Abstract
Head and neck paragangliomas are rare vascular tumors derived from the paraganglionic system, located at the carotid body, jugular vein, tympanic cavity and vagal nerve.
From 2010 to 2020, a cohort of 26 patients divided in two groups, 15 with cervical paragangliomas and 11 with temporal bone paragangliomas, was reviewed by analysing the medical history, the epidemiological and clinical parameters, the imaging results and classification, the modality of treatment and outcome.
Cervical paragangliomas present as firm and pulsatile mass with the characteristic aspect of “salt and pepper” on MRI T1 weighted sequences. The most common type on Shamblin classification was the type II. Total surgical resection was performed in 93,33% of cases. The sensitivity of MRI in the diagnosis of vagal paragangliomas was up to 75%, with a specificity of 90,91% and the correlation of the MRI results and the findings of surgical exploration is significant with p ⩽ 0.02.
Temporal bone paragangliomas appear as pulsatile mass behind the tympanic membrane, causing variable hearing loss in 90,90% of the cases. The facial nerve is the most frequently affected cranial nerve, in 36,36% of the cases. The main type according to FISH classification is the type B. Embolization was performed in all type C tumors. Surgery was the first line treatment while the inoperable patients were considered for radiotherapy.
The aim of this study is to report the main clinical features of head and neck paragangliomas, the imaging tools and findings evaluating their sensitivity and specificity and the treatment protocol and outcome.
Keywords: Paraganglioma, Carotid body, Vagal nerve, Tympanojugular paraganglioma, MR imaging
Highlights
-
•
Head and neck paragangliomas are rare, usually benign hypervascular tumors, characterized by slow growth over time.
-
•
Typical clinical manifestations and imaging evaluation should be considered together to establish the treatment protocol.
-
•
MRI is efficient to detect vagal paragangliomas.
-
•
Surgery provides excellent control of the tumor while radiation therapy is considered for surgery contraindication.
1. Introduction
Head and neck paragangliomas are rare, usually benign hypervascular tumors, derived from cells of the diffuse neuroendocrine system, characterized by slow growth over time [[1], [2], [3]]. They can be classified according to their location as cervical paragangliomas (CP) (vagal (VP) and carotid body paragangliomas (CBP)) and temporal bone paragangliomas (tympanic (TP), tympanomastoid (TMP) and tympanojugular paragangliomas (TJP)) [3].
The aim of this study is to report the main clinical features of head and neck paragangliomas, the imaging tools and findings evaluating their sensitivity and specificity and the treatment protocol and outcome.
2. Patients and methods
We carried out a prospective study in the ENT-Head and neck surgery department of the 20th August hospital, over a period of 10 years, between January 2010 and December 2020, enrolling a cohort of 26 patients with head and neck paragangliomas. Based on the location of paragangliomas, patients were divided into two groups and adequately assessed according to the origin of their tumors. Group 1 consisted of 15 patients with cervical paragangliomas (CP), corresponding either to carotid body (CBP), intrajugular (IJP) or vagal paragangliomas (VP). Group 2 consisted of 11 patients with tympanic (TP) or tympano-jugular paragangliomas (TJP).
Each patient's personal and family medical history was obtained on the basis of their medical files and their direct interrogation. Their epidemiological and clinical characteristics were specified. At presentation, nervous lesions were detected by physical examination as well as other secondary locations.
We performed Ultra sound, CT scan and specially MRI imaging for all patients of group 1. Their results were analysed and were complementary to each other. The degree of attachment to the carotid vessels was assessed based of Shamblin classification, predicting the difficulty of surgical resection. For the group 2: all patients underwent a tonal audiometry in order to assess initial hearing levels, a temporal bone CT scan specifying the location and the degree of osteolysis and cervical MRI to assess the intracranial extension. Then, we typed our patients according to the FISH classification. Laboratory investigations concerned both groups and consisted of urine or serum catecholamine test (per 24 h) (epinephrine and norepinephrine), considered to be abnormally high when> 1.5 times the normal value, resulting in a secretory form of paraganglioma.
We performed an extension work-up (abdominal ultrasound and scintigraphy) in order to look for other secondary localizations, in patients at risk (male, age < 45 years, advanced stage of tumor).The main pre-intervention consideration taken was an embolization of the nourishing arteries for some patients.
For the group 1, the treatment consisted on a surgical resection of the tumor. We specified the procedure, the per-operative findings, the state of the nearby vessels and nerves, the real location of tumor and surgical incidents. Concerning vagal paraganglioma, a correlation between per-operative findings and MRI results were assessed, calculating the specificity of the MRI based on the khi-2 test of Pearson, considering the correlation significant for p < 0,05. For the group 2, we described the different types of chosen treatments and their indications according to the tumor classification, specifying the type of surgery when possible, the surgical approach, and the per-operative findings and incidents. The follow up focused mainly on post-operative complications as nervous lesions and hearing loss for the group 2. Histological examination of the operative specimen confirmed the diagnosis in all patients of both groups.
