ABSTRACT
Deficiencies in the n–3 (ω-3) long-chain PUFAs (LC-PUFAs) EPA and DHA are associated with increased risk for the development of numerous diseases. Although n–3 LC-PUFAs can be obtained by consuming marine products, they are also synthesized endogenously through a biochemical pathway regulated by the Δ-5/Δ-6 desaturase and elongase 2/5 enzymes. This narrative review collates evidence from the past 40 y demonstrating that mRNA expression and activity of desaturase and elongase enzymes are influenced by numerous dietary components, including macronutrients, micronutrients, and polyphenols. Specifically, we highlight that both the quantity and the composition of dietary fats, carbohydrates, and proteins can differentially regulate desaturase pathway activity. Furthermore, desaturase and elongase mRNA levels and enzyme activities are also influenced by micronutrients (folate, vitamin B-12, vitamin A), trace minerals (iron, zinc), and polyphenols (resveratrol, isoflavones). Understanding how these various dietary components influence LC-PUFA synthesis will help further advance our understanding of how dietary patterns, ranging from caloric excesses to micronutrient deficiencies, influence disease risks.
Keywords: Δ-6 desaturase, fatty acid desaturase 2, Δ-5 desaturase, fatty acid desaturase 1, fatty acid elongase 2, fatty acid elongase 5
This review shows that the quality and quantity of macronutrients, as well as micronutrients, trace minerals, and polyphenols, can regulate desaturase and elongase mRNA levels and enzyme activity.
Introduction
Diet-related chronic disease represents one of the strongest risks for morbidity and mortality in Western countries (1). The Western diet is generally characterized as hypercaloric, abundant in refined sugars, saturated fats, n–6 PUFA, and sodium content, while also being low in fiber, micronutrients, and n–3 PUFA (1, 2). This dietary pattern is associated with increased risk of obesity, diabetes, and cardiovascular disease (3). The n–3 and n–6 PUFA content of the Western diet is of interest given that different PUFAs are not equivalent with regard to their influence on chronic disease risk (4).
The main dietary PUFAs in general adult populations from industrialized countries are α-linolenic acid (ALA; 18:3n–3) and linoleic acid (LA; 18:2n–6), which account for 0.02–1% and 3.1–8.6% energy (en) intake, respectively (5). Both ALA and LA are essential dietary fats because humans lack the enzymes necessary for their synthesis (6). These fatty acids are substrates for the production of important long-chain PUFAs (LC-PUFAs), with EPA (20:5n–3), docosapentaenoic acid (DPAn–3; 22:5n–3), and DHA (22:6n–3) derived from ALA and arachidonic acid (AA; 20:4n–6) derived from LA (7). LC-PUFAs are critical bioactive molecules that serve as constituents of cell membrane phospholipids, signaling molecules, and substrates for the production of bioactive lipid mediators (8).
The conversion of ALA and LA into LC-PUFAs occurs through a biochemical pathway (referred to as the desaturation pathway) that is regulated by desaturase [Δ-6 desaturase (Δ6D), Δ-5 desaturase (Δ5D)] and elongase [fatty acid elongase 2 (ELOVL2), fatty acid elongase 5 (ELOVL5)] enzymes. Studies have shown that the conversion of ALA into LC-PUFA occurs in both human infants and adults to a limited extent (9), with estimates indicating that <8% and <1% of ALA is converted into EPA and DHA, respectively (9, 10). The low endogenous conversion of ALA into downstream LC-PUFA places greater emphasis on obtaining EPA and DHA directly from the diet; however, <20% of the global population consumes sufficient EPA and DHA (11). This is germane because low EPA and DHA concentrations are associated with higher risk of nonalcoholic fatty liver disease, Alzheimer's disease, schizophrenia, depression, cancer, type 2 diabetes, and other conditions (12–14). Due to the numerous conditions associated with low EPA and DHA status, the desaturation pathway is of critical importance because it represents the primary source for these LC-PUFAs in most of the population.
Many factors influence LC-PUFA synthesis, including sex, body weight, age, alcohol consumption, smoking, and genetics [reviewed in (15)]; however, nutrients also have the capacity to regulate the desaturation pathway. Although much of the recent discussion surrounding nutrient regulation of this pathway has focused on dietary n–3 and n–6 PUFAs, evidence during the past decades reveals that many other nutrients can directly and/or indirectly regulate this pathway. This narrative review collates evidence showing that macronutrients, micronutrients, and polyphenols are all capable of influencing the desaturases and elongases controlling LC-PUFA synthesis. Whenever possible, we address potential mechanisms by which nutrients influence desaturation pathway activity. The prevailing complexity surrounding diet regulation of LC-PUFA synthesis reinforces the need for continued investigations to further clarify how different nutrients regulate essential fatty acid metabolism and how this may contribute to chronic disease risk.
LC-PUFA Synthesis
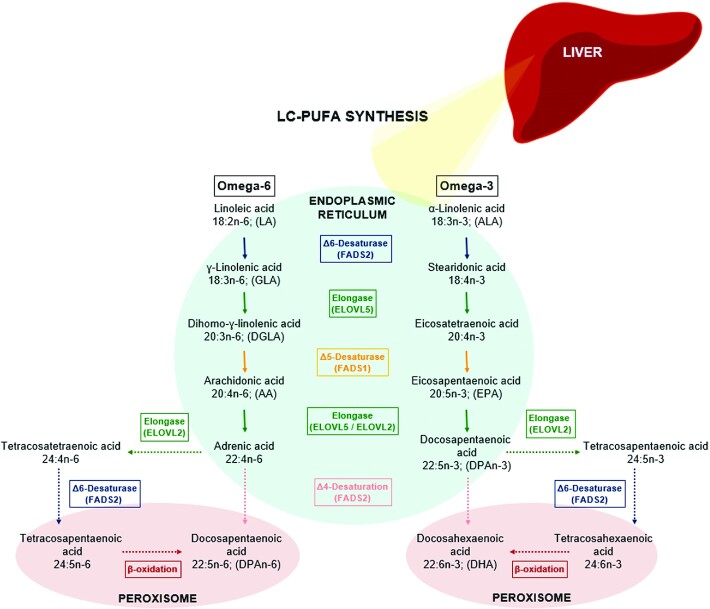
The conversion of essential fatty acids into LC-PUFAs occurs through a series of desaturation and elongation steps (Figure 1). Δ6D and Δ5D are encoded by the fatty acid desaturase 2 (Fads2) and fatty acid desaturase 1 (Fads1) genes, respectively (16). Although the primary tissue for LC-PUFA synthesis is the liver, Fads1 and Fads2 mRNA is detected in most tissues (17). Δ6D and Δ5D are microsomal membrane-bound “front-end” desaturases that insert double bonds at specific positions in a fatty acid chain in a reaction that requires molecular oxygen and 3 enzymes: NAD(P)H cytochrome b5 reductase, cytochrome b5, and the desaturase (18, 19). ELOVL2 and ELOVL5 are encoded by the Elovl2 and Elovl5 genes, respectively (20). Elovl2 was reported to be highly expressed in rat liver, brain, lung, and kidney, whereas Elovl5 appears to be expressed more ubiquitously (21). Elongases extend a fatty acid by 2 carbons as part of 4 sequential reactions that depend on fatty acyl CoA, malonyl CoA, and NAD(P)H (22). The final step in the synthesis of DHA can occur through either a peroxisomal route (23) or Fads2-mediated Δ-4 desaturation in the endoplasmic reticulum (24).
FIGURE 1.
The LC-PUFA synthesis pathway. LC, long chain.
Transcriptional Regulation of Desaturases and Elongases
A comprehensive discussion regarding the transcriptional regulation of LC-PUFA synthesis is beyond the scope of the current review but is well described elsewhere (25, 26). Briefly, the regulation of desaturase and elongase gene expression involves several transcription factors, including peroxisome proliferator–activated receptor α (PPAR-α), sterol regulatory element binding protein 1c (SREBP-1c), retinoid X receptor (RXR), and carbohydrate response element binding protein (ChREBP) (27, 28). Elucidating the transcriptional regulation of LC-PUFA synthesis has been facilitated by using transgenic mouse models and synthetic agonists. For example, Fads1 and Fads2 expression is increased with SREBP-1c overexpression (29), as well as in mice treated with synthetic PPAR-α agonists (21, 29, 30). Elovl5 mRNA levels are augmented with a PPAR-α agonist, but not Elovl2 (21). Considerable complexity surrounds the transcriptional regulation of desaturases and elongases, as LC-PUFAs produced through the desaturation pathway feed back to suppress SREBP-1c and activate PPAR-α (25, 26). These transcription factors act as sensors for many nutrients and related metabolites, intimating that numerous dietary components can influence LC-PUFA synthesis.
Macronutrients
Lipids
The relation between dietary fat and cardiometabolic health is of intense interest (4, 31, 32), with growing recognition that different fatty acids have different associations with health and disease. Because dietary oils used in feeding studies vary significantly in their fatty acid composition, it is challenging to conclusively attribute changes in desaturation pathway activity to a specific fatty acid. Nevertheless, research shows that both the quantity of fat in the diet and its composition can influence LC-PUFA synthesis.
High-fat diets
Several rodent studies report that desaturase and elongase expression and/or activity are altered with a high-fat diet (HFD). Importantly, the studies discussed here used the same oils when making HFDs and low-fat control diets (LFDs), thus minimizing changes in fatty acid composition. However, a notable caveat is that increasing dietary fat content can also change carbohydrate and protein content in the diet; these macronutrients also regulate LC-PUFA synthesis (discussed later).
Wang et al. (33) showed that hepatic Elovl5 mRNA was suppressed in male C57BL/6 mice fed an HFD (∼55% en from lard, ∼5% en from soybean oil) for 10 wk compared with an LFD (∼5% en from lard, ∼5% en from soybean oil), with no change in desaturase expression. The HFD reduction in Elovl5 expression aligned with a lower liver AA:LA ratio. Similarly, Valenzuela et al. (34) reported lower hepatic n–3 and n–6 PUFAs in male mice fed an HFD (∼55% en from lard, ∼5% en from soybean oil) for 10 wk compared with an LFD (∼5% en from lard, ∼5% en from soybean oil). Unlike Wang and colleagues (33), Valenzuela et al. (34) showed that an HFD suppressed both liver Δ5D and Δ6D activities. Differences in dietary carbohydrate content (i.e., sucrose compared with starch) in the LFDs may reconcile discrepancies between these studies; nevertheless, both showed that an HFD generally suppressed the desaturation pathway. Critically, dietary protein content was held constant in both studies, which eliminated a potential confounder. In contrast, an HFD (71% en fat) had no effect on FADS1 and ELOVL5 protein expression in rat white adipose tissue compared with a moderate fat diet (35% en fat) (35). These diets varied only in the amount of corn oil used, thus minimizing differences in fatty acid composition. Together, these results suggest potential tissue-specific regulation of desaturases and elongases in response to an HFD. Finally, a randomized crossover trial in postmenopausal women showed that 8 wk of consuming an HFD (40% en fat) compared with an LFD (20% en fat) suppressed Δ6D activity, as estimated with product-to-precursor ratios from plasma phospholipids (36). A notable strength of this trial was the rigorous control of dietary fatty acid content due to the preparation of foods by trained metabolic kitchen staff.
Collectively, these studies indicate that desaturation pathway activity is sensitive to the quantity of fat in the diet. Given that an HFD is well known to alter hepatic lipid metabolism, studies targeting key transcription factors such as PPAR-α and SREBP-1c will help further define the mechanisms underlying HFD-associated reductions in desaturase and elongase activities.
Polyunsaturated fats
Essential fatty acid–deficient diets
It is well established that an essential fatty acid deficiency (EFAD) promotes fatty acid synthesis and desaturation pathway activity. Animals fed a diet devoid of all n–3 and n–6 PUFAs show increases in mead acid, which is a Δ-6 desaturation product of oleic acid (37). Increased production of mead acid by Δ6D occurs not only in the absence of competition from ALA and LA but also with the increase in Δ6D activity caused by an EFAD. Using labeled LA (i.e., Δ6D substrate), Δ6D activity was increased in liver microsomes isolated from rats fed an EFAD diet for 15 d from weaning (38). Similarly, De Schrijver and Privett (39) also reported that rats fed an EFAD diet for 9 wk had increased Δ5D and Δ6D activities compared with rats fed a safflower-enriched diet. Interestingly, the inclusion of hydrogenated coconut oil (rich in saturated fats) did not influence the increased desaturase activities observed with an EFAD diet. Studies such as these suggest that an EFAD diet may promote desaturation pathway activity to help preserve the unsaturated-to-saturated fatty acid ratios in biological membranes.
