Abstract
Interleukin 6 (IL-6) is an established biomarker of inflammation with one of the earliest responses in sepsis. Serum levels can easily be measured within a few hours. The clinical significance of IL-6 in the early stage of sepsis in burned patients has not yet been confirmed. The purpose of our research was to investigate the predictive value of IL-6 for positive blood cultures in comparison to Procalcitonin (PCT), white blood cell (WBC) count, body temperature and the Sequential Organ Failure Assessment (SOFA) score in the presence of suspected sepsis in burn patients. In a retrospective study, we included all patients admitted to a regional burn centre in a 7-year period. Patients with a clinical suspicion of sepsis and complete laboratory tests underwent further analysis. Patients were categorized following culture results into either positive or negative bloodstream infection (BSI or non-BSI) groups. 39 of the 101 included patients had positive blood cultures (BSI). The serum IL-6 levels were significantly higher in the BSI group [1047 (339.9; 9000.5) vs. 198.5 (112.4; 702.5) ng/l; P = 0.001]. Receiver operating characteristic (ROC) curve analysis showed an AUC of 0.7 (59; 80.8%). The optimal IL-6 cut-off level was 312.8 ng/l (sensitivity 79.5%, specificity 56.5%). Other biomarkers (PCT, WBC), the maximum body temperature and increase of SOFA score were not different between the groups. IL-6 can be used to predict a positive blood culture even in the early stage of suspected sepsis in burned patients. In this context, other biomarkers (PCT, WBC) and body temperature are of limited clinical utility.
Keywords: Burn injury, blood stream infection, sepsis, IL-6, PCT
Introduction
In the severely burned patient the differentiation between sepsis and inflammation represents a challenge with implications for appropriate clinical management. The dilemma includes unnecessary antibiosis in inflammation or delayed treatment for sepsis with consequent poorer outcomes.
No single score, biomarker or clinical parameter exists to reliably inform the physician of early diagnosis of sepsis [1,2]. Traditional indicators of infection, such as body temperature and white blood cell (WBC) count, have been found to be unsuitable as sepsis markers [3,4]. C-reactive protein (CRP) has also been shown to be a poor marker of sepsis [4,5]. Procalcitonin (PCT) is established with a wide clinical application. Due to the late onset of changes and the weak predictive value, a recent comparative review questioned the reliability of PCT for the early diagnosis of sepsis [2].
The aim of the present study was to investigate the predictive value of IL-6 for positive blood cultures in comparison to PCT, WBC count, body temperature and the Sequential Organ Failure Assessment (SOFA) score in the presence of suspected sepsis in burn patients.
Materials and methods
Subjects and study design
Ethics approval was issued by the Saxon State Chamber of Medicine on January 8, 2020 (EK-BR-112/19-1). This retrospective study included all patients admitted to the intensive care unit (ICU) of a regional burn centre within a 7-year period (01.01.2012-31.12.2018).
Patient records were screened for a specific point in time during which there was a clinical event of suspected sepsis. Patients with the complete pallet of laboratory tests (blood cultures, levels of IL-6, PCT and WBC count) at that point underwent further analysis. Patients were categorized following culture results into either positive or negative bloodstream infection (BSI or non-BSI) groups in which IL-6, PCT, WBC count, body temperature and the SOFA score were then compared. If multiple septic episodes occurred in one patient, only the first episode was included in the data set. Patients < 18 years, with coexisting polytrauma, immunosuppressive therapy and those considered for palliative care were primarily excluded from the study (Figure 1).
Figure 1.
