ABSTRACT
Testosterone concentrations in males tend to decline with advancing age. Low testosterone, also known as androgen deficiency (AD), is associated with an increased risk of morbidity and mortality. Currently, the primary treatment for AD is testosterone replacement therapy (TRT), which may exacerbate pre-existing medical conditions. Therefore, the use of alternative options, such as herbs, spices, plants, or their extracts, has been explored as a potential treatment option for AD. The aim of this systematic review was to summarize and critically evaluate randomized controlled trials published on the efficacy of single herbal ingredients on testosterone concentrations, in addition to its fractions or binding proteins, in men (≥18 y). From the 4 databases searched, there were 13 herbs identified in 32 studies, published between 2001 and 2019. The main findings of this review indicate that 2 herbal extracts, fenugreek seed extracts and ashwagandha root and root/leaf extracts, have positive effects on testosterone concentrations in men. Also, some evidence exists for another herb and herbal extract, Asian red ginseng and forskohlii root extract. Overall, 9 out of 32 studies demonstrated statistically significant increases in testosterone concentrations. Moreover, 6 studies out of 32 were judged as having a low risk of bias. Current evidence is largely based on young, nonclinical populations, with 16 out of 32 studies using men <40 y of age. Conclusions are moderated by the paucity of research for many herbs, the variation in dosages and extracts used, small sample sizes, and the heterogeneity of study characteristics. Also, further research is required before definitive conclusions on efficacy and safety can be made. This systematic review was registered at PROSPERO as CRD42020173623.
Keywords: testosterone, sex hormone-binding globulin, SHBG, male, adult, supplement, herbal, extract
This systematic review includes randomized controlled trials examining the effects of herbs, spices, plants, and their extracts on testosterone concentrations in men.
Introduction
From the age of 30 onwards, testosterone concentrations in men tend to decline at the rate of ∼1% per year (1–6). Low testosterone, also known as androgen deficiency (AD), or late-onset hypogonadism (LOH) when it occurs in men >40 y of age, is associated with a range of morbidities including major depressive disorder (7), type 2 diabetes (8), obesity (9), and cardiovascular disease (CVD) (10). AD is also associated with a reduced quality of life (11) and an increased risk of mortality in men (12–14). For example, Laughlin et al. (12) observed over an 11.8-y period that men with the lowest total testosterone (TT) concentrations had a 40% greater likelihood of dying compared with men with higher testosterone concentrations, even after controlling for age, obesity, and lifestyle, whereas Pye et al. (13) noted a 5-fold greater risk of all-cause mortality in men with severe LOH compared with men without the condition, after controlling for age, BMI, current smoking, and poor general health. Testosterone replacement therapy (TRT), on the other hand, was found to significantly increase survival rates of hypogonadal men (15, 16).
Despite TRT being the primary treatment for men with AD, its use remains controversial due to its association with an increased risk of exacerbating pre-existing medical conditions. Accordingly, men with existing prostate disease, elevated hematocrit concentrations, high CVD risk, or obstructive sleep apnea may be contraindicated for TRT (17–20). Moreover, according to the US FDA, TRT is only recommended for cases of “classic” or irreversible AD rather than “functional”, age, or comorbidity-related AD (21). This recommendation is endorsed by the Endocrine Society of Australia (20). Therefore, strategies to increase testosterone production and testosterone concentrations in men, without the potential drawbacks associated with TRT, are highly desirable and are key clinical objectives.
Plants and plant-based products, including herbs and spices, have been used for millennia to improve the flavor of food as well as to treat disease and improve overall health and well-being (22). For example, saffron has demonstrated antidepressant and anxiolytic effects (23), curcumin appears to offer pain relief for sufferers of arthritis (24), and cinnamon may support blood-sugar regulation in type 2 diabetics (25). The popularity of herbal products in the United States continues to rise steadily, with annual sales increasing every year for over a decade, with approximately US$8.8 billion worth of products being sold in 2018 (26). The growth in popularity of herbal products is reportedly due to several factors, including perceived efficacy associated with a long history of traditional use and apparent safety due to the perceived absence of serious side effects (27). Given the increasing body of research on herbal supplementation to support natural hormone production, it presents as a potential treatment option for AD. A recent narrative review by Clemesha et al. (28) concluded that many supplements claiming “testosterone-boosting” properties, including formulations using herbs, spices, plants, or their extracts, do not appear to be supported by scientific evidence. However, the review of Clemesha et al. (28) had several limitations such as using only a single search term, “testosterone booster,” using only Google to search for existing research, and examining only the top 50 supplements. Therefore, the aim of this systematic review was to summarize and critically evaluate randomized controlled trials conducted to assess the efficacy of single herbal ingredients on testosterone concentrations, in addition to their fractions or binding proteins, in men. “Herbs, spices, plants, or their extracts” will henceforth be referred to as “herbs.”
Methods
Protocol, registration, data sources, and searches
The preparation of this systematic review followed the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines (www.prisma-statement.org) and was registered with the International Prospective Register of Systematic Reviews (PROSPERO CRD42020173623) (29). The strategy for preparing this systematic review used the PICOS (population, intervention, comparator, outcomes, and setting) approach, and was as follows:
Population: adult male humans
Intervention: herbs, spices, plants, or their extracts
Comparator: placebo or control
Outcomes: saliva, serum, or plasma testosterone concentrations
Setting: any
The PICOS statement/question was as follows: “In adult human males, does supplementation with herbs, spices, plants, or their extracts affect testosterone concentrations compared to a placebo?” Publications for this review were obtained by searching the following databases: PubMed, Scopus, Cochrane library, and the Cumulative Index to Nursing and Allied Health Literature (CINAHL). Databases were searched from inception until March 2020. The literature search strategy was designed around the search terms of 1) testosterone or androgen and 2) herb* or spice or plant or extract or Ayurved*, with the Title and Abstract limiters applied on the PubMed database. The search terms were modified for each database using filters and limiters as necessary. The reference lists of relevant papers were also examined to locate additional studies that were not identified by the initial database search.
Eligibility criteria
Inclusion criteria for eligible studies were as follows: 1) randomized controlled study design (including crossover study design); 2) recruited an adult (≥18 y), male, human population, or if both genders were recruited, conducted subgroup analyses on males; 3) examined the effects of a single herb, spice, plant, or extract on serum, plasma, or saliva testosterone concentrations (monotherapy); 4) included a placebo or control as a comparator; 5) published in English; 6) had pre- and post-testing of testosterone concentrations in male participants.
Data extraction and quality assessment
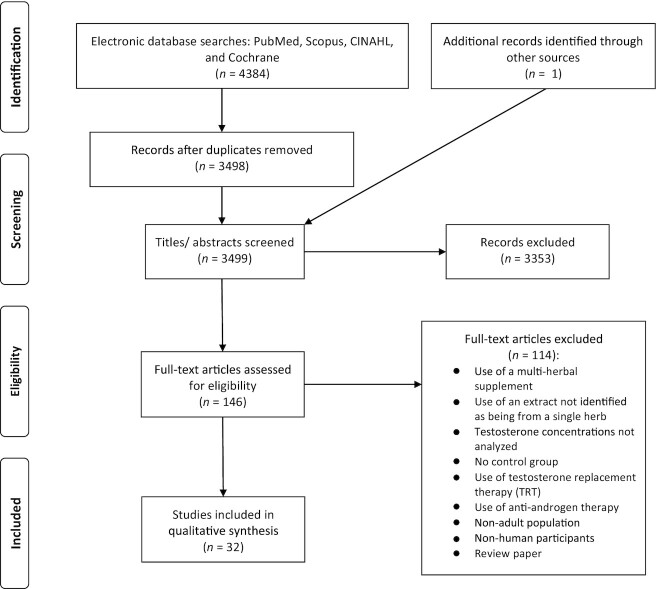
Two authors (SJS and ALL) completed the initial screening to exclude irrelevant titles. The remaining abstracts and subsequent remaining full-text articles were independently screened by 2 authors (SJS and ALL) for eligibility. All eligible studies were included in this review (Figure 1). The data were extracted from each study and entered into a template, which included the following parameters: study, year (reference), study characteristics (design, duration, country), participant characteristics (number of participants, status, age, BMI), supplement characteristics (ingredient and dosage), outcome measures, main results, and study outcomes. The results of the data extraction are detailed in Table 1. The results were detailed based on the change in outcome measures between the intervention and placebo groups with a probability value of P < 0.05 considered statistically significant. Due to the heterogeneity of study methodology (i.e., intervention time frame), study population, types and dose of herbal ingredients, and the limited number of replicable studies, a meta-analysis was not conducted.
FIGURE 1.
Study selection process.
TABLE 1.
