Abstract
Background
Three-dimensional mapping systems and the use of ultra-low dose radiation protocols have supported minimization of radiation dose during left atrial ablation procedures. By using optimal shielding, scattered radiation reaching the operator can be further reduced. This prospective study was designed to determine the remaining operator radiation exposure during left atrial catheter ablations using real-time dosimetry.
Methods
Radiation dose was recorded using real-time digital dosimetry badges outside the lead apron during 201 consecutive left atrial fibrillation ablation procedures. All procedures were performed using the same X‑ray system (Siemens Healthineers Artis dBc; Siemens Healthcare AG, Erlangen, Germany) programmed with ultra-low dose radiation settings including a low frame rate (two frames per second), maximum copper filtration, and an optimized detector dose. To reduce scattered radiation to the operators, table-suspended lead curtains, ceiling-suspended leaded plastic shields, and radiation-absorbing shields on the patient were positioned in an overlapping configuration.
Results
The 201 procedures included 139 (69%) pulmonary vein isolations (PVI) (20 cryoballoon ablations, 119 radiofrequency ablations, with 35 cases receiving additional ablation of the cavotricuspid isthmus) and 62 (31%) PVI plus further left atrial substrate ablation.
Mean radiation dose measured as dose area product for all procedures was 128.09 ± 187.87 cGy ∙ cm2 with a mean fluoroscopy duration of 9.4 ± 8.7 min. Real-time dosimetry showed very low average operator doses of 0.52 ± 0.10 µSv. A subanalysis of 51 (25%) procedures showed that the radiation burden for the operator was highest during pulmonary vein angiography.
Conclusion
The use of ultra-low dose radiation protocols in combination with optimized shielding results in extremely low scattered radiation reaching the operator.
Keywords: Dosimetry, Ablation, Radiation dose, Frame-rate reduction, Radiation protection
Abstract
Hintergrund
Der Einsatz von dreidimensionalen Mapping-Systemen und von Niedrigdosiseinstellungen der Röntgenanlage führte zu einer Minimierung der Strahlenbelastung bei linksatrialen Ablationen. Optimierte Abschirmung kann die Streustrahlung als Strahlenbelastung des Untersuchers weiter reduzieren. In dieser prospektiven Studie wurde untersucht, welcher Strahlenbelastung der Untersucher unter Anwendung dieser Maßnahmen während linksatrialer Ablationen noch ausgesetzt ist.
Methoden
Die Strahlenbelastung wurde mittels Echtzeitdosimetrie an der Außenseite der Bleischürze während 201 konsekutiven linksatrialen Ablationen gemessen. Alle Prozeduren wurden mit demselben Röntgensystem (Siemens Healthineers Artis dBc; Siemens Healthcare AG, Erlangen, Deutschland) und mit strahlensparenden Einstellungen durchgeführt, unter anderem mit einer niedrigen Bildrate von 2 Bildern/s, maximaler Kupferfilterung und angepasster Detektoreingangsdosis. Um Streustrahlung zu reduzieren, wurden die Seitenlamellen, die mobile Acrylscheibe und die strahlenabsorbierenden Schilde auf dem Patienten überlappend angeordnet.
Ergebnisse
Die 201 Prozeduren umfassten 139 (69 %) Pulmonalvenenisolationen (PVI; 20 Kryoballonablationen, 119 Radiofrequenzablationen, in 35 Fällen mit zusätzlicher Ablation des kavotrikuspidalen Isthmus) und 62 (31 %) PVI mit zusätzlicher linksatrialer Substratmodifikation. Die Strahlendosis als Dosis-Flächen-Produkt (DAP) betrug durchschnittlich 128,09 ± 187,87 cGy ∙ cm2 bei einer Fluoroskopiedauer von im Mittel 9,4 ± 8,7 min. Die per Echtzeitdosimetrie erhobene mittlere Strahlendosis des Untersuchers zeigte sich mit 0,52 ± 0,10 µSv als sehr gering. Eine Subanalyse bei 51 (25 %) Prozeduren zeigte, dass die Strahlendosis des Untersuchers während der Pulmonalvenenangiographie am höchsten war.
Schlussfolgerung
Die Kombination von Niedrigdosiseinstellungen und optimierter Abschirmung führt zu einer extrem niedrigen Streustrahlung als Strahlenbelastung des Untersuchers.