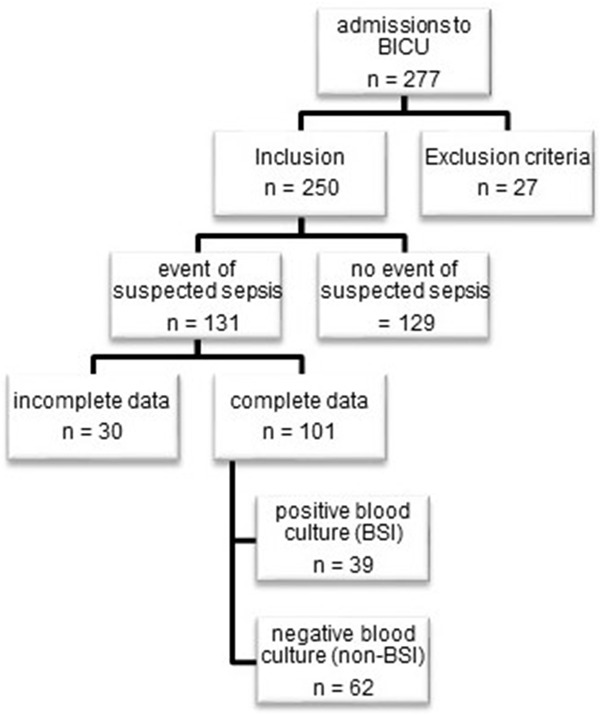
Flowchart of patient inclusion, BICU = burn intensive care unit, BSI = bloodstream infection.
Infection criteria and clinical management
The suspicion of sepsis within our institution is based on clinical experience, including observation of the following: deterioration of the patient’s general condition, a rapid increase in temperature, new tachycardia, haemodynamic instability, increasingly laboured breathing, altered mental state without another reason, and the inability to receive enteral feeding in combination with altered laboratory values (new alteration of blood glucose, increase in biomarkers such as the WBC count, increase in lactate and negative base excess).
Suspected sepsis triggered a standardized protocol. After the vascular access lines were immediately changed, blood, urine and burn wound cultures were taken. Laboratory parameters include WBC count, PCT and IL-6. According to SSC guidelines, two pairs of peripheral blood samples are standard for obtaining a blood culture sample. Chest X-ray and bronchoscopy with microbiological cultures of bronchoscopy specimens were also acquired. The initiation of empirical antibiotic therapy with piperacillin/tazobactam was standard protocol. Upon receiving the microbiological results, antibiotic therapy was adjusted accordingly. In cases of suspected wound-induced sepsis, early surgical debridement was executed. No prophylactic antibiotics were given.
Burn care protocol
Initial fluid infusion, according to Parkland’s formula was administrated at 4 ml/kg bodyweight (BW)/% burned total body surface area (TBSA). Infusions were thereafter adapted to maintain a urine output of 0.5 ml/kg BW/h. Vasopressor support and/or 20% human albumin infusion was used for cases of unstable cardiovascular function. Serum levels of albumin were maintained above 25.0 g/l. Administration of human albumin was avoided in the first 8 hours after injury. Enteral nutrition was initiated within 6 hours of admission or at the earliest possible moment thereafter. Completion of burn wound excision and grafting was performed within 72 hours. No more than a maximum of 20% of the burned surface area was excised in a single sitting. Thereafter, surgical excisions were repeated every 2-3 days as required.
Data collection
The patient data management system (ICU Data, IMESO, Gießen) and patient medical records were used to collect and record data. The collected data included demographic and injury characteristics, results of blood cultures, WBC, PCT, IL-6, SOFA score and maximal body temperature (Tmax). SOFA scores were recorded at 06:00 hours at 24 h intervals. The Tmax was noted in a period 4 hours before and 4 hours after blood culture sampling.
Biomarker measurement
IL-6 levels were determined by an electrochemiluminescence immunoassay, with 200 µl of serum. Our laboratory uses < 6 ng/l as the reference level for IL-6. According to the literature, the reference serum level of IL-6 for the 95th percentile of healthy individuals is < 4.45 ng/l [7]. Cut-off levels of IL-6 for the detection of sepsis have not been established. Serum PCT levels were determined by an electrochemiluminescence immunoassay, with 100 µl of serum. Our laboratory uses < 0.5 µg/l as the reference level for PCT. Normal levels of PCT in the healthy population are < 0.05 µg/l [8]. PCT levels ≥ 0.5 µg/l can be considered clinically relevant for the diagnosis of sepsis [9].