Summary of randomized controlled trials examining the effects of herbs on testosterone concentrations in men1
| Study, year (reference) | Study characteristics | Participant characteristics | Supplement characteristics | Main results | Study outcomes (P) | ||
|---|---|---|---|---|---|---|---|
| Outcome measures | Control | Intervention | |||||
| Panax ginseng (Asian ginseng) | |||||||
| Youl Kang et al., 2002(65) | Design: CrossoverDuration: 2 hCountry: Korea | Participants (commenced, completed): 8 males (CON: 8, 8; INT: 8, 8)Status: No known clinical conditionAge (y): 20 to 24; mean 21.3 (0.7)BMI (kg/m2): DNS | CON: Placebo (water only); INT: 20 g Korean red ginseng root extract taken once daily immediately postexercise | Plasma testosterone concentrations (nmol/L) | BASE: DNSPost-15: DNSPost-30: DNSPost-60: DNSPost-120: DNS | BASE: DNSPost-15: DNSPost-30: DNSPost-60: DNSPost-120: DNS | ↔(>0.05) |
| Choi et al., 2013 (36) | Design: ParallelDuration: 8 wkCountry: Korea | Participants (commenced, completed): 119 males (CON: 59, 59; INT: 60, 59)Status: Erectile dysfunctionAge (y): CON: 57.32 (8.41); INT: 57.49 (7.94)BMI (kg/m2): DNS | CON: Placebo; INT: Standardized Korean ginseng berry extract (SKGB) an Asian ginseng berry ethanolic dry extract standardized to 10% ginsenoside Re (made from dried de-seeded berries from 4-y-old ginseng plants) 350 mg/tablet), 4 tablets daily | Serum testosterone concentrations (ng/dL) | BASE: 482.05 (171.83)POST: 469.57 (154.02) | BASE: 500.53 (189.58)POST: 499.32 (168.00) | ↔(0.649) |
| Jung et al., 2016 (47) | Design: ParallelDuration: 4 wkCountry: Korea | Participants (commenced, completed): 72 males (CON: 36, 30; INT: 36, 32)Status: Metabolic syndromeAge (y): CON: 45.2 (9.7); INT: 48.2 (10.9)BMI (kg/m2): CON: 27.9 (6.8); INT: 29.7 (6.9) | CON: Placebo (corn starch); INT: Red (Asian) ginseng (RG) (500 mg/capsule), 3 capsules, twice daily | Serum testosterone concentrations (ng/dL) | BASE: 431.4 (95.8)POST: 419.1 (101.9) | BASE: 396.2 (152.9)POST: 418.3 (162.2) | ↑(<0.05) |
| Kim et al., 2009 (49) | Design: ParallelDuration: 8 wkCountry: Korea | Participants (commenced, completed): 143 males (CON: 68, 21; INT: 75, 65)Status: Erectile dysfunctionAge (y): CON: 60.19 (2.02); INT: 57.51 (1.24)BMI (kg/m2): DNS | CON: Placebo; INT: Tissue-cultured mountain (Asian) ginseng (root) extract (TMGE) (1000 mg/tablet), 1 tablet, twice daily | Serum testosterone concentrations (ng/mL) | BASE: 4.02 (0.87)POST: 4.21 (1.78) | BASE: 4.22 (1.17)POST: 4.74 (1.64) | ↔(>0.05) |
| de Andrade et al., 2007(37) | Design: ParallelDuration: 12 wkCountry: Brazil | Participants (commenced, completed): 60 males (CON: 30, 30; INT: 30, 30)Status: Erectile dysfunctionAge (y): CON: 54.3 (DNS); INT: 52.6 (DNS)BMI (kg/m2): DNS | CON: Placebo (starch); INT: Korean red ginseng (KRG) (1000 mg/capsule), 1 capsule, 3 times daily | Serum testosterone concentrations (ng/dL) | BASE: 540.3 (109.8)POST: 508.8 (103.0) | BASE: 552.0 (120.7)POST: 560.0 (112.5) | ↔(>0.05) |
| Gaffney et al., 2001 (38) | Design: ParallelDuration: 6 wkCountry: Australia | Participants (commenced, completed): 30 males [CON: 10, 6; INT (PG): 10, 6; INT (ES): 10, 6]Status: No known clinical conditionAge (y): 18 to 40BMI (kg/m2): DNS | CON: Placebo (flavored and colored ethanol); INT: 2 g Asian ginseng (Panax ginseng) or 4 g Eleutherococcus senticosus, 8 mL/d | Serum testosterone concentrations (nmol/L) | BASE: 16.9 (1.2)POST: 17.7 (2.3) | BASE: 15.1 (1.1)POST: 17.7 (1.8) | ↔(>0.05) |
| Park et al., 2016 (56) | Design: ParallelDuration: 12 wkCountry: Korea | Participants (commenced, completed): 80 males [CON (non-V+P): 20, 20; CON (V+P): 20, 19; INT (Non-V+KRG): 20, 20; INT (V+KRG): 20, 18] | CON: Placebo; INT: Korean red ginseng (6-y-old ginseng, steamed, dried, and powdered) (KRG) (500 mg/capsule), 3 capsules daily | Serum testosterone concentrations (ng/dL) | Non-V+P:BASE: 481.1 (50.5)POST: 490.7 (10.1)V+P:BASE: 501.2 (52.8)POST: 506.8 (9.8) | Non-V+KRG:BASE: 493.4 (60.1)POST: 513.9 (14.5)V+KRGBASE: 488.1 (55.3)POST: 507.4 (12.5) | ↔(>0.05) |
| Status: InfertileAge (y): CON (non-V+P): 33.5 (5.9); CON (V+P): 35.1 (6.1); INT (non-V+KRG): 33.3 (5.3); INT (V+KRG): 34.7 (5.4)BMI (kg/m2): DNS | |||||||
| Trigonella foenum-graecum (fenugreek) | |||||||
| Rao et al., 2016 (58) | Design: ParallelDuration: 12 wkCountry: Australia | Participants: (commenced, completed): 120 males (CON: 60, 50; INT: 60, 46)Status: Overweight and obeseAge (y): CON: 56.4 (7.4); INT: 54.8 (6.9)BMI (kg/m2): CON: 29.3 (4.4); INT: 30.1 (5.1) | CON: Placebo; INT: Fenugreek seed extract (Testofen®, manufactured by GencorTM) (TFG), 600 mg taken daily | Serum total testosterone (nmol/L)Calculated FT (pmol/L)Sex hormone–binding globulin concentrations (nmol/L) | BASE: 13.2 (5.1)MID: 13.5 (5.5)POST: 12.4 (5.2)BASE: 254 (93.6)MID: 256 (93.3)POST: 231 (72.9)BASE: 36.6 (19.5)MID: 37.6 (17.7)POST: 37.6 (21.3) | BASE: 12.3 (4.4)MID: 12.9 (4.9)POST: 13.8 (5.1)BASE: 241 (68.9)MID: 245 (80.6)POST: 264 (78.9)BASE: 34.5 (14.6)MID: 36.7 (15.3)POST: 37.4 (15.6) | ↑(=0.001)↑(=0.002)↔(>0.05) |
| Mokashi et al., 2014 (53) | Design: CrossoverDuration: 10 hCountry: India | Participants (commenced, completed): 16 males (CON: 16, 16; INT: 16, 16)Status: No known clinical conditionAge (y): Sequence AB: 24.50 (5.63); Sequence BA: 27.62 (4.4)BMI (kg/m2): Sequence AB: 22.26 (DNS); Sequence BA: 24.59 (DNS) | CON: Placebo (lactose); INT: Fenugreek seed extract (IND9, manufactured by Indus Biotech®) (300 mg/capsule) 2 capsules, once daily | Serum total testosterone (ng/dL)Measurement of FT (pg/dL)Calculated FT (pg/dL)Bioavailable testosterone concentrations (ng/dL) | BASE: 473.6 (185.3)POST: 501.1 (144.6)BASE: 13.6 (4.8)POST: 13.2 (3.8)BASE: 8.4 (3.0)POST: 9.7 (2.3)BASE: 222.6 (81.9)POST: 242.1 (59.7) | BASE: 405.5 (142.9)POST: 519.0 (189.3) BASE: 11.7 (2.6)POST: 13.5 (5.0)BASE: 7.2 (2.1)POST: 9.9 (2.8)BASE: 184.5 (49.6)POST: 246.9 (77.7) | ↑(0.018)↔(>0.05)↑(0.038)↑(0.025) |
| Wankhede et al., 2016(63) | Design: ParallelDuration: 8 wkCountry: India | Participants: (commenced, completed): 60 males (CON: 30, 26; INT: 30, 29)Status: No known clinical conditionAge (y): CON: 21.62 (3.96); INT: 23.21 (3.73)BMI (kg/m2): CON: 22.36 (3.39); INT: 23.73 (2.32) | CON: Placebo; INT: Fenugreek seed extract (Fenu-FG, active component of Testofen®) (300 mg/capsule), 1 capsule, twice daily | Serum total testosterone (ng/dL)FT concentrations (ng/dL) | BASE: 387.52 (86.19)POST: 421.27 (93.36)BASE: 21.30 (12.24)POST: 31.70 (19.48) | BASE: 404.95 (83.57)POST: 452.60 (107.87)BASE: 17.76 (10.98)POST: 35.29 (15.01) | ↔(>0.05)↑(<0.05) |
| Rao and Grant, 2020 (57) | Design: ParallelDuration: 12 wkCountry: Australia | Participants (commenced, completed): 100 males (CON: 50, 42; INT: 50, 42)Status: Apparently healthy with BPHAge (y): CON: 64.0 (7.7), INT: 61.8 (8.0)BMI (kg/m2): CON: 26.7 (3.9); INT: 28.2 (2.8) | CON: Placebo; INT: Fenugreek seed extract (Testofen®) (300 mg/capsule), 1 capsule, twice daily | Serum total testosterone (nmol/L)FT (nmol/L)Sex hormone–binding globulin concentrations (nmol/L) | BASE: 16.4 (6.0)POST: 15.8 (5.2)BASE: 0.27 (0.07)POST: 0.27 (0.08)BASE: 47.9 (17.9)POST: 47.3 (16.3) | BASE: 16.0 (4.7)POST: 15.5 (4.6)BASE: 0.28 (0.08)POST: 0.28 (0.07)BASE: 46.1 (20.1)POST: 44.3 (19.0) | ↔(0.36)↔(0.44)↔(>0.05) |
| Guo et al., 2018 (42) | Design: ParallelDuration: 12 wkCountry: USA | Participants (commenced, completed): 40 males (CON: 20, NA; INT: 20, NA; 35 completed the study)Status: No known clinical conditionAge (y): 18 to 30, mean 24.02 (3.9)BMI (kg/m2): DNS | CON: Placebo; INT: Fenugreek seed extract (Furosap®, manufactured by Chemical Resources, CHERESO) (250 mg/capsule) 1 capsule, once daily | Serum testosterone (ng/dL) | BASE: 608.0 (50.0)POST: 596 (59.0) | BASE: 545.6 (59.0)POST: 669.6 (54.0) | ↑(<0.05) |
| Wilborn et al., 2010 (64) | Design: ParallelDuration: 8 wkCountry: USA | Participants (commenced, completed): 30 males (CON: 13, 13; INT: 17, 17)Status: No known clinical conditionAge (y): CON: 21.0 (3.0), INT: 21.0 (2.8) | CON: Placebo (maltodextrin); INT: Fenugreek seed extract (standardized for Grecunin, manufactured by Indus Biotech®) (500 mg/capsule), 1 capsule, once daily | Serum total testosterone (ng/mL)Bioavailable testosterone concentrations (ng/mL) | BASE: 15.80 (4.91)POST: 13.70 (3.27)BASE: 11.80 (5.40)POST: 10.11 (3.29) | BASE: 14.76 (3.97)POST: 15.73 (3.62)BASE: 10.77 (4.11)POST: 12.09 (4.16) | ↑(<0.05)↑(<0.05) |
| BMI (kg/m2): CON: 25.93 (DNS); INT: 26.83 (DNS) | |||||||
| Withania somnifera (ashwagandha) | |||||||
| Lopresti et al., 2019 (50) | Design: CrossoverDuration: 16 wkCountry: Australia | Participants (commenced, completed): 57 malesG1 (placebo): 1st block: 29, 25G1 (ashwagandha): 2nd block: 25, 23G2 (ashwagandha): 1st block: 28, 25G2 (placebo): 2nd block: 25, 20Status: Overweight males with mild fatigueAge (y): G1: 51.66 (1.19); G2: 50.07 (1.26)BMI (kg/m2): G1: 27.93 (0.65); G2: 26.72 (0.55) | CON: Placebo (rice powder); INT: Ashwagandha root and leaf extract tablets (10.5 mg (35%) of withanolide glycosides) (Shoden®, manufactured by Arjuna Natural), 2 tablets (21 mg of withanolide glycoside), once daily | Salivary testosterone concentrations (pmol/L) | Mean group 1: 324.57 (18.44)Mean group 2: 295.41 (25.25)Overall mean: 309.99 (15.29) | Mean group 1: 332. 77 (35.59)Mean group 2: 378.38 (27.22)Overall mean: 355.57 (22.02) | ↑(<0.01) |
| Lopresti et al., 2019 (51) | Design: ParallelDuration: 60 dCountry: India | Participants (commenced, completed): 60 (M:F; 37:23) (CON: 18, 12; INT: 19, 11)Status: Adults with high stressAge (y): CON: 40.23 (2.43); INT: 42.23 (2.44)BMI (kg/m2): CON: 26.04 (0.65); INT: 24.68 (0.60) | CON: Placebo (rice powder); INT: Ashwagandha root and leaf extract tablets (240 mg ashwagandha extract/tablet) [35% withanolide glycosides (Shoden®)], 1 capsule (84 mg withanolide glycosides), once daily | Serum total testosterone (ng/dL) | BASE (overall): 341.75 (55.17)BASE (males only): 543.47 (51.43)POST (overall): 341.97 (53.42)POST (males only): 544.17 (49.33) | BASE (overall): 320.72 (45.60)BASE (males only): 472.88 (39.76)POST (overall): 354.88 (53.42)POST (males only): 526.89 (48.01) | Overall↔(0.15)Males only↔(0.16) |
| Wankhede et al., 2015(62) | Design: ParallelDuration: 8 wkCountry: India | Participants (commenced, completed): 57 males (CON: 28, 25; INT: 29, 25)Status: No known clinical condition | CON: Placebo (starch); INT: Ashwagandha root extract (KSM-66®, a water-based extraction process, standardized to 5% | Serum testosterone concentrations (ng/dL) | BASE: 675.12 (157.02)POST: 693.12 (115.04) | BASE: 630.45 (231.88)POST: 726.64 (171.55) | ↑(0.004) |
