Abstract
Purpose
To analyze preclinical bone regeneration studies employing mesenchymal stromal cell (MSC)- derived extracellular vesicles (EVs) and highlight any commonalities in EV biomarker expression, miRNA cargo(s) or pathway activation that will aid in understanding the underlying therapeutic mechanisms.
Methods
Articles employing EVs derived from either MSCs or MSC-like osteogenic stromal cells in preclinical bone regeneration studies are included in this review.
Results
EVs derived from a variety of MSC types were able to successfully induce bone formation in preclinical models. Many studies failed to perform in-depth EV characterization. The studies with detailed EV characterization data report very different miRNA cargos, even in EVs isolated from the same species and cell types. Few preclinical studies have analyzed the underlying mechanisms of MSC-EV therapeutic action.
Conclusion
There is a critical need for mechanistic preclinical studies with thorough EV characterization to determine the best therapeutic MSC-EV source for bone regeneration therapies. Issues including controlled EV delivery, large scale production, and proper storage also need to be addressed before EV-based bone regeneration therapies can be translated for clinical bone repair.
Keywords: Extracellular vesicles, Bone regeneration, Mesenchymal stromal cells, Tissue engineering, miRNA, Biomaterials
Highlights
-
•
EVs from different MSC sources successfully regenerate bone in preclinical models.
-
•
Studies were reviewed to find commonalities in EV cargo(s)/pathways activated in MSC-EV-based bone regeneration therapies.
-
•
Issues that need to be overcome to enable clinical translation of EV-based therapies were addressed.
1. Introduction
Every year in the United States, more than half a million patients are treated for bone defects, resulting in health care costs of more than $2.5 billion (Amini et al., 2012). For large defects, the complex process of bone healing, involving the coordination of osteogenesis and angiogenesis, often requires further clinical intervention with autografts, allografts or synthetic bone graft substitutes and/or extenders (Amini et al., 2012). However, mesenchymal stromal cell (MSCs)-based therapies have emerged as a promising alternative and are beginning to be applied clinically (Amini et al., 2012).
MSCs are multipotent stromal cells obtained from tissue sources such as bone marrow, adipose, and umbilical cord, or generated from induced pluripotent stem cells (iPS-MSCs) (Qi et al., 2016; Liu et al., 2017; Zhou et al., 2019; Qin et al., 2016; Takeuchi et al., 2019). While MSC transplantation has induced both osteogenesis and angiogenesis in preclinical bone regeneration models (Takeuchi et al., 2019), it is also associated with disadvantages such as limited donor numbers, invasive harvesting procedures, reduced therapeutic potential with increasing donor age, possible immunogenicity, and the risk of emboli formation (Amini et al., 2012). As the therapeutic effectiveness of transplanted MSCs has been attributed to their paracrine activity (Liang et al., 2014; Tao et al., 2018), research has recently focused on the MSC secretome as an alternative treatment.
Extracellular vesicles (EVs), which are nanoscale vesicles encapsulated by a lipid bilayer membrane that are secreted by almost all types of cells, have been identified as one of the main elements within the MSC secretome which induce osteogenic differentiation (Furuta et al., 2016). EVs transport cargo in the form of lipids, proteins and ribonucleic acids (RNAs) between cells (Tao et al., 2018; Liang et al., 2014) and thus play important roles in intercellular communication by either stimulating receptors on the target cell surface and/or by fusing with the target cells and releasing their contents (Tao et al., 2018; Liang et al., 2014). Offering several advantages over their respective cell sources, MSC-EVs exhibit low immunogenicity, as most of them lack expression of the major histocompatibility complex, and can home to bone upon transplantation while minimizing accumulation in the liver (Wei et al., 2019). MSC-derived EVs may thus be effective mediators of osteogenic differentiation, angiogenesis, and other therapeutic effects.
As EV cargo varies significantly depending on the type, age, and state of the source cell, and the surrounding in vitro culture environment (Luo et al., 2019; Zhang et al., 2016; Narayanan et al., 2016), characterization is crucial for understanding the underlying mechanisms which influence their therapeutic efficacy. A key element of EV cargo is microRNAs (miRNAs), which are small non-coding RNAs that play crucial roles in gene expression and thus influence bone healing via regulation of cell proliferation, differentiation, and apoptosis (Hadjiargyrou and Komatsu, 2019). Expression of miRNAs also influences several key signaling pathways involved in bone regeneration, including the Phosphatidylinositol-3-Kinase (PI3k)/ Protein kinase B(AKT), Wingless/Integrated (Wnt), Ras/ Extracellular receptor kinase (ERK), and mammalian target of rapamycin(mTOR) (Liu et al., 2017; Zhou et al., 2019; Zhang et al., 2016). While various in vitro studies have identified EV miRNAs involved in osteogenic differentiation (for adipose-derived MSCs (ASCs): miR-100-5p, let-7 g-5p, miR-21-5p, miR-24-3p and miR-148a-3p (Weilner et al., 2015); and for bone marrow-derived MSCs (BM-MSCs): miR-27a, miR-21, miR-217, miR-26a, miR-148a, miR-200b, miR-335-5p, miR-92a, miR-9 and miR-199b-5p (Hadjiargyrou and Komatsu, 2019)), results from in vivo preclinical studies have been less clear, with differential expression of various miRNAs reported with very little overlap (Hadjiargyrou and Komatsu, 2019). Indeed, very few mechanisms of therapeutic EV action have been tested in pre-clinical osteogenic models.
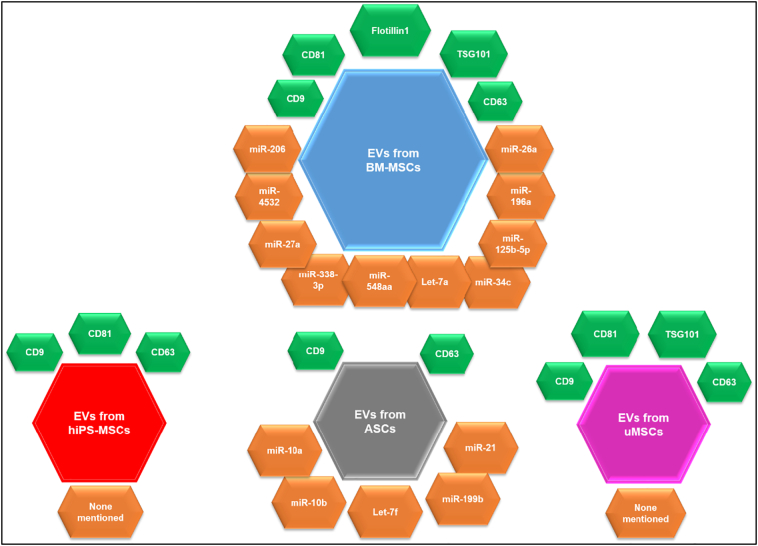
This review focuses on pre-clinical studies of EV-based bone regeneration therapies, searching for any trends in EV biomarker expression, miRNA cargo or pathway activation that could aid in understanding the mechanisms underlying MSC-EVs' therapeutic activity. We will discuss the bone healing potential of EVs isolated from various MSC sources, emphasizing their in vivo effects and underlying molecular mechanisms (see Fig. 1). While EVs are often sub-classified as either exosomes, microvesicles (MVs), oncosomes, or apoptotic bodies (Van der Pol et al., 2012), they are often difficult to separate, as there is some overlap in their size ranges, markers, and cargos. Thus, in this review, we will solely use the term EV and include characterization details were available.
Fig. 1.
Overview of common marker (green hexagons) and miRNA (orange hexagons) cargos carried by EVs derived from different MSC and MSC-like cell sources that demonstrated osteogenic potential in preclinical animal models.
2. Methods
2.1. Literature search
A literature search for this review was performed based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines, using the search terms [(stem cell derived) “Exosome” OR “Extracellular vesicle” OR “Microvesicle” AND “bone fracture” OR “bone healing” OR “bone regeneration” OR “bone defects” OR “osteogenesis” OR “osteogenic differentiation”]. The bibliographies of examined articles were further reviewed for more relevant studies. This web-based search was conducted using PubMed and included articles published until May 21st, 2020.
2.2. Inclusion and exclusion criteria
Inclusion criteria were original studies in the English language which employed EVs, exosomes or MVs derived from either bone marrow-derived MSCs (BM-MSCs), adipose-derived MSCs (ASCs), umbilical cord derived MSCs (uMSCs), or MSC-like stem cells (i.e., iPS-MSCs) with demonstrated osteogenic potential in preclinical animal models of bone regeneration. Exclusion criteria included: review articles; articles written in languages other than English; original studies that involved only in vitro analysis; in vivo bone regeneration studies that involved EVs derived from other cell types; and in vivo bone regeneration studies that employed stem cell-derived “soluble factors”, “matrix vesicles”, “secretion factors”, or “conditioned media” without further characterization. Also, studies involving EVs derived from cells transfected to produce EVs enriched in certain miRNAs were excluded.
2.3. Data extraction
Data extracted from the articles which met the inclusion criteria included: EV source cell type, species, and passage number; EV size range(s); EV content/cargo (i.e. proteins and miRNAs); in vitro effects (if any) of EVs on osteogenesis and/or angiogenesis; preclinical animal model used; EV delivery mechanism (i.e. direct injection or carrier based-delivery); EV concentration/amount used; in vivo effects on bone formation; and, in vivo signaling pathways involved.
