |||
| <10 y | 8 | 0.97 (0.87–1.07) | 38.1 |
| ≥10 y | 5 | 0.96 (0.88–1.06) | 5.8 |
| P between-group heterogeneity | 0.94 | ||
| Geographic location | |||
| Europe | 5 | 1.07 (0.96–1.20) | 0.0 |
| USA | 7 | 0.93 (0.86–1.01) | 38.0 |
| P between-group heterogeneity | 0.12 | ||
| Number of cases | |||
| <1000 | 6 | 1.08 (0.97–1.19) | 40 |
| ≥1000 | 7 | 0.99 (0.98–1.0) | 35.9 |
| P between-group heterogeneity | 0.04 | ||
| Fish, 100 grams/day | |||
| Menopause status | |||
| Premenopause | 6 | 1.02 (0.92–1.14) | 0.0 |
| Postmenopause | 9 | 0.99 (0.93–1.04) | 0.0 |
| P between-group heterogeneity | 0.52 | ||
| Follow-up | |||
| <10 y | 5 | 1.05 (0.87–1.26) | 64.7 |
| ≥10 y | 12 | 0.98 (0.91–1.07) | 0.0 |
| P between-group heterogeneity | 0.87 | ||
| Geographic location | |||
| Europe | 9 | 0.99 (0.88–1.12) | 20.6 |
| USA | 6 | 1.01 (0.87–1.16) | 52.2 |
| Asia | 3 | 1.12 (0.85–1.47) | 0.0 |
| P between-group heterogeneity | 0.63 | ||
| Number of cases | |||
| <1000 | 9 | 1.15 (0.82–1.60) | 49.1 |
| ≥1000 | 8 | 0.98 (0.93–1.03) | 0.0 |
| P between-group heterogeneity | 0.24 | ||
| Egg, 50 grams/day | |||
| Menopause status | |||
| Premenopause | 4 | 1.06 (0.91–1.23) | 39.5 |
| Postmenopause | 4 | 1.01 (0.94–1.09) | 0.0 |
| P between-group heterogeneity | 0.71 | ||
| Follow-up | |||
| <10 y | 3 | 1.0 (0.95–1.06) | 0.0 |
| ≥10 y | 8 | 1.04 (0.93–1.16) | 61.7 |
| P between-group heterogeneity | 0.32 | ||
| Geographic location | |||
| Europe | 5 | 1.03 (0.98–1.08) | 68.5 |
| USA | 3 | 1.02 (0.93–1.12) | 0.0 |
| Asia | 2 | 0.87 (0.70–1.08) | 75 |
| P between-group heterogeneity | 0.34 | ||
| Number of cases | |||
| <1000 | 6 | 1.11 (0.82–1.50) | 70.3 |
| ≥1000 | 5 | 1.01 (0.97–1.06) | 0.0 |
| P between-group heterogeneity | 0.34 | ||
| Total meat, 100 grams/day | |||
| Menopause status | |||
| Premenopause | 4 | 1.04 (0.93–1.16) | 48.3 |
| Postmenopause | 7 | 1.04 (0.98–1.10) | 83.9 |
| P between-group heterogeneity | 0.66 | ||
| Follow-up | |||
| <10 y | 7 | 1.13 (1.03–1.23) | 73.1 |
| ≥10 y | 7 | 1.02 (0.96–1.08) | 55.2 |
| P between-group heterogeneity | <0.001 | ||
| Geographic location | |||
| Europe | 7 | 1.06 (1.02–1.10) | 73.4 |
| USA | 6 | 1.0 (0.98–1.02) | 78.9 |
| P between-group heterogeneity | 0.03 | ||
| Number of cases | |||
| <1000 | 8 | 1.29 (1.10–1.51) | 59.7 |
| ≥1000 | 6 | 1.01 (0.97–1.06) | 72.7 |
| P between-group heterogeneity | <0.001 | ||
| Processed meat, 50 grams/day | |||
| Menopause status | |||
| Premenopause | 6 | 1.03 (0.92–1.16) | 0.0 |
| Postmenopause | 11 | 1.11 (1.01–1.22) | 32.2 |
| P between-group heterogeneity | 0.49 | ||
| Follow-up | |||
| <10 y | 8 | 1.19 (1.03–1.38) | 35.6 |
| ≥10 y | 7 | 1.17 (0.94–1.46) | 76.9 |
| P between-group heterogeneity | 0.29 | ||
| Geographic location | |||
| Europe | 10 | 1.37 (1.16–1.62) | 46.1 |
| USA | 6 | 1.005 (0.93–1.08) | 0.0 |
| P between-group heterogeneity | 0.001 | ||
| Number of cases | |||
| <1000 | 9 | 1.29 (1.01–1.64) | 60.0 |
| ≥1000 | 8 | 1.07 (0.96–1.20) | 45.8 |
| P between-group heterogeneity | 0.001 | ||
| Dairy, 200 grams/day | |||
| Menopause status | |||
| Premenopause | 7 | 0.96 (0.90–1.02) | 54.8 |
| Postmenopause | 7 | 0.99 (0.96–1.02) | 27.1 |
| P between-group heterogeneity | 0.39 | ||
| Follow-up | |||
| <10 y | 4 | 0.96 (0.95–0.97) | 0.0 |
| ≥10 y | 6 | 0.99 (0.97–1.01) | 50.3 |
| P between-group heterogeneity | 0.004 | ||
| Geographic location | |||
| Europe | 4 | 0.96 (0.85–1.09) | 42.5 |
| USA | 5 | 0.97 (0.94–0.997) | 58.3 |
| P between-group heterogeneity | 0.02 | ||
| Number of cases | |||
| <1000 | 4 | 0.91 (0.82–1.02) | 0.0 |
| ≥1000 | 6 | 0.97 (0.96–0.98) | 72.4 |
| P between-group heterogeneity | 0.34 | ||
| Milk, 200 grams/day | |||
| Menopause status | |||
| Premenopause | 4 | 1.0 (0.92–1.09) | 0.0 |
| Postmenopause | 8 | 1.0 (0.96–1.03) | 33.0 |
| P between-group heterogeneity | 0.89 | ||
| Type of milk | |||
| Low fat | 5 | 1.01 (0.97–1.06) | 57.3 |
| Whole milk | 6 | 1.03 (0.99–1.08) | 0.0 |
| P between-group heterogeneity | 0.35 | ||
| Follow-up | |||
| <10 y | 6 | 0.98 (0.92–1.05) | 49.8 |
| ≥10 y | 7 | 0.99 (0.96–1.03) | 60.5 |
| P between-group heterogeneity | 0.97 | ||
| Geographic location | |||
| Europe | 8 | 0.99 (0.95–1.03) | 63.1 |
| USA | 4 | 0.98 (0.95–1.01) | 0.0 |
| P between-group heterogeneity | 0.10 | ||
| Number of cases | |||
| <1000 | 8 | 0.95 (0.88–1.03) | 67.1 |
| ≥1000 | 5 | 1.0 (0.98–1.01) | 0.0 |
| P between-group heterogeneity | 0.37 | ||
| Cheese, 30 grams/day | |||
| Menopause status | |||
| Premenopause | 4 | 0.93 (0.70–1.25) | 53.7 |
| Postmenopause | 7 | 0.90 (0.82–0.99) | 76.3 |
| P between-group heterogeneity | 0.29 | ||
| Follow-up | |||
| <10 y | 5 | 0.96 (0.87–1.06) | 60.4 |
| ≥10 y | 5 | 0.94 (0.87–1.01) | 75.1 |
| P between-group heterogeneity | 0.37 | ||
| Geographic location | |||
| Europe | 8 | 0.95 (0.91–0.99) | 77.9 |
| USA | 2 | 1.04 (0.49–2.19) | 76.1 |
| P between-group heterogeneity | 0.63 | ||
| Number of cases | |||
| <1000 | 5 | 0.95 (0.81–1.12) | 81 |
| ≥1000 | 5 | 0.98 (0.95–1.01) | 60.1 |
| P between-group heterogeneity | 0.025 | ||
| Fruit, 100 grams/day | |||
| Menopause status | |||
| Premenopause | 3 | 0.97 (0.93–1.0) | 0.0 |
| Postmenopause | 10 | 0.98 (0.96–1.0) | 62 |
| P between-group heterogeneity | 0.50 | ||
| Estrogen-progesterone receptor | |||
| Er+Pr+ | 4 | 0.97 (0.96–0.99) | 0.0 |
| Er−Pr− | 4 | 0.98 (0.94–1.02) | 0.0 |
| Er+Pr− | 5 | 0.99 (0.94–1.04) | 43.6 |
| P between-group heterogeneity | 0.65 | ||
| Geographic location | |||
| Europe | 6 | 0.967 (0.94–0.99) | 68.6 |
| North America | 5 | 0.98 (0.97–0.99) | 0.0 |
| Asia | 4 | 0.87 (0.73–1.05) | 86.1 |
| P between-group heterogeneity | 0.70 | ||
| Number of cases | |||
| <1000 | 9 | 0.96 (0.92–0.998) | 70.8 |
| ≥1000 | 6 | 0.97 (0.95–0.99) | 64.1 |
| P between-group heterogeneity | 0.51 | ||
| Vegetable, 100 grams/day | |||
| Menopause status | |||
| Premenopause | 4 | 1.01 (0.96–1.06) | 0.0 |
| Postmenopause | 10 | 1.01 (0.995–1.022) | 0.0 |
| P between-group heterogeneity | 0.98 | ||
| Estrogen receptor | |||
| Er+ | 4 | 0.99 (0.96–1.01) | 1.1 |
| Er− | 4 | 0.94 (0.90–0.997) | 52.7 |
| P between-group heterogeneity | 0.11 | ||
| Geographic location | |||
| Europe | 6 | 0.97 (0.95–0.99) | 0.0 |
| USA | 5 | 0.99 (0.95–1.02) | 63.3 |
| Asia | 3 | 0.92 (0.84–1.01) | 45.4 |
| P between-group heterogeneity | 0.004 | ||
| Number of cases | |||
| <1000 | 10 | 0.97 (0.94–1.001) | 62.4 |
| ≥1000 | 4 | 0.97 (0.95–1.0) | 0.0 |
| P between-group heterogeneity | 0.10 | ||
| Fruit and vegetable, 100 grams/day | |||
| Geographic location | |||
| Europe | 3 | 0.99 (0.98–1.0) | 0.0 |
| USA | 3 | 0.98 (0.97–0.99) | 0.0 |
| Asia | 2 | 0.94 (0.78–1.12) | 90.2 |
| P between-group heterogeneity | 0.22 | ||
| Number of cases | |||
| <1000 | 3 | 0.95 (0.89–1.01) | 67.8 |
| ≥1000 | 5 | 0.99 (0.97–1.0) | 46.9 |
| P between-group heterogeneity | 0.19 | ||
| Total cereals, 20 grams/day | |||
| Menopause status | |||
| Premenopause | 5 | 1.0 (0.99–1.01) | 0.0 |
| Postmenopause | 7 | 1.0 (0.99–1.01) | 22.3 |
| P between-group heterogeneity | 0.40 | ||
| Grain, 20 grams/day | |||
| Whole/refined | |||
| Whole grain | 5 | 1.0 (0.99–1.01) | 30.8 |
| Refined grain | 7 | 1.0 (0.99–1.01) | 0.0 |
| P between-group heterogeneity | 0.025 | ||
| Follow-up | |||
| <10 y | 6 | 1.0 (0.99–1.01) | 31.7 |
| ≥10 y | 8 | 0.99 (0.98–1.01) | 0.0 |
| P between-group heterogeneity | 0.15 | ||
| Geographic location | |||
| Europe | 6 | 1.0 (0.99–1.01) | 0.0 |
| North America | 4 | 0.99 (0.95–1.02) | 75.7 |
| Asia | 3 | 1.0 (0.98–1.02) | 0.0 |
| P between-group heterogeneity | 0.76 | ||
| Number of cases | |||
| <1000 | 9 | 1.0 (0.99–1.01) | 15.4 |
| ≥1000 | 5 | 1.0 (0.99–1.01) | 32.7 |
| P between-group heterogeneity | 0.83 | ||
+, positive/present; −, negative/not present; Er, estrogen receptor; Pr, progesterone receptor; RR, risk ratio.
