Abstract
Objectives
This study sets out to ascertain if recognition of delirium impacts on patient outcomes.
Design
Retrospective cohort study.
Setting
Unscheduled admissions to acute care trust/secondary care UK hospitals.
Participants
Six hundred and fifty-six older adults aged ≥65 years admitted on 14 September 2018.
Measurements
Delirium was ascertained retrospectively from case notes using medical notes. Documented delirium was classified as recognised delirium and retrospectively ascertained delirium was classified as unrecognised delirium.
Primary and secondary outcome measures
Primary outcome measure: inpatient mortality. Secondary outcome measures: length of stay, discharge destination.
Results
Delirium was present in 21.1% (132/626) of patients at any point during admission. The presence of delirium was associated with increased mortality (HR 2.65, CI 1.40 to 5.01). Recognition of delirium did not significantly impact on outcomes.
Conclusions
Delirium is associated with adverse outcomes in hospitalised older adults. However, there is insufficient evidence that recognition of delirium affects outcomes. However, delirium recognition presents an opportunity to discuss a person’s overall prognosis and discuss this with the patient and their family. Further research is needed to assess the pathophysiology of delirium to enable development of targeted interventions towards improved outcomes in patients with delirium.
Keywords: delirium & cognitive disorders, geriatric medicine, medical education & training
Strengths and limitations of this study.
Our unique retrospective approach has enabled true determination of the effect of delirium recognition on outcomes.
Collaborative research has enabled collection of data from multiple sites across the UK.
We adjusted for variables including age, gender, dementia status, frailty and specialty; we were unable to adjust for disease or delirium severity.
Introduction
Delirium is a common neuropsychiatric manifestation of physical precipitants (including acute illness, medications, trauma and surgery) and systemic inflammation.1 The presence of delirium is known to be associated with increased risk of adverse outcomes (twofold increased mortality,2–4 increased length of hospital stay3–5 and increased risk of care home placement2 3). Outcomes have been shown to be worse with longer duration of delirium.6 There is good quality evidence that multifactorial interventions can be used to prevent delirium in at-risk individuals,7 8 but there is no known single intervention for the treatment of delirium, beyond treating recognised precipitants.8 9
Despite this, delirium is known to be frequently under-recognised10; only a third of cases were recognised in our previous prospective study of delirium point prevalence in unscheduled admissions of older adults.4 International programmes strive to increase delirium recognition.11 This is important to enable explanation of diagnosis and prognosis to patients, relatives and carers. However, the effect of recognition of delirium on clinical outcomes has been unknown. One previous single-site study demonstrated increased adverse outcomes for patients with delirium who were discharged early.12 Prospective studies often lead to recognition of delirium by nature of their study design; thus, the effect of recognition cannot truly be evaluated.4 This study aimed to evaluate the effect of recognition of delirium on adverse outcomes using a retrospective cohort design.
Methods
Cohort identification
This was a multicentre retrospective cohort study within the UK using case notes review. Patients were identified through consult with site patient record and informatics teams. We included patients aged 65 years and older, who were admitted on 14 September 2018 across all sites as unscheduled admissions, with lengths of stay of 2 days or greater. This project was performed as a substudy within a larger quality improvement project; the date were chosen as it was 6 months before and after the dates of separate prospective data collection. We excluded patients who were admitted to critical care during their admission or who were admitted electively. Delirium is known to be common in patients admitted to critical care but requires a separate screening process,13 and our retrospective ascertainment has not been validated in this group.
Retrospective delirium ascertainment
Data collectors were clinicians with expertise in delirium diagnosis. Data collectors reviewed case notes from the admission to assess for documentation of a delirium diagnosis by the clinical team. If a delirium diagnosis was made at any stage, this was classified as recognised delirium and assumed to be a true diagnosis. If there was no diagnosis of delirium, data collectors proceeded to retrospectively ascertain if there was evidence of delirium through the clinical notes. Probable delirium diagnosed from case notes vignettes has been shown to be sensitive to identification of prospectively diagnosed delirium.14 Ascertainment of delirium status was based on the Diagnostic and Statistical Model of Diseases, fifth edition (DSM-5) (figure 1).15 Change in awareness is not required as part of the relaxed DSM-5 definition.16 As inattention is more difficult to identify retrospectively if screening has not been performed, we used a relaxed definition requiring the presence of disturbances in either attention or awareness. Our approach was previously piloted in a single site as part of another study, with excellent agreement between multiple data collectors.17 If patients met some but not all criteria for DSM-5 delirium, then a diagnosis of possible delirium was recorded.14 Data collectors recorded whether delirium was prevalent (present on admission) or incident (acquired during their hospital stay). The subtype and delirium duration were recorded from case notes review where possible. Delirium ascertained retrospectively was classified as unrecognised delirium. Data collectors also recorded if patients had been screened for delirium within 48 hours of admission (regardless of presence/ absence of delirium) and whom screening was performed by (training doctor below registrar level/geriatric medicine registrar or consultant/general medicine registrar or consultant/surgical registrar or consultant/nurse or allied health professional). We did not collect information on the screening tools used.