This work has been approved by the ethical committee of our department. All the patients gave their consent for the surgery and the follow up leading to the results of this study. The paper was written meeting the STROCSS criteria [4] and registered under the following registration number “researchregistry6728”.
3. Results
3.1. Group 1: cervical paragangliomas
Our study reports a group of 15 patients presenting cervical paragangliomas.
-
-
Epidemiology
The median age in our population of CP was 56,67 years (range from 33 to 77 years) with a feminine predominance and a sex ratio of 0,25.
-
-
Clinical features
No relevant medical history was noticed in our patients except for one who was already operated for a pheochromocytoma in 2014. The consultation time varied from 1 to 15 years, with a median of 3,6 years and was motivated by the appearance of a lateral cervical mass in all patients except for one when VP was discovered preoperatively during thyroidectomy procedure.
The mass was described as firm and pulsatile in 86,6% of all cases, painless in 73,3%, mobile in 26% and fixed in 40%. The cervical mass was right-sided in 7 patients and left-sided in 6 patients. However, two patients presented bilateral neck masses, firm, pulsatile and asymmetric. The otoscopy was normal in all our patients. Clinical signs of nervous lesions already settled were found in 4 patients (26,6%) represented mainly by vagus nerve lesion, causing unilateral vocal fold paralysis in all of them and translated by dysphonia in two. One patient had also a hypoglossal nerve lesion, with lateral deviation of the tongue apex.
The presence of more than one paraganglioma was found in 4 patients. Multiple cervical locations were observed in 3 cases, 2 patients presented a bilateral CBP asymmetric in size and extension, and one patient associated an IJP with no tympanic component and a VP invading largely the external carotid artery (ECA), both in the same side. Also, a second distant location was observed in the one patient who was previously operated for a pheochromocytoma.
-
-
Radiological features
Neck ultrasound (US) was performed in 46,7% of our patients revealing a hypervascular mass, near the major vessels in 57,14% of cases.
The cervical CT scan was performed in 40% of all the cases. It showed a vascular mass, describing its exact location and measurements, specifying its proximity with the major vessels, their permeability and the apparent state of the internal jugular vein (IJV) and gets very useful to highlight the extension and erosion of bony structures of the skull base specifically.
The MRI was carried out in 80% of all cases, showing a vascular mass, with the characteristic aspect of “salt and pepper” on T1 weighted sequences made of punctate hyperintense regions and small flow voids. The injection of Gadolinium demonstrated a rapid wash-in and out with an important enhancement (Fig. 1). It also diagnosed presumptively 4 cases of VP based on the location of the mass in the parapharyngeal space, displacing the internal and external carotid arteries forwards and the IJV backwards.
Fig. 1.
MRI T1 weighted sequences with injection of Gadolinium showing the tumor dividing the two carotid arteries, classified as Shamblin type 2.
The Shamblin classification was applied to 10 of our patients, based on the involvement of the carotid artery (CA): type I presented in 1 patient, type II in 7 and type III in 2 patients with extension to the skull base which made them type IIIb.
-
-
Biology examination
Urine catecholamine test (per 24 h) and/or serum catecholamine screening (epinephrine AND norepinephrine) were normal except in one patient whose levels were elevated (>1.5 times the normal value) resulting in a secretory form of paraganglioma. He required pre and postoperative management of his hemodynamic state.
-
-
Extension evaluation:
In order to look for other potential locations, a radiological assessment was carried out specifically to detect a silent pheochromocytoma. In 2 patients, abdominal ultrasound and scintigraphy were performed and were found to be normal. In the one case of the IJP, a temporal bone CT scan was performed to look for a tympanic component which was not found.
-
-
Embolization
Not systematic in cervical paragangliomas. Thus, it was performed in a single patient with a large IJP extended to the skull base (jugular golf).
-
-
Surgical procedure
We performed a total surgical resection of the tumour in 93,33% of all cases (14 patients). However, in one case, given the large extent of the tumour, encompassing the vascular axis, invading the vagus, the spinal and the hypoglossal nerves and the parotid gland, up to the skull base and causing preoperative nervous damages, the surgery consisted on a partial resection with the ligation of the IJV and the ECA. Then, the patient was sent for adjuvant radiotherapy.
The surgical exploration found 10 cases (66,66%) of CBP, focused on the carotid bifurcation (Fig. 2). The vagal nerve was preserved in 90% of cases. Only one case required vagal nerve sacrifice, given the total invasion of the nerve which was not functional as long as the patient had already a vocal fold paralysis. The surgical exploration also exposed VP in 5 patients (33,33%) detailed in the dedicated paragraph.