Statistical analysis
The results are presented in a table as the median value (50% quartile) and the interquartile range (interval of the 25% and 75% quartile). Whether the groups (BSI and non-BSI) were comparable with regard to certain parameters (burned TBSA, age, sex, inhalation trauma and Abbreviated Burn Severity Index (ABSI) score) was determined by using logistic regression for the matching parameters. This resulted in a probability (propensity score) for the members assigned to the BSI or non-BSI group. The logistic regression showed that using the assignments based on the propensity score was not significantly better than using the raw data. We concluded that the raw data could be used. Metric parameters were tested for the normality of their distributions using normal Q-Q plots and the Shapiro-Wilk test. The results contradicted the assumption of a normal distribution. The differences between groups were determined using the non-parametric Mann-Whitney U test. Associated categorical parameters were tested using Fisher’s exact test. Receiver operating characteristic (ROC) curve analysis was used to assess the quality of the biomarkers for the prediction of a positive or negative blood culture and to determine reasonable cut-off values. The alpha level of the study was P = 0.05. Consequently, significant test results were obtained at an P value of < 0.05. The analyses were performed using R for Windows version 3.51 software.
Results
Patients, injury characteristics, and outcome parameters
In total, 101 patients met the inclusion criteria. According to the blood culture results, the patients were divided into two groups: no blood stream infection (non-BSI), with negative blood culture (n = 62) and blood stream infection (BSI), with positive blood culture (n = 39). The groups showed no significant differences in age, burned TBSA, full-thickness burn percent, sex, inhalation injury or mortality. The BSI group had a significantly longer hospital stay and a higher continuous renal replacement therapy (CRRT) rate (Table 1).
Table 1.
Baseline patient and injury characteristics and outcome parameters in patients with negative (non-BSI) and positive (BSI) blood cultures
| Non-BSI (n = 62) | BSI (n = 39) | P-value | |
|---|---|---|---|
| Median [Range], n (%) | Median [Range], n (%) | ||
| Age (y) | 55 [43.75; 74] | 57 [44; 75.5] | 0.848 |
| Male, n | 46 (74.2%) | 31 (79.5%) | 0.543 |
| Burned TBSA (%) | 24.75 [16; 34] | 25 [14; 42.25] | 0.389 |
| Full-thickness burn (%) | 18 [4; 24] | 20 [9.5; 29] | 0.285 |
| ABSI | 8 [6; 9] | 8 [7; 10] | 0.068 |
| Inhalation injury | 20 (32.3%) | 14 (35.9%) | 0.706 |
| Days to suspected sepsis | 7 [4; 9] | 5.5 [2; 9] | 0.433 |
| Days of hospital stay | 30 [21.25; 43.75] | 41 [26; 64] | 0.013 |
| Days of hospital stay for survivors | 31 [22; 44.5] | 43 [29; 72] | 0.0044 |
| CRRT | 8 (12.9%) | 13 (33.3%) | 0.014 |
| Mortality | 8 (12.9%) | 10 (25.6%) | 0.103 |
BSI, blood stream infection; TBSA, total body surface area; ABSI, Abbreviated Burn Severity Index; CRRT, continuous renal replacement therapy.
Infection characteristics
In total, 39 positive blood cultures were detected with 16 (41%) gram-negative and 18 (46.2%) gram-positive bacteria. In 5 samples (12.8%), both gram-positive and gram-negative pathogens were found. In 8 blood culture samples, more than one pathogen was found.