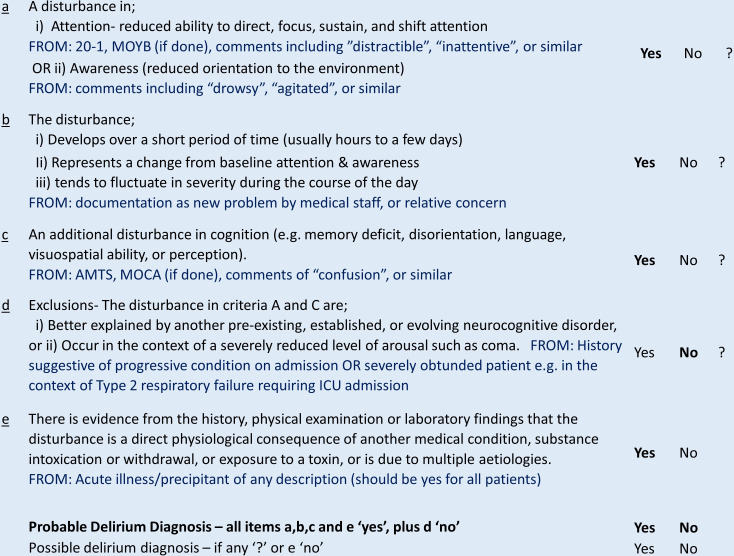
Figure 1.
Criteria used for retrospective delirium diagnosis as adapted from DSM-5. A diagnosis of probable delirium was made in retrospective case notes reviewed in patients who satisfied criteria of ‘yes’ to a, b, c and e, and ‘no’ to d. DSM-5, Diagnostic and Statistical Model of Diseases, fifth edition. AMTS = Abbreviated Mental Test Score; MOCA = Montreal Cognitive Assessment
Other variables recorded
Age, gender, frailty, dementia and main specialty during admission were all recorded from inpatient notes or local hospital electronic data collection. Clinical Frailty Scale (CFS)18 was retrospectively ascertained by data collectors from the inpatient clinical notes using information available on social and functional history recorded by the clinical team (doctors, nurses or allied health professionals involved in their care). Dementia status was recorded based on documentation of known history or high clinical probability considered by data collectors. Data collectors made a clinical diagnosis of probable dementia if there was documentation of pre-existent cognitive decline affecting the patient’s activities of daily living, but a formal diagnosis had not been made. Specialty was recorded as one of seven groups: acute medicine, geriatric medicine, stroke medicine, other medicine, orthopaedic surgery, general surgery or other surgery. Data on length of hospital stay, mortality and discharge location were collected up until 1 month after admission. Each site also provided data on if their site had a specialised delirium team, a geriatric medicine service embedded into the admissions unit, a delirium assessment tool in the clerking booklet, local delirium guidelines or a local delirium patient/carer leaflet at the time of the study.
Central data collation
Individual hospital sites were required to register to participate in this study via REDCap; REDCap is a secure browser-based web application that ensures enables protected collation of data. All data collected via REDCap were fully anonymised. Data upload forms were formatted, so that data could only be uploaded in the prespecified formats.
Statistical analysis
Statistical analysis was performed using IBM SPSS Statistics for Windows, V.22 (IBM, Armonk, New York, USA). Descriptive statistics were reported as mean, SD and frequencies. Probable dementia was considered as dementia for data analysis. Frailty was analysed as CFS 1–3 (robust), 4–6 (prefrailty/ frailty) and 7–9 (advanced frailty/end of life). We used logistic regression to determine factors that were predictive of screening and recognition. We used binary logistic regression and Cox regression to assess if the presence of delirium was predictive of inpatient death compared with no delirium. We then used binary logistic regression and Cox regression to assess whether unrecognised delirium was predictive of inpatient death as compared with recognised delirium. The same approach was used to assess if recognition of delirium was predictive of new institutionalisation (discharge to a new care home), by first assessing the effect of delirium overall and then recognition. Length of stay was log10 transformed to obtain a normal distribution, and linear regression was used to analyse the effect of delirium and the effect of delirium recognition. Delirium duration was also log transformed with linear regression used to assess the effect of delirium recognition on delirium duration. Variables included in multivariable analysis were age, gender, dementia status, frailty and specialty. Additional models were analysed with subtype and duration of delirium as additional variables.
Patient and public involvement
Prior to conduct of this study, the investigators held multiple discussion groups with both healthy older adults and older adults who had previously been hospitalised as well as their carers. Healthy older adults expressed that delirium would be a condition of particular concern and one of their greatest anxieties around being admitted to hospital. Relatives of patients who had been hospitalised with delirium reported that it was a frightening experience, with concerns about how long it would continue and whether their relative was likely to improve.19 Data collection was performed from case notes, so there was no increased burden to patients in this study. Results were disseminated alongside increasing awareness of delirium to members of the general public on World Delirium Awareness Day using local stands at participating sites.