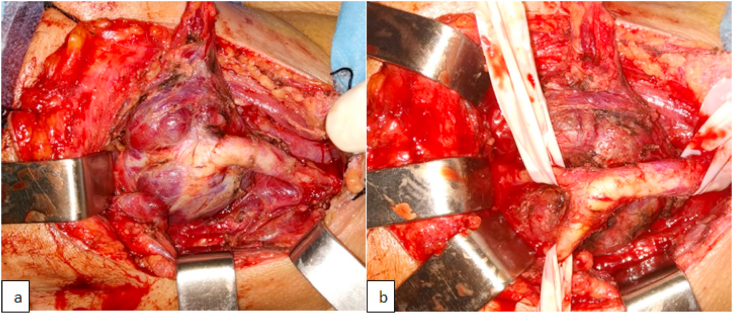
Fig. 2.
Peroperative images of CBP before (a) and after (b) complete resection.
Surgical incidents were represented by the ligation of the ECA in 50% of cases and the ligation of the IJV in 13,33% when the tumour was very extensive invading completely the vein.
-
-
Postoperative data
The hospital stay duration varied between 3 and 5 days, with a median of 3,6 days. Nerve complications were represented mainly by symptoms related to vagal nerve injury, occurring in all patients with VP and in one patient with extensive CBP. Symptoms were dysphonia, swallowing disturbances in 80% of cases and a curtain sign in 20% of cases.
-
-
Histopathology
The diagnosis of paraganglioma was confirmed in 100% of all cases after histopathological examination.
-
-
Follow up
Adjuvant radiotherapy was considered in one patient with an extensive tumor in whom surgical procedure consisted on a partial resection. For bilateral paragangliomas, only the side with a large tumor was operated. We continued to follow and observe the patients, with repetitive MRI scans every 6 months or year. As long as the tumor size remains stable and the nerves functioning, we continue to monitor.
-
-
Vagal paraganglioma, a sub-group of its own
We report in our study 5 cases of VP, all occurring in women, with a median age of 58,4 years and no medical history except for the one who was operated for a total thyroidectomy exposing the confused nodal metastasis as a vagal tumor. The consultation time was on average 2,4 years and was motivated by the appearance of a pulsatile and painless cervical mass, right sided in 3 patients and left sided in 2, associated in 2 patients with ipsilateral vocal fold paralysis and a clinical dysphonia.
The cervical US done in 60% of the cases showed a hypervascular mass, while the CT scan done in only one patient showed a mass of the parapharyngeal space, sheathing the internal carotid artery and the IJV, in contact with the skull base with no bone erosion. The MRI remains the most specific imaging to determine the vagal origin of these tumors and was then performed in all the patients. With a position that may be similar to the CBP, the MRI concluded to VP in all the cases where the mass appeared highly enhanced, displacing the internal and external carotid arteries anteriorly and the IJV posteriorly, without widening the carotid bifurcation. However, in 2 cases, the lesion seemed to be located in the carotid bifurcation (Fig. 3).
Fig. 3.
MRI coronal section (a), T1 axial sequence (b) and T1 axial injected sequence (c) showing highly enhanced mass, displacing the internal and external carotid arteries anteriorly and the IJV posteriorly, without widening the carotid bifurcation.
According to the staging system for VP, 4 patients had a type 1 tumor, lying in the parapharyngeal space without invasion of the jugular foramen and 1 patient had a type 2, invading the foramen without bone destruction. Urine catecholamine test (per 24 h) and/or serum catecholamine screening (epinephrine AND norepinephrine) were normal for all the patients. And the assessment of potential secondary locations based of abdominal US and full body scintigraphy was found normal.
The surgical procedure allowed a total resection of the tumor in all the patients. One of them has an associated IJP arriving to the jugular foramen without a tympanic component. The nerve was sacrificed in 80% of cases, even though we tried to preserve the nerve in one patient, she still had symptoms of postoperative vagal injury (Fig. 4).
Fig. 4.
Peroperative images of VP before (a) and after (b) complete resection.
Comparing the MRI imaging results to the surgical exploration, we observed that the number of VP detected by MRI and confirmed by surgery was 3 out of 5 (60%), while only one false positive case was noted where the MRI concluded to VP that turned to be CBP in surgery. Finally, a single case of VP was not revealed by MRI and discovered peroperatively, corresponding to the case of two tumors on the same side (IJP and vagal VP). Thus, the sensitivity of cervical MRI in the diagnosis of VP was up to 75%, with a specificity of 90,91%. The correlation of the MRI results and the findings of surgical exploration of VP is significant with p ⩽ 0.02.
The main complication was related to the sacrifice of the ipsilateral vagus nerve causing dysphonia and swallowing disorders requiring re-education sessions.
3.2. Group 2: Tympanic and tympano-jugular paragangliomas
Our study reports a group of 11 patients presenting tympanic and tympano-jugular paragangliomas.
-
-
Epidemiology
The median age in our group of TP and TJP was 54,72 years (range from 34 to 80 years) with a feminine predominance and a sex ratio of 0,37.