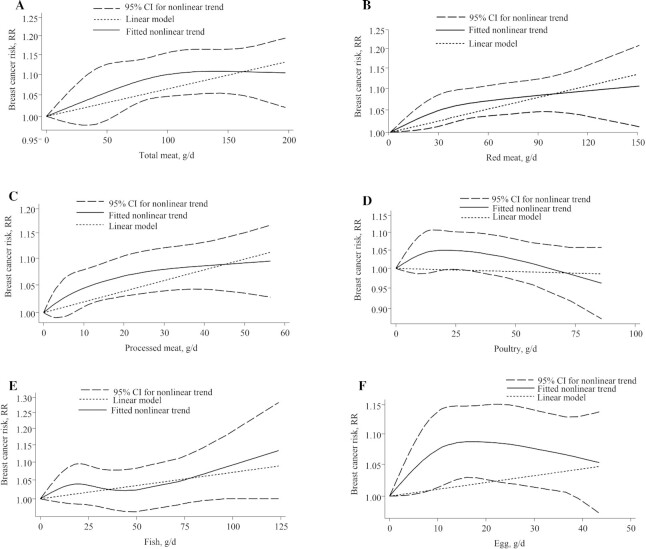
We found no evidence of a nonlinear dose-response association (P-nonlinearity, 0.21; n = 11 studies; Figure 2A).
FIGURE 2.
Nonlinear dose-response relationship between daily intakes of (A) total meat, (B) red meat, (C) processed meat, (D) poultry, (E) fish, and (F) egg and risk of breast cancer. RR = risk ratio.
Red meat
The association of red meat with breast cancer was investigated by 26 articles. We excluded 6 papers because other papers on the same cohort with longer durations were published (14, 29–33); thus, only the most recent studies with the longest follow-ups were included. These 20 studies (15, 16, 18–22, 24, 34–43), with 78 267 breast cancer cases, were included in the linear dose-response meta-analysis. Each 100-g/d increase of red meat was associated with a small increase in the risk of breast cancer (RR, 1.10; 95% CI, 1.03–1.18); however, statistically significant heterogeneity was observed in this model (I2, 60.2%; P-heterogeneity < 0.001; Supplemental Figure 2). The observed positive associations persisted in additional analyses, stratified by follow-up duration, geographic location, number of cases, and menopausal status (Table 2). The subgroup differences were not statistically significant, with the exception of follow-up duration, which showed a stronger inverse association for studies with a duration of <10 y.
There was no evidence of a nonlinear dose-response association (P-nonlinearity, 0.24; n = 14 studies). The risk of breast cancer increased by approximately 10% with increasing intake of red meat, up to 150 g/d (Figure 2B).
Processed meat
From 20 papers that investigated the relationship between processed meat and breast cancer, 3 articles (14, 32, 33) were excluded because the same exposure-outcome pair with a longer duration was published. There were 17 studies, with 34 414 breast cancer cases, included in the linear dose-response meta-analysis (15, 16, 18–20, 22, 23, 25, 27, 34, 37, 39–41, 44, 45). A positive association was observed for each additional 50-g/d increase of processed meat (RR, 1.18; 95% CI, 1.04–1.33; I2, 63.5%; P-heterogeneity < 0.001; Supplemental Figure 3). Subgroup analyses by menopause status and follow-up duration indicated no significant association (Table 2). However, subgroup analyses by number of cases and geographic location revealed a stronger positive association for studies with case numbers of <1000 and studies conducted in Europe (Table 2).
No evidence of a nonlinear dose-response association was detected (P-nonlinearity = 0.10; n = 15 studies). The risk of breast cancer increased by approximately 10% with an increasing intake of processed meat, up to 50 g/d (Figure 2C).
Poultry
From 14 studies that investigated the association of poultry with breast cancer, 1 study was excluded (32) because a paper on the same cohort with a longer duration was published. Therefore, 13 studies, with 27 445 breast cancer cases, were included in a linear dose-response meta-analysis (15, 16, 19, 22–26, 36, 37, 39, 40, 46). No association was observed for each 100-g/d increase of poultry (RR, 0.97; 95% CI, 0.91–1.03; I2, 22.9%; P-heterogeneity, 0.21; Supplemental Figure 4). Subgroup analyses by menopause status, follow-up duration, and geographic location indicated no significant association in the subgroups (Table 2). A subgroup analysis by number of cases revealed a stronger positive association for studies with case numbers of <1000. There was no evidence of a nonlinear dose-response association (P-nonlinearity = 0.08; n = 10 studies; Figure 2D).
Fish
From 18 studies that investigated the association of fish with breast cancer, 1 study was excluded (32) because a study on the same cohort with a longer duration was published. Thus, 17 studies, with 28 818 breast cancer cases, were included in a linear dose-response meta-analysis (15, 16, 18, 19, 23, 24, 26, 35, 36, 39, 42, 44, 46, 53, 57, 65, 80). No association was observed for each additional 100-g/d increase of fish (RR, 1.0; 95% CI, 0.93–1.08; I2, 22.6%; P-heterogeneity, 0.19; Supplemental Figure 5). Subgroup analyses by menopause status, follow-up duration, number of cases, and geographic location indicated no significant association in the subgroups (Table 2).
There was no evidence of a nonlinear dose-response association (P-nonlinearity, 0.39; n = 11 studies). The risk of breast cancer increased by approximately 10% with increasing intake of fish, up to 110 g/d (Figure 2E).
Egg
There were 11 studies, with 53 310 breast cancer cases, included in the linear dose-response meta-analysis of the association between egg intake and breast cancer risk (15, 17, 19, 21, 23, 24, 36, 39, 40, 42, 44). No association was found for each additional 50-g/d increase of egg (RR, 1.03; 95% CI, 0.96–1.12; I2, 48.8%; P-heterogeneity, 0.03; Supplemental Figure 6). Subgroup analyses by menopause status, follow-up duration, geographic location, and number of cases indicated no significant difference between the subgroups (Table 2).
Although a nonlinear dose-response association was detected, the shape of the curve did not provide any valuable information (P-nonlinearity, 0.03; n = 7 studies; Figure 2F).
Fruit
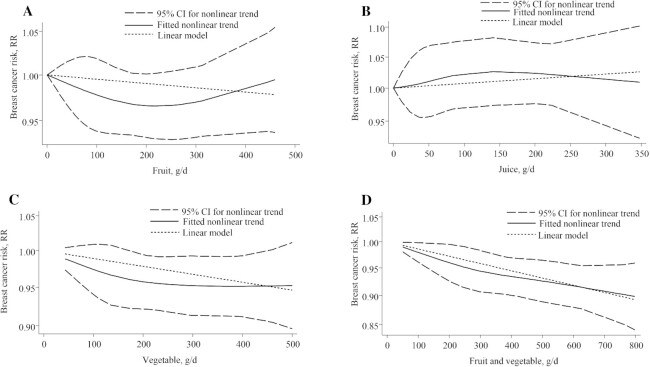
Only 1 study assessed the association between combined fruit and vegetable intake and breast cancer, so it was not included in the analyses (87).
From 21 papers that investigated the relationship between fruit and breast cancer, 6 were excluded because papers on the same cohorts with longer durations were published (31, 72, 88–91). However, 4 of these studies were included in the subgroup analyses according to menopause status (31, 72) and presence of estrogen receptor (89, 90), since the updated papers on the same studies did not report the results according to these factors (52, 56).
There were 15 studies, with 7071 breast cancer cases, included in the linear dose-response meta-analysis (15, 21, 24, 29, 35, 44, 48, 49, 52, 56, 59, 74, 75, 78, 81, 83). A small, inverse association was observed for each additional 100-g/d increase of fruit (RR, 0.97; 95% CI, 0.95–0.99; I2, 66.5%; P-heterogeneity < 0.001; Supplemental Figure 7). Also, in subgroup analyses by geographic location, number of cases, menopause status, and presence of estrogen progesterone receptor, no significant difference was found between the subgroups (Table 2).