Substrate regulation of desaturase activity
n–3 and n–6 PUFAs compete for desaturase pathway enzymes; however, Δ6D appears to have greater affinity for ALA than for LA (10). ALA supplementation increases EPA and DPAn–3 content in blood and tissues in animal and human studies, whereas the increases in DHA are smaller and less consistent (10). The conversion of ALA into LC-PUFA is sensitive to dietary LA content. Specifically, high LA intake reduces n–3 PUFA content in circulation and tissues; however, the effect on desaturases and elongases is less consistent. Kim et al. (40) reported that rats fed diets containing ALA (1% en) for 12 wk had lower liver Δ5D, Δ6D, ELOVL2, and ELOVL5 protein content when the diet contained high LA (24.5% en) compared with low LA (9.1% en); however, desaturase activities were not assessed. In contrast, Blanchard et al. (41) used activity assays to show that rats fed diets containing ALA (0.6% en) had higher Δ6D activity with a trend for increased Δ5D activity after 8 wk of consuming a diet with 4.8% en from LA compared with a diet with 2.5% en from LA, with no change in Fads1 and Fads2 mRNA. Similarly, Sheaff et al. (42) reported higher desaturation pathway activity in rats fed a high-LA diet compared with a low-LA diet. Although these studies all showed that increased dietary LA reduced n–3 PUFA content overall, the different conclusions regarding desaturation pathway activity highlight the challenge of integrating gene/protein data with activity assays. Interestingly, edible vegetable oils (e.g., soy and safflower) with lower LA content are being developed and becoming more readily available (43). It is presumed that the consumption of these low-LA oils will increase n–3 PUFA tissue content, but how they influence desaturation pathway activity remains unknown.
Several studies have examined desaturation pathway activity when dietary LA is held constant but ALA concentrations vary. Igarashi et al. (44) reported that rats fed a diet containing LA (2.6% en) but deficient in ALA (0.016% en) had increased hepatic Fads1, Fads2, Elovl2, and Elovl5 mRNA expression after 15 wk compared with rats fed a sufficient amount of ALA (0.5% en). In agreement, the corresponding enzyme activities were all increased in rats fed the ALA-deficient diet. In contrast, Tu and colleagues (45) did not observe any effect on the expression of the previously mentioned genes in rats fed diets with LA (1% en) but ranging in ALA content (0.2–2.9% en) after 21 d. This suggests that the reprogramming of desaturase and elongase gene expression may necessitate longer dietary challenges.
LC-PUFA regulation of desaturase activity
Desaturases and elongases are also influenced through feedback regulation by products of the desaturation pathway. Several studies provide evidence that EPA, DHA, or a combination of both (i.e., fish oil) feeds back to inhibit this pathway at the level of both gene expression and enzyme activity. Wang et al. (21) reported that male rats fed a fish oil diet for 7 d had reduced Fads1 and Fads2 mRNA expression, with varying effects on Elovl2 and Elovl5 expression. Similarly, a dose-dependent suppression on hepatic mRNA levels for Fads1, Fads2, and Elovl5 was noted in C57BL/6J mice fed diets with a constant amount of ALA (5% w:w) and increasing amounts of EPA (0.25–1% w:w) (46). Although not examined in these studies, it would be interesting to determine if these effects are mediated by PPAR-α and SREBP-1c. Other studies have also shown that the consumption of preformed DHA feeds back to inhibit desaturation pathway activity. Humans receiving an oral mixture of deuterated ALA and LA after consuming a DHA-rich diet for 90 d showed lower concentrations of most desaturated and elongated n–3 and n–6 LC-PUFAs, suggesting a general suppression of desaturation pathway activity (47). Studies in rats have shown a similar inhibition in response to increased DHA intake. For example, Christiansen et al. (48) reported that rats fed a fish oil diet for 3 wk had lower microsomal Δ5D and Δ6D activity. In addition, DeMar et al. (49) showed that deuterated DHA content was reduced in male rats fed a milk formula containing deuterium-labeled ALA + unlabeled DHA for 8 d compared with labeled ALA alone. Importantly, both formulas had a similar percentage fatty acid content for both ALA and LA that helped mitigate the confounding effect of substrates on pathway activity. Interestingly, EPA content was increased in rats fed DHA-supplemented milk, which aligns with other studies (50–52). Although this increase in EPA is commonly attributed to retroconversion from DHA, it was recently shown that EPA conversion into DHA slows when preformed DHA is in the diet, thus causing EPA to accumulate (53). It is notable that the effects of DHA supplementation on fatty acid profiles differed across tissues (49), hinting at tissue-specific responses in fatty acid desaturation and/or accretion.
Fewer studies have examined whether the consumption of preformed AA also inhibits the desaturation pathway, and the available evidence is less conclusive than that observed with EPA/DHA. Trials in piglets fed diets varying in AA content have shown little or no effect on hepatic desaturase and elongase gene expression or pathway activity (54, 55). This suggests that EPA and DHA are more potent feedback regulators of the desaturation pathway compared with AA. However, more studies in rodents fed AA-enriched diets are needed to better compare results with those from n–3 LC-PUFA feeding studies.
Saturated and monounsaturated fats
Few studies have examined if other dietary fats modulate the desaturation pathway. Dang et al. (56) showed that rats fed a diet supplemented with SFA (20% w:w partially hydrogenated coconut oil) for 1 mo had significantly reduced Δ5D activity compared with a nonpurified diet (5% w:w fat), as determined using liver microsomes incubated with labeled dihomo-γ-linolenic acid (DGLA; i.e., Δ5D substrate). However, it is important to note that the SFA diet had very low concentrations of n–3 and n–6 PUFAs compared with the nonpurified diet, which may confound the interpretation of these results. This is particularly important given in vitro evidence that excess palmitate, in the context of low ALA and LA, competes for Δ6D activity (57). Thus, the impact of SFA on LC-PUFA synthesis is minor in the presence of adequate dietary ALA and LA but may exacerbate LC-PUFA deficiencies in the context of an EFAD.
Picklo and Murphy (58) conducted a study in which mice consumed HFDs (50% en fat) that varied in SFA and MUFA content but had relatively constant ALA and LA. In comparison to an LFD (16% en fat), the high-SFA diet reduced liver Fads1, Fads2, Elovl2, and Elovl5 mRNA levels. In contrast, the high-MUFA diet elevated Fads1 and Fads2 mRNA, with no effect on elongase gene expression. The high-SFA diet increased ALA hepatic phospholipid content, as well as EPA, DPAn–3, and DHA, compared with the high-MUFA diet, which does not align with the gene expression results. Therefore, additional studies in which fatty acid tracers are used to assess individual steps in the desaturation pathway will help address this discrepancy between gene expression and fatty acid composition.
Vessby et al. (59) conducted a 3-mo randomized controlled trial to examine the effect of MUFA compared with SFA on desaturase activities (assessed using product-to-precursor ratios based on serum fatty acids). Importantly, the isoenergetic diets were carefully formulated to alter only the SFA and MUFA content while keeping all other nutrients relatively constant. Moderate HFDs (37% en fat) composed of 17%, 14%, and 6% of SFA, MUFA, and PUFA for the SFA diet, respectively, and 8%, 23%, and 6% of SFA, MUFA, and PUFA for the MUFA diet, respectively, were investigated. No changes in estimated Δ5D and Δ6D activities were found with either diet, in relation to baseline estimates. In contrast, individuals who received an EPA/DHA supplement showed a significant reduction in estimated Δ6D activity and a significant increase in estimated Δ5D activity. Thus, the effects of altering dietary SFA and MUFA content appear to be considerably less impactful on desaturation pathway activity compared with PUFA; however, additional studies are needed due to the known limitations of product-to-precursor estimates.
Cholesterol
Several studies conducted in the late 1980s and early 1990s revealed that increases in dietary cholesterol concentrations suppress LC-PUFA synthesis (60–65). In one of the first studies, Leikin and Brenner (61) fed male rats a control diet or a control diet supplemented with 1% (w:w) cholesterol and 0.5% (w:w) cholic acid for either 2 or 21 d and then examined desaturase activities using labeled DGLA and LA. Dietary cholesterol suppressed microsomal Δ5D activity, as reflected by lower concentrations of AA, at both 2 and 21 d, as well as impaired microsomal Δ6D activity at 2 d, as reflected by lower concentrations of GLA. The cholesterol-induced suppression in desaturase activity was further reflected in the AA:LA ratio, as well as in EPA and DHA concentrations. Additional studies investigated whether desaturase enzyme activity was altered in male rats fed diets containing either linseed oil (rich in ALA) or linseed oil with added cholesterol (2% w:w). The desaturation rate of labeled DGLA in liver microsomes isolated from rats after 4 wk of the experimental diets revealed that added dietary cholesterol suppressed both Δ5D (64) and Δ6D (63) activities. Interestingly, DHA content was also reduced; however, the increased EPA concentrations further reinforces Δ6D's preference for ALA compared with LA. An independent study by this same group showed that microsomal results were similarly reflected in plasma and tissue phospholipid fatty acid composition in rats fed a diet containing linseed oil with 2% (w:w) cholesterol for 4 wk (60). In accordance with cholesterol-supplementation studies, a cholesterol-depleted diet showed the opposite effects, where liver microsomal Δ5D and Δ6D activities were increased in conjunction with higher AA (66).
Collectively, these studies provide evidence that dietary cholesterol modulates LC-PUFA synthesis; however, the precise mechanism of action remains equivocal. Brenner et al. (62) reported that both Δ5D and Δ6D activities were suppressed when 1% (w:w) cholesterol was added to an EFAD diet. However, no change in Fads2 mRNA was found, and they did not report on Fads1 gene expression. Regardless, an effect on desaturase gene expression remains plausible given that high cholesterol inhibits nuclear translocation of SREBP-1c (67, 68). It is equally plausible that the excess dietary cholesterol affects the physical properties of membranes and consequently influences endoplasmic reticulum–bound desaturase enzymes. Irrespective of the mechanism(s) at play, cholesterol's ability to suppress desaturase enzyme activity is critical to consider because this has the potential to exacerbate cardiovascular risk, particularly in the context of an EFAD diet.
Protein
Consumption of a high-protein diet decreases hepatic lipid accumulation by inhibiting fatty acid synthesis (69, 70). Previous reports show that high dietary protein has antisteatotic effects, mainly due to the reduction of lipogenic gene expression (71, 72). Furthermore, several studies have reported that the type of dietary protein (e.g., soy, casein) can differentially impact lipid metabolism. Compared to casein, soy protein decreases Srebp-1 gene expression, as well as that of its target genes (73–76). However, the effects of a high-protein diet on essential fatty acid metabolism remain poorly described.
High-protein diets
Mercuri et al. (77) recorded a decrease in microsomal Δ5D and Δ6D activities (as determined using labeled fatty acids) in rat dams fed a low-protein diet (5% w:w casein) during pregnancy compared with a control diet (25% w:w casein). Although fat content was held constant between the diets, carbohydrate content changed significantly (88% compared with 66% w:w dextrin). De Tomás et al. (78) extended this work and showed reduced Δ5D and Δ6D activities at age 31 d in pups fed the same protein-deficient diet as dams. Using an alternate approach, Peluffo and Brenner (79) observed that 1-y consumption of a diet containing ≥35% (w:w) protein increased Δ6D activity in rat liver microsomes compared with a diet containing ≤25% (w:w) protein. Collectively, these results show that high dietary protein promotes desaturase activity. However, it is important to note that carbohydrate intake changed in these studies, thus introducing a potential confounder in the interpretation of findings.
Protein-energy malnutrition in children has also been associated with reduced Δ5D activity, although no effect was seen with Δ6D (80). These conclusions, which were based on blood fatty acid product-to-precursor ratios, align with rodent studies. Although this study represents one of the first forays examining the effect of dietary protein on the desaturation pathway in humans, several limitations are present, including differences in body weight, the use of blood fatty acids to estimate desaturase activity, and the fact that total energy intake was also lower in addition to the protein deficiency. Additional studies in humans in which protein intake is reduced, but daily energy intake is maintained, will prove insightful.