Results
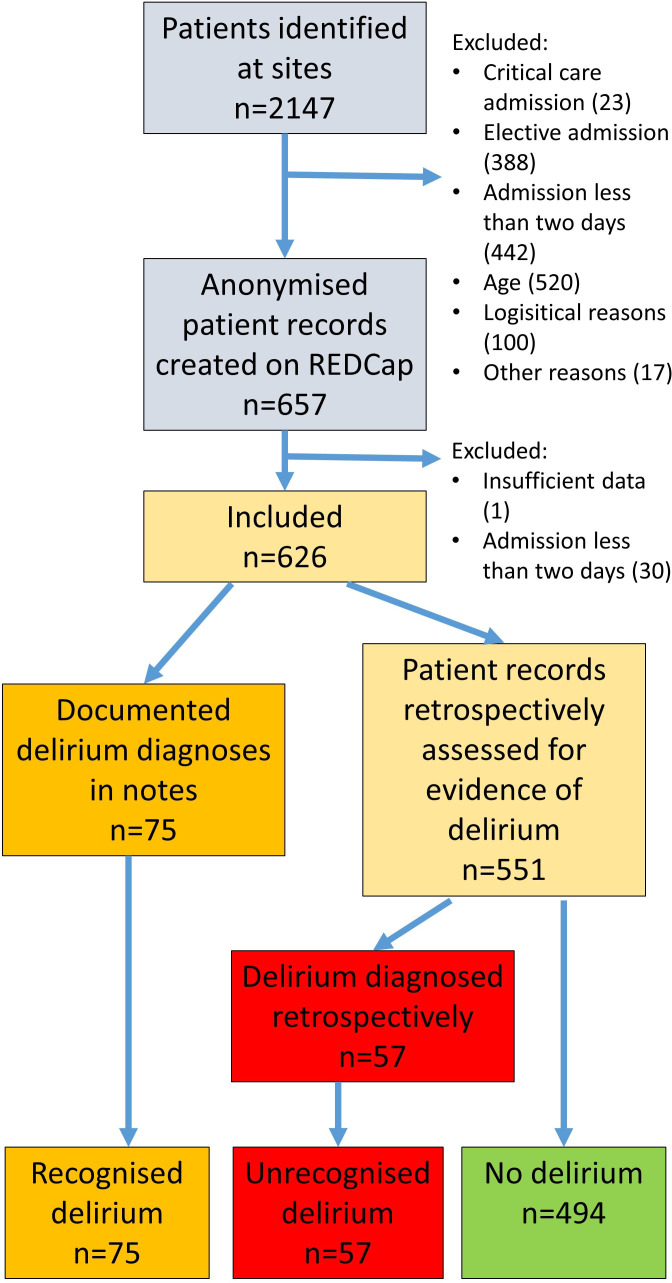
A total of 2147 patients were identified across 27 different UK hospitals. Reasons for exclusion were critical care admission (23), elective admission (388), admission less than 2 days (442), age (520), logistical reasons (100) and other nondeclared reasons (17). A further patient was excluded from analysis due to incomplete data upload and 30 patients due to admission less than 2 days. A total of 626 patients from 27 different hospitals were included. Figure 2 shows the flowchart of patient data inclusion within this study.
Figure 2.
Flowchart of patient identification and delirium diagnosis. Case notes were reviewed in 656 patients, of whom 75 had a recognised diagnosis of delirium and a further 57 were considered to have unrecognised delirium.
The mean age was 80.3 (SD 8.2) and 53.8% (337/626) was women. The majority (77.0%; 482/626) was admitted under medical specialties, the remainder being admitted under surgical specialties (30.0%; 144/626). Considering frailty, 29.6% (185/625) were classified as robust (CFS 1–3), 52.9% (331/625) were classified as prefrail/frail (CFS 4–6) and 17.4% (109/625) were classified as advanced frail/end of life (CFS 7–9). Dementia was present in 17.3% (108/626) and a further 2.9% (18/626) had probable dementia. Demographics for all patients overall and separated by delirium status are shown in table 1.
Table 1.
Demographics of patients included in study
| All | No delirium | Delirium | P value | |
| Age (mean, SD) | 80.3 (8.2) | 79.6 (8.2) | 82.9 (8.1) | <0.001 |
| Gender (% female) | 53.8% (337) | 54.9% (271) | 50.0% (66) | 0.320 |
| Dementia (known/probable %) | 20.2% (126) | 14.4% (71) | 41.7% (55) | <0.001 |
| Clinical frailty scale | ||||
| 1–3 | 29.6% (185) | 34.9% (172) | 9.8% (13) | <0.001 |
| 4–6 | 53.0% (331) | 52.1% (257) | 56.1% (74) | |
| 7–9 | 17.4% (109) | 13.0% (64) | 34.1% (45) | |
| Specialty | ||||
| Acute medicine | 19.5% (122) | 19.0% (94) | 21.2% (28) | <0.001 |
| Geriatric medicine | 25.7% (161) | 20.4% (101) | 45.5% (60) | |
| Stroke | 4.3% (27) | 5.3% (26) | 0.8% (1) | |
| Other medicine | 27.5% (172) | 29.1% (144) | 21.2% (28) | |
| Other surgery | 6.7% (42) | 8.3% (41) | 0.8% (1) | |
| General surgery | 7.7% (48) | 8.5% (42) | 4.5% (6) | |
| Orthopaedic surgery | 8.6% (54) | 9.3% (46) | 6.1% (8) | |
Patients with delirium were older, more likely to have dementia, and more likely to be frail compared with those without delirium. The prevalence of delirium in patients admitted other surgical specialties other than general or orthopaedic was lower than across other specialties.
