Abstract
Takotsubo syndrome (TS) is an acute cardiac disease entity, characterized by a transient myocardial stunning in a distinctive predominantly regional circumferential pattern. One of the discussed pathological mechanisms of TS is coronary ischemia including coronary microvascular dysfunction (CMVD). Many studies have revealed invasive or non-invasive signs of CMVD in patients with TS, and therefore some investigators believe that CMVD is the primary cause of TS. Nevertheless, other studies have not shown any sign of CMVD. In addition, those studies, which have shown signs of CMVD, do not reveal such signs in all the three coronary vessel distribution; some of the patients show signs of CMVD in two or only one coronary artery territory. Moreover, signs of CMVD in TS are more prevalent and more pronounced in the left anterior descending artery (LAD) distribution. The CMVD in TS is reversible in a pattern parallel to the improvement of myocardial wall motion abnormality. In this review, substantial evidences challenging CMVD as the primary cause of TS and supporting the concept that CMVD is a secondary or epiphenomenon in TS are provided. Furthermore, convincing explanation is given for the causes of the more prevalent and the more pronounced signs of CMVD observed in the LAD distribution.
Keywords: Takotsubo syndrome, microvascular dysfunction, myocardial stunning, cardiac cramp, neurocardiogenic syndrome
Introduction
Takotsubo syndrome (TS), also called broken heart syndrome or neurogenic stunned myocardium, is a recognized acute cardiac disease entity characterized by a transient left ventricular (may also be right) myocardial stunning [1,2]. The term “Tsubo” or “Takotsubo” was introduced in the early 1990s by Sato and Dote to describe the shape of the left ventricle during systole in patients presenting with a clinical picture resembling that of an acute coronary syndrome with no obstructive coronary artery disease [3,4]. The defining feature of TS is the regional left ventricular wall motion abnormality (LVWMA) with an exceptional circumferential pattern resulting in a conspicuous ballooning of the left ventricle during systole [1,2,4]. The LVWMA is incongruent with the coronary artery supply territories and is reversible with almost complete resolution of ventricular dysfunction in hours to weeks [1]. The left ventricular ballooning pattern may be apical, mid-apical, mid-ventricular, mid-basal, basal, or focal [1,2]. A global left ventricular contractile abnormality has also been reported [5]. The right ventricle is involved in about 30% of TS cases [6]. A trigger factor (an emotional or a physical) may precede the onset of TS in about 70% of TS cases [2,7]. Several pathophysiological mechanisms for the development of TS have been discussed. The main proposed mechanisms are myocardial ischemia, blood-borne catecholamine myocardial toxicity, left ventricular outlet tract obstruction, epinephrine-induced switch in signal-trafficking, and autonomic nervous system dysfunction with sympathetic nervous system hyper-activation including local cardiac sympathetic disruption and norepinephrine seethe and spillover [2,8-11]. Among the discussed causes of myocardial ischemia as a pathophysiological mechanism for the development of TS are: multi-vessel coronary artery spasm, aborted myocardial infarction caused by a transient thrombosis in a long wrap-around left anterior descending artery (LAD), and coronary microvascular dysfunction (CMVD) [2,10,12]. Many studies, but not all, have shown invasive or non-invasive signs of CMVD in patients with TS leading to the belief that CMVD may be the primary cause of TS by some researchers [13-17]. However, adequate evidences challenging the hypothesis that CMVD is the primary cause of TS and supporting the notion that CMVD is a secondary or an epiphenomenon in TS are provided in this review. Furthermore, a reasonable explanation for the cause of the more prevalent and the more pronounced CMVD in the LAD are also provided.
Evidences for CMVD in TS
Invasive coronary angiography (CAG) reveals normal coronary arteries in most of the patients with TS [2,10]. However, the inability of CAG to visualize the coronary microcirculation (pre-arterioles and arterioles of less than 500 µm) is a significant limitation of CAG. Different invasive and non-invasive techniques have been used as substitution measures to study the coronary microcirculation. From CAG, Thrombolysis in Myocardial Infarction (TIMI) frame-count (TFC) is used to measure coronary flow velocity. Other invasive techniques as corrected TIMI frame count (CTFC), TIMI myocardial perfusion grade (TMPG), coronary flow reserve (CFR), index of microcirculatory resistance (IMR), and hyperemic microvascular resistance (HMR) have also been used to study the coronary microcirculation [13,18,19]. Non-invasive methods as Doppler transthoracic echocardiography (TTE) and myocardial contrast echocardiography (MCE) can provide analysis of both LVWMA and coronary flow [19]. Noninvasive evaluation of myocardial blood flow can also be done by positron emission tomography (PET), cardiac magnetic resonance (CMR) imaging and dynamic myocardial perfusion computer tomography [13,19].
By using doppler guide wire, Sadamutsu et al [20] could document a diminished CFR in 2 patients with a clinical picture consistent with TS. Other investigators demonstrated elevated TFC and abnormal TIMI myocardial perfusion grades in patients with TS [16,21]. Kurisu et al [22] also measured TFC (defined as the number of frames required for the contrast material to travel from the coronary ostium to the distal end of the coronary artery) in 28 patients with apical ballooning pattern of TS compared to 20 control subjects. The TFC was abnormally high in all 3 epicardial vessels during early index CAG compared to normal TFC in the control subjects. Bybee et al [21] reported on TFC in 16 patients with apical ballooning pattern of TS. All patients had significantly increased (more than 30 cine-frames) TFC in one or more epicardial coronary vessel. By assessing TFC, Fazio et al [23] reported slow coronary flow in 23 of 24 patients with TS during the acute stage. De Caterina et al [17] studied TIMI flow, TFC, and both quantitative and qualitative myocardial blush grade in 25 patients with TS compared to 25 normal control groups, 25 patients with STEMI undergoing primary PCI, and 25 patients with microvascular obstruction. The TIMI flow in LAD was significantly lower and the TFC was significantly higher in TS compared to control group and STEMI patients, while it did not differ compared to patients with microvascular obstruction. The myocardial blush grade was significantly lower than that in control and STEMI patients and higher than in patients with microvascular obstruction. Novo et al [24] analyzed TFC in LAD, left circumflex artery (LCx) and right coronary artery (RCA) in 71 patients with TS compared with 70 controls and 71 patients with microvascular angina. TFC was significantly higher in all the coronary arteries in TS compared to control groups (25.70±5.34 frames vs. 16.70±3.76). The CMVD was diffuse in TS as well as in microvascular angina. The authors concluded that there were diffuse but mild signs of CMVD detected by TFC in TS.