Types of protein
Several studies have demonstrated that LC-PUFA synthesis is affected by the type of protein consumed (81–86). For example, casein-fed rats have increased microsomal Δ6D activity (as determined using labeled precursors), concomitant with lower LA, ALA, DPAn–3, and DPAn–6 and higher DGLA, AA, and EPA in phospholipids, compared with soy-fed rats (83, 85). Differences between casein and soy may arise from their amino acid content. To determine if differences in methionine content could explain these differences, Sugiyama and colleagues (87) found that supplementing a soy-based diet with l-methionine restored the microsomal AA:LA ratio to that seen with casein. However, desaturase activities were not measured in this study. It is well known that methionine is limiting in soy (88); thus, these findings suggest that the lesser impact of soy on the desaturation pathway may relate, in part, to its amino acid content. Separately, Shimada and colleagues (89) fed rats a diet with 10% (w:w) casein supplemented with 0.43% l-methionine w:w, 0.34% l-cystine, 3% glycine, or 0.43% l-methionine + 3% glycine for 14 d. Supplementation with l-methionine increased microsomal Δ6D activity compared with a control casein diet. Supplementation with l-cystine and glycine also increased Δ6D activity compared with the control, but to a lesser extent than that seen with l-methionine. Interestingly, the Δ6D activation observed with l-methionine was ablated in the l-methionine + glycine group, suggesting that glycine interferes with the stimulatory ability of l-methionine. Peluffo et al. (90) also reported that different types of amino acids influence Δ6D activity. Briefly, rats were fed diets containing 10% (en) sunflower oil, 25% casein, and 65% sucrose, where 20% of the casein was substituted for phenylalanine, tyrosine, or tryptophan. After 24 h, rats fed diets containing 5% (en) casein and either 20% (en) phenylalanine or tyrosine had lower hepatic microsomal Δ6D activities compared with the 25% (en) casein diet. Interestingly, rats fed a diet containing 5% casein and 20% tryptophan showed no difference in microsomal Δ6D activity compared with the control casein group. Extending this work and conducting studies in animals fed sufficient ALA to avoid any potential confounding effects of the n–3 PUFA deficiency imparted by the use of sunflower oil as the sole source of dietary fat are necessary.
Altogether, these findings suggest that LC-PUFA synthesis is modulated by dietary protein, as well as specific amino acids. Future work should investigate if the effect of different protein sources on desaturation pathway activity stems from the amino acid content influencing desaturase and elongase protein translation.
Carbohydrates
The relation between dietary carbohydrate and fatty acid metabolism is well established and supported by research showing that high-carbohydrate diets stimulate de novo lipogenesis (DNL) (91–94). Dietary carbohydrate regulates DNL by activating ChREBP, which then translocates into the nucleus to stimulate lipogenic gene expression. This process is rapid, as evidenced by increased fatty acid synthesis following a single high-carbohydrate meal (95). Evidence also suggests that the type of dietary carbohydrate (i.e., fructose, sucrose, starch) differentially impacts DNL (96); however, knowledge of how a high-carbohydrate diet affects desaturation pathway activity remains incomplete.
High-carbohydrate diets
Drąg et al. (97) investigated the impact of a high-carbohydrate diet (68% w:w sucrose) on desaturase and elongase expression and activity in male rats compared with a standard diet. Importantly, rats in both groups were weight matched at the time of euthanization to avoid confounding effects related to differences in body weight. Elovl5 and Fads2 gene expression was increased with the high-carbohydrate diet, with no change in Elovl2 and Fads1. Whereas Δ6D activity increased in accordance with Fads2 mRNA, both ELOVL5 and Δ5D activities decreased. Results from an independent study in mice also suggested that Δ6D activity was increased with a high-carbohydrate diet (composed of a mixture of starch and sucrose) (98). However, the authors of these 2 studies estimated enzyme activity using product-to-precursor indices with hepatic fatty acids—an approach that is less accurate than using fatty acid tracers. This may partially explain the discrepancy between gene expression and enzyme activity. Further experiments using tracers and isolated liver microsomes will help clarify the effects of a high-carbohydrate diet on the desaturation pathway.
Types of carbohydrates
Independent studies have demonstrated that sucrose inhibits hepatic desaturases. For example, male rats that consumed 30% (w:v) sucrose in their drinking water for 20 wk showed reduced Δ5D activity, as reflected by lower conversion of labeled DGLA into AA in liver microsomes (99). A more recent study that also examined the effects of 30% (w:v) sucrose in drinking water in rats showed small, but statistically significant, reductions in Fads1 and Elovl5 hepatic gene expression but no change in Fads2 (100). Although supplementing drinking water with carbohydrates helps avoid alterations in diet macronutrient composition, it remains difficult to conclusively attribute changes to carbohydrates given that these studies both reported metabolic changes in the sucrose-supplemented groups (e.g., increased insulin, adiposity, and/or steatosis) that also alter desaturase expression and activity. Future studies that supplement drinking water with equivalent amounts of sucrose, glucose, or fructose will help further define the independent effects of different carbohydrates.
To date, only a few studies have performed head-to-head comparisons of different carbohydrates on LC-PUFA synthesis. De Schrijver and Privett (39) evaluated the effects of sucrose or glucose on Δ5D and Δ6D activities in rats fed isocaloric diets varying in fat content. After 9 wk and irrespective of fat content, sucrose and glucose did not differentially regulate desaturase activity in liver microsomes. In contrast, Hein et al. (101) reported that a 62.5% sucrose-rich diet increased Fads1 and Fads2 hepatic gene expression compared with an isocaloric diet containing starch as the carbohydrate source. The increase in Fads2 mRNA levels in rats fed the sucrose-rich diet was mirrored by an increase in Δ6D activity; however, the increasing trend seen with Δ5D activity did not achieve significance. Together, these results indicate that further head-to-head comparative investigations of the effects of different dietary carbohydrates on LC-PUFA synthesis are warranted.
Micronutrients
Vitamin A
Vitamin A is an essential nutrient required for growth and development, reproduction, and vision. Retinoic acid isomers are derivatives of vitamin A that activate RXR, which heterodimerizes with other nuclear hormone receptors, including PPARs, to regulate gene expression. However, only a few studies have investigated whether vitamin A influences the desaturation pathway (102–107).
Alam et al. (107) fed rats a purified diet supplemented with either β-carotene (vitamin A provitamin) or different amounts of 13-cis retinoic acid. β-carotene had no effect on Δ6D activity in liver microsomes, whereas a dose-dependent increase in Δ6D activity was observed with 13-cis retinoic acid. DGLA content in hepatic microsomes increased with 13-cis retinoic acid, consistent with increased Δ6D activity. In a separate study, these same authors did not find a change in microsomal Δ6D activity in rats fed a vitamin A–deficient diet (106). It is not clear why rats consuming a vitamin A–deficient diet did not show the opposite effect in Δ6D activity as that found in rats supplemented with 13-cis retinoic acid supplementation. A possible explanation is that the high dose of 13-cis retinoic acid may have caused lipidemic side effects, as previously reported (108). In the absence of additional biochemical investigations, it is difficult to interpret these findings.
Independent work by Zolfaghari et al. (105) showed that hepatic Fads1 mRNA was significantly reduced in rats fed a diet containing 4 mg retinol (as retinyl palmitate)/kg diet compared with a vitamin A–deficient diet. A subset of rats fed a vitamin A–deficient diet received an intraperitoneal injection of 20 μg all-trans retinoic acid shortly prior to euthanization. Interestingly, rats receiving 2 injections showed greater suppression of Fads1 mRNA compared with rats receiving only a single injection, suggesting a dose-dependent suppression of Fads1 gene expression by vitamin A. However, this study did not measure hepatic fatty acid composition. This is notable because work by Zhou et al. (103) showed that vitamin A increased hepatic phospholipid Δ5D and Δ6D activities, as assessed with product-to-precursor ratios. Direct comparisons between these studies are not possible due to different measurement outcomes (gene expression compared with fatty acid concentrations) as well as differences in experimental design (e.g., amount of vitamin A in the diet and rat models used). Nevertheless, these intriguing reports reinforce the need to further explore the role of vitamin A on desaturation pathway activity and determine whether these effects are mediated by RXR.
Folate and vitamin B-12
Folate and vitamin B-12 are important micronutrients in 1-carbon metabolism, in which they function as the primary methyl donor for methylation of DNA, protein, and lipids. The effects of these nutrients on LC-PUFA synthesis are relevant because recent reports show associations between DNA methylation in 5′ regulatory regions of human FADS1 and FADS2 with estimated enzyme activities (109, 110). Increased Fads2 methylation was also shown to reduce hepatic Δ6D activity and lower AA and DHA concentrations in mice (111). Furthermore, Fads2 hypermethylation was associated with altered liver phospholipid content in conjunction with lower phosphatidylethanolamine N-methyltransferase activity. Thus, investigating micronutrients that influence methylation of desaturase genes is important with regard to not only LC-PUFA synthesis but also phospholipid production.
Only a few studies have examined the effect of these micronutrients on desaturases and elongases. In all instances, these studies varied the amount of folate and/or vitamin B-12 in the maternal diet and then examined desaturase enzymes in the mother and/or offspring. Results to date are equivocal. Burdge et al. (112) examined liver fatty acid composition in offspring from rat dams fed diets containing either 1 or 5 mg folic acid/kg diet from conception until delivery, with vitamin B-12 concentrations held constant. All offspring were fed the same diet containing 1 mg folic acid/kg diet until killed on postnatal day 28. No differences related to maternal diet were found in offspring hepatic lipids; however, the effects of varying dietary folic acid on the mothers were not investigated.
Wadhwani et al. (113) examined the effects of low and high folic acid (2 and 8 mg folic acid/kg diet, respectively) in the presence or absence of vitamin B-12 on liver fatty acids and desaturase mRNA levels in rat dams. The authors showed that excess folic acid in the absence of vitamin B-12 caused increased Δ5D and reduced Δ6D liver activities (based on product-to-precursor ratios) but decreased Fads1 mRNA levels with no effect on Fads2. These results reinforce the disconnect between mRNA measurements and desaturase activity. Finally, a recent mouse study fed both dams (starting 2 wk before mating) and offspring either a normal diet containing folic acid (2 mg/kg diet) and vitamin B-12 (25 μg/kg diet) or an unbalanced diet containing folic acid (8 mg/kg diet) and vitamin B-12 (5 μg/kg diet) (114). Offspring consumed the same diet as their mothers for 60 d. Fads1 mRNA abundance was increased in dams fed the unbalanced diet (no effect on Fads2 expression), but both Δ5D and Δ6D activities were lower. In contrast, offspring in the unbalanced diet group showed increases in both Δ5D and Δ6D activities. The discrepancy between mother and offspring may suggest a compensatory adaptation occurred in offspring. Future studies should consider similar maternal diets but provide all offspring with a common diet to elucidate any possible compensations in essential fatty acid metabolism. Importantly, none of these feeding trials examined the methylation status of Fads1 and Fads2. Such measurements will help clarify whether folate and/or vitamin B-12 epigenetically regulate the desaturation pathway and may point to a mechanism underlying heritability of LC-PUFA synthesis.
Zinc
Zinc (Zn) is an essential trace element required for the activity of >300 enzymes involved in protein synthesis, fatty acid metabolism, and reproduction (115, 116). Due to similarities between Zn deficiency and EFAD, interactions between Zn and desaturase activity are relevant (117, 118). Discrepant findings from rodent and human studies exist in the literature; however, an important limitation in some of the early work was the use of Zn-free diets that impacted food intake (119, 120). Subsequent studies with rats force-fed Zn-deficient diets by intragastric tubes helped resolve these discrepancies and showed that LC-PUFA synthesis is indeed impaired with Zn deficiency.