Schlüsselwörter: Dosimetrie, Ablation, Strahlendosis, Reduktion der Bildrate, Strahlenschutz
Introduction and background
Despite the widespread use of non-fluoroscopic catheter visualization, fluoroscopy remains an essential part of catheter and wire localization. One of the drawbacks of the ever-increasing number of complex and prolonged ablation procedures is the higher radiation burden to the patient and the operator; it is well documented that even low-dose radiation can lead to potentially fatal radiation damage [13].
Increased awareness lead to the development of ultra-low dose programs with optimized image processing and system settings, resulting in average doses that equal as little as 1% of an ablation procedure performed 6 years ago [2, 6].
Most dose reduction studies [2, 3, 5, 9, 19] used the dose area product (DAP) or some variation thereof as primary endpoint, which mainly serves to measure the patient’s radiation exposure [13]. However, the DAP cannot precisely determine the radiation burden of the operator, since it depends on factors like shielding, angulation, and distance to the X‑ray system.
Therefore, the purpose of this study was to determine the amount of radiation received by the operator as measured by dosimeters during atrial fibrillation ablation procedures.
The authors hypothesized that by using overlapping shielding and an up-to-date ultra-low-dose protocol, scattered radiation reaching the operator would be vastly reduced.
They also sought to determine the moments during the ablation procedure in which radiation exposure to the operator is particularly high.
Methods
Study design
This study is an observational single-center study including 201 patients that underwent left atrial catheter ablation (radiofrequency or cryoballon) due to atrial fibrillation. All patients were consecutively enrolled at the Charité University Hospital, Campus Benjamin Franklin, Berlin, Germany. All patients were treated by the same two senior electrophysiologists using the same Siemens Artis dBc systems (Siemens Healthcare AG, Erlangen, Germany).
Demographic parameters, including body mass index (BMI), age, gender, and comorbidities were documented. Relevant procedural parameters for this study included total procedure time, fluoroscopy time, total radiation dose (measured as DAP), as well as acute procedural success and major periprocedural complications.
Standard radiation protection
Emphasis was put on general “as low as reasonably achievable” (ALARA) measurements, which the authors previously described [24]. These included avoiding magnification and unnecessary fluoroscopy/cine loops, maximal collimation, preferring anteroposterior (AP) and right anterior oblique (RAO) views over steep left anterior oblique (LAO) angulation, and minimizing the distance between patient and detector.
Standard shielding included the use of thyroid and body aprons with 0.5-Pb equivalent. As shown in Fig. 1, table-suspended lead curtains and transparent, ceiling-suspended plastic shields were positioned in an overlapping configuration to ensure maximum protection against scattered radiation. Also, radiation-absorbing shields were placed on the patient.
Fig. 1.
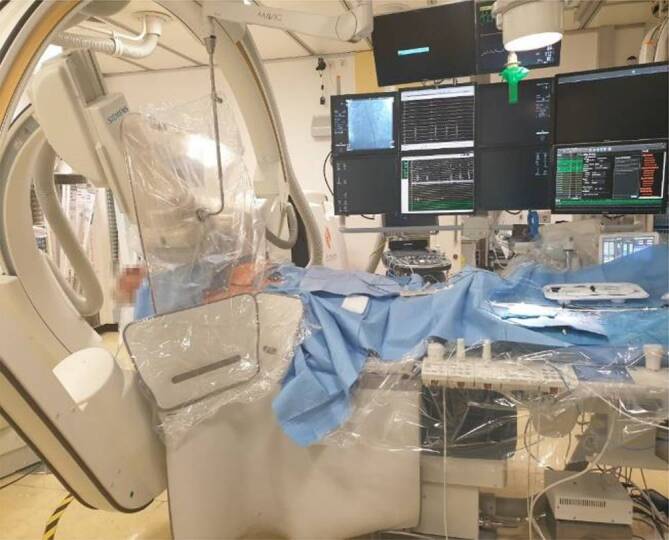
Overlapping shielding with table-suspended lead curtains and ceiling-suspended leaded plastic shields during pulmonary vein isolation
Furthermore, an ultra-low-dose protocol was uniformly used for all ablation procedures. This consists of the use of a low frame rate (two frames per second, FPS), maximum copper filtration, low detector entrance dose (8 nGy/pulse for fluoroscopy), and short pulse width (4 ms). As previously described, the antiscatter grid was not used in any cases in order to further minimize radiation exposure [1]. For left atrial angiography, short (3-s) cine loops with 7.5 FPS were used during fast left ventricular pacing (200 bpm) via the ablation catheter. The pictures were recorded in biplane mode in RAO 30° or LAO 30° view. No preprocedural imaging was performed.
