ABSTRACT
Several randomized clinical trials have investigated the effect of dietary advanced glycation end products (AGEs) on metabolic syndrome risk factors in adults. However, the results of these studies were conflicting. Therefore, our aim was to assess the effect of dietary AGEs on metabolic syndrome risk factors. We searched the PubMed-MEDLINE, Scopus, Cochrane Databases, Google Scholar, Web of Science, and Embase databases for papers published up to October 2019 that investigated the effect of dietary AGEs on metabolic syndrome risk factors. From the eligible trials, 13 articles were selected for inclusion in this systematic review and meta-analysis. The meta-analysis was performed using a random-effects model. Heterogeneity was determined by I2 statistics and Cochrane Q test. Pooled results from the random-effects model showed a significant reduction for insulin resistance [weighted mean difference (WMD): −1.204; 95% CI: −2.057, −0.358; P = 0.006], fasting insulin (WMD: −5.472 μU/mL; 95% CI: −9.718, −1.234 μU/mL; P = 0.011), total cholesterol (WMD: −5.486 mg/dL; 95% CI: −10.222, −0.747 mg/dL; P = 0.023), and LDL (WMD: −6.263 mg/dL; 95% CI: −11.659, −0.866 mg/dL; P = 0.023) in the low-AGEs groups compared with the high-AGEs groups. There were no changes in the other components of the metabolic syndrome. The results of this review suggest that a diet with a low AGEs content has beneficial effects on insulin resistance, fasting insulin, total cholesterol, and LDL. Moreover, following a diet low in AGEs may be a helpful strategy to decrease the burden of metabolic syndrome risk factors in adults and particularly in patients with diabetes.
Keywords: meta-analysis, dietary advanced glycation end products, randomized controlled trials, systematic review, metabolic syndrome
Introduction
The prevalence of the metabolic syndrome (MetS) and its related complications has been increasing worldwide at a worrisome pace (1). The disease is characterized by a cluster of interrelated cardiometabolic risk factors—for example, abdominal obesity, high blood pressure, hyperglycemia, and dyslipidemia (2). These factors are major contributors to the development of cardiovascular diseases, type 2 diabetes mellitus (T2DM), and other chronic diseases (3, 4). In addition to genetic factors, environmental factors such as physical activity and diet play key roles in the development of MetS and related complications (5, 6). In both high-income and middle-/low-income countries, the consumption of highly processed food has dramatically increased (7). Processed foods are rich in fat, sugar, salt, and potentially toxic compounds known as advanced glycation end products (AGEs) (8, 9). AGEs are a group of sugar modification products that are formed through the nonenzymatic reaction of sugars with free amino groups of proteins, lipids, or nucleic acids (9, 10). AGEs have noticeable pro-inflammatory and pro-oxidant effects (11). In addition, studies have discovered a link between the intake of diet-derived AGEs and serum AGE concentrations, insulin resistance, and visceral fat; this association might suggest an important contribution of AGEs in the pathogenesis of MetS (12, 13). Although the average total daily intake of AGEs in an adult's regular diet is ∼1600 AGEs kU/d, the consumption of a diet high in heat-processed foods, sugar, and fats can increase the daily intake of AGEs to >200,000 Ku/d (14). Moreover, AGEs lead to increased oxidative stress levels and chronic inflammation in the body, both of which are relevant actors in the installment of MetS and its related complications. Thus, we may hypothesize that a diet with a low AGE content might be helpful in preventing the development of chronic diseases (15). Several clinical trials have reported that the consumption of a diet with a low AGE content reduced inflammation and decreased the presence of cardiometabolic risk factors (16–18). However, the results from the literature regarding the impact of dietary AGEs are controversial. Inconsistency has arisen from the different health conditions of the subjects included in these studies or from the amount of AGEs derived from the diets received by participants. Although previous systematic reviews have investigated the impact of low-AGEs and high-AGEs diets on metabolic risk factors such as insulin resistance, inflammatory indices, and oxidative stress, their effects on other cardiovascular risk factors have not been assessed (12, 15, 19). Furthermore, the previous reviews did not contain a meta-analysis aimed at evaluating the impact of diet-derived AGEs on the clinical indices of cardiometabolic risk. In addition, a meta-analysis conducted by Baye et al. (14) assessed the effects of AGEs on several biochemical parameters, but their search was limited to articles published until 2016; no studies published in more recent years were included. Moreover, the results of this review are limited because necessary subgroup analyses—for example, subgroup analyses based on the health status of the subjects (overweight, obese, or diabetic) or the duration of the intervention—were been performed and there are several limitations related to the quantity and quality of the assessed publications. Therefore, the aim of the current systematic review and meta-analysis of published randomized controlled trials (RCTs) was to assess the effects of dietary AGEs on MetS risk factors and to quantify the influence of AGEs on fasting serum glucose, fasting serum insulin, HOMA-IR, lipid profile, waist circumference (WC), and blood pressure in both healthy individuals and patients diagnosed with several disorders.
Methods
Search strategy
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement was used as a guideline during all stages of the design, implementation, and reporting of the current systematic review and meta-analysis (20). Systematic computerized literature searches of PubMed/MEDLINE (https://pubmed.ncbi.nlm.nih.gov/), Clarivate Analytics Web of Science (http://www.webofknowledge.com), Scopus (http://www.scopus.com), the Cochrane Central Register of Controlled Trials (http://www.cochranelibrary.com), and Google Scholar (http://scholar.google.com) were performed up to October 2019. Search terms included [glycosylation OR “advanced glycation end product” OR “advanced glycation end product receptor” OR “advanced glyc*” OR “maillard reaction product” OR “maillard reaction product” OR “browning reaction” OR glycation OR “advanced lipox*” OR “nepsilon carboxymethyllysine” OR “methyl glyoxal” OR srage OR “endogenous secretory receptor for advanced glycation end products” OR esrage OR thermal OR “soluble receptor of advanced glycation end products” OR glycat*] and [(random* OR factorial* OR crossover*) AND (cross over*) OR (placebo* OR doubl*) AND blind* OR (singl* blind*) OR assign* OR allocat* OR volunteer* OR “cross over procedure”/exp OR “cross over procedure” OR “double blind procedure” OR “double blind procedure” OR “randomized controlled trial” OR “randomized controlled trial” OR “single blind procedure” OR “single blind procedur”]. To identify relevant studies, the titles and abstracts of the retrieved publications were separately reviewed by 2 authors (MHS and AL). Moreover, the references of the included literature and related reviews were screened to discover potentially relevant studies.