3. Results and discussion
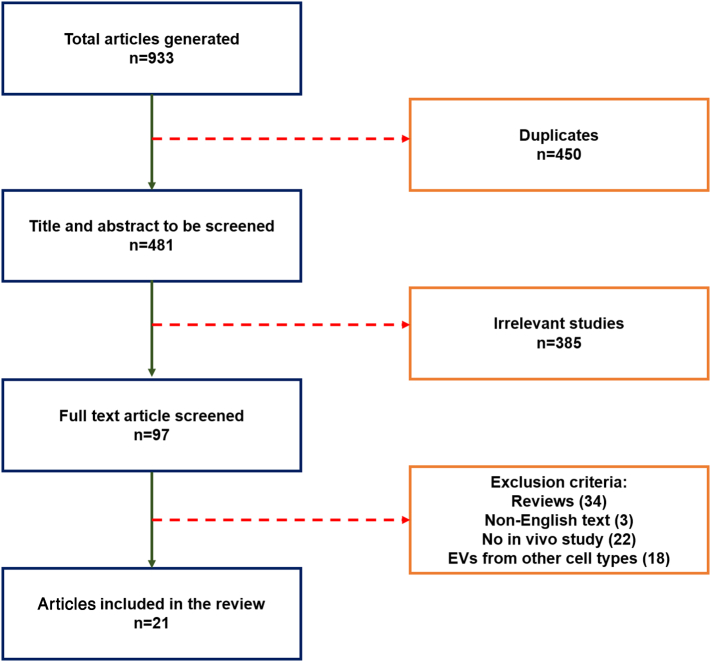
The literature search identified a total of 933 articles. Following the exclusion criteria, 21 articles were included in this review (see Fig. 2). The majority (13) of studies that met the inclusion criteria employed EVs derived from BM-MSCs, while three studies tested EVs from iPS-MSCs, two from uMSCs, and three from ASCs. The bone regeneration effects of EVs are detailed and discussed in subsequent sections, organized by cell type of origin.
Fig. 2.
Literature search results and screening process, based on PRISMA guidelines.
3.1. BM-MSC-EVs
BM-MSCs are the most commonly used MSC-type in clinical and preclinical studies, particularly for bone regeneration applications. One of the first preclinical studies to evaluate human BM-MSC (hBM-MSC)-derived EVs (Table 1) demonstrated the osteogenic potential of both BM-MSC conditioned medium and EVs in rat calvarial defects (Qin et al., 2016). In CD9−/− mice, which exhibit reduced EV production, injection of BM-MSC-EVs, but not culture supernatant devoid of EVs, rescued impaired fracture healing (Furuta et al., 2016). BM-MSC-EVs were subsequently shown to accelerate bone healing in other murine and rodent defect models (Furuta et al., 2016; Zhang et al., 2020) and promote bone formation when seeded onto collagen sponges and polycaprolactone (PCL)-alginate scaffolds in rat periodontal defects and the back pouches of nude mice, respectively (Chew et al., 2019; Xie et al., 2016). In a murine model of osteogenesis imperfecta, BM-MSC-EVs effectively promoted osteogenesis. Interestingly, the EVs lost their osteogenic potential upon in vitro miRNA depletion via prolonged incubation with RNases, highlighting the role of miRNA in bone formation (Otsuru et al., 2018).
Table 1.
Studies of BM-MSC derived EVs for promoting bone regeneration.
| EV cell origin |
EV size (nm) | Content profile |
In vitro effects |
In vivo |
Pathway(s) involved | Ref | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Species | Passage | Markers | miRNA | Model | Delivery mechanism | Amount of EV delivered | In vivo effects | ||||
| Human | 3rd to 5th | Not mentioned | CD63 | 196a, 27a, 206 | Internalized into the endoplasmic reticulum, Golgi apparatus and lysosomes of human osteoblasts. Marginal increase in proliferation. Increased ALP, OCN, OPN & RUNX2 expression | 5 mm rat calvarial defect | Hystem®-Heparin hydrogel | 100 μg | 8 wk- Significantly increased amount and area density of newly formed bone in the EV group as compared to hydrogel and blank control | Not mentioned | (Qin et al., 2016) |
| Human | 3rd to 6th | 80-100 | CD9, CD63, CD81 | Not mentioned | Increased proliferation, mineral deposition, and expression levels of COL1, ALP, OCN, OPN, VEGF, ANG1&2 in MSCs | 5 mm rat calvarial defect | Atelocollagen sponges | 30 μg | 4 wk- Increased area of newly formed bone in the MSC-EV group than in the MSC-CM group. More cells stained for OCN, VEGF, CD31 and CD44 | Not mentioned | (Takeuchi et al., 2019) |
| Human | 4th to 6th | ~80 | CD9, CD81 and flotillin1 | miR-4532, miR-125b-5p, miR-338-3p, miR-548aa. | None | Murine femoral fracture | Local injection | 100 μl | 2 wk- Accelerated formation of hypertrophic chondrocytes, more woven bone and improved fracture healing, many TRAP+ cells in callus of EV injected group. | MCP-1, MCP-3, SDF-1 and VEGF (although effects not solely attributable to these factors) | (Furuta et al., 2016) |
| Human | 4th | ~100 | CD63 | Not mentioned | Increased expression of ALP, OSX, RUNX2 and ARS staining in iliac BM-MSCs incubated with EVs from maxillary BM-MSCs | 4 mm murine (nude) calvarial defect | PLGA scaffold | Not mentioned | 12 wk- More new bone formation in group implanted with iliac BM-MSC treated with maxillary BM-MSC EVs than group implanted with iliac BM-MSC treated with iliac BM-MSC EVs | siRNA: Rab26a | (Li et al., 2019) |
| Human | 4th–6th | 50-150 | CD9, CD63, Hsp70 | MALAT1, miR-34c | Increased osteoblast proliferation, ALP activity and calcium nodule formation | Mouse OVX model | Periostial injection | 20 μl | 3wk- Bone formation rate increased in mice injected with oe-MALAT1 and decreased in mice injected with miR-34c agomir and sh-SATB2. | miRNA-34c/SATB2 axis | (Yang et al., 2019) |
| Human (specific source of MSC not mentioned) | Not mentioned | 100-200 | CD81, Alix, TSG101 | Not mentioned | Enhanced PLSCs proliferation and migration | Rat periodontal defect | Collagen sponges | 40 μg | 4wk- increased bone formation, aligned PDL fibers, and closure of bone gap compared to collagen group | AKT and ERK pathways in PLSCs | (Chew et al., 2019) |
| Rat | 3rd to 4th | 100-1000 | CD73, 105, 29, 44, 90, 34, 45 | Not mentioned | Dose-dependent increase in growth in HUVECs. Increased HUVEC migration and tube formation. No effect on MSC proliferation, apoptosis and differentiation. | Nude mice SQ implantation | DBM scaffold coated with fibronectin | 20 μg | 1&2 m- Increased BV and BV/TV in cells + EV scaffold, more bone regeneration and osteoblast like cells. More CD31 labeling in cells + EV group | Not mentioned | (Xie et al., 2017) |
| Rat | 2nd-5th | 122 | CD9, CD63, CD81 | Not mentioned | Promoted proliferation and migration of HUVECs and MC3T3 cells. Promoted tube formation in HUVECs and osteogenic differentiation of MC3T3s | Rat femoral fracture | Injected at fracture site | 1010 | 20wk- Significantly increased callus formation, BV/TV, vascular branching, and expression of CD31, VEGF, HIF-1α, BMP2, Smad1/5, RUNX2, OGN, OPN and OCN in EV group compared to control | BMP2/Smad1/RUNX2 signaling pathway | (Zhang et al., 2020) |
| Rat | 3rd-4th | 100-1000 | CD73, CD105, CD29, CD44, and CD90 | Not mentioned | Promoted tube formation of HUVECs | SQ Nude mice | PCL-alginate scaffold | 1 μg/μl | 8wk- Increased bone and blood vessel formation in cell+EV+ Scaffold group as compared to cell + Scaffold, EV + Scaffold or scaffold groups | Not mentioned | (Xie et al., 2016) |
| Rat (young-4wk vs old-72wk) | 3rd-5th | 50-150 | CD63, CD81 | miR-128-3p in old EVs | Young EVs induced higher expression of RUNX2, ALP and COL1 in BM-MSCs than old EVs | Rat femoral fracture | Injection at fracture site | 200 μg | 2wk- young EVs stimulated more callus formation in fracture gap with increased expression of RUNX2, ALP and COL1 as compared to old EVs | Not mentioned | (Xu et al., 2020) |
| Rat (2wk old) |
3rd-5th | 60-130 | CD9, CD63, TSG101 | Not mentioned | Increased proliferation, calcium deposition and expression of ALP, RUNX2, and OCN in old MSCs | Old Rat (60wk) tibial DO | Injection at distraction site | 1 × 1010 | 5wk- Increased BV/TV, BMD, ultimate load, and energy to failure in EV group than control group. | Not mentioned | (Jia et al., 2020) |
| Mouse | Not mentioned | 35-105 | CD63, CD81, TSG101 | miR-26a | Increased internalization by BM-MSCs and RAW264.7 cells after aptamer functionalization. Dose-dependent increase in OCN, RUNX2 & ALP levels and matrix mineralization. Did not affect osteoclastic differentiation of RAW264.7 cells. | OVX murine femoral fracture | Tail vein injection | 100 μg | OVX only model: 8 wk. – Significant increase in trabecular number, thickness, and volume and OCN stained area in aptamer EV group with no effect on osteoclastic differentiation. Fracture model: 6 wk- Increased width and area of callus and increased BV/TV in the aptamer EV group. |
Not mentioned | (Luo et al., 2019) |
| Mouse | Not mentioned | 170.3 ± 8.6 | CD9, CD29, CD44, CD90 and Sca-1 | Let-7a | EVs promoted chondrocyte proliferation | G610C OI mice | Tail vein injection | Not mentioned | 2wk - longer femora and tibiae in EV group than control group | Not mentioned | (Otsuru et al., 2018) |
BM-MSC: Bone marrow mesenchymal stromal cells; OCN: Osteocalcin; RUNX: Runt-related transcription factor; ALP: Alkaline phosphatase; OPN: Osteopontin; COL: Collagen; EV: Extracellular vesicle; CM: Conditioned medium; VEGF: Vascular endothelial growth factor; ANG: Angiopoietin; OVX: Ovariectomized; BV/TV: Bone volume/total volume; HUVEC: Human umbilical vein endothelial cells; BMP: Bone Morphogenetic Protein; SQ: subcutaneous; TRAP: Tartrate-resistant acid phosphatase; OSX: Osterix; ARS: Alizarin red S; PLGA: Poly lactic- co-glycolic acid; DBM: Demineralized bone matrix; BMD: Bone mineral density; DO: Distraction osteogenesis; PLSCs: Periodontal ligament stem cells; MCP: Monocyte chemotactic protein; SDF: Stromal cell-derived factor; MALAT: Metastasis associated lung adenocarcinoma transcript; SATB: Special AT-rich sequence-binding protein; PDL: Periodontal ligament; AKT: Protein kinase B; ERK: Extracellular receptor kinase; HIF: Hypoxia inducible factor; PCL: Poly caprolactone; OGN: Osteoglycin.
