Abstract
Purpose
Cather ablation is known to influence the autonomic nervous system. This study sought to investigate the association of sinus heart rate pre-/post-ablation and recurrences in patients with atrial fibrillation undergoing pulmonary vein isolation (PVI).
Methods
Between January 2012 and December 2017, data of 482 patients undergoing their first PVI were included. Sinus heart rate was recorded before (PRE), directly post-ablation (POST) and 3 months post-ablation (3 M). All patients were screened for atrial tachyarrhythmia recurrences during the one-year follow-up.
Results
In the total study cohort, the mean resting sinus heart rate at PRE [mean 57.9 bpm (95% CI 57.1–58.7 bpm)] increased by over 10 bpm to POST [mean 69.4 bpm (95% CI 68.5–70.3 bpm); p < 0.001] followed by a slight decrease at 3 M [mean 67.3 bpm (95% CI 66.4–68.2 bpm)] but still remaining higher compared to PRE (p < 0.001). This pattern was observed in patients with and without recurrences at POST and 3 M (both p < 0.001 compared to PRE). However, at 3 M the mean sinus heart rate was significantly lower in patients with compared to patients without recurrences (p = 0.031). In this regard, patients with a heart rate change < 11 bpm (PRE to 3 M) or, as an alternative parameter, patients with a heart rate < 60 bpm at 3 M had a significantly higher risk of recurrences compared to the remaining patients (Hazard ratio (HR) 1.82 (95% CI 1.32–2.49), p < 0.001 and HR 1.64 (95% CI 1.20–2.25), p = 0.002, respectively).
Conclusion
Our study confirms the impact of PVI on cardiac autonomic function with a significant sinus heart rate increase post-ablation. Patients with a sinus heart rate change < 11 bpm (PRE to 3 M) are at higher risk for recurrences during one-year post-PVI.
Electronic supplementary material
The online version of this article (10.1007/s00392-020-01765-z) contains supplementary material, which is available to authorized users.
Keywords: Atrial fibrillation, Pulmonary vein isolation, Parasympathetic denervation, Sinus heart rate, Recurrence
Introduction
Atrial fibrillation is the most common arrhythmia of clinical significance and its prevalence is rising worldwide [1]. Catheter ablation aiming at pulmonary vein isolation (PVI) has become an effective treatment option in patients with symptomatic atrial fibrillation. However, recurrences of atrial tachyarrhythmias after initially successful catheter ablation are common and often require re-do ablations [2]. Despite increasing knowledge about the risk factors for recurrences, identifying patients at risk for recurrence that would benefit from close monitoring remains challenging. The autonomic nervous system has been shown to play a pivotal role in the pathogenesis of atrial fibrillation [3]. In this regard, it has been shown that patients with atrial fibrillation and a low resting sinus heart rate prior to ablation seem to carry a higher risk for recurrence of atrial fibrillation post-ablation [4, 5]. It has been suggested that catheter ablation has an attenuating effect on parasympathetic activity [6, 7] leading to an increased resting heart rate post-ablation. In this context, it has been shown that a low sinus heart rate post-ablation is associated with a significantly higher recurrence risk of atrial fibrillation during follow-up [8–10]. However, studies were either limited by sample size or timepoints for significant changes in sinus heart rate differed. Hence, the aim of this study was to investigate the association of sinus heart rate pre-/post-ablation and recurrences in patients undergoing PVI for atrial fibrillation in a large cohort.
Methods
Study population
All patients who underwent catheter ablation for atrial fibrillation at the Karolinska University Hospital between January 2012 and December 2017 were included provided that a first-time PVI procedure using the radiofrequency technique was performed without major complications such as cardiac tamponade, cerebrovascular events, major bleeding and AV fistula. Patients subjected to additional ablation lines in the right/left atrium or ablation of complex fractionated atrial electrograms were excluded. Patients with a pacemaker or implantable cardioverter defibrillator were also excluded. Complete follow-up information had to be available for 12 months post-PVI. Relevant patient characteristics and procedural details were prospectively collected at the time of the ablation procedure and recorded in a computerized database. All patient data and follow-up information were derived from the digital medical record system (TakeCare, CompuGroup Medical Sweden, Uppsala, Sweden) which covers most of the hospitals and medical practices in Stockholm county.
According to the 2016 ESC guidelines for the management of atrial fibrillation [11], atrial fibrillation was classified as paroxysmal if self-terminating (in most cases within 48 h) or if atrial fibrillation episodes were cardioverted within 7 days. Atrial fibrillation was classified as persistent if it lasted longer than 7 days, including episodes that were terminated by cardioversion (either with drugs or by direct current cardioversion) after 7 days or more.
Catheter ablation procedure
Oral anticoagulation therapy was prescribed at least one month before the procedure. Transesophageal echocardiography prior to the procedure was performed to exclude left atrial appendage thrombus in all patients. The ablation procedures were performed under conscious sedation and analgesia. Throughout the procedure, a continuous infusion of heparin was maintained to achieve an activated clotting time (ACT) of > 300 s and ACT measurements were routinely done every 30 min. All patients underwent sole circumferential PVI as described before at our institution [12]. In brief, vascular access was obtained using the right and/or left femoral vein. Under fluoroscopic guidance trans-septal access to the left atrium was established through which the radiofrequency ablation catheter (Biosense-Webster Inc., Diamond Bar, CA, USA) and circular mapping catheter (Lasso, Biosense-Webster Inc., Diamond Bar, CA, USA) guided by a 3-dimensional mapping system (Carto, Biosense-Webster Inc., Diamond Bar, CA, USA or NavX, St. Jude Medical Inc., St. Paul, MN, USA) were advanced into the left atrium. Circumferential lesions were created to surround the right and left pulmonary veins (PV) with a 3.5 mm irrigated-tip catheter (Biosense-Webster Inc., Diamond Bar, CA, USA). RF energy was applied with a power between 25 and 35 W, with an irrigation rate of 10 to 40 mL/min.