-
-
Clinical features
No particular medical history was noted in our patients, except for 2 patients: one was already operated for TJP in 2009 (without documents) and another patient in whom TJP was diagnosed 25 years ago, without additional exploration or treatment. No other similar case in their families was noticed.
The consultation time varied from 1 to 10 years, with a median of 3,95 years. All our patients complained of unilateral pulsatile tinnitus associated in 90,9% of the cases to a progressive hearing loss, otorrhea in 45,45%, otorrhagia in 18,18% and headaches in 18,18% of all cases. A cervical mass was described in one patient.
The otoscopy revealed a pulsatile mass behind the tympanic membrane in 63,63% of all cases, a mass elevating the inferior wall (the tympanic bone) of the external auditory canal (EAC) and a reddish budding mass in the EAC in 18,18% of cases each (Fig. 5).
Fig. 5.
Otoscopy revealing pulsatile reddish mass behind the tympanic membrane (a), mass elevating the inferior wall of EAC (b) and a reddish budding mass in the EAC (c).
The tumour occurred mainly on the right side, on 8 patients, with a rate of 72,72%, versus 27,27% on the left side. No second or other locations were found.
Clinical signs of nervous lesions were seen in 4 patients. The facial nerve is the most frequently affected cranial nerve, affected in all of these 4 patients (36,36% of the group 2) with an ipsilateral facial palsy and classified according to the House and Brackmann scale as grade II in one patient, grade III in another one and grade V in two patients. The hypoglossal nerve is the second most commonly affected nerve. Its lesion was found in 2 patients presenting a lesion of the facial nerve, expressed by a deviation of the apex of the tongue. One of these patients presented with a lesion of the glossopharyngeal nerve (velar palsy causing swallowing disorder), paralysis of the vagal nerve with dysphonia (due to ipsilateral vocal cord palsy) and dysphagia and a lesion of the spinal nerve which complements the Collet-Sicard syndrome.
-
-
Audiometry findings:
Hearing assessment was realised by a tonal audiometry, performed in all our patients, showing a normal hearing in one patient, a mild hearing loss in one, a moderate hearing loss in 2, a severe hearing loss in 3 and a profound hearing loss in 4 patients.
-
-
Radiological features
A temporal bone CT scan was carried out in 10 patients (90,90% of the cases). It showed soft tissue material filling only the middle ear in one patient, the middle ear and the mastoid cells in 4 patients, a petrous bone lesion invading the jugular foramen (JF) and the carotid canal (CC) in 3 patients and a lesion invading the skull base with cervical extension in 2 patients (Fig. 6).
Fig. 6.
CT scan imaging in axial section (a) and coronal section (b) showing TJP invading the tympanic cavity with osteolysis and dehiscent jugular bulb.
The MRI was performed in 8 patients (72,72% of the cases). Four patients had no intracranial invasion, while one patient had a process invading the pontocerebellar angle with a mass effect on the posterior cerebral fossa structures. The invasion of the temporal lobe was noticed in 2 patients and the infratemporal fossa in one patient. Usually, the tumour appears in a low or iso signal in T1 sequence and in a high signal in T2 and FLAIR sequences, enhanced remarkably after Gadolinium injection. The typical salt and pepper sign was described once. And the internal ear structures were affected in the 3 most extensive cases with a partial lysis of the semicircular canals and the cochlea; the internal auditory canal (IAC) was affected in one case (Fig. 7).
Fig. 7.
MRI T1 weighted axial sequence (a), T2 weighted axial sequence (b), coronal section (c) and Flair axial sequence (d) showing the tumor centred on the right jugular foramen in iso signal T1 and high signal T2 and FLAIR with the typical “salt and pepper” sign.
The classification of these tumors according to FISH was as follow: type A (glomus tympanicum) in 1 case; type B (glomus hypotympanicum) in 4 cases; type C1 in 2 cases; type C2 De1 in 2 cases; Type C3 Di3 in 2 cases.
-
-
Biology examination
Urine catecholamine test (per 24 h) and/or serum catecholamine screening were normal in all the 7 patients tested.
-
-
Extension workup
A cerebral and thoraco-abdominal CT scan was done in two males, under 45 years old with extensive paragangliomas, and did not reveal a second location.
-
-
Embolization
Performed in 3 patients from the 8 operated ones and 27,27% of all the cases, all FISH type C paragangliomas, 2 of them classified as C1 and one as De1C2.
-
-
Surgical procedure
The surgical approach varied according to the extension of the tumor, the involvement of the internal carotid artery (ICA), the state of the facial nerve and options of whether or not preserving the middle ear. It consisted on a retro-auricular approach in 4 patients, a type A infratemporal approach in 3 patients, an approach through the external auditory canal was chosen for one patient with a type A TP but a mastoidectomy was also performed. Three patients were inoperable.
Surgical procedure allowed a preservation of the ossicular chain in 3 patients, a reconstruction of the ossicular chain by an ossiculoplasty in one patient, while 4 patients underwent an exclusion of the middle ear. The three inoperable patients were considered for radiotherapy.
