ABSTRACT
Caloric restriction (CR) has been shown repeatedly to prolong the lifespan in laboratory animals, with its benefits dependent on molecular targets forming part of the nutrient signaling network, including the NAD-dependent deacetylase silent mating type information regulation 2 homologue 1 (SIRT1). It has been hypothesized that the stilbene resveratrol (RSV) may counteract age- and obesity-related diseases similarly to CR. In yeast and worms, RSV-promoted longevity also depended on SIRT1. While it remains unclear whether RSV can prolong lifespans in mammals, some studies in rodents supplemented with RSV have reported lowered body weight (BW) and fat mass, improved insulin sensitivity, lowered cholesterol levels, increased fitness, and mitochondrial biogenesis. Molecular mechanisms possibly leading to such changes include altered gene transcription and activation of SIRT1, AMP-activated kinase (AMPK), and peroxisome proliferator–activated receptor gamma coactivator 1-alpha (PPARGC1A). However, some mouse models did not benefit from RSV treatment to the same extent as others. We conducted a literature search on PubMed (15 April, 2020) for trials directly comparing RSV application to CR feeding in mice. In most studies retrieved by this systematic PubMed search, mice supplemented with RSV did not show significant reductions of BW, glucose, or insulin. Moreover, in some of these studies, RSV and CR treatments affected molecular targets differently and/or findings on RSV and CR impacts varied between trials. We discuss those RSV-induced changes in gene transcription hypothesized to partly counteract age-related alterations. Although there may be a moderate effect of RSV supplementation on parameters such as insulin sensitivity toward a more CR-like profile in mice, data are inconsistent. Likewise, RSV supplementation trials in humans report controversial findings. While we consider that RSV may, under certain circumstances, moderately mimic some aspects of CR, current evidence does not fully support its use to prevent or treat age- or obesity-related diseases.
Keywords: sirtuin, polyphenol, healthspan, diabetes mellitus type II, lunularin, dihydroresveratrol
Impact of caloric restriction on hormonal, transcriptional and other molecular changes in mice is compared to resveratrol application. Findings are related to data from human supplementation trials.
Introduction
Resveratrol sources and structure
Resveratrol (RSV) is a secondary plant metabolite found in dietary sources such as berries and other fruits, including peanuts and cocoa (1−4), with considerable amounts in grapes and, consequently, in wine (5). The stilbene RSV forms cis and trans derivatives that are mostly found as glucosides in plants (5). While wine drinkers can consume more than 1 mg/d RSV (6), in the US population the average RSV uptake is estimated to be around 80 μg/d (7). Supplementation of synthetic RSV up to 150 mg/d has been regarded as safe by the European Food Safety Authority (8). However, at doses of 2.5 g or higher, RSV supplementation may cause mild to moderate gastrointestinal symptoms (9). RSV is metabolized by gut microbiota (to dihydroresveratrol and possibly other metabolites, such as lunularin) and is quickly sulfonated or glucuronidated after resorption from the intestines, leading to very low levels of nonconjugated RSV in mice and humans (10, 11).
Caloric restriction as putatively lifespan-extending intervention
Studies employing model organisms ranging from yeast to mice found that RSV may possibly prolong the lifespan [reviewed in Barnett et al. (12)]. The only nongenetic intervention that has repeatedly promoted longevity is a reduction of feed intake [caloric restriction (CR)]. Compared to ad libitum feeding, CR prolonged lifespans in model organisms, including yeast, mice, and rhesus monkeys (13−15). In mammals, CR was shown to reduce adipose tissue mass and body weight (BW) and decrease leptin, insulin, insulin-like growth factor I (IGF-I), and cholesterol plasma levels. Furthermore, CR lowered markers of inflammation such as TNF, which is expressed under the control of nuclear factor kappa B (15, 16) and may even favor healthy aging in humans (17). The WHO defines healthy aging as “the process of developing and maintaining the functional ability that enables wellbeing in older age” (18). Aging, however, increases susceptibility toward many common diseases (19, 20), such as coronary artery disease, type II diabetes mellitus (T2DM), cancers, dementia, and painful conditions (21). Chronic inflammation may further contribute to the pathogenesis of age-related diseases (22) and, late in life, increased frailty (23).
Pathways implicated in aging
Nutrients activate insulin and IGF-I receptors (IGF-IR), as well as downstream signaling pathways. Interestingly, heterogenetic knockout of Igfr1 increased the lifespan in mice (24). Upon inhibition of IGF-IR downstream mechanistic target of rapamycin, the lysosomal degradation pathway autophagy is induced (25). Of interest, genetically inducing autophagy made mice longer-lived, as well as more insulin-sensitive and more tolerant toward oxidative damage (26). Another molecular nutrient−sensing enzyme is NAD-dependent deacetylase silent mating type information regulation 2 homologue 1 (SIRT1) (27). Its overexpression in yeast, worms, and mice increased their lifespans (28−32). Moreover, SIRT1 can activate peroxisome proliferator–activated receptor gamma coactivator 1-alpha (PPARGC1A), a major regulator for mitochondrial biogenesis (27).
Consistent with CR counteracting age-related changes in cellular signaling, it improved insulin sensitivity and autophagy in aged organisms (33). Remarkably, the phenotypes of CR and transgenic SIRT1 mice resemble each other (28), and SIRT1 was necessary for CR-induced lifespan extensions (32, 34, 35). In long-lived mice with genetically distorted signaling of growth hormone, which controls IGF-I production, CR could not further promote lifespan extensions (36). Thus, CR appears to activate SIRT1 and inhibit IGF-I, thereby affecting their downstream signaling (37).
Since the number of overweight humans worldwide and, consequently, cases of premature onset of age-related diseases are increasing (38), molecules possibly mimicking CR [caloric restriction mimetics (CRMs)] without having to decrease nutrient intake may seem like a hopeful measure that can prolong the human lifespan. According to National Institute on Aging−based researchers, a CRM candidate substance should not “significantly reduce long-term food intake”; mimic “metabolic, hormonal, and physiological effects of CR”; activate stress response pathways observed in CR; and promote CR-like effects on the lifespan and healthspan (39). In Figure 1, such effects and further desired properties (i.e., safety/lack of side effects) of ideal CRM candidates are depicted.
FIGURE 1.
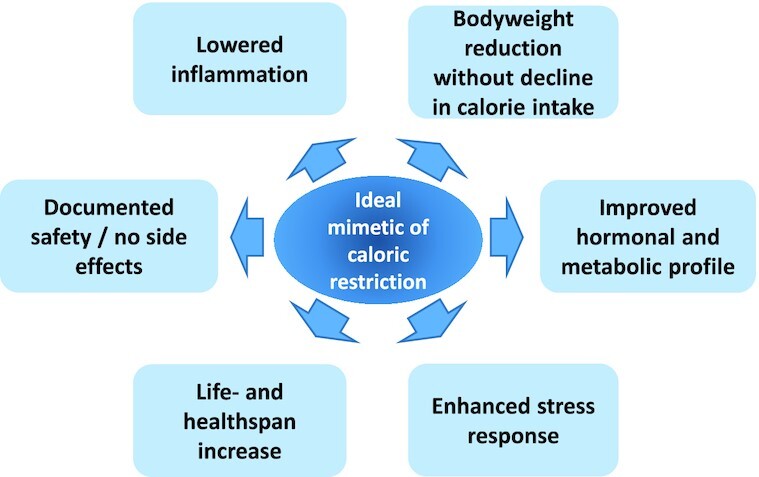
Desired properties of an "ideal" caloric restriction mimetic that would lead to health- and lifespan increases.
Resveratrol and putative lifespan extension
Of interest, RSV-induced lifespan increases in yeast, worms, and flies depended on their SIRT1 homologue (30, 32). Because RSV supplementation resembled CR-induced lifespan extensions in these settings, it has been hypothesized that RSV may mimic CR (32). However, it remains unclear whether RSV supplementation can increase the lifespan in mice (40−45). Therefore, we have reviewed the data from studies directly comparing CR with RSV that analyze aging- and obesity-related parameters. Since the number of studies in mammalian non-mouse models was too small to compare trials reasonably, we have limited our systematic search to CR-RSV trials in mice. Prior to reviewing this data, we give an overview of studies that have supplemented RSV in mice without including a CR control. At the end of this narrative review with a systematic component, we relate (inconsistent) findings from studies in rodents to data from human trials.