Acute procedural success was defined as an entrance and exit block at least 20 min after initial PV isolation, documented with a circular mapping catheter. Those PVs with acute reconnection were re-isolated. Application of adenosine to assess for dormant PV conduction after ablation was not performed. All patients who underwent ablation were treated with the same approach.
Post-ablation follow-up
Post-ablation, patients were closely monitored for post-procedural complications and discharged home after 24 h. Oral anticoagulation was continued for at least 3 months post-ablation. Further use of oral anticoagulation was determined according to the ESC guidelines [11]. At the discretion of the treating physician, prescription of anti-arrhythmic drugs (AADs) during the 3 months blanking period was performed to favor reverse electrical and structural atrial remodelling. AADs were stopped in all symptom-free patients not later than the end of the blanking period.
For heart rate assessment, resting 12-lead electrocardiograms (ECGs) were recorded within 24 h before ablation (PRE), within 24 h post-ablation (POST) and 3 months post-ablation (3 M). Patients were only included when sinus rhythm was present during these ECG measurements.
For ECG registration, patients had to lay down in supine position. After 3–5 min of rest, the ECG was recorded. ECG registration was at a paper speed of 50 mm/s (PHILIPS, Amsterdam, Netherlands) and digitally stored in the digital medical record system.
Patients were followed-up for one year with clinical visits in the outpatient clinic and at medical practices scheduled at 3-, 6- and 12-months post-procedure. An ambulatory ECG and/or 24-h Holter ECG (at least one Holter ECG during follow-up) was routinely obtained during follow-up visits as well as during unscheduled ambulatory visits related to arrhythmia recurrences. Documentation of arrhythmic episodes was based on ECG, Holter-ECG or event loop recorder (when available). Recurrences were defined as any documented atrial tachyarrhythmia (atrial fibrillation, atrial flutter, atrial tachycardia) lasting > 30 sec occurring after the 3 months blanking period.
Statistical analysis
Continuous variables are presented as mean ± standard deviation or 95% confidence intervals and were compared using Student’s t-tests and ANOVAs. Continuous variables presented as median and interquartile range were compared using Mann–Whitney tests. Categorical variables are expressed as frequencies/percentages and were compared by Chi-square tests. The Kaplan–Meier method was used for building event curves. Hazard ratios with 95% confidence intervals and p values from Cox regression and Log-Rank analyses are provided. All statistical tests and confidence intervals were 2-sided, with a significance level of 0.05. Univariate and multivariable backward logistic regression analyses were performed. The multivariable model considered factors associated with a p value < 0.05 in univariate analysis. Log-rank optimization was performed to determine a cutoff which may have prognostic significance for recurrence. The optimal cut-off was considered as the sinus heart rate change (from PRE to 3 M) separating the population in two groups of which the log-rank comparison revealed the highest chi-square statistic. This was performed using the R package ‘maxstat’ Version 0.7–25 in R (Version 3.6.1) [13]. All other statistical analyses were performed using SPSS software, version 25 (IBM Corp., Armonk, New York).
Results
Between January 2012 and December 2017, 1836 patients underwent ablation for atrial fibrillation at the Karolinska University Hospital. From this patient cohort, 482 patients fulfilled the inclusion criteria and were included in the analysis (Figure 1, supplementary). Patients were divided into two groups dependent on experiencing recurrences [173 patients (35.9%)] vs. no recurrences [309 patients (64.1%)]. Patients with recurrences compared to patients without recurrences had a significantly higher body mass index (27.4 vs. 26.4 kg/m2, p = 0.002), more persistent than paroxysmal atrial fibrillation (46.8% vs. 15.5%, p < 0.001), a longer history of atrial fibrillation (5.0 vs. 4.0 years, p < 0.001), a higher CHA2DS2-VASc Score (p = 0.033), more arterial hypertension (53.2% vs. 38.8%, p = 0.003), more diabetes mellitus (9.2% vs. 4.2%, p = 0.029), a larger left atrial size in the parasternal long axis (39.9 vs. 38.9 mm, p = 0.012), received more beta-blocker at PRE (69.4% vs. 60.2%, p = 0.049) as well as at 3 M (71.1% vs. 57.9%, p = 0.004) and received more other AADs at 3 M (p = 0.047). All baseline and procedural characteristics of patients with and without recurrences are presented in Table 1.
Table 1.