IMR has been measured in the LAD in case descriptions in patients with TS, and was remarkably high indicating microvascular dysfunction [25-27]. Warisawa et al [25] reported on a 77-years-old woman with typical mid-ventricular TS with normal CAG and TIMI flow 3. She was further investigated to determine coexistence of microvascular dysfunction. Fractional flow reserve (FFR) was normal 0.94, CFR was decreased 1.8, and IMR was increased 66.0 in LAD. These results indicate that the microvascular function was highly impaired. Morrow et al [28] reported on a 82-years old woman with sepsis induced mid-apical TS. She had slow flow in the LAD during CAG. This prompted performance of invasive coronary physiology, which showed an FFR 0.91, low CFR 1.6 and marked increase in the IMR 117. The combination of these results is indicative of CMVD in the absence of significant epicardial coronary stenosis.
Non-invasive techniques have shown lower myocardial blood flow in dysfunctional left ventricular segments in patients with TS compared with left ventricular segments showing normal wall motion in the acute phase [13,29]. TTE-CFR has also been used to study coronary flow in the acute phase of the disease with similar results [13]. Based on these results, some investigators suggested that CMVD may contribute to the pathogenesis of TS [30,31]. Kume et al [32] studied the coronary flow velocity and coronary flow velocity reserve (CFVR) recorded by doppler guidewire in 8 patients with TS. They found decreased CFVR and short deceleration time of diastolic velocity in the acute phase of the disease. These findings improved 3 weeks later. The authors concluded that the CMVD may be a causative mechanism of TS. Abdelmoneim et al [29] performed real time myocardial contrast echocardiography in 9 patients with TS within 24 hrs. of angiographic diagnosis of TS. They found significantly lower myocardial blood flow velocity and lower myocardial blood flow in myocardial segments with ventricular wall motion abnormality compared with those with normal ventricular wall motion. The authors concluded that these findings are indicative for microvascular dysfunction in TS. In addition to high IMR, Warisawa et al [25] reported in the same case that there was slightly decreased myocardial perfusion during thallium-201 (201T1) myocardial single-photon emission computed tomography (SPECT) and highly decreased uptake of iodine-123-β-methyl-p-iodophenyl penta-decanoic acid (123I-BMIPP) in the midventricular segments. These findings suggested a localized transient ischemia. Anderson et al [33] reported on 16 patients with TS who underwent radionuclide perfusion studies, positron emission tomography in 8, SPECT with radionuclide tracer Tc99m SestaMIBI in 6 and SPECT with thaliun-201 in 2. The radionuclide perfusion studies were abnormal in 11 (69%) of patients in regions matching the LVWMA. The author’s conclusion was that the study supports the evidence that CMVD is a major causal element in the pathogenesis of TS. Consequently, substantial numbers of patients with TS have invasive or non-invasive signs of CMVD leading to the belief of some investigators that CMVD may be the primary cause of TS.
The distribution of CMVD in the coronary artery territories in TS
Many studies have shown signs of CMVD in patients with TS as discussed above [17,20-22,24,25]. Nevertheless, there are studies, which have shown signs of normal microvascular function in patients with TS [14,15]. Abe et al [14] reported on 17 patients with TS. They found no significant signs of slow flow in epicardial coronary arteries. There was also no significant abnormality in the coronary microcirculation in patients investigated with doppler guidewire or contrast echocardiography. Sganzerla et al [15] evaluated the coronary microvascular function in 5 patients with TS by means of transthoracic doppler ultrasound imaging of the LAD. The CFR value was in the normal range and was not significantly different from age and sex matched control subjects. Furthermore, patients who had shown signs of CMVD, not all of them had shown CMVD in all the three coronary artery distribution, some of them had CMVD in 2 or one coronary artery distribution. Bybee et al [21] reported on TFC in 16 patients with apical ballooning pattern of TS. All patients had significantly increased (more than 30 cine-frames) TFC in one or more epicardial coronary vessel. Ten (62.5%) patients had significantly increased TFC in the distribution of the three major epicardial coronary arteries, which implies that more one third of patients had increased TFC in only one or 2 coronary artery territories. In evaluation of 42 consecutive patients with TS and technically adequate CAG, Elesber et al [16] assessed the TIMI myocardial perfusion grade (TMPG), an index of myocardial perfusion. They observed abnormal myocardial perfusion in 29 (69%) patients. Thirteen (31%) patients had normal TMPG. Abnormal perfusion was found in all 3 coronary artery territories in 18, in 2 coronary artery territories in 7, and in 1 coronary artery territories in 4. Abnormal perfusion was limited to the apical and mid-apical regions. Sharkey et al [34] studied TFC in 59 patients with TS and found markedly prolonged frame counts (30 or more) in one epicardial coronary artery in 19 patients (34%), 2 arteries in 12 patients (21%) and in all 3 epicardial coronary arteries (5%). TIMI frame counts in the 59 patients with TS were only modestly prolonged compared with those in control subjects. Despite signs of slow coronary flow in 23 of 24 patients with TS during the acute stage in a study by Fazio et al [23], only 9 patients had slow flow in all three epicardial coronary arteries. Several other studies discussed below have not shown signs of microvascular dysfunction in all 3 coronary artery territories.