| Age (y): CON: 29.0 (9.0); INT: 28.0 (8.0)BMI (kg/m2): DNS | withanolides, manufactured by Ixoreal) (300 mg/capsule), 1 capsule, twice daily | ||||||
| Ambiye et al., 2013 (35) | Design: ParallelDuration: 90 dCountry: India | Participants (commenced, completed): 46 males (CON: 21, 21; INT: 25, 25)Status: InfertileAge (y): CON: 35.28 (5.49); INT: 32.38 (4.31)BMI (kg/m2): CON: 26.61 (DNS); INT: 25.45 (DNS) | CON: Placebo (maltodextrin); INT: Ashwagandha root extract (KSM-66®, a water-based extraction process, standardized to 5% withanolides) (225 mg/capsule), 1 capsule, 3 times daily | Serum testosterone concentrations (ng/mL) | BASE: 4.42 (1.5)POST: 4.59 (1.48) | BASE: 4.45 (1.41)POST: 5.22 (1.39) | ↑(0.01) |
| Tribulus terrestris (tribulus) | |||||||
| Santos et al., 2014 (61) | Design: ParallelDuration: 30 dCountry: Brazil | Participants (commenced, completed): 30 males (CON: 15, 15; INT: 15, 15)Status: No known clinical conditionAge (y): CON: 62.9 (7.9); INT: 60 (9.4)BMI (kg/m2): CON: (normal): 53.3%, (overweight): 33.3%, (obese): 13.3%; INT: (normal): 26.6%; (overweight): 40.0%, (obese): 33.3% | CON: Placebo (pharmaceutical talc); INT: Tribulus dry extract (400 mg/capsule), 1 capsule, twice daily | Serum testosterone concentrations (ng/dL) | BASE: 442.7 (range: 301.0–609.1)POST: 466.3 (range: 264.3–934.3) | BASE: 417.1 (range: 270.7–548.4)POST: 409.3 (range: 216.9–760.8) | ↔(0.36) |
| Neychev and Mitev,2005 (54) | Design: ParallelDuration: 4 wkCountry: Bulgaria | Participants (commenced, completed): 21 males [CON: 7,7; INT1 (TT1): 7, 7; INT2 (TT2): 7, 7]Status: No known clinical conditionAge (y): 20 to 36BMI (kg/m2): DNS | CON: Placebo; INT: TT1: Tribulus dry extract 20 mg . kg−1 . d−1 (3 divided doses); TT2: Tribulus terrestris dry extract 10 mg . kg−1 . d−1 (3 divided doses) | Serum testosterone concentrations (nmol/L) | BASE: DNSPOST: 17.74 (1.09) | BASE (TT1): DNSPOST (TT1): 15.75 (1.75)BASE (TT2): DNSPOST (TT2): 16.32 (1.57) | TT1 vs. CON↔(>0.05)TT2 vs. CON↔(>0.05) |
| GamalEl Din et al., 2019(39) | Design: ParallelDuration: 3 moCountry: Egypt | Participants (commenced, completed): 70 males (CON: 35, 35; INT: 35, 35)Status: Erectile dysfunction and lower urinary tract symptomsAge (y): CON: 58.38 (9.71); INT: 55.69 (9.35)BMI (kg/m2): CON: DNS; INT: DNS | CON: Placebo (starch granules); INT: Tribulus (Trib Gold, manufactured by Nerhadou International), containing no more than 45% steroidal saponins (250 mg tribulus/capsule), 1 capsule, 3 times daily | Total testosterone concentrations (ng/mL) | BASE: 2.01 (2.18)POST: 2.06 (1.3) | BASE: 2.15 (0.24)POST: 2.73 (0.70) | NA |
| Kamenov et al., 2017 (48) | Design: ParallelDuration: 12 wkCountry: Bulgaria | Participants (commenced, completed) 180 males (CON: 90, 86; INT: 90, 86)Status: Erectile dysfunctionAge (y): CON: 41.18 (12.36); INT: 44.11 (12.37)BMI (kg/m2): CON: 28.04 (4.49); INT: 27.47 (4.65) | CON: Placebo; INT: Tribulus extract (overground parts) (Tribestan, manufactured by Sopharma) (250 mg, containing 112.5 mg furostanol saponins/tablet), 2 tablets, 3 times daily | Serum total testosterone (nmol/L)FT (nmol/L)Sex hormone–binding globulin concentrations (nmol/L) | BASE: 16.01 (5.48)POST: 13.91 (5.20)BASE: 0.31 (0.09)POST: 0.26 (0.08)BASE: 35.20 (14.97)POST: 35.41 (15.28) | BASE: 15.42 (6.04)POST: 13.93 (5.86)BASE: 0.31 (0.10)POST: 0.27 (0.12)BASE: 31.34 (14.46)POST: 33.95 (15.35) | ↔(>0.05)↔(>0.05)↔(>0.05) |
| Lepidium meyenii (maca) | |||||||
| Melnikovova et al., 2015(52) | Design: ParallelDuration: 12 wkCountry: Czech Republic | Participants (commenced, completed): 20 males (CON: 9, 7; INT: 11, 11)Status: No known clinical conditionAge (y): 20 to 40BMI (kg/m2): DNS | CON: Placebo; INT: Maca (dried hypocotyls of maca were rehydrated and pressurized under moist conditions) (350 mg/capsule), 5 capsules (1750 mg Maca) taken daily | Serum testosterone concentrations (nmol/L) | BASE: 19.43 (2.25)POST: 18.63 (2.15) | BASE: 19.92 (1.75)POST: 20.10 (2.24) | ↔(>0.05) |
| Zenico et al., 2009 (66) | Design: ParallelDuration: 12 wkCountry: Italy | Participants (commenced, completed): 50 males (CON: 25, 25; INT: 25, DNS)Status: Erectile dysfunctionAge (y): 36 (5)BMI (kg/m2): DNS | CON: Placebo; INT: Maca (pulverized, dehydrated maca root) (1200 mg/tablet), 1 tablet, twice daily (2400 mg maca) | Serum total testosterone (ng/mL)FT concentrations (pg/mL) | BASE: 6.2 (0.7)POST: 6.0 (0.9)BASE: 12.1 (1.1)POST: 11.8 (0.9) | BASE: 5.9 (0.8)POST: 6.1 (0.9)BASE: 12.4 (1.2)POST: 12.5 (1.0) | ↔(>0.05)↔(>0.05) |
| Gonzales et al., 2003 (41) | Design: ParallelDuration: 12 wkCountry: Peru | Participants (commenced, completed): 56 malesCON:Placebo 1 (4, 4)Placebo 2 (4, 4)Placebo 3 (4, 4)INT:Maca 1 (15, 15)Maca 2 (15, 14)Maca 3 (15, 15)Status: No known clinical conditionAge (y): 21 to 56BMI (kg/m2): DNS | CON:Placebo 1: 1 tablet, 3 times/dPlacebo 2: 2 tablets, 3 times/dPlacebo 3: 3 tablets, once/dayINT (gelatinized maca, method not described):Maca 1: 500 mg 3 times/dMaca 2: 1000 mg 3 times/dMaca 3: 1500 mg maca, once/day | Serum testosterone concentrations (ng/mL) | BASE: DNSPOST: DNS | BASE: DNSPOST: DNS | ↔(>0.05) |
| Rhodiola rosea (rhodiola) | |||||||
| Jowko et al., 2018 (46) | Design: ParallelDuration: 4 wkCountry: Poland | Participants (commenced, completed): 26 males (CON: 13, 13; INT: 13, 13)Status: No known clinical conditionAge (y): CON: 20.5 (0.3); INT: 20.9 (0.2)BMI (kg/m2): CON:23.77 (DNS); INT: 23.85 (DNS) | CON: Placebo; INT: Rhodiola extract, standardized to 3% rosavins (200 mg/tablet), 1 tablet, 3 times daily | Plasma testosterone concentrations (nmol/L) | BASE: 24.7 (2.1)POST: 26.5 (2.5) | BASE: 21.7 (2.2)POST: 21.6 (1.2) | ↔(>0.05) |
| Chlorophytum borivilianum (musali) | |||||||
| Rath et al., 2013 (59) | Design: ParallelDuration: 12 wkCountry: India | Participants (commenced, completed): 30 males (CON: 15, 15; INT: 15, 15)Status: No known clinical conditionAge (y): 20 to 40BMI (kg/m2): DNS | Control: Placebo (barley powder); INT: 500 mg musali root extract (water extract of dried root tubers) capsules, 2 tablets taken daily | Serum testosterone concentrations (ng/mL) | BASE: 477.07 (DNS)POST: 481.73 (DNS) | BASE: 475.13 (DNS)POST: 481.13 (DNS) | ↔(>0.1) |
| Garcinia cambogia (garcinia) | |||||||
| Hayamizu et al., 2008(43) | Design: ParallelDuration: 12 wkCountry: USA | Participants (commenced, completed): 44 (M:F; 25:19) (CON: 23, 21; INT: 21, 18)Status: Overweight/obeseAge (y): 20 to 65BMI (kg/m2): 28.7 (0.7) | Control: Placebo (cellulose); INT: garcinia (270 mg/tablet; 185.25 mg Garcinia cambogia; active ingredient, hydroxycitric acid, 111.11 mg), 3 tablets, 3 times daily | Serum testosterone concentrations (ng/dL) (data were provided as a figure) | BASE: DNSPOST: DNS | BASE: DNSPOST: DNS | ↔(>0.05) |
| Coleus forskohlii (forskohlii) | |||||||
| Godard et al., 2005 (40) | Design: ParallelDuration: 12 wkCountry: USA | Participants (commenced, completed): 30 males (CON: 15, 15; INT: 15, 15)Status: Overweight/obeseAge (y): CON: 28.7 (8.6); INT: 24.4 (5.9)BMI (kg/m2): CON: 32.6 (3.8); INT: 32.5 (4.1) | CON: Placebo; INT: 500 mg forskohlii root extract, manufactured to contain a forskolin concentration of 10% (Forslean), 2 tablets daily | Serum total testosterone (ng/mL)FT concentrations (pg/mL) | BASE: 4.12 (0.82)MID: 3.97 (0.85)POST: 4.00 (0.89)BASE: 13.28 (7.26)MID: 12.28 (7.44)POST: 12.77 (7.30) | BASE: 5.06 (1.21)MID: 5.27 (1.03)POST: 5.75 (1.50)BASE: 15.90 (13.39)MID: 15.67 (13.68)POST: 16.36 (13.32) | ↑(<0.05)↔(>0.05) |
| Ganoderma lucidum (reishi) | |||||||
| Noguchi et al., 2008 (55) | Design: ParallelDuration: 16 wkCountry: Japan | Participants (commenced, completed): 88 males (CON: 44, 39; INT: 44, 41)Status: LUTSAge (y): CON: 64.0 (8.0); INT: 64.0 (6.9)BMI (kg/m2): DNS | CON: Placebo; INT: reishi extract (dried and chipped with 30% ethanol) (3 mg/tablet), 2 tablets, once daily | Serum testosterone concentrations (ng/mL) | BASE: 3.9 (1.4)POST: 4.1 (DNS) | BASE: 3.9 (1.2)POST: 3.93 (DNS) | ↔(0.31) |
| Urtica dioca (stinging nettle) | |||||||
| Safarinejad, 2005(60) | Design: ParallelDuration: 6 moCountry: Iran | Participants (commenced, completed): 620 males (CON: 315, 231; INT: 305, 240)Status: LUTSAge (y): CON: 62.0 (DNS); INT: 64.0 (DNS)BMI (kg/m2): DNS | CON: Placebo; INT: stinging nettle root extract (synthesised from the roots via a fractional percolation process and standardization) (120 mg/liquid preparation), 120 mg 3 times daily | Serum testosterone concentrations (ng/dL) | BASE: 651 (27)POST: 645 (30) | BASE: 645 (31)POST: 649 (29) | ↔(>0.05) |
| Eurycoma longifolia (longjack) | |||||||
| Ismail et al., 2012 (45) | Design: ParallelDuration: 12 wkCountry: Malaysia | Participants (commenced, completed): 109 males (CON: 55, 51; INT: 54, 52)Status: No known clinical conditionAge (y): CON: 42.8 (6.73); INT: 43.6 (6.52)BMI (kg/m2): CON: 25.78 (DNS); INT: 26.41 (DNS) | CON: Placebo (maltodextrin); INT: longjack (Physta®, freeze-dried water extract, manufactured by Biotropics) (75 mg/capsule), 4 capsules taken daily | Serum total testosterone (nmol/L)FT (nmol/L)Sex hormone–binding globulin concentrations (nmol/L) | BASE: DNSPOST: DNSBASE: DNSPOST: DNSBASE: DNSPOST: DNS | BASE: DNSPOST: DNSBASE: DNSPOST: DNSBASE: DNSPOST: DNS | ↔(>0.05)↔(>0.05)↔(>0.05) |
| Cordyceps sinensis (cordyceps) | |||||||
| Hsu et al., 2011 (44) | Design: ParallelDuration: 8 wkCountry: Taiwan | Participants (commenced, completed): 16 males (CON: 8, 8; INT: 8, 8)Status: No known clinical conditionAge (y): 19 to 25BMI (kg/m2): CON: 23.9 (0.1); INT: 24.3 (1.4) | CON: Placebo (maltodextrin); INT: Cordyceps (manufactured by a submerged culture technique and spray-dried to obtain a powder) (400 mg/capsule), 6 capsules taken daily | Serum testosterone concentrations (nmol/L) | BASE: 21.4 (1.6)POST: 22.5 (1.6) | BASE: 23.2 (1.8)POST: 24.0 (2.9) | ↔(>0.05) |
Values are means (SDs) unless otherwise indicated. AB, active-placebo; BA, placebo-active; BASE, baseline; BPH, benign prostate hyperplasia; CON, control group; DNS, did not state in manuscript; ES, Eleutherococcus senticosus; FT, free testosterone; INT, supplement intervention group; LUTS, lower urinary tract symptoms; MID, mid-intervention; NA, not applicable; Non-V+KRG, non-varicocelectomy plus Korean red ginseng; Non-V+P, non-varicocelectomy plus placebo group; PG, Panax ginseng; POST, post-intervention; TT1, Tribulus terrestris 20 mg/kg/d; TT2, Tribulus terrestris 10 mg/kg/d; V+KRG, varicocelectomy plus Korean red ginseng; V+P, varicocelectomy plus placebo group; ↔, no significant difference reported between conditions; ↑ or ↓, significant difference between exercise and control conditions.