Eligibility criteria
Retrieved studies were included in our review if they met the following criteria: They 1) were original articles with an RCT design, 2) evaluated the effects of AGE diets in humans (either healthy or suffering from a disease), and 3) assessed the presence of risk factors for MetS. Studies were excluded if 1) they were conducted in children, 2) they evaluated single diet components rather than a whole diet, 3) the duration of the intervention was <24 h, or 4) they did not report the targeted outcomes.
Data extraction
Two independent reviewers (MHS and SF) extracted the relevant data, which were double-checked by other authors (FS and AL). We extracted the following data from each included study: first author's name, year of publication, country where the study was performed, study design, study period, participants’ characteristics (number, age, sex, and health status), and the mean changes with corresponding SDs of the measured outcomes in the intervention and control arms.
Quality assessment
Two reviewers (SF and FS) independently evaluated the methodological quality of the eligible studies via the Cochrane Collaboration's tool, which includes the following 6 domains: 1) random sequence generation (selection bias), 2) allocation concealment (selection bias), 3) blinding of participants and personnel (performance bias), 4) blinding of outcome assessment (detection bias), 5) incomplete outcome data (attrition bias), and 6) selective reporting (reporting bias). Because blinding is not possible in dietary interventions, the studies were judged based on the fulfillment of the other 5 items. Each domain was classified into 3 categories: low risk of bias, high risk of bias, and unclear risk of bias. According to the aforementioned domains, the overall quality of each individual study was considered as good (low risk for >2 items), fair (low risk for 2 items), or weak (low risk for <2 items).
Data synthesis and subgroup analysis
The statistical analysis was conducted using RevMan version 5.3 software (Cochran Collaboration) and STATA version 12.0 (Stata Corp). If data were expressed in a different format, standard calculations were executed to obtain the means and SDs (21, 22). For instance, if the SDs of the change were not stated in the trials, we derived them using the following formula: SD changes = square root [(SD baseline 2 + SD final 2) − (2 × R × SD baseline × SD final)]. Also, for trials that only reported SEM, SDs were obtained using the following formula: SD = SEM × √n, where n is the number of subjects in each group. Heterogeneity was examined using the I2 statistic, in which the source of heterogeneity was determined if the I2 value was >50% or if there was any inconsistency in the data across the RCTs (23). In order to identify potential sources of heterogeneity, a predefined subgroup analysis based on the duration of the intervention and the health status of the subjects was performed. A sensitivity analysis was applied to assess the contribution of each study to the overall mean difference. We assessed the presence of publication bias using the formal Egger's test (24).
Results
Search results
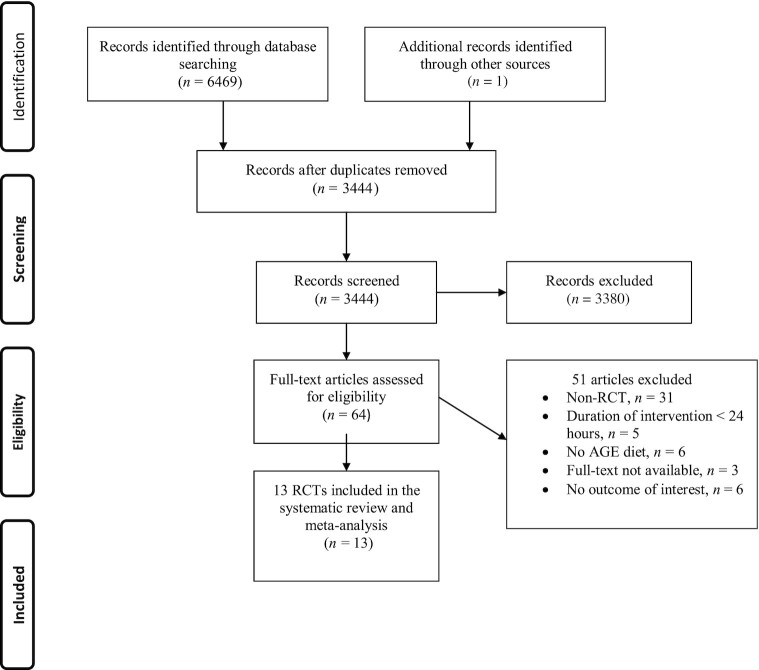
A total of 6469 papers were identified via the database searches. Two studies were retrieved from the other sources. After excluding the duplicates, 3444 papers remained for the additional screening based on title and abstract. Twenty-one studies that were not published in English were discarded, leaving 64 potentially eligible studies for further evaluation. Fifty-one publications were excluded for the following reasons: non-RCT (n = 31), duration of intervention <24 h (n = 5), no AGEs diet (n = 6), full text not available (n = 3), and no outcome of interest (n = 6). Therefore, 13 RCT studies were included in this systematic review and meta-analysis (Figure 1).
FIGURE 1.
Flowchart of study selection process. AGE, advanced glycation end product; RCT, randomized controlled trial.
Study characteristics
The detailed characteristics of the included articles are presented in Table 1. Eleven studies used a parallel design and 2 used a crossover design. The intervention duration varied from 2 to 48 wk. Five studies investigated the effects of dietary AGEs in patients with T2DM, 5 studies examined the effects in obese or overweight people, 2 studies examined the effects in healthy individuals, and 1 study examined the effects in women with polycystic ovary syndrome. Our primary outcome measures in this study were the components of MetS: waist circumference, triglycerides, HDL cholesterol, LDL cholesterol, total cholesterol, blood pressure (systolic and diastolic), and fasting blood sugar. Our secondary outcome measures were fasting insulin, HOMA-IR, and glycated hemoglobin (HbA1c) as variables related to glucose metabolism.
TABLE 1.
