Abstract
In recent years, immunotherapy has revolutionised the treatment landscape for oncology patients with improved survival rates in cancers which previously had a dismissal prognosis. These agents target specific pathways of inhibition such as programmed cell death -1 (PD-1), PD ligand-1 and cytotoxic T-lymphocyte-associated antigen 4 resulting in stimulation of T cell activity. This results in enabling an individual’s own immune system to fight against cancer, a different modality of treatment when compared with traditional chemotherapy. While attacking the tumour cells, there is an increased chance of host tissue immune reactions.
We report a case of a patient who received immunotherapy for metastatic malignant melanoma. During the course of the treatment, development of a sarcoid-like reaction was histologically confirmed in the mediastinal lymph nodes. The patient had no respiratory symptoms and continued on the immunotherapy treatment with good clinical and radiological response.
Keywords: skin cancer, immunology, respiratory system
Background
Immunotherapy has changed the way we treat melanoma and indeed many other malignancies. The ability to allow the body’s natural immune system to detect and kill cancer cells is a very exciting prospect. While chemotherapy predisposes a patient to side effects related to an immunosuppressed state, patients on immunotherapy may present with conditions mimicking an autoimmune condition. Clinicians need to be aware of these unique presentations as they may be a sign of treatment response rather than an adverse event attributed to the immunotherapy.
Case presentation
This is a case about a 56-year-old woman who was incidentally diagnosed with metastatic malignant melanoma. She initially presented with dysfunctional uterine bleeding. As part of her management, she underwent a subtotal hysterectomy and bilateral salphingo-oophorectomy in 2014. She did not have a total hysterectomy due to a large cervical fibroid which could not be resected during her surgery.
She continued to have dysfunctional uterine bleeding and was referred for resection of the cervical fibroid. She underwent laparotomy in 2018 where she had the fibroid resected. However, during surgery, a strange black nodule was found in the omentum which was resected and send for histology. The fibroid histology came back as benign but unfortunately the histology from the omental nodule came back as consistent with metastatic melanoma.
Investigations
Following the discovery of the malignant nodule, she underwent extensive workup including skin mapping. A positron emission tomography (PET) CT scan unfortunately showed extensive metastatic disease involving the subcutaneous tissue in the neck, anterior and posterior chest wall, pleural deposits, liver metastasis, bone metastasis and omental disease. Surprisingly no primary was found. Further workup of her malignant nodule showed no mutation in her BRAF gene.
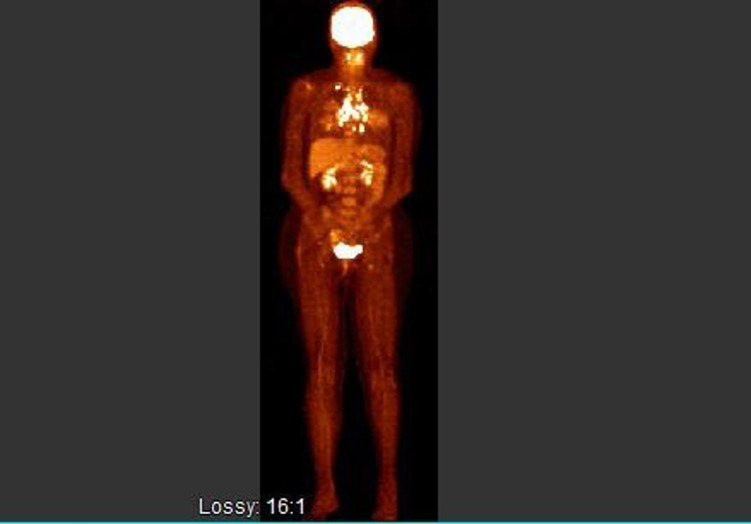
Given her extensive stage, BRAF wild type malignant melanoma she was commenced on combination immunotherapy with ipilimumab and nivolumab. She had a repeat PET CT after four cycles of treatment. This showed fluorodeoxyglucose (FDG) avid mediastinal, perivascular, peritracheal and subcarinal lymphadenopathy. There was also a new mediastinal lymph node which was not present in her baseline PET CT. There was no evidence of any FDG avid uptake anywhere else (figure 1).
Figure 1.
PET CT response after four cycles of immunotherapy. PET, positron emission tomography.
She went on to have an endoscopic bronchial ultrasound (EBUS) and biopsy of her new mediastinal lymph node which came back as showing borderline cellularity with few benign reactive bronchial epithelial cells present in a background of lymphocytes (figure 2). There was no evidence of malignancy but it was involved by non-necrotising granulomatous inflammation. This was suggestive of sarcoidosis or a sarcoid-like reaction (SLR).
Figure 2.