Infection parameters and SOFA score
IL-6 serum levels were above normal in all patients. IL-6 levels were significantly higher in the BSI group than in the non-BSI group (1047 [339.9; 9000.5] vs 198.5 [112.4; 702.5], P = 0.001). The subgroup with gram-negative blood cultures had significantly higher levels of IL-6 (2123.5 [356.6; 10375]) than the non-BSI group (P = 0.001). The IL-6 level in patients with gram-positive blood cultures was 646.25 [238.5; 4334.7]. The difference narrowly missed statistical significance in comparison with the non-BSI group (P = 0.06). There was no statistical difference in IL-6 levels between the gram-positive and gram-negative groups (P = 0.247) (Table 2).
Table 2.
Infection markers and SOFA scores in patients with negative (non-BSI) and positive (BSI) blood cultures
| Median [Range] | P values | |||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| non-BSI n = 62 | BSI n = 39 | Gram-negative n = 16 | Gram-positive n = 18 | non-BSI vs BSI | non-BSI vs Gram-negative | non-BSI vs Gram-positive | Gram positive vs Gram negative | |
| IL-6 (ng/l) | 198.5 [112.4; 702.5] | 1047 [339.9; 9000.5] | 2123.5 [356.6; 10375] | 646.25 [238.5; 4334.7] | 0.001 | 0.001 | 0.06 | 0.247 |
| PCT (µg/l) | 0.58 [0.18; 1.35] | 0.82 [0.24; 3.7] | 0.82 [0.28; 1.74] | 0.64 [0.15; 2.54] | 0.182 | 0.307 | 0.936 | 0.501 |
| WBC count (109/l) | 12.06 [8.22; 18.44] | 10.02 [4.76; 14.88] | 7.36 [5.47; 12.87] | 13.29 [7.86; 15.91] | 0.032 | 0.018 | 0.653 | 0.184 |
| Tmax (°C) | 38.75 [38; 39.1] | 38.6 [37.9; 39.2] | 38.75 [37.9; 39.3] | 38.5 [37.95; 39] | 0.856 | 0.651 | 0.548 | 0.500 |
| SOFA pre | 3 [2; 5] | 3 [2; 7.5] | 3 [2; 4] | 3 [2; 7.5] | 0.231 | 0.946 | 0.525 | 0.596 |
| SOFA post | 3 [2; 6] | 6 [3; 7.5] | 6 [3; 7] | 4.5 [3; 7] | 0.008 | 0.107 | 0.093 | 1.00 |
| SOFA delta | 0 [0; 1] | 1 [0; 3] | 1.5 [0; 3.25] | 0.5 [0; 2.5] | 0.086 | 0.039 | 0.610 | 0.764 |
BSI, blood stream infection, IL-6, interleukin 6; PCT, procalcitonin; WBC, white blood cell; T max, maximum temperature; SOFA, Sequential Organ Failure Assessment, SOFA pre = SOFA score on the day before the clinical suspicion of sepsis was established, SOFA post = SOFA score on the day the clinical suspicion of sepsis was established, SOFA delta = differences in SOFA scores on the day before and the day the clinical suspicion of sepsis was established.
Different cut-off levels of IL-6 were tested for their sensitivity and specificity for predicting blood culture positivity in an ROC curve analysis (Table 3). The cut-off value of 312.8 ng/l had a sensitivity of 79.5% and a specificity of 56.5%. The ROC curve for IL-6 had an area under the curve (AUC) of 0.7 (CI 0.59; 0.8) (Figure 2).
Table 3.
Specificity and sensitivity of different IL-6 cut-off levels for positive blood cultures
| IL-6 cut-off ng/l | 104.5 | 180.5 | 312.8 | 419.3 |
| Specificity % | 22.6 | 48.3 | 56.5 | 67.7 |
| Sensitivity % | 87.1 | 84.6 | 79.5 | 66.6 |
Figure 2.