Molecular targets of resveratrol in rodents
In an early study on lifespans and RSV in male C57BL/6 mice, the stilbene prolonged the lifespan when supplemented at 0.4 g/kg to a high-fat diet (HFD) with 60% energy from fat. Moreover, stilbene supplementation lowered fed and fasted insulin, as well as fasted glucose levels, while increasing the number of mitochondria. In line with this finding, after 6 months of supplementation, hepatic PPARGC1A had a lower acetylation status in RSV-supplemented mice than in nonsupplemented mice. Yet, feed consumption and body temperature were unchanged (43). Moreover, RSV attenuated obesity-induced damage to the heart and liver in those mice on a HFD [60% calories from fat (43)]. Lagouge et al. (46) also found an increase in muscular mitochondria and brown adipose tissue, as well as improved insulin sensitivity, in RSV-supplemented C57BL/6 mice on a HFD with 40% energy from fat. In contrast to Baur et al. (43), Lagouge and colleagues (46) used a 10-time higher dose of RSV (0.4 g/kg and 4 g/kg diets, respectively) and found lower BWs in RSV-treated mice compared to the controls, since the stilbene-supplemented mice gained weight more slowly than the control mice. The RSV-fed Lagouge male C57Bl/6J mice (46) also had more active muscular PPARGC1A, showing higher mRNA and protein levels alongside with SIRT-mediated deacetylation and therefore activation of PPARGC1A While RSV supplementation of mice on a standard diet (in contrast to mice on 60% energy from a HFD) did not increase the lifespan, it induced transcriptional changes similar to the pattern detected in animals following an every other day feeding regimen (EOD) (47).
Using Ampk knockout mice, Um et al. (48) showed that RSV-induced benefits relied on this kinase responding to a lowered nutrient supply and forming part of the SIRT1 regulatory network (49). In contrast to Ampk knockout mice, RSV-supplemented wild-type mice showed an increased metabolic rate and a lowered BW, as well as improved insulin sensitivity and glucose tolerance. In skeletal muscle, mitochondrial biogenesis and Ppargca1 mRNA levels were increased in RSV-supplemented mice as compared to nonsupplemented animals (48). However, phosphorylation of AMPK as a measure of its activation shows high interindividual differences within experimental groups when analyzed in vivo and was not significantly altered in the liver, muscle, or white adipose tissue (WAT) of mice receiving RSV at 4 g/kg for 4 or 13 weeks (50). In vitro studies observed AMPK phosphorylation when RSV was administered at 25 μM to Chinese hamster ovarian cells (43). This concentration is not reached in vivo since RSV fed at 4 g/kg led to concentrations below 2 μM (41, 46).
In order to study whether RSV may slow down aging, the stilbene was applied to a mouse model for the premature-aging Werner syndrome. A mutation of the gene Wrn in a C57BL/6 background led to increased BW, visceral fat, liver steatosis, and triglyceride (TG) and glucose levels, as well as insulin resistance, when compared to wild-type mice (51). In the Werner mice, applying RSV at 0.4 g/kg from weaning to 5 or 9 months of age attenuated liver steatosis, hyperglycemia, and insulin resistance without lowering TG levels, visceral fat mass, or BW compared to nonsupplemented mutant mice. Moreover, RSV supplementation did not attenuate inflammation in the prematurely aging mice and did not expand their lifespan (51).
In a study with organ-specific Ppargca1 knockout mice, RSV-supplemented (4 g/kg diet) animals depended on muscular PPARGCA1 for increased mitochondrial biogenesis. In wild-type mice, RSV showed a rather moderate influence on insulin and glucose levels, decreased total cholesterol plasma levels, and possibly decreased hepatic TGs. Dietary application of RSV (4 g/kg diet) was further compared to feeding a chemical SIRT1 inducer. Interestingly, improved glucose homeostasis after supplementing the SIRT1 inducer did not depend on PPARGCA1. This indicates that RSV may not be a sole SIRT1 inducer. The effect of RSV in wild-type mice was highly tissue dependent, and Ppargca1 mRNA levels in muscle did not seem to be affected significantly. While Ppargca1 transcription was decreased in liver, it was increased in WAT (50).
Conversely, in another study in mice on a HFD, feeding RSV at 4 g/kg did not increase the mitochondria number or affect PPARGCA1 protein levels in the muscle. When the same authors used rats as a model, they similarly observed no changes in the mitochondria number or PPARGCA1 (41).
In a Wistar-based steatosis rat model, RSV at 0.2 g/kg BW for 18 weeks attenuated the HFD-induced rises in total cholesterol, TGs, and BW. Additionally, downregulation of autophagy-related Map1lc3b mRNA levels in animals on an HFD as compared to a standard diet was counteracted, and hepatic Sirt1 mRNA levels were elevated by RSV feeding (52). An overview of molecular targets putatively affected by RSV, as well as RSV's microbial products and host-modified products, is depicted in Figure 2.
FIGURE 2.
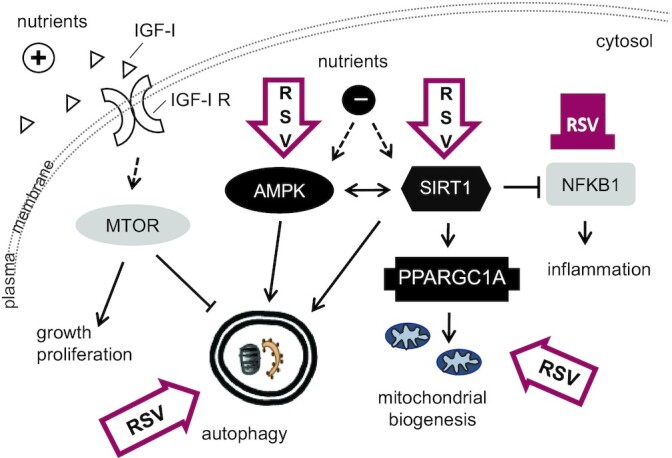
Molecular targets and cellular processes identified as being affected directly or indirectly by resveratrol. Contoured block arrows depict activation, the color-filled blunt arrow shows inhibition. AMPK, AMP-activated kinase; IGF-I, insulin-like growth factor I; IGF-IR, IGF-I receptor; MTOR, mechanistic target of rapamycin; NFKB1, nuclear factor kappa B; PPARGC1A, peroxisome proliferator-activated receptor gamma coactivator 1-alpha; RSV, resveratrol; SIRT1, silent mating type information regulation 2 homologue 1.
Neuroprotective properties of RSV have also been observed. CR in elderly humans may improve memory (53), and activation of SIRT1 could possibly counteract neurodegenerative disease (54). However, data on RSV improving neurodegeneration in mouse brains is not consistent (55).
Changes in phenotype, protein, and mRNA levels in mice
On 15 April, 2020, we searched PubMed (pubmed.ncbi.nlm.nih.gov) for original research articles using the search terms “restriction AND (mouse OR mice) AND resveratrol.” We screened the articles retrieved for studies that compared ad libitum−fed nonsupplemented animals to an RSV-supplemented group and to mice on CR and that were describing aging- or obesity-related parameters beyond changes in BW. We excluded genetic disease models and studies focusing on the brain. Since outcomes from studies on RSV often yield contradicting data, we concentrated on parameters that had been analyzed by at least 2 of these studies (Figure 3). This led to finding 7 studies that had evaluated BW, body composition, insulin sensitivity, serum hormone and/or lipid levels, activation of known molecular targets of CR, and/or changes in gene transcription (Table 1). Of these, 5 studies used C57BL/6 strains and the other 2 studies employed the F1 generation obtained from crossing C57BL/6 x C3H/He mice. All but 1 study, which included male and female rodents, studied male mice (56−62). Diets provided 10−60% of calories from fat. The RSV supplementation dose and duration varied from 18.6 mg to 4 g/kg and from 8 weeks to 16 months, respectively. In the majority of these studies, RSV application showed little to no effect on mouse phenotype. In contrast to CR, RSV did not decrease BW and only slightly blunted an adipose tissue mass increase in 1 out of 3 studies. The analyzed feed intake was not changed by RSV application. Cholesterol was measured in 3 studies, of which 2 showed reductions by CR. However, RSV did not affect cholesterol levels (58, 59, 62). In 1 study, RSV increased TG levels but CR did not (62). However, in the mice from studies by Günther et al. (58) and Pallauf et al. (59), TG levels remained unchanged by RSV as well as by CR (63). Fasting glucose levels were monitored in 6 studies and decreased by most CR interventions. RSV tended to decrease glucose levels in genetically heterogeneous mice but increased them in C57BL/6 mice on a low-dose RSV-supplemented standard diet (56, 62). The other 4 studies found no significant changes in blood glucose by RSV (56, 58−60, 63). Fasted insulin levels were measured in 5 studies, 2 of which showed a reduction by RSV as well as CR (58, 60). For fasting insulin, 2 trials revealed no influence by CR or RSV and 1 showed an increase by RSV supplementation but not by CR (56 ,57, 62). Insulin levels after glucose application as an indicator of insulin sensitivity were decreased and thus improved in the CR groups of both studies that measured challenged insulin (58, 59). However, RSV only improved insulin sensitivity in 1 of these 2 studies (58). CR lowered IGF-I levels in 1 out of 2 studies, while RSV did not affect the growth factor (57, 62). In 30-month-old mice, both CR and RSV decreased muscular SIRT1 (57), while in 4–5-month-old mice, CR increased SIRT1 levels (61). SIRT1 protein in the muscle was not impacted by 2 g/kg RSV and was upregulated by 4 g/kg RSV, similar to the results of CR in the same study (61) (Table 1).
FIGURE 3.