Risk of bias of the included studies was assessed using the Cochrane Collaboration's tool for assessing the risk of bias in randomized trials (30). The risk-of-bias assessment was independently conducted by 2 authors (SJS and SYMT). Each study was scored based upon its risk of bias being low, with some concerns, or high for each criterion. The results of the assessment are summarized in Supplemental Tables 1 and 2.
Outcome measures
Testosterone, its fractions, and binding proteins were all examined in this systematic review. These included TT, (calculated) free testosterone (FT), measurement of FT, bioavailable testosterone (BT), and sex hormone–binding globulin (SHBG). Measurements were provided per specific unit of plasma, serum, or saliva. The inclusion of the testosterone fractions and binding proteins were deemed important since only ∼2% is “free” testosterone, with the remaining testosterone “bound” to proteins (predominantly SHBG, ∼40–45%) (31–33) and controversy existing regarding the relative importance of “bound” and “unbound” testosterone (34). FT or BT (the combination of FT and albumin-bound, “weakly-bound” testosterone) have been suggested as more effective measures for diagnosing AD, particularly as men age (32).
Results
The initial search identified 4384 unique studies, of which 32 randomized controlled trials were identified as eligible following assessment using the selection criteria (Figure 1) (35–66). Only the outcome measures from men were used in this review. Included trials were published between 2001 and 2019 and used either a parallel (35–49, 51, 52, 54–64, 66) (n = 29) or crossover (50, 53, 65) (n = 3) design.
Study characteristics
Sample sizes ranged from 7 to 620 and ages of the male participants ranged from 18 to 79 y. Two studies out of 32 included both male and female participants (2488 participants in total; 2446 males and 42 females). The mean BMI (in kg/m2) of the participants ranged from 22.26 to 32.6. Study durations were from 2 h up to 6 mo, with 2 studies having durations of 2 h and 10 h, respectively, and the remaining studies having durations from 4 wk to 6 mo. Details of studies included in this review are outlined in Table 1.
Adverse events and tolerability
All herbal extracts were well tolerated, and the incidence of adverse events was low. Rao et al. (58) reported that there were 5 minor adverse events. These included headaches, dizziness, nausea, and increased asthma symptoms. However, they may not have been attributed to the treatment. Rao and Grant (57) reported 4 incidents of reflux during their study. Ismail et al. (45) reported a total of 31 adverse events, with all of them being assessed as unlikely related to the treatment.
Panax ginseng (Asian ginseng)
Seven studies were conducted examining the effects of Asian ginseng on testosterone concentrations (36–38, 47, 49, 56, 65). All studies used berry or root extracts of Asian ginseng (Panax ginseng), with the root reported as Korean red ginseng, a processed form (67). Six studies used a randomized, double-blind, placebo-controlled study design (36–38, 47, 49, 56), and 1 study used a randomized, controlled crossover study design (65). A total of 512 male participants were recruited for the studies involving Asian ginseng, with ages ranging from 18 to 79 y and samples sizes ranging from 8 to 143 participants.
Only 1 study demonstrated positive effects of Asian ginseng supplementation on testosterone (47), while 6 studies showed no effect of supplementation (Table 1). The positive study by Jung et al. (47), recruited 72 overweight men (intervention group: 48.2 ± 10.9 y; control group: 45.2 ± 9.7 y) with metabolic syndrome. Participants supplemented with 3.0 g of Asian red ginseng (details of extract preparation not provided) daily for 4 wk significantly increased their TT concentrations (5.6%) compared with those in the placebo group (−2.9%) (P < 0.05). Two studies (38, 65) recruited healthy young (age range: 18–40 y) men. In an acute study (2 h) conducted in 8 men with a mean age of 21.3 ± 0.7 y, supplementation of 20 g of Korean red ginseng root extract did not increase testosterone concentrations (65), while in 30 healthy, endurance-trained men, aged between 18 and 40 y, Panax ginseng (an ethanolic liquid extract equivalent to 2 g of dried root, daily) did not increase testosterone concentrations after 6 wk of supplementation (38). Three studies recruited participants with erectile dysfunction (36, 37, 49). In 119 men with erectile dysfunction, aged between 20 and 70 y, an Asian ginseng berry ethanolic dry extract standardized to 10% ginsenoside Re (made from dried de-seeded berries from 4-y-old ginseng plants) at a daily dosage of 1.4 g for 8 wk was not associated with a significant between-group difference in testosterone concentrations (36). This outcome was similar to that of an 8-wk study in 143 men (33–79 y) with erectile dysfunction, using a slightly higher dose (2 g/d) of tissue-cultured mountain (Asian) ginseng (root) extract (49), as well as another study using a longer intervention (12 wk, 3 g/d, Korean red ginseng; details of extract preparation not provided) in 60 men (26–70 y) with mild-to-moderate erectile dysfunction (37). The final study using Asian ginseng was conducted in 80 infertile men, aged between 25 and 45 y (56). Daily supplementation with 1.5 g Korean red ginseng (6-y-old ginseng, steamed, dried, and powdered) over 12 wk resulted in no significant between-group differences in testosterone concentrations.
Trigonella foenum-graecum (fenugreek)
Six studies (42, 53, 57, 58, 63, 64) were conducted examining the effects of fenugreek seed extracts on testosterone concentrations. Three studies used the Testofen® extract, manufactured by GencorTM (also known as Fenu-FG) (details of extract preparation not provided) (57, 58, 63) and the other 3 studies used the extracts IND9, manufactured by Indus Biotech® (53), Furosap®, manufactured by Chemical Resources (CHERESO) (42), and an extract standardized for Grecunin, manufactured by Indus Biotech® (64) (details of all extract preparations not provided). Five studies were randomized, double-blind, placebo-controlled trials (42, 57, 58, 63, 64) and one was a randomized, double-blind, placebo-controlled crossover study (53). A total of 366 male participants were recruited for the studies, with ages ranging from 18 to 72 y and samples sizes ranging from 16 to 120 participants.
Four of the 6 studies demonstrated increased testosterone concentrations with fenugreek supplementation (42, 53, 58, 64), while 2 studies (57, 63) showed no effect of supplementation (Table 1). Three out of 4 studies also demonstrated positive effects on (calculated) FT (53, 58, 63), and the 2 fenugreek studies that included BT as an outcome measure demonstrated positive effects (53, 64). The acute study by Mokashi et al. (53) recruited 16 healthy men aged between 18 and 45 y (sequence active-placebo (AB): 24.5 ± 5.63 y; sequence placebo-active (BA): 27.62 ± 4.4 y). Participants supplemented with a fenugreek seed extract (IND9), at a daily dosage of 600 mg for 10 h, increased their TT concentrations (IND9: 28.0%; placebo: 5.8%; P = 0.018), calculated FT (cFT) concentrations (IND9: 37.5%; placebo: 15.5%; P = 0.038), and BT (IND9: 33.8%; placebo: 8.8%; P = 0.025). However, there were no between-group differences in the concentration of the directly measured FT (P > 0.05). Two studies (42, 64) recruited healthy, young (18–30 y) men. A fenugreek seed extract, Furosap® (250 mg daily), supplemented for 12 wk was associated with a significant increase in TT concentrations (22.7%) compared with the placebo group (−2.0%) in young men (n = 40; 18–30 y; mean: 24.02 ± 3.9 y) (42), while in 30 healthy men, aged between 18 and 24 y, a fenugreek seed extract (standardized for Grecunin), at a daily dosage of 500 mg, was associated with a significant increase in TT concentrations (6.6%) compared with the placebo group (−13.3%), and a significant increase in BT concentrations (12.3%) compared with the placebo group (−14.3%) after 8 wk of supplementation (64). In a 12-wk study conducted in 120 overweight or obese men, aged between 43 and 70 y (intervention group: 54.8 ± 6.9 y; control group: 56.4 ± 7.4 y), supplementing with 600 mg daily of a specialized fenugreek seed extract, Testofen®, was associated with a significant increase in TT concentrations (12.2%) compared with the placebo group (−6.1%) (P = 0.001) and an increase in cFT concentrations (9.5%) compared with the placebo group (−9.1%) (P = 0.002). There was no statistically-significant difference in SHBG concentrations between the 2 groups (58). The 2 studies that showed no effect of fenugreek supplementation on TT concentrations (57, 63) recruited healthy men, with participants in the Rao and Grant (57) study including men with benign prostate hyperplasia (BPH). In the 8-wk study conducted by Wankhede et al. (63), the researchers examined the effects of a fenugreek seed extract, Fenu-FG, on testosterone concentrations in 60 healthy men aged between 18 and 35 y (intervention group: 23.21 ± 3.73 y; control group: 21.62 ± 3.96 y). A daily dose of 600 mg of the fenugreek seed extract was associated with a significant increase in FT concentrations (98.7%) compared with the placebo group (48.8%) (P < 0.05). However, no significant difference in TT concentrations was found between the groups. In the 12-wk study conducted by Rao and Grant (57) in 100 men with BPH aged between 53 and 72 y (intervention group: 61.8 ± 8.0 y; control group: 64.0 ± 7.7 y), the 12-wk administration of Testofen® (600 mg/d) was associated with no significant difference in any hormone concentrations between the intervention and placebo groups.