CMVD in TS is more prevalent and more pronounced in LAD supply territories
Microvascular dysfunction has been reported in a substantial number of patients with TS; it has been demonstrated in one, two, or the three coronary vessel distribution as detailed above. However, it has been reported that signs of CMVD are more prevalent and more pronounced in LAD distribution [35-37] as demonstrated in Figure 1 where CSF is clearly seen in LAD compared with LCx in a patient with mid-apical ballooning pattern of TS. Khalid et al [35] studied 16 patients with TS and found that corrected TFC in LAD was significantly higher than control patients; no difference in the corrected TFC was observed between the 2 groups in LCx or RCA. Loffi et al [36] studied coronary flow (TIMI flow and TFC) in the three coronary arteries of 27 patients with TS compared to 27 patients with microvascular angina and 27 control subjects. TFC revealed flow impairment in the 3 arteries in patients with TS compared to control subjects, the impairment was highest in LAD. Myocardial perfusion was also studied with blush score and Quantitative Blush evaluator (QuBE). The QuBE showed myocardial perfusion impairment in the LAD territory. Consequently, the microvascular impairment was highest in LAD supply region and the myocardial perfusion defect was detected in the LAD area. In a case with typical mid-apical pattern of TS, Bayon et al [37] measured HMR (defined as the ratio between the pressure in the distal part of the coronary artery and the mean peak velocity at the same part of the coronary artery; the normal value is less than 2). The measurement revealed high HMR in both LAD and LCx but the degree of increase was strikingly higher in LAD compared to LCx (15.6 in LAD vs 5.5 in LCx). There was also a greater proportional response to intracoronary infusion of adenosine in LAD compared to LCx. Cases of TS with remarkably high IMR were also determined in the LAD [25,28]. However, it was not compared to the LCx and RCA in the same patients. Montone et al [38] studied 101 patients with TS and found CSF (defined as TIMI-2 flow [requiring three or more beats to opacify the distal vasculature] in angiographically normal or near normal [<50% stenosis]) in 18 (17.8%) patients. Seventeen of the 18 patients (94%) had CSF lonely in LAD. Only one patient had CSF in both LAD and LCx. Furthermore, patients with CSF had worse myocardial perfusion with significantly reduced myocardial blush grade and quantitative myocardial blush score in LAD territory compared with patients with normal coronary flow. Consequently, in this study only a limited number of patients with TS had signs of CMVD and this was mainly observed in the LAD territory. Ozaki et al [39] analyzed quantitative flow ratio (QFR) in both LAD and LCx in 30 patients with TS. QFR was obtained by using 3-dimentional quantitative CAG (3D-QCA) and contrast flow by thrombolysis in myocardial infarction frame count. They reported that vessel QFR was significantly reduced in the LAD compared with that in the LCx. The authors concluded that this finding suggested that the pathophysiology of TS might indirectly point to microvascular dysfunction in the LAD territory. Consequently, substantial evidences exist for the fact that CMVD is more prevalent and more pronounced in LAD territory in patients with TS.
Figure 1.
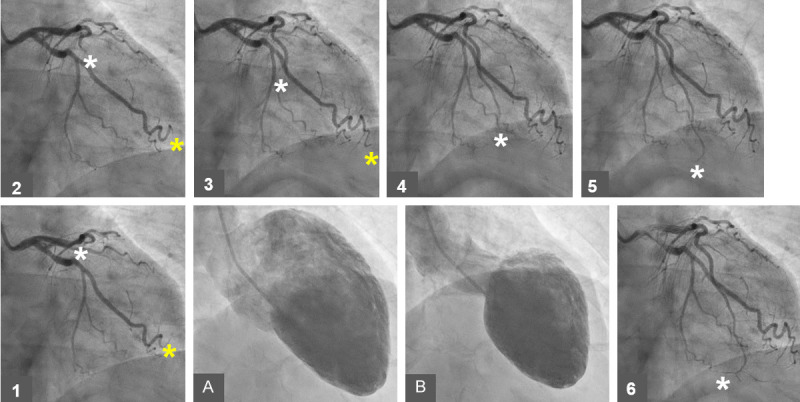
A case of mid-apical ballooning pattern of TS showing the left ventricle during diastole (A) and during systole (B). A series of the left coronary angiography (LCA) from 1 to 6 showing clearly slower flow in the left anterior descending artery (LAD, white Asterix) compared to flow in the left circumflex artery (LCx, yellow Asterix). Note that the contrast has reached the end left circumflex-obtuso-marginal branch at the image number 3 (yellow Asterix 1 to 3) while the contrast has reached the middle of LAD at the image 3 (white Asterix 1 to 3) and reached the end of LAD at the image 6 (white Asterix 4 to 6).