-
-
Postoperative data
The hospital stay duration varied between 2 and 7 days, with a median of 4,2 days. Besides the previous nervous lesions, postoperative complications where mainly represented by vertigo and tinnitus. The postoperative tonal audiometry showed hearing levels preservation for the 4 patients whose middle ear was preserved.
-
-
Histopathology examination
The diagnosis of paraganglioma was confirmed in 100% of patients after histopathological examination.
4. Discussion
Paragangliomas are rare, usually benign hypervascular tumors, derived from cells of the diffuse neuroendocrine system - from the skull to the pelvis, characterized by slow growth over time. However, there remains the risk of local compression, involvement of important neurovascular structures, such as the internal carotid artery (ICA), the facial nerve (VII) and the lower cranial nerves (LCNs: IX, X, XI, XII) and associated malignancy [[1], [2], [3]].
With an overall incidence of 1 in 30,000–100,000 and a strong female predilection, head and neck PGs (HNPG) arise, in decreasing order of frequency, from the carotid body (CBP), jugular bulb (IJP), vagus nerve (VP) (5%), the tympanic branch of the glossopharyngeal nerve or auricular branch of the vagus nerve (TP), and the cervical sympathetic chain (SCP) [1,5].
It seems that 30–40% of cases of HNPG are known to occur in patients with hereditary predisposition [1,2]. The most commonly implicated are germline mutations in one of the four subunits (A-D) of the succinate dehydrogenase complex of the mitochondrial electron transport chain and its flavination co-factor (SDHA-D, SDHAF2, collectively referred to as SDHx) [1]. Screening for pathologic SDHx mutations in all patients with HNPG diagnosis should be a standard of care regarding the considerable prevalence (6–25%) of mutations in patients with apparently sporadic forms [1,2] and the higher risk of malignancy, specially, in case of SDHB gene mutation [6]. However, the financial burden in our context prevented us to perform genetic testing for our patients.
The common clinical presentation of CP is a pulsatile lateral cervical mass, usually with restricted vertical mobility as seen in our group 1 of patients [2]. VP develop in the retrostyloid compartment of the parapharyngeal space. Depending on their size, they may appear as a latero-cervical mass. They are often monofocal but they may be part of a multifocal disease that develops either metachronically or synchronously [7]. Cranial nerve deficits mainly concern the vagus nerve and can be presented with dysphonia and dysphagia.
On another hand, the presence of a pulsatile middle ear mass is pathognomonic of a TBP. Whitening of the middle ear component during pneumatic otoscopy (Brown's sign) can be seen in 20% of the cases as well as the external extension of the tumor through the tympanic membrane can be mistaken for an inflammatory polyp causing otorrhagia. The main complaints of 60–80% of patients with TJP are pulsatile tinnitus with hearing loss, usually conductive secondary to the tumor compression, effusion or erosion of or the ossicular chain, or sensoneurinal hearing loss with vestibular symptoms if the inner ear is invaded [3].
Cranial nerve deficits can be masked for a long time, due to slow progression of the tumor, gradually compensated by the contralateral functional nerves. Palsies of cranial nerves (IX and X) are present in 35–40% of cases, clinically presented as vocal fold paralysis and palatal asymmetry. Palsies of cranial nerves (XI and XII) are seen in 21–30% of cases. Facial palsy following facial nerve invasion is reported in 10–39% of cases. Complete cranial nerves examination, including palpation of the neck and upper aero-digestive tract endoscopy, is an integral part of the examination for TBPs. Jugular fossa pathology must be kept in mind for patients with isolated or multiple LCN palsies [3].
The appearance of the tumor on imaging helps to determine Shamblin's classification: grade I is defined as a small, localized tumor with minimal attachment to the carotid arteries (internal and/or external) which is easy to resect. Grade II tumor is partially attached without fully encasing the carotid arteries. Grade III tumor largely encases at least one of the carotid arteries. This classification enables to predict the operative time, the difficulty of resection, the blood loss, the risk of operative vascular lesion but not the occurrence of postoperative complications. [2, 8]
Vagal paraganglioma appears on MRI similar to CBP, in the same location given the proximity of the Vagus nerve to the carotid artery, although, it is highly enhanced, displacing the internal and external carotid arteries anteriorly and the internal jugular vein posteriorly, without widening the carotid bifurcation. Imaging also distinguishes others parapharyngeal tumors such as pleomorphic adenoma and schwannoma [7].
Temporal CT scan is the mainstay of TJP diagnosis, where bone erosion is classically described as a “moth eaten” appearance and concerns the margins of the jugular fossa, the carotico-jugular crest or the entire jugular foramen in addition to the component of the middle ear. TMP are differentiated from TJP when identifying the margins of the jugular foramen which are large and free [3].