In rat calvarial defects, hBM-MSC-derived EVs induced greater vascularization and increased osteocalcin (OCN) and Vascular Endothelial Growth Factor (VEGF) immunostaining (Takeuchi et al., 2019). Xie et al. more closely evaluated the angiogenic potential of MSC-derived EVs during bone regeneration (Xie et al., 2017). MSC-EVs adhered onto MSC-seeded decalcified bone matrix scaffolds were implanted subcutaneously in nude mice and yielded significant new bone formation with increased vascularization. Interestingly, although the EV only group had more CD31 positive cells, no significant bone was formed (Xie et al., 2017).
Differences in cell origin can contribute to differences in the therapeutic capacity of BM-MSC-EVs (Bugueno, 2014). While most studies of BM-MSC-EVs isolated cells from long bones, Li et al. compared the osteogenic potential of BM-MSC-EVs derived from the jaw bone (BMSC-J) and iliac crest (BMSC-I) of alveolar cleft patients (Li et al., 2019). Implantation of BMSC-I cells treated with EVs derived from BMSC-J cells in rat calvarial defects resulted in greater bone mineral density (BMD) and bone volume than cells treated with BMSC-I (Li et al., 2019). However, scaffolds seeded with EVs and cells promoted more bone formation than those loaded with EVs alone (Li et al., 2019). In another study, while BM-MSC-derived EVs isolated from both normal (nBMSCs) and type 1 diabetic (dBMSCs) rats supported more new bone formation and vascularization than the control, the effects of nBMSC-EVs were greater than those of dBMSC-EVs (Zhu et al., 2019).
Variations in the donor and cellular age from which BM-MSC-EVs are obtained may also have an important effect on their therapeutic efficacy. While comparing BM-MSC-EVs isolated from four-week and 72-week-old rats, the younger EVs stimulated more callus formation with increased expression of osteogenic genes in a rat fracture model (Xu et al., 2020). In another study, EVs isolated from BM-MSCs of two-week old rats promoted better bone healing in tibial distraction osteogenesis in 15 month old rats (Jia et al., 2020). Interestingly, while studying EVs derived from cells grown for two and four weeks, it was found that the four week old EVs upregulated more osteogenic gene expression in hBM-MSCs than the two week old EVs (Narayanan et al., 2016). However, no further studies were performed to confirm these results in vivo.
Pre-clinical research has shown that BM-MSC-derived EVs have effects on both vasculogenesis and osteogenesis, however, in vitro studies have shown conflicting data on which of these mechanisms are likely to dominate. For example, some studies with rat BM-MSC-derived EVs showed that EVs promoted proliferation, migration, and tube formation in Human umbilical vein endothelial cells (HUVECs), but had no effect on MSC proliferation, apoptosis or osteogenic differentiation (Xie et al., 2017). This was in direct contrast to other studies with hBM-MSC-EVs, where EVs stimulated MSCs to express osteogenic as well as angiogenic genes including Alkaline phosphatase (ALP), Osteopontin (OPN), OCN, collagen (COL)1A1, VEGF, and Angiopoietin (ANG)1 and 2 (Takeuchi et al., 2019; Narayanan et al., 2016). However, in this study, the EVs lost their bone stimulating properties in the presence of an angiogenesis inhibitor, highlighting the role of vascularization in EV stimulated osteogenesis (Takeuchi et al., 2019).
Different miRNAs and pathways are known to be involved in bone regeneration and have thus been analyzed in BM-MSC-derived EVs. EVs carry a variety of angiogenic factors including VEGF, interleukin-6, Fibroblast growth factor (FGF)b, angiogenin, and monocyte chemotactic protein (MCP)-1 (Chen et al., 2014). RNA sequencing by Qin et al. indicated the upregulation of three osteogenic-related miRNAs- miR-196a, miR-27a and miR-207 in BM-MSC-EVs, with miR-196a being the most osteogenic (Qin et al., 2016). Meanwhile, another study revealed upregulation of 104 miRNAs in BM-MSC-EVs as compared to their source MSCs, many of which were pro-osteogenic. Among these, miR-26a was found to play a key role; as it was enriched approximately 35-fold in the EVs as compared to the cells, and its silencing completely abolished the in vitro osteogenic potential of the EVs (Luo et al., 2019). Interestingly, in contrast to previous studies, cytokine and antibody arrays revealed that proteins, including VEGF, MCP-1, MCP-3 and Stromal cell-derived factor-1, were expressed at much lower levels in the MSC-EV group as compared to the culture supernatant group (Furuta et al., 2016), suggesting that the fracture healing capacity of MSC-EVs might be influenced by several other factors present in the supernatant.
Few other miRNAs and pathways have also been shown to play important roles in osteogenesis induced by BM-MSC-derived EVs. For example, miRNA characterization by Nakamura et al. showed significant expression of miR-21, miR-4532, miR-125b-5p and miR-338-3p in EVs, among which miR-21 is an antiapoptotic miRNA known to promote osteogenic differentiation of MSCs (Nakamura et al., 2015). Further, Yang et al. demonstrated the roles of EV-Metastasis Associated Lung Adenocarcinoma Transcript 1 (MALAT1) and the Special AT-rich sequence-binding protein 2 (SATB2)/miR-34c axis in promoting bone formation in ovariectomized (OVX) mice (Yang et al., 2019). While osteoporotic mice displayed increased levels of miR-34c and decreased levels of MALAT1 and SATB2 (Yang et al., 2019), injection of MALAT1-over-expressing-EVs reversed these expression patterns and increased bone formation (Yang et al., 2019). The AKT/ERK and Bone morphogenetic protein (BMP)2/Smad1/Runt-related transcription factor (RUNX)2 pathways were also found to play crucial roles in EV-induced in vivo bone formation (Zhang et al., 2020) and the in vitro proliferation and differentiation of periodontal ligament stem cells (Chew et al., 2019). While studying the effect of aging on EV therapeutic efficiency, EVs from aged rats upregulated miR-128-3p and showed a lowered capacity to stimulate bone formation in vivo (Xu et al., 2020). Crucially, in vivo silencing of miR-128-3p, via injection of miR-128-3p antagomir, demonstrated increased bone healing and expression of osteogenic genes and Smad5 in fractured rat femurs, compared to the control antagomir (Xu et al., 2020).