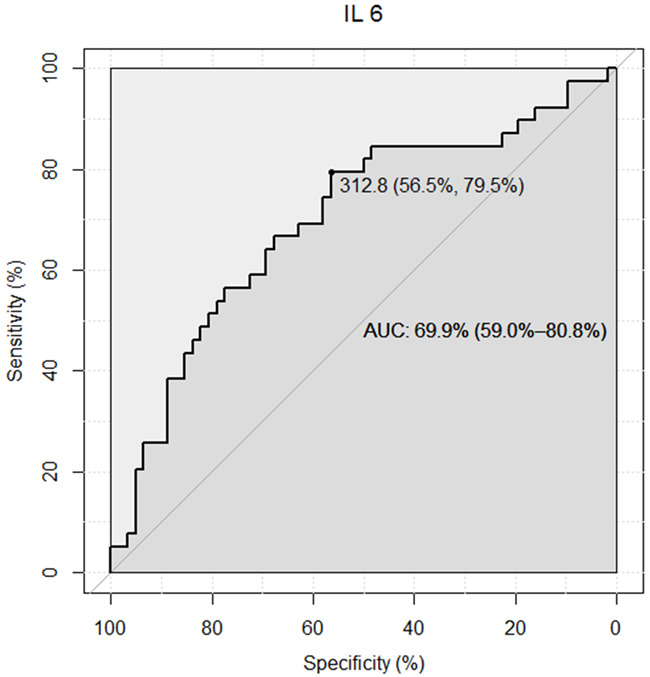
Receiver operating characteristic (ROC) curve for Interleukin 6 (IL-6) for detection of a positive blood culture.
PCT levels were not significantly different between the groups. The WBC count was significantly higher in the non-BSI group. This was also true for the gram-negative subgroup but not the gram-positive subgroup. T max was comparable in all groups (Table 2). The SOFA score on the day before the clinical suspicion of sepsis (SOFA pre) was comparable between groups. On the day the clinical suspicion of sepsis was established, the SOFA score was significantly higher in the BSI group (SOFA post: 6 [3; 7.5] vs 3 [2; 6], P = 0.008). The median difference in the SOFA score between the day before and the day the of clinical suspicion of sepsis (SOFA delta) was significantly higher only in the gram-negative subgroup compared to the non-BSI group (0 [0; 1] vs 1.5 [0; 3.25], P = 0.039) (Table 2).
Discussion
The main finding of the present study is that IL-6 may predict positive blood cultures in patients with clinical suspicion of sepsis. ROC curve analysis revealed a moderate diagnostic performance with an AUC of 0.7. A chosen cut-off level of 312.8 ng/l provided a specificity of 56.5% and sensitivity of 79.5%, which can support decision making regarding the use of empirical antibiotics and other diagnostic and therapeutic measures (change in devices, microbiological samples, CT scans, surgery). This may permit a more restrictive and deliberate approach especially in patients in a less critical condition. Thus, IL-6 levels might facilitate the appropriate usage of antibiotics, reduce the risk of antibiotic resistance and optimise the use of hospital resources.
The results are consistent with those in the literature in a non-burned population, which showed a good correlation between elevated IL-6 levels and sepsis [6,10]. A meta-analysis of a non-burned population found an AUC of 0.8 for IL-6 to differentiate patients with sepsis from those with non-infectious systemic inflammatory response syndrome [11]. There is no relevant available data for burn patients to date.
We found a strong predictive ability of the IL-6 level for blood stream infection caused by gram-negative pathogens. However, for patients with gram-positive blood stream infections, IL-6 levels were also higher compared to those in the non-BSI group, but the difference narrowly missed statistical significance (Table 2). One cell line study [12] showed different biomarker release patterns in gram-positive vs gram-negative bacteria, with extensive IL-6 release in gram-negative pathogens. One clinical study in non-burned paediatric patients confirmed significantly higher levels of IL-6 in those with gram-negative sepsis than in those with gram-positive sepsis [13]. The present study does not reflect these findings showing no statistical differences in IL-6 levels in patients with gram-positive and gram-negative blood cultures.