Withania somnifera (ashwagandha)
Four studies were conducted examining the effects of ashwagandha on testosterone concentrations (35, 50, 51, 62). Two studies used a patented ashwagandha root extract, KSM-66®, manufactured by Ixoreal (using a water-based extraction process, standardized to 5% withanolides) (35, 62), and the other 2 studies used another patented root and leaf extract, Shoden®, manufactured by Arjuna Natural (using a 70:30 ethanol:water extraction process, standardized to 35% withanolide glycosides) (50, 51). Three studies used a randomized, double-blind, placebo-controlled study design (35, 51, 62), and 1 study used a randomized, double-blind, placebo-controlled, crossover study design (50). A total of 197 male participants were recruited for the studies, with ages ranging from 18 to 70 y and sample sizes ranging from 46 to 60 participants.
Three of the 4 studies demonstrated positive effects of ashwagandha supplementation on testosterone concentrations in men (35, 50, 62), while 1 study (51) showed no effect of supplementation (Table 1). Two of the studies demonstrating positive effects recruited men with no known clinical conditions (50, 62). The study by Lopresti et al. (50), recruited 57 overweight men with mild fatigue, aged between 40 and 70 y (group 1, placebo-active: 51.66 ± 1.19 y; group 2, active-placebo: 50.07 ± 1.26 y). The 8-wk administration of an ashwagandha extract (Shoden®), delivering 21 mg of withanolide glycosides/d, and which was preceded by 8 wk of supplementation with a placebo, was associated with significantly-higher testosterone concentrations (16.6%) compared with the opposite protocol, ashwagandha followed by placebo (−11.2%) (P < 0.01). While in 57 young males, with a mean age of 28 y, an ashwagandha extract (KSM-66®), at a daily dosage of 600 mg for 8 wk, was associated with a significant increase in testosterone concentrations (15.3% increase) compared with the placebo group (2.7% increase) (P < 0.001) (62). The other positive study examined the effect of KSM-66® on 46 infertile men with a mean age of 34 y (intervention group: 32.38 ± 5.49 y; control group: 35.28 ± 4.31 y). Supplementing with 675 mg/d of KSM-66® was associated with a significant increase in testosterone concentrations (17.3% increase) after 90 d compared with the placebo group (3.8% increase) (P < 0.01) (35). The 1 study that showed no effect of an ashwagandha extract, Shoden®, on testosterone concentrations in men was conducted by Lopresti et al. (51). The researchers examined testosterone concentrations in 60 stressed, healthy men and women with a mean age of 41 y. In the male participants (n = 19), daily intake of 240 mg of the ashwagandha extract was associated with no statistically-significant difference in testosterone concentrations compared with the placebo group after 60 d of treatment. However, the ashwagandha group did significantly increase testosterone concentrations (11.4%) compared with baseline values (P = 0.038).
Tribulus terrestris (tribulus)
Four studies (39, 48, 54, 61) examined the effects of Tribulus terrestris on testosterone concentrations. Two studies used patented extracts, Trib Gold, manufactured by Nerhadou International, standardized to contain up to 45% steroidal saponins (extract source not provided) (39), and Tribestan, manufactured by Sopharma, delivering a minimum of 675 mg furostanol saponins (from overground parts of the herb) (48). Santos et al. (61) and Neychev and Mitev (54) both used a dry extract of tribulus. Three studies were randomized controlled trials (39, 48, 54) and 1 study was a randomized, double-blind, placebo-controlled trial (61). A total of 301 participants were recruited for the studies involving Tribulus terrestris, with ages ranging from 18 to 65 y and sample sizes ranging from 21 to 180 participants.
There were no significant between-group differences in any of the 4 studies; however, in a 3-mo study conducted by GamalEl Din et al. (39), the researchers found that TT concentrations were significantly higher in the tribulus group after 3 mo of treatment (27.0%) with a daily intake of 750 mg of a Tribulus terrestris extract (Trib Gold), compared with their baseline results (P < 0.001). A between-group analysis was not conducted on this study. GamalEl Din et al. (39) recruited 70 middle- to older-age men with erectile dysfunction and lower urinary tract symptoms (intervention group: 55.69 ± 9.35 y; control group: 58.38 ± 9.71 y). Kamenov et al. (48) also recruited men with mild-to-moderate erectile dysfunction. In this 12-wk study with 180 men (intervention group: 44.11 ± 12.37 y; control group: 41.18 ± 12.36 y), the administration of 1.5 g of a tribulus extract (Tribestan) daily was associated with no significant difference in TT concentrations between the groups (48). Two studies (54, 61) recruited healthy men but with varying ages. The study conducted by Santos et al. (61) recruited an older cohort of 30 healthy men (intervention group: 60.0 ± 9.4 y; control group: 62.9 ± 7.9 y). Daily supplementation with 800 mg of a tribulus extract for 30 d did not increase testosterone concentrations, while in the Neychev and Mitev study (54) in 21 participants aged 20 to 36 y, a tribulus extract at 2 doses, 10 mg ⸱ kg−1 ⸱ d−1 (3 divided doses) or 20 mg ⸱ kg−1 ⸱ d−1 (3 divided doses), did not increase testosterone concentrations after 4 wk of supplementation.
Lepidium meyenii (maca)
Three randomized, double-blind, placebo-controlled studies (41, 52, 66) examined the effects of maca on testosterone concentrations. Two studies used gelatinized maca (41, 52) and 1 study used a dried maca extract (66). A total of 126 male participants were recruited for the studies, with ages ranging from 20 to 56 y and sample sizes ranging from 20 to 56 participants.
There were no statistically-significant increases in testosterone concentrations after 12 wk of supplementation in any of the trials. The study by Zenico et al. (66) recruited 50 men with mild-to-moderate erectile dysfunction (mean age: 36 ± 5 y). Participants supplemented with 2.4 g of maca (pulverized, dehydrated maca root) daily did not increase testosterone concentrations compared with the placebo group. Two studies (41, 52) recruited healthy men (age range: 20–56 y). In a study conducted in 20 healthy men between the ages of 20 and 40 y, supplementing with 1.75 g of gelatinized maca (dried hypocotyls of maca were rehydrated and pressurized under moist conditions) daily was associated with no significant difference in testosterone concentrations compared with the placebo group (52), while in a study of 56 healthy men aged between 21 and 56 y, 3 gelatinized (method not described) maca doses and schedules (1500 mg/d, 3 divided doses; 3000 mg/d, 3 divided doses; 1500 mg/d, 1 dose) were associated with no significant difference in testosterone concentrations compared with the equivalent placebo doses and schedules.
Rhodiola rosea (rhodiola)
One randomized, double-blind, placebo-controlled study was conducted examining the effects of a rhodiola extract (standardized to 3% rosavins) on testosterone concentrations (46). Four weeks of supplementation with 600 mg/d of a standardized rhodiola extract in 26 healthy men (intervention group: 20.9 ± 0.2 y; control group: 20.5 ± 0.3 y) did not significantly increase testosterone concentrations compared with the placebo group.
Chlorophytum borivilianum (musali)
One randomized, double-blind, placebo-controlled study was conducted examining the effects of a musali root extract (water extract of dried root tubers) on testosterone concentrations (59). Twelve weeks of daily supplementation with 1.0 g of a musali extract in 40 healthy men, aged between 20 and 40 y, did not significantly increase testosterone concentrations compared with the placebo group.
Garcinia cambogia (garcinia)
One randomized, double-blind, placebo-controlled study was conducted examining the effects of a garcinia extract (standardized to contain 60% hydroxycitric acid; details of extraction process not provided) on testosterone concentrations (43). Twelve weeks of daily supplementation with 1667 mg of garcinia, equivalent to 1000 mg of hydroxycitric acid, in 25 overweight or obese men aged between 20 and 65 y did not significantly increase testosterone concentrations compared with men in the placebo group.
Coleus forskohlii (forskohlii)
One randomized, double-blind, placebo-controlled study was conducted examining the effects of a forskohlii root extract, manufactured to contain a forskolin concentration of 10% (Forslean) daily (extraction process not provided) on testosterone concentrations (40). Twelve weeks of supplementation with 500 mg forskohlii in 30 overweight or obese men aged between 18 and 37 y (intervention group: 24.4 ± 5.9 y; control group: 28.7 ± 8.6 y) was associated with a significant increase in testosterone concentrations (13.6%) compared with the placebo group (−2.9%) (P < 0.05). However, there was no significant difference in FT concentrations between the groups.
Ganoderma lucidum (reishi)
One randomized, double-blind, placebo-controlled study was conducted examining the effects of reishi extract (dried and chipped with 30% ethanol) on testosterone concentrations (55). Sixteen weeks of daily supplementation with 6 mg/d of reishi extract in 88 men with slight-to-moderate lower urinary tract symptoms and aged >49 y (intervention group: 64.0 ± 6.9 y; control group: 64.0 ± 8.0 y) did not significantly increase testosterone concentrations compared with the placebo group.
Urtica dioca (stinging nettle)
One randomized, double-blind, placebo-controlled, partial-crossover study was conducted examining the effects of a stinging nettle root extract (synthesized from the roots via a fractional percolation process and standardization) on testosterone concentrations (60). Six months of daily supplementation with 360 mg of stinging nettle root extract in 620 men with lower urinary tract symptoms and a mean age of 63 y (intervention group: 64.0 y; control group: 62.0 y) did not significantly increase testosterone concentrations compared with the placebo group.
Eurycoma longifolia (longjack)
One randomized, double-blind, placebo-controlled study was conducted examining the effects of a longjack extract (Physta®, manufactured by Biotropics; freeze-dried water extract) on testosterone concentrations (45). Twelve weeks of daily supplementation with 300 mg of a longjack extract in 109 healthy men (or men with stable chronic medical conditions) and aged between 30 and 55 y (intervention group: 43.6 ± 6.52 y; control group: 42.8 ± 6.73 y) did not significantly increase testosterone concentrations compared with the placebo group.
Cordyceps sinensis (cordyceps)
One randomized, double-blind, placebo-controlled study was conducted examining the effects of cordyceps (manufactured by a submerged culture technique with the mycelia spray-dried to obtain a powder) on testosterone concentrations (44). Eight weeks of daily supplementation with 2.4 g of a cordyceps extract in 16 healthy men aged between 19 and 25 y did not significantly increase testosterone concentrations compared with the placebo group.
Assessment of studies’ risk of bias
The risk-of-bias assessment (Supplemental Tables 1 and 2) revealed some concerns for most of the included studies. Six studies (50, 52, 55, 56, 61, 63) out of 32 were judged to have a low risk of bias due to their strong methodological designs. Conversely, the study by Rath and Panja (59) was judged to have a high risk of bias since it had some concerns arising from its randomization process (domain 1) and a high risk of bias for its measurement of the outcome (domain 4) and selection of the reported result (domain 5). The remaining studies (25/32) were judged as having some concerns for their risk of bias due to potential biases arising from the randomization process (domain 1) (35–49, 51, 53, 54, 57, 58, 60, 62, 64–66), with Gaffney et al. (38) also being judged as having some concerns for its risk of bias due to missing outcome data (domain 3).
Discussion
The main findings of this review indicate that some herbs, particularly fenugreek seed extracts and ashwagandha extracts, have positive effects on testosterone concentrations in men. Overall, 9 out of 32 studies demonstrated significant increases in testosterone concentrations. Fenugreek seed extracts (positive findings in 4 out of 6 studies) and ashwagandha root and root/leaf extracts (positive findings in 3 out of 4 studies) demonstrated the most consistent increases in testosterone concentrations. Fenugreek seed extracts also demonstrated efficacy for increasing (calculated) FT and BT concentrations. While limited to support from a single trial, a forskohlii root extract was associated with increased TT. Asian ginseng also had 1 study demonstrating its efficacy in increasing testosterone (extract details not provided), but there were 6 studies demonstrating no effect. The paucity of high-quality randomized controlled trials examining the effects of herbs on testosterone concentrations in men, along with the heterogeneous cohorts assessed in these trials, precludes definitive conclusions being made.