Included randomized controlled trial study characteristics of the impact of low AGEs diet on metabolic risk factors
| Study, year (reference) | Study design | Participants | Sample size, n | Intervention diet | Control diet | Duration | Dietary AGE content | Outcomes |
|---|---|---|---|---|---|---|---|---|
| Harcourt et al., 2011 (27) | Crossover | Overweight men aged 18–50 y and BMI (in kg/m2) 26–39 | 11 | LAGE diet | HAGE diet | 2 wk | HAGE = 14,090, LAGE = 3302 kU AGE/d | Fasting blood sugar |
| Uribarri et al., 2011 (25) | Parallel | Type 2 diabetic patients | 18 randomised, 18 assessed: 12 LAGE and 6 HAGE diet | LAGE diet | HAGE diet | 4 mo | HAGE = >2 0, LAGE = <10 AGE Eq/d | Fasting blood sugarHbA1cFasting insulinHOMA-IR |
| Luévano-Contreras et al., 2013 (37) | Parallel | Type 2 diabetic patients | 34 randomised, 26 assessed: 13 HAGE and 13 LAGE diet | LAGE diet | HAGE diet | 6 wk | HAGE = 9910 ± 4164, LAGE = 8956 ± 3587 kU CML/d | Fasting blood sugarHbA1cFasting insulinHOMA-IRTriglyceridesHDL cholesterolTotal cholesterol |
| Mark et al., 2014 (26) | Parallel | Overweight women aged 20–50 y | 99 randomised, 74 assessed: 36 LAGE and 37 HAGE diet | LAGE diet | HAGE diet | 4 wk | HAGE = 24.6, LAGE = 10.7 mg CML/d | Fasting blood sugarWaist circumferenceFasting insulinHOMA-IRTriglyceridesHDL cholesterolLDL cholesterolTotal cholesterol |
| Macías-Cervantes et al., 2015 (33) | Parallel | Overweight men (BMI >25) aged 30–55 y | 75 randomized, 45 assessed: 15 in the diet plus exercise group, 14 in the exercise group, and 14 in the diet group | LAGE diet | HAGE diet | 12 wk | HAGE = 13,284 ± 4983, LAGE = 13,019 ± 4526 kU AGE/d | Fasting blood sugarWaist circumferenceTriglyceridesHDL cholesterolLDL cholesterolSystolic blood pressureDiastolic blood pressure |
| Tantalaki et al., 2014 (31) | Crossover | PCOS; age range: 18–40 y | 34 randomised, 23 assessed | LAGE diet | HAGE diet | 8 wk | HAGE = 1869.6 ± 114.6, LAGE = 1869.6 ± 114.6 kU AGE/d | Fasting blood sugarFasting insulinHOMA-IR |
| Di Pino et al., 2016 (36) | Parallel | Adults with prediabetes; age range: 35–65 y; BMI: 18.5–40 | 62 randomised, 57 assessed: 29 LAGE and 28 HAGE diet | LAGE diet | HAGE diet | 24 wk | NA | Fasting blood sugarHbA1cTriglyceridesHDL cholesterolLDL cholesterolTotal cholesterolSystolic blood pressureDiastolic blood pressure |
| Vlassara et al., 2016 (35) | Parallel | Obese subjects with metabolic syndrome aged ≥50 y | 138 randomised, 100 assessed: 51 LAGE and 49 HAGE diet | LAGE diet | HAGE diet | 48 wk | HAGE = 20 ± 11, LAGE = 7 ± 6 AGE Eq/d | Fasting blood sugarHbA1cWaist circumferenceFasting insulinHOMA-IRTriglyceridesHDL cholesterol |
| Cai et al., 2004 (29) | Parallel | Diabetic patients with normal lipid profile and renal function | 24 randomised: 11 HAGE and 13 LAGE | LAGE diet | HAGE diet | 6 wk | HAGE = 5 times higher than LAGE | Fasting blood sugarHbA1cTriglyceridesHDL cholesterolLDL cholesterolTotal cholesterol |
| Vlassara et al., 2002 (30) | Parallel | Diabetic subjects with good glycemic control and normal renal function | 11 for crossover and 13 for parallel trials (6 in HAGE and 7 in LAGE diet) | LAGE diet | HAGE diet | 6 wk | HAGE = 5 times higher than LAGE | Fasting blood sugarTriglyceridesHDL cholesterolLDL cholesterolTotal cholesterolSystolic blood pressureDiastolic blood pressure |
| Baye et al., 2017 (34) | Parallel | Overweight and obese otherwise healthy adults | 24 randomised: 11 HAGE and 13 LAGE | LAGE diet | HAGE diet | 2 wk | HAGE = 3 times higher than LAGE | TriglyceridesHDL cholesterolLDL cholesterolTotal cholesterolSystolic blood pressureDiastolic blood pressure |
| Semba et al., 2014 (32) | Parallel | Healthy adults aged 50–69 y | 24 randomised, 24 assessed: 12 in each group | LAGE diet | HAGE diet | 6 wk | HAGE = 4 times higher than LAGE | Fasting blood sugarTriglyceridesHDL cholesterolLDL cholesterolTotal cholesterol |
| Uribarri et al., 2014 (28) | Parallel | Healthy participants aged >60 y | 18 randomised, 18 assessed: 10 LAGE and 8 HAGE | LAGE diet | HAGE diet | 16 wk | HAGE = >1 5, LAGE = <10 AGE Eq/d | HOMA-IRTriglyceridesHDL cholesterolLDL cholesterol |
AGE, advanced glycation end product; CML, carboxymethyllysine; HAGE, high advanced glycation end product; LAGE, low advanced glycation end product; NA, not applicable; PCOS, polycystic ovary syndrome.