In the present study, the PCT level was elevated over the threshold (PCT>0.5 µg/ml) in all patients, suggesting an inflammatory state. In the present study, the IL6 and PCT levels were slightly elevated in all patients, suggesting burn-induced subliminal inflammatory state. In contrast to IL-6, we did not find significantly different levels of PCT between the BSI and non-BSI group. The lack of a significant peak in PCT levels in BSI patients could be explained by the latency in PCT release. Blood sampling was performed at time of clinical suspicion of sepsis. Elevated values of PCT become measurable only 2-4 hours after the onset of the infectious process, peaking at 24-30 hours, and rapidly subsiding with recovery [14]. Previous studies reported only a daily measurement of PCT rather than a measurement at the exact time of appearance of sepsis [4,15-17]. In a recent study, we demonstrated a small but significant increase in PCT levels on the day of infection, but a peak PCT level was only noted one day after the infection occurred [18]. This observation may also explain the partially negative results regarding the suitability of PCT for the diagnosis of sepsis in previous studies. In this context, PCT seems to be unsuitable as a biomarker for the early diagnosis of blood culture-positive sepsis in burn patients.
WBC count levels had no clinically relevant alterations among the groups, although statistically significant differences between them were observed. In agreement with the literature, our results do not support the use of the WBC count as a relevant indicator of blood stream infection [19,20]. Elevated temperature was found in all groups, including the non-BSI group. Again this corresponds to the literature [3,4] and illustrates the non-specific reaction of body temperature to inflammatory triggers independent of their origin. Nevertheless, changes in body temperature often trigger the suspicion of sepsis, and they are still incorporated into the sepsis criteria of the American Burn Association (ABA) [21].
Sepsis is now defined as a life-threatening organ dysfunction caused by a dysregulated host response to an infection (Sepsis-3 definition) [22]. Organ dysfunction is recorded using the SOFA score. In our study, SOFA scores were significantly higher in the BSI than in the non-BSI group, suggesting more severe illness in the BSI group. Nevertheless, a SOFA score increase of more than 2 points, as required by the Sepsis-3 definition, did not occur. This could be explained by the fact that negative blood cultures do not exclude sepsis. Our approach in assigning patients to groups according to blood culture positivity may have weakened the statistical importance of the SOFA score. Thus, we can neither confirm nor ally the significance of the SOFA score in burn sepsis. With our findings, we confirm the claims made in previous studies that the best pathway to achieving an early diagnosis seems to be a daily assessment of sepsis by a burn team informed by substantial number of clinical parameters and scores [23,24]. In this context, although far from being ideal, IL-6 might be an additional tool for identifying blood culture-positive sepsis.
The present study has several limitations. As the study had a retrospective design, some patients with suspected or confirmed infections could not be included due to incomplete data. Although we included a reasonable number of patients for a single burn centre, some questions regarding subgroups (i.e., the role of gram-positive vs gram-negative pathogens) could not be sufficiently investigated. The results should be further investigated in a larger number of patients in a prospective study. The present study defined blood sampling and parameter measurement as the same time point of suspected clinical sepsis. Further sampling with a narrow boundaries measured in hours may shed light on the dynamics of sepsis in the acute period rather than a summation average of the period of the first day.
Conclusion
In the acute burn setting the treating physician is faced with the challenge of differentiation between sepsis and posttraumatic inflammation and applying the timely therapeutic pathway to ensure optimal outcome. IL-6 enables early signalling of blood stream infection prior to the availability of blood culture results. Larger studies are required to more clearly define the parameters at which IL-6 levels may become diagnostic.
Acknowledgements
The authors are particularly grateful for the statistical support from Martin Mogk, MoReData (statistical office) GmbH, Kerkrader Street 11, 35394 Giessen, Germany.
Disclosure of conflict of interest
None.