Delirium prevalence and incidence
Delirium was present in 21.1% (132/626) at some point during their admission; 4.5% (28/626) incident cases and 16.6% (105/626) prevalent cases. Prevalence at individual sites is available online (online supplemental table S1). Delirium was documented in the notes of 56.8% (75/132) of cases, the remainder (43.2%; 57/132) being diagnosed retrospectively. A further 2.4% (15/626) had evidence of possible delirium on retrospective notes analysis. Considering subtype, 33.3% (44/132) were hypoactive, 31.1% (41/132) were hyperactive, 12.9% (17/132) were mixed and 22.7% (30/132) had no clear motor subtype. The median duration of delirium was 5 days (IQR 3–11). In adjusted models, the presence of dementia (OR 2.51, CI 1.53 to 4.13; p<0.001) and increasing frailty status (CFS 4–6: OR 2.61, CI 1.34 to 5.05; p=0.004; CFS 7–9: OR 4.04, CI 1.88 to 8.71; p<0.001) were associated with increased odds of delirium (online supplemental table S2). There were reduced odds of delirium in patients admitted to other surgery specialties (not general or orthopaedic) (OR 0.10, CI 0.01 to 0.75; p=0.026) and stroke specialties (OR 0.12, CI 0.02 to 0.92; p=0.042).
bmjopen-2020-042440supp001.pdf (105.1KB, pdf)
Screening and recognition
Overall, 30.4% (190/626) were screened for delirium within 48 hours of admission. Where screening was performed, 46.2% (85/184) were performed by a doctor less senior to registrar level (foundation year 1 through to core medical training year 2), 33.2% (61/184) were performed by a nurse or allied health professional, 9.8% (18/184) were performed by a geriatric medicine registrar or consultant, 8.7% (16/184) were performed by a registrar or consultant in another medical specialty and 2.2% (4/184) were performed by a surgery registrar or consultant. The presence of dementia (OR 1.63, CI 1.01 to 2.61; p=0.044) and increased age (OR 1.03 per year of life, CI 1.00 to 1.05; p=0.031) were associated with increased odds of delirium screening. Admission under general surgery (OR 0.41, CI 0.18 to 0.98; p=0.045) and other medicine specialties (OR 0.56, CI 0.33 to 0.97; p=0.039) were associated with reduced odds of screening (online supplemental table S3).
Of those patients who were considered to have delirium through either documentation in the notes or retrospective identification, delirium was considered to be recognised in 56.8% (75/132). Recognition rates at individual sites are available online (online supplemental table S1). Screening for delirium was associated with increased odds of recognition (OR 5.05, CI 2.19 to 11.65; p<0.001) and this was not affected by grade or profession of screener. Recognition was not affected by age, gender, dementia status, frailty or specialty (table 2).
Table 2.
Logistic regression of variables predictive of delirium being recognised
| Beta | SE | Wald | Freedom | OR | CI | P value | ||
| Lower | Upper | |||||||
| Screening | 1.62 | 0.43 | 14.43 | 1 | 5.05 | 2.19 | 11.65 | <0.001* |
| Grade of screener | 1.60 | 3 | 0.659 | |||||
| Age | −0.03 | 0.03 | 1.03 | 1 | 0.97 | 0.92 | 1.03 | 0.309 |
| Gender | 0.53 | 0.41 | 1.65 | 1 | 1.70 | 0.76 | 3.80 | 0.199 |
| Dementia | 0.19 | 0.48 | 0.15 | 1 | 1.20 | 0.47 | 3.09 | 0.700 |
| Frailty | 1.348 | 2 | 0.510 | |||||
| Specialty | 7.00 | 6 | 0.324 | |||||
Screening for delirium was associated with nearly five-fold increased likelihood of delirium recognition. The grade or profession of the screener did not impact on the chances of delirium being recognised. Recognition was not affected by age, gender, dementia, frailty or specialty.
Effect of delirium on outcomes
Delirium was associated with increased odds of inpatient mortality in both univariable (OR 4.74, CI 2.56 to 8.76; p<0.001) and multivariable (OR 3.27, CI 1.65 to 6.48; p<0.001) analysis (online supplemental table S4). These results were duplicated in time to event analysis in multivariable analysis (HR 2.65, CI 1.40 to 5.01; p<0.001) (table 3). The presence of delirium was associated with increased odds of new discharge to a care home in univariable (OR 2.57, CI 1.08 to 6.14; p=0.033) but not multivariable (OR 1.26, CI 0.48 to 3.36; p=0.639) analysis (online supplemental table S5). Length of stay did not significantly differ in patients with delirium compared with those without (online supplemental tables S6, S7).
Table 3.
Cox regression for the association of delirium and delirium recognition with inpatient mortality
| Beta | SE | Wald | Freedom | HR | CI | P value | ||
| Lower | Upper | |||||||
| Delirium unadjusted | 1.14 | 0.30 | 14.43 | 1 | 3.13 | 1.74 | 5.65 | <0.001* |
| Delirium adjusted* | 0.98 | 0.33 | 9.02 | 1 | 2.65 | 1.40 | 5.01 | 0.003* |
| Recognition unadjusted | −0.61 | 0.41 | 2.17 | 1 | 0.55 | 0.24 | 1.22 | 0.141 |
| Recognition adjusted† | −0.38 | 0.47 | 0.68 | 1 | 0.68 | 0.27 | 1.70 | 0.411 |
| Recognition adjusted‡ | −0.33 | 0.55 | 0.36 | 1 | 0.72 | 0.24 | 2.12 | 0.547 |
The presence of delirium was associated with increased risk of inpatient death in both univariable and multivariable analyses. Recognition of delirium did not statistically significantly impact on risk of inpatient mortality. The ORs represent the likelihood of death with recognised delirium compared with unrecognised delirium.
