Abstract
Scaphoid fractures are the most prevalent type of carpal bone fractures. High-spatial-resolution sonography detects direct signs of scaphoid fractures such as scaphoid cortical disruption; nevertheless, indirect signs such as radiocarpal effusion and scapho-trapezium-trapezoid effusion can also be visible. The diagnosis is performed when both direct and indirect signs of scaphoid fracture are presented. The presence of indirect signs alone is not enough to complete the diagnosis, for which more advanced imaging modalities are usually required. Here, we review the anatomy of the scaphoid, the clinical manifestations of scaphoid fractures, as well as ultrasonographic findings and differential diagnosis.
Abbreviations: US, high-spatial-resolution sonography; CT, computed tomography; MRI, magnetic resonance imaging; ASB, anatomical snuffbox; ST, scaphoid tubercle; LC, longitudinal compression; NPV, negative predictive value; PPV, positive predictive value
Keywords: Scaphoid bone, Ultrasonography, Diagnostic imaging, Fractures, Bone
1. Introduction
Scaphoid fractures are the most prevalent type of carpal bone fractures, which affect mainly young people [1]. Diagnosis is based on clinical examination and images, with wrist radiography being the most commonly used method due to its high availability and low price. Nevertheless, sensitivity and specificity of radiography in the acute phase is not optimal, presenting between 20–25 % of false negatives results, and missing close to 16 % of fractures, for which preventive immobilization, and sometimes additional images such as magnetic resonance imaging (MRI) and computed tomography (CT) are required [[1], [2], [3], [4], [5]]. Consequently, unnecessary wrist immobilization represents a high sanitary cost due to prolonged time off from work [6]. If a scaphoid fracture is left untreated, complications such as non-union, delayed healing, pseudoarthrosis, avascular necrosis, arthrosis, and secondary displacement of the wrist may appear [5,6].
High-spatial-resolution sonography (US) has become a debated diagnostic method for occult scaphoid fractures, showing the capacity to detect fractures even when radiography has not, allowing to accurately select which patients require immobilization or more advanced image modalities, and which patients do not.
Currently, there are rising concerns about the use of US for the diagnosis of occult scaphoid fractures because it is an accessible, fast, nonionizing, cost-effective method that may help avoid overtreatment [1,2,5,7].
This narrative literature review seeks to provide resources to radiologists and non-radiologist in the musculoskeletal field (orthopedics, emergency physician, physiatrists) to accurately perform and comprehend the basics of scaphoid US in order to diagnose scaphoid fractures.
2. Scaphoid anatomy
The scaphoid is the most prominent bone of the first row of the carpal bones, which articulates proximally with the scaphoid fossa of the radius, and with the lunate bone, and distally with the head of the capitate, trapezium, and trapezoid. The scaphoid bone can be divided into a distal pole, a waist, and a proximal pole. Anteriorly and laterally, the scaphoid tubercle can be observed, in which the external lateral ligament is attached [8]. Due to the large number of bones with which it articulates, the scaphoid is covered 90 % by cartilage (just exposing the tubercle area and a dorsal bony ridge); also, the scaphoid has an absence of periosteum, for which the development of periosteal callus is not observed when the fracture heals [9]. The scaphoid's blood supply is guaranteed by small branches of the radial artery, which enter the scaphoid at its distal pole and from there runs proximally toward its base, irrigating the distal bone segments and the waist (Fig. 1). The proximal pole is supplied by an intraosseous collateral network, which plays a crucial role in the scaphoid pathology; these collateral arteries can be easily disrupted by a fracture, leading to nutritional deficiency and hypoxia, which may end up in pseudoarthrosis or osteonecrosis of the proximal pole [[8], [9], [10], [11], [12]].
Fig. 1.
Blood supply of the scaphoid coming from the distal pole. (A) Distal pole. (B) Scaphoid tubercle. (C) The waist of the scaphoid. (D) Proximal pole. (E) Radial artery.