Radiological parameters allow determining the Fisch classification which is essential for choosing the adequate operative approach. Class A is a PG that arises along the tympanic plexus on promontory. Class B: PG invades the hypotympanum leaving the cortical bone over jugular bulb intact. Class C1: PG erodes the carotid foramen. Class C2: PG destroys the vertical carotid canal. Class C3: PG involves the horizontal carotid canal and in C4, PG invades the foramen lacerum and cavernous sinus. Class De concern PG with intracranial but extradural extension (De1 < 2 cm, De2> 2 cm, according to the displacement of the dura). Class Di represent PG with intracranial and intradural extension (Di1: less than 2 cm, Di 2 = between 2 and 4 cm, Di3 = more than 4 cm, according to depth of invasion into the posterior cranial fossa) [9]. On MRI, PG appears with low to intermediate intensity in T1 signal and high intensity in T2 signal with the classic “salt and pepper” pattern appearance in lesions >2 cm, especially in T2 images due to areas of hyperintensity because of slow flow within the tumor and the presence of intratumoral vessels appearing as flow voids [3]. Magnetic Resonance angiography allows the mapping of dominant tumor feeding vessels and demonstrates characteristic blush and rapid venous diffusion, the degree of ICA involvement, the contralateral cerebral blood flow, the venous drainage. It is an important for pre-operative preparation since it guides the endovascular embolization [1,3].
Given a sensitivity of 22% and considering the fact that HNPG are infrequent secretors (<3%), plasma and/or urine metanephrines is more useful for detecting concomitant catecholamine-secreting PG at other sites in SDHx mutation carriers or, most importantly, a concomitant pheochromocytoma, with sensitivity near to 100% [1].
The endovascular embolization of PG prior to surgical resection reduces operative blood loss and associated morbidity. It consists on the occlusion of all feeding vessels with an intentional delay of 1–2 days before surgery allowing time for edema to resolve without providing time for reconstitution or recruitment of feeding arteries [10].
The embolization in CBP, even if still controversial since it increases peritumoral inflammation, may facilitate the resection of voluminous and very adherent tumors, particularly if close to the skull base and reduces the time of vascular clamping and the blood loss [11,12]. However, it became an essential tool in the surgical management of TJP easing tumor dissection and hemostasis in the temporal bone complex anatomy. The main major complications of preoperative embolization are the occurrence of a stroke with accidental introduction of emboli into the vertebrobasilar system via the ECA or its anastomoses with the ICA, or cranial nerve palsies if embolic material is introduced into the vessels which supply them [10].
Complete surgical is the only curative treatment [2]. In CBP surgery, vascular risk has decreased over the past few decades and the risk of postoperative stroke is actually very low. Vascular manipulation and repair has become safer with the early control of proximal and distal carotid arteries, sub-adventitial tumor dissection, effective heparin therapy, precise hemodynamic control and reduced clamping time [2]. Peritumoral lymph nodes can be resected to identify possible occult metastasis. Boedeker et al. [13] recommended selective neck dissection for levels II and III, but the extension of the neck dissection remains to be defined. Lamblin et al. study reported a rate of malignancy around 6% [2]. For VP, small tumors ⩽2 cm are usually removed with predictable morbidity concerning only the vagus nerve, while larger tumors carry higher surgical risks for additional cranial nerve palsies and internal carotid artery damage [7].
The main factors to consider in TJP surgery are whether the facial nerve needs to be mobilized and whether the middle ear can be preserved, in addition to the degree of ICA involvement and the intracranial extension [3]. Surgical removal is the treatment of choice of TPs, categorized as Fisch A and B, through transmeatal approach or mastoidectomy, with posterior tympanotomy and exposure of the facial nerve. TJP categorized as Fisch C and D are usually resected via an infratemporal approach. Tumors classified as Fisch C1, C2 and De, Di1/2 may need a variant of the juxtacondylar approach in association with further surgical procedures to correct cranial nerves injuries. Finally, tumors classified as Fisch Di1/2 should be resected in a two-stage, team-approach procedure involving neurosurgeons. This therapeutic option is not available for patients categorized as Fisch Di3. In these cases, palliative radiotherapy is advocated [9]. A review on long-term control of operated TJP enrolling 176 patients between 1972 and 1998 observed complete surgical control in 85% of cases. The main complications were cerebrospinal fluid leakage in 4,5% of cases with intracranial extension and new cranial nerves deficits (IX, X, XI, XII) in 39%, 25%, 26% and 21% of cases respectively [14].
In order to detect and preserve the facial nerve, specifically on TP, surgery should be performed under facial nerve neural integrity monitoring [15]. Also, facial nerve rerouting techniques were developed to enhance exposure of the jugular foramen and internal carotid artery with safe management of the nerve [16].
In case of multiplicity, functional tumors (pheochromocytoma) are treated first [2]. If there are signs of LCN deficits on the side of the larger tumor, surgery is recommended on that side first, following which the smaller tumor is either operated or irradiated. On the contrary, if the patients present with LCN deficits on the side of the smaller tumor, surgery is performed on the smaller tumor [3].