Measurement of radiation dose
Two parameters aiming to quantify radiation burden were obtained for each procedure. To evaluate dose to the patient, DAP (cGy ∙ cm2) was automatically measured by an ionization chamber. Measurement of DAP is routinely used to compare radiation burden of the patient and correlates well with skin dose [18].
Additionally, electronic dosimetry badges (RaySafe i3, Unfors, Hopkinton, MA, USA) were mounted outside the lead apron at the left side of the chest to measure the operator’s dose (µSv) for each procedure. Both operators were blinded to the measured doses.
This dosimeter is able to measure radiation every second and has high sensitivity (<30 µSv/h). RaySafe dosimeters also have good reproducibility [14] and have already been successfully used in neuroradiological [22], orthopaedic [15, 25], and peripheral endovascular procedures [16].
The RaySafe badges were collected and analyzed every week with DoseViewer software in order to prevent loss of sensitivity. For the last 51 (25%) procedures, radiation burden was analyzed for three different segments of the ablation procedure to assess the sequences with the highest radiation burden.
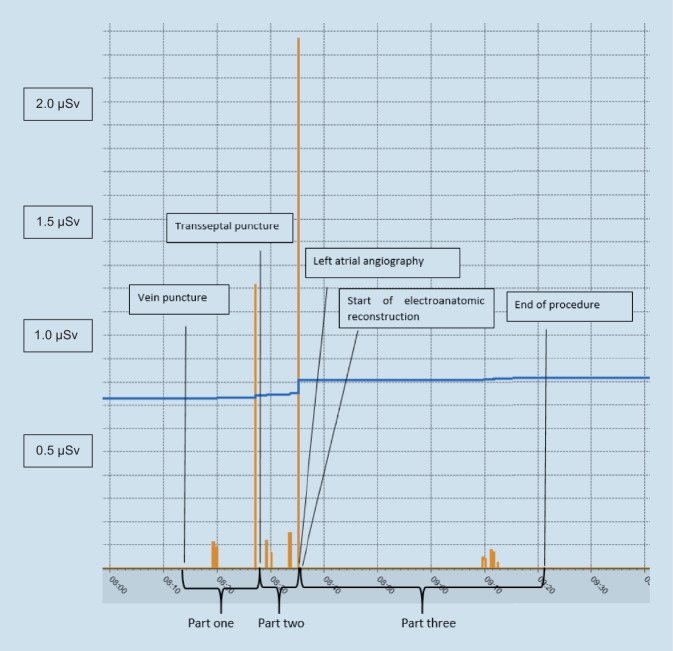
The procedures were divided into three parts as follows (see Fig. 2):
Part one: femoral vein puncture, coronary sinus (CS) catheter placement, transseptal puncture
Part two: catheter placement, left atrial angiography
Part three: three-dimensional (3D) mapping, catheter ablation
Fig. 2.
Radiation dispersion during a standard pulmonary vein isolation ablation procedure. The highest peaks can be observed during transseptal puncture and left atrial angiography
Left atrial ablation procedures
After exclusion of intracardiac thrombi by transesophageal echocardiography, pulmonary vein isolation (PVI) or PVI plus further left atrial substrate modification or PVI plus ablation of the cavotricuspid isthmus (CTI) were performed under conscious sedation with midazolam and propofol. A diagnostic 10-poled steerable electrophysiology catheter (Inquiry, St Jude Medical, Saint Paul, MN, USA) was placed in the CS. Double transseptal puncture was performed in all cases and two catheters were introduced via two long left atrial sheaths (Swartz Braided SL0 Curve 8.5 F, St. Jude Medical, Saint Paul, MN, USA, and Agilis Nxt Small Curl 8.5F, Abbott, MN, USA):
A circular 12-pole pulmonary vein mapping catheter (Lasso Nav Variable, Biosense Webster, Diamond Bar, CA, USA; via the Swartz sheath)
An open-irrigated 3.5-mm tip Thermocool Smarttouch SF (Biosense Webster) as ablation catheter (via Agilis)
After electroanatomical reconstruction of the left atrium, catheter ablation was performed guided by ablation index (AI) with irrigated radiofrequency energy at a power of 20–25 W at the posterior left atrial wall (target AI, 400) and 30–35 W at all other sites (target AI, 550).