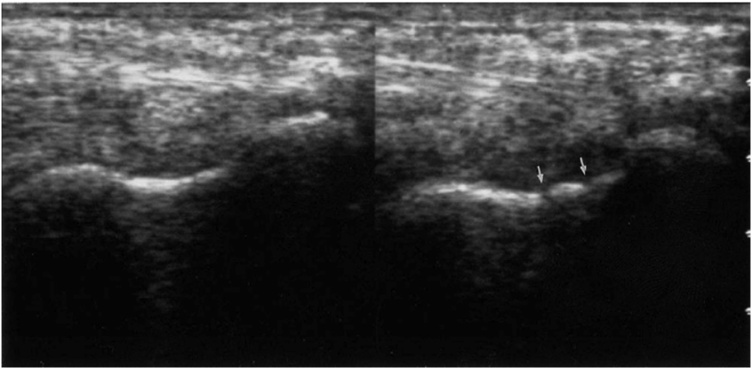
In US, the scaphoid has a peanut-shaped appearance in the volar view and a pyramid appearance in the dorsal view (Fig. 2). The volar cortex is seen as a hyperechogenic line; however, anterior to it, a hypoechogenic line is visible, which represents the joint capsule (Fig. 3) [12].
Fig. 2.
(A) Peanut-shaped appearance of the scaphoid (arrow) in the volar view. (B) Pyramid appearance of the scaphoid (arrow) in the dorsal view.
Fig. 3.
A normal scaphoid. The palmar cortex of the scaphoid (S), radius palmar cortex (R), joint capsule (c), and flexor carpi radialis tendon (t).
3. Clinical findings of scaphoid fractures
It is essential to understand the clinical findings of scaphoid fractures since it could improve the sensitivity and specificity of US. Diagnosis should be focused on a deep anamnesis and a detailed physical examination, followed by diagnostic images [5,[13], [14], [15], [16]].
The primary traumatic mechanism of scaphoid fractures is a fall on the outstretched hand with the wrist extended in radial deviation, which results in extreme dorsiflexion of the wrist and compression of the radial side of the hand. This mechanism causes the scaphoid bone to impact against the distal radius concavity, transmitting the forces from the hand to the arm through the scaphoid bone, causing a fracture most likely to occur in the middle of the scaphoid. On the other hand, when the impact occurs with the wrist in abduction, the chances of a proximal pole scaphoid fracture rise [16,17].
The physician must be aware of intense pain in the distal radius that increases with palpation. Edema, ecchymosis, and fullness in the anatomical snuffbox (ASB) are also important signs that may suggest scaphoid fracture [16].
The clinical examination should include the three most commonly used clinical tests, which are: 1. palpation on the ASB with the wrist in ulnar deviation, 2. palpation over the scaphoid tubercle (ST) with the wrist in slight extension, and 3. longitudinal compression (LC) of the thumb (5) [15,18,19]. The ASB tenderness is the most sensitive clinical test, followed by the ST test and the LC test. If an experienced physician conducts all tests, the sensitivity and negative predictive value (NPV) have been reported to be close to 100 %, substantially decreasing if performed by an inexperienced physician [5,16,18,19].
Haugher et al. defined three clinical criteria to evaluate the level of suspicion of scaphoid fractures: 1. Tenderness on palpation on the ASB, 2. Tenderness on axial loading of the first ray, and 3. swelling at the ASB. If the three clinical criteria were presented, a high clinical suspicion was considered. Suspicion was considered moderate when two criteria were presented. If just one sign was presented, the suspicion was low [2,20,21].
The absence of ASB tenderness may substantially reduce the probability of scaphoid fracture when conducted by an experienced physician. Nevertheless, physical examination alone is not enough to rule out a scaphoid fracture, for which imaging modalities are usually required. [14,15,17,18].