Microvascular dysfunction in TS is reversible
Patients, who have been investigated for CMVD during follow up of TS, have shown improvement or normalization of microvascular function [21,25,40-42]. Among the 16 patients with TS who had significantly increased TFC during the index presentation, the abnormal TFC were completely normalized in all 3 epicardial vessel distribution in the 2 patients who underwent repeat CAG about 4 weeks after the initial CAG [21]. Other investigators have observed that the LVWMA improved in parallel with the dynamic improvement of the microcirculation [40-42]. Meimoun et al [40] studied 12 patients with TS using Doppler TTE-CFR (calculated as the ratio of hyperemic to basal mean diastolic flow velocity) in the distal part of the LAD using intravenous adenosine infusion at the acute phase and 25 days later. Doppler echocardiography-CFR increased significantly from 2.2 at the acute phase to 2.9 at the follow up whereas wall motion score decreased significantly. The authors concluded that the LVWMAs improved in parallel with the dynamic improvement of the microcirculation. Rigo et al [41] studied 30 patients with TS, which were evaluated with transthoracic dipyridamole stress echocardiography and pulsed doppler CFR assessment on mid-distal LAD and posterior descending artery of RCA in addition to evaluation of wall motion score index. They observed signs of profound diffuse microcirculatory disturbance in the acute phase, with early reversal to near normal values on discharge days, paralleling the functional recovery in regional wall motion abnormality. Interestingly the CFR reached almost normal values on discharge in almost all patients, whereas the wall motion score index kept on improving over time with complete normalization at 6-month follow-up. Galiuto et al [42] studied myocardial perfusion assessed by contrast score index (CSI) and myocardial dysfunction by wall motion score index (WMSI) in 15 patients with TS who underwent contrast echocardiography at baseline during adenosine infusion and at 1-month follow-up. In patients with TS, perfusion defect was evident at myocardial contrast echocardiography within the dysfunctional myocardial area, which was transiently reduced by adenosine. There was also transient improvement of regional wall motion abnormality during adenosine administration. At 1-month, there was complete recovery of both myocardial perfusion and myocardial wall motion abnormalities. One month after the index presentation, Warisawa et al [25] have demonstrated complete normalization of microvascular dysfunction and remarkable improvement of uptake in the mid ventricular area during the 201T1 and the BMIPP SPECT in a patient with typical mid-ventricular TS with normal CAG and TIMI flow 3. Rivero et al [43] studied CFR and IMR in LAD in 14 patients with TS where 78.6% were of apical ballooning’s pattern. The mean CFR was 1.4 and the mean IMR was 53 indicating microvascular dysfunction. They also observed a significant negative linear correlation between the extent of microvascular dysfunction and the time from symptom onset to the IMR measurement indicating improvement of CMVD with time after the acute onset of TS. Heyse et al [44] reported on a 53-years-old woman with recurrent mid-apical TS. CAG showed a 50% stenosis in LAD. Functional assessment under maximal myocardial hyperemia in the LAD revealed FFR 0.92, which was normal and IMR was increased to 43 indicating microvascular dysfunction. Functional assessment at 1 month after the index presentation showed FFR 0.89 and normalization of IMR to 10 indicating reversible microvascular dysfunction. All these studies indicate that CMVD in TS is transient and normalizes with the normalization of left ventricular function.
Evidences challenging CMVD as a primary cause of TS
The body of evidences challenging CMVD as a primary cause of TS are substantial and are as follows:
First; not all patients with TS have signs of CMVD as detailed above [14,15]. Even in the studies with signs of CMVD, some patients in the same study had no signs of CMVD [16]. Furthermore, in patients with CMVD, most of them hadn’t CMVD in all the 3 coronary artery territories [16,23]. Further evidences for these points are already provided in the sections above. Second, despite ST-elevation MI (STEMI)-like ECG changes in more than one third and long-lasting STEMI-like ECG changes in more than one fourth of patients with TS who usually have extensive LVWMA, patients with TS have modest troponin elevation [1,10,38]. This argues strongly against ischemia, causing STEMI-like ECG changes, induced by microvascular dysfunction as a cause of TS. In the 18 TS-patients with CSF studied by Montone et al [38] 44% had STMI-like ECG changes (compared with 31% of TS-patients with normal coronary flow) and 27.7% (compared with 12%) had persistent STEMI-like ECG changes at 48 hrs. Despite these findings, patients with CSF did not have higher peak troponin value, it was rather lower 2.4 ng/ml in TS-patients with CSF compared to 3.2 ng/ml in TS-patients with normal coronary flow. This argues strongly against any type of coronary ischemia as a primary cause of TS including CMVD. Third, the first pass perfusion in CMR imaging has not shown any evidence of focal perfusion abnormalities in some patients with TS [45]. Absence of late gadolinium enhancement with the pattern of MI during CMR imaging argues against any ischemia as a primary cause of TS including CMVD [2,10]. Fourth, TS may be induced by dobutamine, which in fact has a vasodilatory effect [46] a finding which challenge the role of CMVD in the pathogenesis of TS. Fifth, Patients with symptoms of stable angina, non-obstructive coronary arteries during CAG and invasive or non-invasive signs of microvascular dysfunction are classified as ischemia with non-obstructive coronary arteries (INOCA) [18]. If CMVD is a primary cause of TS so INOCA should be the most common cause of TS and this is not the case. All studies reporting on CMVD in patients with TS during the index presentation and follow-up have revealed normalization of microvascular function during follow-up parallel with normalization of ventricular wall motion abnormalities [21,25,40,42,44]. Sixth, the main and consistent histopathological finding in TS are the hypercontracted sarcomeres and contraction band necrosis which is distinct from the coagulation necrosis caused by protracted ischemia [47]. This argues against any ischemia including that due to the CMVD as a cause of TS.
CMVD is secondary to TS, more prevalent and more pronounced, and sometimes restricted to LAD territories, mechanism?
As discussed above, there are enough evidences for that CMVD may occur in some patients with TS and is more pronounced and sometimes limited mainly to the LAD supply territory. Evidences are provided that CMVD is not a primary cause of TS. Nevertheless, CMVD in TS may be an epiphenomenon caused by the same pathogenesis of TS that is the cardiac sympathetic hyperactivation [9,11]. However, the CMVD in TS is mainly secondary to some pathological processes, which occurs in TS. Myocardial edema secondary to intramyocardial catecholamine inflammatory process may occur in some patients with TS [6] and this may lead to compression of the microvascular system resulting in microvascular dysfunction. This mechanism may involve the microvascular circulation of all the three coronary artery territories. However, myocardial edema does not occur in all patients with TS. Because the microvascular dysfunction occurs mainly in LAD territory, so the myocardial edema is not the main mechanism of microvascular dysfunction in TS. The main mechanism of microvascular compression is most probably caused by the cardiac cramp (myocardial stunning) which occurs in TS [9,48]. The cardiac cramp can also explain the more pronounced and sometimes restriction of microvascular dysfunction to the LAD territory [36]. Evidences for the occurrence of cardiac cramp in TS are discussed elsewhere [9,48,49]. There are explanations for the more pronounced and sometimes restriction for the occurrence of signs of CMVD in LAD territory. The myocardial territory supplied by LAD has not only intramyocardial resistance vessels (micro-circulation) but also has intramyocardial conductance vessels (macro-circulation) that can also be compressed by cardiac cramp. The intramyocardial conductance vessels in LAD territory are the septal branches (1 to 8 branches) in all patients and the LAD segments with myocardial bridging in some of the patients [50]. The compression of these vessels during the acute and subacute stages of TS may be the main cause of CSF in LAD (Figure 1). Systo-diastolic compression of LAD segments with myocardial bridging during the subacute stage of TS has been described [48]. Figure 2 reveals a long segment of LAD with myocardial bridging compressed during both systole and diastole. The relief of systo-diastolic compression has been documented during resolution of left ventricular dysfunction [48]. Compression of septal branch and even occlusion of it during systole has been reported in patients with TS [50]. Figure 3 demonstrates a patient with mid-apical TS, where the septal branches are well-seen and well-filled at the proximal part of LAD while they are scanty and poorly filled at the level of LAD covering the left ventricular segments with myocardial stunning. At the same time, the microvascular circulation is compressed during systole and diastole during the acute stage of the disease. This compression will be relieved during recovery of the myocardial stunning explaining the reversibility of CMVD as previously mentioned [25,43,44]. Consequently, adequate evidences provided that CMVD in patients with TS is a secondary phenomenon mainly explained by cardiac cramp (myocardial stunning) in TS; the CMVD is more prevalent and more pronounced in LAD territory because of compression of both macro- and microcirculation by the cardiac cramp during the acute stage of the disease.