Fractioned radiation (RT) and stereotactic radiosurgery (SRS) are safe alternatives to surgery. They are best suited for patients with large PGs with high morbidity to lower cranial nerves or contraindications to surgery [1]. It is also mainly indicated as adjuvant treatment after incomplete surgical resection, slowing tumor growth and improving survival. However, there is no evidence that it improves the 5-year overall survival in case of metastasis [17,18]. While some studies reported comparable 10-year control rates, specific survival, and distant-metastasis free survival, between surgery and RT and SRS [5,7]
Regarding the very slow and indolent tumor growth pattern and exceedingly low rates of malignant degeneration, “watchful waiting” seems to be a conservative alternative to consider for appropriately selected cases like elderly patients with small tumor size. Nevertheless, this “wait and see” protocol is switched to active treatment in front of radiologic growth, pain, or new cranial nerve dysfunction [1,2].
The main challenge facing the surgeon is the management of LCN injuries. These deficits may result in varying degrees of lingual, palatal, pharyngeal, and laryngeal dysfunction requiring early rehabilitation. Most patients compensate, but deficits involving more than one nerve extend the rehabilitation period for speech and swallowing [5]. In Lamblin and al. study on CBP [2], 68% of peripheral neurologic deficit was transient, with permanent deficit at 18 months seen in 17% of the cases.
According to Lee and al. [17] and Sethi and al. [18] studies on malignant HNPG, patients with regional metastasis have better 5-year overall survival (76.8–82.4% respectively) in comparison to those with distant metastases (11.8–41.4% respectively). Elshaikh and al. [19] reported long median time to recurrence of HNPG of 36 months (range from 15 to 350 months). Thus, long follow up is mandatory to detect late recurrence and was the main challenge in our study, overcame by frequently calling up the patients for clinical examination.
5. Conclusion
Head and neck paragangliomas are mainly benign, slow-growing tumors, commonly delayed in diagnosis. Typical clinical manifestations and imaging evaluation should be considered together to establish the treatment protocol. MRI is efficient to detect VP. According to the stage, surgery can provide excellent control of the tumor while radiation therapy may be considered for patients with surgery contraindication such as advanced age, comorbidity or aggressive or unresectable tumors.
The authors recommend a more personal and adapted management protocol to each case regarding the patient-related factors including age, goals of care, medical comorbidities and genetic status, in addition to the potential anatomical damages caused by the growth of the PG, its size, site, multcentricity and its seldom malignant potential.
Ethical approval
Written informed consent was obtained from the patients for publication of these results and accompanying images.
A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Source of funding
None.
Author contribution
Boutaina Merzouqi: Corresponding author, writing the paper.
Khadija El Bouhmadi: Writing the paper.
Youssef Oukesou: Study concept.
Sami Rouadi: Study concept.
Redallah Larbi Abada: Study concept.
Mohamed Roubal: Correction of the paper.
Mohamed Mahtar: Correction of the paper.
Research registration unique identifying number (UIN)
Researchregistry6728.
Trial registry number
Not needed.
Guarantor
Merzouqi Boutaina.
Provenance and peer review
Not commissioned, externally peer-reviewed.
Declaration of competing interest
The authors declare having no conflicts of interest for this article.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.amsu.2021.102412.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Smith J.D., Harvey R.N., Darr O.A., Prince M.E., Bradford C.R., Wolf G.T., Else T., Basura G.J. Head and neck paragangliomas: a two-decade institutional experience and algorithm for management. Laryngoscope Investig. Otolaryngol. 2017 Nov 11;2(6):380–389. doi: 10.1002/lio2.122. PMID: 29299512; PMCID: PMC5743157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lamblin E., Atallah I., Reyt E., Schmerber S., Magne J.L., Righini C.A. Neurovascular complications following carotid body paraganglioma resection. Eur. Ann. Otorhinolaryngol. Head Neck Dis. 2016 Nov;133(5):319–324. doi: 10.1016/j.anorl.2016.05.006. Epub 2016 Jun 11. PMID: 27297087. [DOI] [PubMed] [Google Scholar]