Baseline and procedural characteristics of patients with and without recurrences
| Baseline characteristics | All patients (n = 482) | No recurrence (n = 309) | Recurrence (n = 173) | p value |
|---|---|---|---|---|
| Age (years) | 59.3 ± 10.1 | 59.1 ± 10.3 | 59.5 ± 9.8 | 0.650 |
| Male, n (%) | 319 (66.2) | 214 (69.3) | 105 (60.7) | 0.071 |
| BMI (kg/m2) | 26.8 ± 3.5 | 26.4 ± 3.4 | 27.4 ± 3.6 | 0.002 |
| Type of atrial fibrillation | < 0.001 | |||
| Paroxysmal, n (%) | 353 (73.2) | 261 (84.5) | 92 (53.2) | |
| Persistent, n (%) | 129 (26.8) | 48 (15.5) | 81 (46.8) | |
| Duration of atrial fibrillation in the past, years | 4.2 (2.0; 7.3) | 4.0 (2.1; 6.0) | 5.0 (2.9; 10.0) | < 0.001 |
| Number of failed AADs | 1.1 ± 0.7 | 0.9 ± 0.6 | 1.3 ± 0.8 | < 0.001 |
| CHA2DS2-VASc Score, n (%) | 0.033 | |||
| 0 | 133 (27.6) | 89 (28.8) | 44 (25.4) | |
| 1 | 158 (32.8) | 112 (36.2) | 46 (26.6) | |
| 2 | 108 (22.4) | 59 (19.1) | 49 (28.3) | |
| > = 3 | 83 (17.2) | 49 (15.9) | 34 (19.7) | |
| Congestive heart failure, n (%) | 32 (6.6) | 19 (6.1) | 13 (7.5) | 0.571 |
| Structural heart disease | 0.792 | |||
| Ischemic cardiomyopathy, n (%) | 15 (3.1) | 11 (3.6) | 4 (2.3) | |
| Dilated cardiomyopathy, n (%) | 8 (1.7) | 6 (1.9) | 2 (1.2) | |
| Hypertrophic cardiomyopathy, n (%) | 6 (1.2) | 4 (1.3) | 2 (1.2) | |
| Arterial hypertension, n (%) | 212 (44.0) | 120 (38.8) | 92 (53.2) | 0.003 |
| Diabetes mellitus, n (%) | 29 (6.0) | 13 (4.2) | 16 (9.2) | 0.029 |
| Hyperlipidemia, n (%) | 80 (16.6) | 50 (16.2) | 30 (17.3) | 0.799 |
| Left atrial size, parasternal long axis (mm) | 39.3 ± 4.3 | 38.9 ± 4.1 | 39.9 ± 4.6 | 0.012 |
| Left ventricular ejection fraction (%) | 58.1 ± 4.5 | 58.4 ± 4.4 | 57.7 ± 4.6 | 0.108 |
| Time of RF energy delivery (sec) | 2677.0 ± 946.8 | 2642.5 ± 891.6 | 2736.1 ± 1038.3 | 0.354 |
| Procedure time (min) | 172.2 ± 50.5 | 167.4 ± 47.5 | 179.5 ± 54.7 | 0.009 |
| Fluoroscopy time (min) | 15.6 ± 9.6 | 15.1 ± 9.5 | 16.4 ± 9.5 | 0.096 |
| Beta-blocker at PRE, n (%) | 306 (63.5) | 186 (60.2) | 120 (69.4) | 0.049 |
| Other AADs at PRE | 0.871 | |||
| Dronedarone, n (%) | 60 (12.4) | 37 (12.0) | 23 (13.3) | |
| Flecainid, n (%) | 56 (11.6) | 33 (10.7) | 23 (13.3) | |
| Sotalol, n (%) | 43 (8.9) | 29 (9.4) | 14 (8.1) | |
| Amiodarone, n (%) | 24 (5.0) | 16 (5.2) | 8 (4.6) | |
| Disopyramid, n (%) | 8 (1.7) | 4 (1.3) | 4 (2.3) | |
| Propafenon, n (%) | 1 (0.2) | 1 (0.3) | 0 (0.0) | |
| Beta-blocker at 3 M, n (%) | 302 (62.7) | 179 (57.9) | 123 (71.1) | 0.004 |
| Other AADs at 3 M | 0.047 | |||
| Dronedarone, n (%) | 27 (5.6) | 13 (4.2) | 14 (18.1) | |
| Flecainid, n (%) | 19 (3.9) | 7 (2.3) | 12 (6.9) | |
| Sotalol, n (%) | 21 (4.4) | 12 (3.9) | 9 (5.2) | |
| Amiodarone, n (%) | 13 (2.7) | 8 (2.6) | 5 (2.9) | |
| Disopyramid, n (%) | 2 (0.4) | 1 (0.3) | 1 (0.6) | |
| Propafenon, n (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
BMI body mass index, AAD antiarrhythmic drug, RF radiofrequency
aNon-normally distributed continuous variables are expressed as the median and interquartile range (25th and 75th percentile)
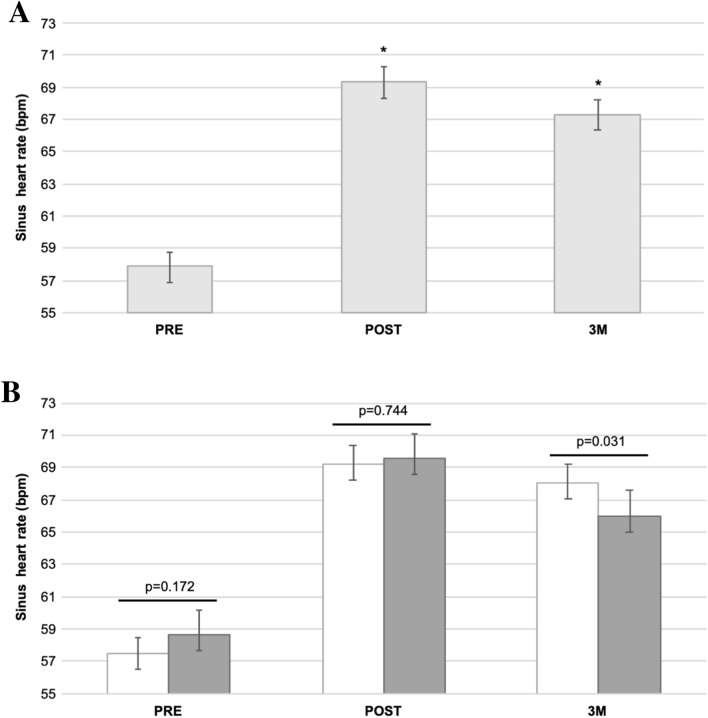
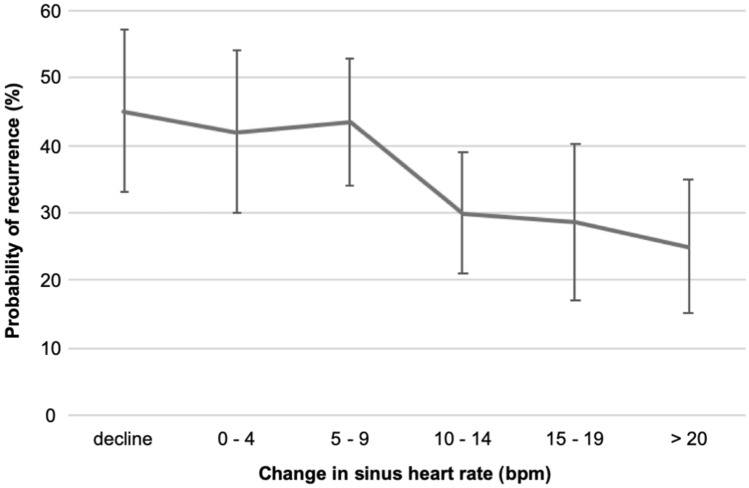
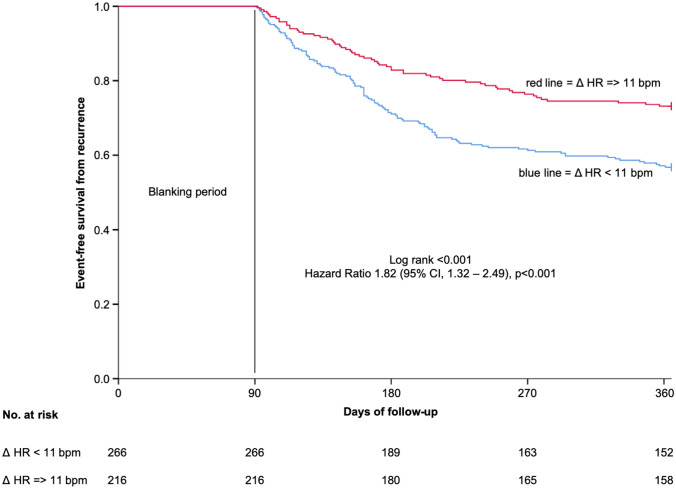