3.2. iPS-MSC-EVs
iPS-MSCs, which have properties similar to both MSCs and iPSCs, demonstrated bone regeneration capacity (Villa-Diaz et al., 2012) and promoted angiogenesis (Liao et al., 2019b) in pre-clinical models. These cells exhibited a similar surface profile to BM-MSCs (CD73, 90 and 105), could be passaged more than 40 times in culture while sustaining self-renewal, and demonstrated greater proliferative capacity and immunoregulatory function than BM-MSCs (Villa-Diaz et al., 2012). iPS-MSC-EVs were thus investigated as a promising candidate for bone regeneration applications (Table 2). Human iPS-MSC (hiPS-MSC)-EVs increased bone regeneration in a dose-dependent manner when implanted in rat calvarial defects along with β-tricalcium phosphate (Zhang et al., 2016). This effect was likely due to their in vitro capacity to stimulate proliferation, migration and osteogenic differentiation of human BM-MSCs and induce upregulation of Platelet derived growth factor (PDGF)A, FGF1/2, and FGF Receptor (FGFR)1, and down regulation of Glycogen synthase kinase (GSK)3β. hiPS-MSC-EVs also increased the expression of COL1A1 and BCL2L1 and decreased Phosphatase and tensin homolog (PTEN) expression (Zhang et al., 2016). Similarly, when calvarial defects in OVX rats were treated with hiPS-MSC-EVs, they promoted bone formation and vascularization, with higher concentrations of EVs yielding higher expression of OCN, OPN and CD31 (Liu et al., 2017). The PI3k/AKT signaling pathway was found to be critical in hiPS-MSC derived EV-induced osteogenesis, as addition of a PI3k/AKT inhibitor reduced in vitro expression of osteogenic genes and differentiation of BM-MSCs (Zhang et al., 2016), and blocking this pathway in HUVECs abolished the angiogenic potential of EVs (Liu et al., 2017).
Table 2.
Studies of human pluripotent stem cell-MSC-derived EVs for promoting bone regeneration.
| EV cell origin |
EV size (nm) |
Content profile |
In vitro effects |
In vivo |
Pathway (s) involved | Ref | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Species | Passage | Markers | miRNA | Model | Delivery mechanism | Amount of EVs delivered | In vivo effects | ||||
| Human | Not mentioned | 50-150 | CD9, CD63, CD81 | Not mentioned | Dose dependent increase in proliferation, ALP activity and mineral deposition in BM-MSCs. Increased levels of RUNX2, COL1 and ALP. | 5 mm rat (osteopenic) calvarial defect | β-Tricalcium phosphate scaffold | 100, 200 μg/ml | 8 wk- Dose dependent increase in BMD, BV/TV, neovascularization. More OCN, OPN & CD31 expression in 200 μg/ml group | Not mentioned | (Qi et al., 2016) |
| Human | Not mentioned | 30-100 | CD9, CD63, CD81 | Not mentioned | Internalized by HUVECs. Increased expression of BCL2A1, Sphk1, MYC in HUVECs | Steroid induced ONFH in rats | tail vein injection | 1 × 1010/ml or 1 × 1011/ml | 3 wk- Fewer empty lacunae and necrotic medullary hematopoietic and adipose cells, increased trabecular bone structural integrity, higher BV/TV, bone surface area/bone volume, Tb.Th and Tb.N, more vascular branches, increased expression of VEGFR2 & CD31 | PI3k/AKT | (Liu et al., 2017) |
| Human | Not mentioned | 50-150 | CD9, CD63, CD81 | Not mentioned | Dose dependent increase in proliferation and ALP activity in BM-MSCs. Increased levels of PDGFA, FGF1/2, FGFR1, COL1A1 & BCL2L1 | 5 mm rat calvarial defects | β-Tricalcium phosphate scaffold | 5 × 1011 particles/ml or 1 × 1011 particles/ml | 8 wk- Dose-dependent increase in bone regeneration; more tetracycline, alizarin red, calcein and OCN staining | PI3k/AKT signaling increase in PDGFA, FGF1/2, FGFR1, COL1A1, BCL2L1 and decrease in GSK3β, PTEN. | (Zhang et al., 2016) |
BMD: Bone mineral density; PDGF: Platelet derived growth factor; FGF: Fibroblast growth factor; FGFR: FGF receptor; Tb. Th: Trabecular thickness; Tb.N: trabecular number; ONFH: Osteonecrosis femoral head; ALP: Alkaline phosphatase; BM-MSC: Bone marrow derived mesenchymal stromal cell; RUNX: Runt-related transcription factor; COL: Collagen; BV/TV: Bone volume/Total volume; OCN: Osteocalcin; OPN: Osteopontin; HUVECs: Human umbilical vein endothelial cells; VEGFR: Vascular endothelial growth factor receptor; BCL2L1: B-lymphoma-2 like protein-1; AKT: Protein kinase B; PI3K: Phosphoinositide 3-kinase; GSK: Glycogen synthase kinase; PTEN: Phosphatase and tensin homolog.
3.3. uMSC-EVs
uMSCs are easy to obtain, display low immunogenicity, and possess enhanced proliferation and osteogenic differentiation capacity, making them an appealing alternative therapeutic cell source (Baksh et al., 2007). These cells have also promoted bone regeneration in animal models, mostly by stimulating vascularization (Liao et al., 2009), making their EVs a promising candidate for bone regeneration therapies (Table 3). In a rat femoral fracture model, treatment with uMSC-EVs increased bone healing and local expression of Wnt3a and β-catenin (Zhou et al., 2019), which are known to play crucial roles in skeletal development and fracture healing (Church and Francis-West, 2004). uMSC-EVs increased fracture healing compared to EVs from HEK293 cells, with the resulting healed bone displaying higher BMD, more CD31 positive cells, and better mechanical properties (Zhang et al., 2019).
Table 3.
Studies of uMSC-derived EVs for promoting bone regeneration.
| EV cell origin |
EV size (nm) |
Content profile |
In vitro effects |
In vivo |
Pathway(s) involved | Ref | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Species | Passage | Markers | miRNA | Model | Delivery mechanism | Amount of EVs delivered | In vivo effects | ||||
| Human | Not mentioned | 30-100 | CD9, CD63, CD81 | Not mentioned | None | SD rat femoral fracture | HyStem®-Heparin hydrogel | 100 μg | 3 wk- Increased fracture healing in EV group as compared to control groups; higher expression of β-catenin, Wnt3a, COL1, OPN and RUNX2 | β-catenin and Wnt 3a | (Zhou et al., 2019) |
| Human | 2nd to 5th | 100 | CD9, CD63, CD81 | Not mentioned | Internalization by HUVECs. Increased proliferation, migration and tube formation by HUVECs with increased expression of VEGF and HIFα1. No effect on osteoblast proliferation or differentiation. | Rat femoral fracture | Hystem®-Heparin hydrogel | 100 μg/ml | 4 wk-Larger callus volume, increased bone mineral density, BV and BV/TV; increased CD31+ blood vessels, enhanced maximum load at failure and bending stiffness | HIF1α | (Zhang et al., 2019) |
HIF: Hypoxia inducible factor; SD: Sprague Dawley; COL: Collagen; OPN: Osteopontin; RUNX: Runt-related transcription factor; HUVECs: Human umbilical vein endothelial cells; VEGF: Vascular endothelial growth factor; BV: Bone volume; TV: total volume; EV: Extracellular vesicle.
The bone regeneration efficacy of uMSC-EVs appears to be mainly due to their ability to induce angiogenesis and vasculogenesis. uMSC-EVs stimulated HUVEC proliferation and migration, and upregulation of VEGF and Hypoxia inducible factor (HIF)-1α expression while not having any effects on the osteogenic differentiation capacity of primary osteoblasts (Zhang et al., 2019). Using specific small interfering RNA (siRNA), uMSC-EVs were shown to upregulate HIF-1α and VEGF gene expression in endothelial cells, which are known to play important roles during angiogenesis (Ahluwalia and Tarnawski, 2012; Peng et al., 2005). This pro-angiogenic capability of uMSC-EVs was enhanced when the source cells were exposed to a hypoxic environment (hypoxic uMSC-EVs) (Zhang et al., 2019). While performing miRNA microarray analysis to understand the mechanism(s) underlying the angiogenic effects of hypoxic uMSCs-EVs, it was found that miR-126, miR-8855-5p, miR-146b, miR-223 and miR-451 were significantly upregulated (Liu et al., 2019). Specifically, miR-126, which exhibits a positive effect on angiogenesis (Fish et al., 2008; Wang et al., 2008), was found to have been transferred to the HUVECs from the uMSC-EVs (Liu et al., 2019). Further, knockdown of miR-126 inhibited hypoxic EV mediated angiogenesis in vitro, and reduced callus volume and number of blood vessels in vivo (Liu et al., 2019).
3.4. Adipose tissue-derived-EVs
MSCs are also found in the stromal vascular fraction of adipose tissue, which is widely available and can be collected using less invasive procedures (Amini et al., 2012). Human ASCs (hASCs) have osteogenic potential and their EVs are effective in stimulating angiogenesis and wound healing in vitro and in vivo (Table 4). In murine calvarial defects, hASC-EVs delivered via poly glycolic-co-lactic acid/ poly dopamine scaffolds resulted in much higher new bone formation compared to non-EV scaffolds (Li et al., 2018). In a subcutaneous implantation model in nude mice, hASC-EVs functionalized onto MG63 cell seeded titanium substrates stimulated greater osteogenic differentiation of the seeded cells as compared to controls (Chen et al., 2019a). Interestingly, while these EVs did not support the osteogenic differentiation of ASCs in vitro, they expressed high levels of miR-21, let-7f, miR-10a&b and miR-199b, all of which are involved in maintaining bone homeostasis or promoting osteogenic differentiation via SMAD, RUNX2, GSK3β/catenin, Axin2 and/or Krüppel-like factor (KLF)4 signaling (Chen et al., 2019a).