There was no evidence of a nonlinear dose-response association detected (P-nonlinearity, 0.20; n = 7 studies; Figure 3A).
FIGURE 3.
Nonlinear dose-response relationship between daily intakes of (A) fruit, (B) juice, (C) vegetables, and (D) fruits and vegetables and risk of breast cancer. RR = risk ratio.
Fruit juice
There were 6 studies, with 4463 breast cancer cases, included in the linear dose-response meta-analysis of fruit juice intake and breast cancer risk (15, 56, 58, 62, 67, 78). No significant association was observed for each additional 50-g/d increase of fruit juice (RR, 1.0; 95% CI, 0.99–1.01; I2, 26.7%; P-heterogeneity, 0.23; Supplemental Figure 8).
There was no evidence of a nonlinear dose-response association (P-nonlinearity, 0.78; n = 5 studies; Figure 3B).
Vegetable
From 18 papers that investigated the relationship between vegetable intake and breast cancer, 4 were excluded because papers on the same cohorts with longer durations were published (31, 72, 88, 91); however, 1 of these 4 studies (72) was included in a subgroup analysis since the updated paper on the same cohort (52) did not report the results according to menopause status.
There were 14 studies, with 54 845 breast cancer cases, included in the linear dose-response meta-analysis (15, 21, 24, 29, 48, 49, 52, 56, 59, 74, 75, 78, 81, 83). A small, inverse association was observed for each additional 100-g/d increase of vegetable intake (RR, 0.97; 95% CI, 0.953–0.995; I2, 55.8%; P-heterogeneity, 0.006; Supplemental Figure 9). Subgroup analyses by menopause status, presence of estrogen receptor, geographical location, and number of cases indicated no significant difference in the effect sizes between the subgroups (Table 2). The follow-up durations of all studies, except 1, were longer than 10 y.
There was no evidence of a nonlinear dose-response association (P-nonlinearity, 0.37; n = 6, studies; Figure 3C).
Fruit and vegetable
There were 8 studies with breast cancer cases included in the linear dose-response meta-analysis of fruit and vegetable intake (24, 48, 52, 56, 58, 78, 81, 87). A small, inverse association was observed for each additional 100-g/d increase of fruit and vegetable intake (RR, 0.98; 95% CI, 0.97–0.996; I2, 54.7%; P-heterogeneity, 0.03; Supplemental Figure 10). Subgroup analyses by geographical location and number of cases indicated no significant difference between the subgroups (Table 2). Follow up durations of all studies, except 1, were longer than 10 y.
There was no evidence of a nonlinear dose-response association (P-nonlinearity, 0.67; n = 8 studies; Figure 3D).
Dairy
There were 10 studies, with 16 175 breast cancer cases (15, 24, 26, 31, 35, 55, 63, 66, 73, 76), that reported the association of dairy intake as a whole with breast cancer risk. Studies that assessed dairy products separately were not included in this category. A linear dose-response meta-analysis indicated no significant association for each additional 200-g/d increase of dairy intake (RR, 0.97; 95% CI, 0.95–1.003; I2, 55.6%; P-heterogeneity, 0.02; Supplemental Figure 11). Subgroup analyses by menopause status and number of cases indicated no significant difference between the subgroups (Table 2). Geographic location and follow-up duration were the sources of heterogenity (Table 2).
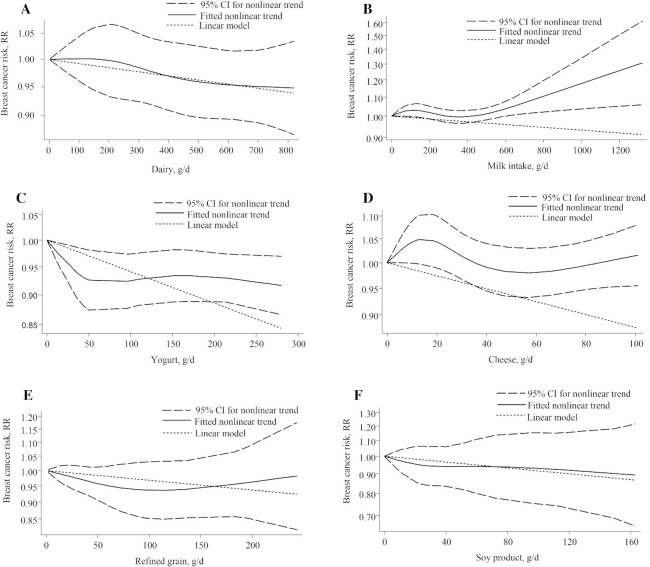
There was no evidence of a nonlinear dose-response association (P-nonlinearity, 0.83; n = 8 studies; Figure 4A).
FIGURE 4.
Nonlinear dose-response relationship between daily intakes of (A) dairy, (B) milk, (C) yogurt, (D) cheese, (E) refined grains, and (F) soy product and risk of breast cancer. RR = risk ratio.
Milk
There were 13 studies, with 47 729 breast cancer cases, included in the linear dose-response meta-analysis of milk intake and breast cancer risk (8, 17, 18, 21, 23, 39, 40, 42, 45, 63, 64, 68, 77). No significant association was observed for each additional 200-g/d increase of milk intake (RR, 0.99; 95% CI, 0.96–1.02; I2, 52.3%; P-heterogeneity, 0.01; Supplemental Figure 12). Subgroup analyses by menopause status, follow-up duration, geographical location, type of milk, and number of cases indicated no significant difference between the subgroups (Table 2).
We found evidence of a nonlinear dose-response association (P-nonlinearity, 0.04; n = 12 studies). The association of milk intake with breast cancer risk was not significant for intakes of up to 450 g/d, but in amounts greater than 450 g/d, up to 1300 g/d, the risk increased by approximately 30% (Figure 4B).
Yogurt
There were 6 studies, with 28 291 breast cancer cases, included in the linear dose-response meta-analysis of yogurt intake and breast cancer risk (8, 21, 39, 42, 64, 92). No significant association was observed for each additional 200-g/d increase of yogurt intake (RR, 0.91; 95% CI, 0.79–1.05; I2, 71.5%; P-heterogeneity, 0.004; Supplemental Figure 13).
There was no evidence of a nonlinear dose-response association (P-nonlinearity, 0.06; n = 4 studies). The risk of breast cancer decreased by approximately 7.5% with an increasing intake of yogurt, up to 100 g/d (Figure 4C).
Cheese
There were 10 studies, with 39 703 breast cancer cases, included in the linear dose-response meta-analysis of cheese intake and breast cancer risk (8, 18, 21, 23, 39, 40, 42, 45, 63, 64). A small, inverse association was observed for each additional 30-g/d increase of cheese intake (RR, 0.95; 95% CI, 0.91–0.996; I2, 75.1%; P-heterogeneity < 0.001; Supplemental Figure 14). Subgroup analyses for menopause status, follow-up duration, and geographical location were not statistically significant (Table 2). A subgroup analysis for the number of cases revealed a stronger inverse association for studies with case numbers of <1000 (Table 2).
There was no evidence of a nonlinear dose-response association (P-nonlinearity, 0.07; n = 9 studies; Figure 4D).
Total cereals
There were 14 studies, with 16 857 breast cancer cases, included in the linear dose-response meta-analysis of total cereal intake and breast cancer risk (9, 15, 24, 31, 35, 44, 51, 60, 69, 70, 74, 78, 86, 90). No significant association was observed for each additional 20-g/d increase of total cereal intake (RR, 1.0; 95% CI, 0.99–1.01; I2, 17.4%; P-heterogeneity = 0.26; Supplemental Figure 15). In subgroup analyses by menopause status, follow-up duration, geographic location, number of cases, and refined- vs. whole-grain cereal intake, no significant difference was found in the effect sizes between the subgroups (Table 2). Although evidence of a nonlinear dose-response association was detected (P-nonlinearity 0.04; n = 7 studies), the shape of the curve did not yield any valuable information (Figure 4E).
Soy and soy products
There were 7 studies, with 4055 breast cancer cases, included in the linear dose-response meta-analysis of soy and soy product intake and breast cancer risk (15, 24, 44, 49, 69, 71, 84). A significant association was observed for each additional 30-g/d increase of soy and/or soy product intake (RR, 0.965; 95% CI, 0.94–0.99; I2, 0.0%; P-heterogeneity = 0.64; Supplemental Figure 16). There was no evidence of a nonlinear dose-response association (P-nonlinearity, 0.87; n = 5 studies; Figure 4F).
Nuts
There were 6 studies, with 9219 breast cancer cases, included in the linear dose-response meta-analysis of nut intake and breast cancer risk (15, 36, 58, 67, 78, 82). No significant association was observed for each additional 28-g/d increase of nut intake (RR, 0.92; 95% CI, 0.83–1.01; I2, 9.7%; P-heterogeneity, 0.35; Supplemental Figure 17).
Legumes
There were 4 studies that investigated the association of legumes, besides soy, with breast cancer risk (15, 24, 47, 58). No significant association was observed for each additional 50-g/d increase of legume intake (RR, 0.95; 95% CI, 0.87–1.05; I2, 32.1%; P-heterogeneity, 0.22).
Publication bias
Based on Egger's test, publication bias was evident only for total meat (P = 0.007), red meat (P = 0.002), and fish (P = 0.03) intakes, and their funnel plots (Supplemental Figure 18A, B, and E) were asymmetric. There was no publication bias and the associated funnel plots were symmetrical for processed meat (Supplemental Figure 18C), poultry (Supplemental Figure 18D), fruit (Supplemental Figure 19A), vegetable (Supplemental Figure 19B), dairy (Supplemental Figure 19C), milk (Supplemental Figure 19D), cheese (Supplemental Figure 19E), and cereal (Supplemental Figure 19F) intakes.