The mean resting sinus heart rate of the total study population was 57.9 beats per minute (bpm) before ablation (PRE) (95% CI 57.1–58.7 bpm). Directly post-ablation (POST) it significantly increased by 11.5 bpm (mean 69.4 bpm (95% CI 68.5–70.3 bpm); p < 0.001) (Fig. 1a) with a distribution of heart rate change (PRE to POST) as provided in Fig. 2, supplementary. After 3 months post-ablation (3 M), the heart rate slightly decreased but remained markedly higher as compared to PRE (mean 67.3 bpm (95% CI 66.4–68.2 bpm); p < 0.001) (Fig. 1a) with a distribution of heart rate change (PRE to 3 M) as provided in Figure 2, supplementary. This increasing pattern was observed in patients with as well as without recurrences at POST and 3 M respectively (both p < 0.001 as compared to PRE). However, in patients with recurrences the mean heart rate was significantly lower compared to patients with no recurrences at 3 M (mean 66.0 bpm (64.4–67.6 bpm) vs. mean 68.1 bpm (95% CI 67.0–69.2 bpm); p = 0.031) (Fig. 1b). Due to this difference at 3 M, we analyzed the heart rate change (PRE to 3 M) and the corresponding probability of recurrence. In this analysis, an increase of sinus heart rate change went along with a decreased probability of recurrence (Hazard ratio (HR) 0.85 (95% CI 0.78–0.94); p = 0.001) (Fig. 2). In addition, we performed a Log-Rank optimization to determine a cut-off of heart rate change (PRE to 3 M) which maximized the discrimination for recurrences. This analysis revealed a cut-off of 11 bpm. According to this cut-off, 266 patients (55.2%) had a heart rate change < 11 bpm and 216 patients (44.8%) >= 11 bpm. Treatments with beta-blocker and other antiarrhythmic drugs (AADs) as well as procedural characteristics were similar in both groups (Table 2). Recurrences occurred in 115 patients (43.2%) in the heart rate change < 11 bpm group compared to 58 patients (26.9%) in the heart rate change >= 11 bpm group (HR 1.82 (95% CI 1.32–2.49), p < 0.001) during a one-year follow-up. The corresponding Kaplan–Meier-curve is provided in Fig. 3. In multivariable analysis, a heart rate change < 11 bpm (PRE to 3 M) remained independently associated with recurrences (Table 1, supplementary).
Fig. 1.
a Mean sinus heart rate (with 95% confidence interval) of the total study population at PRE, POST and 3 M. b Mean sinus heart rate (with 95% confidence interval) at PRE, POST and 3 M in patients without (white bars) and with (grey bars) recurrence. * p < 0.001 as compared to PRE
Fig. 2.
Sinus heart rate change (PRE to 3 M) with a corresponding probability of recurrence (with 95% confidence interval)
Table 2.
Treatment with beta-blocker and other antiarrhythmic drugs as well as procedural characteristics in patients with a sinus heart rate change < 11 bpm and >= 11 bpm (PRE to 3 M)
| Variable | Sinus heart rate change < 11 bpm (PRE to 3 M) (n = 266) | Sinus heart rate change > = 11 bpm (PRE to 3 M) (n = 216) | p value |
|---|---|---|---|
| Beta-blocker at PRE, n (%) | 170 (63.9) | 136 (63.0) | 0.850 |
| Other AADs at PRE | 0.408 | ||
| Dronedarone, n (%) | 38 (14.3) | 22 (10.2) | |
| Flecainid, n (%) | 26 (9.8) | 30 (13.9) | |
| Sotalol, n (%) | 23 (8.6) | 20 (9.3) | |
| Amiodarone, n (%) | 16 (6.0) | 8 (3.7) | |
| Disopyramid, n (%) | 5 (1.9) | 3 (1.4) | |
| Propafenon, n (%) | 0 (0.0) | 1 (0.5) | |
| Beta-blocker at 3 M, n (%) | 176 (66.2) | 126 (58.3) | 0.088 |
| Other AADs at 3 M | 0.093 | ||
| Dronedarone, n (%) | 19 (7.1) | 8 (3.7) | |
| Flecainid, n (%) | 11 (4.1) | 8 (3.7) | |
| Sotalol, n (%) | 14 (5.3) | 7 (3.2) | |
| Amiodarone, n (%) | 11 (4.1) | 2 (0.9) | |
| Disopyramid, n (%) | 1 (0.4) | 1 (0.5) | |
| Propafenon, n (%) | 0 (0.0) | 0 (0.0) | |
| Time of RF energy delivery (sec) | 2710.2 ± 979.1 | 2634.2 ± 906.1 | 0.446 |
| Procedure time (min) | 174.4 ± 50.8 | 168.4 ± 49.9 | 0.145 |
| Fluoroscopy time (min) | 15.3 ± 8.7 | 15.9 ± 10.4 | 0.826 |
AAD antiarrhythmic drug, RF radiofrequency
Fig. 3.