Statistical analysis
All analyses were performed using SPSS software version 25 (SPSS Inc., Chicago, IL, USA). Data are presented as mean ± standard deviation (SD) for continuous variables or absolute numbers and percentages for categorical variables. For normally distributed data, correlation values were calculated with Pearson’s correlation coefficient and Spearman for skewed data. A p-value of <0.05 was considered statistically significant.
Results
The 201 procedures included 139 (69%) PVI (20 cryoballoon ablations and 119 radiofrequency ablations with 35 cases receiving additional ablation of the CTI) and 62 (31%) PVI plus further extra pulmonary substrate ablations. Table 1 shows baseline characteristics, Table 2 shows procedural characteristics of the study cohort.
Table 1.
Baseline characteristics
| Female, n (%) | 86 (43%) |
|---|---|
| Age, mean (±SD) | 68.31 (±10.04) |
| BMI, mean (±SD) (kg/m2) | 27.83 (±4.85) |
| LVEF (±SD) (%) | 55.96 (±9.95) |
| Paroxysmal atrial fibrillation, n (%) | 85 (42%) |
| Diabetes mellitus, n (%) | 33 (16%) |
| Arterial hypertension, n (%) | 154 (77%) |
| COLD, n (%) | 7 (3%) |
| CAD, n (%) | 52 (26%) |
SD standard deviation, BMI body mass index, LVEF left ventricular ejection fraction, COLD chronic obstructive lung disease, CAD coronary artery disease
Table 2.
Procedural characteristics
| DAP, mean (±SD) (µGy ∙ m2) | 128.09 (±187.87) |
| Dose to the operator, mean (±SD) (µSv) | 0.52 (±0.10) |
| Procedural duration, mean (±SD) (h:min) | 1:37 (±0:30) |
| Fluoroscopy time, mean (±SD) (min) | 9.4 (±8.7) |
| DAP per minute, mean (±SD) | 14.09 (±15.77) |
| Acute procedural success, n (%) | 201 (100%) |
| Complications, n (%) | 9 (4%) |
DAP dose area product, SD standard deviation
Mean radiation dose measured as DAP for all procedures was 128.09 + 187.87 cGy ∙ cm2 with a mean fluoroscopy duration of 9.4 + 8.7 min. The 10 highest recorded doses predominantly (90%) occurred during ablation procedures of patients with a BMI >30. We observed a significant positive relationship between the patients BMI and operator dose/DAP (R = 0.348, p < 0.001 and R = 0.506, p < 0.001).
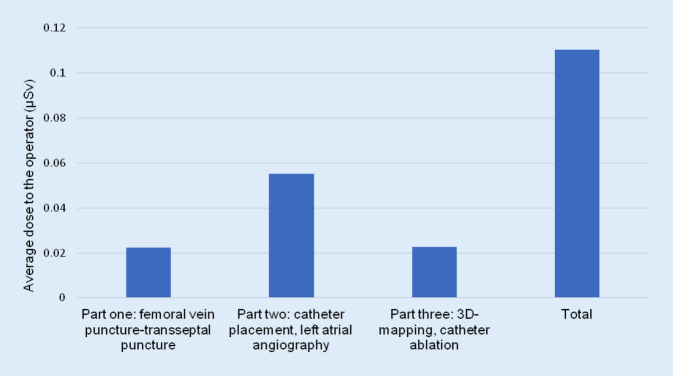
As mentioned earlier, for the last 51 procedures, a subanalysis was performed to determine which part of the procedure showed the highest radiation exposure to the operator. Figure 3 shows that most radiation (55%) towards the operator occurs during left atrial angiography.
Fig. 3.