Table 4.
Studies of human adipose stem cell and perivascular stem cell -derived EVs for promoting bone regeneration.
| EV cell origin |
EV size (nm) | Content profile |
In vitro effects |
In vivo |
Pathway(s) involved | Ref | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Species | Cell type | Passage | Markers | miRNA | Model | Delivery mechanism | Amount of EVs delivered | In vivo effects | ||||
| Human | Adipose stem cells | Not mentioned | 33-17 | CD9, CD63 | not mentioned | Internalized by BM-MSCs. Increased ALP activity and mineralization with increased RUNX2, COL1A1 and ALP expression (when cultured in OM) | 4 mm murine calvarial defect | PLGA-pDA scaffold | 1 μg/ml | 6 wk- PLGA/pDA-EV group showed some bone formation along the margins of the defect, increased mature collagen deposition, and increased immunostaining for RUNX2 & OCN. Other groups showed no bone and/or fibrotic tissue formation | Not mentioned | (Li et al., 2018) |
| Human | Adipose stem cell | 4th to 6th | 100-800 | CD9, CD81 | miR-21, 10a, 10b, 199b, let-7f | Increased viability, calcium deposition and expression of ALP, OCN & COL1 gene expression in MG63 cells | SQ nude mice model | Biotin doped polypyrrole coated titanium substrates | 10 μg/ml | 4 wk- More OCN positive cells in EV group as compared to control groups | Not mentioned | (Chen et al., 2019a) |
| Human | Adipose derived-PSCs | 3rd to 9th | 100 | Not mentioned | Not mentioned | Internalized by BM-MSCs. Increased ALP Activity. | 1.8 mm murine calvarial defect | Percutaneous injection over the defect region | 1 or 2.5 mg | 4 wk- Increased osteoblastic proliferation at the bone defect edge; significant narrowing of the gap between bony fronts and an enrichment of OCN+ cells at the leading edges of the defect site. | IGSF8 or PTGFRN KD nullified the pro-migratory effects of PSC-EVs | (Xu et al., 2019) |
pDA: polydopamine; PSC: Perivascular stem cells; KD: Knock down; IGSF: Immunoglobulin superfamily; PTGFRN: Prostaglandin F2 receptor negative regulator; BM-MSC: Bone marrow derived mesenchymal stromal cell; ALP: Alkaline phosphatase; COL: collagen; OM: Osteogenic medium; OCN: Osteocalcin; PLGA: Poly lactic-co-glycolic acid; RUNX: Runt-related transcription factor; EV: Extracellular vesicle; SQ: Subcutaneous.
Perivascular stem cells (PSCs) are another class of stem cell isolated from adipose tissue. While the relationship between PSCs and ASCs is unclear, some reports show evidence that ASCs might actually be derived from the PSC niche (James et al., 2012). PSCs differentiate into chondroblasts, osteoblasts and osteocytes and induce bone healing in a manner similar to ASCs (James et al., 2012). PSC-EVs shared the pro-osteogenic properties of their parent cells and resulted in a significant increase in new bone formation and healing compared to control groups when implanted in murine calvarial defects (Xu et al., 2019). Interestingly, while these PSC-EVs stimulated a dose-dependent increase in RUNX2 and osterix levels in BM-MSCs in vitro, no such effect was seen in ASCs (Xu et al., 2019). Also, while comparing the efficacy of PSC-EVs and ASC-EVs, PSC-EVs promoted higher cellular proliferation and osteogenic differentiation than ASC-EVs (Xu et al., 2019). Despite some differences, PSC-EVs shared many commonalities with ASC-EVs, including upregulation of several positive regulators of cellular proliferation (JMJD1C, NRIP1 and TRPS1) and cellular migration (JMJD1C, TCF4 and KLF7) (Xu et al., 2019).
When tested in vitro, the uptake and bioactivity of PSC-EVs was found to be dependent on the interactions between membrane-bound proteins and host cells. When EV membrane bound proteins were digested by trypsinization, their pro-migratory and osteogenic properties were abolished (Xu et al., 2019). Further, neutralizing the surface marker CD9, partially reversed the pro-osteogenic effect of PSC-EVs in BM-MSCs, thus highlighting its role in this process (Xu et al., 2019).
4. Challenges to clinical translation
Translation of EVs to clinical bone regeneration therapies faces several roadblocks. One of the most critical issues is the large variations reported to date in MSC-EV characterization (i.e. techniques, parameters measured) and composition (e.g. proteins, miRNAs) (Baglio et al., 2015). While most studies have reported similar sets of surface markers, including in EVs isolated from different MSC sources, very different miRNA cargos have been reported; even in EVs isolated from same species and cell type (Table 1). For example, miRNA profiles of BM-MSC EVs have been reported in multiple studies, but no two cases observed the upregulation of similar miRNAs (see Table 1).
The different miRNAs expressed by EVs influence a variety of pathways which are important in bone biology, including PI3k/AKT, transforming growth factor (TGF)β-BMP, Ras/ERK and Wnt signaling (Zhou et al., 2019; Zhang et al., 2016; Zhang et al., 2020). However, most studies analyzing the underlying mechanisms of EV action were performed in static in vitro culture environments, which are very different from complex-dynamic in vivo systems. Thus, there is a critical need to perform more mechanistic preclinical experiments, involving the silencing or overexpression of specific miRNAs and pathways of interest, to ascertain the best therapeutic targets for bone regeneration.
To improve the therapeutic efficacy of EVs, the source cells are often grown in vitro in media supplemented with factors known to promote osteogenesis and/or angiogenesis, such as BMP2 (Huang et al., 2020), dexamethasone (Zhao et al., 2018; Narayanan et al., 2016) or dimethyloxalylglycine (Liang et al., 2019), or in hypoxic conditions (Liu et al., 2019). Several attempts have also been made to enrich EVs with miRNAs known to promote osteogenic differentiation. For example, BM-MSC-EVs have been transfected to over-express miR-122-5p, a possible biomarker of osteoporosis (Liao et al., 2019a), and hASC-EVs were enriched with miR-375, a positive regulator of MSC osteogenic differentiation (Chen et al., 2019b). In all these cases, the altered EVs promoted better bone healing than regular EVs in various animal models (Chen et al., 2019b; Liao et al., 2019a; Zhao et al., 2018; Huang et al., 2020; Liu et al., 2019; Liang et al., 2019; Narayanan et al., 2016).
The age of the cells producing EVs, including their in vitro culture passage number, is another important factor influencing the EV cargo. Most of the studies discussed here use cells from a relatively early passage number, but many do not report this information (Luo et al., 2019; Narayanan et al., 2016; Liang et al., 2019). Since the properties of MSCs and other stem cells are known to change with increasing passaging (Jia et al., 2020; Xu et al., 2020), it is important to document the passage number of the cells from which the EVs were isolated. Alternatively, the MSC source cells for EV production can be immortalized to circumvent issues associated with cell aging and heterogeneity. However, these immortalized clonal cell lines would have to be stringently tested to ensure reproducibility in the production of EVs with stable characteristics (genotypically and phenotypically) (Katsuda and Ochiya, 2015; Buzas et al., n.d.). EVs derived from immortalized cell lines may prove clinically translatable, even though immortalized cells themselves cannot, provided the EVs are free from any products of the immortalization procedure (Buzas et al., n.d.).
The amount of EVs used within a study also influenced EV bone regeneration capacity. For example, in studies conducted by Qi et al. and Zhang et al., increasing EV dosage was associated with increased bone regeneration in calvarial defect models (Qi et al., 2016; Zhang et al., 2016). However, there is significant variation between studies in the units of measurement used to report EV quantity, including: number of EVs; number of EVs/ unit volume; weight of EVs (in μg or mg); and, volume of EVs (μl), thereby making it difficult to draw direct comparisons (Zhang et al., 2016; Xie et al., 2016; Qin et al., 2016; Furuta et al., 2016; Jia et al., 2020). To enable determination of therapeutically effective EV dosages for clinical translation, there is thus a critical need for standardizing the units used to report EV amount, to be able to determine therapeutically effective EV dosage.
The route of EV administration can also significantly affect biodistribution and its therapeutic efficacy. Studies employing systemic delivery have documented the accumulation of EVs in the lungs, liver, spleen, and kidney within 30 min of injection (Lai et al., 2014; Lai et al., 2015). Systemic delivery of EVs might also cause off-target toxicity, early degradation and early clearance from the system (Shahabipour et al., 2017; Smyth et al., 2015). Therefore, developing biomaterial-based strategies for the local delivery of EVs has been investigated as an option to increase EV dosage at the bone regeneration site. Such localized delivery strategies need to carefully consider carrier characteristics, such as degradation profile and physical architecture (pore size, porosity, etc.), to ensure that EVs are uniformly loaded and released in a controllable manner appropriate to the given therapeutic application. A few studies have begun investigating the use of polymeric and calcium based scaffolds for localized EV delivery for bone regeneration, however, further research needs to be conducted to understand the loading and release profile of these EVs, particularly in vivo (Pizzicannella et al., 2019; Diomede et al., 2018a; Diomede et al., 2018b; Qin et al., 2016; Xie et al., 2017).