Figure 2.
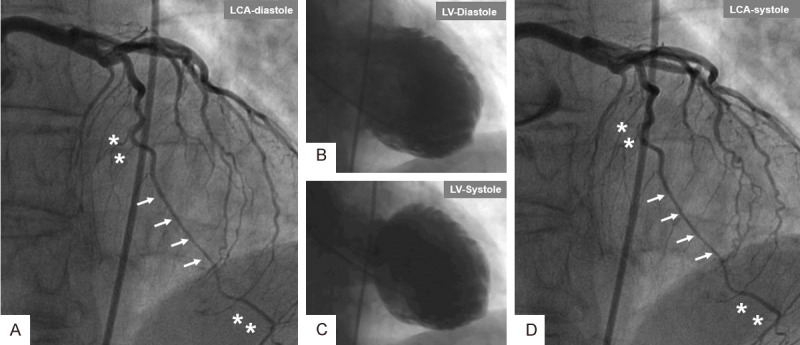
A case of emotional-triggered mid-apical with apical-tip-sparing Takotsubo syndrome (TS) showing compression of a long segment of left anterior descending artery (LAD) with myocardial bridging during both diastole and systole. Left coronary artery (LCA) during diastole (A) reveals a long segment of LAD with myocardial bridging (arrows); note that this segment is narrower compared to the segments proximal and distal (Asterix). Left ventriculography during diastole (B) and systole (C). LCA (D) during systole shows that the segment of LAD with myocardial bridging is as narrow as that during diastole (arrows) compared to the segments of LAD proximal and distal to the affected segment (Asterix).
Figure 3.
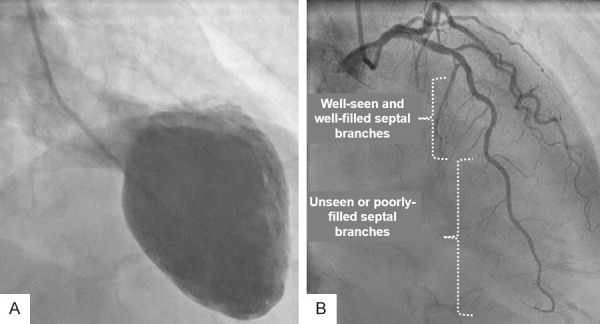
Mid-apical ballooning pattern of TS during systole (A) of the same patient in Figure 1. The left coronary artery (LCA) in cranial right anterior oblique projection (B) display the LAD clearly. Observe the septal branches in the proximal part of LAD are well-seen and well filled compared to the distal half of LAD where the septal branches are scanty and poorly filled due to compression of these septal branches by the myocardial stunning caused by TS.
Conclusion
Reversible invasive and non-invasive signs of CMVD exist in a substantial number of patients with TS. CMVD may occur in 3, 2, or 1 coronary vessel distribution and are usually more prevalent and more pronounced in LAD distribution. Persuasive evidences challenging CMVD as a primary cause for TS and arguing for that CMVD if present is an epiphenomenon or mainly secondary to TS are provided. Furthermore, the mechanisms of secondary CMVD in TS and the reasons for the more prevalent and more pronounced signs of CMVD in LAD territory are explained.
Disclosure of conflict of interest
None.