Several studies in the 1980s reported impaired LA metabolism in Zn-deficient rats (121–123); however, the effect of Zn was dependent on essential fatty acid status. Indeed, in the context of an EFAD, Zn supplementation had no effect on fatty acid composition of plasma and liver (121). Interestingly, Zn-deficient rats administered primrose oil (which contains GLA), but not safflower oil (which contains LA), showed improvements in many of the changes observed with Zn deficiency. This suggests that the outcomes on LC-PUFA synthesis associated with Zn deficiency may be circumvented by providing a dietary source containing fatty acid products of Δ6D. Clejan et al. (122) pair-fed rats either Zn-sufficient or Zn-deficient diets and examined liver microsomal desaturase activity. Although the authors did not report a change in Δ6D activity, Δ5D activity was significantly impaired with Zn deficiency. Finally, Ayala and Brenner (123) showed that Δ6D and Δ5D activities were reduced in liver and testes microsomes isolated from rats fed a Zn-deficient diet. Nearly a decade later, studies by Eder and Kirchgessner (120, 124) expanded on the role of Zn in essential fatty acid metabolism using a force-fed rat model to prevent Zn-deficiency associated hypophagia. An initial study in which rats were fed Zn-deficient diets varying in fat content (coconut/safflower compared with linseed) found no effect on desaturase activities (124). However, when rats were initially fed Zn-deficient fat-free diets prior to the consumption of a Zn-deficient + 5% (w:w) safflower diet, desaturase activity was impaired (120). This implies that the outcomes seen with Zn deficiency may be particularly important in the context of an EFAD.
The relation between Zn and estimated desaturase activities has also been alluded to in human studies. Yary et al. (125) reported that high serum Zn was associated with low Δ5D and high Δ6D activities in men. Both Knez et al. (126) and Chimhashu et al. (127) reported positive associations between plasma Zn and DGLA concentrations and inverse relations between plasma Zn and the LA:DGLA ratio (126). Together, these studies highlight the association between Zn and Δ6D activity, although the association with Δ5D remains uncertain. Future clinical studies using stable isotope fatty acid tracers coupled with Zn supplementation will help further solidify the relation between Zn and LC-PUFA synthesis.
Iron
Iron is an essential mineral involved in redox reactions, DNA synthesis, and oxygen transport (128). Desaturase enzymes are non-heme iron–containing enzymes (18, 129). Okayasu et al. (18) reported that an iron chelator reduced Δ6D activity, implying a catalytic role for the non-heme iron. Given the global prevalence of iron deficiency (130), investigations regarding the associations between iron and LC-PUFA synthesis have widespread implications.
Studies in rats and humans have demonstrated that dietary iron status affects desaturase activities. When rats were fed an iron-deficient diet (10 mg/kg) for 12 wk, both the AA:LA ratio and the Δ5D ratio (AA:DGLA) were lower in plasma, erythrocytes, and liver phospholipids compared with those of control (35 mg/kg) and iron-supplemented (250 mg/kg) rats (131). Additional studies by independent groups (132–134) generally report similar outcomes, indicating that iron deficiency impairs LC-PUFA synthesis. Results from iron-deficient children (135) and adults (136), compared with age-matched controls, are mostly in agreement with findings from rat studies. Moreover, when iron-deficient children were provided iron-fortified soup for 15 wk, erythrocyte concentrations of n–3 LC-PUFA increased to levels comparable to those of age-matched controls, showing that iron supplementation can help rescue impaired desaturase activity (135). Interestingly, reductions in estimated desaturase activities and blood fatty acids in these human studies were detected in n–3 PUFA and not n–6 PUFA. A plausible explanation may lie with the more variable concentrations of n–6 PUFA in participants’ diets compared with n–3 PUFA, which would make it more difficult to detect significant differences. Irrespective, these studies reinforce the essential role of iron as a critical cofactor of desaturase enzymes.
Polyphenols
Polyphenols are a large group of bioactive phytochemicals found abundantly in foods such as fruits, vegetables, whole grains, chocolate, tea, and wine (137, 138). Although initial interest in polyphenols focused on their antioxidant activity, these molecules also regulate signaling pathways, gene expression, and energy metabolism (139, 140). Evidence shows that polyphenols can regulate LC-PUFA synthesis directly or indirectly, depending on the molecule. Results from in vivo and in vitro studies suggest that these effects may occur via regulation of PPAR-α activity.
Resveratrol
Resveratrol (RSV) is a type of stilbene found in the skin and seeds of grapes, as well as in red wine (141). Since its discovery, there has been considerable interest in RSV due to its widespread actions and benefits on numerous metabolic outcomes, such as dyslipidemia and inflammation (142). However, most of this past research was conducted in cell and rodent models using micromolar concentrations of RSV that are not readily achievable in humans (142, 143), primarily due to low RSV bioavailability in humans (144, 145). Furthermore, RSV is rapidly metabolized, leading to only nanomolar amounts of the native molecule being detected in circulation (144, 145). Consequently, research investigating the role of RSV on the desaturation pathway will have little direct translational value for humans but can help elucidate the transcriptional mechanisms underlying desaturase regulation.
For example, Kühn et al. (146) treated HepG2 cells with RSV (40 μM) and showed significant increases in Fads1 and Fads2 mRNA. However, Δ5D protein was slightly reduced and no change was seen for Δ6D protein concentrations after 24 h. Interestingly, when HepG2 cells were cotreated with RSV and ALA, trends for reduced Fads1 and Fads2 gene and protein expression were observed compared with ALA alone. Importantly, concentrations of EPA and DHA were significantly reduced with this dual treatment. This suggests that RSV, which is known to bind PPAR-α (147), may mimic the transcriptional feedback inhibition observed with EPA and DHA. Future studies using PPAR-α agonists and antagonists in conjunction with n–3 PUFA fatty acid treatments will help determine to what extent polyphenol regulation of LC-PUFA synthesis occurs via this critical transcription factor.
Anthocyanins
Anthocyanins (ACNs) are water-soluble pigments found in the flesh, skin, and roots of many plants and vegetables, such as pomegranates, berries, and beets (148). Past observational studies report increases in n–3 LC-PUFA status following the consumption of wine (which is rich in ACNs) (149, 150). However, few studies have investigated whether these increases are related to ACN content.
In a comprehensive study, Vauzour et al. (149) reported no effect of ACNs on EPA and DHA concentrations in HepG2 cells, as well as in rodent and human investigations. HepG2 cells were treated with ALA (50 μM) with or without various ACNs (5 μM)—delphinidin-3-O-glucoside (D3G), cyanidin-3-O-glucoside, or malvidin-3-O-glucoside—or their metabolites (gallic acid, syringic acid, or p-coumaric acid). Whereas cells treated with ALA showed increased Fads2 expression and corresponding increases in EPA, pretreatment with D3G reduced Fads2 mRNA levels and EPA content. None of the other ACNs had any impact on these primary outcomes. In the same study, the authors fed rats a diet containing rapeseed oil (rich in ALA) supplemented with a mixture of ACNs extracted from blueberries and reported no effect on serum or liver n–3 PUFA content or hepatic Fads2 gene expression. Finally, plasma fatty acids were analyzed from a previous randomized, placebo-controlled trial examining the effects of an ACN-rich elderberry extract in postmenopausal women (151); however, no differences in n–3 PUFA were found (149). Collectively, the cell culture work suggested that only D3G inhibited Fads2 mRNA levels and EPA content, but these effects were lost in vivo. This study highlights the challenge of translating in vitro findings using purified compounds to in vivo contexts in which ACNs are part of a complex food matrix.
Gallegos et al. (152) separately reported that in rats fed a chia oil–enriched diet (ALA enriched) supplemented with ACNs extracted from purple corn, there was no effect on hepatic EPA, DHA, and AA concentrations. Although liver Fads1 mRNA was reduced in the ACN-supplemented group compared with control, with no effect seen on Fads2 mRNA levels, both the product-to-precursor ratio estimates for Δ5D and Δ6D activities were increased with ACN supplementation. Interestingly, these authors also examined the expression of Ppar-α and Srebp-1c, and their results suggest that PPAR-α may have a dominant effect on Fads2 expression, whereas SREBP-1c may have a greater influence on Fads1 expression. This suggests potential differences in the transcriptional regulation of desaturase genes. Together, these studies suggest that our understanding of ACN regulation of LC-PUFA synthesis remains incomplete, but the limited evidence available indicates that more work is necessary before making any human dietary recommendations.
Isoflavones
Isoflavones are a class of molecules that are rich in legumes such as soybean, mung beans, and green beans. Isoflavones are also referred to as phytoestrogens due to their structural similarities to 17β-estradiol and their ability to interact with the estrogen receptor (153). The effect of isoflavones on LC-PUFA synthesis has been studied due to their potent estrogenic activity (154). Similar to RSV, isoflavones are thought to bind PPAR-α to induce its transcriptional activity (155–157).
Kühn et al. (158) conducted a series of in vitro experiments to investigate the effects of different isoflavones on Fads1 and Fads2 (but not elongases) gene expression in cell lines corresponding to liver (HepG2), muscle (C2C12), and adipose (3T3-L1). Only quercetin increased Fads1 mRNA in HepG2 cells; however, Fads2 mRNA levels were strongly affected by fenretinide (reduction) and genistein (increase). Interestingly, both quercetin and fenretinide induced Ppar-α gene expression, indicating that their divergent effects on desaturase gene expression are not solely mediated by PPAR-α. In C2C12 cells, apigenin, luteolin, genistein, and fenretinide all reduced Fads1 expression, with similar reductions in Fads2 expression also seen with apigenin and luteolin. Finally, both Fads1 and Fads2 mRNA was increased by pratensein and fenretinide in differentiated 3T3-L1 adipocytes. These results suggest that different tissues may respond differently to these isoflavones, which makes it difficult to appreciate how these molecules will compete with one another to influence whole-body LC-PUFA synthesis. It is also notable that the isoflavone concentrations used in this study were supraphysiological. Furthermore, as seen with other nutrients, effects at the mRNA level did not always translate to effects at the protein and/or enzyme activity levels.
A recent study in chickens showed that the inclusion of isoflavones in a linseed oil (ALA-rich) –enriched diet increased EPA and DHA content in breast muscle compared with a linseed oil diet alone (159). In conjunction, Fads2 and Elovl2 gene expression was also higher in the isoflavone-supplemented chickens compared with controls, with no difference seen in Fads1 or Elovl5 mRNA levels. These in vivo findings using soybean (which is rich in genistein, daidzein, and glycitein) differ from those of the previously discussed in vitro study, but supplementation with a mixture of isoflavones is more relevant to the human diet.
Conclusions
This article demonstrates that numerous components of the diet can regulate desaturases and elongases (Supplemental Table 1), identifies important methodological limitations, and highlights considerations for population health. Different nutrients can affect desaturation pathway activity through various mechanisms, including the regulation of gene expression, enzyme activity, and methylation (Figure 2). Consequently, this highlights the importance of obtaining several measurement outcomes to fully appreciate how dietary components regulate LC-PUFA synthesis. This is particularly pertinent given that many of the studies presented in this article show that changes in gene expression often do not align with changes in enzyme activities. Furthermore, because many nutrients are capable of regulating LC-PUFA synthesis, careful consideration must be made when designing rodent diets to minimize differences in composition. Finally, as illustrated with the case of RSV, findings from in vitro studies may have limited relevance for humans due to various issues, including nutrient bioavailability and the use of supraphysiological doses.
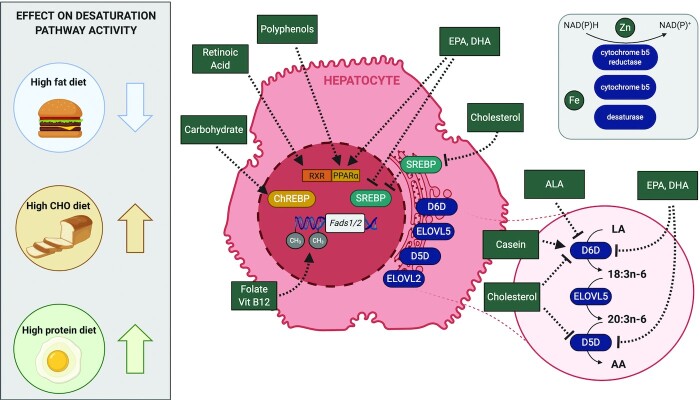
FIGURE 2.