Other factors essential for the clinical translation of EV-based therapies include large scale production, proper storage, and controlled delivery (Xie et al., 2017; Buzas et al., n.d.). Although a variety of EV isolation methods are currently being employed (Bjørge et al., 2018), the most common approach involves sequential ultracentrifugation followed by filtration and/or purification steps (Qi et al., 2016; Qin et al., 2016; Zhu et al., 2019; Zhang et al., 2016; Cui et al., 2019). Recently, commercially available isolation kits which are based on EV precipitation, such as ExoQuick, have also gained popularity (Diomede et al., 2018b; Luo et al., 2019; Narayanan et al., 2016). However, these methods are time consuming, exhibit low yields and may potentially damage EV integrity (Momen-Heravi et al., 2013; Rekker et al., 2014). Though strategies such as growing cells in serum-free media or under oxidative stress and hypoxic conditions have increased the cellular yield of EVs, they are also likely to induce a stress response in the cells, potentially altering the EV cargo (Zhang et al., 2012; Atienzar-Aroca et al., 2016).
Documentation and precise control of factors, including thorough detailing of cell source (passage number, donor safety and qualification criteria) and chemically defined culture media, will be key to large scale production. Further, implementation of a EV purity metric to enable more stringent and systematic assessment of EV therapeutic potential and possible mechanism(s) of action, needs to be established (Buzas et al., n.d.). Therefore, more research into developing metrics and methods to reliably produce, isolate and purify large volumes of therapeutically effective EVs is critically needed.
5. Conclusions
EVs from a variety of osteogenic stem cell types have been shown to promote bone regeneration in preclinical models, presumably due to their miRNA cargos and their resulting effects on osteogenic and angiogenic signaling pathways. However, depending on the cell source, the miRNA profile of EVs varied greatly, and no conclusion could be reached on the EV cargos that were most important for bone regeneration. Thus, in order to determine the best source of EVs for clinical translation, thorough characterization, and a more comprehensive understanding of the underlying mechanism(s) of action in orthotopic preclinical models will be critical.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
CRediT authorship contribution statement
Christina Holmes: Conceptualization, Methodology, Supervision, Writing- Review & Editing Vishnu Priya Murali: Data curation, Methodology, Writing- Original draft & review.
Declaration of competing interest
None.
Contributor Information
Vishnu Priya Murali, Email: vmurali@eng.famu.fsu.edu.
Christina A. Holmes, Email: caholmes@eng.famu.fsu.edu.
References
- Ahluwalia A., Tarnawski A.S. Critical role of hypoxia sensor-HIF-1α in VEGF gene activation. Implications for angiogenesis and tissue injury healing. Curr. Med. Chem. 2012;19:90–97. doi: 10.2174/092986712803413944. [DOI] [PubMed] [Google Scholar]
- Amini Ami R., Laurencin Cato T., Nukavarapu Syam P. Bone tissue engineering: recent advances and challenges. Crit. Rev. Biomed. Eng. 2012;40:363. doi: 10.1615/critrevbiomedeng.v40.i5.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atienzar-Aroca Sandra, Flores-Bellver Miguel, Serrano-Heras Gemma, Martinez-Gil Natalia, Barcia Jorge M., Aparicio Silvia, Perez-Cremades Daniel, Garcia-Verdugo Jose M., Diaz-Llopis Manuel, Romero Francisco J. Oxidative stress in retinal pigment epithelium cells increases exosome secretion and promotes angiogenesis in endothelial cells. J. Cell. Mol. Med. 2016;20:1457–1466. doi: 10.1111/jcmm.12834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baglio Serena Rubina, Rooijers Koos, Koppers-Lalic Danijela, Verweij Frederik J., Lanzón M. Pérez, Zini Nicoletta, Naaijkens Benno, Perut Francesca, Niessen Hans W.M., Baldini Nicola. Human bone marrow-and adipose-mesenchymal stem cells secrete exosomes enriched in distinctive miRNA and tRNA species. Stem Cell Res Ther. 2015;6:127. doi: 10.1186/s13287-015-0116-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baksh Dolores, Yao Raphael, Tuan Rocky S. Comparison of proliferative and multilineage differentiation potential of human mesenchymal stem cells derived from umbilical cord and bone marrow. Stem Cells. 2007;25:1384–1392. doi: 10.1634/stemcells.2006-0709. [DOI] [PubMed] [Google Scholar]
- Bjørge I.M., Kim S.Y., Mano J.F., Kalionis B., Chrzanowski W. Extracellular vesicles, exosomes and shedding vesicles in regenerative medicine–a new paradigm for tissue repair. Biomater. Sci. 2018;6:60–78. doi: 10.1039/c7bm00479f. [DOI] [PubMed] [Google Scholar]
- Bugueno, Juan M. 2014. 'Characterization of Mandible and Femur Canine Mesenchymal Stem Cells: A Pilot Study'.
- Buzas, Edit, Giovanni Camussi, Nathalie Chaput, Devasis Chatterjee, Felipe A Court, Hernando A del Portillo, Lorraine O'Driscoll, Stefano Fais, Juan M Falcon-Perez, and Ursula Felderhoff-Mueser. 'Applying extracellular vesicles based therapeutics in clinical trials Á an ISEV position paper'. [DOI] [PMC free article] [PubMed]
- Chen, Jianying, Zhenjun Liu, Mian Ming Hong, Hongzhe Zhang, Can Chen, Mengyuan Xiao, Junxian Wang, Feng Yao, Mingchuan Ba, and Jinghu Liu. 2014. 'Proangiogenic compositions of microvesicles derived from human umbilical cord mesenchymal stem cells', PloS one, 9. [DOI] [PMC free article] [PubMed]
- Chen Lifeng, Mou Shan, Li Fangying, Zeng Yuyang, Yang Sun, Horch Raymund E., Wei Wei, Wang Zhenxing, Sun Jiaming. Self-assembled human adipose-derived stem cell-derived extracellular vesicle-functionalized biotin-doped polypyrrole titanium with long-term stability and potential osteoinductive ability. ACS Appl. Mater. Interfaces. 2019;11:46183–46196. doi: 10.1021/acsami.9b17015. [DOI] [PubMed] [Google Scholar]
- Chen Si, Tang Yiman, Liu Yunsong, Zhang Ping, Lv Longwei, Zhang Xiao, Jia Lingfei, Zhou Yongsheng. Exosomes derived from miR-375-overexpressing human adipose mesenchymal stem cells promote bone regeneration. Cell Prolif. 2019;52 doi: 10.1111/cpr.12669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chew Jacob Ren Jie, Chuah Shang Jiunn, Teo Kristeen Ye Wen, Zhang Shipin, Lai Ruenn Chai, Fu Jia Hui, Lim Lum Peng, Lim Sai Kiang, Toh Wei Seong. Mesenchymal stem cell exosomes enhance periodontal ligament cell functions and promote periodontal regeneration. Acta Biomater. 2019;89:252–264. doi: 10.1016/j.actbio.2019.03.021. [DOI] [PubMed] [Google Scholar]
- Church Vicki L., Francis-West Philippa. Wnt signalling during limb development. Int. J. Dev. Biol. 2004;46:927–936. [PubMed] [Google Scholar]