In support of the findings of this review, a recent meta-analysis of clinical trials on fenugreek seed extract supplementation reported a significant increase in testosterone concentrations (68). This meta-analysis included 4 studies (42, 58, 63, 64) but did not include 2 of the studies included in this review because it was conducted in late 2018 and excluded studies with a duration <4 wk. Similar to the findings of the current review, Qureshi et al. (69) conducted a systematic review on all tribulus studies examining various forms of testosterone in animals and humans and concluded that the evidence indicated that it is ineffective at increasing testosterone concentrations in humans. Additionally, all studies examining the effect of maca on testosterone concentrations have previously been reviewed by Gonzales et al. (70) and were determined to not affect serum concentrations of testosterone.
Despite a lack of robust support for herbal supplementation increasing testosterone concentrations in men, there are potential mechanisms of action. These include anti-inflammatory and antioxidant properties of some herbs (69–74); decreasing the main counterregulatory hormones of testosterone, such as cortisol (75–78); or changing activity of key enzymes associated with testosterone production (72, 74, 77–80). For example, inflammation and oxidative stress have an inverse relationship with testosterone, as demonstrated by several human and animal trials (9, 81–83). Therefore, herbs with anti-inflammatory and antioxidant properties may have a positive effect on testosterone concentrations. For example, ashwagandha's antioxidant and anti-inflammatory effects, which have been demonstrated in animal and in vitro studies (71–73), may be associated with increased testosterone concentrations if the underlying cause of low testosterone is inflammation or oxidative stress. Asian ginseng also has antioxidant properties, which reduce lipid peroxidation and increase glutathione peroxidase concentrations (74), and it is noteworthy that the only study demonstrating benefits of Asian ginseng recruited individuals with metabolic syndrome (47). Moreover, the testosterone-enhancing effects identified in the single study on forskohlii (40) are consistent with the results of an animal study where reproductive toxicity in rats, induced by the pro-oxidant mancozeb, was attenuated by forskohlii supplementation, potentially resulting from its antioxidant properties (84).
Another potential mechanism through which herbs may increase testosterone concentrations in males is by ameliorating cortisol production. Since cortisol, the body's major stress hormone, is inversely correlated with testosterone concentrations (85), reducing its production may elevate testosterone concentrations. In several human trials, ashwagandha supplementation was associated with reduced cortisol concentrations (75, 76), potentially contributing to the testosterone-enhancing effects identified in 3 out of 4 studies herein. Moreover, in animal studies, the administration of 4-hydroxyisoleucine, an extract from fenugreek seeds, lowered serum cortisol concentrations (77). In this review, Asian ginseng only had 1 study out of 7 demonstrating a positive effect on testosterone concentrations in men; however, its administration reduced cortisol concentrations in adults experiencing high work stress (78).
Herbs may also influence the activity of enzymes associated with testosterone production. For example, in an animal study, Asian ginseng reduced the activity of 5α-reductase, the enzyme responsible for the conversion of testosterone into dihydrotestosterone (DHT) (80). Similarly, in vitro studies demonstrated reishi's ability to inhibit the activity of 5α-reductase (79, 86, 87).
Moreover, fenugreek seed extracts also demonstrated a positive effect on BT concentrations. This means that testosterone may have a greater physiological effect in the body when concentrations of these biomarkers are elevated. If future, robustly-designed studies demonstrate consistent positive effects of some of these herbs on testosterone production, further research will also be required to elucidate their potential mechanisms of action.
In this systematic review, many studies were underpowered, making it difficult to obtain statistically significant, between-group differences. Moreover, explanations were not provided for anomalous results. For example, reasons for 2 of the 6 fenugreek seed extract studies and 1 of the 4 ashwagandha root/leaf studies not achieving significant differences between groups were not discussed. However, it is possible that the populations examined (young men and men with BPH) may have been a factor. Also, the quality of studies identified in this review was variable, with 1 study assessed as having a high risk of bias due to concerns associated with randomization and the measurement and reporting of the outcome. Most included studies (n = 26) were assessed as having some concerns as their overall risk of bias. Only 6 out of 32 studies were assessed as having a low risk of bias due to their strong methodological study designs.
Strengths and limitations
A strength of this systematic review is the inclusion of only randomized controlled trials investigating the effect of a single herb on testosterone concentrations in men. In addition, given the existing controversy regarding the relative importance of “bound” and “unbound” testosterone (34), data for testosterone fractions and binding proteins were extracted and presented in the current review to increase the scope of findings. A limitation of this review is the exclusion of non-English studies. In addition, many of the studies included in this review have design flaws that adversely affect the strength of conclusions derived from this systematic review. The heterogeneity of herbal extracts, various dosages used, and differences in sample types (serum, plasma, and saliva samples) make comparing studies and conducting a meta-analysis difficult. For example, the studies on ashwagandha used dosages ranging from 240 to 675 mg/d, with varying standardization and extraction processes and different sample types. Also, the studies on fenugreek used 4 different extracts, with dosages ranging from 250 mg/d up to 600 mg/d. Moreover, several studies did not provide specific details of extracts as recommended by the National Center for Complementary and Integrative Health (88), making duplication of those studies difficult.
Directions for future research
The paucity of high-quality studies investigating the effects of herbs on testosterone production in men means further research is required. This involves increasing sample sizes to ensure studies are adequately powered, ensuring reliable and valid testing methods are used to measure testosterone concentrations, elucidating mechanisms of action, and assessing the efficacy and safety associated with acute and chronic herbal supplementation. The dosages used in the included studies varied widely, making an evaluation of safety and efficacy difficult. Moreover, there was significant variability in the herbal extracts used, which may impact safety and potency. Using standardized, replicable herbal extracts or, where appropriate, reporting the concentration of the active ingredient, can ameliorate some of these problems since different parts of the plant (e.g., leaf, stem, or root), the time of the year the herbs are harvested, how they are stored, and how they are processed can all affect concentrations of active ingredients (89). Even though some included studies demonstrated positive effects on testosterone concentrations, it is important that these effects are associated with meaningful health-enhancing benefits. This may be determined by examining concurrent improvements in mood, quality of life, and/or physical function; changes in biomarkers associated with comorbid conditions (inflammatory markers, oxidative stress markers, etc.); and/or changes in anatomical markers (muscle girths, body composition, microbial diversity, etc.). For example, studies in this review, such as Guo et al. (42), identified a significant increase in lean body mass in addition to a significant increase in testosterone concentrations from 12 wk of supplementation with a fenugreek seed extract. Moreover, 8 wk of supplementation with an ashwagandha root extract resulted in a significant increase in both muscle strength and testosterone concentrations (62). In addition to identifying the effects of herbal supplementation during use, it will be useful to determine the duration of effects once supplementation ceases. Moreover, how quickly herbs influence testosterone concentrations in men needs to be elucidated. This review included studies with a wide range of durations, from 2 h up to 6 mo. This may moderate the conclusions of the review since an herb's efficacy may only be identified with long-term use. However, this review identified 1 study having significant positive effects on testosterone concentrations with acute use (10 h, fenugreek seed extract) (53). Examining the safety and efficacy of an herb in different populations, such as healthy, aging men; men with comorbid medical conditions such as metabolic syndrome, obesity, or diabetes; men engaging in regular exercise; and in men taking concurrent medications, will also help to determine the most suitable groups to target for its use. Finally, matching treatment based on presentation may also clarify populations in whom herbal supplementation may be most beneficial. For example, given that specific herbs have anti-inflammatory and antioxidant activity, these may provide the greatest benefits for men presenting with excess premorbid inflammation or oxidative stress.
Conclusions
This systematic review provides some evidence that certain herbs and herbal extracts increase testosterone concentrations in men. Currently, the strongest evidence is for fenugreek seed extracts (Trigonella foenum-graecum; details of extract preparation not provided) and ashwagandha roots and leaves (Withania somnifera; water-based or ethanol:water-based, 70:30, extracts). However, conclusions are moderated by the paucity of research for many herbs, the variation in dosages and extracts used, small sample sizes, and the heterogeneity of study characteristics. Further research is required before definitive conclusions on efficacy and safety can be made. Moreover, making specific recommendations for certain herbs should be done cautiously until further robust research is conducted.
Supplementary Material
Acknowledgments
The authors thank Colin Smith for reviewing and editing the final copy of the manuscript. The authors’ responsibilities were as follows—All authors: are responsible for the design, writing, and final content of the manuscript and read and approved the final manuscript.
Notes
The authors reported no funding received for this study.
Author disclosures: SJS and ALL are employed by Clinical Research Australia, which has received funding to conduct research on herbal extracts. The other authors report no conflicts of interest.
Supplemental Tables 1 and 2 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/advances.
Abbreviations used: AD, androgen deficiency; BPH, benign prostate hyperplasia; BT, bioavailable testosterone; cFT, calculated free testosterone; CVD, cardiovascular disease; FT, free testosterone; LOH, late-onset hypogonadism; SHBG, sex hormone-binding globulin; TRT, testosterone replacement therapy.
Contributor Information
Stephen J Smith, Clinical Research Australia, Perth, Western Australia, Australia; College of Science, Health, Engineering and Education, Murdoch University, Perth, Western Australia, Australia.
Adrian L Lopresti, Clinical Research Australia, Perth, Western Australia, Australia; College of Science, Health, Engineering and Education, Murdoch University, Perth, Western Australia, Australia.
Shaun Y M Teo, College of Science, Health, Engineering and Education, Murdoch University, Perth, Western Australia, Australia.
Timothy J Fairchild, College of Science, Health, Engineering and Education, Murdoch University, Perth, Western Australia, Australia.
