Abstract
A 69-year-old retired miner with stage 4 non-small-cell lung cancer presented with a 2-month history of obstructive liver function tests following nivolumab immunotherapy. His case had not responded to high dose prednisolone or mycophenolate and he was admitted for investigation. MR cholangiopancreatography demonstrated areas of intrahepatic biliary tree beading and stricturing, in keeping with sclerosing cholangitis. Prednisolone and mycophenolate were stopped and ursodeoxycholic acid commenced with subsequent partial improvement of the patient’s liver function tests.
Keywords: lung cancer (oncology), unwanted effects / adverse reactions, radiology, liver disease
Background
Programmed cell death-1 (PD-1) inhibitors are a relatively novel class of immune checkpoint inhibitors (CPIs) that have been hailed as ‘wonder drugs’ in the field of oncology. CPIs were initially used in the treatment of metastatic melanoma, but their application has been broadened to other solid organ tumours including non-small-cell lung cancer (NSCLC), renal cell, colorectal and urothelial carcinoma.1 Nivolumab was the first CPI to receive Scottish Medicines Consortium (SMC) approval for use in stage 4 NSCLC as a second line therapy in 2016.2
The PD-1 receptor is present on CD28+ cytotoxic T-cells. In normal physiology, it binds to programmed cell death ligand-1, which induces programmed death of the T-cell. When the PD-1 receptor is blocked via drugs such as nivolumab this leads to T-cell proliferation and migration to the tumour site to perform a cytotoxic role.1
CPI medications are well known to cause a spectrum of so-called immune-related adverse events (irAEs). PD-1 inhibitors are particularly associated with irAEs such as hypophysitis, colitis and hepatic injury.1 3 PD-1 inhibitor-associated hepatic injury may occur via several mechanisms. Most often the histological findings are of a lobular hepatitis with patchy necrosis; while less commonly the pattern of injury may be purely cholestatic.1 4 Importantly, the former tends to respond well to high-dose steroid therapy, with or without mycophenolate mofetil, and cessation of the PD-1 inhibitor, whereas these therapies are at least ineffective and at worst may in fact be harmful in the latter cholestatic form.4 Sclerosing cholangitis, a distinct, emerging variant of PD-1 inhibitor-induced hepatic injury, is the focus of this case report.
Sclerosing cholangitis is a pattern of cholestatic liver injury characterised by biliary tract stricturing and dilatation, that is, classified as either primary or secondary in aetiology. Primary sclerosing cholangitis (PSC) tends to present in the 3rd–4th decade, and approximately 80% of patients with PSC are also diagnosed with inflammatory bowel disease (IBD), predominantly ulcerative colitis (UC). While PSC alone occurs equally commonly in men and women, female sex has been shown to confer a more favourable prognosis, and concurrent IBD is less likely in female patients with PSC.5 6 The aetiology of PSC remains uncertain but an interaction between a genetic predisposition and environmental factors such as gut microbiota appears likely.7 The biliary tract damage seen in PSC is predominantly caused by T-cells, although again the precise mechanism is unclear.7 Patients may have positive autoantibodies, but none are specific for PSC.8 Liver biopsy may show characteristic periductal ‘onion skin’ fibrosis and imaging of the biliary system shows multifocal beading due to intrahepatic and extrahepatic strictures, or non-specific evidence of cholestasis.9 PSC is complicated by recurrent cholangitis, development of dominant strictures, cholangiocarcinoma and progression to cirrhosis. In addition to supportive care, monitoring for and managing complications, ursodeoxycholic acid (UDCA) is frequently used at a low dose (10–15 mg/kg/day). This appears to improve liver function tests (LFTs), but has not been shown to improve clinical outcomes as measured by need for liver transplantation, death or development of hepatobiliary malignancy. There is no role for immunosuppressive therapy in PSC.10
Case presentation
A 69-year-old retired coal miner presented with a 2-month history of deranged LFTs following seven cycles of nivolumab which were not improving with high dose oral steroids (2 mg/kg/day).