Kaplan–Meier analysis of event-free survival from recurrence in patients with a sinus heart rate change < 11 bpm compared to >= 11 bpm (PRE to 3 M) after a one-year follow-up
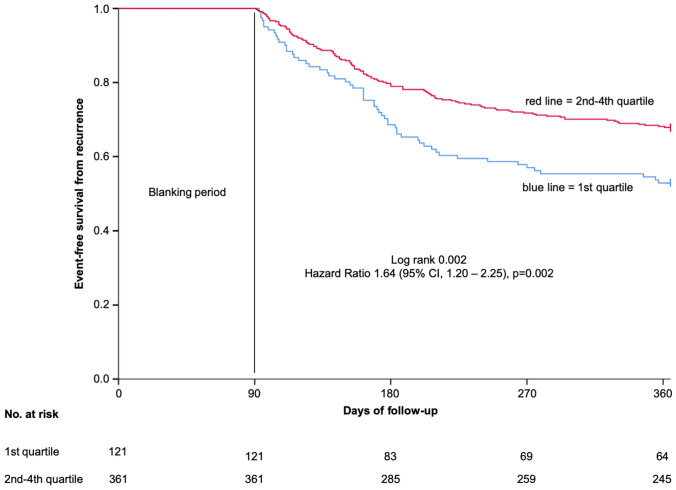
For further analysis, we divided all patients into quartiles regarding to their sinus heart rate at 3 M. Patients in the 1st quartile had a significantly increased risk of recurrences compared to the 4th quartile (HR 1.59, 95% CI 1.05–2.38, p = 0.027) while the other quartiles showed a comparable recurrence risk (2nd quartile HR 1.02, 95% CI 0.66–1.58, p = 0.938 and 3rd quartile HR 0.88, 95% CI 0.56–1.39, p = 0.588) during a one-year follow-up (Table 3; corresponding Kaplan–Meier curve Figure 3, supplementary). In a direct comparison, patients in the 1st quartile had a significantly higher risk of recurrences compared to patients in the 2nd—4th quartile (57 patients (47.1%) vs. 116 patients (32.1%); HR 1.64 (95% CI 1.20–2.25), p = 0.002) (Fig. 4). Treatments with beta-blocker and other AADs as well as procedural characteristics were similar in patients of the 1st quartile compared to patients in the 2nd—4th quartile (Table 4). In multivariable analysis, a heart rate < 60 bpm at 3 M remained independently associated with recurrences (Table 2, supplementary).
Table 3.
Patients divided into quartiles regarding their sinus heart rate at 3 M and their risk of recurrence development
| 3 M | Hazard ratio | 95% CI | p value |
|---|---|---|---|
| 1st quartile: heart rate 43—59 bpm | 1.59 | 1.05–2.38 | 0.027 |
| 2nd quartile: heart rate 60—66 bpm | 1.02 | 0.66–1.58 | 0.938 |
| 3rd quartile: heart rate 67—72 bpm | 0.88 | 0.56–1.39 | 0.588 |
| 4th quartile: heart rate 73—102 bpm | reference | – | – |
CI confidence interval
Fig. 4.
Kaplan–Meier analysis of event-free survival from recurrence in patients within the 1st quartile compared to 2nd—4th quartile at 3 M after a one-year follow-up
Table 4.
Treatment with beta-blocker and other antiarrhythmic drugs as well as procedural characteristics in patients of the 1st sinus heart rate quartile and 2nd—4th sinus heart rate quartile at 3 M
| Variable | Sinus heart rate 1st quartile (3 M) (n = 121) | Sinus heart rate 2nd—4th quartile (3 M) (n = 361) | p value |
|---|---|---|---|
| Beta-blocker at PRE, n (%) | 76 (62.8) | 230 (62.7) | 0.913 |
| Other AADs at PRE | 0.910 | ||
| Dronedarone, n (%) | 14 (11.6) | 46 (12.7) | |
| Flecainid, n (%) | 16 (13.2) | 40 (11.1) | |
| Sotalol, n (%) | 12 (9.9) | 31 (8.6) | |
| Amiodarone, n (%) | 7 (5.8) | 17 (4.7) | |
| Disopyramid, n (%) | 3 (2.5) | 5 (1.4) | |
| Propafenon, n (%) | 0 (0.0) | 1 (0.3) | |
| Beta-blocker at 3 M, n (%) | 81 (66.9) | 221 (61.2) | 0.279 |
| Other AADs at 3 M | 0.126 | ||
| Dronedarone, n (%) | 7 (5.8) | 20 (5.5) | |
| Flecainid, n (%) | 9 (7.4) | 10 (2.8) | |
| Sotalol, n (%) | 7 (5.8) | 14 (3.9) | |
| Amiodarone, n (%) | 5 (4.1) | 8 (2.2) | |
| Disopyramid, n (%) | 1 (0.8) | 1 (0.3) | |
| Propafenon, n (%) | 0 (0.0) | 0 (0.0) | |
| Time of RF energy delivery (sec) | 2599.3 ± 910.0 | 2701.3 ± 958.3 | 0.273 |
| Procedure time (min) | 173.8 ± 49.0 | 171.0 ± 51.0 | 0.586 |
| Fluoroscopy time (min) | 15.7 ± 8.7 | 15.5 ± 9.7 | 0.972 |
AAD antiarrhythmic drug, RF radiofrequency
Consistency analysis
To investigate whether the observed findings are independent of antiarrhythmic drug effects, we performed a consistency analysis only considering patients without any treatment of beta-blocker or other antiarrhythmic drugs (n = 79). In this patient cohort, the mean resting sinus heart at PRE (59.2 bpm at (95% CI 56.9–61.6 bpm) increased by over 10 bpm to POST (mean 69.6 bpm (95% CI 67.3–71.9 bpm); p < 0.001) and remained high also at 3 M [mean 69.4 bpm (95% CI 66.9–72.0 bpm)] compared to PRE (p < 0.001). (Figure 4, supplementary). Of these patients, 62 patients (78.5%) remained free from recurrences and 17 patients (21.5%) developed recurrences.