Risk of bias assessment
As shown in Table 2, 6 studies were rated with unclear risk in random sequence generation because they did not explicitly mention the random sequence generation methods (25, 26, 27, 28, 29, 30), and 1 was rated as high risk (31). Two studies were rated as low risk in allocation concealment (32, 33), and 1 was rated as high risk (31). The other 10 studies were rated as unclear risk of bias. Only 1 study reported that both the participants and the researchers were blinded and was thus identified as having a low risk of bias for the blinding step (34). Four of the trials provided a clear description of the blinding of outcome assessment (32, 33, 34, 35). Six studies were clear on providing incomplete outcome data and then were considered as having a low risk of bias (25, 26, 32, 34, 35, 36). Six studies were assessed as having an unclear risk of bias in selective reporting (27, 28, 29, 30, 34, 37), and the other 7 studies were rated as having a low risk of bias. Except for 1 study that was considered as having a high risk of bias in the quality stage (31), the other 12 studies were considered as having an unclear risk of bias because we found ≥1 of their 6 key domains of quality as having an unclear risk of bias.
TABLE 2.
Risk of bias assessment according to the Cochrane Collaboration's Risk of Bias assessment tool
| Study, year (reference) | Random sequence generation | Allocation concealment | Blinding of participants and personnel | Blinding of outcome assessment | Incomplete outcome data | Selective reporting | Overall assessment of risk of bias |
|---|---|---|---|---|---|---|---|
| Uribarri et al., 2011 (25) | Unclear | Unclear | Unclear | Unclear | Low | Low | Unclear |
| Vlassara et al., 2016 (35) | Low | Unclear | Unclear | Low | Low | Low | Unclear |
| Uribarri et al., 2014 (28) | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear |
| Di Pino et al., 2016 (36) | Low | Unclear | Unclear | Unclear | Low | Low | Unclear |
| Baye et al., 2017 (34) | Low | Unclear | Low | Low | Low | Unclear | Unclear |
| Semba et al., 2014 (32) | Low | Low | Unclear | Low | Low | Low | Unclear |
| Mark et al., 2014 (26) | Unclear | Unclear | Unclear | Unclear | Low | Low | Unclear |
| Harcourt et al., 2011 (27) | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear |
| Macías-Cervantes et al., 2015 (33) | Low | Low | Unclear | Low | Unclear | Low | Unclear |
| Luévano-Contreras et al., 2012 (37) | Low | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear |
| Cai et al., 2004 (29) | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear |
| Vlassara et al., 2002 (30) | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear | Unclear |
| Tantalaki et al., 2014 (31) | High | High | Unclear | Unclear | Unclear | Low | High |
Effects of dietary AGEs on MetS risk factors
Waist circumference and blood pressure measurements
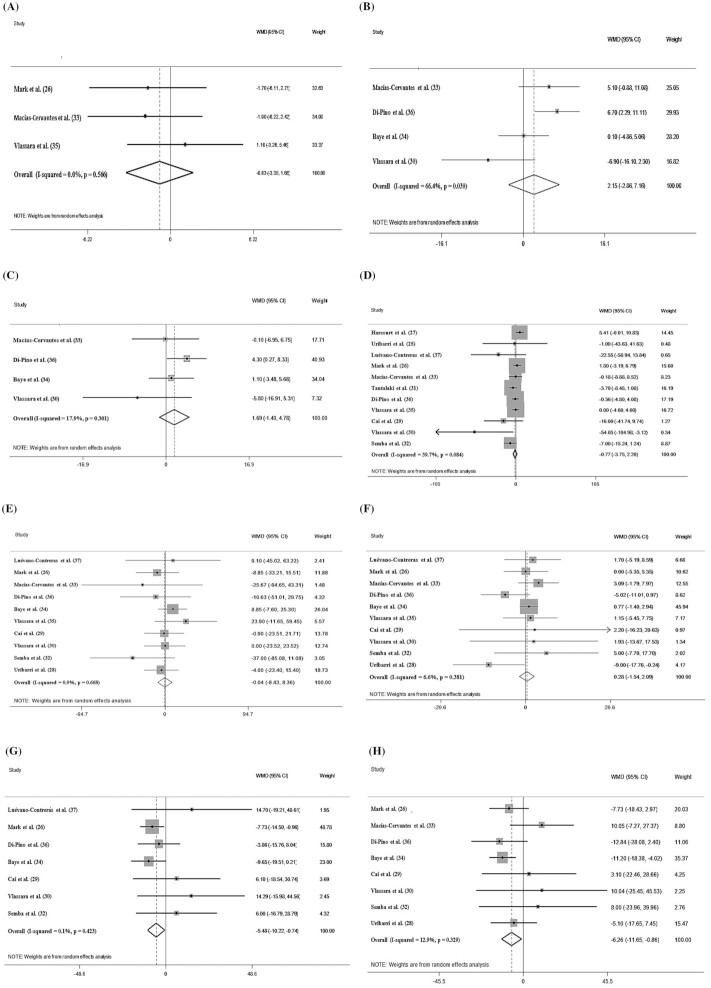
Among the included studies, 3 assessed the effects of AGEs on WC and 4 assessed the effects on blood pressure. After pooling effect sizes, there was no significant effect of a low-AGEs diet on WC [weighted mean difference (WMD): −0.832 cm; 95% CI: −3.353, 1.682 cm; P = 0.516], systolic blood pressure (WMD: 2.153 mm Hg; 95% CI: −2.864, 7.163 mm Hg; P = 0.400), and diastolic blood pressure (WMD: 1.696 mm Hg; 95% CI: −1.401, 4.784 mm Hg; P = 0.283) (Figure 2A–C). The subgroup analysis for blood pressure based on the length of follow-up did not display any differences in these measurements between a low- and a high-AGEs diet (Supplemental Table 1). However, the subgroup analysis for WC was not possible because there were not enough studies (<2) in each group.
FIGURE 2.
Forest plots of the impact of low advanced glycation end products diet on primary outcomes using a random-effects model. (A) Waist circumference (cm); (B) systolic blood pressure (mm Hg); (C) diastolic blood pressure (mm Hg); (D) fasting blood sugar (mg/dL); (E) triglycerides (mg/dL); (F) HDL cholesterol (mg/dL); (G) cholesterol (mg/dL); (H) LDL cholesterol (mg/dL). WMD, weighted mean difference.