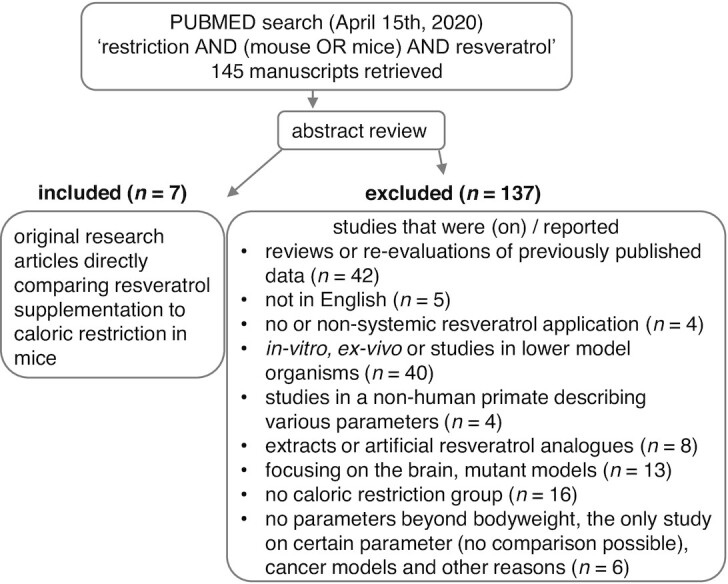
Selection of studies on resveratrol application compared to caloric restriction in mice.
TABLE 1.
Studies comparing resveratrol supplementation to caloric restriction and ad libitum control in mice
| Mice and diet | Male 14-mo-old C57BL/6xC3H/He F1 hybrid on AIN-93M; 10% energy from fat (57) | Male 2-mo-old C57BL/6xC3H/He F1 hybrid on AIN-93M; 10% energy from fat (56) | Male 7-wk-old C57BL/6J on 60% energy from fat; CR, RSV, and controls (61) | Female & male 6-mo-old C57Bl/6J on a standard diet (62) | Male 1-y-old C57BL/6Rj on 40% energy from fat (59) | Male 18-wk-old C57BL/6Rj on 40% energy from fat (58) | Male 4-mo-old C57BL/6Rj on AIN-93G (17% energy from fat) (60) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Duration | 16 mo | 3 mo | 12 wk | 6 mo | 8 wk | 8 wk | 8 mo | |||||||||
| Treatment | CR | RSV | CR | RSV | LFD | CR | RSV | CR | RSV | CR | RSV | CR | RSV | CR | RSV | |
| Dose | 75%3 | 0.05 g/kg diet | 75%3 | 0.05 g/kg diet | 70% HFD | 2 g/kg HFD | 4 g/kg HFD | 58% +8%4 | 18.6 mg/kg diet | 60% | i.p. 24 mg/kg BW5 | 60% | 0.35 g/kg diet6 | 60% | 0.1 g/kg diet | |
| Phenotype | ||||||||||||||||
| BW | n.a. | n.a. | 0.71,7 | ≈ | 0.81,7 | 0.61,7 | ≈ | ≈ | 0.91,7 | 1.12¸7 | 0.61 | ≈ | 0.61 | ≈ | n.a. | n.a. |
| ATM | n.a. | n.a. | n.a. | n.a. | 0.51 | 0.31 | ≈ | ≈ | n.a. | n.a. | 0.21 | ≈ | 0.21 | 0.81 | n.a. | n.a. |
| Energy intake | CR | n.a. | CR | n.a. | ≈ | CR | ≈ | ≈ | n.a. | n.a. | CR | ≈ | CR | ≈ | CR | n.a. |
| Cholesterol | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | ≈ | ≈ | 0.51 | ≈ | 0.71 | ≈ | n.a. | n.a. |
| Glucose8 | 0.82¸7,9 | 0.92¸7,9 | 0.82¸7 | ≈ | n.a. | n.a. | n.a. | n.a. | ≈ | 1.31,7 | 0.71,10 | ≈10 | ≈10 | ≈10 | 0.81,7 | ≈ |
| Insulin | ≈ 8,9 | ≈ 8,9 | ≈8 | ≈8 | n.a. | n.a. | n.a. | n.a. | ≈8 | 1.21,7,8 | 0.51,11 | ≈11 | 0.31,8,10 0.21,11 | 0.51,8,10 0.51,11 | 0.21,7,8 | 0.41,7,8 |
| IGF-I | 0.71,7,9 | ≈ 9 | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | ≈ | ≈ | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. |
| mRNA levels | ||||||||||||||||
| Liver | ||||||||||||||||
| Ppargc1a | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | 3.61 | ≈ | 2.71 | ≈ | n.a. | n.a. |
| Sirt1 | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | 3.21 | ≈ | 1.91 | ≈ | n.a. | n.a. |
| Muscle | ||||||||||||||||
| Ppargc1a | 3.41,7 | ≈ | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | ≈ | 1.42 | n.a. | n.a. |
| Heart | ||||||||||||||||
| Ppargc1a | ≈ | ≈ | ≈ | ≈ | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | 1.41,10 | ≈10 | n.a. | n.a. |
| Protein levels | ||||||||||||||||
| Muscle | ||||||||||||||||
| SIRT1 | 0.71,7 | 0.61,7 | n.a. | n.a. | ≈ | 1.31,7 | ≈ | 1.41,7 | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. |
| Liver | ||||||||||||||||
| SIRT1 | ≈ | ≈ | n.a. | n.a. | 1.51,7 | 1.61,7 | ≈ | ≈ | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. |
Endpoints with assumed relevance to aging measured in more than 1 study are listed. Values are given related to the controls and therefore do not show units. Values <1 show decreases and values >1 show increases by the treatment. Since many values were derived from graphs rather than the original values, they are approximations and no SDs or SEMs can be shown. The amount of feed consumed by the CR group is given as the percentage of feed consumed by the controls. The LFD had 10% energy from fat. The ≈ symbol indicates data that are not statistically significant different compared to nonsupplemented/non-CR animals. Abbreviations: AIN-93M, American Institute of Nutrition (AIN) diet formulation 93 for maintanance (M); ATM, adipose tissue mass; BW, body weight; CR, caloric restriction; HFD, high-fat diet; IGF-I, insulin-like growth factor I; LFD, low-fat diet; n.a., not analyzed or value not given; Ppargc1a, peroxisome proliferator-activated receptor gamma coactivator 1-alpha; RSV, resveratrol; Sirt1, sirtuin-1 (murine mRNA); SIRT1, NAD-dependent protein deacetylase sirtuin-1 (murine protein).
1Statistically significant difference to the control at P < 0.05
P value is < 0.1.
Compared to intake of the control group, control was -10% ad libitum.
The diet for the CR group consisted of 58% control diet + 34% dietary fiber + 8% soy.
24 mg/kg BW injected 3 times per week.
With increasing BW of the mice, the RSV concentration in the diet was increased from 0.3 g/kg to 0.4 g/kg halfway through the trial.
Approximated from figure.
Measured in fasted mice.
Measured in 5-month-old mice after 3 months of supplementation.
Previously unpublished data obtained by using methods and mice described in Günther et al. (58) and Pallauf et al. (59).
Measured in glucose-challenged mice (after application of a glucose bolus).
Supplementation of RSV was shown to attenuate the damage of a HFD on the mouse heart (43), possibly by somewhat mimicking CR (56). Since CR induced phosphorylation of the putative RSV target AMPK (64), we analyzed phosphorylated AMPK (p-AMPK) levels in the hearts of mice described in Günther et al. (58). While RSV-fed mice showed high variations of AMPK phosphorylation within the group, ad libitum control mice, CR mice, and RSV mice did not differ from each other in cardiac levels of activated AMPK [unpublished results (63)].
Various studies explored the changes in gene expression induced by aging and investigated whether CR and/or RSV could partly attenuate these changes. In the hearts of 30-month-old C57BL/6xC3H/He F1 hybrid mice after 16 months of dietary intervention, CR as well as RSV [50 mg/kg American Institute of Nutrition diet formulation 93 for maintenance (AIN-93M) diet or 4.9 mg/(kg mouse BW · d)] opposed the majority of age-related changes in gene transcription. Differential gene expression because of aging was observed for 1029 genes (57). However, in younger (5-month-old) mice supplemented with 1.25 mg RSV/(kg mouse BW · d), transcription of less than 10% of the 304 genes differentially transcribed in corresponding CR mice was changed toward a more CR-like pattern (56). Park et al. (65) studied the variations in gene expression in 5- and 25-month-old mice of 7 inbred strains. They found that age changed 6–15% of more than 22 000 gene transcripts measured in the hearts of the mice. However, which genes were affected by age depended greatly on the strain. Of the differentially regulated genes, only 20 genes were altered consistently over at least 6 out of 7 strains. Interestingly, in a study with Balb/C mice at 4 and 28 months of age, individual variation in hepatic gene transcription increased significantly with age. In studies by Park et al. (65) and White et al. (66), gene ontology terms obtained by functional annotation analysis of the transcriptome indicated that the immune response was upregulated in aged mice. Analyzing cardiac gene transcription, RSV at 50 mg/kg was as effective as CR in inhibiting age-dependent gene upregulation in the majority of the strains studied when applied from 15–30 months of age. Yet in the cerebellum, when measuring the transcription of 5 genes that were analyzed as markers for dietary interventions attenuating age-related changes in mice, RSV supplementation only affected the transcription of complement C1q subcomponent subunit A, which codes for a protein involved in the complement system (65). Moreover, PCR data from mouse livers showed CR-mediated upregulation of Ppargca1, Sirt1, and phosphoenolpyruvate carboxykinase 1 (a gene that codes for a central protein in gluconeogenesis), while RSV supplementation left the mRNA levels of these genes unaffected (Table 1) (58, 59). Of interest, Svensson et al. (50) found decreased Ppargca1 mRNA levels in the livers of RSV-supplemented mice (4 g/kg diet for 4 and 13 weeks), implying that RSV may even act contrarily to CR on Ppargca1 gene regulation. In the heart, Ppargca1 transcription showed no changes by CR in old, genetically heterogeneous mice on a standard diet (56); in young C57BL/6 mice on a HFD (40% energy from fat), Ppargca1 was transcriptionally upregulated by CR (Table 1) (58, 63). However, RSV supplementation did not influence Ppargca1 transcription in young or old mouse hearts (56, 57, 63). The pyruvate dehydrogenase lipoamide kinase isozyme 4 (Pdk4; a gene coding for an enzyme that inhibits the use of glucose for metabolism) and mitochondrial uncoupling protein 3 (Ucp3) were transcriptionally upregulated in the hearts of old and young genetically heterogeneous mice under CR. Young but not old RSV-supplemented mice also showed increased cardiac mRNA levels of Pdk4 and Ucp3 (56, 57). However, in young C57BL/6 mice on an HFD, neither CR nor RSV affected cardiac Pdk4 mRNA levels (63). These findings indicate that, similar to CR-induced transcriptional changes, RSV supplementation effects may also depend on mouse strain, age, diet, and dose.