We further analyzed the heart rate change and the corresponding probability of recurrence. The relation between sinus heart rate change (PRE to 3 M) and the probability of recurrence [HR 0.86 (95% CI 0.65–1.15); p = 0.306] as well as the risk increase according to the sinus heart rate change cut-off of 11 bpm [HR 1.80 (95% CI 0.67–4.88), p = 0.246 (Figure 5, supplementary) were similar compared to the primary analysis. However, they lacked statistical significance most likely due to the small sample size and number of events.
Discussion
In this study of patients undergoing a first-time PVI procedure, we found that the mean resting heart rate significantly increased at POST and 3 M compared to PRE. However, only at 3 M, there was a significant difference in mean heart rate being lower in patients with compared to patients without recurrences. In this regard, patients with a heart rate change < 11 bpm (PRE to 3 M) had a significantly higher risk of recurrences compared to the remaining patients. As an alternative marker, a heart rate < 60 bpm at 3 M was associated with an unfavorable outcome in terms of recurrences. Both variables remained independently associated with multivariable analysis.
Our study confirms the significant impact of PVI on cardiac autonomic function with a mean heart rate increase by over 10 bpm at POST which is in line with previously published results [5, 7–9]. This effect is suggested to be induced by parasympathetic denervation owing to PVI ablation [6, 7] which is known to influence the sinus rate, the atrial refractory period and atrioventricular conduction [14]. Other contributing factors may be postoperative stress and pain as well as increased fluid load associated with the use of irrigated ablation catheters.
Interestingly, at 3 M the mean heart rate was lower among patients with recurrent atrial fibrillation compared to those without while we did not observe any difference in mean heart rate at PRE or POST. This is in contrast to published results, where this difference in mean heart rate among patients with and without recurrence was already observed directly post-ablation [8]. While the effect of parasympathetic denervation of PVI might be similar directly post-ablation provided that same ablation techniques have been applied to all patients, lesion maturation with potential restitution during the first weeks or months post-ablation might modify the initially successful impact of parasympathetic denervation. Hence, the discriminatory effect of persistently effective parasympathetic denervation might rather be seen later i.e. after 1 month [9] or three months post-ablation as performed in our study.
Patients should be well-informed about the likelihood of an increased heart rate post-PVI since a heart rate increase post-ablation was observed in over 80% of the patients in our study. However, while an increased heart rate post-ablation seems to be protective with respect to recurrences, it has also been connected to adverse cardiovascular outcomes in patients with but also without cardiovascular risk factors [15–18]. In this context, the extent but also the duration of an elevated heart rate might play an important role. Interestingly, in a study of Pappone et al. changes in heart rate returned to pre-ablation levels at six months [7]. In another study of Nilsson et al., however, heart rate increases post-ablation persisted during the total follow-up period of 12 months (mean heart rate 70 bpm at 12 months compared to 58 bpm pre-ablation) [9]. Hence, those with persistently elevated heart rates and cardiovascular risk factors might benefit from a more intense beta-blocker therapy.
Treatment with beta-blocker as well as other AADs can modulate the sinus heart rate and, thus, are potential confounders for this study. However, the treatments with beta-blocker and other ADDs were similar in the analyzed patient groups without statistically significant differences (heart rate change < 11 bpm vs. >= 11 bpm and 1st quartile vs. 2nd–4th quartile at 3 M, respectively). Moreover, the results from the consistency analysis indicate that the observed association between heart rate change and late recurrences are independent of the use of beta-blocker or other AADs.
According to our data, a heart rate change < 11 bpm (PRE to 3 M) at 3 M is linked to an unfavorable outcome in terms of recurrences during one-year post-PVI. As an alternative parameter, patients with a heart rate < 60 bpm at 3 M are at higher risk for recurrences. This alternative marker might be relevant especially in settings where a PRE ECG is not available. Both parameters are clinically easily applicable tools which aid in identifying patients at high risk for recurrences benefitting from close monitoring. However, a combination with other ECG based markers is to be proven.
Limitations
This is a registry-based cohort study. Moreover, as all data have been collected at a single electrophysiology center with local routines for ablation procedure, results may differ from other centres. Only about a fourth of the screened patients were included in the final analysis which could have led to a potential selection bias. All ablation procedures were performed applying radiofrequency energy and our results may not be applicable to other forms of energy delivery such as cryoablation or more complex ablation techniques. Patients were only selected when sinus rhythm was present at PRE, POST and 3 M. Therefore, we cannot firmly conclude on the ablation effect on heart rate in those with ongoing atrial fibrillation at PRE, POST and/or 3 M. Since follow-up post-ablation did not include intensive monitoring by transtelephonic monitoring or implantable loop recorder it is possible that asymptomatic recurrences may have been missed.