- 3.Prasad SC, Paties CT, Schiavi F, et al. Tympanojugular Paragangliomas: Surgical Management and Clinicopathological Features. In: Mariani-Costantini R, editor. Paraganglioma: A Multidisciplinary Approach [Internet]. Brisbane (AU): Codon Publications; 2019 Jul 2. Chapter 6. Available from: https://www.ncbi.nlm.nih.gov/books/NBK543222/# doi: 10.15586/paraganglioma.2019.ch6. [PubMed]
- 4.Agha R.A., Abdall-Razak A., Crossley E., Dowlut N., Iosifidis C., Mathew G., for the STROCSS Group The STROCSS 2019 guideline: strengthening the reporting of cohort studies in surgery. Int. J. Surg. 2019;72:156–165. doi: 10.1016/j.ijsu.2019.11.002. [DOI] [PubMed] [Google Scholar]
- 5.Netterville J.L., Jackson C.G., Miller F.R., Wanamaker J.R., Glasscock M.E. Vagal paraganglioma: a review of 46 patients treated during a 20-year period. Arch. Otolaryngol. Head Neck Surg. 1998;124(10):1133–1140. doi: 10.1001/archotol.124.10.1133. [DOI] [PubMed] [Google Scholar]
- 6.Van Hulsteijn L.T., Dekkers O.M., Hes F.J., Smit J.W.A., Corssmit E.P.M. Risk ofmalignant paraganglioma in SDHB-mutation and SDHD-mutation carriers: asystematic review and meta-analysis. J. Med. Genet. 2012;49(12):768–776. doi: 10.1136/jmedgenet-2012-101192. [DOI] [PubMed] [Google Scholar]
- 7.Zanoletti E., Mazzoni A. Vagal paraganglioma. Skull Base. 2006 Aug;16(3):161–167. doi: 10.1055/s-2006-949519. PMID: 17268589; PMCID: PMC1586175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Law Y., Chan Y., Cheng S. vol. 25. 2017. Surgical Management of Carotid Body Tumor – Is Shamblin Classification Sufficient to Predict Surgical Outcome? Vascular; pp. 184–189. 2. [DOI] [PubMed] [Google Scholar]
- 9.Offergeld C., Brase C., Yaremchuk S., Mader I., Rischke H.C., Gläsker S., Schmid K.W., Wiech T., Preuss S.F., Suárez C., Kopeć T., Patocs A., Wohllk N., Malekpour M., Boedeker C.C., Neumann H.P. Head and neck paragangliomas: clinical and molecular genetic classification. Clinics. 2012;67(Suppl 1):19–28. doi: 10.6061/clinics/2012(sup01)05. PMID: 22584701; PMCID: PMC3328838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.White J.B., Link M.J., Cloft H.J. Endovascular embolization of paragangliomas: a safe adjuvant to treatment. J. Vasc. Interv. Neurol. 2008 Apr;1(2):37–41. PMID: 22518217; PMCID: PMC3317314. [PMC free article] [PubMed] [Google Scholar]
- 11.Power A.H., Bower T.C., Kasperbauer J. Impact of preoperative embolizationon outcomes of carotid body tumor resections. J. Vasc. Surg. 2012;56(4):979–989. doi: 10.1016/j.jvs.2012.03.037. [DOI] [PubMed] [Google Scholar]
- 12.Zhang T., Jiang W., Li Y., Li B., Yamakawa T. Perioperative approach in the surgicalmanagement of carotid body tumors. Ann. Vasc. Surg. 2012;26(6):775–782. doi: 10.1016/j.avsg.2012.01.020. [DOI] [PubMed] [Google Scholar]
- 13.Boedeker C.C. Paragangliomas and paraganglioma syndromes. GMS Curr. Top. Otorhinolaryngol., Head Neck Surg. 2011;10:3. doi: 10.3205/cto000076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jackson C.G., McGrew B.M., Forest J.A., Netterville J.L., Hampf C.F., Glasscock M.E., 3rd Lateral skull base surgery for glomus tumors: long-term control. Otol. Neurotol. 2001 May;22(3):377–382. doi: 10.1097/00129492-200105000-00018. PMID: 11347643. [DOI] [PubMed] [Google Scholar]
- 15.Forest J.A., 3rd, Jackson C.G., McGrew B.M. Long-term control of surgically treated glomus tympanicum tumors. Otol. Neurotol. 2001 Mar;22(2):232–236. doi: 10.1097/00129492-200103000-00020. PMID: 11300275. [DOI] [PubMed] [Google Scholar]
- 16.Bacciu A., Ait Mimoune H., D'Orazio F., Vitullo F., Russo A., Sanna M. Management of facial nerve in surgical treatment of previously untreated fisch class C tympanojugular paragangliomas: long-term results. J. Neurol. Surg. B Skull Base. 2014;75(1):1–7. doi: 10.1055/s-0033-1349061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee J.H., Barich F., Karnell L.H. National cancer data base report on malignantparagangliomas of the head and neck. Cancer. 2002;94(3):730–737. doi: 10.1002/cncr.10252. [DOI] [PubMed] [Google Scholar]
- 18.Sethi RV, Sethi RKV, Herr MW, Deschler DG. Malignant head and neck para-gangliomas: treatment efficacy and prognostic indicators. Am. J. Otolaryngol.;34(5):431–438. [DOI] [PubMed]
- 19.Elshaikh M.A., Mahmoud-Ahmed A.S., Kinney S.E. Recurrent head-and-neckchemodectomas: a comparison of surgical and radiotherapeutic results. Int. J. Radiat. Oncol. 2002;52(4):953–956. doi: 10.1016/s0360-3016(01)02751-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.