- Cui Yigong, Shenglong Fu, Dong Sun, Xing Junchao, Hou Tianyong, Xuehui Wu. EPC-derived exosomes promote osteoclastogenesis through Lnc RNA-MALAT 1. J. Cell. Mol. Med. 2019;23:3843–3854. doi: 10.1111/jcmm.14228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diomede Francesca, D’aurora Marco, Gugliandolo Agnese, Merciaro Ilaria, Ettorre Valeria, Bramanti Alessia, Piattelli Adriano, Gatta Valentina, Mazzon Emanuela, Fontana Antonella. A novel role in skeletal segment regeneration of extracellular vesicles released from periodontal-ligament stem cells. Int. J. Nanomedicine. 2018;13:3805. doi: 10.2147/IJN.S162836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diomede Francesca, Gugliandolo Agnese, Cardelli Paolo, Merciaro Ilaria, Ettorre Valeria, Traini Tonino, Bedini Rossella, Scionti Domenico, Bramanti Alessia, Nanci Antonio. Three-dimensional printed PLA scaffold and human gingival stem cell-derived extracellular vesicles: a new tool for bone defect repair. Stem Cell Res Ther. 2018;9:104. doi: 10.1186/s13287-018-0850-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fish Jason E., Santoro Massimo M., Morton Sarah U., Yu Sangho, Yeh Ru-Fang, Wythe Joshua D., Ivey Kathryn N., Bruneau Benoit G., Stainier Didier Y.R., Srivastava Deepak. miR-126 regulates angiogenic signaling and vascular integrity. Dev. Cell. 2008;15:272–284. doi: 10.1016/j.devcel.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuta Taisuke, Miyaki Shigeru, Ishitobi Hiroyuki, Ogura Toshihiko, Kato Yoshio, Kamei Naosuke, Miyado Kenji, Higashi Yukihito, Ochi Mitsuo. Mesenchymal stem cell-derived exosomes promote fracture healing in a mouse model. Stem Cells Transl. Med. 2016;5:1620–1630. doi: 10.5966/sctm.2015-0285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadjiargyrou Michael, Komatsu David E. The therapeutic potential of microRNAs as orthobiologics for skeletal fractures. J. Bone Miner. Res. 2019;34:797–809. doi: 10.1002/jbmr.3708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, Chun-Chieh, Miya Kang, Yu Lu, Sajjad Shirazi, Jose Iriarte Diaz, Lyndon F Cooper, Praveen Gajendrareddy, and Sriram Ravindran. 2020. 'Functionally engineered extracellular vesicles improve bone regeneration', Acta Biomater. [DOI] [PMC free article] [PubMed]
- James Aaron W., Zara Janette N., Zhang Xinli, Askarinam Asal, Goyal Raghav, Chiang Michael, Yuan Wei, Le Chang Mirko Corselli, Shen Jia. Perivascular stem cells: a prospectively purified mesenchymal stem cell population for bone tissue engineering. Stem Cells Transl. Med. 2012;1:510–519. doi: 10.5966/sctm.2012-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia, Yachao, Shuo Qiu, Jia Xu, Qinglin Kang, and Yimin Chai. 2020. 'Exosomes secreted by young mesenchymal stem cells promote new bone formation during distraction Osteogenesis in older rats', Calcif. Tissue Int.: 1-9. [DOI] [PubMed]
- Katsuda Takeshi, Ochiya Takahiro. Molecular signatures of mesenchymal stem cell-derived extracellular vesicle-mediated tissue repair. Stem Cell Res Ther. 2015;6:212. doi: 10.1186/s13287-015-0214-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai Charles P., Mardini Osama, Ericsson Maria, Prabhakar Shilpa, Maguire Casey A., Chen John W., Tannous Bakhos A., Breakefield Xandra O. Dynamic biodistribution of extracellular vesicles in vivo using a multimodal imaging reporter. ACS Nano. 2014;8:483–494. doi: 10.1021/nn404945r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai Charles P., Kim Edward Y., Badr Christian E., Weissleder Ralph, Mempel Thorsten R., Tannous Bakhos A., Breakefield Xandra O. Visualization and tracking of tumour extracellular vesicle delivery and RNA translation using multiplexed reporters. Nat. Commun. 2015;6:1–12. doi: 10.1038/ncomms8029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Wenyue, Liu Yunsong, Zhang Ping, Tang Yiman, Zhou Miao, Jiang Weiran, Zhang Xiao, Wu Gang, Zhou Yongsheng. Tissue-engineered bone immobilized with human adipose stem cells-derived exosomes promotes bone regeneration. ACS Appl. Mater. Interfaces. 2018;10:5240–5254. doi: 10.1021/acsami.7b17620. [DOI] [PubMed] [Google Scholar]
- Li, Xiaobei, Yunfei Zheng, Liyu Hou, Zhibo Zhou, Yiping Huang, Yixin Zhang, Lingfei Jia, and Weiran Li. 2019. 'Exosomes derived from maxillary BMSCs enhanced the osteogenesis in iliac BMSCs', Oral Dis.. [DOI] [PubMed]
- Liang Bo, Liang Jia-Ming, Ding Jia-Ning, Xu Jia, Xu Jian-Guang, Chai Yi-Min. Dimethyloxaloylglycine-stimulated human bone marrow mesenchymal stem cell-derived exosomes enhance bone regeneration through angiogenesis by targeting the AKT/mTOR pathway. Stem Cell Res Ther. 2019;10:335. doi: 10.1186/s13287-019-1410-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Xiaoting, Ding Yue, Zhang Yuelin, Tse Hung-Fat, Lian Qizhou. Paracrine mechanisms of mesenchymal stem cell-based therapy: current status and perspectives. Cell Transplant. 2014;23:1045–1059. doi: 10.3727/096368913X667709. [DOI] [PubMed] [Google Scholar]
- Liao Songyan, Zhang Yuelin, Ting Sherwin, Zhen Zhe, Luo Fan, Zhu Ziyi, Yu Jiang, Sun Sijia, Lai Wing-Hon, Lian Qizhou. Potent immunomodulation and angiogenic effects of mesenchymal stem cells versus cardiomyocytes derived from pluripotent stem cells for treatment of heart failure. Stem Cell Res Ther. 2019;10:78. doi: 10.1186/s13287-019-1183-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao Wen, Yu Ning, Xu Hai-Jia, Zou Wen-Zhong, Hu Jing, Liu Xiang-Zhong, Yang Yi, Li Zhang-Hua. BMSC-derived exosomes carrying microRNA-122-5p promote proliferation of osteoblasts in osteonecrosis of the femoral head. Clin. Sci. 2019;133:1955–1975. doi: 10.1042/CS20181064. [DOI] [PubMed] [Google Scholar]
- Liao Wenbin, Zhong Jian, Yu Jingxia, Xie Jiang, Liu Yongjun, Lei Du, Yang Shaoguang, Liu Pengxia, Xu Jie, Wang Jiming. Therapeutic benefit of human umbilical cord derived mesenchymal stromal cells in intracerebral hemorrhage rat: implications of anti-inflammation and angiogenesis. Cell. Physiol. Biochem. 2009;24:307–316. doi: 10.1159/000233255. [DOI] [PubMed] [Google Scholar]
- Liu, Wei, Linwei Li, Yuluo Rong, Dingfei Qian, Jian Chen, Zheng Zhou, Yongjun Luo, Dongdong Jiang, Lin Cheng, and Shujie Zhao. 2019. '.Hypoxic mesenchymal stem cell-derived exosomes promote bone fracture healing by the transfer of miR-126', Acta Biomater. [DOI] [PubMed]
- Liu Xiaolin, Li Qing, Niu Xin, Hu Bin, Chen Shengbao, Song Wenqi, Ding Jian, Zhang Changqing, Yang Wang. Exosomes secreted from human-induced pluripotent stem cell-derived mesenchymal stem cells prevent osteonecrosis of the femoral head by promoting angiogenesis. Int. J. Biol. Sci. 2017;13:232. doi: 10.7150/ijbs.16951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Zhong-Wei, Liu Yi-Wei, Rao Shan-Shan, Yin Hao, Huang Jie, Chen Chun-Yuan, Hu Yin, Zhang Yan, Tan Yi-Juan, Yuan Ling-Qing. Aptamer-functionalized exosomes from bone marrow stromal cells target bone to promote bone regeneration. Nanoscale. 2019;11:20884–20892. doi: 10.1039/c9nr02791b. [DOI] [PubMed] [Google Scholar]
- Momen-Heravi Fatemeh, Balaj Leonora, Alian Sara, Mantel Pierre-Yves, Halleck Allison E., Trachtenberg Alexander J., Soria Cesar E., Oquin Shanice, Bonebreak Christina M., Saracoglu Elif. Current methods for the isolation of extracellular vesicles. Biol. Chem. 2013;394:1253–1262. doi: 10.1515/hsz-2013-0141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura Yoshihiro, Miyaki Shigeru, Ishitobi Hiroyuki, Matsuyama Sho, Nakasa Tomoyuki, Kamei Naosuke, Akimoto Takayuki, Higashi Yukihito, Ochi Mitsuo. Mesenchymal-stem-cell-derived exosomes accelerate skeletal muscle regeneration. FEBS Lett. 2015;589:1257–1265. doi: 10.1016/j.febslet.2015.03.031. [DOI] [PubMed] [Google Scholar]
- Narayanan, Raghuvaran, Chun-Chieh Huang, and Sriram Ravindran. 2016. 'Hijacking the cellular mail: exosome mediated differentiation of mesenchymal stem cells', Stem Cells Int., 2016. [DOI] [PMC free article] [PubMed]
- Otsuru Satoru, Desbourdes Laura, Guess Adam J., Hofmann Ted J., Relation Theresa, Kaito Takashi, Dominici Massimo, Iwamoto Masahiro, Horwitz Edwin M. Extracellular vesicles released from mesenchymal stromal cells stimulate bone growth in osteogenesis imperfecta. Cytotherapy. 2018;20:62–73. doi: 10.1016/j.jcyt.2017.09.012. [DOI] [PubMed] [Google Scholar]
- Peng Hairong, Usas Arvydas, Olshanski Anne, Ho Andrew M., Gearhart Brian, Cooper Gregory M., Huard Johnny. VEGF improves, whereas sFlt1 inhibits, BMP2-induced bone formation and bone healing through modulation of angiogenesis. J. Bone Miner. Res. 2005;20:2017–2027. doi: 10.1359/JBMR.050708. [DOI] [PubMed] [Google Scholar]