Data quality
The quality of most of the studies was classified as good, while 13 studies were classified as being of fair quality (23–26, 34, 37, 62, 64, 70, 71, 75, 77, 83) and 2 studies were classified as being of poor quality (44, 74) (Supplemental Table 2). To discern whether study quality had an effect on the results, we excluded the studies rated as being of fair or poor quality from the analysis; no statistically significant changes were seen, except for in the analysis of total meat. After excluding studies with fair quality, the association of total meat intake with the risk of breast cancer was not significant.
Additionally, the NutriGrade meta-evidence rating indicated moderate confidence in the effect estimates for all of the food categories, except poultry, fish, cereals, and legumes, which had low confidence ratings (Table 3).
TABLE 3.
NutriGrade assessment of confidence in estimate effect of studies evaluated the association between various food groups and risk of breast cancer
| Outcomes | Risk of bias1 | Precision2 | Indirectness | Heterogeneity3 | Publication bias4 | Effect size5 | Dose response | Funding bias | Total score | Confidence of evidence6 |
|---|---|---|---|---|---|---|---|---|---|---|
| Total meat | 2 | 1 | 1 | 0.5 | 0 | 0 | 1 | 1 | 6.5 | Moderate |
| Red meat | 2 | 1 | 1 | 0.5 | 0 | 0 | 1 | 1 | 6.5 | Moderate |
| Processed meat | 1 | 1 | 1 | 0.5 | 1 | 0 | 1 | 1 | 6.5 | Moderate |
| Poultry | 1 | 1 | 1 | 0.5 | 1 | 0 | 0 | 1 | 5.5 | Low |
| Fish | 2 | 1 | 1 | 0.5 | 0 | 0 | 0 | 1 | 5.5 | Low |
| Egg | 2 | 1 | 1 | 0.5 | 1 | 0 | 0 | 1 | 6.5 | Moderate |
| Dairy | 2 | 1 | 1 | 0.5 | 1 | 0 | 0 | 1 | 6.5 | Moderate |
| Milk | 2 | 1 | 1 | 0.5 | 1 | 0 | 1 | 1 | 7.5 | Moderate |
| Yogurt | 2 | 0 | 1 | 0.5 | 0.5 | 0 | 0 | 1 | 7 | Moderate |
| Cheese | 2 | 1 | 1 | 0.5 | 1 | 0 | 1 | 1 | 6.5 | Moderate |
| Fruit | 2 | 1 | 1 | 0.5 | 1 | 0 | 1 | 1 | 6.5 | Moderate |
| Vegetable | 2 | 1 | 1 | 0.5 | 1 | 0 | 1 | 1 | 7.5 | Moderate |
| Fruit + vegetable | 2 | 1 | 1 | 0.5 | 0.5 | 0 | 1 | 1 | 7 | Moderate |
| Juice | 2 | 1 | 1 | 0.5 | 0.5 | 0 | 0 | 1 | 6 | Moderate |
| Cereals | 1 | 1 | 1 | 0.5 | 1 | 0 | 0 | 1 | 5.5 | Low |
| Soy | 1 | 1 | 1 | 0.5 | 0.5 | 0 | 1 | 1 | 6 | Moderate |
| Nut | 2 | 1 | 1 | 0.5 | 0.5 | 0 | 0 | 1 | 6 | Moderate |
| Legume | 1 | 1 | 1 | 0.5 | 0 | 0 | 0 | 1 | 4.5 | Low |
NutriGrade, Nutrition Grading of Recommendations Assessment, Development and Evaluation; RR, risk ratio.
Risk of bias was based on the Newcastle-Ottawa Scale, where ≥7 = 2 points; 4–6.9 = 1 point; and 0–3.9 = 0 points.
Precision is 1 point if the number of events ≥500 and the 95% CI excludes the null value; precision is 0 points if the number of events <500 or number of events ≥500, but 95% CI includes the null value (i.e., CI includes RR of 1.0) and 95% CI fails to exclude an important benefit (RR of 0.8) or harm (RR of 1.2).
When I2 was <40% or I2 was ≥40% but the source of heterogeneity was found by subgroup analysis 1 point was assigned; otherwise, 0 points were assigned.
Based on the funnel plots, Egger or Begg's test. For the outcomes with small number of studies (n < 10), the risk of publication bias was not formally assessed.
If the RR or HR <0.80–0.50 and >1.20–2.00, respectively, 1 point is assigned and the corresponding test is statistically significant; if the RR or HR <0.50 and >2.00, respectively, 0 points are assigned and the corresponding test is statistically significant.
6Moderate quality indicates that we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality indicates that our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect.
Discussion
In the present systematic review and meta-analyses, the associations of preselected foods and food groups—total meat, red meat, poultry, fish, processed meat, egg, fruits, vegetables, dairy, milk, yogurt, cheese, grains, soybeans, nuts, and legumes—and the risk of breast cancer were evaluated using data reported within and across prospective studies. We identified decreased risks of breast cancer with increased intakes of fruits, vegetables, soybeans, and cheese, and there was a positive association between red meat and processed meat consumption and the risk of breast cancer. No linear dose-response associations were observed for egg, dairy, milk, yogurt, grain, nut, and legume intakes and breast cancer risks, whilst a nonlinear dose-response association was observed for milk intake. We observed moderate confidence in the effect estimates for all food items, except poultry, fish, cereal, and legumes, which had low confidence ratings.
Lifestyle and environmental factors, including diet, are considered as important factors in the prevention of breast cancer (93). The International Agency for Research on Cancer reported that red meat and processed meat may be potential carcinogens for humans (94); indeed, in the present meta-analysis, the risk of breast cancer increased by 10% for red meat, 7% for total meat, and 18% for processed meat. Similarly, a previous meta-analysis reported a significant, positive association between processed meat consumption and the risk of breast cancer (95), but the authors only compared the highest category with the lowest category of red and processed meat consumption.
The carcinogenicity of red meat and processed meat may be attributed to mutagenic compounds, such as polyaromatic hydrocarbons and heterocyclic amines, which are by-products of cooking red meat at high temperatures (96, 97). Also, heme iron, fat, and animal sugar molecule N-glycolylneuraminic acid, found in red meat, are posited to potentially increase inflammation, oxidative stress, and tumor formation (96), and in some countries, hormone residue of the exogenous hormones used to stimulate the growth of beef cattle has also been suggested as an independent risk factor of breast cancer (96).
To ameliorate the cancer risk, fish and poultry represent good substitutes for red meat in the dietary composition. As in the present meta-analysis, poultry and fish had no significant association with the breast cancer risk. Indeed, red meat and poultry differ in their relative percentages of heme iron and saturated fat content. Also, consumption of poultry has been associated with less mutagenic activity, oxidative stress, and DNA damage (93).
Breast cancer has a heterogeneous etiology, so, in the present study, subgroup analyses were conducted based on several factors. In a subgroup analysis, the association of red meat, total meat, and processed meat consumption and breast cancer risk was stronger in the studies from Europe. Indeed, this stronger association might be attributed to the fact that breast cancer is the most common cancer type in Europe (98), and such differences might be manifest from the prevalence and distribution of known risk factors of breast cancer in European countries (98).
Considering the association between red meat and total meat intake and the breast cancer risk, larger and significant effects were seen in follow-up durations of more than 10 y, which might be attributed to the higher number of cancer cases that occurred in longer follow-ups. Also, cumulative effects of risk factors concomitant to increasing age and an increasing number of post-menopause cancer cases in long follow-ups could be considered, notwithstanding the fact that the effect of red meat consumption on the breast cancer risk was not significant in a subgroup analysis of menopausal status. While some studies revealed that the risks of breast cancer following red meat consumption are different in pre- and postmenopausal women (29), in the present study such differences were seen for processed meat intake, which had a stronger association with breast cancer risk in postmenopausal women. Regarding case numbers, larger and significant effects were identified for total and processed meat consumption and breast cancer risk in the subgroup of studies with case numbers <1000; however, it is conceivable that such differences might be attributed to the higher level of bias in lower case numbers.
A contentious issue in the relationship between diet and breast cancer risk is dairy consumption. In the present study, a null association was seen between dairy product, milk, and yogurt consumption and breast cancer risks in linear dose-response analyses. A positive nonlinear dose-dependent association was seen for milk intake, although no association was observed for consumption of less than 450 g/d of milk, but in amounts greater than 450 g/d, the risk of breast cancer increased by approximately 30%, with increasing milk intake up to 1300 g/d. In a subgroup analysis, the association of dairy consumption with breast cancer risk was larger and was significant in follow-ups of <10 y. The results of a previous meta-analysis investigating the association between milk consumption and breast cancer risk did not provide consistent evidence for such an association (99). Indeed, in the aforementioned study, the authors only assessed the relationship between the highest versus lowest intakes of milk; moreover, their literature search was limited to PubMed and Chinese biomedicine databases up to 2009 (99), so a number of studies were missing. The association between milk consumption and breast cancer risk might be related to the presence of fat-soluble hormones in the milk, which come from pregnant cows, leading to an increased risk of hormone-dependent cancers, such as breast, ovarian, and corpus uteri cancers (100). Milk consumption is among the most important routes of human exposure to estrogens; in fact, milk is considered as the predominant source of animal-derived estrogens in the human diet, accounting for 60–80% of the estrogens consumed (100). Moreover, milk contains insulin-like growth factor I (IGF-I), which stimulates cell proliferation and neoplasm formation (101). The association between milk consumption and IGF-I tumorigenesis was only suggested for milk and not for other dairy products (101). Indeed, considering these mechanisms, it is noteworthy to mention that previous meta-analyses showed significant, positive associations between milk consumption and reproductive cancers, such as ovarian cancer (102) and prostate cancer (101).
In the present study, a small, inverse association was seen between cheese consumption and breast cancer risk, such that the breast cancer risk decreased by 5%. In a subgroup analysis, this association was stronger in studies from Europe. To the best of our knowledge, no meta-analysis has been conducted regarding the association of cheese consumption with breast cancer risk. Cheese is a good dietary source of proteins; several vitamins, such as A, B6, B12, D, and K; and minerals, including calcium, iodine, magnesium, potassium, phosphorus, and zinc. The ameliorative effects of cheese on the breast cancer risk might be because cheese consumption is representative of a relatively healthy diet (103). Also, desaturase inhibitors in cheese, which inhibit triglyceride synthesis, reduce the pathogenic effects of fat (102), whilst cancer-protective properties of fermented products, such as cheese, might be attributed to live microorganisms acting as probiotics (102, 104).