Distribution of radiation throughout the different parts of the ablation procedure
Discussion
Main findings
This single-center study demonstrated that, with the use of an ultra-low-dose program and optimized shielding, operator doses can be kept at very low levels during catheter ablation of atrial fibrillation. The operator’s exposure was highest during left atrial angiography, making shielding and further dose reduction particularly valuable assets at this moment in the procedure. To our knowledge, this is the first analysis of the operator’s exposure with the use of ultra-low-dose programs for catheter ablation of atrial fibrillation.
Previously published studies
Scant data exist on personal dosimetry regarding atrial fibrillation ablation. A 2019 study reported mean values of 3 µSv with a mean DAP of 429.6 cGy ∙ cm2 [10]. Those results were achieved by using a frame rate of 4 FPS. By using a lower frame rate of 2 FPS, the authors were able to achieve lower average doses while maintaining a low rate of complications with a reasonable procedural time. Reducing the frame rate is arguably one of the simplest and most effective ways to lower radiation burden, given that the relationship between dose and frame rate is linear. Despite this fact, only 50% of European ablation centers use a frame rate of less than 6 FPS for electrophysiology (EP) procedures [9]. The reason for this might be that a considerable number of EP labs use settings also used in coronary interventions. However, the demand for image quality in EP studies is usually far more modest, even in complex procedures. Therefore, the authors advise contacting the X‑ray system’s manufacturer to establish a low-dose radiation setup tailored to the operators’ requirements.
Since scattered radiation emitted from the patient’s body is the main source of the operator’s radiation burden, methods to absorb scattered radiation have been tested: sterile radiation-absorbing drapes placed around the puncture site can reduce the operator’s radiation exposure by more than half [12]. Mobile full-body protection systems can provide protection against scattered radiation, except for the arms, and can reduce radiation to the operator close to background radiation values [8]. High costs, limited availability, and structural preconditions have hindered their widespread use.
Radiation put into context
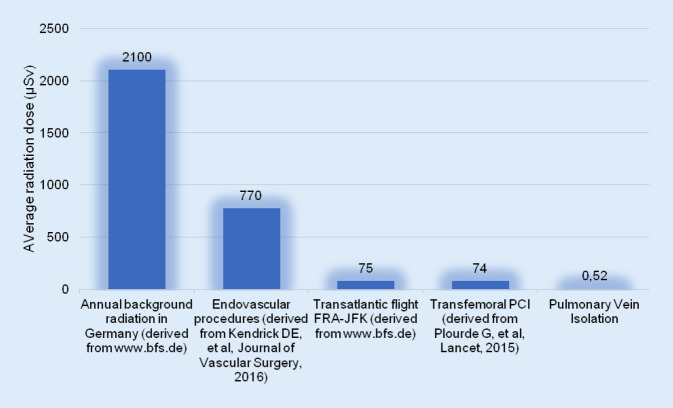
Despite various efforts to minimize operator radiation, interventional cardiologists in most centers are still subjected to a relevant amount of radiation throughout their career, with an annual estimated dose of 5 mSv [13]. Reducing radiation burden minimizes the detrimental effects of ionizing radiation, some of which include cancer, inherited diseases, and the formation of cataracts [21]. Figure 4 shows previously published radiation exposure for different diagnostic and therapeutic interventions, as well annual background radiation and radiation during an intercontinental flight.
Fig. 4.
Comparison between annual background radiation and different interventions. PCI percutaneous coronary intervention, FRA-JFK Frankfurt-John F. Kennedy
Minimizing radiation during left atrial angiography
Knowledge of pulmonary vein anatomy and its variations (common ostia, accessory pulmonary vein, pulmonary vein fusion) is a key factor for a safe and efficient ablation procedure. This information is most frequently obtained by intraprocedural angiography. Preprocedural computed tomograhy left atrial angiography might be helpful to avoid intraprocedural angiography and thereby reduce operator X‑ray exposure. However, this method is associated with a higher radiation burden to the patient [4, 17]. The authors suggest implementing the following measures to ensure low radiation burden:
Using short cine loops with low frame rates (for example, 3 s and 7.5 FPS) with fast left ventricular pacing to lower use of contrast agent [23]
Strict collimation to the left atrium
Avoiding additional angiography of single pulmonary veins
The feasibility and safety of numerous fluoroless approaches for catheter-based pulmonary vein ablation and LA visualization have been demonstrated in smaller studies [7]. These include magnetic resonance imaging-guided ablation procedures [26], as well as intracardiac echocardiography [11] and transesophageal echocardiography [20] without any subsequent LA angiography. However, limited experience and availability, high costs, and possible complications have hindered more widespread use.