Summary of potential mechanisms by which various dietary components regulate LC-PUFA synthesis. Arrows represent activation, while flat lines indicate inhibition. For simplicity, only n–6-PUFA desaturation is shown. AA, arachidonic acid; ALA, α-linolenic acid; ChREBP, carbohydrate response element binding protein; D5D, Δ-5 desaturase; D6D, Δ-6 desaturase; ELOVL2, fatty acid elongase 2; ELOVL5, fatty acid elongase 5; Fads, fatty acid desaturase; LA, linoleic acid; LC, long chain; PPARα, peroxisome proliferator–activated receptor α; RXR, retinoid X receptor; SREBP, sterol regulatory element binding protein. Created with BioRender.com.
Despite these challenges, this line of investigation is clearly important to continue due to its implications for human health and disease. Humans do not consume nutrients but, rather, diets whose nutrient composition varies according to the foods eaten. Thus, it becomes critical to study LC-PUFA synthesis in the context of nutrient deficiencies and excesses. Indeed, the global prevalence of micronutrient deficiencies could have an underappreciated impact on LC-PUFA synthesis, exacerbating deficiencies in these important fatty acids that are widely related to chronic disease risks. Moreover, HFDs deficient in EPA and DHA (e.g., the typical Western diet) may exaggerate this deficiency by inhibiting the endogenous production of these important n–3 LC-PUFAs. Given the important changes in dietary patterns that have arisen during the past 40 y, it is crucial that we continue to advance our understanding regarding diet regulation of LC-PUFA synthesis.
Supplementary Material
Acknowledgments
Both authors read and approved the final manuscript.
Notes
MG-S is supported by a scholarship from Consejo Nacional de Ciencia y Tecnología . DMM has received research funding from the Canola Council of Canada and Dairy Farmers of Canada.
Author disclosures: The authors report no conflicts of interest.
Supplemental Table 1 is available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/advances/.
Abbreviations used: AA, arachidonic acid; ACN, anthocyanin; ALA, α-linolenic acid; DGLA, dihomo-γ-linolenic acid; DNL, de novo lipogenesis; DPA, docosapentaenoic acid; D3G, delphinidin-3-O-glucoside; EFAD, essential fatty acid deficiency; ELOVL2, fatty acid elongase 2; ELOVL5, fatty acid elongase 5; FADS1, fatty acid desaturase 1; FADS2, fatty acid desaturase 2; HFD, high-fat diet; LA, linoleic acid; LC-PUFA, long-chain PUFA; LFD, low-fat diet; PPAR-α, peroxisome proliferator–activated receptor α; RSV, resveratrol; RXR, retinoid X receptor; SREBP-1c, sterol regulatory element binding protein 1c; Zn, zinc; Δ5D, Δ-5 desaturase; Δ6D, Δ-6 desaturase.
Contributor Information
Melissa Gonzalez-Soto, Department of Human Health and Nutritional Sciences, University of Guelph, Guelph, Canada.
David M Mutch, Department of Human Health and Nutritional Sciences, University of Guelph, Guelph, Canada.
References
- 1. Cordain L, Eaton SB, Sebastian A, Mann N, Lindeberg S, Watkins BA, O'Keefe JH, Brand-Miller J. Origins and evolution of the Western diet: health implications for the 21st century. Am J Clin Nutr. 2005;81(2):341–54. [DOI] [PubMed] [Google Scholar]
- 2. Tapsell LC, Neale EP, Satija A, Hu FB. Foods, nutrients, and dietary patterns: interconnections and implications for dietary guidelines. Adv Nutr. 2016;7(3):445–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Simopoulos AP. An increase in the ω-6/ω-3 fatty acid ratio increases the risk for obesity. Nutrients. 2016;7(3):128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wu JHY, Micha R, Mozaffarian D. Dietary fats and cardiometabolic disease: mechanisms and effects on risk factors and outcomes. Nat Rev Cardiol. 2019;16:(10):581–601. [DOI] [PubMed] [Google Scholar]
- 5. Harika RK, Eilander A, Alssema M, Osendarp SJ, Zock PL. Intake of fatty acids in general populations worldwide does not meet dietary recommendations to prevent coronary heart disease: a systematic review of data from 40 countries. Ann Nutr Metab. 2013;63(3):229–38. [DOI] [PubMed] [Google Scholar]
- 6. Nguyen QV, Malau-Aduli BS, Cavalieri J, Malau-Aduli AEO, Nichols PD. Enhancing ω-3 long-chain polyunsaturated fatty acid content of dairy-derived foods for human consumption. Nutrients. 2019;11(4):743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Murff HJ, Edwards TL. Endogenous production of long-chain polyunsaturated fatty acids and metabolic disease risk. Curr Cardiovasc Risk Rep. 2014;; 8(12):418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Calder PC. Omega-3 fatty acids and inflammatory processes: from molecules to man. Biochem Soc Trans. 2014;45(5):1105–15. [DOI] [PubMed] [Google Scholar]
- 9. Metherel AH, Bazinet RP. Updates to the n–3 polyunsaturated fatty acid biosynthesis pathway: DHA synthesis rates, tetracosahexaenoic acid and (minimal) retroconversion. Prog Lipid Res. 2019;76:101008. [DOI] [PubMed] [Google Scholar]
- 10. Brenna JT, Salem N, Sinclair AJ, Cunnane SC. α-Linolenic acid supplementation and conversion to n–3 long-chain polyunsaturated fatty acids in humans. Prostaglandins, Leukotrienes Essent Fatty Acids. 2009;80(2–3):85–91. [DOI] [PubMed] [Google Scholar]
- 11. Micha R, Khatibzadeh S, Shi P, Fahimi S, Lim S, Andrews KG, Engell RE, Powles J, Ezzati M, Mozaffarian D. Global, regional, and national consumption levels of dietary fats and oils in 1990 and 2010: a systematic analysis including 266 country-specific nutrition surveys. BMJ. 2014;348:g2272. doi:10.1136/bmj.g2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jump DB, Depner CM, Tripathy S, Lytle KA. Potential for dietary ω-3 fatty acids to prevent nonalcoholic fatty liver disease and reduce the risk of primary liver cancer. Adv Nutr. 2015;6(6):694–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Janssen CIF, Kiliaan AJ. Long-chain polyunsaturated fatty acids (LCPUFA) from genesis to senescence: the influence of LCPUFA on neural development, aging, and neurodegeneration. Prog Lipid Res. 2014;53(6):1–17. [DOI] [PubMed] [Google Scholar]
- 14. O'Neill CM, Minihane AM. The impact of fatty acid desaturase genotype on fatty acid status and cardiovascular health in adults. Proc Nutr Soc. 2017;76(1):64–75. [DOI] [PubMed] [Google Scholar]
- 15. De Groot RHM, Emmett R, Meyer BJ. Non-dietary factors associated with n–3 long-chain PUFA levels in humans: a systematic literature review. Br J Nutr. 2019;121(7):793–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Patterson E, Wall R, Fitzgerald GF, Ross RP, Stanton C. Health implications of high dietary ω-6 polyunsaturated fatty acids. J Nutr Metab. 2012;2012:539426. doi:10.1155/2012/539426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Reynolds LM, Howard TD, Ruczinski I, Kanchan K, Seeds MC, Mathias RA, Chilton FH. Tissue-specific impact of FADS cluster variants on FADS1 and FADS2 gene expression. PLoS One. 2018;13(3)e0194610. doi:10.1371/journal.pone.0194610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Okayasu T, Nagao M, Ishibashi T, Imai Y. Purification and partial characterization of linoleoyl-CoA desaturase from rat liver microsomes. Arch Biochem Biophys. 1981;206(1):21–8. [DOI] [PubMed] [Google Scholar]
- 19. Napier JA, Michaelson LV, Sayanova O. The role of cytochrome b5 fusion desaturases in the synthesis of polyunsaturated fatty acids. Prostaglandins, Leukotrienes Essent Fatty Acids. 2003;68(2):135–43. [DOI] [PubMed] [Google Scholar]
- 20. Guillou H, D'Andrea S, Rioux V, Barnouin R, Dalaine S, Pedrono F, Jan S, Legrand P. Distinct roles of endoplasmic reticulum cytochrome b5 and fused cytochrome b5-like domain for rat Δ6-desaturase activity. J Lipid Res. 2004;45(1):32–40. [DOI] [PubMed] [Google Scholar]
- 21. Wang Y, Botolin D, Christian B, Busik J, Xu J, Jump DB. Tissue-specific, nutritional, and developmental regulation of rat fatty acid elongases. J Lipid Res. 2005;46(4):706–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jakobsson A, Westerberg R, Jacobsson A. Fatty acid elongases in mammals: their regulation and roles in metabolism. Prog Lipid Res. 2006;45:237–49. [DOI] [PubMed] [Google Scholar]
- 23. Burdge GC. Metabolism of α-linolenic acid in humans. Prostaglandins, Leukotrienes Essent Fatty Acids. 2006;75(3):161–8. [DOI] [PubMed] [Google Scholar]
- 24. Park HG, Park WJ, Kothapalli KS, Brenna JT. The fatty acid desaturase 2 (FADS2) gene product catalyzes Δ4 desaturation to yield n–3 docosahexaenoic acid and n–6 docosapentaenoic acid in human cells. FASEB J. 2015;29(9):3911–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jump DB, Botolin D, Wang Y, Xu J, Christian B, Demeure O. Fatty acid regulation of hepatic gene transcription. J Nutr. 2005;135(11):2503–6. [DOI] [PubMed] [Google Scholar]
- 26. Nakamura MT, Nara TY. Essential fatty acid synthesis and its regulation in mammals. Prostaglandins, Leukotrienes Essent Fatty Acids. 2003;68(2):145–50. [DOI] [PubMed] [Google Scholar]
- 27. Miyazaki M, Ntambi JM. Fatty acid desaturation and chain elongation in mammals. In: Vance DE, Vance JE, editors. Biochemistry of lipids, lipoproteins and membranes. 5th ed.New York:Elsevier; 2008. pp. 191–211. [Google Scholar]
- 28. Jump DB. Fatty acid regulation of hepatic lipid metabolism. Curr Opin Clin Nutr Metab Care. 2011;14(2):115–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Matsuzaka T, Shimano H, Yahagi N, Amemiya-Kudo M, Yoshikawa T, Hasty AH, Tamura Y, O J-i, Okazaki H, Iizuka Yet al. Dual regulation of mouse Δ(5)- and Δ(6)-desaturase gene expression by SREBP-1 and PPARα. J Lipid Res. 2002;43(1):107–14. [PubMed] [Google Scholar]
- 30. Tang C, Cho HP, Nakamura MT, Clarke SD. Regulation of human Δ-6 desaturase gene transcription: identification of a functional direct repeat-1 element. J Lipid Res. 2003;44(4):686–95. [DOI] [PubMed] [Google Scholar]
- 31. Forouhi NG, Krauss RM, Taubes G, Willett W. Dietary fat and cardiometabolic health: evidence, controversies, and consensus for guidance. BMJ. 2018;k2139. doi:10.1136/bmj.k2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wali JA, Jarzebska N, Raubenheimer D, Simpson SJ, Rodionov RN, O'Sullivan JF. Cardio-metabolic effects of high-fat diets and their underlying mechanisms: a narrative review. Nutrients. 2020;12(5):1505. doi:10.3390/NU12051505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wang Y, Botolin D, Xu J, Christian B, Mitchell E, Jayaprakasam B, Nair M, Peters JM, Busik J, Olson LKet al. Regulation of hepatic fatty acid elongase and desaturase expression in diabetes and obesity. J Lipid Res. 2006;47(9):2028–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Valenzuela R, Barrera C, Espinosa A, Llanos P, Orellana P, Videla LA. Reduction in the desaturation capacity of the liver in mice subjected to high fat diet: relation to LCPUFA depletion in liver and extrahepatic tissues. Prostaglandins, Leukotrienes Essent Fatty Acids. 2015;98:7–14. doi:10.1016/j.plefa.2015.04.002. [DOI] [PubMed] [Google Scholar]