The patient had a history of T4 N3 M1a squamous cell carcinoma of the lung for which he had previously received palliative chemotherapy with carboplatin and paclitaxel. He had also completed his seventh cycle of nivolumab 4 months prior to the onset of his deranged LFTs. Following his last cycle of nivolumab, the patient developed grade 2 immune related colitis for which he received a tapering course of prednisolone. During this time, he developed steroid induced diabetes for which he was started on gliclazide.
His other medical history included moderate chronic obstructive pulmonary disease, hypertension and peripheral vascular disease. His Eastern Cooperative Oncology Group (ECOG) performance status was 0 at the time of presentation with deranged LFTs. He was a current smoker and previously drank alcohol to excess. Three years previously, he had been referred to the gastroenterology clinic with alcohol excess and mildly elevated alanine aminotransferase (ALT). At this time, a chronic liver disease screen was negative and fibroscan was normal (5.9 kPa), suggesting no significant liver fibrosis.
On admission, he was clinically well with normal vital signs, and on review of systems his only complaint was of fatigue and chronic cough. There were no features of hepatic decompensation.
Investigations
Prior to admission the patient had been having his LFTs monitored in the community under review of the oncology team. These are shown in table 1. As can be seen, there was a steady worsening of LFTs over the course of 2 months before the patient was admitted to our unit.
Table 1.
Chart of liver function tests (LFTs) over time in relation to administration of cycle 7 of nivolumab, with key medication changes highlighted
| Time relative to cycle 7 of nivolumab |
0 weeks | +14 weeks | +20 weeks | +22 weeks | +23 weeks | +27 weeks |
| Key medications and timepoints | Cycle 7 Nivolumab | Abnormal LFTs identified | Commencement of high-dose steroids | Admission to hospital | Discharge from hospital | 1-month post discharge |
| Prednisolone | – | – | 140 mg daily | 60 mg daily | 20 mg daily | 10 mg |
| Mycophenolate mofetil | – | – | – | 1 g two times per day | – | - |
| Ursodeoxycholic acid | – | – | – | – | 250 mg three times per day | 250 mg three times per day |
| Investigation (reference range) | ||||||
| Bilirubin (<20 µmol/L) | 3 | 7 | 13 | 14 | 16 | 17 |
| ALT (<50 U/L) | 19 | 121 | 381 | 443 | 187 | 50 |
| AST (<40 U/L) | 22 | 49 | 144 | 158 | 48 | – |
| ALK P (30–130 U/L) | 95 | 258 | 826 | 857 | 544 | 251 |
| Albumin (35–50 g/L) | 36 | 30 | 28 | 30 | 25 | 24 |
| PT (9–13 s) | 10 | 10 | 14 | 14 | – | – |
ALK P, alkaline phosphatas; ALT, alanine aminotransferase; AST, aspartate aminotransferase; PT, prothrombin time.
Portal venous phase CT abdomen was performed to exclude biliary obstruction and showed mild intrahepatic biliary dilatation, with no obstructing lesion identified. This was a new finding since the CT 3 months previously. There was no extrahepatic biliary dilatation or evidence of hepatic metastases.
At the time of admission, LFTs were as follows: bilirubin 14 mmol/L (0–20), ALT 443 U/L (5 – 55), aspartate aminotransferase 158 U/L (5 – 45), alkaline phosphatase (ALP) 857 U/L (35 – 135), albumin 30 g/L (35 – 50). A coagulation screen on admission was normal. A panel of investigations for chronic liver disease and acute hepatitis did not reveal the aetiology of his abnormal LFTs. These are summarised in table 2. Liver biopsy was not performed in this case, nor were any further fibroscan measurements of liver stiffness.
Table 2.