Glucose metabolism
Fasting blood sugar and HbA1c
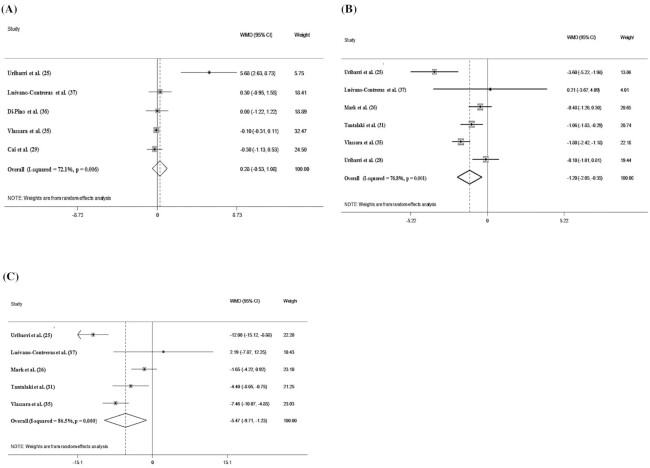
The pooled WMD of 11 effect sizes showed a nonsignificant effect of a low-AGEs diet on fasting blood sugar (WMD: −0.778 mg/dL; 95% CI: −3.755, 2.205 mg/dL; P = 0.611) (Figure 2D) and HbA1c (WMD: 0.283, 95% CI: −0.533, 1.084, P = 0.502) (Figure 3A). However, in the stratified studies, we observed a significant difference in the fasting blood sugar concentrations based on the length of follow-up and on the health status of the participants. In addition, the effects of a low-AGEs compared with a high-AGEs diet were more pronounced when the follow-up was >8 wk (WMD: −9.655 mg/dL; 95% CI: −14.098, −5.200 mg/dL; I2 = 79.1%) and when it was prescribed to diabetic subjects (WMD: −9.654 mg/dL; 95% CI: −14.091, −5.202 mg/dL; I2 = 79.1%). The results also showed that the duration of the intervention is a source of heterogeneity (Supplemental Table 1).
FIGURE 3.
Forest plots of the impact of low advanced glycation end products diet on secondary outcomes using a random-effects model. (A) HbA1c (%); (B) HOMA-IR; (C) fasting insulin (μU/mL).
Insulin resistance and secretion
The quantitative analysis of HOMA-IR (WMD: −1.204; 95% CI: −2.057, −0.358; P = 0.006) and fasting insulin (WMD: −5.472 μU/mL; 95% CI: −9.718, −1.234 μU/mL; P = 0.011) concentrations indicated a significant reduction in the low-AGEs compared with the high-AGEs groups (Figure 3B, C). The duration of the intervention (≤8/>8 wk) was considered a heterogeneity factor on the overall effect size on fasting insulin concentrations and HOMA-IR. In the subgroup analyses based on the length of follow-up, our findings also demonstrated that the effects of a low-AGEs diet on fasting insulin (WMD: −9.651 μU/mL; 95% CI: −14.095, −5.207 μU/mL; I2 = 79.1%) and HOMA-IR (WMD: −1.716; 95% CI: −3.320, −0.113; I2 = 87.9%) were more noticeable for a length of follow-up >8 wk compared with ≤8 wk.
Lipid profile
Pooled results from the random-effects model showed nonsignificant changes in triglyceride (TG; WMD: −0.041 mg/dL; 95% CI: −8.433, 8.365 mg/dL; P = 0.993) and HDL cholesterol (WMD: 0.287 mg/dL; 95% CI: −1.547, 2.093 mg/dL; P = 0.766) concentrations in the low-AGEs compared with the high-AGEs groups (Figure 2E, F). The subgroup analyses revealed that in terms of HDL cholesterol or TG concentrations, the effects of a low-AGEs diet were not influenced by the duration of the intervention (≤8/>8 wk) or the baseline health status of the participants. However, after the intake of a low-AGEs diet, there was a significant reduction in total cholesterol (WMD: −5.486 mg/dL; 95% CI: −10.222, −0.747 mg/dL; P = 0.023) and LDL cholesterol (WMD: −6.263 mg/dL; 95% CI: −11.659, −0.866 mg/dL; P = 0.023) (Figure 2G, H). The results of the subgroup analyses showed the reduction in total cholesterol (TC) was greatest in overweight or obese subjects (WMD: −13.879 mg/dL; 95% CI: −25.211, −2.548 mg/dL; I2 = 0.0%; P = 0.016). In addition, in the studies with a duration of intervention ≤8 wk, a low-AGEs diet reduced LDL cholesterol more effectively (WMD: −10.566 mg/dL; 95% CI: −20.753, −0.379 mg/dL; I2 = 0.0%; P = 0.041) (Supplemental Table 1).
Publication bias and sensitivity analysis
A review of the funnel plots used to assess publication bias is shown in Supplemental Figure 1. The funnel plots showed no publication bias for HOMA-IR, HbA1c, fasting insulin, WC, systolic blood pressure, HDL cholesterol, and triglycerides concentrations; this was also confirmed using Egger's regression test. However, based on Egger's regression test, we detected a publication bias for fasting glucose (P = 0.063), LDL cholesterol (P = 0.025), diastolic blood pressure (P = 0.094), and total cholesterol (P = 0.001), but the results of the meta trim-and-fill analysis did not identify a study responsible for the publication bias. The sensitivity analysis was conducted by removing each RCT 1 by 1 to estimate the effectiveness of an individual study on the pooled effect size. The leave-1-out sensitivity analysis showed that leaving out 1 study at a time had no significant effect on the pooled effect size (Supplemental Figure 1).