- 35. Rocha-Rodrigues S, Rodríguez A, Gonçalves IO, Moreira A, Maciel E, Santos S, Domingues MR, Frühbeck G, Ascensão A, Magalhães J. Impact of physical exercise on visceral adipose tissue fatty acid profile and inflammation in response to a high-fat diet regimen. Int J Biochem Cell Biol. 2017;87:114–24. doi:10.1016/j.biocel.2017.04.008. [DOI] [PubMed] [Google Scholar]
- 36. Raatz SK, Young LR, Picklo MJ, Sauter ER, Qin W, Kurzer MS. Total dietary fat and fatty acid content modifies plasma phospholipid fatty acids, desaturase activity indices, and urinary prostaglandin E in women. Nutr Res. 2012;32(1):1–7. [DOI] [PubMed] [Google Scholar]
- 37. Li Y, Nara TY, Nakamura MT. Peroxisome proliferator–activated receptor α is required for feedback regulation of highly unsaturated fatty acid synthesis. J Lipid Res. 2005;46(11):2432–40. [DOI] [PubMed] [Google Scholar]
- 38. Peluffo RO, Nervi AM, Brenner RR. Linoleic acid desaturation activity of liver microsomes of essential fatty acid deficient and sufficient rats. Biochim Biophys Acta. 1976;441(1):25–31. [DOI] [PubMed] [Google Scholar]
- 39. De Schrijver R, Privett OS. Hepatic fatty acids and acyl desaturases in rats: effects of dietary carbohydrate and essential fatty acids. J Nutr. 1983;113(11):2217–22. [DOI] [PubMed] [Google Scholar]
- 40. Kim D, Choi JE, Park Y. Low-linoleic acid diet and oestrogen enhance the conversion of α-linolenic acid into DHA through modification of conversion enzymes and transcription factors. Br J Nutr. 2019;121(2):137–45. [DOI] [PubMed] [Google Scholar]
- 41. Blanchard H, Pédrono F, Boulier-Monthéan N, Catheline D, Rioux V, Legrand P. Comparative effects of well-balanced diets enriched in α-linolenic or linoleic acids on LC-PUFA metabolism in rat tissues. Prostaglandins, Leukotrienes Essent Fatty Acids. 2013;88(5):383–9. [DOI] [PubMed] [Google Scholar]
- 42. Sheaff RC, Su HM, Keswick LA, Brenna JT. Conversion of α-linolenate to docosahexaenoate is not depressed by high dietary levels of linoleate in young rats: tracer evidence using high precision mass spectrometry. J Lipid Res. 1995;36(5):998–1008. [PubMed] [Google Scholar]
- 43. Jandacek RJ. Linoleic acid: a nutritional quandary. Healthcare. 2017;5(2). doi:10.3390/healthcare5020025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Igarashi M, Ma K, Chang L, Bell JM, Rapoport SI. Dietary n–3 PUFA deprivation for 15 weeks upregulates elongase and desaturase expression in rat liver but not brain. J Lipid Res. 2007;48(11):2463–70. doi:10.1194/jlr.M700315-JLR200. [DOI] [PubMed] [Google Scholar]
- 45. Tu WC, Cook-Johnson RJ, James MJ, Mühlhäusler BS, Gibson RA. ω-3 long chain fatty acid synthesis is regulated more by substrate levels than gene expression. Prostaglandins, Leukotrienes Essent Fatty Acids. 2010;83(2):61–8. doi:10.1016/j.plefa.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 46. Wang SH, Pan Y, Li J, Chen HQ, Zhang H, Chen W, Gu ZN, Chen YQ. Endogenous ω-3 long-chain fatty acid biosynthesis from α-linolenic acid is affected by substrate levels, gene expression, and product inhibition. RSC Adv. 2017;7(65):40946–51. [Google Scholar]
- 47. Emken EA, Adlof RO, Duval SM, Nelson GJ. Effect of dietary docosahexaenoic acid on desaturation and uptake in vivo of isotope-labeled oleic, linoleic, and linolenic acids by male subjects. Lipids. 1999;34(8):785–91. [DOI] [PubMed] [Google Scholar]
- 48. Christiansen EN, Lund JS, Rørtveit T, Rustan AC. Effect of dietary n–3 and n–6 fatty acids on fatty acid desaturation in rat liver. Biochim Biophys Acta. 1991;1082(1):57–62. [DOI] [PubMed] [Google Scholar]
- 49. DeMar JC, DiMartino C, Baca AW, Lefkowitz W, Salem N. Effect of dietary docosahexaenoic acid on biosynthesis of docosahexaenoic acid from α-linolenic acid in young rats. J Lipid Res. 2008;49(9):1963–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Arterburn LM, Hall EB, Oken H. Distribution, interconversion, and dose response of n–3 fatty acids in humans. Am J Clin Nutr. 2006;83(6 Suppl):1467S–76S. [DOI] [PubMed] [Google Scholar]
- 51. Brossard N, Croset M, Pachiaudi C, Riou JP, Tayot JL, Lagarde M. Retroconversion and metabolism of [13C]22:6n–3 in humans and rats after intake of a single dose of [13C]22:6n–3-triacylglycerols. Am J Clin Nutr. 1996;64(4):577–86. [DOI] [PubMed] [Google Scholar]
- 52. Park HG, Lawrence P, Engel MG, Kothapalli K, Brenna JT. Metabolic fate of docosahexaenoic acid (DHA; 22:6n–3) in human cells: direct retroconversion of DHA to eicosapentaenoic acid (20:5n–3) dominates over elongation to tetracosahexaenoic acid (24:6n–3). FEBS Lett. 2016;590(18):3188–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Metherel AH, Irfan M, Klingel SL, Mutch DM, Bazinet RP. Compound-specific isotope analysis reveals no retroconversion of DHA to EPA but substantial conversion of EPA to DHA following supplementation: a randomized control trial. Am J Clin Nutr. 2019;110(4):823–31. [DOI] [PubMed] [Google Scholar]
- 54. Wijendran V, Downs I, Srigley CT, Kothapalli KSD, Park WJ, Blank BS, Zimmer JP, Butt CM, Salem N, Brenna JT. Dietary arachidonic acid and docosahexaenoic acid regulate liver fatty acid desaturase (FADS) alternative transcript expression in suckling piglets. Prostaglandins, Leukotrienes Essent Fatty Acids. 2013;89(5):345–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Jacobi SK, Lin X, Corl BA, Hess HA, Harrell RJ, Odle J. Dietary arachidonate differentially alters desaturase–elongase pathway flux and gene expression in liver and intestine of suckling pigs. J Nutr. 2011;141(4):548–53. [DOI] [PubMed] [Google Scholar]
- 56. Dang AQ, Kemp K, Faas FH, Carter WJ. Effects of dietary fats on fatty acid composition and Δ5 desaturase in normal and diabetic rats. Lipids. 1989;24(10):882–9. [DOI] [PubMed] [Google Scholar]
- 57. Park HG, Kothapalli KSD, Park WJ, DeAllie C, Liu L, Liang A, Lawrence P, Brenna JT. Palmitic acid (16:0) competes with ω-6 linoleic and ω-3 α-linolenic acids for FADS2 mediated Δ6-desaturation. Biochim Biophys Acta. 2016;1861(2):91–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Picklo MJ, Murphy EJ. A high-fat, high-oleic diet, but not a high-fat, saturated diet, reduces hepatic α-linolenic acid and eicosapentaenoic acid content in mice. Lipids. 2016;51(5):537–47. [DOI] [PubMed] [Google Scholar]
- 59. Vessby B, Gustafsson IB, Tengblad S, Berglund L. Indices of fatty acid desaturase activity in healthy human subjects: effects of different types of dietary fat. Br J Nutr. 2013;110(5):871–9. [DOI] [PubMed] [Google Scholar]
- 60. Garg ML, Wierzbicki A, Keelan M, Thomson ABR, Clandinin MT. Fish oil prevents change in arachidonic acid and cholesterol content in rat caused by dietary cholesterol. Lipids. 1989;24(4):266–70. [DOI] [PubMed] [Google Scholar]
- 61. Leikin AI, Brenner RR. Cholesterol-induced microsomal changes modulate desaturase activities. Biochim Biophys Acta. 1987;922(3):294–303. [DOI] [PubMed] [Google Scholar]
- 62. Brenner RR, Bernasconi AM, González MS, Rimoldi OJ. Dietary cholesterol modulates Δ6 and Δ9 desaturase mRNAs and enzymatic activity in rats fed a low-EFA diet. Lipids. 2002;37(4):375–83. [DOI] [PubMed] [Google Scholar]
- 63. Garg ML, Sebokova E, Thomson ABR, Clandinin MT. Δ6-Desaturase activity in liver microsomes of rats fed diets enriched with cholesterol and/or ω3 fatty acids. Biochem J. 1988;249(2):351–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Garg ML, Thomson AB, Clandinin MT. Effect of dietary cholesterol and/or ω3 fatty acids on lipid composition and Δ5-desaturase activity of rat liver microsomes. J Nutr. 1988;118(6):661–8. [DOI] [PubMed] [Google Scholar]
- 65. Huang YS, Manku MS, Horrobin DF. The effects of dietary cholesterol on blood and liver polyunsaturated fatty acids and on plasma cholesterol in rats fed various types of fatty acid diet. Lipids. 1984;19(9):664–72. [DOI] [PubMed] [Google Scholar]
- 66. Leikin AI, Brenner RR. In vivo cholesterol removal from liver microsomes induces changes in fatty acid desaturase activities. Biochim Biophys Acta. 1988;963(2):311–9. [DOI] [PubMed] [Google Scholar]
- 67. Jump DB, Tripathy S, Depner CM. Fatty acid-regulated transcription factors in the liver. Annu Rev Nutr. 2013;33(1):249–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Shimomura I, Bashmakov Y, Shimano H, Horton JD, Goldstein JL, Brown MS. Cholesterol feeding reduces nuclear forms of sterol regulatory element binding proteins in hamster liver. Proc Natl Acad Sci U S A. 1997;94(23):12354–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Garcia-Caraballo SC, Comhair TM, Verheyen F, Gaemers I, Schaap FG, Houten SM, Hakvoort TBM, Dejong CHC, Lamers WH, Koehler SE. Prevention and reversal of hepatic steatosis with a high-protein diet in mice. Biochim Biophys Acta. 2013;1832(5):685–95. [DOI] [PubMed] [Google Scholar]
- 70. Yeh YY, Leveille GA. Effect of dietary protein on hepatic lipogenesis in the growing chick. J Nutr. 1969;98(3):356–66. [DOI] [PubMed] [Google Scholar]
- 71. Stepien M, Gaudichon C, Fromentin G, Even P, Tomé D, Azzout-Marniche D. Increasing protein at the expense of carbohydrate in the diet down-regulates glucose utilization as glucose sparing effect in rats. PLoS One. 2011;6(2):e14664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Margolis LM, Rivas DA, Ezzyat Y, Gaffney-Stomberg E, Young AJ, McClung JP, Fielding RA, Pasiakos SM. Calorie restricted high protein diets downregulate lipogenesis and lower intrahepatic triglyceride concentrations in male rats. Nutrients. 2016;8(9):571. doi:10.3390/nu8090571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Torre-Villalvazo I, Gonzalez F, Aguilar-Salinas CA, Tovar AR, Torres N. Dietary soy protein reduces cardiac lipid accumulation and the ceramide concentration in high-fat diet-fed rats and ob/ob mice. J Nutr. 2009;139(12):2237–43. [DOI] [PubMed] [Google Scholar]
- 74. Shukla A, Brandsch C, Bettzieche A, Hirche F, Stangl GI, Eder K. Isoflavone-poor soy protein alters the lipid metabolism of rats by SREBP-mediated down-regulation of hepatic genes. J Nutr Biochem. 2007;18(5):313–21. [DOI] [PubMed] [Google Scholar]