Histology from EBUS showing sarcoid-like reaction. EBUS, endoscopic bronchial ultrasound.
Treatment
The patient had no respiratory symptoms at all. There were no other systemic features of sarcoidosis. As the patient had completed four cycles of combination immunotherapy with ipilimumab and nivolumab with complete radiological response of her metastatic disease, she was put on maintenance therapy with Nivolumab as a single agent every 4 weeks.
Outcome and follow-up
The patient will continue on nivolumab with restaging scans every 3–6 months to assess for any progression. There are no data to suggest how long immunotherapy should be continued in metastatic cancerpatients who are responding to the treatment. The current consensus is to continue treatment until disease progression or unacceptable side effects.
Discussion
In recent times, SLR, a rare complication of the immune checkpoint inhibitors, has been reported in patients receiving these treatments. SLR has histological and clinical features similar to those seen in sarcoidosis.1
SLR has previously been described in relation to other treatments such as cisplatin and α-interferon and is characterised by the presence of non-caseating granulomas.2
Development of a granulomatous reaction in the distant lymph nodes is possibly due to the immunological response to T cells. CD4 +T cells interact with antigen presenting cells to form granulomas. These activated CD4 +cells differentiate into type 1 helper T-like cells which secrete interleukin-2 and interferon γ. This results in increased TNF-α production and the amplification of the local cellular immune response.3 The role of natural killer cells remains undefined and controversial in the formation of granulomas in sarcoidosis. They have been shown to be in dominance in certain lymph nodes but not in the skin lesions.4
Imbalance between the Th17 and regulatory T cells (Treg) both of which originate from CD4 T cells have been implicated in the development of inflammation and autoimmunity.5 Increased Th17 in the peripheral blood of patients receiving anticytotoxic T-lymphocyte-associated antigen 4 (CTLA4) antibodies for melanoma has been documented and their presence linked to toxicities with these therapies.6
Ipilimumab is an anti-CTLA4 antibody and elicits an anti-tumour immune response by unlocking the effects of inhibition on T cells. Studies have shown Ipilimumab enhances both cellular and humoral immunity by T cells.7 Emergence of increased lymphocytes and increased expression of Th1 is associated with treatment response and can potentially induce an SLR. Th1 cells are abundant in sarcoidosis and deemed integral to the development of an SLR.1
Nivolumab, a human IgG4 anti-PD1 monoclonal antibody targets the PD1 receptor on tumour cells or antigen presenting cells and has demonstrated an improved overall survival and progression-free survival in BRAF wild-type metastatic melanoma patients.8 These inhibitory receptors have shown to play an important role in pathogenesis of sarcoidosis. Increased number of programmed cell death-1 (PD-1) and CD4 +T cells were found in the blood compartment and bronchoalveolar lavage in sarcoidosis patients.9 Similarly, PD ligand-1 levels were seen to be upregulated in patients with sarcoidosis. These associations are a marker of the paradoxical relationship to immune checkpoint inhibition therapy.1
It is important to be aware of these manifestations since they can mimic disease recurrence or immune-related adverse events where there is lymph node enlargement. Mediastinoscopy and/or EBUS play an important role in evaluating and assessing the pathology of the lymph nodes in patients receiving the immunotherapy. These are safe, minimally invasive procedures which are used to obtain tissue for histological analysis.10
Role of 18F-FDG/PET scan for the detection of these lesions cannot be underestimated. The radiologists should be aware of development of SLRs in the context of immunotherapy to avoid the misdiagnosis of disease progression.11
A recent study looked at a cohort of 200 melanoma patients receiving BRAF/MEK inhibitors as well as immune checkpoint inhibitors. They observed that eight patients had developed an SLR (4%).12 The summary of product characteristics for Ipilimumab lists the incidence at 1%. Treatment is not necessary if the patient remains asymptomatic and has not developed any organ dysfunction. If required, corticosteroids can help alleviate the symptoms.13 Immunosuppression is not advised for prolonged periods since there is an associated risk of opportunistic infections. SLRs persist if immunotherapy is continued but regress on discontinuation of the treatment.1
Patient’s perspective.
In July 2018 I attended a post-operative review following surgery to resect a cervical fibroid. I was feeling well and had travelled to the appointment on my own. My gynaecologist revealed that during the surgery he had removed a ‘nodule’, which after a subsequent PET CT scan turned out to be a diagnosis of Stage 4 Malignant Melanoma.
That was the day my life changed forever. Would I survive this? I was heartbroken for my children. My future seemed uncertain.
I commenced Immunotherapy treatment in July 2018. The side effects I developed were joint pain, fatigue, thyroid issues and the Sarcoidosis (SLR) like reaction. Fortunately I had no respiratory issues with the SLR. The other side effects were manageable and I was able to live a normal life and look after my family.