Discussion
The current systematic review and meta-analysis of 13 RCTs was performed to assess the effects of a low-AGEs diet on metabolic risk factors. Our results revealed no significant effects of a low intake of dietary AGEs on fasting blood sugar, HbA1c, TG, HDL cholesterol, WC, and blood pressure in comparison with a high intake of dietary AGEs. However, for HOMA-IR, fasting insulin, LDL cholesterol, and TC, significant decreases were observed following the consumption of diets low in AGEs compared with diets high in AGEs. The results of the subgroup analysis based on the length of follow-up and on the health status of the participants showed that the reduction of fasting blood sugar after a low AGEs intake was more pronounced in patients with T2DM and when the length of follow-up was >8 wk. This result may indicate that a low intake of dietary AGEs can reduce fasting blood sugar only in individuals with a higher baseline fasting blood sugar. For fasting insulin and HOMA-IR, a longer follow-up duration had more noticeable effects on the changes in these variables. This finding might indicate that a long-term restriction of dietary AGEs intake can be more effective in reducing insulin resistance. In a systematic review, Clarke et al. (19) showed that the consumption of low-AGEs diets improved insulin sensitivity. However, no significant changes in fasting blood sugar and HbA1c concentrations in healthy or diabetic subjects were detected. In addition, the results of a meta-analysis conducted by Baye et al. (14) demonstrated that low-AGEs diets had no significant effects on fasting blood sugar, HbA1c, TG, HDL cholesterol, blood pressure, and anthropometric indices. Their results also showed significant improvements in insulin sensitivity, regardless of an individual's health status, and decreased fasting insulin concentrations in T2DM individuals. Thus, the authors postulated that the beneficial effects of low-AGEs diets on fasting insulin may be more pronounced in subjects with higher baseline insulin concentrations. Studies demonstrated that AGEs can induce insulin resistance via interaction with the receptors for AGEs (RAGEs) (38, 39). Monden et al. (39) reported that an increased expression of RAGEs leads to adipocyte hypertrophy, lower expression of glucose transporter type 4, reduction of insulin-stimulated glucose uptake, and decreased phosphorylation of insulin receptor substrate-1. Consequently, it is assumed that the consumption of low-AGEs diets is likely to be associated with improvement of insulin sensitivity. However, it is still unclear how severe the restriction in dietary AGEs must be in order to achieve insulin sensitivity. Due to the differences regarding the diets prescribed in each study and also due to the variation in the baseline levels of diet AGEs, it is difficult to recommend an acceptable daily dietary restriction of AGEs. The results of this review regarding LDL cholesterol and TC indicated significant decreases following the administration of a diet low in AGEs compared with a diet high in AGEs. Subgroup analysis revealed that the TC-lowering effect of a low-AGEs diets was more noticeable in overweight and obese individuals. Obesity increases one's cardiometabolic risk and accentuates dyslipidemia. The effects of a low-AGEs diet may be more significant in individuals with potential cardiometabolic risk factors. In addition, the differences in composition of the 2 dietary interventions (low-AGEs compared with high-AGEs diets) and the baseline characteristics of the participants in each study may be factors contributing to the significant effects observed in subgroups (34). Baye and colleagues (14) showed that a low dietary consumption of AGEs, for any duration, could reduce TC in individuals without T2DM. The beneficial effects of a long-term dietary restriction of AGEs on the serum concentrations of LDL cholesterol in patients with T2DM and chronic kidney diseases have been reported in previous studies (12, 19). However, in this review, the subgroup analysis for the length of follow-up showed that low-AGEs diets were more effective in decreasing LDL cholesterol when the duration of the intervention was ≤8 wk compared with >8 wk. This controversy may be related to the small number of studies allocated to each subgroup.
In the current study, we performed a systematic review and meta-analysis of the effect of dietary AGEs intake on cardiometabolic factors such as measures of insulin sensitivity, HbA1c, fasting insulin, WC, blood pressure, and lipid profile. This study included 13 RCTs with 530 participants. The meta-analysis was conducted for all of the outcomes of MetS risk factors. In addition, we performed subgroup analyses to assess the effect of AGEs on each risk factor. Moreover, a low degree of heterogeneity was detected for most studies assessing cardiometabolic factors. Also, because our study only involved RCTs, the causal inference of our findings is strong. However, the effects of some confounding factors, such as dietary intake and physical activity, were not considered in most of the studies. Furthermore, in some studies included in this systematic review, the intervention lasted <24 h. Therefore, we could not perform a meta-analysis for these studies.
In conclusion, this meta-analysis of RCTs showed that a low intake of dietary AGEs exhibits a statistically significant effect on insulin resistance, fasting insulin, TC, and LDL cholesterol. Given the results of our study, because diets with a low AGEs content have effectively improved insulin sensitivity and reduced insulin resistance and cholesterol levels, it seems that the use of such diets might be beneficial in patients with metabolic disorders (e.g., diabetes and hyperlipidemia). Our results can be considered as an important implication for dietary guidelines and for strategies to prevent the development of noncommunicable chronic diseases linked to high-AGEs diets. Therefore, a reduction in the consumption of processed foods that have high AGEs and changing food preparation methods are good strategies to promote health. However, because a high heterogeneity has been observed for many outcome variables, conducting future prospective studies with a correct methodology and increased quality would be useful and clarify how these diets should be introduced in clinical practice.
Supplementary Material
Acknowledgments
The authors’ responsibilities were as follows—MHS, SF, and AL: contributed to the search strategy and initial work on conceptualizing the manuscript; M-AG and FS: designed the study and wrote the manuscript; MHS, ES-Z, HOS, NT, BP, and HK-V: conducted the review and meta-analyses; FS: was responsible for final content; and all authors: read and approved the final manuscript. The lead author affirms that this article is an honest, accurate, and transparent account of the reported analyses. No important aspects of the study have been omitted. No discrepancies from the study as originally planned took place. All outcomes are reported.
Notes
The authors reported no funding received for this study.
Author disclosures: The authors report no conflicts of interest.
Supplemental Figure 1 and Supplemental Table 1 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/advances/.
MHS and SF contributed equally to this work.
Abbreviations used: AGE, advanced glycation end product; HbA1c, glycated hemoglobin; MetS, metabolic syndrome; RAGE, receptor for advanced glycation end product; RCT, randomized controlled trial; TC, total cholesterol; TG, triglyceride; T2DM, type 2 diabetes mellitus; WC, waist circumference; WMD, weighted mean difference.
Contributor Information
Mohammad Hasan Sohouli, Student Research Committee, Faculty of Public Health Branch, Iran University of Medical Sciences, Tehran, Iran; Department of Nutrition, School of Public Health, Iran University of Medical Sciences, Tehran, Iran.