Limitations
This study represents a single-center analysis with two senior electrophysiologists performing all procedures. Therefore, the observed results may not be valid for other ablation centers with different dose settings. Although standardized shielding was aimed for, different placement of the shields may have altered the results.
Conclusion
This study demonstrates that by using an ultra-low-dose setting and optimal shielding, radiation reaching the operator can be largely reduced. Therefore, table-suspended lead curtains and ceiling-suspended shields should be positioned in an overlapping configuration to ensure maximum protection against scattered radiation, especially during left atrial angiography.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Declarations
Conflict of interest
T. Schreiber, N. Kähler, S. Biewener, V. Tscholl, P. Nagel, P. Attanasio, U. Landmesser, and M. Huemer declare that they have no competing interests.
For this article no studies with human participants or animals were performed by any of the authors. All studies mentioned were in accordance with the ethical standards indicated in each case.
References
- 1.Attanasio P, Schreiber T, Parwani A, et al. The effect of an ultra-low frame rate and antiscatter grid-less radiation protocol for cardiac device implantations. Pacing Clin Electrophysiol. 2017;40:1380–1383. doi: 10.1111/pace.13229. [DOI] [PubMed] [Google Scholar]
- 2.Attanasio P, Schreiber T, Pieske B, et al. Pushing the limits: establishing an ultra-low framerate and antiscatter grid-less radiation protocol for left atrial ablations. Europace. 2017;20(4):604–607. doi: 10.1093/europace/eux010. [DOI] [PubMed] [Google Scholar]
- 3.Bourier F, Reents T, Ammar-Busch S, et al. Evaluation of a new very low dose imaging protocol: feasibility and impact on X-ray dose levels in electrophysiology procedures. Europace. 2016;18:1406–1410. doi: 10.1093/europace/euv364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bourier F, Reents T, Ammar-Busch S, et al. Transseptal puncture guided by CT-derived 3D-augmented fluoroscopy. J Cardiovasc Electrophysiol. 2016;27:369–372. doi: 10.1111/jce.12885. [DOI] [PubMed] [Google Scholar]
- 5.Christoph M, Wunderlich C, Moebius S, et al. Fluoroscopy integrated 3D mapping significantly reduces radiation exposure during ablation for a wide spectrum of cardiac arrhythmias. Europace. 2015;17:928–937. doi: 10.1093/europace/euu334. [DOI] [PubMed] [Google Scholar]
- 6.De Buck S, La Gerche A, Ector J, et al. Asymmetric collimation can significantly reduce patient radiation dose during pulmonary vein isolation†. Europace. 2011;14:437–444. doi: 10.1093/europace/eur346. [DOI] [PubMed] [Google Scholar]
- 7.Doshi RN. Atrial fibrillation ablation without fluoroscopy: because we Can. J Innov Cardiac Rhythm Manag. 2018;9:3391–3394. doi: 10.19102/icrm.2018.091103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dragusin O, Weerasooriya R, Jaïs P, et al. Evaluation of a radiation protection cabin for invasive electrophysiological procedures. Eur Heart J. 2007;28:183–189. doi: 10.1093/eurheartj/ehl420. [DOI] [PubMed] [Google Scholar]
- 9.Estner HL, Grazia Bongiorni M, Chen J, et al. Use of fluoroscopy in clinical electrophysiology in Europe: results of the European Heart Rhythm Association Survey. Europace. 2015;17:1149–1152. doi: 10.1093/europace/euv223. [DOI] [PubMed] [Google Scholar]
- 10.Faroux L, Daval C, Lesaffre F, et al. Physicians’ exposure to radiation during electrophysiology procedures. J Interv Cardiac Electrophysiol. 2019;55:233–237. doi: 10.1007/s10840-019-00568-1. [DOI] [PubMed] [Google Scholar]