References
- 1.Y-Hassan S, Tornvall P. Epidemiology, pathogenesis, and management of Takotsubo syndrome. Clin Auton Res. 2018;28:53–65. doi: 10.1007/s10286-017-0465-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ghadri JR, Wittstein IS, Prasad A, Sharkey S, Dote K, Akashi YJ, Cammann VL, Crea F, Galiuto L, Desmet W, Yoshida T, Manfredini R, Eitel I, Kosuge M, Nef HM, Deshmukh A, Lerman A, Bossone E, Citro R, Ueyama T, Corrado D, Kurisu S, Ruschitzka F, Winchester D, Lyon AR, Omerovic E, Bax JJ, Meimoun P, Tarantini G, Rihal C, Y-Hassan S, Migliore F, Horowitz JD, Shimokawa H, Luscher TF, Templin C. International expert consensus document on Takotsubo syndrome (Part I): clinical characteristics, diagnostic criteria, and pathophysiology. Eur Heart J. 2018;39:2032–46. doi: 10.1093/eurheartj/ehy076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Y-Hassan S, Yamasaki K. History of Takotsubo syndrome: is the syndrome really described as a disease entity first in 1990? Some inaccuracies. Int J Cardiol. 2013;166:736–7. doi: 10.1016/j.ijcard.2012.09.183. [DOI] [PubMed] [Google Scholar]
- 4.Dote K, Sato H, Tateishi H, Uchida T, Ishihara M. Myocardial stunning due to simultaneous multivessel coronary spasms: a review of 5 cases. J Cardiol. 1991;21:203–14. [PubMed] [Google Scholar]
- 5.Y-Hassan S, Tornvall P, Tornerud M, Henareh L. Capecitabine caused cardiogenic shock through induction of global Takotsubo syndrome. Cardiovasc Revasc Med. 2013;14:57–61. doi: 10.1016/j.carrev.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 6.Eitel I, von Knobelsdorff-Brenkenhoff F, Bernhardt P, Carbone I, Muellerleile K, Aldrovandi A, Francone M, Desch S, Gutberlet M, Strohm O, Schuler G, Schulz-Menger J, Thiele H, Friedrich MG. Clinical characteristics and cardiovascular magnetic resonance findings in stress (Takotsubo) cardiomyopathy. JAMA. 2011;306:277–86. doi: 10.1001/jama.2011.992. [DOI] [PubMed] [Google Scholar]
- 7.Sharkey SW, Windenburg DC, Lesser JR, Maron MS, Hauser RG, Lesser JN, Maron MS, Hauser RG, Lesser JN, Haas TS, Hodges JS, Maron BJ. Natural history and expansive clinical profile of stress (tako-tsubo) cardiomyopathy. J Am Coll Cardiol. 2010;55:333–41. doi: 10.1016/j.jacc.2009.08.057. [DOI] [PubMed] [Google Scholar]
- 8.Lyon AR, Bossone E, Schneider B, Sechtem U, Citro R, Underwood SR, Sheppard MN, Figtree GA, Parodi G, Akashi YJ, Ruschitzka F, Filippatos G, Mebazaa A, Omerovic E. Current state of knowledge on Takotsubo syndrome: a position statement from the taskforce on Takotsubo syndrome of the heart failure association of the European Society of Cardiology. Eur J Heart Fail. 2016;18:8–27. doi: 10.1002/ejhf.424. [DOI] [PubMed] [Google Scholar]
- 9.Y-Hassan S. Autonomic neurocardiogenic syndrome is stonewalled by the universal definition of myocardial infarction. World J Cardiol. 2020;12:231–47. doi: 10.4330/wjc.v12.i6.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Y-Hassan S, De Palma R. Contemporary review on the pathogenesis of Takotsubo syndrome: the heart shedding tears: norepinephrine churn and foam at the cardiac sympathetic nerve terminals. Int J Cardiol. 2017;228:528–36. doi: 10.1016/j.ijcard.2016.11.086. [DOI] [PubMed] [Google Scholar]
- 11.Y-Hassan S. Insights into the pathogenesis of Takotsubo syndrome, which with persuasive reasons should be regarded as an acute cardiac sympathetic disease entity. ISRN Cardiol. 2012;2012:593735. doi: 10.5402/2012/593735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ghadri JR, Wittstein IS, Prasad A, Sharkey S, Dote K, Akashi YJ, Cammann VL, Crea F, Galiuto L, Desmet W, Yoshida T, Manfredini R, Eitel I, Kosuge M, Nef HM, Deshmukh A, Lerman A, Bossone E, Citro R, Ueyama T, Corrado D, Kurisu S, Ruschitzka F, Winchester D, Lyon AR, Omerovic E, Bax JJ, Meimoun P, Tarantini G, Rihal C, Y-Hassan S, Migliore F, Horowitz JD, Shimokawa H, Luscher TF, Templin C. International expert consensus document on Takotsubo syndrome (Part II): diagnostic workup, outcome, and management. Eur Heart J. 2018;39:2047–62. doi: 10.1093/eurheartj/ehy077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vitale C, Rosano GM, Kaski JC. Role of coronary microvascular dysfunction in Takotsubo cardiomyopathy. Circ J. 2016;80:299–305. doi: 10.1253/circj.CJ-15-1364. [DOI] [PubMed] [Google Scholar]
- 14.Abe Y, Kondo M, Matsuoka R, Araki M, Dohyama K, Tanio H. Assessment of clinical features in transient left ventricular apical ballooning. J Am Coll Cardiol. 2003;41:737–42. doi: 10.1016/s0735-1097(02)02925-x. [DOI] [PubMed] [Google Scholar]
- 15.Sganzerla P, Alioto G, Funaro A, Passaretti B, Borghini E. Coronary microvascular function in Takotsubo cardiomyopathy: results of non-invasive evaluation. Int J Cardiol. 2009;137:181–3. doi: 10.1016/j.ijcard.2008.05.033. [DOI] [PubMed] [Google Scholar]