Somaye Fatahi, Student Research Committee, Faculty of Public Health Branch, Iran University of Medical Sciences, Tehran, Iran; Pediatric Gastroenterology, Hepatology and Nutrition Research Center, Research Institute for Children's Health, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
Elham Sharifi-Zahabi, Department of Nutrition, School of Public Health, Iran University of Medical Sciences, Tehran, Iran.
Heitor O Santos, School of Medicine, Federal University of Uberlândia, Uberlândia, Brazil.
Nishant Tripathi, University of Kentucky College of Medicine, Lexington, KY, USA.
Abolfazl Lari, Student Research Committee, Faculty of Public Health Branch, Iran University of Medical Sciences, Tehran, Iran; Department of Nutrition, School of Public Health, Iran University of Medical Sciences, Tehran, Iran.
Behnaz Pourrajab, Student Research Committee, Faculty of Public Health Branch, Iran University of Medical Sciences, Tehran, Iran; Department of Nutrition, School of Public Health, Iran University of Medical Sciences, Tehran, Iran.
Hamed Kord-Varkaneh, Department of Clinical Nutrition and Dietetics, Faculty of Nutrition and Food Technology, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
Mihnea-Alexandru Găman, Carol Davila University of Medicine and Pharmacy, Bucharest, Romania; Center of Hematology and Bone Marrow Transplantation, Fundeni Clinical Institute, Bucharest, Romania.
Farzad Shidfar, Department of Nutrition, School of Public Health, Iran University of Medical Sciences, Tehran, Iran; Colorectal Research Center, Iran University of Medical Sciences, Tehran, Iran.
References
- 1. Obeidat AA, Ahmad MN, Haddad FH, Azzeh FS. Alarming high prevalence of metabolic syndrome among Jordanian adults. Pak J Med Sci. 2015;31(6):1377–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lusis AJ, Attie AD, Reue K. Metabolic syndrome: from epidemiology to systems biology. Nat Rev Genet. 2008;9(11):819–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Watanabe J, Kotani K. Metabolic syndrome for cardiovascular disease morbidity and mortality among general Japanese people: a mini review. VHRM. 2020;16:149–55.. doi: 10.2147/vhrm.S245829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, Fruchart JC, James WP, Loria CM, Smith SC Jr.. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120(16):1640–5. [DOI] [PubMed] [Google Scholar]
- 5. Ottum MS, Mistry AM. Advanced glycation end-products: modifiable environmental factors profoundly mediate insulin resistance. J Clin Biochem Nutr. 2015;57(1):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Galbete C, Toledo J, Martinez-Gonzalez MA, Martinez JA, Guillen-Grima F, Marti A. Lifestyle factors modify obesity risk linked to PPARG2 and FTO variants in an elderly population: a cross-sectional analysis in the SUN Project. Genes Nutr. 2013;8(1):61–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. de Courten B, de Courten MP, Soldatos G, Dougherty SL, Straznicky N, Schlaich M, Sourris KC, Chand V, Scheijen JL, Kingwell BAet al. . Diet low in advanced glycation end products increases insulin sensitivity in healthy overweight individuals: a double-blind, randomized, crossover trial. Am J Clin Nutr. 2016;103(6):1426–33. [DOI] [PubMed] [Google Scholar]
- 8. Henle T. Protein-bound advanced glycation endproducts (AGEs) as bioactive amino acid derivatives in foods. Amino Acids. 2005;29(4):313–22. [DOI] [PubMed] [Google Scholar]
- 9. Uribarri J, Woodruff S, Goodman S, Cai W, Chen X, Pyzik R, Yong A, Striker GE, Vlassara H. Advanced glycation end products in foods and a practical guide to their reduction in the diet. J Am Diet Assoc. 2010;110(6):911–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Poulsen MW, Hedegaard RV, Andersen JM, de Courten B, Bugel S, Nielsen J, Skibsted LH, Dragsted LO. Advanced glycation endproducts in food and their effects on health. Food Chem Toxicol. 2013;60:10–37.. doi: 10.1016/j.fct.2013.06.052. [DOI] [PubMed] [Google Scholar]
- 11. Negrean M, Stirban A, Stratmann B, Gawlowski T, Horstmann T, Gotting C, Kleesiek K, Mueller-Roesel M, Koschinsky T, Uribarri Jet al. . Effects of low- and high-advanced glycation endproduct meals on macro- and microvascular endothelial function and oxidative stress in patients with type 2 diabetes mellitus. Am J Clin Nutr. 2007;85(5):1236–43. [DOI] [PubMed] [Google Scholar]
- 12. Kellow NJ, Savige GS. Dietary advanced glycation end-product restriction for the attenuation of insulin resistance, oxidative stress and endothelial dysfunction: a systematic review. Eur J Clin Nutr. 2013;67(3):239–48. [DOI] [PubMed] [Google Scholar]
- 13. Uribarri J, Cai W, Woodward M, Tripp E, Goldberg L, Pyzik R, Yee K, Tansman L, Chen X, Mani Vet al. . Elevated serum advanced glycation endproducts in obese indicate risk for the metabolic syndrome: a link between healthy and unhealthy obesity?. J Clin Endocrinol Metab. 2015;100(5):1957–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Baye E, Kiriakova V, Uribarri J, Moran LJ, de Courten B. Consumption of diets with low advanced glycation end products improves cardiometabolic parameters: meta-analysis of randomised controlled trials. Sci Rep. 2017;7(1):2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ribeiro PV, Tavares JF, Costa MA, Mattar JB, Alfenas RC. Effect of reducing dietary advanced glycation end products on obesity-associated complications: a systematic review. Nutr Rev. 2019;77(10):725–34. [DOI] [PubMed] [Google Scholar]
- 16. Birlouez-Aragon I, Saavedra G, Tessier FJ, Galinier A, Ait-Ameur L, Lacoste F, Niamba C-N, Alt N, Somoza V, Lecerf J-M. A diet based on high-heat-treated foods promotes risk factors for diabetes mellitus and cardiovascular diseases. Am J Clin Nutr. 2010;91(5):1220–6. [DOI] [PubMed] [Google Scholar]