Investigations for chronic liver disease and acute hepatitis, performed on admission to hospital
| Investigation (units) | Result (reference range) |
| Immunology | |
| Immunoglobulin G (g/L) | 3.2 (6.0–16.0) |
| Immunoglobulin A (g/L) | 2.1 (0.8–4.0) |
| Immunoglobulin M (g/L) | 0.2 (0.4–2.4) |
| Immunoglobulin G-4 (g/L) | 0.2 (0.0–1.3) |
| Antinuclear antibody | Negative |
| Anti-mitochondrial antibody | Negative |
| Smooth muscle antibody | Negative |
| Gastric parietal antibody | Negative |
| Liver kidney microsomal antibody | Negative |
| Liver cytosol one antibody | Negative |
| Nuclear antibody | Negative |
| Biochemistry | |
| Alpha-1-antitrypsin (g/L) | 1.4 (1.1–2.1) |
| Caeruloplasmin (g/L) | 0.32 (0.16–0.47) |
| Ferritin (g/L) | 373 (20–300) |
| Thyroid stimulating hormone (mU/L) | 1.5 (0.3–5.0) |
| Free T4 (pmol/L) | 12.4 (9.0–21.0) |
| Alfa fetoprotein (kU/L) | 6 (<6) |
| Virology | |
| Glandular fever screening test | Negative |
| Hepatitis A virus IgM | Negative |
| Hepatitis B virus surface antigen | Negative |
| Hepatitis C virus IgM | Negative |
| Hepatitis E virus IgM | Negative |
| HIV antigen | Negative |
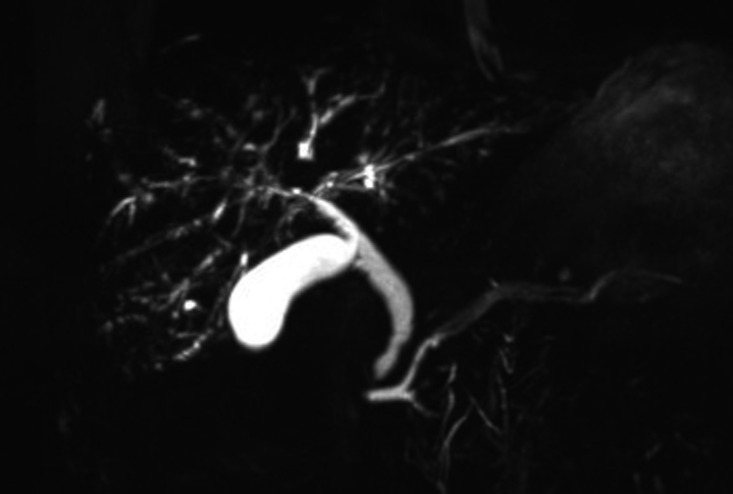
During the admission, MR cholangiopancratography (MRCP) was performed (figure 1). This demonstrated an irregular intrahepatic biliary tree with focal areas of beading and stricturing consistent with sclerosing cholangitis. The common bile duct (CBD) was not significantly dilated but was slightly irregular just above ampulla. There were no focal hepatic lesions, ductal calculi or abnormalities of the gallbladder, pancreatic duct or pancreas.
Figure 1.
Coronal T2-weighted maximum intensity projection MR cholangiopancreatography showing beading and stricturing of the intrahepatic biliary system, with only mild extrahepatic irregularity at the common bile duct.
Differential diagnosis
Based on the primarily cholestatic LFTs and appearances of the biliary tree on MRCP, a diagnosis of sclerosing cholangitis was made. There was nothing in his previous history, LFTs or imaging to suggest a diagnosis of PSC, and specifically he did not have any personal or family history of IBD or liver disease. Causes of secondary sclerosing cholangitis were therefore considered.8–12 These are summarised in table 3.
Table 3.
Differential diagnosis for secondary sclerosing cholangitis
| Secondary causes of sclerosing cholangitis | Description |
| Infection | Ascending cholangitis, recurrent pyogenic cholangitis, echinococcosis, HIV cholangiopathy |
| Ischaemia | Iatrogenic, critical illness, transplant related |
| Drug induced | Ketamine, non-steroidal anti-inflammatory drugs, sevoflurane, trans-arterial chemotherapy, coamoxiclav, amiodarone, infliximab, venlafaxine, atorvastatin, green tea extract |
| Chronic obstruction | Choledocholithiasis, gallbladder/pancreatic adenocarcinoma, portal biliopathy, hilar lymphadenopathy |
| Inflammatory | IgG4 disease, eosinophilic cholangitis, sarcoidosis, Behçet’s disease, systemic mastocytosis |
The most likely culprit in this case was felt to be nivolumab given that the patient tested negative for HIV, IgG4 levels were normal, and there was no history of recent changes to other medications, clinical or biochemical evidence of infection, previous hepatobiliary surgery or intercurrent critical illness. A panel of investigations for chronic liver disease and acute hepatitis, were normal on admission to our unit; however, it is important to note that the patient had already been commenced on high-dose immunosuppression prior to this which does limit the diagnostic utility of some of these investigations. These investigations are shown in table 2.