*Delirium adjusted for age, gender, dementia status, frailty and specialty.
†Recognition adjusted for age, gender, dementia status, frailty and specialty.
‡Recognition adjusted for variables above, duration, and subtype.
Effect of delirium recognition on outcomes
Recognition of delirium did not impact on the risk of inpatient mortality in univariable or multivariable analysis in either logistic regression or time to event analysis in a statistically significant manner (HR 0.72, CI 0.24 to 2.12; p=0.547) (table 3 and online supplemental table S8). Similarly, recognition did not statistically significantly impact on the odds of new discharge to a care home (OR 2.59, CI 0.16 to 41.43; p=0.501) (online supplemental table S9) or length of hospital stay in univariable or multivariable analyses (online supplemental tables S10 and S11). However, recognition of delirium was associated with an increased duration of delirium compared with unrecognised delirium (+1.55 days, CI 1.10 to 2.19; p=0.012) (online supplemental tables S12 and S13). Inclusion of delirium duration and subtype in multivariable analysis did not affect the impact of recognition on mortality, length of stay or new discharge to a care home.
Discussion
This study has confirmed previous findings that the prevalence of delirium was associated with increased risk of adverse outcomes2–4 and increased risk of inpatient mortality. This effect is demonstrated even when accounting for other variables, suggesting that all things being equal, a patient with delirium is more likely to suffer from adverse outcomes just through way of having delirium. Previous research has also shown that delirium is associated with increased risk of a later life diagnosis of dementia,20 and importantly it can be highly distressing for the patient and their relative.19 21 Delirium can be a devastating condition and prevention should be of the utmost importance, particularly in frail vulnerable older adults. Our results differed from our previous study of delirium prevalence in not showing a significant increased length of stay4; this likely relates to the exclusion of patients with lengths of stay less than 2 days.
Despite this, the results of our study did not show any significant impact of recognition of delirium on outcomes. However, in patients where confusion and disorientation were named and recognised, but not specifically diagnosed as delirium, healthcare professionals may have been able to implement similar treatment strategies as they would had the term delirium been used. Patients in whom a new change in cognition, alertness or attentiveness was not recognised to any extent to have been documented represent a particular subset of under-recognition that may be at heightened risk. It is also important to note that we did not measure illness severity or delirium severity in this study. It is possible that more severe cases of delirium may have been more likely to be recognised, and previous research suggests that increasing severity of delirium may be associated with increased risk of adverse outcomes.22 Thus, if recognised cases of delirium presented more severe cases, any positive effect of recognition may have been ameliorated by higher risk related to severity.
In addition, it was found that recognition was associated with increased delirium duration. It is probable that, rather than recognition causing delirium to last longer, longer lasting cases of delirium were more likely to be recognised. As longer delirium duration has been shown to be associated with worse outcomes,6 this may have tempered our results, although inclusion of delirium duration in multivariable analysis did not affect the overall impact of recognition on outcomes. There is currently no known treatment for delirium in itself and the mainstay of treatment focuses on treatment of the underlying precipitant(s). There is currently insufficient evidence that multicomponent interventions, which have been shown to prevent delirium, are effective for treatment of delirium.8 At present, the focus of quality improvement strategies should be on prevention of incident delirium; further research to determine the pathophysiology of delirium may enable targeted treatment in the future.23
We acknowledge that our study may have been underpowered to detect a statistically significant difference in mortality between recognised and unrecognised delirium. In a post hoc power calculation, a sample size of 75 would detect a 10% difference in mortality between groups with power of 0.67% and 10% alpha. We encourage the development of further studies to assess whether these results are duplicated in larger powered studies, in other settings, and in the incorporation of our results into future systematic reviews on this subject.
The overall prevalence of delirium was higher than our previous UK multicentre study of delirium4 and is more closely concordant with prevalence studies performed elsewhere.10 This may be related to the inclusion of incident as well as prevalent cases of delirium; our previous UK study included only prevalent cases, whereas point prevalence studies elsewhere have included both incident and prevalent cases. The incidence of delirium in this study was actually lower than has been shown in previous studies.7 24 This may relate to implementation of multicomponent interventions to prevent delirium at individual sites or may relate to differences in population. Many incidence studies have previously been conducted on elective patients,25 where all cases of delirium are considered incident. Screening rates were similar to that which has been shown previously although recognition rates were higher.4
We recognise that there are limitations to the use of retrospective methodology to diagnose delirium, although this approach has been previously validated against expert diagnosis.14 Overall, this approach is more likely to miss cases rather than lead to false diagnoses; the true delirium prevalence may be even greater. In addition, where delirium was documented in the notes, we assumed this to be a true diagnosis. However, it is possible that some of these may not have met full criteria for delirium through prospective expert review. It would be unethical to conduct a prospective study to evaluate the effects of delirium recognition. However, our previous prospective study did not show any impact of recognition by the usual care team prior to screening by study staff.4
As described, the diagnosis of delirium is based on psychiatric criteria, although delirium itself is caused by physical precipitants. The psychiatric presentation of delirium may not correlate with the underlying biological processes. Thus, identification of the underlying biological processes may be more beneficial in enabling targeting of interventions. Further research evaluating the use of techniques such as electroencephalogram studies is needed.26 Nevertheless, we consider that our methodology demonstrates feasibility in diagnosing delirium retrospectively from medical notes, which would not be possible using a biological definition. This enables the determination of the effect of delirium on outcomes in studies where this was not measured prospectively.17
Conclusion
This study has demonstrated novel and important results. Our finding that recognition of delirium did not impact on outcomes demonstrates why prevention of delirium is vitally important,8 as the negative effects of delirium are not easily ameliorated once it occurs. Although we have not shown any effect of recognition on the outcomes measured, we emphasise that recognition remains important to offer an opportunity to explain the nature of the diagnosis to the patient and their relatives19 21 and assist with prognostication.20 We recommend that clinicians should use the word delirium rather than words such as confusion or agitation in order to ensure consistency in language and to enable clinical coding of diagnosis. Further research is needed to assess the pathophysiology of delirium to enable development of targeted interventions towards improved outcomes in patients with delirium.