Conclusion
Our study confirms the impact of PVI on cardiac autonomic function with a significant sinus heart rate increase post-ablation. Patients with a sinus heart rate change < 11 bpm (PRE to 3 M) are at higher risk for recurrences during one-year post-PVI. Hence, an extra-careful follow-up of these patients is advisable.
Data availability statement
The data underlying this article cannot be shared publicly due to the privacy of individuals that were investigated in the study. The data will be shared on reasonable request to the corresponding author provided that this in accordance with the institutional ethical guidelines as well as regulation and legislation.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary file1 (PDF 22 KB) Figure 1 PRISMA flow diagram showing the selection process for all patients
Supplementary file2 (TIFF 13683 KB) Figure 2 Sinus heart rate change from PRE to POST (white bars) and PRE to 3M (grey bars)
Supplementary file3 (TIFF 13683 KB) Figure 3 Kaplan–Meier analysis of event-free survival from recurrence in patients within the 1st,2nd, 3rd and 4th quartile of sinus heart rate at 3M after a one-year follow-up
Supplementary file4 (TIFF 17718 KB) Figure 4 Mean sinus heart rate (with 95% confidence interval) of patients without any treatment of beta-blocker or AADs * p < 0.001 as compared to PRE, AAD = antiarrhythmic drug
Supplementary file5 (TIFF 17711 KB) Figure 5 Kaplan–Meier analysis of event-free survival from recurrence in patients not treated with any beta-blocker or AADs and a sinus heart rate change <11 bpm compared to >= 11 bpm (PRE to 3M) after a one-year follow-up
Supplementary file6 (DOCX 17 KB) Table 1 Logistic regression analysis for recurrence. BMI = body mass index, CI = confidence interval. Table 2 Logistic regression analysis for recurrence. BMI = body mass index, CI = confidence interval
Funding
Open access funding provided by Karolinska Institute. This project was supported by the Deutsche Forschungsgemeinschaft (OL 605/1-1) to G. O.
Compliance with ethical standards
Conflicts of interest
M. J.-U. is a consultant for Medtronic and Johnson and Johnson and has received research grants from Medtronic. F. B. is a consultant for Medtronic and Biotronik and has received speaker fees from Biotronik, Boston Scientific, Abbott, Pfizer and Orion. For the remaining authors, no conflicts of interest are declared.
Ethical approval
All data collection was approved by the regional ethics committee and patient data were collected in accordance with the institutional ethics guidelines.
Footnotes
Gesa von Olshausen and Ott Saluveer contributed equally.
References
- 1.Chugh SS, Havmoeller R, Narayanan K, Singh D, Rienstra M, Benjamin EJ, Gillum RF, Kim YH, McAnulty JH, Jr, Zheng ZJ, Forouzanfar MH, Naghavi M, Mensah GA, Ezzati M, Murray CJ. Worldwide epidemiology of atrial fibrillation: a global burden of disease 2010 study. Circulation. 2014;129(8):837–847. doi: 10.1161/CIRCULATIONAHA.113.005119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Calkins H, Hindricks G, Cappato R, Kim YH, Saad EB, Aguinaga L, Akar JG, Badhwar V, Brugada J, Camm J, Chen PS, Chen SA, Chung MK, Nielsen JC, Curtis AB, Davies DW, Day JD, d'Avila A, de Groot N, Di Biase L, Duytschaever M, Edgerton JR, Ellenbogen KA, Ellinor PT, Ernst S, Fenelon G, Gerstenfeld EP, Haines DE, Haissaguerre M, Helm RH, Hylek E, Jackman WM, Jalife J, Kalman JM, Kautzner J, Kottkamp H, Kuck KH, Kumagai K, Lee R, Lewalter T, Lindsay BD, Macle L, Mansour M, Marchlinski FE, Michaud GF, Nakagawa H, Natale A, Nattel S, Okumura K, Packer D, Pokushalov E, Reynolds MR, Sanders P, Scanavacca M, Schilling R, Tondo C, Tsao HM, Verma A, Wilber DJ, Yamane T. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Heart Rhythm. 2017;14(10):e275–e444. doi: 10.1016/j.hrthm.2017.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen PS, Chen LS, Fishbein MC, Lin SF, Nattel S. Role of the autonomic nervous system in atrial fibrillation: pathophysiology and therapy. Circ Res. 2014;114(9):1500–1515. doi: 10.1161/CIRCRESAHA.114.303772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu J, Fan X, Yang H, Yan L, Xu X, Duan H, Wang S, Chu Y. Usefulness of a low resting heart rate to predict recurrence of atrial fibrillation after catheter ablation in people >/=65 years of age. Am J Cardiol. 2018;122(1):97–101. doi: 10.1016/j.amjcard.2018.03.025. [DOI] [PubMed] [Google Scholar]
- 5.Wagner L, Darche FF, Thomas D, Lugenbiel P, Xynogalos P, Seide S, Scholz EP, Katus HA, Schweizer PA. Cryoballoon pulmonary vein isolation-mediated rise of sinus rate in patients with paroxysmal atrial fibrillation. Clin Res Cardiol. 2020 doi: 10.1007/s00392-020-01659-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hamdan MH, Page RL, Wasmund SL, Sheehan CJ, Zagrodzky JD, Ramaswamy K, Joglar JA, Adamson MM, Barron BA, Smith ML. Selective parasympathetic denervation following posteroseptal ablation for either atrioventricular nodal reentrant tachycardia or accessory pathways. Am J Cardiol. 2000;85(7):875–878. doi: 10.1016/s0002-9149(99)00885-1. [DOI] [PubMed] [Google Scholar]