- Pizzicannella Jacopo, Gugliandolo Agnese, Orsini Tiziana, Fontana Antonella, Ventrella Alessia, Mazzon Emanuela, Bramanti Placido, Diomede Francesca, Trubiani Oriana. Engineered extracellular vesicles from human periodontal-ligament stem cells increase VEGF/VEGFR2 expression during bone regeneration. Front. Physiol. 2019;10:512. doi: 10.3389/fphys.2019.00512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi Xin, Zhang Jieyuan, Yuan Hong, Xu Zhengliang, Li Qing, Niu Xin, Hu Bin, Yang Wang, Li Xiaolin. Exosomes secreted by human-induced pluripotent stem cell-derived mesenchymal stem cells repair critical-sized bone defects through enhanced angiogenesis and osteogenesis in osteoporotic rats. Int. J. Biol. Sci. 2016;12:836. doi: 10.7150/ijbs.14809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin Yunhao, Wang Lian, Gao Zhengliang, Chen Genyin, Zhang Changqing. Bone marrow stromal/stem cell-derived extracellular vesicles regulate osteoblast activity and differentiation in vitro and promote bone regeneration in vivo. Sci. Rep. 2016;6:1–11. doi: 10.1038/srep21961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rekker Kadri, Saare Merli, Roost Anne Mari, Kubo Anna-Liisa, Zarovni Natasa, Chiesi Antonio, Salumets Andres, Peters Maire. Comparison of serum exosome isolation methods for microRNA profiling. Clin. Biochem. 2014;47:135–138. doi: 10.1016/j.clinbiochem.2013.10.020. [DOI] [PubMed] [Google Scholar]
- Shahabipour Fahimeh, Barati Nastaran, Johnston Thomas P., Derosa Giuseppe, Maffioli Pamela, Sahebkar Amirhossein. Exosomes: Nanoparticulate tools for RNA interference and drug delivery. J. Cell. Physiol. 2017;232:1660–1668. doi: 10.1002/jcp.25766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth Tyson, Kullberg Max, Malik Noeen, Smith-Jones Peter, Graner Michael W., Anchordoquy Thomas J. Biodistribution and delivery efficiency of unmodified tumor-derived exosomes. J. Control. Release. 2015;199:145–155. doi: 10.1016/j.jconrel.2014.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi, Ryoko, Wataru Katagiri, Satoshi Endo, and Tadaharu Kobayashi. 2019. 'Exosomes from conditioned media of bone marrow-derived mesenchymal stem cells promote bone regeneration by enhancing angiogenesis', PLoS One, 14. [DOI] [PMC free article] [PubMed]
- Tao Shi-Cong, Guo Shang-Chun, Zhang Chang-Qing. Modularized extracellular vesicles: the dawn of prospective personalized and precision medicine. Adv. Sci. 2018;5:1700449. doi: 10.1002/advs.201700449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Pol Edwin, Böing Anita N., Harrison Paul, Sturk Augueste, Nieuwland Rienk. Classification, functions, and clinical relevance of extracellular vesicles. Pharmacol. Rev. 2012;64:676–705. doi: 10.1124/pr.112.005983. [DOI] [PubMed] [Google Scholar]
- Villa-Diaz L.G., Brown S.E., Liu Y., Ross A.M., Lahann J., Parent J.M., Krebsbach P.H. Derivation of mesenchymal stem cells from human induced pluripotent stem cells cultured on synthetic substrates. Stem Cells. 2012;30:1174–1181. doi: 10.1002/stem.1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Shusheng, Aurora Arin B., Johnson Brett A., Qi Xiaoxia, McAnally John, Hill Joseph A., Richardson James A., Bassel-Duby Rhonda, Olson Eric N. The endothelial-specific microRNA miR-126 governs vascular integrity and angiogenesis. Dev. Cell. 2008;15:261–271. doi: 10.1016/j.devcel.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Yan, Tang Cuizhu, Zhang Jinglun, Li Zhihao, Zhang Xiaoxin, Miron Richard J., Zhang Yufeng. Extracellular vesicles derived from the mid-to-late stage of osteoblast differentiation markedly enhance osteogenesis in vitro and in vivo. Biochem. Biophys. Res. Commun. 2019;514:252–258. doi: 10.1016/j.bbrc.2019.04.029. [DOI] [PubMed] [Google Scholar]
- Weilner Sylvia, Skalicky Susanna, Salzer Benjamin, Keider Verena, Wagner Michael, Hildner Florian, Gabriel Christian, Dovjak Peter, Pietschmann Peter, Grillari-Voglauer Regina. Differentially circulating miRNAs after recent osteoporotic fractures can influence osteogenic differentiation. Bone. 2015;79:43–51. doi: 10.1016/j.bone.2015.05.027. [DOI] [PubMed] [Google Scholar]
- Xie Hui, Wang Zhenxing, Zhang Liming, Lei Qian, Zhao Aiqi, Wang Hongxiang, Li Qiubai, Chen Zhichao, Zhang WenJie. Development of an angiogenesis-promoting microvesicle-alginate-polycaprolactone composite graft for bone tissue engineering applications. PeerJ. 2016;4 doi: 10.7717/peerj.2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Hui, Wang Zhenxing, Zhang Liming, Lei Qian, Zhao Aiqi, Wang Hongxiang, Li Qiubai, Cao Yilin, Zhang Wen Jie, Chen Zhichao. Extracellular vesicle-functionalized decalcified bone matrix scaffolds with enhanced pro-angiogenic and pro-bone regeneration activities. Sci. Rep. 2017;7:45622. doi: 10.1038/srep45622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, Jiajia, Yiyun Wang, Ching-Yun Hsu, Yongxing Gao, Carolyn Ann Meyers, Leslie Chang, Leititia Zhang, Kristen Broderick, Catherine Ding, and Bruno Peault. 2019. 'Human perivascular stem cell-derived extracellular vesicles mediate bone repair', Elife, 8. [DOI] [PMC free article] [PubMed]
- Xu Tao, Luo Yongjun, Wang Jiaxing, Zhang Ning, Changjiang Gu, Li Linwei, Qian Dingfei, Cai Weihua, Fan Jin, Yin Guoyong. Exosomal miRNA-128-3p from mesenchymal stem cells of aged rats regulates osteogenesis and bone fracture healing by targeting Smad5. J. Nanobiotechnol. 2020;18:1–18. doi: 10.1186/s12951-020-00601-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Xucheng, Yang Junxiao, Lei Pengfei, Wen Ting. LncRNA MALAT1 shuttled by bone marrow-derived mesenchymal stem cells-secreted exosomes alleviates osteoporosis through mediating microRNA-34c/SATB2 axis. Aging (Albany NY) 2019;11:8777. doi: 10.18632/aging.102264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Hong-Chao, Liu Xin-Bin, Huang Shu, Bi Xiao-Yun, Wang Heng-Xiang, Xie Li-Xian, Wang Yong-Qi, Cao Xiao-Fang, Lv Jun, Xiao Feng-Jun. Microvesicles derived from human umbilical cord mesenchymal stem cells stimulated by hypoxia promote angiogenesis both in vitro and in vivo. Stem Cells Dev. 2012;21:3289–3297. doi: 10.1089/scd.2012.0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Jieyuan, Liu Xiaolin, Li Haiyan, Chen Chunyuan, Hu Bin, Niu Xin, Li Qing, Zhao Bizeng, Xie Zongping, Yang Wang. Exosomes/tricalcium phosphate combination scaffolds can enhance bone regeneration by activating the PI3K/Akt signaling pathway. Stem Cell Res Ther. 2016;7:136. doi: 10.1186/s13287-016-0391-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Lu, Jiao Guangjun, Ren Shanwu, Zhang Xiaoqian, Li Ci, Wu Wenliang, Wang Hongliang, Liu Haichun, Zhou Hongming, Chen Yunzhen. Exosomes from bone marrow mesenchymal stem cells enhance fracture healing through the promotion of osteogenesis and angiogenesis in a rat model of nonunion. Stem Cell Res Ther. 2020;11:1–15. doi: 10.1186/s13287-020-1562-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Yuntong, Hao Zichen, Wang Panfeng, Xia Yan, Wu Jianghong, Xia Demeng, Fang Shuo, Shuogui Xu. Exosomes from human umbilical cord mesenchymal stem cells enhance fracture healing through HIF-1α-mediated promotion of angiogenesis in a rat model of stabilized fracture. Cell Prolif. 2019;52 doi: 10.1111/cpr.12570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, Mingyan, Peng Li, Haijia Xu, Qunwen Pan, Rong Zeng, Xiaotang Ma, Zhanghua Li, and Hao Lin. 2018. 'Dexamethasone-activated MSCs release MVs for stimulating osteogenic response', Stem Cells Int., 2018. [DOI] [PMC free article] [PubMed]
- Zhou J., Liu H.X., Li S.H., Gong Y.S., Zhou M.W., Zhang J.H., Zhu G.Y. Effects of human umbilical cord mesenchymal stem cells-derived exosomes on fracture healing in rats through the Wnt signaling pathway. Eur. Rev. Med. Pharmacol. Sci. 2019;23:4954–4960. doi: 10.26355/eurrev_201906_18086. [DOI] [PubMed] [Google Scholar]
- Zhu Yu, Jia Yachao, Wang Yanmao, Xu Jia, Chai Yimin. Impaired bone regenerative effect of exosomes derived from bone marrow mesenchymal stem cells in type 1 diabetes. Stem Cells Transl. Med. 2019;8:593–605. doi: 10.1002/sctm.18-0199. [DOI] [PMC free article] [PubMed] [Google Scholar]