- 17. Prasad A, Bekker P, Tsimikas S. Advanced glycation end products and diabetic cardiovascular disease. Cardiol Rev. 2012;20(4):177–83. [DOI] [PubMed] [Google Scholar]
- 18. Yamagishi S-i, Ueda S, Okuda S. Food-derived advanced glycation end products (AGEs): a novel therapeutic target for various disorders. Curr Pharm Des. 2007;13(27):2832–6. [DOI] [PubMed] [Google Scholar]
- 19. Clarke RE, Dordevic AL, Tan SM, Ryan L, Coughlan MT. Dietary advanced glycation end products and risk factors for chronic disease: a systematic review of randomised controlled trials. Nutrients. 2016;8(3):125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010;8(5):336–41. [DOI] [PubMed] [Google Scholar]
- 21. Higgins J. Cochrane handbook for systematic reviews of interventions. Version 5.1. [Internet]. The Cochrane Collaboration ; 2011. Available from: http://www.cochrane-handbook.org. [Google Scholar]
- 22. Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5(1):13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Uribarri J, Cai W, Ramdas M, Goodman S, Pyzik R, Chen X, Zhu L, Striker GE, Vlassara H. Restriction of advanced glycation end products improves insulin resistance in human type 2 diabetes: potential role of AGER1 and SIRT1. Diabetes Care. 2011;34(7):1610–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mark AB, Poulsen MW, Andersen S, Andersen JM, Bak MJ, Ritz C, Holst JJ, Nielsen J, de Courten B, Dragsted LO. Consumption of a diet low in advanced glycation end products for 4 weeks improves insulin sensitivity in overweight women. Diabetes Care. 2014;37(1):88–95. [DOI] [PubMed] [Google Scholar]
- 27. Harcourt BE, Sourris KC, Coughlan MT, Walker KZ, Dougherty SL, Andrikopoulos S, Morley AL, Thallas-Bonke V, Chand V, Penfold SA. Targeted reduction of advanced glycation improves renal function in obesity. Kidney Int. 2011;80(2):190–8. [DOI] [PubMed] [Google Scholar]
- 28. Uribarri J, Cai W, Pyzik R, Goodman S, Chen X, Zhu L, Ramdas M, Striker GE, Vlassara H. Suppression of native defense mechanisms, SIRT1 and PPARγ, by dietary glycoxidants precedes disease in adult humans; relevance to lifestyle-engendered chronic diseases. Amino Acids. 2014;46(2):301–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cai W, He JC, Zhu L, Peppa M, Lu C, Uribarri J, Vlassara H. High levels of dietary advanced glycation end products transform low-density lipoprotein into a potent redox-sensitive mitogen-activated protein kinase stimulant in diabetic patients. Circulation. 2004;110(3):285–91. [DOI] [PubMed] [Google Scholar]
- 30. Vlassara H, Cai W, Crandall J, Goldberg T, Oberstein R, Dardaine V, Peppa M, Rayfield EJ. Inflammatory mediators are induced by dietary glycotoxins, a major risk factor for diabetic angiopathy. Proc Natl Acad Sci USA. 2002;99(24):15596–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tantalaki E, Piperi C, Livadas S, Kollias A, Adamopoulos C, Koulouri A, Christakou C, Diamanti-Kandarakis E. Impact of dietary modification of advanced glycation end products (AGEs) on the hormonal and metabolic profile of women with polycystic ovary syndrome (PCOS). Hormones. 2014;13(1):65–73. [DOI] [PubMed] [Google Scholar]
- 32. Semba RD, Gebauer SK, Baer DJ, Sun K, Turner R, Silber HA, Talegawkar S, Ferrucci L, Novotny JA. Dietary intake of advanced glycation end products did not affect endothelial function and inflammation in healthy adults in a randomized controlled trial. J Nutr. 2014;144(7):1037–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Macías-Cervantes MH, Rodríguez-Soto JMD, Uribarri J, Díaz-Cisneros FJ, Cai W, Garay-Sevilla ME. Effect of an advanced glycation end product-restricted diet and exercise on metabolic parameters in adult overweight men. Nutrition. 2015;31(3):446–51. [DOI] [PubMed] [Google Scholar]
- 34. Baye E, De Courten MP, Walker K, Ranasinha S, Earnest A, Forbes JM, De Courten B. Effect of dietary advanced glycation end products on inflammation and cardiovascular risks in healthy overweight adults: a randomised crossover trial. Sci Rep. 2017;7(1):1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Vlassara H, Cai W, Tripp E, Pyzik R, Yee K, Goldberg L, Tansman L, Chen X, Mani V, Fayad ZA. Oral AGE restriction ameliorates insulin resistance in obese individuals with the metabolic syndrome: a randomised controlled trial. Diabetologia. 2016;59(10):2181–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Di Pino A, Currenti W, Urbano F, Mantegna C, Purrazzo G, Piro S, Purrello F, Rabuazzo AM. Low advanced glycation end product diet improves the lipid and inflammatory profiles of prediabetic subjects. J Clin Lipidol. 2016;10(5):1098–108. [DOI] [PubMed] [Google Scholar]
- 37. Luévano-Contreras C, Garay-Sevilla ME, Wrobel K, Malacara JM, Wrobel K. Dietary advanced glycation end products restriction diminishes inflammation markers and oxidative stress in patients with type 2 diabetes mellitus. J Clin Biochem Nutr. 2013;52(1):22–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Unoki H, Bujo H, S-i Y, Takeuchi M, Imaizumi T, Saito Y. Advanced glycation end products attenuate cellular insulin sensitivity by increasing the generation of intracellular reactive oxygen species in adipocytes. Diabetes Res Clin Pract. 2007;76(2):236–44. [DOI] [PubMed] [Google Scholar]
- 39. Monden M, Koyama H, Otsuka Y, Morioka T, Mori K, Shoji T, Mima Y, Motoyama K, Fukumoto S, Shioi A. Receptor for advanced glycation end products regulates adipocyte hypertrophy and insulin sensitivity in mice: involvement of Toll-like receptor 2. Diabetes. 2013;62(2):478–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.