References
- 1.Lopez ON, Cambiaso-Daniel J, Branski LK, Norbury WB, Herndon DN. Predicting and managing sepsis in burn patients: current perspectives. Ther Clin Risk Manag. 2017;13:1107–1117. doi: 10.2147/TCRM.S119938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stanojcic M, Vinaik R, Jeschke MG. Status and challenges of predicting and diagnosing sepsis in burn patients. Surg Infect (Larchmt) 2018;19:168–175. doi: 10.1089/sur.2017.288. [DOI] [PubMed] [Google Scholar]
- 3.Murray CK, Hoffmaster RM, Schmit DR, Hospenthal DR, Ward JA, Cancio LC, Wolf SE. Evaluation of white blood cell count, neutrophil percentage, and elevated temperature as predictors of bloodstream infection in burn patients. Arch Surg. 2007;142:639–642. doi: 10.1001/archsurg.142.7.639. [DOI] [PubMed] [Google Scholar]
- 4.Lavrentieva A, Kontakiotis T, Lazaridis L, Tsotsolis N, Koumis J, Kyriazis G, Bitzani M. Inflammatory markers in patients with severe burn injury. What is the best indicator of sepsis? Burns. 2007;33:189–194. doi: 10.1016/j.burns.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 5.Jeschke MG, Finnerty CC, Kulp GA, Kraft R, Herndon DN. Can we use C-reactive protein levels to predict severe infection or sepsis in severely burned patients? Int J Burns Trauma. 2013;3:137–143. [PMC free article] [PubMed] [Google Scholar]
- 6.Hou T, Huang D, Zeng R, Ye Z, Zhang Y. Accuracy of serum interleukin (IL)-6 in sepsis diagnosis: a systematic review and meta-analysis. Int J Clin Exp Med. 2015;8:15238–15245. [PMC free article] [PubMed] [Google Scholar]
- 7.Todd J, Simpson P, Estis J, Torres V, Wub AH. Reference range and short- and long-term biological variation of interleukin (IL)-6, IL-17A and tissue necrosis factor-alpha using high sensitivity assays. Cytokine. 2013;64:660–665. doi: 10.1016/j.cyto.2013.09.018. [DOI] [PubMed] [Google Scholar]
- 8.Morgenthaler NG, Struck J, Fischer-Schulz C, Seidel-Mueller E, Beier W, Bergmann A. Detection of procalcitonin (PCT) in healthy controls and patients with local infection by a sensitive ILMA. Clin Lab. 2002;48:263–270. [PubMed] [Google Scholar]
- 9.Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA, Schein RM, Sibbald WJ. American college of chest physicians/society of critical care medicine consensus conference: definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit Care Med. 1992;20:864–874. [Google Scholar]
- 10.Song J, Park DW, Moon S, Cho HJ, Park JH, Seok H, Choi WS. Diagnostic and prognostic value of interleukin-6, pentraxin 3, and procalcitonin levels among sepsis and septic shock patients: a prospective controlled study according to the Sepsis-3 definitions. BMC Infect Dis. 2019;19:968. doi: 10.1186/s12879-019-4618-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ma L, Zhang H, Yin YL, Guo WZ, Ma YQ, Wang YB, Shu C, Dong LQ. Role of interleukin-6 to differentiate sepsis from non-infectious systemic inflammatory response syndrome. Cytokine. 2016;88:126–135. doi: 10.1016/j.cyto.2016.08.033. [DOI] [PubMed] [Google Scholar]
- 12.Hessle CC, Andersson B, Wold AE. Gram-positive and gram-negative bacteria elicit different patterns of pro-inflammatory cytokines in human monocytes. Cytokine. 2005;30:311–318. doi: 10.1016/j.cyto.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 13.Shao WX, Yu DJ, Zhang WY, Wang XJ. Clinical significance of interleukin-6 in the diagnosis of sepsis and discriminating sepsis induced by gram-negative bacteria. Pediatr Infect Dis J. 2018;37:801–805. doi: 10.1097/INF.0000000000001904. [DOI] [PubMed] [Google Scholar]