Supplementary Material
Footnotes
Twitter: @Gemresearchuk
Collaborators: Geriatric Medicine Research Collaborative: Phillipa Adams (Gateshead Health NHS Foundation Trust) Olugbenro Akintade (North West Anglia NHS Foundation Trust) Mustafa Alsahab (Oxford University Hospitals NHS Trust) Thomas Arkle (Scarborough General Hospital) Imola Bargaoanu (Scarborough General Hospital) Amr Bazaraa (Bradford Teaching Hospitals NHS Foundation Trust) Laura J Beeley (Derriford Hospital) Jenni Burton (University of Glasgow) Swetha Byravan (Royal Wolverhampton NHS Trust) Natalie Cox (University of SouthamptonJodie Crofts (Barnsley District General Hospital) Elizabeth J Ellis (North West Anglia Foundation Trust) Rinata Farah (Great Western Hospital) Victoria Gaunt (Gloucestershire Hospitals NHS Trust) Jane Giddings (University Hospitals of Leicester NHS Trust) Kumudhini Giridharan (East Kent Hospitals University NHS Foundation Trust) Robert Grange (Great Western Hospital)Olivia Handley (Poole Hospital) Alexander Harbinson (Bradford Teaching Hospitals NHS Foundation Trust) John Headlam (Bradford Teaching Hospitals NHS Foundation Trust) Roisin Healy (South Eastern Trust) Fiona Herbert (Great Western Hospital) Elisabeth Hunter (Bradford Teaching Hospitals NHS Foundation Trust) Sophie J Irwin (Gateshead Health NHS Foundation Trust) Chioma Iwu (North West Anglia NHS Trust) Thomas A Jackson (University of Birmingham) Sarah Jagdeo (Royal Wolverhampton NHS Trust) Benjamin Jelley (Cardiff and Vale University Health Board) Lindsay Jones (University of Exeter) Vee-Han Lim (Bolton NHS Foundation Trust) Elizabeth Lonsdale-Eccles (Great Western Hospital) James E Lucocq (NHS Tayside) Fiona E Macdonald (Poole Hospital) Al Wakkass Mahmood (Bradford Teaching Hospitals NHS Foundation Trust) Zeinab Majid (University of Birmingham) Jane Masoli (University of Exeter)Yathu Matheswaran (University Hospitals Birmingham NHS TrustLauren McCluskey (University of Birmingham) Gerry McGonigal (York District Hospital) Emily McNicholas (Barnsley District General Hospital) Christopher Miller (University Hospitals of Leicester NHS Trust) Shonit Nagumantry (North West Anglia NHS Foundation Trust) Nader Nashed (North West Anglia NHS Foundation Trust) Liji Ng (Bradford Teaching Hospitals NHS Foundation Trust) Mary Ni Lochlainn (Guy’s and St Thomas’ NHS Trust) Philip Nwabufor (North West Anglia NHS Foundation Trust) Ijeoma T Obi (North West Anglia NHS Foundation Trust) Minal D Patel (University Hospitals of Leicester NHS Trust) Anna Reay (York District Hospital) Sarah Richardson (University of Newcastle) Hannah J Robinson (Barnsley District General Hospital) Anekea Ross (University Hospitals Birmingham NHS Trust) Aidan Ryan (Guy’s and St Thomas’ NHS Trust) Dhruv Sarma (Guy’s and St Thomas’ NHS Trust) Lahiru Satharasinghe (University Hospitals of Leicester NHS Trust) Hussun-Ara Shah (University Hospital North Durham) Simon M Stapley (York District Hospital) Srividya Sundara (Bradford Teaching Hospitals NHS Foundation Trust) Joanne Taylor (University of Manchester) Thyn Thyn (Barnsley District General Hospital)Oliver Todd (Bradford Teaching Hospitals NHS Foundation Trust) Kelli Torsney (University of Cambridge) Philippa K Traill (NHS Tayside) Qurrat Ul Ain (Great Western Hospital) Tarunya Vedutla (University Hospitals Birmingham NHS Trust) Amy Walter (NHS Tayside) Hannah Watson (Great Western Hospital) Carly Welch (University of Birmingham) Alice Wheeler (Bradford Teaching Hospitals NHS Foundation Trust) Michael SJ Wilson (NHS Tayside) Daisy Wilson (University Hospitals Birmingham NHS Trust) Ruth Willott (University of Nottingham) Abigail Wrathall (NHS Tayside) Sophie Wright (Bradford Teaching Hospitals NHS Foundation Trust) Chenxian Wu (Guy’s and St Thomas’ NHS Trust).