Treatment
Before admission, the patient had been treated presumptively for immune related hepatitis, being commenced on prednisolone 40 mg once daily. Following deterioration in his LFTs, this was escalated to 140 mg (2 mg/kg) oral prednisolone once daily, with a view to tapering the dose down if LFTs improved. Mycophenolate mofetil 1 g two times per day was added to facilitate reduction of his steroid dose.
On admission, the patient was taking 60 mg oral prednisolone once daily and 1 g oral mycophenolate mofetil two times per day. This was continued initially while further investigations were performed.
Once the diagnosis of nivolumab induced sclerosing cholangitis was established, it was decided that mycophenolate should be stopped immediately, and prednisolone gradually withdrawn over the following weeks in the community, under supervision of the endocrine team. UDCA was commenced at a dose of 250 mg three times per day (12 mg/kg/day based on a weight of 62 kg).
Outcome and follow-up
The patient in this case was discharged from our unit shortly after adjustment of his medications, once a decline in his liver enzymes was seen. These continued to be monitored in the community by his general practitioner. His steroid dose was reduced under the supervision of the endocrine team due to concerns of steroid induced adrenal suppression and possible undiagnosed hypophysitis due to nivolumab and the possibility of secondary adrenal insufficiency. There was a marked decrease in ALP and ALT on introduction of UDCA and the levels of these stabilised at around one-third of peak values (table 1) on a dose of 10 mg oral prednisolone once daily. In keeping with previous studies, this was classified as a moderate response to treatment (ie, improvement but not normalisation of LFTs).
The patient’s steroid induced diabetes was better controlled following reduction in the prednisolone dose and gliclazide therapy was able to be titrated down in dose. Clinically, the patient was more frail on discharge from hospital and was ECOG performance status 2. He was seen by the oncology team who discussed further chemotherapy for his progressive lung cancer which the patient declined. The patient died approximately 2 months after discharge from hospital with the primary cause of death recorded by his general practitioner as ‘squamous cell carcinoma of the lung’.
Discussion
Sclerosing cholangitis is a rarely reported irAE of PD-1 inhibitors whose exact features, clinical course and optimal treatment are not yet agreed on. The disease was first reported by Gelsomino et al following treatment of NSCLC with nivolumab.13 In a subsequent case series, Kawakami et al identified three cases of nivolumab related SC out of 91 patients with recurrent or metastatic NSCLC who received nivolumab, generating an estimated incidence of 3.3% in these patients.14 Cases have since been reported following treatment with other PD-1 inhibitors including pembrolizumab.15–23 There have been efforts to define diagnostic criteria and optimal management, but no consensus has been reached due to the emerging variety in presentation, imaging and histological findings and response to steroid therapy.23
The pathophysiology of PD-1 inhibitor-related sclerosing cholangitis is not known although it is generally felt to be a cytotoxic T-cell-mediated reaction against biliary epithelium driven by anti-PD-1 monoclonal antibodies.14 There appears to be a predominance of CD8 +T cell infiltration in PD-1 inhibitor-related hepatobiliary disease, including a significant proportion of sclerosing cholangitis; however, this is absent in approximately half of reported cases.15 23 Other findings on histology include lobular hepatitis and predominance of other inflammatory cell lines. In our case, liver biopsy was not performed as the benefits of a pathology sample in helping to confirm the diagnosis was not felt to outweigh the potential risks, and the patient was treated empirically.
Although not the primary focus of this report, it is interesting to note that this patient also suffered previously with nivolumab related colitis, given the strong association between PSC and IBD. PD-1 inhibitor related colitis demonstrates a number of histopathological features that may also be seen in UC, Crohn’s disease and microscopic colitis: crypt abscesses, cryptitis and lymphoplasmacytic infiltrate of the lamina propria; granuloma formation; and lymphocytic or more rarely collagenous colitis, respectively.24 Current data do not support the same degree of association between PD-1 inhibitor related sclerosing cholangitis and colitis as is seen between PSC and IBD. However, as the damage in PSC is primarily mediated by T-cells, which are upregulated in PD-1 inhibitor therapies, further investigation may yield important discoveries about any shared pathogenesis of PD-1 inhibitor iRAEs, PSC and IBD, which may in turn guide diagnosis and management of each condition.