- 75. Ascencio C, Torres N, Isoard-Acosta F, Gómez-Pérez FJ, Hernández-Pando R, Tovar AR. Soy protein affects serum insulin and hepatic SREBP-1 mRNA and reduces fatty liver in rats. J Nutr. 2004;134(3):522–9. [DOI] [PubMed] [Google Scholar]
- 76. Ronis MJ, Chen Y, Badeaux J, Badger TM. Dietary soy protein isolate attenuates metabolic syndrome in rats via effects on PPAR, LXR, and SREBP signaling. J Nutr. 2009;139(8):1431–8. [DOI] [PubMed] [Google Scholar]
- 77. Mercuri O, de Tomás ME, Itarte H. Prenatal protein depletion and Δ9, Δ6 and Δ5 desaturases in the rat. Lipids. 1979;14(9):822–5. [DOI] [PubMed] [Google Scholar]
- 78. De Tomás ME, Mercuri O, Rodrigo A. Effects of dietary protein and EFA deficiency on liver Δ5, Δ6 and Δ9 desaturase activities in the early developing rat. J Nutr. 1980;110(4):595–9. [DOI] [PubMed] [Google Scholar]
- 79. Peluffo RO, Brenner RR. Influence of dietary protein on 6- and 9-desaturation of fatty acids in rats of different ages and in different seasons. J Nutr. 1974;104(7):894–900. [DOI] [PubMed] [Google Scholar]
- 80. Decsi T, Molnár D, Koletzko B. The effect of under- and overnutrition on essential fatty acid metabolism in childhood. Eur J Clin Nutr. 1998;52(8):541–8. [DOI] [PubMed] [Google Scholar]
- 81. Lindholm M, Sjöblom L, Nordborg C, Östlund-Lindqvist AM, Eklund A. Comparison of dietary casein and soybean protein effects on plasma lipid and gastrin levels, hepatic ∆6-desaturase activity and coronary arteriosclerosis in male Sprague–Dawley rats. Ann Nutr Metab. 1993;37(6):302–10. [DOI] [PubMed] [Google Scholar]
- 82. Madani S, Lopez S, Blond JP, Prost J, Belleville J. Highly purified soybean protein is not hypocholesterolemic in rats but stimulates cholesterol synthesis and excretion and reduces polyunsaturated fatty acid biosynthesis. J Nutr. 1998;128(7):1084–91. [DOI] [PubMed] [Google Scholar]
- 83. Koba K, Wakamatsu K, Obata K, Sugano M. Effects of dietary proteins on linoleic acid desaturation and membrane fluidity in rat liver microsomes. Lipids. 1993;28(5):457–64. [DOI] [PubMed] [Google Scholar]
- 84. Brandsch C, Shukla A, Hirche F, Stangl GI, Eder K. Effect of proteins from beef, pork, and turkey meat on plasma and liver lipids of rats compared with casein and soy protein. Nutrition. 2006;22(11–12):1162–70. [DOI] [PubMed] [Google Scholar]
- 85. Ikeda A, Koba K, Sugano M. Impact of dietary protein on polyunsaturated fatty acid desaturation in rats fed diets rich in α-linolenic acid. Biosci Biotechnol Biochem. 1993;57(1):61–4. [DOI] [PubMed] [Google Scholar]
- 86. Noguchi A, Takita T, Suzuk K, Nakamura K. Effects of casein and soy-protein on α-linolenic acid metabolism in rats. J Nutr Sci Vitaminol. 1992;38(6):579–91. [DOI] [PubMed] [Google Scholar]
- 87. Sugiyama K, Yamakawa A, Kumazawa A, Saeki S. Methionine content of dietary proteins affects the molecular species composition of plasma phosphatidylcholine in rats fed a cholesterol-free diet. J Nutr. 1997;127(4):600–7. [DOI] [PubMed] [Google Scholar]
- 88. Yi H, Ravilious GE, Galant A, Krishnan HB, Jez JM. From sulfur to homoglutathione: thiol metabolism in soybean. Amino Acids. 2010;39(4):963–78. [DOI] [PubMed] [Google Scholar]
- 89. Shimada Y, Morita T, Sugiyama K. Increased response of liver microsomal Δ6-desaturase activity to dietary methionine in rats. Biosci Biotechnol Biochem. 2003;67(4):743–51. [DOI] [PubMed] [Google Scholar]
- 90. Peluffo RO, Nervi AM, Gonzalez MS, Brenner RR. Effect of different amino acid diets on δ5, δ6 and δ9 desaturases. Lipids. 1984;19(2):154–7. [DOI] [PubMed] [Google Scholar]
- 91. Schwarz J-M, Linfoot P, Dare D, Aghajanian K. Hepatic de novo lipogenesis in normoinsulinemic and hyperinsulinemic subjects consuming high-fat, low-carbohydrate and low-fat, high-carbohydrate isoenergetic diets. Am J Clin Nutr. 2003;77(1):43–50. [DOI] [PubMed] [Google Scholar]
- 92. Chong MFF, Hodson L, Bickerton AS, Roberts R, Neville M, Karpe F, Frayn KN, Fielding BA. Parallel activation of de novo lipogenesis and stearoyl-CoA desaturase activity after 3 d of high-carbohydrate feeding. Am J Clin Nutr. 2008;87(4):817–23. [DOI] [PubMed] [Google Scholar]
- 93. Schwarz JM, Neese RA, Turner S, Dare D, Hellerstein MK. Short-term alterations in carbohydrate energy intake in humans: striking effects on hepatic glucose production, de novo lipogenesis, lipolysis, and whole-body fuel selection. J Clin Invest. 1995;96(6):2735–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Hudgins LC, Hellerstein M, Seidman C, Neese R, Diakun J, Hirsch J. Human fatty acid synthesis is stimulated by a eucaloric low fat, high carbohydrate diet. J Clin Invest. 1996;97(9):2081–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Marques-Lopes I, Ansorena D, Astiasaran I, Forga L, Martínez JA. Postprandial de novo lipogenesis and metabolic changes induced by a high-carbohydrate, low-fat meal in lean and overweight men. Am J Clin Nutr. 2001;73(2):253–61. [DOI] [PubMed] [Google Scholar]
- 96. Chiu S, Mulligan K, Schwarz JM. Dietary carbohydrates and fatty liver disease: de novo lipogenesis. Clin Nutr Metab Care. 2018;21(4):277–82. [DOI] [PubMed] [Google Scholar]
- 97. Drąg J, Goździalska A, Knapik-Czajka M, Gawędzka A, Gawlik K, Jaśkiewicz J. Effect of high carbohydrate diet on elongase and desaturase activity and accompanying gene expression in rat's liver. Genes Nutr. 2017;12(1). doi:10.1186/s12263-017-0551-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. da Silva-Santi LG, Antunes MM, Caparroz-Assef SM, Carbonera F, Masi LN, Curi R, Visentainer JV, Bazotte RB. Liver fatty acid composition and inflammation in mice fed with high-carbohydrate diet or high-fat diet. Nutrients. 2016;8(11). doi:10.3390/nu8110682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. El Hafidi M, Cuéllar A, Ramírez J, Baos G. Effect of sucrose addition to drinking water, that induces hypertension in the rats, on liver microsomal Δ9 and Δ5-desaturase activities. J Nutr Biochem. 2001;12(7):396–403. [DOI] [PubMed] [Google Scholar]
- 100. Mašek T, Filipović N, Vuica A, Starčević K. Effects of treatment with sucrose in drinking water on liver histology, lipogenesis and lipogenic gene expression in rats fed high-fiber diet. Prostaglandins, Leukotrienes Essent Fatty Acids. 2017;116:1–8. doi:10.1016/j.plefa.2016.11.001. [DOI] [PubMed] [Google Scholar]
- 101. Hein GJ, Bernasconi AM, Montanaro MA, Pellon-Maison M, Finarelli G, Chicco A, Lombardo YB, Brenner RR. Nuclear receptors and hepatic lipidogenic enzyme response to a dyslipidemic sucrose-rich diet and its reversal by fish oil n–3 polyunsaturated fatty acids. Am J Physiol Endocrinol Metab. 2010;298(3):E429–39. [DOI] [PubMed] [Google Scholar]
- 102. Dziedzic B, Bewicz-Binkowska D, Zgorzynska E, Stulczewski D, Wieteska L, Kaza B, Walczewska A. DHA upregulates FADS2 expression in primary cortical astrocytes exposed to vitamin A. Physiol Res. 2018;67(4):663–8. [DOI] [PubMed] [Google Scholar]
- 103. Zhou D, Zaiger G, Ghebremeskel K, Crawford MA, Reifen R. Vitamin A deficiency reduces liver and colon docosahexaenoic acid levels in rats fed high linoleic and low α-linolenic acid diet. Prostaglandins, Leukotrienes Essent Fatty Acids. 2004;71(6):383–9. [DOI] [PubMed] [Google Scholar]
- 104. Zolfaghari R, Ross AC. Recent advances in molecular cloning of fatty acid desaturase genes and the regulation of their expression by dietary vitamin A and retinoic acid. Prostaglandins, Leukotrienes Essent Fatty Acids. 2003;68(2):171–9. [DOI] [PubMed] [Google Scholar]
- 105. Zolfaghari R, Cifelli CJ, Banta MD, Ross AC. Fatty acid Δ5-desaturase mRNA is regulated by dietary vitamin A and exogenous retinoic acid in liver of adult rats. Arch Biochem Biophys. 2001;391(1):8–15. [DOI] [PubMed] [Google Scholar]
- 106. Alam SQ, Alam BS. Microsomal fatty acid desaturase activities in vitamin A-deficient rat liver. Biochim Biophys Acta. 1985;833(1):175–7. [DOI] [PubMed] [Google Scholar]
- 107. Alam SQ, Alam BS, Ta-Wei C. Activities of fatty acid desaturases and fatty acid composition of liver microsomes in rats fed β-carotene and 13-cis-retinoic acid. Biochim Biophys Acta. 1984;792(2):110–7. [DOI] [PubMed] [Google Scholar]
- 108. Bershad S, Rubinstein A, Paterniti JR, Le NA, Poliak SC, Heller B, Ginsberg HN, Fleischmajer R, Brown WV. Changes in plasma lipids and lipoproteins during isotretinoin therapy for acne. N Engl J Med. 1985;313(16):981–5. [DOI] [PubMed] [Google Scholar]
- 109. Howard TD, Mathias RA, Seeds MC, Herrington DM, Hixson JE, Shimmin LC, Hawkins GA, Sellers M, Ainsworth HC, Sergeant Set al. DNA methylation in an enhancer region of the FADS cluster is associated with FADS activity in human liver. PLoS One. 2014;9(5):e97510. doi:10.1371/journal.pone.0097510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Walle P, Männistö V, De Mello VD, Vaittinen M, Perfilyev A, Hanhineva K, Ling C, Pihlajamäki J. Liver DNA methylation of FADS2 associates with FADS2 genotypex. Clin Epigenet. 2019;11(1). doi:10.1186/s13148-019-0609-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Devlin AM, Singh R, Wade RE, Innis SM, Bottiglieri T, Lentz SR. Hypermethylation of Fads2 and altered hepatic fatty acid and phospholipid metabolism in mice with hyperhomocysteinemia. J Biol Chem. 2007;282(51):37082–90. [DOI] [PubMed] [Google Scholar]
- 112. Burdge GC, Slater-Jefferies JL, Grant RA, Chung WS, West AL, Lillycrop KA, Hanson MA, Calder PC. Sex, but not maternal protein or folic acid intake, determines the fatty acid composition of hepatic phospholipids, but not of triacylglycerol, in adult rats. Prostaglandins, Leukotrienes Essent Fatty Acids. 2008;78(1):73–9. [DOI] [PubMed] [Google Scholar]
- 113. Wadhwani NS, Manglekar RR, Dangat KD, Kulkarni AV, Joshi SR. Effect of maternal micronutrients (folic acid, vitamin B12) and ω3 fatty acids on liver fatty acid desaturases and transport proteins in Wistar rats. Prostaglandins, Leukotrienes Essent Fatty Acids. 2012;86(1–2):21–7. [DOI] [PubMed] [Google Scholar]
- 114. Castaño-Moreno E, Castillo V, Peñailillo R, Llanos MN, Valenzuela R, Ronco AM. Fatty acid and lipid metabolism in liver of pregnant mice and their offspring is influenced by unbalanced folates/vitamin B12 diets. Prostaglandins, Leukotrienes Essent Fatty Acids. 2020;154:102057. doi:10.1016/j.plefa.2020.102057. [DOI] [PubMed] [Google Scholar]
- 115. Stefanidou M, Maravelias C, Dona A, Spiliopoulou C. Zinc: a multipurpose trace element. Arch Toxicol. 2006;80(1):1–9. [DOI] [PubMed] [Google Scholar]