References
- 1. Travison TG, Araujo AB, Kupelian V, O'Donnell AB, McKinlay JB. The relative contributions of aging, health, and lifestyle factors to serum testosterone decline in men. J Clin Endocrinol Metab. 2007;92(2):549–55. [DOI] [PubMed] [Google Scholar]
- 2. Lapauw B, Goemaere S, Zmierczak H, Van Pottelbergh I, Mahmoud A, Taes Y, De Bacquer D, Vansteelandt S, Kaufman JM. The decline of serum testosterone levels in community-dwelling men over 70 years of age: descriptive data and predictors of longitudinal changes. Eur J Endocrinol. 2008;159(4):459–68. [DOI] [PubMed] [Google Scholar]
- 3. Liu PY, Beilin J, Meier C, Nguyen TV, Center JR, Leedman PJ, Seibel MJ, Eisman JA, Handelsman DJ. Age-related changes in serum testosterone and sex hormone binding globulin in Australian men: longitudinal analyses of two geographically separate regional cohorts. J Clin Endocrinol Metab. 2007;92(9):3599–603. [DOI] [PubMed] [Google Scholar]
- 4. Andersson AM, Jensen TK, Juul A, Petersen JH, Jorgensen T, Skakkebaek NE. Secular decline in male testosterone and sex hormone binding globulin serum levels in Danish population surveys. J Clin Endocrinol Metab. 2007;92(12):4696–705. [DOI] [PubMed] [Google Scholar]
- 5. Yeap BB, Almeida OP, Hyde Z, Norman PE, Chubb SA, Jamrozik K, Flicker L. In men older than 70 years, total testosterone remains stable while free testosterone declines with age. The Health in Men Study. Eur J Endocrinol. 2007;156(5):585–94. [DOI] [PubMed] [Google Scholar]
- 6. Harman SM, Metter EJ, Tobin JD, Pearson J, Blackman MR. Longitudinal effects of aging on serum total and free testosterone levels in healthy men. Baltimore Longitudinal Study of Aging. J Clin Endocrinol Metab. 2001;86(2):724–31. [DOI] [PubMed] [Google Scholar]
- 7. Joshi D, van Schoor NM, de Ronde W, Schaap LA, Comijs HC, Beekman AT, Lips P. Low free testosterone levels are associated with prevalence and incidence of depressive symptoms in older men. Clin Endocrinol (Oxf). 2010;72(2):232–40. [DOI] [PubMed] [Google Scholar]
- 8. Ding EL, Song Y, Malik VS, Liu S. Sex differences of endogenous sex hormones and risk of type 2 diabetes: a systematic review and meta-analysis. JAMA. 2006;295(11):1288–99. [DOI] [PubMed] [Google Scholar]
- 9. Traish AM, Zitzmann M. The complex and multifactorial relationship between testosterone deficiency (TD), obesity and vascular disease. Rev Endocr Metab Disord. 2015;16(3):249–68. [DOI] [PubMed] [Google Scholar]
- 10. Corona G, Rastrelli G, Monami M, Guay A, Buvat J, Sforza A, Forti G, Mannucci E, Maggi M. Hypogonadism as a risk factor for cardiovascular mortality in men: a meta-analytic study. Eur J Endocrinol. 2011;165(5):687–701. [DOI] [PubMed] [Google Scholar]
- 11. Maggi M, Schulman C, Quinton R, Langham S, Uhl-Hochgraeber K. The burden of testosterone deficiency syndrome in adult men: economic and quality-of-life impact. J Sex Med. 2007;4(4 Pt 1):1056–69. [DOI] [PubMed] [Google Scholar]
- 12. Laughlin GA, Barrett-Connor E, Bergstrom J. Low serum testosterone and mortality in older men. J Clin Endocrinol Metab. 2008;93(1):68–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pye SR, Huhtaniemi IT, Finn JD, Lee DM, O'Neill TW, Tajar A, Bartfai G, Boonen S, Casanueva FF, Forti Get al. . Late-onset hypogonadism and mortality in aging men. J Clin Endocrinol Metab. 2014;99(4):1357–66. [DOI] [PubMed] [Google Scholar]
- 14. Lehtonen A, Huupponen R, Tuomilehto J, Lavonius S, Arve S, Isoaho H, Huhtaniemi I, Tilvis R. Serum testosterone but not leptin predicts mortality in elderly men. Age Ageing. 2008;37(4):461–4. [DOI] [PubMed] [Google Scholar]
- 15. Shores MM, Smith NL, Forsberg CW, Anawalt BD, Matsumoto AM. Testosterone treatment and mortality in men with low testosterone levels. J Clin Endocrinol Metab. 2012;97(6):2050–8. [DOI] [PubMed] [Google Scholar]
- 16. Muraleedharan V, Marsh H, Kapoor D, Channer KS, Jones TH. Testosterone deficiency is associated with increased risk of mortality and testosterone replacement improves survival in men with type 2 diabetes. Eur J Endocrinol. 2013;169(6):725–33. [DOI] [PubMed] [Google Scholar]
- 17. Bhasin S, Cunningham GR, Hayes FJ, Matsumoto AM, Snyder PJ, Swerdloff RS, Montori VM; Endocrine Society Task Force. Testosterone therapy in men with androgen deficiency syndromes: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2010;95(6):2536–59. [DOI] [PubMed] [Google Scholar]
- 18. Grossmann M. Low testosterone in men with type 2 diabetes: significance and treatment. J Clin Endocrinol Metab. 2011;96(8):2341–53. [DOI] [PubMed] [Google Scholar]
- 19. Snyder PJ, Bhasin S, Cunningham GR, Matsumoto AM, Stephens-Shields AJ, Cauley JA, Gill TM, Barrett-Connor E, Swerdloff RS, Wang Cet al. . Effects of testosterone treatment in older men. N Engl J Med. 2016;374(7):611–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yeap BB, Grossmann M, McLachlan RI, Handelsman DJ, Wittert GA, Conway AJ, Stuckey BG, Lording DW, Allan CA, Zajac JDet al. . Endocrine Society of Australia position statement on male hypogonadism (part 2): treatment and therapeutic considerations. Med J Aust. 2016;205(5):228–31. [DOI] [PubMed] [Google Scholar]
- 21. Corona G, Maggi M. Perspective: regulatory agencies' changes to testosterone product labeling. J Sex Med. 2015;12(8):1690–3. [DOI] [PubMed] [Google Scholar]
- 22. Panickar KS. Beneficial effects of herbs, spices and medicinal plants on the metabolic syndrome, brain and cognitive function. Cent Nerv Syst Agents Med Chem. 2013;13(1):13–29. [DOI] [PubMed] [Google Scholar]
- 23. Marx W, Lane M, Rocks T, Ruusunen A, Loughman A, Lopresti A, Marshall S, Berk M, Jacka F, Dean OM. Effect of saffron supplementation on symptoms of depression and anxiety: a systematic review and meta-analysis. Nutr Rev. 2019. doi: 10.1093/nutrit/nuz023. [DOI] [PubMed] [Google Scholar]
- 24. Daily JW, Yang M, Park S. Efficacy of turmeric extracts and curcumin for alleviating the symptoms of joint arthritis: a systematic review and meta-analysis of randomized clinical trials. J Med Food. 2016;19(8):717–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Deyno S, Eneyew K, Seyfe S, Tuyiringire N, Peter EL, Muluye RA, Tolo CU, Ogwang PE. Efficacy and safety of cinnamon in type 2 diabetes mellitus and pre-diabetes patients: a meta-analysis and meta-regression. Diabetes Res Clin Pract. 2019;156:107815. [DOI] [PubMed] [Google Scholar]
- 26. Smith T, Gillespie M, Eckl V, Knepper J, Reynolds C. Herbal supplement sales in US increase by 9.4% in 2018. HerbalGram. 2019;123:62–73. [Google Scholar]
- 27. Schiff PL Jr, Srinivasan VS, Giancaspro GI, Roll DB, Salguero J, Sharaf MH. The development of USP botanical dietary supplement monographs, 1995–2005. J Nat Prod. 2006;69(3):464–72. [DOI] [PubMed] [Google Scholar]
- 28. Clemesha CG, Thaker H, Samplaski MK. “Testosterone boosting” supplements composition and claims are not supported by the academic literature. World J Mens Health. 2020;38(1):115–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, Shekelle P, Stewart LA. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JAet al. . The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Liu PY, Veldhuis JD. Hypothalamo-pituitary unit, testis, and male accessory organs. In: Yen and Jaffe's reproductive endocrinology. 8th ed. Elsevier; 2019. p. 285–300. [Google Scholar]
- 32. Faix JD. Principles and pitfalls of free hormone measurements. Best Pract Res Clin Endocrinol Metab. 2013;27(5):631–45. [DOI] [PubMed] [Google Scholar]
- 33. Colombo S, Buclin T, Decosterd LA, Telenti A, Furrer H, Lee BL, Biollaz J, Eap CB, Swiss H. Orosomucoid (alpha1-acid glycoprotein) plasma concentration and genetic variants: effects on human immunodeficiency virus protease inhibitor clearance and cellular accumulation. Clin Pharmacol Ther. 2006;80(4):307–18. [DOI] [PubMed] [Google Scholar]
- 34. Goldman AL, Bhasin S, Wu FCW, Krishna M, Matsumoto AM, Jasuja R. A reappraisal of testosterone's binding in circulation: physiological and clinical implications. Endocr Rev. 2017;38(4):302–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ambiye VR, Langade D, Dongre S, Aptikar P, Kulkarni M, Dongre A. Clinical evaluation of the spermatogenic activity of the root extract of Ashwagandha (Withania somnifera) in oligospermic males: a pilot study. Evid Based Complement Alternat Med. 2013;2013:571420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Choi YD, Park CW, Jang J, Kim SH, Jeon HY, Kim WG, Lee SJ, Chung WS. Effects of Korean ginseng berry extract on sexual function in men with erectile dysfunction: a multicenter, placebo-controlled, double-blind clinical study. Int J Impot Res. 2013;25(2):45–50. [DOI] [PubMed] [Google Scholar]
- 37. de Andrade E, de Mesquita AA, Claro Jde A, de Andrade PM, Ortiz V, Paranhos M, Srougi M. Study of the efficacy of Korean red ginseng in the treatment of erectile dysfunction. Asian J Andrology. 2007;9(2):241. [DOI] [PubMed] [Google Scholar]
- 38. Gaffney BT, Hugel HM, Rich PA. The effects of Eleutherococcus senticosus and Panax ginseng on steroidal hormone indices of stress and lymphocyte subset numbers in endurance athletes. Life Sci. 2001;70(4):431–42. [DOI] [PubMed] [Google Scholar]
- 39. GamalEl Din SF, Abdel Salam MA, Mohamed MS, Ahmed AR, Motawaa AT, Saadeldin OA, Elnabarway RR. Tribulus terrestris versus placebo in the treatment of erectile dysfunction and lower urinary tract symptoms in patients with late-onset hypogonadism: a placebo-controlled study. Urologia J. 2019;86(2):74–8. [DOI] [PubMed] [Google Scholar]
- 40. Godard MP, Johnson BA, Richmond SR. Body composition and hormonal adaptations associated with forskolin consumption in overweight and obese men. Obes Res. 2005;13(8):1335–43. [DOI] [PubMed] [Google Scholar]
- 41. Gonzales GF, Cordova A, Vega K, Chung A, Villena A, Gonez C. Effect of Lepidium meyenii (Maca), a root with aphrodisiac and fertility-enhancing properties, on serum reproductive hormone levels in adult healthy men. J Endocrinol. 2003;176(1):163–8. [DOI] [PubMed] [Google Scholar]
- 42. Guo R, Wang Q, Nair RP, Barnes SL, Smith DT, Dai B, Robinson TJ, Nair S. Furosap, a novel Fenugreek seed extract improves lean body mass and serum testosterone in a randomized, placebo-controlled, double-blind clinical investigation. Funct Food Health Disease. 2018;8(11):519–30. [Google Scholar]
- 43. Hayamizu K, Tomi H, Kaneko I, Shen M, Soni MG, Yoshino G. Effects of Garcinia cambogia extract on serum sex hormones in overweight subjects. Fitoterapia. 2008;79(4):255. [DOI] [PubMed] [Google Scholar]
- 44. Hsu C, Lin Y, Su B, Li J, Huang H, Hsu M. No effect of cordyceps sinensis supplementation on testosterone level and muscle strength in healthy young adults for resistance training. Biol Sport. 2011;28(2):107. [Google Scholar]
- 45. Ismail SB, Wan Mohammad WM, George A, Nik Hussain NH, Musthapa Kamal ZM, Liske E. Randomized clinical trial on the use of PHYSTA® freeze-dried water extract of Eurycoma longifolia for the improvement of quality of life and sexual well-being in men. Evid Based Complement Alternat Med. 2012;2012:429268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Jowko E, Sadowski J, Dlugolecka B, Gierczuk D, Opaszowski B, Cieslinski I. Effects of Rhodiola rosea supplementation on mental performance, physical capacity, and oxidative stress biomarkers in healthy men. J Sport Health Sci. 2018;7(4):473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Jung DH, Lee YJ, Kim CB, Kim JY, Shin SH, Park JK. Effects of ginseng on peripheral blood mitochondrial DNA copy number and hormones in men with metabolic syndrome: a randomized clinical and pilot study. Complement Ther Med. 2016;24:40. [DOI] [PubMed] [Google Scholar]