In C57BL/6NIA mice on an AIN-93G diet supplemented with 0.4 g RSV/kg, Pearson et al. (47) detected transcriptional changes in the liver, heart, muscle, and WAT of mice supplemented from 12–18 and 12–27 months of age. They found that RSV, similar to EOD, counteracted age-related changes in the liver. However, they did not detect such antiaging transcriptional alterations by RSV or EOD in the heart. In muscle, RSV but not EOD slowed down age-related changes. Contrarily, in adipose tissue, RSV and EOD (67) enhanced age-related changes. Yet, RSV improved parameters that decline with age and obesity. In old mice, supplementation of the stilbene increased bone strength (tissue mineral density in the distal femur) and improved cataracts (30-month-old mice), prolonged the time before falling off a rotarod (21- and 24-month-old mice), and, at doses of 0.24%, enhanced endothelial function, measured as acetylcholine relaxation and reduced oxidative stress (18-month-old mice). However, in mice with a leptin-deficient background, RSV supplementation could not improve murine motor functions and possibly decreased endurance, as was shown in a treadmill test (68).
RSV supplementation in humans renders inconclusive data
Future and ongoing studies could possibly show a moderate impact of RSV on human health, yet discouraging findings from studies in rodents are somewhat reflected in humans. A PubMed search on 30 August, 2020, for recent (published 2014 or later) meta-analyses of human trials supplementing RSV and using glucose, insulin, TG, cholesterol, BW, or inflammatory markers as endpoints retrieved 19 publications [search terms “resveratrol AND (glucose OR insulin OR lipid OR inflammation OR body weight)”]. Of these 19 publications, 2 were on preclinical models and were thus not considered. Total cholesterol levels were analyzed by 8 articles. No influence of RSV supplementation was found in 4 reports (69−72), while 2 found a reduction (73, 74) and 2 focusing on patients with obesity and nonalcoholic fatty liver disease concluded that RSV could increase cholesterol levels (75, 76). Of 7 meta-analyses examining the impact of RSV supplementation on TG levels, only 1 study discovered reliable evidence for a reduction after treatments longer than 6 months in diabetic patients (70−74, 76, 77). Waist circumference (WC) and/or BW were investigated by 4 studies (69, 77−79). While 3 of these found a reduction of WC by RSV supplementation, 2 studies also included fat mass in their analyses. Although both reports detected reduced BWs, only Tabrizi et al. (79) furthermore observed a decreased fat mass. In contrast, Elgebaly et al. (77) found unchanged BW upon RSV supplementation. There were 7 meta-analyses that covered data on glucose levels after RSV supplementation. Of these, 3 reported no influence on glucose levels after stilbene application (71, 72, 77) and 3 analyses concluded that RSV could decrease blood glucose (69, 74, 80). However, Liu et al. (80) observed only diabetic patients to be responsive. Intriguingly, the authors of a meta-analysis from 2020 in patients with T2DM stated that data were insufficient for the evaluation of health benefits, since, after excluding trials with incomparable interventions and controls, only 3 studies remained for their assessment (81). Further glucose homeostasis−related parameters, such as insulin levels, the HOMA-IR index, or glycated hemoglobin (HbA1c), were analyzed by 4 publications (71, 74, 77, 80). Interestingly, the marker for average glucose levels during the last 3 months, HbA1c, appeared to be responsive toward stilbene treatment. While measuring insulin levels and determining the HOMA index yielded few promising results, all 3 meta-analyses evaluating HbA1 concluded that patients may benefit from RSV supplementation (71, 74, 80).
During aging, low-grade, chronic inflammation occurs (22). By counteracting the expression of inflammatory cytokines such as TNF, IL6, and acute-phase C-reactive protein (CRP), RSV could possibly counteract development of age-related illnesses (82). Of the meta-analyses retrieved by our PubMed search, 6 studied inflammation markers after RSV supplementation. While 4 of 6 studies found reduced CRP levels in RSV-supplemented human subjects (72, 74, 83−86), all 4 studies evaluating IL6 found no difference between stilbene-supplemented and nonsupplemented individuals (83−86). Levels of TNF were decreased by RSV in 2 of 3 analyses (84−86).
The contradicting data on RSV benefits in human trials may indicate that although there are some well-controlled and well-designed studies, many trials lack appropriate controls and are difficult to compare with each other. Furthermore, studying different patients (gender, health status, age) and types of interventions (dose, time point, duration) might have contributed to these controversial outcomes.
In a small number of original research articles, laborious muscle biopsies and analyses of AMPK, SIRT1, and PPARGCA1 (molecular targets that may mediate putative CR-like properties of RSV, as mentioned above in the section “Resveratrol and putative lifespan extension”) were conducted (87−89). In 2 of these trials with 10–11 obese men and/or men suffering from T2DM, CR-like effects were shown by RSV supplementation, with elevated activation of AMPK and SIRT1 in the muscle (87), increased mitochondrial function, improved insulin sensitivity, and lowered plasma levels of proinflammatory cytokines and TGs (88). In contrast, with a larger number (n = 45) of nonobese women, none of these CR-like outcomes were observed after RSV supplementation (89).
Of interest, RSV could also affect patients negatively. In human subjects suffering from nonalcoholic fatty liver disease, 3 g of RSV per day increased hepatic stress (90). High doses may even lead to increased levels of TNF, as did a single dose of 5 g of RSV in healthy men (91). Furthermore, RSV supplementation may blunt the positive effects from health-improving interventions such as exercise (92). A negative impact of supplements on exercise-induced benefits has been reported before for antioxidant vitamins (93). Of interest, application of vitamin C and vitamin E at high doses has also been discussed as putatively favoring the healthspan. However, lifespan studies with these vitamins have yielded discouraging data (94, 95).
Another factor possibly contributing to controversial outcomes in human RSV supplementation trials could be their duration, since weeks or months may not suffice to adequately monitor metabolic, hormonal, and physiological changes caused by an intervention, or to evaluate how these changes may affect aging during years or decades. While studies in laboratory animals can render endpoints such as the lifespan and can provide easy access to organ tissues, choosing the correct biomarker in human trials can be challenging (96). The Targeting Aging with Metformin (TAME) trial, which is evaluating whether metformin may also benefit nondiabetic patients (97), has studied various possible biomarkers for aging research. They emphasize that a suitable biomarker needs to show a measurable change with age and be age-dependently associated with the all-mortality risk. Furthermore, it should be robust across data sets and populations, as well as reliable and reproducible across labs. Of interest, blood IGF-I levels show U-shaped concentration patterns for mortality risk, since both high and low levels of IGF-I are associated with increased risks of cancer and cardiovascular mortality (98). Candidate biomarkers for inflammation such as IL2, IL1B, interferon gamma (IFNG), or TNF may be unstable during storage or present at very low levels (97, 99). Here, IL6, CRP, and TNF receptor II seem more suitable for measuring inflammation. Fasting insulin and IGF-I levels respond to changes in nutrient signaling, and HbA1c appears to be useful for monitoring metabolic aging (97). A recent 6-month supplementation trial in overweight adults found that although insulin sensitivity was not improved, HbA1c levels were decreased by 150 mg of RSV per day (67). Interestingly, nonenzymatically glycated tissue proteins [advanced glycation end products (AGEs)] also reflect increased glucose levels over time. AGEs can induce inflammation and increase oxidative stress. RSV supplementation may lower AGE toxicity (100). Since RSV may, similar to CR, affect numerous pathways implicated in aging, analyzing the serum metabolome as an —omics approach in human subjects (characterizing all metabolites affected by RSV treatment) could also produce relevant and feasibly reproducible data (101).