I realise I have been incredibly fortunate. First, that the melanoma was discovered and second, that I have reacted so positively to the Immunotherapy treatment. I am currently in remission with no evidence of disease.
What has made this journey a little easier is the care and compassion I have received from the oncology team and my consultant. With Immunotherapy I was given hope and ultimately my life back.
This 56-year-old woman is so ‘GLAD TO BE ALIVE’.
Learning points.
Sarcoid-like reactions are a rare but known complication of immunotherapies and it is important to be aware of their diagnosis and presentation to avoid misdiagnosis of disease progression.
Treatment of the sarcoid-like reaction is not necessary if the patient is asymptomatic.
Symptomatic patients can be treated with a course of steroids and standard anti sarcoidosis treatment but avoid long-term immunosuppression.
Acknowledgments
Gavin Calpin: Medical Intern Galway University Hospital. Darrell Martin: Department of Histopathology, University Hospital Galway.
Footnotes
Correction notice: This article has been corrected since it was published Online. The author's name has been corrected from "Asma Patil" to "Asma Patel". Also, "Darrell Martin" has been included in the acknowledgement section.
Contributors: TK: literature research and formatting for this case report. AP: consented the patient and helped with the literature research. AA: assisted with the formatting and literature research. AY: was the mentor and supervisor for the case report.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Obtained.
References
- 1.Gkiozos I, Kopitopoulou A, Kalkanis A, et al. Sarcoidosis-Like reactions induced by checkpoint inhibitors. J Thorac Oncol 2018;13:1076–82. 10.1016/j.jtho.2018.04.031 [DOI] [PubMed] [Google Scholar]
- 2.Cohen PR, Kurzrock R. Sarcoidosis and malignancy. Clin Dermatol 2007;25:326–33. 10.1016/j.clindermatol.2007.03.010 [DOI] [PubMed] [Google Scholar]
- 3.Zissel G, Prasse A, Müller-Quernheim J. Sarcoidosis-Immunopathogenetic concepts. Semin Respir Crit Care Med 2007;28:003–14. 10.1055/s-2007-970329 [DOI] [PubMed] [Google Scholar]
- 4.Mempel M, Flageul B, Suarez F, et al. Comparison of the T cell patterns in leprous and cutaneous sarcoid granulomas. presence of Valpha24-invariant natural killer T cells in T-cell-reactive leprosy together with a highly biased T cell receptor Valpha repertoire. Am J Pathol 2000;157:509–23. 10.1016/s0002-9440(10)64562-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee GR. The balance of Th17 versus Treg cells in autoimmunity. Int J Mol Sci 2018;19:730. 10.3390/ijms19030730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.von Euw E, Chodon T, Attar N, et al. CTLA4 blockade increases Th17 cells in patients with metastatic melanoma. J Transl Med 2009;7:35. 10.1186/1479-5876-7-35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weber JS, Hamid O, Chasalow SD, et al. Ipilimumab increases activated T cells and enhances humoral immunity in patients with advanced melanoma. J Immunother 2012;35:89–97. 10.1097/CJI.0b013e31823aa41c [DOI] [PubMed] [Google Scholar]
- 8.Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med 2015;372:320–30. 10.1056/NEJMoa1412082 [DOI] [PubMed] [Google Scholar]
- 9.Braun NA, Celada LJ, Herazo-Maya JD, et al. Blockade of the programmed death-1 pathway restores sarcoidosis CD4(+) T-cell proliferative capacity. Am J Respir Crit Care Med 2014;190:560–71. 10.1164/rccm.201401-0188OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frohlich M, Wang H, Sakr L. Sarcoid-like reaction discovered on EBUS-TBNA of intrathoracic lymph nodes during immunotherapy for metastatic melanoma. J Immunother 2020;43:75–8. 10.1097/CJI.0000000000000298 [DOI] [PubMed] [Google Scholar]
- 11.Cheshire SC, Board RE, Lewis AR, et al. Pembrolizumab-induced Sarcoid-like reactions during treatment of metastatic melanoma. Radiology 2018;289:564–7. 10.1148/radiol.2018180572 [DOI] [PubMed] [Google Scholar]
- 12.Dimitriou F, Frauchiger AL, Urosevic-Maiwald M, et al. Sarcoid-like reactions in patients receiving modern melanoma treatment. Melanoma Res 2018;28:230–6. 10.1097/CMR.0000000000000437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weber JS, Postow M, Lao CD, et al. Management of adverse events following treatment with Anti-Programmed death-1 agents. Oncologist 2016;21:1230–40. 10.1634/theoncologist.2016-0055 [DOI] [PMC free article] [PubMed] [Google Scholar]