In a recent systematic review by Onoyama et al, the most common finding on imaging of the biliary tract was of biliary dilatation and diffuse hypertrophy.23 Interestingly, 10% of patients had intrahepatic disease only, which the author relates to a suggestion by Gelsomino et al that there may be distinct phenotypes of PD-1 inhibitor related cholangitis.13 If this were shown to be the case, then it may provide an explanation for the observed variations in clinical course and response to treatment. In the case of our patient, MRCP demonstrated classical features of PSC with beading and stricturing involving the intrahepatic ducts and an irregular CBD.
There have been no controlled comparisons of the management of PD-1 inhibitor-related sclerosing cholangitis, and the regimens described in the literature have been initiated on a case-by-case basis. In all cases, PD-1 inhibitor therapy was withheld following presentation with cholangitis, and the majority of cases were treated initially with steroids. Other immunosuppressants including mycophenolate and tacrolimus have been used at variable doses, though outcomes are persistently poor.23 A key feature of early cases of PD-1 inhibitor related sclerosing cholangitis appeared to be a moderate to poor response to steroids, which has been further demonstrated by systematic review of subsequent cases. Indeed, as many as 30% of patients appear to show no response to steroids, while only 11.5% of patients in the review by Onoyama et al had normalisation of LFTs with steroid therapy.23 This differentiates the disease from other immune-mediated hepatobiliary disease, including IgG4 disease and autoimmune hepatitis, which tend to be more steroid responsive.25 26 Importantly however, as with PSC, immunosuppressive therapy has no role in the management of, and can in fact be harmful in patients with predominantly cholestatic hepatitis, which as mentioned previously, is a rare manifestation of PD-1 inhibitor-related hepatic injury. Hence, accurate diagnosis is crucial in patients presenting with hepatic injury as a consequence of PD-1 inhibitor therapy in order to guide treatment and avoid potentially harmful complications of steroid therapy.4
UDCA (10–15 mg/kg) has been shown to be of benefit in improving liver biochemistry in PSC, although clinical and histological outcomes are not significantly improved, and is of use in limiting progression and improving outcomes in PBC.10 27 UDCA, either alone or in combination with steroids, was used in 42% of cases reviewed by Onoyama et al. A moderate response (defined as improvement of LFTs but not normalisation) to UDCA monotherapy was seen in two cases. Moderate response was also seen in three cases which had not responded to steroid monotherapy following the addition of UDCA.23
Our patient had received a total of 95 days of high-dose prednisolone (2 mg/kg/day) and 21 days of mycophenolate mofetil (1 g two times per day) by the time of admission to hospital. Despite this his ALP continued to rise and he was suffering from steroid induced diabetes mellitus. As such, the decision was made to withdraw immunosuppressive therapy with careful monitoring and commence UDCA.
There are many features in our case that are in keeping with previously reported PD-1-related sclerosing cholangitis, including: LFTs derangement with predominantly elevated ALP/GGT; MRCP findings of beading/stricturing of intrahepatic ducts and irregular CBD; absence of clinical or laboratory evidence of alternative aetiology; poor response to steroids and moderate response to UDCA and reduced dose steroids. The diagnostic certainty in this case is limited however by lack of liver biopsy or autoantibody and immunoglobulin levels prior to the commencement of high-dose steroids and mycophenolate. Of particular note, normal IgG4 levels under any circumstances do not exclude IgG4 disease, and this is further confounded by use of steroids at the time of measurement. The absence of extrahepatic findings on MRCP in this case are interesting, and in keeping with a subset of previous cases. The possibility of an intrahepatic phenotype of disease and the implications of this on treatment warrants further investigation. PD-1 inhibitor-related sclerosing cholangitis is certainly a diagnosis of exclusion at present, although early use of MRCP in suspected cases may guide diagnosis and avoid the ineffective and potentially harmful use of high-dose steroid therapy.
Learning points.