- 7.Pappone C, Santinelli V, Manguso F, Vicedomini G, Gugliotta F, Augello G, Mazzone P, Tortoriello V, Landoni G, Zangrillo A, Lang C, Tomita T, Mesas C, Mastella E, Alfieri O. Pulmonary vein denervation enhances long-term benefit after circumferential ablation for paroxysmal atrial fibrillation. Circulation. 2004;109(3):327–334. doi: 10.1161/01.CIR.0000112641.16340.C7. [DOI] [PubMed] [Google Scholar]
- 8.Goff ZD, Laczay B, Yenokyan G, Sivasambu B, Sinha SK, Marine JE, Ashikaga H, Berger RD, Spragg DD, Calkins H. Heart rate increase after pulmonary vein isolation predicts freedom from atrial fibrillation at 1 year. J Cardiovasc Electrophysiol. 2019 doi: 10.1111/jce.14257. [DOI] [PubMed] [Google Scholar]
- 9.Nilsson B, Chen X, Pehrson S, Hilden J, Svendsen JH. Increased resting heart rate following radiofrequency catheter ablation for atrial fibrillation. Europace. 2005;7(5):415–420. doi: 10.1016/j.eupc.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 10.Yu HT, Kim TH, Uhm JS, Kim JY, Joung B, Lee MH, Pak HN. Prognosis of high sinus heart rate after catheter ablation for atrial fibrillation. Europace. 2017;19(7):1132–1139. doi: 10.1093/europace/euw142. [DOI] [PubMed] [Google Scholar]
- 11.Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B, Castella M, Diener HC, Heidbuchel H, Hendriks J, Hindricks G, Manolis AS, Oldgren J, Popescu BA, Schotten U, Van Putte B, Vardas P, Group ESCSD 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016;37(38):2893–2962. doi: 10.1093/eurheartj/ehw210. [DOI] [PubMed] [Google Scholar]
- 12.Akerstrom F, Bastani H, Insulander P, Schwieler J, Arias MA, Jensen-Urstad M. Comparison of regular atrial tachycardia incidence after circumferential radiofrequency versus cryoballoon pulmonary vein isolation in real-life practice. J Cardiovasc Electrophysiol. 2014;25(9):948–952. doi: 10.1111/jce.12423. [DOI] [PubMed] [Google Scholar]
- 13.Hothorn T, Lausen B. On the exact distribution of maximally selected rank statistics. Comput Stat Data Anal. 2003;43(2):121–137. doi: 10.1016/S0167-9473(02)00225-6. [DOI] [Google Scholar]
- 14.Schauerte P, Scherlag BJ, Pitha J, Scherlag MA, Reynolds D, Lazzara R, Jackman WM. Catheter ablation of cardiac autonomic nerves for prevention of vagal atrial fibrillation. Circulation. 2000;102(22):2774–2780. doi: 10.1161/01.cir.102.22.2774. [DOI] [PubMed] [Google Scholar]
- 15.Bohm M, Swedberg K, Komajda M, Borer JS, Ford I, Dubost-Brama A, Lerebours G, Tavazzi L, Investigators S. Heart rate as a risk factor in chronic heart failure (SHIFT): the association between heart rate and outcomes in a randomised placebo-controlled trial. Lancet. 2010;376(9744):886–894. doi: 10.1016/S0140-6736(10)61259-7. [DOI] [PubMed] [Google Scholar]
- 16.Diaz A, Bourassa MG, Guertin MC, Tardif JC. Long-term prognostic value of resting heart rate in patients with suspected or proven coronary artery disease. Eur Heart J. 2005;26(10):967–974. doi: 10.1093/eurheartj/ehi190. [DOI] [PubMed] [Google Scholar]
- 17.Gillman MW, Kannel WB, Belanger A, D'Agostino RB. Influence of heart rate on mortality among persons with hypertension: the Framingham study. Am Heart J. 1993;125(4):1148–1154. doi: 10.1016/0002-8703(93)90128-v. [DOI] [PubMed] [Google Scholar]
- 18.Kannel WB, Kannel C, Paffenbarger RS, Jr, Cupples LA. Heart rate and cardiovascular mortality: the Framingham study. Am Heart J. 1987;113(6):1489–1494. doi: 10.1016/0002-8703(87)90666-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary file1 (PDF 22 KB) Figure 1 PRISMA flow diagram showing the selection process for all patients
Supplementary file2 (TIFF 13683 KB) Figure 2 Sinus heart rate change from PRE to POST (white bars) and PRE to 3M (grey bars)
Supplementary file3 (TIFF 13683 KB) Figure 3 Kaplan–Meier analysis of event-free survival from recurrence in patients within the 1st,2nd, 3rd and 4th quartile of sinus heart rate at 3M after a one-year follow-up
Supplementary file4 (TIFF 17718 KB) Figure 4 Mean sinus heart rate (with 95% confidence interval) of patients without any treatment of beta-blocker or AADs * p < 0.001 as compared to PRE, AAD = antiarrhythmic drug
Supplementary file5 (TIFF 17711 KB) Figure 5 Kaplan–Meier analysis of event-free survival from recurrence in patients not treated with any beta-blocker or AADs and a sinus heart rate change <11 bpm compared to >= 11 bpm (PRE to 3M) after a one-year follow-up
Supplementary file6 (DOCX 17 KB) Table 1 Logistic regression analysis for recurrence. BMI = body mass index, CI = confidence interval. Table 2 Logistic regression analysis for recurrence. BMI = body mass index, CI = confidence interval
Data Availability Statement
The data underlying this article cannot be shared publicly due to the privacy of individuals that were investigated in the study. The data will be shared on reasonable request to the corresponding author provided that this in accordance with the institutional ethical guidelines as well as regulation and legislation.