- 116. Chasapis CT, Spiliopoulou CA, Loutsidou AC, Stefanidou ME. Zinc and human health: an update. Arch Toxicol. 2012;86(4):521–34. [DOI] [PubMed] [Google Scholar]
- 117. Cunnane SC, Huang YS, Horrobin DF, Davignon J. Role of zinc in linoleic acid desaturation and prostaglandin synthesis. Prog Lipid Res. 1981;20(C):157–60. [DOI] [PubMed] [Google Scholar]
- 118. Knez M, Stangoulis JCR, Glibetic M, Tako E. The linoleic acid: dihomo-γ-linolenic acid ratio (LA:DGLA)—an emerging biomarker of Zn status. Nutrients. 2017;9(8):825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Cunnane SC. Evidence that adverse effects of zinc deficiency on essential fatty acid composition in rats are independent of food intake. Br J Nutr. 1988;59(2):273–8. [DOI] [PubMed] [Google Scholar]
- 120. Eder K, Kirchgessner M. Zinc deficiency and the desaturation of linoleic acid in rats force-fed fat-free diets. Biol Trace Elem Res. 1996;54(2):173–83. [DOI] [PubMed] [Google Scholar]
- 121. Huang YS, Cunnane SC, Horrobin DF, Davignon J. Most biological effects of zinc deficiency corrected by γ-linolenic acid (18:3ω6) but not by linoleic acid (18:2ω6). Atherosclerosis. 1982;41(2–3):193–207. [DOI] [PubMed] [Google Scholar]
- 122. Clejan S, Castro-Magana M, Collipp PJ, Jonas E, Maddaiah VT. Effects of zinc deficiency and castration on fatty acid composition and desaturation in rats. Lipids. 1982;17(3):129–35. [DOI] [PubMed] [Google Scholar]
- 123. Ayala S, Brenner RR. Essential fatty acid status in zinc deficiency: effect on lipid and fatty acid composition, desaturation activity and structure of microsomal membranes of rat liver and testes. Acta Physiol Lat Am. 1983;33(3):193–204. [PubMed] [Google Scholar]
- 124. Eder K, Kirchgessner M. Activities of liver microsomal fatty acid desaturases in zinc-deficient rats force-fed diets with a coconut oil/safflower oil mixture of linseed oil. Biol Trace Elem Res. 1995;48(3):215–29. [DOI] [PubMed] [Google Scholar]
- 125. Yary T, Voutilainen S, Tuomainen TP, Ruusunen A, Nurmi T, Virtanen JK. Serum n–6 polyunsaturated fatty acids, Δ5- and Δ6-desaturase activities, and risk of incident type 2 diabetes in men: the Kuopio Ischaemic Heart Disease Risk Factor Study. Am J Clin Nutr. 2016;103(5):1337–43. [DOI] [PubMed] [Google Scholar]
- 126. Knez M, Stangoulis JCR, Zec M, Debeljak-Martacic J, Pavlovic Z, Gurinovic M, Glibetic M. An initial evaluation of newly proposed biomarker of zinc status in humans: linoleic acid:dihomo-γ-linolenic acid (LA:DGLA) ratio. Clin Nutr ESPEN. 2016;15:85–92. doi:10.1016/j.clnesp.2016.06.013. [DOI] [PubMed] [Google Scholar]
- 127. Chimhashu T, Malan L, Baumgartner J, Van Jaarsveld PJ, Galetti V, Moretti D, Smuts CM, Zimmermann MB. Sensitivity of fatty acid desaturation and elongation to plasma zinc concentration: a randomised controlled trial in Beninese children. Br J Nutr. 2018;119(6):610–9. [DOI] [PubMed] [Google Scholar]
- 128. Abbaspour N, Hurrell R, Kelishadi R. Review on iron and its importance for human health. J Res Med Sci. 2014;19(2):164–74. [PMC free article] [PubMed] [Google Scholar]
- 129. Nakamura MT, Nara TY. Structure, function, and dietary regulation of Δ6, Δ5, and Δ9 desaturases. Annu Rev Nutr. 2004;24(1):345–76. [DOI] [PubMed] [Google Scholar]
- 130. Miller JL. Iron deficiency anemia: a common and curable disease. Cold Spring Harb Perspect Med. 2013;3(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Cunnane SC, McAdoo KR. Iron intake influences essential fatty acid and lipid composition of rat plasma and erythrocytes. J Nutr. 1987;117(9):1514–9. [DOI] [PubMed] [Google Scholar]
- 132. Ananda Rao G, Manix M, Larkin EC. Reduction of essential fatty acid deficiency in rats fed a low iron fat free diet. Lipids. 1980;15(1):55–60. [DOI] [PubMed] [Google Scholar]
- 133. Johnson SB, Kramer TR, Briske-Anderson M, Holman RT. Fatty acid pattern of tissue phospholipids in copper and iron deficiencies. Lipids. 1989;24(2):141–5. [DOI] [PubMed] [Google Scholar]
- 134. Stangl GI, Kirchgessner M. Different degrees of moderate iron deficiency modulate lipid metabolism of rats. Lipids. 1998;33(9):889–95. [DOI] [PubMed] [Google Scholar]
- 135. Smuts CM, Tichelaar HY, van Jaarsveld PJ, Badenhorst CJ, Kruger M, Laubscher R, Mansvelt EPG, Benadé AJS. The effect of iron fortification on the fatty acid composition of plasma and erythrocyte membranes in primary school children with and without iron deficiency. Prostaglandins, Leukotrienes Essent Fatty Acids. 1995;52(1):59–67. [DOI] [PubMed] [Google Scholar]
- 136. Krajcovicova-Kudlackova M, Klvanová J, Dušinská M. Polyunsaturated fatty acid plasma content in groups of general population with low vitamin B6 or low iron serum levels. Ann Nutr Metab. 2004;48(2):118–21. [DOI] [PubMed] [Google Scholar]
- 137. Cardona F, Andrés-Lacueva C, Tulipani S, Tinahones FJ, Queipo-Ortuño MI. Benefits of polyphenols on gut microbiota and implications in human health. J Nutr Biochem. 2013;24(8):1415–22. [DOI] [PubMed] [Google Scholar]
- 138. Tsao R. Chemistry and biochemistry of dietary polyphenols. Nutrients. 2010;2(12):1231–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Fraga CG, Croft KD, Kennedy DO, Tomás-Barberán FA. The effects of polyphenols and other bioactives on human health. Food Funct. 2019;10(2):514–28. [DOI] [PubMed] [Google Scholar]
- 140. Williamson G. The role of polyphenols in modern nutrition. Nutr Bull. 2017;42(3):226–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Soleas GJ, Diamandis EP, Goldberg DM. Wine as a biological fluid: history, production, and role in disease prevention. J Clin Lab Anal. 1997;11(5):287–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Tang PC, Ng YF, Ho S, Gyda M, Chan SW. Resveratrol and cardiovascular health: promising therapeutic or hopeless illusion?. Pharmacol Res. 2014;90:88–115. doi:10.1016/j.phrs.2014.08.001. [DOI] [PubMed] [Google Scholar]
- 143. Visioli F. The resveratrol fiasco. Pharmacol Res. 2014;90:87. doi:10.1016/j.phrs.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 144. Rotches-Ribalta M, Andres-Lacueva C, Estruch R, Escribano E, Urpi-Sarda M. Pharmacokinetics of resveratrol metabolic profile in healthy humans after moderate consumption of red wine and grape extract tablets. Pharmacol Res. 2012;66(5):375–82. [DOI] [PubMed] [Google Scholar]
- 145. Walle T, Hsieh F, DeLegge MH, Oatis JE Jr, Walle UK. High absorption but very low bioavailability of oral resveratrol in humans. Drug Metab Dispos. 2004;32(12):1377–82. [DOI] [PubMed] [Google Scholar]
- 146. Kühn G, Pallauf K, Schulz C, Birringer M, Diaz-Rica B, de Pascual-Teresa S, Rimbach G. Resveratrol modulates desaturase expression and fatty acid composition of cultured hepatocytes. Front Nutr. 2018;5:106. doi:10.3389/fnut.2018.00106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Calleri E, Pochetti G, Dossou KSS, Laghezza A, Montanari R, Capelli D, Prada E, Loiodice F, Massolini G, Bernier Met al. Resveratrol and its metabolites bind to PPARs. ChemBioChem. 2014;15(8):1154–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Speer H, D'Cunha NM, Alexopoulos NI, McKune AJ, Naumovski N. Anthocyanins and human health: a focus on oxidative stress, inflammation and disease. Antioxidants. 2020;9(5):366. doi:10.3390/antiox9050366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Vauzour D, Tejera N, O'Neill C, Booz V, Jude B, Wolf IMA, Rigby N, Silvan JM, Curtis PJ, Cassidy Aet al. Anthocyanins do not influence long-chain n–3 fatty acid status: studies in cells, rodents and humans. J Nutr Biochem. 2015;26(3):211–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Di Giuseppe R, De Lorgeril M, Salen P, Laporte F, Di Castelnuovo A, Krogh V, Siani A, Arnout J, Cappuccio FP, Van Dongen Met al. Alcohol consumption and n–3 polyunsaturated fatty acids in healthy men and women from 3 European populations. Am J Clin Nutr. 2009;89(1):354–62. [DOI] [PubMed] [Google Scholar]
- 151. Curtis PJ, Kroon PA, Hollands WJ, Walls R, Jenkins G, Kay CD, Cassidy An. Cardiovascular disease risk biomarkers and liver and kidney function are not altered in postmenopausal women after ingesting an elderberry extract rich in anthocyanins for 12 weeks. J Nutr. 2009;139(12):2266–71. [DOI] [PubMed] [Google Scholar]
- 152. Reyna Gallegos S, Torres Arrunátegui G, Valenzuela R, Rincón-Cervera MÀ, Villanueva Espinoza ME. Adding a purple corn extract in rats supplemented with chia oil decreases gene expression of SREBP-1c and retains Δ5 and Δ6 hepatic desaturase activity, unmodified the hepatic lipid profile. Prostaglandins, Leukotrienes Essent Fatty Acids. 2018;132:1–7. doi:10.1016/j.plefa.2018.03.005. [DOI] [PubMed] [Google Scholar]
- 153. Zaheer K, Humayoun Akhtar M. An updated review of dietary isoflavones: nutrition, processing, bioavailability and impacts on human health. Crit Rev Food Sci Nutr. 2017;57(6):1280–93. [DOI] [PubMed] [Google Scholar]
- 154. Fickler A, Staats S, Rimbach G, Schulz C. Screening dietary biochanin A, daidzein, equol and genistein for their potential to increase DHA biosynthesis in rainbow trout (Oncorhynchus mykiss). PLoS One. 2019;14(1):e0210197–e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Mezei O, Li Y, Mullen E, Ross-Viola JS, Shay NF. Dietary isoflavone supplementation modulates lipid metabolism via PPAR-dependent and -independent mechanisms. Physiol Genomics. 2006;26(1):8–14. [DOI] [PubMed] [Google Scholar]
- 156. Mezei O, Banz WJ, Steger RW, Peluso MR, Winters TA, Shay N. Soy isoflavones exert antidiabetic and hypolipidemic effects through the PPAR pathways in obese Zucker rats and murine RAW 264.7 cells. J Nutr. 2003;133(5):1238–43. [DOI] [PubMed] [Google Scholar]
- 157. Kim S, Shin HJ, Kim SY, Kim JH, Lee YS, Kim DH, Lee MO. Genistein enhances expression of genes involved in fatty acid catabolism through activation of PPARα. Mol Cell Endocrinol. 2004;220(1–2):51–8. [DOI] [PubMed] [Google Scholar]
- 158. Kühn G, Pallauf K, Schulz C, Rimbach G. Flavonoids as putative modulators of Δ4-, Δ5-, and Δ6-desaturases: studies in cultured hepatocytes, myocytes, and adipocytes. Biofactors. 2018;44(5):485–95. [DOI] [PubMed] [Google Scholar]
- 159. Gou ZY, Cui XY, Li L, Fan QL, Lin XJ, Wang YB, Jiang ZY, Jiang SQ. Effects of dietary incorporation of linseed oil with soybean isoflavone on fatty acid profiles and lipid metabolism-related gene expression in breast muscle of chickens. Animal. 2020:1–9. doi:10.1017/S1751731120001020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.