Sclerosing cholangitis is an important emerging form of hepatic injury resulting from treatment with programmed cell death-1 (PD-1) inhibitors, and is likely underdiagnosed.
Diagnosis is primarily based on history, imaging findings in keeping with sclerosing cholangitis, exclusion of alternative causes of sclerosing cholangitis, and histology if felt clinically appropriate.
Response to treatment is variable and corticosteroids appear not to be effective in the majority of cases although different subsets of patients may be more steroid responsive. In this case, liver function tests showed partial response with low-dose ursodeoxycholic acid (12 mg/kg) and reduced dose prednisolone.
Early MR cholangiopancratography imaging in a patient with cholestatic liver injury following PD-1 inhibitors should be performed to appropriately guide therapy and avoid the harmful effects of high-dose steroids.
Further research is needed to determine optimal investigation, stratification and management of this emerging disease.
Footnotes
Contributors: ST was the primary author of this manuscript, and was responsible for obtaining patient consent, collation of images and data, literature review, drafting and submission of the manuscript. VM contributed to and reviewed the manuscript from an oncology perspective. HL supervised and approved the final manuscript for submission, in addition to contributing to and reviewing it from a hepatology perspective.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Karamchandani DM, Chetty R. Immune checkpoint inhibitor-induced gastrointestinal and hepatic injury: pathologists' perspective. J Clin Pathol 2018;71:665–71. 10.1136/jclinpath-2018-205143 [DOI] [PubMed] [Google Scholar]
- 2.SMC . Scottish medicines Consortium. SMC No. (1144/16), 2016: 1–12. [Google Scholar]
- 3.Pérez-De-Lis M, Retamozo S, Flores-Chávez A, et al. Autoimmune diseases induced by biological agents. A review of 12,731 cases (BIOGEAS registry). Expert Opin Drug Saf 2017;16:1255–71. 10.1080/14740338.2017.1372421 [DOI] [PubMed] [Google Scholar]
- 4.Zhang D, Hart J, Ding X, et al. Histologic patterns of liver injury induced by anti-PD-1 therapy. Gastroenterol Rep 2020;8:50–5. 10.1093/gastro/goz044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gochanour E, Jayasekera C, Kowdley K. Primary sclerosing cholangitis: epidemiology, genetics, diagnosis, and current management. Clin Liver Dis 2020;15:125–8. 10.1002/cld.902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weismüller TJ, Trivedi PJ, Bergquist A, et al. Patient age, sex, and inflammatory bowel disease phenotype associate with course of primary sclerosing cholangitis. Gastroenterology 2017;152:1975–84. 10.1053/j.gastro.2017.02.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Karlsen TH, Folseraas T, Thorburn D, et al. Primary sclerosing cholangitis - a comprehensive review. J Hepatol 2017;67:1298–323. 10.1016/j.jhep.2017.07.022 [DOI] [PubMed] [Google Scholar]
- 8.Seo N, Kim SY, Lee SS, et al. Sclerosing cholangitis: clinicopathologic features, imaging spectrum, and systemic approach to differential diagnosis. Korean J Radiol 2016;17:25–38. 10.3348/kjr.2016.17.1.25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vitellas KM, Keogan MT, Freed KS, et al. Radiologic manifestations of sclerosing cholangitis with emphasis on Mr cholangiopancreatography. Radiographics 2000;20:959–75. 10.1148/radiographics.20.4.g00jl04959 [DOI] [PubMed] [Google Scholar]
- 10.Chapman MH, Thorburn D, Hirschfield GM, et al. British Society of gastroenterology and UK-PSC guidelines for the diagnosis and management of primary sclerosing cholangitis. Gut 2019;68:1356–78. 10.1136/gutjnl-2018-317993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abdalian R, Heathcote EJ. Sclerosing cholangitis: a focus on secondary causes. Hepatology 2006;44:1063–74. 10.1002/hep.21405 [DOI] [PubMed] [Google Scholar]