Conclusion
RSV supplementation studies in humans yield less promising data for aging-related benefits than epidemiological data on consumption of RSV-rich food (102, 103). Studies in model organisms comparing CR to RSV and studying age- and obesity-related biomarkers have found small, nonexistent, and even contrary effects of RSV on CR targets. Based on our literature review, we consider that RSV's CR-mimicking properties are rather moderate or are only applicable under certain circumstances. Therefore, we conclude that RSV supplementation cannot replace restricting dietary intake and may not be suitable for the prevention of age- or obesity-related diseases. With diet-derived molecules, hopes were that they might have fewer side effects than pharmaceutical drugs. However, non−plant derived small molecules such as metformin may be superior to polyphenols when counteracting age-related disease (104, 105).
In lifespan studies with RSV in mice, genotype or diet may influence the experimental outcome (42, 43, 47). Responsiveness toward RSV supplementation in humans may depend on the genome and dietary matrix. Genome-wide association studies searching for longevity genes and analyzing gene-diet interactions may identify different alleles of genes coding for proteins in nutrient-sensing cascades that are affected by RSV supplementation. To further evaluate whether RSV promotes healthy aging in human trials, long-term studies with high enough numbers of participants to detect the possible subgroups benefitting or not benefitting from RSV (106), using adequate biomarkers (96), and applying doses that have been regarded as safe (8) are needed. Supplementation of a single polyphenol compared to consumption of polyphenol-rich food may be less effective or even abolish positive effects because of side effects, such as toxicity issues, as well as nutrient-drug interactions (107). Moreover, the health benefits from consuming a plant-based diet appear to not solely rely on secondary plant metabolite uptake but on other factors, such as fiber consumption (108, 109). Therefore, further research on these other components found in plant-derived food and how they may positively affect aging is warranted. To date, to prolong the healthspan, it seems that trying to increase the compliance for known lifespan-prolonging interventions, such as dietary changes and exercise, may yield more success than supplementation of single dietary factors such as RSV.
Acknowledgments
We thank Vivien Schmuck for excellent technical assistance.
The authors’ responsibilities were as follows – KP: designed the article, collected the data, and wrote the manuscript; GR: designed the article and wrote the manuscript; DC, IG, GK, SdP-T: contributed experimental data; and all authors revised the manuscript and read and approved the final manuscript.
Notes
The project was funded by the German Research Foundation Deutsche Forschungsgemeinschaft (project number 274521263).
Author disclosures: KP, IG, GK, DC, SdP-T, and GR, no conflicts of interest.
Abbreviations used: AGE, advanced glycation end products; AMPK, AMP-activated kinase; BW, body weight; CR, caloric restriction; CRM, caloric restriction mimetic; CRP, C-reactive protein; EOD, every other day feeding regimen; HbA1c, glycated hemoglobin; HFD, high-fat diet; IGF-I, insulin-like growth factor I; IGF-IR, IGF-I receptor; Pdk4, pyruvate dehydrogenase lipoamide kinase isozyme 4; PPARGC1A, peroxisome proliferator–activated receptor gamma coactivator 1-alpha; RSV, resveratrol; SIRT1, silent mating type information regulation 2 homologue 1; T2DM, type II diabetes mellitus; TG, triglyceride; Ucp3, uncoupling protein 3; WAT, white adipose tissue; WC, waist circumference.
Contributor Information
Kathrin Pallauf, Institute of Human Nutrition and Food Science, University of Kiel, Kiel, Germany.
Ilka Günther, Institute of Human Nutrition and Food Science, University of Kiel, Kiel, Germany.
Gianna Kühn, Institute of Human Nutrition and Food Science, University of Kiel, Kiel, Germany.
Dawn Chin, Institute of Human Nutrition and Food Science, University of Kiel, Kiel, Germany.
Sonia de Pascual-Teresa, Department of Metabolism and Nutrition, Institute of Food Science, Technology and Nutrition (ICTAN-CSIC), Madrid, Spain.
Gerald Rimbach, Institute of Human Nutrition and Food Science, University of Kiel, Kiel, Germany.
References
- 1. Burns J, Gardner PT, O'Neil J, Crawford S, Morecroft I, McPhail DB, Lister C, Matthews D, MacLean MR, Lean MEet al. . Relationship among antioxidant activity, vasodilation capacity, and phenolic content of red wines. J Agric Food Chem. 2000;48(2):220–30. [DOI] [PubMed] [Google Scholar]
- 2. Chukwumah YC, Walker LT, Verghese M, Bokanga M, Ogutu S, Alphonse K. Comparison of extraction methods for the quantification of selected phytochemicals in peanuts (Arachishypogaea). J Agric Food Chem. 2007;55(2):285–90. [DOI] [PubMed] [Google Scholar]
- 3. Counet C, Callemien D, Collin S. Chocolate and cocoa: New sources of trans-resveratrol and trans-piceid. Food Chem. 2006;98(4):649–57. [Google Scholar]
- 4. Ehala S, Vaher M, Kaljurand M. Characterization of phenolic profiles of Northern European berries by capillary electrophoresis and determination of their antioxidant activity. J Agric Food Chem. 2005;53(16):6484–90. [DOI] [PubMed] [Google Scholar]
- 5. Sato M, Suzuki Y, Okuda T, Yokotsuka K. Contents of resveratrol, piceid, and their isomers in commercially available wines made from grapes cultivated in Japan. Biosci Biotechnol Biochem. 1997;61(11):1800–5. [DOI] [PubMed] [Google Scholar]
- 6. Zamora-Ros R, Andres-Lacueva C, Lamuela-Raventós RM, Berenguer T, Jakszyn P, Martínez C, Sánchez MJ, Navarro C, Chirlaque MD, Tormo M-Jet al. . Concentrations of resveratrol and derivatives in foods and estimation of dietary intake in a Spanish population: European Prospective Investigation into Cancer and Nutrition (EPIC)-Spain cohort. Br J Nutr. 2008;100(1):188–96. [DOI] [PubMed] [Google Scholar]
- 7. Williams LD, Burdock GA, Edwards JA, Beck M, Bausch J. Safety studies conducted on high-purity trans-resveratrol in experimental animals. Food Chem Toxicol. 2009;47(9):2170–82. [DOI] [PubMed] [Google Scholar]
- 8. European Food Safety Authority - Scientific Panel on Nutrition, Novel Foods and Food Allergens . Safety of synthetic trans-resveratrol. EFSA J. 2016;1(14):4368. doi: 10.2903/j.efsa.2016.4368. [Google Scholar]
- 9. Brown VA, Patel KR, Viskaduraki M, Crowell JA, Perloff M, Booth TD, Vasilinin G, Sen A, Schinas AM, Piccirilli Get al. . Repeat dose study of the cancer chemopreventive agent resveratrol in healthy volunteers: Safety, pharmacokinetics, and effect on the insulin-like growth factor axis. Cancer Res. 2010;70(22):9003–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bode LM, Bunzel D, Huch M, Cho GS, Ruhland D, Bunzel M, Bub A, Franz CM, Kulling SE. In vivo and in vitro metabolism of trans-resveratrol by human gut microbiota. Am J Clin Nutr. 2013;97(2):295–309. [DOI] [PubMed] [Google Scholar]
- 11. Yu C, Shin YG, Chow A, Li Y, Kosmeder JW, Lee YS, Hirschelman WH, Pezzuto JM, Mehta RG, van Breemen RB. Human, rat, and mouse metabolism of resveratrol. Pharm Res. 2002;19(12):1907–14. [DOI] [PubMed] [Google Scholar]
- 12. Pallauf K, Rimbach G, Rupp PM, Chin D, Wolf IM. Resveratrol and lifespan in model organisms. Curr Med Chem. 2016;23(41):4639–80. [DOI] [PubMed] [Google Scholar]
- 13. Colman RJ, Anderson RM, Johnson SC, Kastman EK, Kosmatka KJ, Beasley TM, Allison DB, Cruzen C, Simmons HA, Kemnitz JWet al. . Caloric restriction delays disease onset and mortality in rhesus monkeys. Science. 2009;325(5937):201–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jiang JC, Jaruga E, Repnevskaya MV, Jazwinski SM. An intervention resembling caloric restriction prolongs life span and retards aging in yeast. FASEB J. 2000;14(14):2135–7. [DOI] [PubMed] [Google Scholar]
- 15. Speakman JR, Mitchell SE. Caloric restriction. Mol Aspects Med. 2011;32(3):159–221. [DOI] [PubMed] [Google Scholar]