- 11.Ferguson JD, Helms A, Mangrum JM, et al. Catheter ablation of atrial fibrillation without fluoroscopy using intracardiac echocardiography and electroanatomic mapping. Circulation. Circ Arrhythm Electrophysiol. 2009;2:611–619. doi: 10.1161/CIRCEP.109.872093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Germano JJ, Day G, Gregorious D, et al. A novel radiation protection drape reduces radiation exposure during fluoroscopy guided electrophysiology procedures. J Invasive Cardiol. 2005;17:469–472. [PubMed] [Google Scholar]
- 13.Heidbuchel H, Wittkampf FH, Vano E, et al. Practical ways to reduce radiation dose for patients and staff during device implantations and electrophysiological procedures. Europace. 2014;16:946–964. doi: 10.1093/europace/eut409. [DOI] [PubMed] [Google Scholar]
- 14.Inaba Y, Chida K, Kobayashi R, et al. Fundamental study of a real-time occupational dosimetry system for interventional radiology staff. J Radiol Prot. 2014;34:N65. doi: 10.1088/0952-4746/34/3/N65. [DOI] [PubMed] [Google Scholar]
- 15.Francisco J, Lopez N, Ferreira R, Santos J. Orthopaedic surgery occupational exposure using active dosimeters. 2015. [Google Scholar]
- 16.Kendrick DE, Miller CP, Moorehead PA, et al. Comparative occupational radiation exposure between fixed and mobile imaging systems. J Vasc Surg. 2016;63:190–197. doi: 10.1016/j.jvs.2015.08.062. [DOI] [PubMed] [Google Scholar]
- 17.Kriatselis C, Nedios S, Akrivakis S, et al. Intraprocedural imaging of left atrium and pulmonary veins: a comparison study between rotational angiography and cardiac computed tomography. Pacing Clin Electrophysiol. 2011;34:315–322. doi: 10.1111/j.1540-8159.2010.02969.x. [DOI] [PubMed] [Google Scholar]
- 18.Mcfadden SL, Mooney RB, Shepherd PH. X-ray dose and associated risks from radiofrequency catheter ablation procedures. Br J Radiol. 2002;75:253–265. doi: 10.1259/bjr.75.891.750253. [DOI] [PubMed] [Google Scholar]
- 19.Nof E, Lane C, Cazalas M, et al. Reducing radiation exposure in the electrophysiology laboratory: it is more than just fluoroscopy times! Pacing Clin Electrophysiol. 2015;38:136–145. doi: 10.1111/pace.12544. [DOI] [PubMed] [Google Scholar]
- 20.O’brien B, Balmforth DC, Hunter RJ, et al. Fluoroscopy-free AF ablation using transesophageal echocardiography and electroanatomical mapping technology. J Interv Card Electrophysiol. 2017;50:235–244. doi: 10.1007/s10840-017-0288-9. [DOI] [PubMed] [Google Scholar]
- 21.Picano E, Vano E. The radiation issue in cardiology: the time for action is now. Cardiovasc Ultrasound. 2011;9:35. doi: 10.1186/1476-7120-9-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Santos J, Pereira S, Cardoso I, et al. Interventional neuroradiology procedures: patient and staff dose levels. 2015. [Google Scholar]
- 23.Schneider R, Lauschke J, Schneider C, et al. Reduction of radiation exposure during ablation of atrial fibrillation. Herz. 2015;40(6):883–891. doi: 10.1007/s00059-015-4307-2. [DOI] [PubMed] [Google Scholar]
- 24.Schreiber T, Kahler N, Tscholl V, et al. Update on radiation exposure in catheter ablation of atrial fibrillation. Herzschrittmacherther Elektrophysiol. 2019;31(1):84–90. doi: 10.1007/s00399-019-00660-z. [DOI] [PubMed] [Google Scholar]
- 25.Van Rappard JRM, De Jong T, Hummel WA, et al. Radiation exposure to surgeon and assistant during flat panel mini C-arm fluoroscopy in hand and wrist surgical procedures. J Hand Surg. 2019;44:68.e61–68.e65. doi: 10.1016/j.jhsa.2018.05.010. [DOI] [PubMed] [Google Scholar]
- 26.Vergara GR, Vijayakumar S, Kholmovski EG, et al. Real-time magnetic resonance imaging-guided radiofrequency atrial ablation and visualization of lesion formation at 3 Tesla. Heart Rhythm. 2011;8:295–303. doi: 10.1016/j.hrthm.2010.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]