In our study, an inverse association was detected between fruit and vegetable consumption and breast cancer risk, such that the risk of breast cancer decreased by 3% for increased fruit intake, by 4% for increased vegetable intake, and by 2% for increased combined fruit and vegetable consumption. The most recent meta-analysis on the associations of fruit and vegetable intakes with breast cancer risk, which was published in 2012, reported that high intakes of fruits alone and of fruits and vegetables combined, but not of vegetables alone, were associated with weak reductions in the breast cancer risk (105). There were 9 studies included in this previous study (in a linear dose-response analysis), while our results are based on 15 studies. Anti-cancer properties of fruits and vegetables are possibly due to their high content of antioxidant nutrients, including fiber, vitamins C and E, carotenoids, and other bioactive substances (56). In the present study, a subgroup analysis revealed a larger and significant association between vegetable intake and estrogen receptor–negative breast cancer. This might be due to the dominant role of hormonal exposure and hormone-related factors in the etiology of estrogen receptor–positive tumors (56, 106). Moreover, vegetables contain phytochemical compounds that can reduce the levels of epidermal growth factor receptor, nuclear factor kappa B, and cyclin E, which may, in turn, reduce the risk of developing estrogen receptor–negative breast cancer (106).
Besides the antioxidant content of fruits and vegetables, high fiber intake has been shown to interfere with bile acids and decrease estrogen deconjugation, leading to increased fecal excretion of estrogen and reduced plasma concentration of this hormone (107). This explanation might also pertain to the significant differences that were seen in a subgroup analysis between refined- and whole-grain consumption in the present study, while a null association was observed between overall grain consumption and breast cancer risk. Also, high glycemic-index foods were shown to be associated with higher insulin levels, and the insulin–IGF-I axis has been shown to be directly associated with cancer promotion (108). Similar findings were reported by a previous meta-analysis on whole-grain intake and breast cancer risk, suggesting that intermediate and high intake levels of whole grains were associated with modest reductions of breast cancer risks, but this inverse association was only observed in case-control and not cohort studies (109), and the mentioned meta-analysis only assessed studies published specifically on whole grains.
In the present study, soybean consumption was associated with a 3.5% reduction in breast cancer risk. In previous meta-analyses of the association of soy intake and breast cancer, most of the included studies assessed the soy isoflavones. We did not include these studies, since the aim of our study was to investigate the association of food groups, rather than the food component, with breast cancer. There were 2 recent meta-analyses on soy isoflavones that indicated a reduction of breast cancer risk with soy intake, especially in larger amounts. Accordingly, 1 of these studies indicated that high versus low consumption of soy was associated with a lower risk of breast cancer (n = 6 studies), while moderate versus low intake of soy did not significantly affect the breast cancer risk (n = 4 studies) (110). Another study, which evaluated the risk in Chinese women, revealed that every 10 mg/day of soy isoflavone intake was associated with a 3% reduction in breast cancer risk (111). Indeed, the results of previous studies indicate that menopause status may influence the association of soy consumption and breast cancer (112); however, because of the small number of studies included in our meta-analysis, we could not conduct a subgroup analysis based on menopause status.
The novelties of our study compared to previously published meta-analyses on single food groups (95, 99, 105, 109, 113) have been explicated above. The only meta-analysis considering several food groups was conducted by Wu et al. in 2016 (96), on dietary protein sources and breast cancer risk, where the literature search was conducted up to 2015. Thus, given that a substative period of time (5 y) has elapsed and a number of large-scale prospective studies have been published, especially on red meat, processed meat, and fish, an up-to-date synthesis was urgently required. Moreover, former meta-analyses have rarely assessed the quality of evidence that is important for generating guidelines and recommendations, while we comprehensively assessed the quality of meta-evidence using the NutriGrade scoring system.
As a limitation to the present study it is worth noting that, for some of the food groups, residual components may influence the association with breast cancer: for example, in low-fat versus high-fat dairy, lean meat versus high-fat meat, or low-sugar versus sweetened fruit juices. Since the primary studies did not report results according to fat or sugar contents of food, we could not perform subgroup analyses or conduct the analysis separately according to these factors; therefore, a limitation of this study is that the results may be confounded by a component of the food. For legumes, nuts, and soy, the results were derived from a small number of studies. Most of the studies on these food groups assessed their components, such as fiber, protein, and isoflavones, and so were excluded based on our inclusion criteria. Moreover, meta-evidence for poultry, fish, cereals, and legumes was low; therefore, results for these food groups should be interpreted with caution. Moreover, it should be noted that numerous analyses were performed, and it is conceivable that some associations could be statistically significant as a result of multiple comparisons. Finally, it should be acknowledged that due to the observational design of the primary studies, causality cannot be inferred from these statistical correlations.
This meta-analysis has several strengths; for instance, we are moderately confident in the veracity of the results for most of the food groups, as the primary studies were mostly assessed as being of good quality. The adequate number of included studies also allowed us to conduct multiple subgroup analyses for important factors, such as menopausal status and presence of estrogen receptor. Additionally, we conducted both linear and nonlinear dose-response analyses, which provide detailed insight into the associations.
In conclusion, the findings of the present meta-analysis show that high intakes of fruits, vegetables, soybeans, and cheese and low intakes of red meat and processed meat are associated with reduced risks of breast cancer. A null association was noted between poultry, fish, egg, fruit juice, dairy, milk (<450 g/day), yogurt, grain, nut, and legume consumption and breast cancer risk, whilst consumption of milk in amounts more than 450 g/day was associated with an increased risk. Finally, it should be acknowledged that causality cannot be inferred from these statistical correlations, indicating the need for further well-conducted RCTs.
Supplementary Material
Acknowledgments
We thank Dr Dominik Alexander for the internal peer review of the manuscript.
The authors’ responsibilities were as follows—AK: designed the study; AK, RB-B, MA, and SS: literature search and screening; AK, RB-B, ZE, and NM: data extraction; AK and SS: quality assessment; AK, MA, and SB: wrote the initial draft, which was modified after feedback from all coauthors; CCTC: critical revising of the manuscript; AK: had primary responsibility for content; and all authors: read and approved the final manuscript.
Notes
This study was supported by Vice Chancellor of Research, Shiraz University of Medical Sciences (grant number 99-01-106-23236).
Author disclosures: The authors report no conflicts of interest.
Supplemental Tables 1 and 2 and Supplemental Figures 1–19 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/advances/.
Abbreviations used: IGF-I, insulin-like growth factor I; RCTs, randomized controlled trial; RR, risk ratio; SSB, sugar-sweetened beverages.
Contributor Information
Asma Kazemi, Nutrition Research Center, Shiraz University of Medical Sciences, Shiraz, Iran.
Reza Barati-Boldaji, Nutrition Research Center, Shiraz University of Medical Sciences, Shiraz, Iran.
Sepideh Soltani, Yazd Cardiovascular Research Center, Shahid Sadoughi University of Medical Sciences, Yazd, Iran.
Nazanin Mohammadipoor, Nurtition Research Center, School of Nutrition and Food Sciences, Shiraz University of Medical Sciences, Shiraz, Iran.
Zahra Esmaeilinezhad, Nutrition Research Center, Shiraz University of Medical Sciences, Shiraz, Iran.
Cian C T Clark, Centre for Intelligent Healthcare, Coventry University, Coventry, UK.
Siavash Babajafari, Nutrition Research Center, Shiraz University of Medical Sciences, Shiraz, Iran.
Marzieh Akbarzadeh, Nurtition Research Center, School of Nutrition and Food Sciences, Shiraz University of Medical Sciences, Shiraz, Iran.