- 12.Brooling J, Leal R. Secondary sclerosing cholangitis: a review of recent literature. Curr Gastroenterol Rep 2017;19:1–7. 10.1007/s11894-017-0583-8 [DOI] [PubMed] [Google Scholar]
- 13.Gelsomino F, Vitale G, D'Errico A, et al. Nivolumab-induced cholangitic liver disease: a novel form of serious liver injury. Ann Oncol 2017;28:671–2. 10.1093/annonc/mdw649 [DOI] [PubMed] [Google Scholar]
- 14.Kawakami H, Tanizaki J, Tanaka K, et al. Imaging and clinicopathological features of nivolumab-related cholangitis in patients with non-small cell lung cancer. Invest New Drugs 2017;35:529–36. 10.1007/s10637-017-0453-0 [DOI] [PubMed] [Google Scholar]
- 15.Zen Y, Chen Y-Y, Jeng Y-M, et al. Immune-Related adverse reactions in the hepatobiliary system: second-generation check-point inhibitors highlight diverse histological changes. Histopathology 2020;76:470–80. 10.1111/his.14000 [DOI] [PubMed] [Google Scholar]
- 16.Koya Y, Shibata M, Shinohara N, et al. Secondary sclerosing cholangitis with hemobilia induced by pembrolizumab: case report and review of published work. Hepatol Res 2019;49:950–6. 10.1111/hepr.13329 [DOI] [PubMed] [Google Scholar]
- 17.Matsumoto S, Watanabe K, Kobayashi N, et al. Pembrolizumab‐induced secondary sclerosing cholangitis in a non‐small cell lung cancer patient. Respirol Case Rep 2020;8:1–5. 10.1002/rcr2.560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kono M, Sakurai T, Okamoto K, et al. Efficacy and safety of chemotherapy following anti-PD-1 antibody therapy for gastric cancer: a case of sclerosing cholangitis. Intern Med 2019;58:1263–6. 10.2169/internalmedicine.1981-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gelsomino F, Vitale G, Ardizzoni A. A case of nivolumab-related cholangitis and literature review: how to look for the right tools for a correct diagnosis of this rare immune-related adverse event. Invest New Drugs 2018;36:144–6. 10.1007/s10637-017-0484-6 [DOI] [PubMed] [Google Scholar]
- 20.Kashima J, Okuma Y, Shimizuguchi R, et al. Bile duct obstruction in a patient treated with nivolumab as second-line chemotherapy for advanced non-small-cell lung cancer: a case report. Cancer Immunol Immunother 2018;67:61–5. 10.1007/s00262-017-2062-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Izumi H, Kodani M, Kurai J, et al. Nivolumab-induced cholangitis in patients with non-small cell lung cancer: case series and a review of literature. Mol Clin Oncol 2019;11:439–46. 10.3892/mco.2019.1923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ogawa K, Kamimura K, Terai S. Antiprogrammed cell death-1 Immunotherapy-Related secondary sclerosing cholangitis. Hepatology 2019;69:914–6. 10.1002/hep.30189 [DOI] [PubMed] [Google Scholar]
- 23.Onoyama T, Takeda Y, Yamashita T, et al. Programmed cell death-1 inhibitor-related sclerosing cholangitis: a systematic review. World J Gastroenterol 2020;26:353–65. 10.3748/wjg.v26.i3.353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Soularue E, Lepage P, Colombel JF, et al. Enterocolitis due to immune checkpoint inhibitors: a systematic review. Gut 2018;67:2056–67. 10.1136/gutjnl-2018-316948 [DOI] [PubMed] [Google Scholar]
- 25.Mack CL, Adams D, Assis DN, et al. Diagnosis and management of autoimmune hepatitis in adults and children: 2019 practice guidance and guidelines from the American association for the study of liver diseases. Hepatology 2020;72:671–722. 10.1002/hep.31065 [DOI] [PubMed] [Google Scholar]
- 26.European Association for the Study of the Liver . EASL clinical practice guidelines: autoimmune hepatitis. J Hepatol 2015;63:971–1004. 10.1016/j.jhep.2015.06.030 [DOI] [PubMed] [Google Scholar]
- 27.Hirschfield GM, Dyson JK, Alexander GJM, et al. The British Society of Gastroenterology/UK-PBC primary biliary cholangitis treatment and management guidelines. Gut 2018;67:1568–94. 10.1136/gutjnl-2017-315259 [DOI] [PMC free article] [PubMed] [Google Scholar]