Contributors: MA, JB, NC, VG, KG, RH, TAJ, BJ, JM, LM, GM, MNL, SR, HJR, JT, OT, KT, CW, DW and RW significantly contributed towards planning, conception, and design of the study. PA, OA, TA, IB, AB, LJB, SB, JC, EJE, RF, JG, RG, OH, AH, JH, FH, EH, SJI, CI, SJ, LJ, CL, EL, JEL, FEM, AM, ZM, YM, EM, CM, SN, NN, PN, ITO, MDP, AR, AR, AR, DS, LS, HS, SS, SS, TT, PT, QU, TV, AWr, HW, CW, AWh, MSJW, DW, AWa, SW, CW significantly contributed towards study conduct and acquisition of data. CW, LM, DVW significantly contributed towards data analysis. PA, OA, MA, TA, IB, AB, LJB, JB, SB, NC, JC, EJE, RF, VG, JG, KG, RG, OH, AH, JH, RH, FH, EH, SJI, CI, TAJ, SJ, BJ, LJ, CL, EL, JEL, FEM, AM, ZM, JM, YM, LM, GM, EM, CM, SN, NN, MN, PN, ITO, MDP, AR, SR, HJR, AR, AR, DS, LS, HS, SS, SS, JT, TT, OT, KT, PT, QU, TV, AWr, HW, CW, AWh, MSJW, DW, RW, AW, SW, CW significantly contributed towards reporting and interpretation of the data. All authors reviewed and agreed the final submitted version.
Funding: The research was sponsored by the University of Birmingham. This study received support for project delivery and coordination from the Birmingham Surgical Trials Consortium via core funding from the National Institute for Health Research (NIHR) Clinical Research Network West Midlands allocated to help support new research collaboratives. The sponsor was not involved in design, methods, subject recruitment, data collections, analysis or preparation of the manuscript. The views expressed in this manuscript are those of the authors are not the NIHR, National Health Service or Department of Health.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Contributor Information
Collaborators: Abigail Wrathall, Aidan Ryan, Al Wakkass Mahmood, Alexander Harbinson, Alice Wheeler, Amr Bazaraa, Amy Walter, Anekea Ross, Anna Reay, Benjamin Jelley, Carly Welch, Chenxian Wu, Chioma Iwu, Christopher Miller, Daisy Wilson, Dhruv Sarma, Elisabeth Hunter, Elizabeth J Ellis, Elizabeth Lonsdale-Eccles, Emily McNicholas, Fiona E Macdonald, Fiona Herbert, Gerry McGonigal, Hannah J Robinson, Hannah Watson, Hussun-Ara Shah, Ijeoma T Obi, Imola Bargaoanu, James E Lucocq, Jane Giddings, Jane Masoli, Jenni Burton, Joanne Taylor, Jodie Crofts, John Headlam, Kelli Torsney, Kumudhini Giridharan, Lahiru Satharasinghe, Laura J Beeley, Lauren McCluskey, Liji Ng, Lindsay Jones, Mary Ni Lochlainn, Michael SJ Wilson, Minal D Patel, Mustafa Alsahab, Nader Nashed, Natalie Cox, Oliver Todd, Olivia Handley, Olugbenro Akintade, Philip Nwabufor, Philippa K Traill, Phillipa Adams, Qurrat Ul Ain, Rinata Farah, Robert Grange, Roisin Healy, Ruth Willott, Sarah Jagdeo, Sarah Richardson, Shonit Nagumantry, Simon M Stapley, Sophie J Irwin, Sophie Wright, Srividya Sundara, Swetha Byravan, Tarunya Vedutla, Thomas A Jackson, Thomas Arkle, Thyn Thyn, Vee-Han Lim, Victoria Gaunt, Yathu Matheswaran, Zeinab Majid, and Carly Welch
Data availability statement
The anonymised database is available from the corresponding author upon reasonable request.
Ethics statements
Patient consent for publication
Not required.
Ethics approval
This study was registered with local governance teams for approval to participate in national audit led by the Geriatric Medicine Research Collaborative, as part of a wider multi-centre quality improvement project. Anonymised data were collated centrally. Ethical approval was granted from the University of Birmingham Science, Technology, Engineering and Mathematics Ethical Review Committee (ERN_18-1415A) for secondary data analysis of the anonymised database.