References
- 1.World Health Organization, International Agency for Research on Cancer. Latest global cancer data: cancer burden rises to 18.1 million new cases and 9.6 million cancer deaths in 2018. Geneva (Switzerland): World Health Organization; 2018. [Google Scholar]
- 2. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. [DOI] [PubMed] [Google Scholar]
- 3. Coughlin SS. Epidemiology of breast cancer in women. Breast cancer metastasis and drug resistance. In Aamir A, editor. Springer Nature Switzerland: Springer; 2019: pp. 9–29. [Google Scholar]
- 4. 2020 Dietary Guidelines Advisory Committee and Nutrition Evidence Systematic Review Team. Dietary Patterns and Breast, Colorectal, Lung, and Prostate Cancer: A Systematic Review. 2020 Dietary Guidelines Advisory Committee Project [Internet] . Alexandria, VA: U.S. Department of Agriculture, Food and Nutrition Service, Center for Nutrition Policy and Promotion; 2020. Available from: https://nesr.usda.gov/2020-dietary-guidelines-advisory-committee-systematic-reviews. [Google Scholar]
- 5. Dietary Guidelines Advisory Committee. Scientific Report of the 2015 Dietary Guidelines Advisory Committee: Advisory Report to the Secretary of Health and Human Services and the Secretary of Agriculture. [Internet] . Washington, DC: U.S. Department of Agriculture, Agricultural Research Service; 2015. Available from: https://health.gov/our-work/food-nutrition/2015-2020-dietary-guidelines/advisory-report. [Google Scholar]
- 6. Schwingshackl L, Schwedhelm C, Hoffmann G, Lampousi A-M, Knüppel S, Iqbal K, Bechthold A, Schlesinger S, Boeing H. Food groups and risk of all-cause mortality: a systematic review and meta-analysis of prospective studies. Am J Clin Nutr. 2017;105(6):1462–73. [DOI] [PubMed] [Google Scholar]
- 7. Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB. Meta-analysis of observational studies in epidemiology: a proposal for reporting. JAMA. 2000;283(15):2008–12. [DOI] [PubMed] [Google Scholar]
- 8. Nilsson LM, Winkvist A, Esberg A, Jansson JH, Wennberg P, Van Guelpen B, Johansson I. Dairy products and cancer risk in a Northern Sweden population. Nutr Cancer. 2020;72:409–20. [DOI] [PubMed] [Google Scholar]
- 9. Zhang R, Zhang X, Wu K, Wu H, Sun Q, Hu FB, Han J, Willett WC, Giovannucci EL. Rice consumption and cancer incidence in US men and women. Int J Cancer. 2016;138(3):555–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–5. [DOI] [PubMed] [Google Scholar]
- 11. Schwingshackl L, Knüppel S, Schwedhelm C, Hoffmann G, Missbach B, Stelmach-Mardas M, Dietrich S, Eichelmann F, Kontopanteils E, Iqbal K. Perspective: NutriGrade: a scoring system to assess and judge the meta-evidence of randomized controlled trials and cohort studies in nutrition research. Adv Nutr. 2016;7(6):994–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Greenland S, Longnecker M. Methods for trend estimation from summarized dose-response data, with applications to meta-analysis. Am J Epidemiol. 1992;135(11):1301–9. [DOI] [PubMed] [Google Scholar]
- 13. Orsini N, Bellocco R, Greenland SJT. Generalized least squares for trend estimation of summarized dose–response data. SJ. 2006;6(1):40–57. [Google Scholar]
- 14. Kabat GC, Cross AJ, Park Y, Schatzkin A, Hollenbeck AR, Rohan TE, Sinha R. Meat intake and meat preparation in relation to risk of postmenopausal breast cancer in the NIH-AARP diet and health study. Int J Cancer. 2009;124(10):2430–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dunneram Y, Greenwood DC, Cade JE. Diet and risk of breast, endometrial and ovarian cancer: UK Women's Cohort Study. Br J Nutr. 2019;122(5):564–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Egeberg R, Olsen A, Autrup H, Christensen J, Stripp C, Tetens I, Overvad K, Tjønneland A. Meat consumption, N-acetyl transferase 1 and 2 polymorphism and risk of breast cancer in Danish postmenopausal women. Eur J Cancer Prev. 2008;17(1):39–47. [DOI] [PubMed] [Google Scholar]
- 17. Gaard M, Tretli S, Loken EB. Dietary fat and the risk of breast cancer: a prospective study of 25,892 Norwegian women. Int J Cancer. 1995;63(1):13–7. [DOI] [PubMed] [Google Scholar]
- 18. Genkinger JM, Makambi KH, Palmer JR, Rosenberg L, Adams-Campbell LL. Consumption of dairy and meat in relation to breast cancer risk in the Black Women's Health Study. Cancer Causes Control. 2013;24(4):675–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Holmes MD, Colditz GA, Hunter DJ, Hankinson SE, Rosner B, Speizer FE, Willett WC. Meat, fish and egg intake and risk of breast cancer. Int J Cancer. 2003;104(2):221–7. [DOI] [PubMed] [Google Scholar]
- 20. Inoue-Choi M, Sinha R, Gierach GL, Ward MH. Red and processed meat, nitrite, and heme iron intakes and postmenopausal breast cancer risk in the NIH-AARP Diet and Health Study. Int J Cancer. 2016;138(7):1609–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Key TJ, Balkwill A, Bradbury KE, Reeves GK, Kuan AS, Simpson RF, Green J, Beral V. Foods, macronutrients and breast cancer risk in postmenopausal women: a large UK cohort. Int J Epidemiol. 2018;48(2):489–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lo JJ, Park YMM, Sinha R, Sandler DP. Association between meat consumption and risk of breast cancer: findings from the Sister Study. Int J Cancer. 2019;146(8):2156–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mills PK, Beeson WL, Phillips RL, Fraser GE. Dietary habits and breast cancer incidence among Seventh-Day Adventists. Cancer. 1989;64(3):582–90. [DOI] [PubMed] [Google Scholar]
- 24. Shannon J, Ray R, Wu C, Nelson Z, Gao DL, Li W, Hu W, Lampe J, Horner N, Satia J. Food and botanical groupings and risk of breast cancer: a case-control study in Shanghai, China. Cancer Epidemiol Biomarkers Prev. 2005;14(1):81–90. [PubMed] [Google Scholar]
- 25. Taylor EF, Burley VJ, Greenwood DC, Cade JE. Meat consumption and risk of breast cancer in the UK Women's Cohort Study. Br J Cancer. 2007;96(7):1139–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Toniolo P, Riboli E, Shore RE, Pasternack BS. Consumption of meat, animal products, protein, and fat and risk of breast cancer: a prospective cohort study in New York. Epidemiology. 1994;5(4):391–7. [DOI] [PubMed] [Google Scholar]
- 27. Van Der Hel OL, Peeters PHM, Hein DW, Doll MA, Grobbee DE, Ocké M, Bueno De Mesquita HB. GSTM1 null genotype, red meat consumption and breast cancer risk (The Netherlands). Cancer Causes Control. 2004;15(3):295–303. [DOI] [PubMed] [Google Scholar]
- 28. Vatten LJ, Solvoll K, Loken EB. Frequency of meat and fish intake and risk of breast cancer in a prospective study of 14,500 Norwegian women. Int J Cancer. 1990;46(1):12–5. [DOI] [PubMed] [Google Scholar]
- 29. Cho E, Chen WY, Hunter DJ, Stampfer MJ, Colditz GA, Hankinson SE, Willett WC. Red meat intake and risk of breast cancer among premenopausal women. Arch Intern Med. 2006;166(20):2253–9. [DOI] [PubMed] [Google Scholar]
- 30. Harris HR, Bergkvist L, Wolk A. An estrogen-associated dietary pattern and breast cancer risk in the Swedish Mammography Cohort. Int J Cancer. 2015;137(9):2149–54. [DOI] [PubMed] [Google Scholar]
- 31. Trichopoulou A, Bamia C, Lagiou P, Trichopoulos D. Conformity to traditional Mediterranean diet and breast cancer risk in the Greek EPIC (European Prospective Investigation into Cancer and Nutrition) cohort. Am J Clin Nutr. 2010;92(3):620–5. [DOI] [PubMed] [Google Scholar]
- 32. Gertig DM, Hankinson SE, Hough H, Spiegelman D, Colditz GA, Willett WC, Kelsey KT, Hunter DJ. N‐acetyl transferase 2 genotypes, meat intake and breast cancer risk. Int J Cancer. 1999;80(1):13–7. [DOI] [PubMed] [Google Scholar]
- 33. Cross AJ, Leitzmann MF, Gail MH, Hollenbeck AR, Schatzkin A, Sinha R. A prospective study of red and processed meat intake in relation to cancer risk. PLoS Med. 2007;4(12):e325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Anderson JJ, Darwis ND, Mackay DF, Celis-Morales CA, Lyall DM, Sattar N, Gill JM, Pell JP. Red and processed meat consumption and breast cancer: UK Biobank cohort study and meta-analysis. Eur J Cancer. 2018;90:73–82. [DOI] [PubMed] [Google Scholar]
- 35. Couto E, Sandin S, Löf M, Ursin G, Adami HO, Weiderpass E. Mediterranean dietary pattern and risk of breast cancer. PLoS One. 2013;8(2):e55374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Farvid MS, Cho E, Chen WY, Eliassen AH, Willett WC. Dietary protein sources in early adulthood and breast cancer incidence: prospective cohort study. BMJ. 2014;348:3437–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ferrucci L, Cross A, Graubard B, Brinton L, McCarty C, Ziegler R, Ma X, Mayne S, Sinha R. Intake of meat, meat mutagens, and iron and the risk of breast cancer in the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial. Br J Cancer. 2009;101(1):178–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Larsson SC, Bergkvist L, Wolk A. Long-term meat intake and risk of breast cancer by oestrogen and progesterone receptor status in a cohort of Swedish women. Eur J Cancer. 2009;45(17):3042–6. [DOI] [PubMed] [Google Scholar]
- 39. Marcondes LH, Franco OH, Ruiter R, Ikram MA, Mulder M, Stricker BH, Kiefte-De Jong JC. Animal foods and postmenopausal breast cancer risk: a prospective cohort study. Br J Nutr. 2019;122:583–91. [DOI] [PubMed] [Google Scholar]
- 40. Pala V, Krogh V, Berrino F, Sieri S, Grioni S, Tjonneland A, Olsen A, Jakobsen MU, Overvad K, Clavel-Chapelon Fet al. Meat, eggs, dairy products, and risk of breast cancer in the European Prospective Investigation into Cancer and Nutrition (EPIC) cohort. Am J Clin Nutr. 2009;90(3):602–12. [DOI] [PubMed] [Google Scholar]