- 48. Kamenov Z, Fileva S, Kalinov K, Jannini EA. Evaluation of the efficacy and safety of Tribulus terrestris in male sexual dysfunction-A prospective, randomized, double-blind, placebo-controlled clinical trial. Maturitas. 2017;99:20. [DOI] [PubMed] [Google Scholar]
- 49. Kim TH, Jeon SH, Hahn EJ, Paek KY, Park JK, Youn NY, Lee HL. Effects of tissue-cultured mountain ginseng (Panax ginseng CA Meyer) extract on male patients with erectile dysfunction. Asian J Androl. 2009;11(3):356–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Lopresti AL, Drummond PD, Smith SJ. A randomized, double-blind, placebo-controlled, crossover study examining the hormonal and vitality effects of ashwagandha (Withania somnifera) in aging, overweight males. Am J Mens Health. 2019;13(2):155798831983598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Lopresti AL, Smith SJ, Malvi H, Kodgule R. An investigation into the stress-relieving and pharmacological actions of an ashwagandha (Withania somnifera) extract: a randomized, double-blind, placebo-controlled study. Medicine (Baltimore). 2019;98(37):e17186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Melnikovova I, Fait T, Kolarova M, Fernandez EC, Milella L. Effect of Lepidium meyenii Walp. on semen parameters and serum hormone levels in healthy adult men: a double-blind, randomized, placebo-controlled pilot study. Evid Based Complement Alternat Med. 2015;2015:324369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Mokashi M, Singh-Mokashi R, Mohan V, Thakurdesai P. Effects of glycosides based fenugreek seed extract on serum testosterone levels of healthy sedentary male subjects: an exploratory double blind, placebo controlled, crossover study. Asian J Pharm Clin Res. 2014;7(Suppl 2):177‐81. [Google Scholar]
- 54. Neychev VK, Mitev VI. The aphrodisiac herb Tribulus terrestris does not influence the androgen production in young men. J Ethnopharmacol. 2005;101(1–3):319‐23. [DOI] [PubMed] [Google Scholar]
- 55. Noguchi M, Kakuma T, Tomiyasu K, Yamada A, Itoh K, Konishi F, Kumamoto S, Shimizu K, Kondo R, Matsuoka K. Randomized clinical trial of an ethanol extract of Ganoderma lucidum in men with lower urinary tract symptoms. Asian J Androl. 2008;10(5):777‐85. [DOI] [PubMed] [Google Scholar]
- 56. Park HJ, Choe S, Park NC. Effects of Korean red ginseng on semen parameters in male infertility patients: a randomized, placebo-controlled, double-blind clinical study. Chin J Integr Med. 2016;22(7):490. [DOI] [PubMed] [Google Scholar]
- 57. Rao A, Grant R. The effect of Trigonella foenum-graecum extract on prostate-specific antigen, and prostate function in otherwise healthy men with benign prostate hyperplasia. Phytother Res. 2020;34(3):634–9. [DOI] [PubMed] [Google Scholar]
- 58. Rao A, Steels E, Inder WJ, Abraham S, Vitetta L. Testofen, a specialised Trigonella foenum-graecum seed extract reduces age-related symptoms of androgen decrease, increases testosterone levels and improves sexual function in healthy aging males in a double-blind randomised clinical study. Aging Male. 2016;19(2):134–42. [DOI] [PubMed] [Google Scholar]
- 59. Rath SK, Panja AK. Clinical evaluation of root tubers of Shweta Musali (Chlorophytum borivilianum L.) and its effect on semen and testosterone. Ayu. 2013;34(3):273–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Safarinejad MR. Urtica dioica for treatment of benign prostatic hyperplasia: a prospective, randomized, double-blind, placebo-controlled, crossover study. J Herb Pharmacother. 2005;5(4):1–11. [PubMed] [Google Scholar]
- 61. Santos CA Jr, Reis LO, Destro-Saade R, Luiza-Reis A, Fregonesi A. Tribulus terrestris versus placebo in the treatment of erectile dysfunction: a prospective, randomized, double blind study. Actas Urol Esp. 2014;38(4):244–8. [DOI] [PubMed] [Google Scholar]
- 62. Wankhede S, Langade D, Joshi K, Sinha SR, Bhattacharyya S. Examining the effect of Withania somnifera supplementation on muscle strength and recovery: a randomized controlled trial. J Int Soc Sports Nutr. 2015;12:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Wankhede S, Mohan V, Thakurdesai P. Beneficial effects of fenugreek glycoside supplementation in male subjects during resistance training: a randomized controlled pilot study. J Sport Health Sci. 2016;5(2):176–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Wilborn C, Taylor L, Poole C, Foster C, Willoughby D, Kreider R. Effects of a purported aromatase and 5alpha-reductase inhibitor on hormone profiles in college-age men. Int J Sport Nutr Exerc Metab. 2010;20(6):457–65. [DOI] [PubMed] [Google Scholar]
- 65. Youl Kang H, Hwan Kim S, Jun Lee W, Byrne HK. Effects of ginseng ingestion on growth hormone, testosterone, cortisol, and insulin-like growth factor 1 responses to acute resistance exercise. J Strength Cond Res. 2002;16(2):179–83. [DOI] [PubMed] [Google Scholar]
- 66. Zenico T, Cicero AF, Valmorri L, Mercuriali M, Bercovich E. Subjective effects of Lepidium meyenii (Maca) extract on well-being and sexual performances in patients with mild erectile dysfunction: a randomised, double-blind clinical trial. Andrologia. 2009;41(2):95–9. [DOI] [PubMed] [Google Scholar]
- 67. Lee SM, Bae BS, Park HW, Ahn NG, Cho BG, Cho YL, Kwak YS. Characterization of Korean red ginseng (Panax ginseng Meyer): history, preparation method, and chemical composition. J Ginseng Res. 2015;39(4):384–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Mansoori A, Hosseini S, Zilaee M, Hormoznejad R, Fathi M. Effect of fenugreek extract supplement on testosterone levels in males: a meta-analysis of clinical trials. Phytother Res. 2020;34(7):1550–5. [DOI] [PubMed] [Google Scholar]
- 69. Qureshi A, Naughton DP, Petroczi A. A systematic review on the herbal extract Tribulus terrestris and the roots of its putative aphrodisiac and performance enhancing effect. J Diet Suppl. 2014;11(1):64–79. [DOI] [PubMed] [Google Scholar]
- 70. Gonzales GF, Gonzales C, Gonzales-Castaneda C. Lepidium meyenii (Maca): a plant from the highlands of Peru—from tradition to science. Forschende Komplementarmedizin (2006). 2009;16(6):373–80. [DOI] [PubMed] [Google Scholar]
- 71. Kumar A, Kumar R, Rahman MS, Iqubal MA, Anand G, Niraj PK, Ali M. Phytoremedial effect of Withania somnifera against arsenic-induced testicular toxicity in Charles Foster rats. Avicenna J Phytomed. 2015;5(4):355–64. [PMC free article] [PubMed] [Google Scholar]
- 72. Samadi Noshahr Z, Shahraki MR, Ahmadvand H, Nourabadi D, Nakhaei A. Protective effects of Withania somnifera root on inflammatory markers and insulin resistance in fructose-fed rats. Rep Biochem Mol Biol. 2015;3(2):62–7. [PMC free article] [PubMed] [Google Scholar]
- 73. Senthil K, Thirugnanasambantham P, Oh TJ, Kim SH, Choi HK. Free radical scavenging activity and comparative metabolic profiling of in vitro cultured and field grown Withania somnifera roots. PLoS One. 2015;10(4):e0123360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Yun SJ, Bae GS, Park JH, Song TH, Choi A, Ryu BY, Pang MG, Kim EJ, Yoon M, Chang MB. Antioxidant effects of cultured wild ginseng root extracts on the male reproductive function of boars and guinea pigs. Anim Reprod Sci. 2016;170:51–60. [DOI] [PubMed] [Google Scholar]
- 75. Salve J, Pate S, Debnath K, Langade D. Adaptogenic and anxiolytic effects of ashwagandha root extract in healthy adults: a double-blind, randomized, placebo-controlled clinical study. Cureus. 2019;11(12):e6466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Chandrasekhar K, Kapoor J, Anishetty S. A prospective, randomized double-blind, placebo-controlled study of safety and efficacy of a high-concentration full-spectrum extract of ashwagandha root in reducing stress and anxiety in adults. Indian J Psychol Med. 2012;34(3):255–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Kalshetti PB, Alluri R, Mohan V, Thakurdesai PA. Effects of 4-hydroxyisoleucine from fenugreek seeds on depression-like behavior in socially isolated olfactory bulbectomized rats. Pharmacogn Mag. 2015;11(Suppl 3):S388–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Flanagan SD, DuPont WH, Caldwell LK, Hardesty VH, Barnhart EC, Beeler MK, Post EM, Volek JS, Kraemer WJ. The effects of a Korean ginseng, GINST15, on hypo-pituitary-adrenal and oxidative activity induced by intense work stress. J Med Food. 2018;21(1):104–12. [DOI] [PubMed] [Google Scholar]
- 79. Fujita R, Liu J, Shimizu K, Konishi F, Noda K, Kumamoto S, Ueda C, Tajiri H, Kaneko S, Suimi Yet al. . Anti-androgenic activities of Ganoderma lucidum. J Ethnopharmacol. 2005;102(1):107–12. [DOI] [PubMed] [Google Scholar]
- 80. Murata K, Takeshita F, Samukawa K, Tani T, Matsuda H. Effects of ginseng rhizome and ginsenoside Ro on testosterone 5alpha-reductase and hair re-growth in testosterone-treated mice. Phytother Res. 2012;26(1):48–53. [DOI] [PubMed] [Google Scholar]
- 81. Mohamad NV, Wong SK, Wan Hasan WN, Jolly JJ, Nur-Farhana MF, Ima-Nirwana S, Chin KY. The relationship between circulating testosterone and inflammatory cytokines in men. Aging Male. 2019;22(2):129–40. [DOI] [PubMed] [Google Scholar]
- 82. Tostes RC, Carneiro FS, Carvalho MH, Reckelhoff JF. Reactive oxygen species: players in the cardiovascular effects of testosterone. Am J Physiol Regul Integr Comp Physiol. 2016;310(1):R1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Wang Y, Chen F, Ye L, Zirkin B, Chen H. Steroidogenesis in Leydig cells: effects of aging and environmental factors. Reproduction. 2017;154(4):R111–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Girish BP, Reddy PS. Forskolin ameliorates mancozeb-induced testicular and epididymal toxicity in Wistar rats by reducing oxidative toxicity and by stimulating steroidogenesis. J Biochem Mol Toxicol. 2018;32(2):2026. [DOI] [PubMed] [Google Scholar]
- 85. Brownlee KK, Moore AW, Hackney AC. Relationship between circulating cortisol and testosterone: influence of physical exercise. J Sports Sci Med. 2005;4(1):76–83. [PMC free article] [PubMed] [Google Scholar]
- 86. Liu J, Shimizu K, Konishi F, Kumamoto S, Kondo R. The anti-androgen effect of ganoderol B isolated from the fruiting body of Ganoderma lucidum. Bioorg Med Chem. 2007;15(14):4966–72. [DOI] [PubMed] [Google Scholar]
- 87. Liu J, Tamura S, Kurashiki K, Shimizu K, Noda K, Konishi F, Kumamoto S, Kondo R. Anti-androgen effects of extracts and compounds from Ganoderma lucidum. Chem Biodivers. 2009;6(2):231–43. [DOI] [PubMed] [Google Scholar]
- 88. NCCIH Policy: Natural Product Integrity. National Center for Complimentary and Integrative Health 2015; [Internet]. (accessed 2 August 2020). Available from: https://www.nccih.nih.gov/research/nccih-policy-natural-product-integrity. [Google Scholar]
- 89. Sun S, Wang Y, Wu A, Ding Z, Liu X. Influence factors of the pharmacokinetics of herbal resourced compounds in clinical practice. Evid Based Complement Alternat Med. 2019;2019:1983780. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.