References
- 1.Fong TG, Tulebaev SR, Inouye SK. Delirium in elderly adults: diagnosis, prevention and treatment. Nat Rev Neurol 2009;5:210–20. 10.1038/nrneurol.2009.24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Witlox J, Eurelings LSM, de Jonghe JFM, et al. Delirium in elderly patients and the risk of postdischarge mortality, institutionalization, and dementia: a meta-analysis. JAMA 2010;304:443–51. 10.1001/jama.2010.1013 [DOI] [PubMed] [Google Scholar]
- 3.Siddiqi N, House AO, Holmes JD. Occurrence and outcome of delirium in medical in-patients: a systematic literature review. Age Ageing 2006;35:350–64. 10.1093/ageing/afl005 [DOI] [PubMed] [Google Scholar]
- 4.Geriatric Medicine Research Collaborative . Delirium is prevalent in older hospital inpatients and associated with adverse outcomes: results of a prospective multi-centre study on world delirium awareness day. BMC Med 2019;17:229. 10.1186/s12916-019-1458-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fick DM, Steis MR, Waller JL, et al. Delirium superimposed on dementia is associated with prolonged length of stay and poor outcomes in hospitalized older adults. J Hosp Med 2013;8:500–5. 10.1002/jhm.2077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jackson TA, Wilson D, Richardson S, et al. Predicting outcome in older hospital patients with delirium: a systematic literature review. Int J Geriatr Psychiatry 2016;31:392–9. 10.1002/gps.4344 [DOI] [PubMed] [Google Scholar]
- 7.Hsieh S, Schubert S, Hoon C, et al. Validation of the Addenbrooke’s cognitive examination III in frontotemporal dementia and Alzheimer’s disease. Dement Geriatr Cogn Disord 2013;36:242–50. 10.1159/000351671 [DOI] [PubMed] [Google Scholar]
- 8.Abraha I, Trotta F, Rimland JM, et al. Efficacy of non-pharmacological interventions to prevent and treat delirium in older patients: a systematic overview. The SENATOR project ONTOP series. PLoS One 2015;10:e0123090. 10.1371/journal.pone.0123090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marcantonio ER. Delirium in hospitalized older adults. New Eng J Med 2017;377:1456–66. 10.1056/NEJMcp1605501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ryan DJ, O’Regan NA, Caoimh Ronán Ó, et al. Delirium in an adult acute hospital population: predictors, prevalence and detection. BMJ Open 2013;3:e001772. 10.1136/bmjopen-2012-001772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Teodorczuk A, Reynish E, Milisen K. Improving recognition of delirium in clinical practice: a call for action. BMC Geriatr 2012;12:55. 10.1186/1471-2318-12-55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kakuma R, du Fort GG, Arsenault L, et al. Delirium in older emergency department patients discharged home: effect on survival. J Am Geriatr Soc 2003;51:443–50. 10.1046/j.1532-5415.2003.51151.x [DOI] [PubMed] [Google Scholar]
- 13.Page V, Katawala T. Management of ICU delirium. ICU Dir 2011;2:31–5. 10.1177/1944451611405197 [DOI] [Google Scholar]
- 14.Kuhn E, Du X, McGrath K, et al. Validation of a consensus method for identifying delirium from hospital records. PLoS One 2014;9:e111823. 10.1371/journal.pone.0111823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.American Psychiatric Association . Diagnostic and statistical manual of mental disorders. 5th edn, 2013. [Google Scholar]
- 16.Meagher DJ, Morandi A, Inouye SK, et al. Concordance between DSM-IV and DSM-5 criteria for delirium diagnosis in a pooled database of 768 prospectively evaluated patients using the delirium rating scale-revised-98. BMC Med 2014;12:164. 10.1186/s12916-014-0164-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jackson TA, Grudzinska F, Welch C, et al. Reduced neutrophil migratory function is associated with delirium in older people with pneumonia. Age and Ageing 2018;47:ii23–ii. [Google Scholar]
- 18.Rockwood K, Song X, MacKnight C, et al. A global clinical measure of fitness and frailty in elderly people. CMAJ 2005;173:489–95. 10.1503/cmaj.050051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Law J, Welch C, Javanmard-Emamghissi H, et al. Decision-making for older patients undergoing emergency laparotomy: defining patient and clinician values and priorities. Colorectal Dis 2020;22:1694–703. 10.1111/codi.15165 [DOI] [PubMed] [Google Scholar]
- 20.Davis DHJ, Muniz Terrera G, Keage H, et al. Delirium is a strong risk factor for dementia in the oldest-old: a population-based cohort study. Brain 2012;135:2809–16. 10.1093/brain/aws190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Partridge JSL, Martin FC, Harari D, et al. The delirium experience: what is the effect on patients, relatives and staff and what can be done to modify this? Int J Geriatr Psychiatry 2013;28:804–12. 10.1002/gps.3900 [DOI] [PubMed] [Google Scholar]
- 22.Marcantonio E, Ta T, Duthie E, et al. Delirium severity and psychomotor types: their relationship with outcomes after hip fracture repair. J Am Geriatr Soc 2002;50:850–7. 10.1046/j.1532-5415.2002.50210.x [DOI] [PubMed] [Google Scholar]
- 23.Maldonado JR. Delirium pathophysiology: an updated hypothesis of the etiology of acute brain failure. Int J Geriatr Psychiatry 2018;33:1428–57. 10.1002/gps.4823 [DOI] [PubMed] [Google Scholar]
- 24.Pendlebury ST, Lovett NG, Smith SC, et al. Observational, longitudinal study of delirium in consecutive unselected acute medical admissions: age-specific rates and associated factors, mortality and re-admission. BMJ Open 2015;5:e007808. 10.1136/bmjopen-2015-007808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ansaloni L, Catena F, Chattat R, et al. Risk factors and incidence of postoperative delirium in elderly patients after elective and emergency surgery. Br J Surg 2010;97:273–80. 10.1002/bjs.6843 [DOI] [PubMed] [Google Scholar]
- 26.Boord MS, Moezzi B, Davis D, et al. Investigating how electroencephalogram measures associate with delirium: a systematic review. Clin Neurophysiol 2021;132:246–57. 10.1016/j.clinph.2020.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2020-042440supp001.pdf (105.1KB, pdf)
Data Availability Statement
The anonymised database is available from the corresponding author upon reasonable request.