- 41. Pouchieu C, Deschasaux M, Hercberg S, Druesne-Pecollo N, Latino-Martel P, Touvier M. Prospective association between red and processed meat intakes and breast cancer risk: modulation by an antioxidant supplementation in the SU.VI.MAX randomized controlled trial. Int J Epidemiol. 2014;43(5):1583–92. [DOI] [PubMed] [Google Scholar]
- 42. Wirfalt E, Li C, Manjer J, Ericson U, Sonestedt E, Borgquist S, Landberg G, Olsson H, Gullberg B. Food sources of fat and sex hormone receptor status of invasive breast tumors in women of the Malmo diet and cancer cohort. Nutr Cancer. 2011;63(5):722–33. [DOI] [PubMed] [Google Scholar]
- 43. Kabat GC, Miller AB, Jain M, Rohan TE. Dietary iron and heme iron intake and risk of breast cancer: a prospective cohort study. Cancer Epidemiol Biomarkers Prev. 2007;16(6):1306–8. [DOI] [PubMed] [Google Scholar]
- 44. Key TJ, Sharp GB, Appleby PN, Beral V, Goodman MT, Soda M, Mabuchi K. Soya foods and breast cancer risk: a prospective study in Hiroshima and Nagasaki, Japan. Br J Cancer. 1999;81(7):1248–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Voorrips LE, Brants HAM, Kardinaal AFM, Hiddink GJ, Van Den Brandt PA, Alexandra Goldbohm R. Intake of conjugated linoleic acid, fat, and other fatty acids in relation to postmenopausal breast cancer: The Netherlands Cohort Study on Diet and Cancer. Am J Clin Nutr. 2002;76(4):873–82. [DOI] [PubMed] [Google Scholar]
- 46. Daniel CR, Cross AJ, Graubard BI, Hollenbeck AR, Park Y, Sinha R. Prospective investigation of poultry and fish intake in relation to cancer risk. Cancer Prev Res. 2011;4(11):1903–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Adebamowo CA, Cho E, Sampson L, Katan MB, Spiegelman D, Willett WC, Holmes MD. Dietary flavonols and flavonol-rich foods intake and the risk of breast cancer. Int J Cancer. 2005;114(4):628–33. [DOI] [PubMed] [Google Scholar]
- 48. Boggs DA, Palmer JR, Wise LA, Spiegelman D, Stampfer MJ, Adams-Campbell LL, Rosenberg L. Fruit and vegetable intake in relation to risk of breast cancer in the Black women's health study. Am J Epidemiol. 2010;172(11):1268–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Butler LM, Wu AH, Wang R, Koh WP, Yuan JM, Yu MC. A vegetable-fruit-soy dietary pattern protects against breast cancer among postmenopausal Singapore Chinese women. Am J Clin Nutr. 2010;91(4):1013–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Diallo A, Deschasaux M, Latino-Martel P, Hercberg S, Galan P, Fassier P, Alles B, Gueraud F, Pierre FH, Touvier M. Red and processed meat intake and cancer risk: results from the prospective NutriNet-Sante cohort study. Int J Cancer. 2018;142(2):230–7. [DOI] [PubMed] [Google Scholar]
- 51. Egeberg R, Olsen A, Loft S, Christensen J, Johnsen NF, Overvad K, Tjønneland A. Intake of whole grain products and risk of breast cancer by hormone receptor status and histology among postmenopausal women. Int J Cancer. 2009;124(3):745–50. [DOI] [PubMed] [Google Scholar]
- 52. Emaus MJ, Peeters PH, Bakker MF, Overvad K, Tjonneland A, Olsen A, Romieu I, Ferrari P, Dossus L, Boutron-Ruault MCet al. Vegetable and fruit consumption and the risk of hormone receptor-defined breast cancer in the EPIC cohort. Am J Clin Nutr. 2016;103(1):168–77. [DOI] [PubMed] [Google Scholar]
- 53. Engeset D, Alsaker E, Lund E, Welch A, Khaw KT, Clavel-Chapelon F, Thiebaut A, Chajes V, Key TJ, Allen NEet al. Fish consumption and breast cancer risk. Int J Cancer. 2006;119(1):175–82. [DOI] [PubMed] [Google Scholar]
- 54. Farvid MS, Cho E, Eliassen AH, Chen WY, Willett WC. Lifetime grain consumption and breast cancer risk. Breast Cancer Res Treat. 2016;159(2):335–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Farvid MS, Eliassen AH, Cho E, Chen WY, Willett WC. Dairy consumption in adolescence and early adulthood and risk of breast cancer. Cancer Epidemiol Biomarkers Prev. 2018;27(5):575–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Farvid MS, Chen WY, Rosner BA, Tamimi RM, Willett WC, Eliassen AH. Fruit and vegetable consumption and breast cancer incidence: repeated measures over 30 years of follow-up. Int J Cancer. 2019;144(7):1496–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Folsom AR, Demissie Z. Fish intake, marine omega-3 fatty acids, and mortality in a cohort of postmenopausal women. Am J Epidemiol. 2004;160(10):1005–10. [DOI] [PubMed] [Google Scholar]
- 58. Fung TT, Hu FB, Hankinson SE, Willett WC, Holmes MD. Low-carbohydrate diets, dietary approaches to stop hypertension-style diets, and the risk of postmenopausal breast cancer. Am J Epidemiol. 2011;174(6):652–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. George SM, , Park Y, Leitzmann MF, Freedman ND, Dowling EC, Reedy J, Schatzkin A, Hollenbeck A, Subar A. Fruit and vegetable intake and risk of cancer: a prospective cohort study. AJCN. 2009;89(1):347–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Giles GG, Simpson JA, English DR, Hodge AM, Gertig DM, MacInnis RJ, Hopper JL. Dietary carbohydrate, fibre, glycaemic index, glycaemic load and the risk of postmenopausal breast cancer. Int J Cancer. 2006;118(7):1843–7. [DOI] [PubMed] [Google Scholar]
- 61. Haraldsdottir A, Steingrimsdottir L, Valdimarsdottir UA, Aspelund T, Tryggvadottir L, Harris TB, Launer LJ, Mucci LA, Giovannucci EL, Adami HOet al. Early life residence, fish consumption, and risk of breast cancer. Cancer Epidemiol Biomarkers Prev. 2017;26(3):346–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Hirvonen T, Mennen LI, de Bree A, Castetbon K, Galan P, Bertrais S, Arnault N, Hercberg S. Consumption of antioxidant-rich beverages and risk for breast cancer in French women. Ann Epidemiol. 2006;16(7):503–8. [DOI] [PubMed] [Google Scholar]
- 63. Hjartåker A, Thoresen M, Engeset D, Lund E. Dairy consumption and calcium intake and risk of breast cancer in a prospective cohort: the Norwegian Women and Cancer study. Cancer Causes Control. 2010;21(11):1875–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Kesse-Guyot E, Bertrais S, Duperray B, Arnault N, Bar-Hen A, Galan P, Hercberg S. Dairy products, calcium and the risk of breast cancer: results of the French SU.VI.MAX prospective study. Ann Nutr Metab. 2007;51(2):139–45. [DOI] [PubMed] [Google Scholar]
- 65. Kiyabu GY, Inoue M, Saito E, Abe SK, Sawada N, Ishihara J, Iwasaki M, Yamaji T, Shimazu T, Sasazuki Set al. Fish, n-3 polyunsaturated fatty acids and n-6 polyunsaturated fatty acids intake and breast cancer risk: the Japan Public Health Center–based prospective study. Int J Cancer. 2015;137(12):2915–26. [DOI] [PubMed] [Google Scholar]
- 66. Lin J, Manson JE, Lee I-M, Cook NR, Buring JE, Zhang SM. Intakes of calcium and vitamin D and breast cancer risk in women. Arch Intern Med. 2007;167(10):1050–9. [DOI] [PubMed] [Google Scholar]
- 67. Masala G, Assedi M, Bendinelli B, Ermini I, Sieri S, Grioni S, Sacerdote C, Ricceri F, Panico S, Mattiello Aet al. Fruit and vegetables consumption and breast cancer risk: the EPIC Italy study. Breast Cancer Res Treat. 2012;132(3):1127–36. [DOI] [PubMed] [Google Scholar]
- 68. McCullough ML, Rodriguez C, Diver WR, Feigelson HS, Stevens VL, Thun MJ, Calle EE. Dairy, calcium, and vitamin D intake and postmenopausal breast cancer risk in the cancer prevention study II nutrition cohort. Cancer Epidemiol Biomarkers Prev. 2005;14(12):2898–904. [DOI] [PubMed] [Google Scholar]
- 69. Narita S, Inoue M, Saito E, Abe SK, Sawada N, Ishihara J, Iwasaki M, Yamaji T, Shimazu T, Sasazuki Set al. Dietary fiber intake and risk of breast cancer defined by estrogen and progesterone receptor status: the Japan Public Health Center–based prospective study. Cancer Causes Control. 2017;28(6):569–78. [DOI] [PubMed] [Google Scholar]
- 70. Nicodemus KK, Jacobs DR Jr., Folsom AR. Whole and refined grain intake and risk of incident postmenopausal breast cancer (United States). Cancer Causes Control. 2001;12(10):917–25. [DOI] [PubMed] [Google Scholar]
- 71. Nishio K, Niwa Y, Toyoshima H, Tamakoshi K, Kondo T, Yatsuya H, Yamamoto A, Suzuki S, Tokudome S, Lin Yet al. Consumption of soy foods and the risk of breast cancer: findings from the Japan Collaborative Cohort (JACC) study. Cancer Causes Control. 2007;18(8):801–8. [DOI] [PubMed] [Google Scholar]
- 72. Olsen A, Tjonneland A, Thomsen BL, Loft S, Stripp C, Overvad K, Moller S, Olsen JH. Fruits and vegetables intake differentially affects estrogen receptor negative and positive breast cancer incidence rates. J Nutr. 2003;133(7):2342–7. [DOI] [PubMed] [Google Scholar]
- 73. Park Y, Leitzmann MF, Subar AF, Hollenbeck A, Schatzkin A. Dairy food, calcium, and risk of cancer in the NIH-AARP Diet and Health Study. Arch Intern Med. 2009;169(4):391–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Rohan TE, Howe GR, Friedenreich CM, Jain M, Miller AB. Dietary fiber, vitamins A, C, and E, and risk of breast cancer: a cohort study. Cancer Causes Control. 1993;4(1):29–37. [DOI] [PubMed] [Google Scholar]
- 75. Shibata A, Paganini-Hill A, Ross R, Henderson B. Intake of vegetables, fruits, beta-carotene, vitamin C and vitamin supplements and cancer incidence among the elderly: a prospective study. Br J Cancer. 1992;66(4):673–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Shin MH, Holmes MD, Hankinson SE, Wu K, Colditz GA, Willett WC. Intake of dairy products, calcium, and vitamin D and risk of breast cancer. J Natl Cancer Inst. 2002;94(17):1301–11. [DOI] [PubMed] [Google Scholar]