- 16. Mitchell SE, Delville C, Konstantopedos P, Hurst J, Derous D, Green C, Chen L, Han JJ, Wang Y, Promislow DEet al. . The effects of graded levels of calorie restriction: II. Impact of short term calorie and protein restriction on circulating hormone levels, glucose homeostasis and oxidative stress in male C57BL/6 mice. Oncotarget. 2015;6(27):23213–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Walford RL, Mock D, Verdery R, MacCallum T. Calorie restriction in biosphere 2: Alterations in physiologic, hematologic, hormonal, and biochemical parameters in humans restricted for a 2-year period. J Gerontol A Biol Sci Med Sci. 2002;57(6):B211–24. [DOI] [PubMed] [Google Scholar]
- 18. World Health Organization. [Internet], Ageing: Healthy ageing and functional ability. Available from: https://www.who.int/ageing/healthy-ageing/en/. [Google Scholar]
- 19. Melzer D, Pilling LC, Ferrucci L. The genetics of human ageing. Nat Rev Genet. 2020;21(2):88–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Partridge L, Deelen J, Slagboom PE. Facing up to the global challenges of ageing. Nature. 2018;561(7721):45–56. [DOI] [PubMed] [Google Scholar]
- 21. Barnett K, Mercer SW, Norbury M, Watt G, Wyke S, Guthrie B. Epidemiology of multimorbidity and implications for health care, research, and medical education: A cross-sectional study. Lancet North Am Ed. 2012;380(9836):37–43. [DOI] [PubMed] [Google Scholar]
- 22. Franceschi C, Campisi J. Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. J Gerontol A Biol Sci Med Sci. 2014;69(Suppl 1):S4–S9. [DOI] [PubMed] [Google Scholar]
- 23. Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, Seeman T, Tracy R, Kop WJ, Burke Get al. . Frailty in older adults: Evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146–56. [DOI] [PubMed] [Google Scholar]
- 24. Holzenberger M, Dupont J, Ducos B, Leneuve P, Geloen A, Even PC, Cervera P, Le Bouc Y. IGF-1 receptor regulates lifespan and resistance to oxidative stress in mice. Nature. 2003;421(6919):182–7. [DOI] [PubMed] [Google Scholar]
- 25. Mizushima N, Levine B, Cuervo AM, Klionsky DJ. Autophagy fights disease through cellular self-digestion. Nature. 2008;451(7182):1069–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pyo JO, Yoo SM, Ahn HH, Nah J, Hong SH, Kam TI, Jung S, Jung YK. Overexpression of Atg5 in mice activates autophagy and extends lifespan. Nat Commun. 2013;4, doi: 10.1038/ncomms3300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rodgers JT, Lerin C, Haas W, Gygi SP, Spiegelman BM, Puigserver P. Nutrient control of glucose homeostasis through a complex of PGC-1alpha and SIRT1. Nature. 2005;434(7029):113–8. [DOI] [PubMed] [Google Scholar]
- 28. Bordone L, Cohen D, Robinson A, Motta MC, Van Veen E, Czopik A, Steele AD, Crowe H, Marmor S, Luo Jet al. . SIRT1 transgenic mice show phenotypes resembling calorie restriction. Aging Cell. 2007;6(6):759–67. [DOI] [PubMed] [Google Scholar]
- 29. Haigis MC, Sinclair DA. Mammalian sirtuins: Biological insights and disease relevance. Annu Rev Pathol Mech Dis. 2010;5:253–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Howitz KT, Bitterman KJ, Cohen HY, Lamming DW, Lavu S, Wood JG, Zipkin RE, Chung P, Kisielewski A, Zhang LLet al. . Small molecule activators of sirtuins extend Saccharomycescerevisiae lifespan. Nature. 2003;425(6954):191–6. [DOI] [PubMed] [Google Scholar]
- 31. Satoh A, Brace CS, Rensing N, Cliften P, Wozniak DF, Herzog ED, Yamada KA, Imai S. Sirt1 extends life span and delays aging in mice through the regulation of Nk2 homeobox 1 in the DMH and LH. Cell Metab. 2013;18(3):416–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wood JG, Rogina B, Lavu S, Howitz K, Helfand SL, Tatar M, Sinclair D. Sirtuin activators mimic caloric restriction and delay ageing in metazoans. Nature. 2004;430(7000):686–9. [DOI] [PubMed] [Google Scholar]
- 33. Cuervo AM, Bergamini E, Brunk UT, Droge W, Ffrench M, Terman A. Autophagy and aging: The importance of maintaining “clean” cells. Autophagy. 2005;1(3):131–40. [DOI] [PubMed] [Google Scholar]
- 34. Kume S, Uzu T, Horiike K, Chin-Kanasaki M, Isshiki K, Araki S, Sugimoto T, Haneda M, Kashiwagi A, Koya D. Calorie restriction enhances cell adaptation to hypoxia through Sirt1-dependent mitochondrial autophagy in mouse aged kidney. J Clin Invest. 2010;120(4):1043–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Morselli E, Maiuri MC, Markaki M, Megalou E, Pasparaki A, Palikaras K, Criollo A, Galluzzi L, Malik SA, Vitale Iet al. . Caloric restriction and resveratrol promote longevity through the Sirtuin-1-dependent induction of autophagy. Cell Death Dis. 2010;1(1):e10–e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bonkowski MS, Rocha JS, Masternak MM, Al Regaiey KA, Bartke A. Targeted disruption of growth hormone receptor interferes with the beneficial actions of calorie restriction. Proc Natl Acad Sci. 2006;103(20):7901–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Derous D, Mitchell SE, Green CL, Wang Y, Han JD, Chen L, Promislow DE, Lusseau D, Speakman JR, Douglas A. The effects of graded levels of calorie restriction: VII. Topological rearrangement of hypothalamic aging networks. Aging. 2016;8(5):917–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults: Findings from the third National Health and Nutrition Examination Survey. JAMA. 2002;287(3):356–9. [DOI] [PubMed] [Google Scholar]
- 39. Ingram DK, Zhu M, Mamczarz J, Zou S, Lane MA, Roth GS, deCabo R. Calorie restriction mimetics: An emerging research field. Aging Cell. 2006;5(2):97–108. [DOI] [PubMed] [Google Scholar]
- 40. Bass TM, Weinkove D, Houthoofd K, Gems D, Partridge L. Effects of resveratrol on lifespan in Drosophila melanogaster and Caenorhabditiselegans. Mech Ageing Dev. 2007;128(10):546–52. [DOI] [PubMed] [Google Scholar]
- 41. Higashida K, Kim SH, Jung SR, Asaka M, Holloszy JO, Han DH. Effects of resveratrol and SIRT1 on PGC-1alpha activity and mitochondrial biogenesis: A reevaluation. PLOS Biol. 2013;11(7):e1001603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Miller RA, Harrison DE, Astle CM, Baur JA, Boyd AR, de Cabo R, Fernandez E, Flurkey K, Javors MA, Nelson JFet al. . Rapamycin, but not resveratrol or simvastatin, extends life span of genetically heterogeneous mice. J Gerontol A Biol Sci Med Sci. 2011;66A(2):191–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, Prabhu VV, Allard JS, Lopez-Lluch G, Lewis Ket al. . Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444(7117):337–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Porquet D, Casadesús G, Bayod S, Vicente A, Canudas AM, Vilaplana J, Pelegrí C, Sanfeliu C, Camins A, Pallàs Met al. . Dietary resveratrol prevents Alzheimer's markers and increases life span in SAMP8. Age (Dordr). 2013;35(5):1851–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hector KL, Lagisz M, Nakagawa S. The effect of resveratrol on longevity across species: A meta-analysis. Biol Lett. 2012;8(5):790–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lagouge M, Argmann C, Gerhart-Hines Z, Meziane H, Lerin C, Daussin F, Messadeq N, Milne J, Lambert P, Elliott Pet al. . Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell. 2006;127(6):1109–22. [DOI] [PubMed] [Google Scholar]
- 47. Pearson KJ, Baur JA, Lewis KN, Peshkin L, Price NL, Labinskyy N, Swindell WR, Kamara D, Minor RK, Perez Eet al. . Resveratrol delays age-related deterioration and mimics transcriptional aspects of dietary restriction without extending life span. Cell Metab. 2008;8(2):157–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Um J-H, Park S-J, Kang H, Yang S, Foretz M, McBurney MW, Kim MK, Viollet B, Chung JH. AMP-activated protein kinase-deficient mice are resistant to the metabolic effects of resveratrol. Diabetes. 2010;59(3):554–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Canto C, Jiang LQ, Deshmukh AS, Mataki C, Coste A, Lagouge M, Zierath JR, Auwerx J. Interdependence of AMPK and SIRT1 for metabolic adaptation to fasting and exercise in skeletal muscle. Cell Metab. 2010;11(3):213–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Svensson K, Schnyder S, Albert V, Cardel B, Quagliata L, Terracciano LM, Handschin C. Resveratrol and SRT1720 elicit differential effects in metabolic organs and modulate systemic parameters independently of skeletal muscle peroxisome proliferator-activated receptor γ co-activator 1α (PGC-1α). J Biol Chem. 2015;290(26):16059–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Labbé A, Garand C, Cogger VC, Paquet ER, Desbiens M, Le Couteur DG, Lebel M. Resveratrol improves insulin resistance hyperglycemia and hepatosteatosis but not hypertriglyceridemia, inflammation, and life span in a mouse model for Werner syndrome. J Gerontol A Biol Sci Med Sci. 2011;66A(3):264–78. [DOI] [PubMed] [Google Scholar]