- 16.Elesber A, Lerman A, Bybee KA, Murphy JG, Barsness G, Singh M, Rihal CS, Prasad A. Myocardial perfusion in apical ballooning syndrome correlate of myocardial injury. Am Heart J. 2006;152:469, e9–13. doi: 10.1016/j.ahj.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 17.De Caterina AR, Leone AM, Galiuto L, Basile E, Fedele E, Paraggio L, De Maria GL, Porto I, Niccoli G, Burzotta F, Trani C, Rebuzzi AG, Crea F. Angiographic assessment of myocardial perfusion in Tako-Tsubo syndrome. Int J Cardiol. 2013;168:4717–22. doi: 10.1016/j.ijcard.2013.07.172. [DOI] [PubMed] [Google Scholar]
- 18.Kunadian V, Chieffo A, Camici PG, Berry C, Escaned J, Maas A, Prescott E, Karam N, Appelman Y, Fraccaro C, Louise Buchanan G, Manzo-Silberman S, Al-Lamee R, Regar E, Lansky A, Abbott JD, Badimon L, Duncker DJ, Mehran R, Capodanno D, Baumbach A. An EAPCI expert consensus document on ischaemia with non-obstructive coronary arteries in collaboration with european society of cardiology working group on coronary pathophysiology & microcirculation endorsed by coronary vasomotor disorders international study group. Eur Heart J. 2020;41:3504–20. doi: 10.1093/eurheartj/ehaa503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vancheri F, Longo G, Vancheri S, Henein M. Coronary microvascular dysfunction. J Clin Med. 2020;9:2880. doi: 10.3390/jcm9092880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sadamatsu K, Tashiro H, Maehira N, Yamamoto K. Coronary microvascular abnormality in the reversible systolic dysfunction observed after noncardiac disease. Jpn Circ J. 2000;64:789–92. doi: 10.1253/jcj.64.789. [DOI] [PubMed] [Google Scholar]
- 21.Bybee KA, Prasad A, Barsness GW, Lerman A, Jaffe AS, Murphy JG, Wright RS, Rihal CS. Clinical characteristics and thrombolysis in myocardial infarction frame counts in women with transient left ventricular apical ballooning syndrome. Am J Cardiol. 2004;94:343–6. doi: 10.1016/j.amjcard.2004.04.030. [DOI] [PubMed] [Google Scholar]
- 22.Kurisu S, Inoue I, Kawagoe T, Ishihara M, Shimatani Y, Nishioka K, Umemura T, Nakamura S, Yoshida M, Sato H. Myocardial perfusion and fatty acid metabolism in patients with takotsubo-like left ventricular dysfunction. J Am Coll Cardiol. 2003;41:743–8. doi: 10.1016/s0735-1097(02)02924-8. [DOI] [PubMed] [Google Scholar]
- 23.Fazio G, Sarullo FM, Novo G, Evola S, Lunetta M, Barbaro G, Sconci F, Azzarelli S, Akashi Y, Fedele F, Novo S. Tako-Tsubo cardiomyopathy and microcirculation. J Clin Monit Comput. 2010;24:101–5. doi: 10.1007/s10877-009-9217-5. [DOI] [PubMed] [Google Scholar]
- 24.Novo G, Quagliana A, Buccheri D, Rizzo S, Giambanco S, Giambanco F, Evola S, Geraci G, Novo S. Characteristics of coronary microcirculatory function in patients with Takotsubo syndrome. J Thorac Dis. 2017;9:4531–7. doi: 10.21037/jtd.2017.10.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Warisawa T, Naganuma T, Nakamura S. Reversible microvascular dysfunction in Takotsubo syndrome shown using index of microcirculatory resistance. Circ J. 2016;80:750–2. doi: 10.1253/circj.CJ-15-1283. [DOI] [PubMed] [Google Scholar]
- 26.Daniels DV, Fearon WF. The index of microcirculatory resistance (IMR) in Takotsubo cardiomyopathy. Catheter Cardiovasc Interv. 2011;77:128–31. doi: 10.1002/ccd.22599. [DOI] [PubMed] [Google Scholar]
- 27.Cuisset T, Quilici J, Pankert M, Fourcade L, Poyet R, Lambert M, Bonnet JL. Usefulness of index of microcirculatory resistance to detect microvascular dysfunction as a potential mechanism of stress-induced cardiomyopathy (Tako-Tsubo syndrome) Int J Cardiol. 2011;153:e51–3. doi: 10.1016/j.ijcard.2011.02.028. [DOI] [PubMed] [Google Scholar]
- 28.Morrow AJ, Nordin S, O’Boyle P, Berry C. ‘Acute micro-coronary syndrome’: detailed coronary physiology in a patient with Takotsubo cardiomyopathy. BMJ Case Rep. 2019;12:e229618. doi: 10.1136/bcr-2019-229618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abdelmoneim SS, Mankad SV, Bernier M, Dhoble A, Hagen ME, Ness SA, Chandrasekaran K, Pellikka PA, Oh JK, Mulvagh SL. Microvascular function in Takotsubo cardiomyopathy with contrast echocardiography: prospective evaluation and review of literature. J Am Soc Echocardiogr. 2009;22:1249–55. doi: 10.1016/j.echo.2009.07.012. [DOI] [PubMed] [Google Scholar]
- 30.Wittstein IS. Why age matters in Takotsubo syndrome. J Am Coll Cardiol. 2020;75:1878–81. doi: 10.1016/j.jacc.2020.03.030. [DOI] [PubMed] [Google Scholar]
- 31.Niccoli G, Camici PG. Myocardial infarction with non-obstructive coronary arteries: what is the prognosis? Eur Heart J Suppl. 2020;22:E40–E5. doi: 10.1093/eurheartj/suaa057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kume T, Akasaka T, Kawamoto T, Yoshitani H, Watanabe N, Neishi Y, Wada N, Yoshida K. Assessment of coronary microcirculation in patients with Takotsubo-like left ventricular dysfunction. Circ J. 2005;69:934–9. doi: 10.1253/circj.69.934. [DOI] [PubMed] [Google Scholar]
- 33.Anderson JL, Horne BD, Le VT, Bair TL, Min DB, Minder CM, Dhar R, Mason S, Muhlestein JB, Knowlton KU. Spectrum of radionuclide perfusion study abnormalities in Takotsubo cardiomyopathy. J Nucl Cardiol. 2020 doi: 10.1007/s12350-020-02385-w. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 34.Sharkey SW, Lesser JR, Menon M, Parpart M, Maron MS, Maron BJ. Spectrum and significance of electrocardiographic patterns, troponin levels, and thrombolysis in myocardial infarction frame count in patients with stress (Tako-Tsubo) cardiomyopathy and comparison to those in patients with ST-elevation anterior wall myocardial infarction. Am J Cardiol. 2008;101:1723–8. doi: 10.1016/j.amjcard.2008.02.062. [DOI] [PubMed] [Google Scholar]