- 77. Shin W-K, Lee H-W, Shin A, Lee J-K, Kang D. Milk consumption decreases risk for breast cancer in Korean women under 50 years of age: Results from the Health Examinees Study. Nutrients. 2019;12(1):32–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Sonestedt E, Borgquist S, Ericson U, Gullberg B, Landberg G, Olsson H, Wirfalt E. Plant foods and oestrogen receptor alpha- and beta-defined breast cancer: observations from the Malmo Diet and Cancer cohort. Carcinogenesis. 2008;29(11):2203–9. [DOI] [PubMed] [Google Scholar]
- 79. Stolzenberg-Solomon RZ, Chang SC, Leitzmann MF, Johnson KA, Johnson C, Buys SS, Hoover RN, Ziegler RG. Folate intake, alcohol use, and postmenopausal breast cancer risk in the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial. Am J Clin Nutr. 2006;83(4):895–904. [DOI] [PubMed] [Google Scholar]
- 80. Stripp C, Overvad K, Christensen J, Thomsen BL, Olsen A, Møller S, Tjønneland A. Fish intake is positively associated with breast cancer incidence rate. J Nutr. 2003;133(11):3664–9. [DOI] [PubMed] [Google Scholar]
- 81. Suzuki R, Iwasaki M, Hara A, Inoue M, Sasazuki S, Sawada N, Yamaji T, Shimazu T, Tsugane S. Fruit and vegetable intake and breast cancer risk defined by estrogen and progesterone receptor status: the Japan Public Health Center–based prospective study. Cancer Causes Control. 2013;24(12):2117–28. [DOI] [PubMed] [Google Scholar]
- 82. van den Brandt PA, Nieuwenhuis L. Tree nut, peanut, and peanut butter intake and risk of postmenopausal breast cancer: the Netherlands Cohort Study. Cancer Causes Control. 2018;29(1):63–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Verhoeven D, Assen N, Goldbohm R, Dorant E, Van't Veer P, Sturmans F, Hermus R, Van den Brandt P. Vitamins C and E, retinol, beta-carotene and dietary fibre in relation to breast cancer risk: a prospective cohort study. Br J Cancer. 1997;75(1):149–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Wada K, Nakamura K, Tamai Y, Tsuji M, Kawachi T, Hori A, Takeyama N, Tanabashi S, Matsushita S, Tokimitsu Net al. Soy isoflavone intake and breast cancer risk in Japan: from the Takayama study. Int J Cancer. 2013;133(4):952–60. [DOI] [PubMed] [Google Scholar]
- 85. Wilson KM, Mucci LA, Cho E, Hunter DJ, Chen WY, Willett WC. Dietary acrylamide intake and risk of premenopausal breast cancer. Am J Epidemiol. 2009;169(8):954–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Wu H, Kyrø C, Tjønneland A, Boll K, Olsen A, Overvad K, Landberg R. Long-term whole grain wheat and rye intake reflected by adipose tissue alkylresorcinols and breast cancer: a case-cohort study. Nutrients. 2019;11(2):465–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Mattisson I, Wirfält E, Johansson U, Gullberg B, Olsson H, Berglund G. Intakes of plant foods, fibre and fat and risk of breast cancer–a prospective study in the Malmö Diet and Cancer cohort. Br J Cancer. 2004;90(1):122–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Bradbury KE, Appleby PN, Key TJ. Fruit, vegetable, and fiber intake in relation to cancer risk: findings from the European Prospective Investigation into Cancer and Nutrition (EPIC). Am J Clin Nutr. 2014;100(Suppl 1):394S–8S. [DOI] [PubMed] [Google Scholar]
- 89. Fung TT, Chiuve SE, Willett WC, Hankinson SE, Hu FB, Holmes MD. Intake of specific fruits and vegetables in relation to risk of estrogen receptor-negative breast cancer among postmenopausal women. Breast Cancer Res Treat. 2013;138(3):925–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Farvid MS, Chen WY, Michels KB, Cho E, Willett WC, Eliassen AH. Fruit and vegetable consumption in adolescence and early adulthood and risk of breast cancer: population based cohort study. BMJ. 2016;353:i2343–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Zhang S, Hunter DJ, Forman MR, Rosner BA, Speizer FE, Colditz GA, Manson JE, Hankinson SE, Willett WC. Dietary carotenoids and vitamins A, C, and E and risk of breast cancer. J Natl Cancer Inst. 1999;91(6):547–56. [DOI] [PubMed] [Google Scholar]
- 92. Hjartaker A, Laake P, Lund E. Childhood and adult milk consumption and risk of premenopausal breast cancer in a cohort of 48,844 women–the Norwegian Women and Cancer Study. Int J Cancer. 2001;93(6):888–93. [DOI] [PubMed] [Google Scholar]
- 93. Lo JJ, Park YMM, Sinha R, Sandler DP. Association between meat consumption and risk of breast cancer: findings from the Sister Study. Int J Cancer. 2020;146(8):2156–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Han MA, Zeraatkar D, Guyatt GH, Vernooij RWM, El Dib R, Zhang Y, Algarni A, Leung G, Storman D, Valli Cet al. Reduction of red and processed meat intake and cancer mortality and incidence a systematic review and meta-analysis of cohort studies. Ann Intern Med. 2019;171(10):711–20. [DOI] [PubMed] [Google Scholar]
- 95. Farvid MS, Stern MC, Norat T, Sasazuki S, Vineis P, Weijenberg MP, Wolk A, Wu K, Stewart BW, Cho E. Consumption of red and processed meat and breast cancer incidence: a systematic review and meta-analysis of prospective studies. Int J Cancer. 2018;143(11):2787–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Wu J, Zeng R, Huang J, Li X, Zhang J, Ho JCM, Zheng Y. Dietary protein sources and incidence of breast cancer: a dose-response meta-analysis of prospective studies. Nutrients. 2016;8(11):730–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Dai Q, Shu XO, Jin F, Gao YT, Ruan ZX, Zheng W. Consumption of animal foods, cooking methods, and risk of breast cancer. Cancer Epidemiol Biomarkers Prev. 2002;11(9):801–8. [PubMed] [Google Scholar]
- 98. Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, Rosso S, Coebergh JWW, Comber H, Forman D, Bray F. Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. Eur J Cancer. 2013;49(6):1374–403. [DOI] [PubMed] [Google Scholar]
- 99. Chen L, Li M, Li H. Milk and yogurt intake and breast cancer risk: a meta-analysis. Medicine (Baltimore). 2019;98(12):e14900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Ganmaa D, Sato A. The possible role of female sex hormones in milk from pregnant cows in the development of breast, ovarian and corpus uteri cancers. Med Hypotheses. 2005;65(6):1028–37. [DOI] [PubMed] [Google Scholar]
- 101. Harrison S, Lennon R, Holly J, Higgins JPT, Gardner M, Perks C, Gaunt T, Tan V, Borwick C, Emmet Pet al. Does milk intake promote prostate cancer initiation or progression via effects on insulin-like growth factors (IGFs)? A systematic review and meta-analysis. Cancer Causes Control. 2017;28(6):497–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Liao MQ, Gao XP, Yu XX, Zeng YF, Li SN, Naicker N, Joseph T, Cao WT, Liu YH, Zhu Set al. Effects of dairy products, calcium, and vitamin D on ovarian cancer risk: a meta-analysis of 29 epidemiological studies. Br J Nutr. 2020; 124(10):1001–12. [DOI] [PubMed] [Google Scholar]
- 103. Dekker LH, Vinke PC, Riphagen IJ, Minović I, Eggersdorfer ML, van den Heuvel E, Schurgers LJ, Kema IP, Bakker SJL, Navis G. Cheese and healthy diet: associations with incident cardio-metabolic diseases and all-cause mortality in the general population. Front Nutr. 2019;6:185–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Zang J, Shen M, Du S, Chen T, Zou S. The association between dairy intake and breast cancer in Western and Asian populations: a systematic review and meta-analysis. J Breast Cancer. 2015;18(4):313–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Aune D, Chan DSM, Vieira AR, Navarro Rosenblatt DA, Vieira R, Greenwood DC, Norat T. Fruits, vegetables and breast cancer risk: a systematic review and meta-analysis of prospective studies. Breast Cancer Res Treat. 2012;134(2):479–93. [DOI] [PubMed] [Google Scholar]
- 106. Jung S, Spiegelman D, Baglietto L, Bernstein L, Boggs DA, Van Den Brandt PA, Buring JE, Cerhan JR, Gaudet MM, Giles GGet al. Fruit and vegetable intake and risk of breast cancer by hormone receptor status. J Natl Cancer Inst. 2013;105(3):219–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Andersen JLM, Hansen L, Thomsen BLR, Christiansen LR, Dragsted LO, Olsen A. Pre- and post-diagnostic intake of whole grain and dairy products and breast cancer prognosis: the Danish Diet, Cancer and Health cohort. Breast Cancer Res Treat. 2020;179(3):743–53. [DOI] [PubMed] [Google Scholar]
- 108. Castelló A, Pollán M, Buijsse B, Ruiz A, Casas AM, Baena-Cañada JM, Lope V, Antolýn S, Ramos M, Muñoz Met al. Spanish Mediterranean diet and other dietary patterns and breast cancer risk: case-control EpiGEICAM study. Br J Cancer. 2014;111:1454–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Xiao Y, Ke Y, Wu S, Huang S, Li S, Lv Z, Yeoh EK, Lao X, Wong S, Kim JHet al. Association between whole grain intake and breast cancer risk: a systematic review and meta-analysis of observational studies. Nutr J. 2018;17(1). doi:10.1186/s12937-018-0394-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Zhao T-T, Jin F, Li J-G, Xu Y-Y, Dong H-T, Liu Q, Xing P, Zhu G-L, Xu H, Miao Z-F. Dietary isoflavones or isoflavone-rich food intake and breast cancer risk: a meta-analysis of prospective cohort studies. Clin Nutr. 2019;38(1):136–45. [DOI] [PubMed] [Google Scholar]
- 111. Wei Y, Lv J, Guo Y, Bian Z, Gao M, Du H, Yang L, Chen Y, Zhang X, Wang T. Soy intake and breast cancer risk: a prospective study of 300,000 Chinese women and a dose-response meta-analysis. Eur J Epidemiol. 2019;35(6):567–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Bahrom S, Idris NRN. Soy intake and breast cancer risk: a meta-analysis of epidemiological studies. AIP Conf Proc; 2016;75–82. [Google Scholar]
- 113. Si R, Qu K, Jiang Z, Yang X, Gao P. Egg consumption and breast cancer risk: a meta-analysis. Breast Cancer. 2014;21(3):251–61. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.