- 14.Schuetz P, Raad I, Amin DN. Using procalcitonin-guided algorithms to improve antimicrobial therapy in ICU patients with respiratory infections and sepsis. Curr Opin Crit Care. 2013;19:453–460. doi: 10.1097/MCC.0b013e328363bd38. [DOI] [PubMed] [Google Scholar]
- 15.Lavrentieva A, Papadopoulou S, Kioumis J, Kaimakamis E, Bitzani M. PCT as a diagnostic and prognostic tool in burn patients. Whether time course has a role in monitoring sepsis treatment. Burns. 2012;38:356–363. doi: 10.1016/j.burns.2011.08.021. [DOI] [PubMed] [Google Scholar]
- 16.Cabral L, Afreixo V, Meireles R, Vaz M, Chaves C, Caetano M, Almeida L, Paiva JA. Checking procalcitonin suitability for prognosis and antimicrobial therapy monitoring in burn patients. Burns Trauma. 2018;6:10. doi: 10.1186/s41038-018-0112-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gille J, Schmidt J, Kremer T, Sablotzki A. Evaluation of MR-proANP and copeptin for sepsis diagnosis after burn injury. J Crit Care. 2019;52:149–155. doi: 10.1016/j.jcrc.2019.04.031. [DOI] [PubMed] [Google Scholar]
- 18.Gille J, Ostermann H, Dragu A, Sablotzki A. MR-proADM: a new biomarker for early diagnosis of sepsis in burned patients. J Burn Care Res. 2017;38:290–298. doi: 10.1097/BCR.0000000000000508. [DOI] [PubMed] [Google Scholar]
- 19.Su CP, Chen TH, Chen SY, Ghiang WC, Wu GH, Sun HY, Lee CC, Wang JL, Chang SC, Chen YC, Yen AM, Chen WJ, Hsueh PR. Predictive model for bacteremia in adult patients with blood cultures performed at the emergency department: a preliminary report. J Microbiol Immunol Infect. 2011;44:449–455. doi: 10.1016/j.jmii.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 20.Cavallazzi R, Bennin CL, Hirani A, Gilbert C, Marik PE. Is the band count useful in the diagnosis of infection? An accuracy study in critically ill patients. J Intensive Care Med. 2010;25:353–357. doi: 10.1177/0885066610377980. [DOI] [PubMed] [Google Scholar]
- 21.Greenhalgh DG, Saffle JR, Holmes JH, Gamelli RL, Palmieri TL, Horton JW, Tompkins RG, Traber DL, Mozingo DW, Deitch EA, Goodwin CW, Herndon DN, Gallagher JJ, Sanford AP, Jeng JC, Ahrenholz DH, Neely AN, O’Mara MS, Wolf SE, Purdue GF, Garner WL, Yowler CJ, Latenser BA. American burn association consensus conference to define sepsis and infection in burns. J Burn Care Res. 2007;28:776–790. doi: 10.1097/BCR.0b013e3181599bc9. [DOI] [PubMed] [Google Scholar]
- 22.Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche JD, Coopersmith CM, Hotchkiss RS, Levy MM, Marshall JC, Martin GS, Opal SM, Rubenfeld GD, van der Poll T, Vincent JL, Angus DC. The third International consensus definitions for sepsis and septic shock (sepsis-3) JAMA. 2016;315:801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Greenhalgh DG. Defining sepsis in burn patients: still a long way to go. J Burn Care Res. 2017;38:e990–e991. doi: 10.1097/BCR.0000000000000551. [DOI] [PubMed] [Google Scholar]
- 24.Yan J, Hill WF, Rehou S, Pinto R, Shahrokhi S, Jeschke MG. Sepsis criteria versus clinical diagnosis of sepsis in burn patients: a validation of current sepsis scores. Surgery. 2018;164:1241–1245. doi: 10.1016/j.surg.2018.05.053. [DOI] [PMC free article] [PubMed] [Google Scholar]