- 35.Khalid N, Iqbal I, Coram R, Raza T, Fahsah I, Ikram S. Thrombolysis in myocardial infarction frame count in Takotsubo cardiomyopathy. Int J Cardiol. 2015;191:107–8. doi: 10.1016/j.ijcard.2015.04.192. [DOI] [PubMed] [Google Scholar]
- 36.Loffi M, Santangelo A, Kozel M, Kocka V, Budesinsky T, Lisa L, Tousek P. Takotsubo cardiomyopathy: one more angiographic evidence of microvascular dysfunction. Biomed Res Int. 2018;2018:5281485. doi: 10.1155/2018/5281485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bayon J, Santas-Alvarez M, Ocaranza-Sanchez R, Gonzalez-Juanatey C. Assessment with intracoronary pressure and flow guidewire, at baseline and after intracoronary adenosine infusion, in a patient with Takotsubo syndrome. Rev Port Cardiol. 2019;38:829, e1–e3. doi: 10.1016/j.repc.2017.08.014. [DOI] [PubMed] [Google Scholar]
- 38.Montone RA, Galiuto L, Meucci MC, Del Buono MG, Vergni F, Camilli M, Sanna T, Pedicino D, Buffon A, D’Amario D, Giraldi L, Trani C, Liuzzo G, Rebuzzi AG, Niccoli G, Crea F. Coronary slow flow is associated with a worse clinical outcome in patients with Takotsubo syndrome. Heart. 2020;106:923–30. doi: 10.1136/heartjnl-2019-315909. [DOI] [PubMed] [Google Scholar]
- 39.Ozaki Y, Gonzalo N, Salazar CH, Kuku KO, Mejia-Renteria H, Hideo-Kajita A, Nunez-Gil IJ, Escaned J, Waksman R, Garcia-Garcia HM. Comparison of quantitative flow ratio value of left anterior descending and circumflex coronary artery in patients with Takotsubo syndrome. Int J Cardiovasc Imaging. 2020;36:3–8. doi: 10.1007/s10554-019-01703-9. [DOI] [PubMed] [Google Scholar]
- 40.Meimoun P, Malaquin D, Sayah S, Benali T, Luycx-Bore A, Levy F, Zemir H, Tribouilloy C. The coronary flow reserve is transiently impaired in tako-tsubo cardiomyopathy: a prospective study using serial Doppler transthoracic echocardiography. J Am Soc Echocardiogr. 2008;21:72–7. doi: 10.1016/j.echo.2007.05.024. [DOI] [PubMed] [Google Scholar]
- 41.Rigo F, Sicari R, Citro R, Ossena G, Buja P, Picano E. Diffuse, marked, reversible impairment in coronary microcirculation in stress cardiomyopathy: a Doppler transthoracic echo study. Ann Med. 2009;41:462–70. doi: 10.1080/07853890903022793. [DOI] [PubMed] [Google Scholar]
- 42.Galiuto L, De Caterina AR, Porfidia A, Paraggio L, Barchetta S, Locorotondo G, Rebuzzi AG, Crea F. Reversible coronary microvascular dysfunction: a common pathogenetic mechanism in Apical Ballooning or Tako-Tsubo syndrome. Eur Heart J. 2010;31:1319–27. doi: 10.1093/eurheartj/ehq039. [DOI] [PubMed] [Google Scholar]
- 43.Rivero F, Cuesta J, Garcia-Guimaraes M, Bastante T, Alvarado T, Antuna P, Alfonso F. Time-related microcirculatory dysfunction in patients with Takotsubo cardiomyopathy. JAMA Cardiol. 2017;2:699–700. doi: 10.1001/jamacardio.2016.5993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Heyse A, Milkas A, Van Durme F, Barbato E, Lazaros G, Vanderheyden M, Bartunek J. Pitfalls in coronary artery stenosis assessment in Takotsubo syndrome: the role of microvascular dysfunction. Hellenic J Cardiol. 2018;59:290–2. doi: 10.1016/j.hjc.2017.10.003. [DOI] [PubMed] [Google Scholar]
- 45.Gerbaud E, Montaudon M, Leroux L, Corneloup O, Dos Santos P, Jais C, Coste P, Laurent F. MRI for the diagnosis of left ventricular apical ballooning syndrome (LVABS) Eur Radiol. 2008;18:947–54. doi: 10.1007/s00330-008-0853-9. [DOI] [PubMed] [Google Scholar]
- 46.Brewington SD, Abbas AA, Dixon SR, Grines CL, O’Neill WW. Reproducible microvascular dysfunction with dobutamine infusion in Takotsubo cardiomyopathy presenting with ST segment elevation. Catheter Cardiovasc Interv. 2006;68:769–74. doi: 10.1002/ccd.20514. [DOI] [PubMed] [Google Scholar]
- 47.Nef HM, Mollmann H, Kostin S, Troidl C, Voss S, Weber M, Dill T, Rolf A, Brandt R, Hamm CW, Elsasser A. Tako-Tsubo cardiomyopathy: intraindividual structural analysis in the acute phase and after functional recovery. Eur Heart J. 2007;28:2456–64. doi: 10.1093/eurheartj/ehl570. [DOI] [PubMed] [Google Scholar]
- 48.Y-Hassan S. Tight coronary artery stenosis and Takotsubo syndrome triggered each other: well-illustrated in a case. Cardiovasc Revasc Med. 2018;19:2–4. doi: 10.1016/j.carrev.2018.05.012. [DOI] [PubMed] [Google Scholar]
- 49.Y-Hassan S. Acute cardiac sympathetic disruption and left ventricular wall motion abnormality in Takotsubo syndrome. Acute Card Care. 2015;17:24–5. doi: 10.3109/17482941.2014.989858. [DOI] [PubMed] [Google Scholar]
- 50.Migliore F, Maffei E, Perazzolo Marra M, Bilato C, Napodano M, Corbetti F, Zorzi A, Andres AL, Sarais C, Cacciavillani L, Favaretto E, Martini C, Seitun S, Cademartiri F, Corrado D, Iliceto S, Tarantini G. LAD coronary artery myocardial bridging and apical ballooning syndrome. JACC Cardiovasc Imaging. 2013;6:32–41. doi: 10.1016/j.jcmg.2012.08.013. [DOI] [PubMed] [Google Scholar]