- 52. Ding S, Jiang J, Zhang G, Bu Y, Zhang G, Zhao X. Resveratrol and caloric restriction prevent hepatic steatosis by regulating SIRT1-autophagy pathway and alleviating endoplasmic reticulum stress in high-fat diet-fed rats. PLOS One. 2017;12(8):e0183541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Witte AV, Fobker M, Gellner R, Knecht S, Flöel A. Caloric restriction improves memory in elderly humans. Proc Natl Acad Sci. 2009;106(4):1255–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kim D, Nguyen MD, Dobbin MM, Fischer A, Sananbenesi F, Rodgers JT, Delalle I, Baur JA, Sui G, Armour SMet al. . SIRT1 deacetylase protects against neurodegeneration in models for Alzheimer's disease and amyotrophic lateral sclerosis. EMBO J. 2007;26(13):3169–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Varamini B, Sikalidis AK, Bradford KL. Resveratrol increases cerebral glycogen synthase kinase phosphorylation as well as protein levels of drebrin and transthyretin in mice: An exploratory study. Int J Food Sci Nutr. 2014;65(1):89–96. [DOI] [PubMed] [Google Scholar]
- 56. Barger JL, Kayo T, Pugh TD, Prolla TA, Weindruch R. Short-term consumption of a resveratrol-containing nutraceutical mixture mimics gene expression of long-term caloric restriction in mouse heart. Exp Gerontol. 2008;43(9):859–66. [DOI] [PubMed] [Google Scholar]
- 57. Barger JL, Kayo T, Vann JM, Arias EB, Wang J, Hacker TA, Wang Y, Raederstorff D, Morrow JD, Leeuwenburgh Cet al. . A low dose of dietary resveratrol partially mimics caloric restriction and retards aging parameters in mice. PLOS One. 2008;3(6):e2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Günther I, Rimbach G, Mack CI, Weinert CH, Danylec N, Lüersen K, Birringer M, Bracher F, Soukup ST, Kulling SEet al. . The putative caloric restriction mimetic resveratrol has moderate impact on insulin sensitivity, body composition, and the metabolome in mice. Mol Nutr Food Res. 2020;64(6):1901116. doi: 10.1002/mnfr.201901116. [DOI] [PubMed] [Google Scholar]
- 59. Pallauf K, Chin D, Günther I, Birringer M, Lüersen K, Schultheiß G, Vieten S, Krauß J, Bracher F, Danylec Net al. . Resveratrol, lunularin and dihydroresveratrol do not act as caloric restriction mimetics when administered intraperitoneally in mice. Sci Rep. 2019;9(1):4445. doi: 10.1038/s41598-019-41050-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Scheibye-Knudsen M, Mitchell SJ, Fang EF, Iyama T, Ward T, Wang J, Dunn CA, Singh N, Veith S, Hasan-Olive MMet al. . A high-fat diet and NAD(+) activate Sirt1 to rescue premature aging in Cockayne syndrome. Cell Metab. 2014;20(5):840–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Tauriainen E, Luostarinen M, Martonen E, Finckenberg P, Kovalainen M, Huotari A, Herzig KH, Lecklin A, Mervaala E. Distinct effects of calorie restriction and resveratrol on diet-induced obesity and fatty liver formation. J Nutr Metab. 2011;2011:525094. doi: 10.1155/2011/525094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Xu BL, Wang R, Ma LN, Dong W, Zhao ZW, Zhang JS, Wang YL, Zhang X. Comparison of the effects of resveratrol and caloric restriction on learning and memory in juvenile C57BL/6J mice. Iran J Basic Med Sci. 2015;18(11):1118–23. [PMC free article] [PubMed] [Google Scholar]
- 63. Pallauf K, Günther I, Rimbach G. AMPK, Ppargca1 and Pdk4 in the heart as well as triglyceride and fasting glucose levels in the blood of young and middle-aged C57BL/6Rj mice supplemented with resveratrol compared to mice on caloric restriction. Unpublished results.2020. [Google Scholar]
- 64. Miller BF, Robinson MM, Bruss MD, Hellerstein M, Hamilton KL. A comprehensive assessment of mitochondrial protein synthesis and cellular proliferation with age and caloric restriction. Aging Cell. 2012;11(1):150–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Park SK, Kim K, Page GP, Allison DB, Weindruch R, Prolla TA. Gene expression profiling of aging in multiple mouse strains: Identification of aging biomarkers and impact of dietary antioxidants. Aging Cell. 2009;8(4):484–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. White R, Milholland B, MacRae S, Lin M, Zheng D. Comprehensive transcriptional landscape of aging mouse liver. BMC Genomics. 2015;16:899. doi: 10.1186/s12864-015-2061-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. de Ligt M, Bergman M, Fuentes RM, Essers H, Moonen-Kornips E, Havekes B, Schrauwen-Hinderling VB, Schrauwen P. No effect of resveratrol supplementation after 6 months on insulin sensitivity in overweight adults: a randomized trial. Am J Clin Nutr. 2020;112(4), 1029–1038.. doi: 10.1093/ajcn/nqaa125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Mayers JR, Iliff BW, Swoap SJ. Resveratrol treatment in mice does not elicit the bradycardia and hypothermia associated with calorie restriction. FASEB J. 2009;23(4):1032–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Asgary S, Karimi R, Momtaz S, Naseri R, Farzaei MH. Effect of resveratrol on metabolic syndrome components: A systematic review and meta-analysis. Rev Endocr Metab Disord. 2019;20(2):173–86. [DOI] [PubMed] [Google Scholar]
- 70. Haghighatdoost F, Hariri M.. Effect of resveratrol on lipid profile: An updated systematic review and meta-analysis on randomized clinical trials. Pharmacol Res. 2018;129:141–50. [DOI] [PubMed] [Google Scholar]
- 71. Hausenblas HA, Schoulda JA, Smoliga JM. Resveratrol treatment as an adjunct to pharmacological management in type 2 diabetes mellitus−systematic review and meta-analysis. Mol Nutr Food Res. 2015;59(1):147–59. [DOI] [PubMed] [Google Scholar]
- 72. Sahebkar A, Serban C, Ursoniu S, Wong ND, Muntner P, Graham IM, Mikhailidis DP, Rizzo M, Rysz J, Sperling LSet al. . Lack of efficacy of resveratrol on C-reactive protein and selected cardiovascular risk factors−Results from a systematic review and meta-analysis of randomized controlled trials. Int J Cardiol. 2015;189:47–55. [DOI] [PubMed] [Google Scholar]
- 73. Akbari M, Tamtaji OR, Lankarani KB, Tabrizi R, Dadgostar E, Haghighat N, Kolahdooz F, Ghaderi A, Mansournia MA, Asemi Z. The effects of resveratrol on lipid profiles and liver enzymes in patients with metabolic syndrome and related disorders: A systematic review and meta-analysis of randomized controlled trials. Lipids Health Dis. 2020;19(1):25. doi: 10.1186/s12944-020-1198-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Guo XF, Li JM, Tang J, Li D. Effects of resveratrol supplementation on risk factors of non-communicable diseases: A meta-analysis of randomized controlled trials. Crit Rev Food Sci Nutr. 2018;58(17):3016–29. [DOI] [PubMed] [Google Scholar]
- 75. Zhang C, Yuan W, Fang J, Wang W, He P, Lei J, Wang C. Efficacy of resveratrol supplementation against non-alcoholic fatty liver disease: A meta-analysis of placebo-controlled clinical trials. PLOS One. 2016;11(8):e0161792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Zhao H, Song A, Zhang Y, Shu L, Song G, Ma H. Effect of resveratrol on blood lipid levels in patients with type 2 diabetes: A systematic review and meta-analysis. Obesity. 2019;27(1):94–102. [DOI] [PubMed] [Google Scholar]
- 77. Elgebaly A, Radwan IA, AboElnas MM, Ibrahim HH, Eltoomy MF, Atta AA, Mesalam HA, Sayed AA, Othman AA. Resveratrol supplementation in patients with non-alcoholic fatty liver disease: Systematic review and meta-analysis. J Gastrointestin Liver Dis. 2017;26(1):59–67. [DOI] [PubMed] [Google Scholar]
- 78. Mousavi SM, Milajerdi A, Sheikhi A, Kord-Varkaneh H, Feinle-Bisset C, Larijani B, Esmaillzadeh A. Resveratrol supplementation significantly influences obesity measures: A systematic review and dose-response meta-analysis of randomized controlled trials. Obes Rev. 2019;20(3):487–98. [DOI] [PubMed] [Google Scholar]
- 79. Tabrizi R, Tamtaji OR, Lankarani KB, Akbari M, Dadgostar E, Dabbaghmanesh MH, Kolahdooz F, Shamshirian A, Momen-Heravi M, Asemi Z. The effects of resveratrol intake on weight loss: A systematic review and meta-analysis of randomized controlled trials. Crit Rev Food Sci Nutr. 2020;60(3):375–90. [DOI] [PubMed] [Google Scholar]
- 80. Liu K, Zhou R, Wang B, Mi M-T. Effect of resveratrol on glucose control and insulin sensitivity: A meta-analysis of 11 randomized controlled trials. Am J Clin Nutr. 2014;99(6):1510–9. [DOI] [PubMed] [Google Scholar]
- 81. Jeyaraman MM, Al-Yousif NSH, Singh Mann A, Dolinsky VW, Rabbani R, Zarychanski R, Abou-Setta AM. Resveratrol for adults with type 2 diabetes mellitus. Cochrane Database Syst Rev. 2020;1:CD011919. doi: 10.1002/14651858.CD011919.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Lainampetch J, Panprathip P, Phosat C, Chumpathat N, Prangthip P, Soonthornworasiri N, Puduang S, Wechjakwen N, Kwanbunjan K. Association of tumor necrosis factor alpha, interleukin 6, and C-reactive protein with the risk of developing type 2 diabetes: A retrospective cohort study of rural Thais. J Diabetes Res. 2019;2019:1. doi: 10.1155/2019/9051929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Custodero C, Mankowski RT, Lee SA, Chen Z, Wu S, Manini TM, Hincapie Echeverri J, Sabbà C, Beavers DP, Cauley JAet al. . Evidence-based nutritional and pharmacological interventions targeting chronic low-grade inflammation in middle-age and older adults: A systematic review and meta-analysis. Ageing Res Rev. 2018;46:42–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Haghighatdoost F, Hariri M.. Can resveratrol supplement change inflammatory mediators? A systematic review and meta-analysis on randomized clinical trials. Eur J Clin Nutr. 2019;73(3):345–55. [DOI] [PubMed] [Google Scholar]
- 85. Koushki M, Dashatan NA, Meshkani R. Effect of resveratrol supplementation on inflammatory markers: A systematic review and meta-analysis of randomized controlled trials. Clin Ther. 2018;40(7):1180–92. [DOI] [PubMed] [Google Scholar]
- 86. Tabrizi R, Tamtaji OR, Lankarani KB, Mirhosseini N, Akbari M, Dadgostar E, Peymani P, Asemi Z. The effects of resveratrol supplementation on biomarkers of inflammation and oxidative stress among patients with metabolic syndrome and related disorders: A systematic review and meta-analysis of randomized controlled trials. Food Funct. 2018;9(12):6116–28. [DOI] [PubMed] [Google Scholar]
- 87. Goh KP, Lee HY, Lau DP, Supaat W, Chan YH, Koh AF. Effects of resveratrol in patients with type 2 diabetes mellitus on skeletal muscle SIRT1 expression and energy expenditure. Int J Sport Nutr Exerc Metab. 2014;24(1):2–13. [DOI] [PubMed] [Google Scholar]
- 88. Timmers S, Konings E, Bilet L, Houtkooper RH, van de Weijer T, Goossens GH, Hoeks J, van der Krieken S, Ryu D, Kersten Set al. . Calorie restriction-like effects of 30 days of resveratrol supplementation on energy metabolism and metabolic profile in obese humans. Cell Metab. 2011;14(5):612–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Yoshino J, Conte C, Fontana L, Mittendorfer B, Imai S, Schechtman KB, Gu C, Kunz I, Rossi Fanelli F, Patterson BWet al. . Resveratrol supplementation does not improve metabolic function in nonobese women with normal glucose tolerance. Cell Metab. 2012;16(5):658–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Chachay VS, Macdonald GA, Martin JH, Whitehead JP, O'Moore-Sullivan TM, Lee P, Franklin M, Klein K, Taylor PJ, Ferguson Met al. . Resveratrol does not benefit patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2014;12(12):2092–103. [DOI] [PubMed] [Google Scholar]
- 91. Gualdoni GA, Kovarik JJ, Hofer J, Dose F, Pignitter M, Doberer D, Steinberger P, Somoza V, Wolzt M, Zlabinger GJ. Resveratrol enhances TNF-alpha production in human monocytes upon bacterial stimulation. Biochim Biophys Acta. 2014;1840(1):95–105. [DOI] [PubMed] [Google Scholar]
- 92. Gliemann L, Schmidt JF, Olesen J, Bienso RS, Peronard SL, Grandjean SU, Mortensen SP, Nyberg M, Bangsbo J, Pilegaard Het al. . Resveratrol blunts the positive effects of exercise training on cardiovascular health in aged men. J Physiol. 2013;591(20):5047–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Ristow M, Zarse K, Oberbach A, Kloting N, Birringer M, Kiehntopf M, Stumvoll M, Kahn CR, Bluher M. Antioxidants prevent health-promoting effects of physical exercise in humans. Proc Natl Acad Sci. 2009;106(21):8665–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Ernst IM, Pallauf K, Bendall JK, Paulsen L, Nikolai S, Huebbe P, Roeder T, Rimbach G. Vitamin E supplementation and lifespan in model organisms. Ageing Res Rev. 2013;12(1):365–75. [DOI] [PubMed] [Google Scholar]
- 95. Pallauf K, Bendall JK, Scheiermann C, Watschinger K, Hoffmann J, Roeder T, Rimbach G. Vitamin C and lifespan in model organisms. Food Chem Toxicol. 2013;58:255–63. [DOI] [PubMed] [Google Scholar]
- 96. Gonzalez-Freire M, Diaz-Ruiz A, Hauser D, Martinez-Romero J, Ferrucci L, Bernier M, de Cabo R. The road ahead for health and lifespan interventions. Ageing Res Rev. 2020;59:101037. doi: 10.1016/j.arr.2020.101037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Justice JN, Ferrucci L, Newman AB, Aroda VR, Bahnson JL, Divers J, Espeland MA, Marcovina S, Pollak MN, Kritchevsky SBet al. . A framework for selection of blood-based biomarkers for geroscience-guided clinical trials: Report from the TAME Biomarkers Workgroup. Geroscience. 2018;40(5-6):419–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Burgers AMG, Biermasz NR, Schoones JW, Pereira AM, Renehan AG, Zwahlen M, Egger M, Dekkers OM. Meta-analysis and dose-response metaregression: Circulating insulin-like growth factor I (IGF-I) and mortality. J Clin Endocrinol Metab. 2011;96(9):2912–20. [DOI] [PubMed] [Google Scholar]
- 99. Barron E, Lara J, White M, Mathers JC. Blood-borne biomarkers of mortality risk: Systematic review of cohort studies. PLOS One. 2015;10(6):e0127550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Hajizadeh-Sharafabad F, Sahebkar A, Zabetian-Targhi F, Maleki V. The impact of resveratrol on toxicity and related complications of advanced glycation end products: A systematic review. Biofactors. 2019;45(5):651–65. [DOI] [PubMed] [Google Scholar]
- 101. Aon MA, Bernier M, Mitchell SJ, Di Germanio C, Mattison JA, Ehrlich MR, Colman RJ, Anderson RM, de Cabo R. Untangling determinants of enhanced health and lifespan through a multi-omics approach in mice. Cell Metab. 2020;32(1):100–16.. doi:10.1016/j.cmet.2020.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Tresserra-Rimbau A, Medina-Remon A, Lamuela-Raventos RM, Bullo M, Salas-Salvado J, Corella D, Fito M, Gea A, Gomez-Gracia E, Lapetra Jet al. . Moderate red wine consumption is associated with a lower prevalence of the metabolic syndrome in the PREDIMED population. Br J Nutr. 2015;113:S121–30. [DOI] [PubMed] [Google Scholar]
- 103. Tresserra-Rimbau A, Rimm EB, Medina-Remon A, Martinez-Gonzalez MA, Lopez-Sabater MC, Covas MI, Corella D, Salas-Salvado J, Gomez-Gracia E, Lapetra Jet al. . Polyphenol intake and mortality risk: A re-analysis of the PREDIMED trial. BMC Med. 2014;12:77. doi: 10.1186/1741-7015-12-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Evans JM, Donnelly LA, Emslie-Smith AM, Alessi DR, Morris AD. Metformin and reduced risk of cancer in diabetic patients. BMJ. 2005;330(7503):1304–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Martin-Montalvo A, Mercken EM, Mitchell SJ, Palacios HH, Mote PL, Scheibye-Knudsen M, Gomes AP, Ward TM, Minor RK, Blouin MJet al. . Metformin improves healthspan and lifespan in mice. Nat Commun. 2013;4:2192. doi: 10.1038/ncomms3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Fogacci F, Fogacci S, Cicero A. Resveratrol for high blood pressure: A total failure or the need to identify the right patient?. High Blood Press Cardiovasc Prev. 2019;26(5):421–3. [DOI] [PubMed] [Google Scholar]
- 107. Egert S, Rimbach G.. Which sources of flavonoids: Complex diets or dietary supplements?. Adv Nutr. 2011;2(1):8–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Budhathoki S, Sawada N, Iwasaki M, Yamaji T, Goto A, Kotemori A, Ishihara J, Takachi R, Charvat H, Mizoue Tet al. . Association of animal and plant protein intake with all-cause and cause-specific mortality in a Japanese cohort. JAMA Intern Med. 2019;179(11):1509–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Liu L, Wang S, Liu J. Fiber consumption and all-cause, cardiovascular, and cancer mortalities: A systematic review and meta-analysis of cohort studies. Mol Nutr Food Res. 2015;59(1):139–46. [DOI] [PubMed] [Google Scholar]