4. Imaging technique
US with a linear high-frequency probe (>12 Mhz) is required to evaluate the scaphoid bone [2,3,7,13,20,22,23]. Comparative sonography of both normal and painful wrist should be performed, assessing the normal side before the injured side [16]. The patient must be seated in front of the radiologist with the wrist placed on a cushion; scaphoid tuberosity should be identified by palpation. The probe must be placed on the hand over the area between the scaphoid's tuberosity and the radius [16].
The scaphoid must be evaluated in the longitudinal and transverse planes from the dorsal, lateral, and palmar directions in both normal and ulnar deviation positions to elongate the scaphoid bone and acquire an optimal visualization of the scaphoid waist (Fig. 4) [2,3,24].
Fig. 4.
Photograph of the exact location of the linear probe used to image the scaphoid bone. Position of the linear probe when scanning the scaphoid bone from the dorsal (a), dorsal with ulnar deviation (b), and volar surface (c).
R: radius, T: scaphoid tuberosity
As a general rule, the waist of the scaphoid is technically easier to assess by US examination; thus, the radiologist can identify the direct and indirect signs of scaphoid fractures reliably. However, assessing the distal pole of the scaphoid bone is more technically challenging, usually presenting more false-negative results [13]. Herneth et al. and Platon et al. reported that the scapholunate area and the tubercle of the scaphoid are the most complicated structures to evaluate by US, due to the impossibility of assessing the integrality of the bone contour [2,3,20].
5. Ultrasound findings
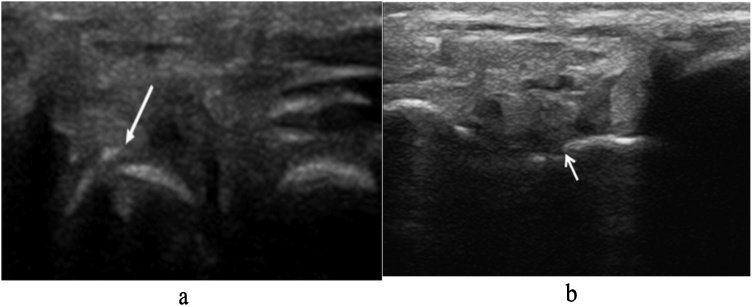
Multiple studies have described US findings of scaphoid fractures [2,3,6,21,25,26]. The most commons radiographic findings are disruption of the scaphoid cortical (direct sign) (Fig. 5, Fig. 6), hypoechoic radiocarpal fluid due to hemarthrosis (sometimes showing mixed echogenicity depending on the stage of degradation blood) (Fig. 7, Fig. 8), and scapho-trapezium-trapezoid effusion (indirect signs) [2,3,6,21,25, 26, 27].
Fig. 5.
A Split-Screen comparison of the fractured scaphoid (right) and healthy scaphoid (left). The arrows distinguish two cortical fractures in the palmar cortex. Reprinted with permission from Senall et al. (2004).
Fig. 6.
Scaphoid US showing cortical disruption. (a) Disruption in the cortex of the scaphoid (arrow) consistent with a scaphoid fracture. (b) Scaphoid fracture with minor cortical defect (arrow). Reprinted with permission from Simard et al. (2020) and Dickman et al. (2014), respectively.
Fig. 7.
A Split-Screen comparison of the US image of the harmed (I) and the healthy side (II). The scaphoid's cortex (arrow A) shows a disruption line (arrow B) on the harmed side. The space from the broken scaphoid's cortex to the skin (arrow C) is enlarged in contrast to the healthy side. There is a hematoma (small arrows) on the affected side. The cortex of the radius is marked (arrow D). Reprinted with permission from Munk et al. (2000).
Fig. 8.
A sagittal oblique image through the scaphoid's waist shows a fracture with cortical step-off (larger 2 arrows), shown magnified in the inset. The proximal pole is to the left, and the tuberosity is to the right. A fluid collection is seen anterior to the fractured palmar-radial cortex (smaller 3 arrows). The fibrillar pattern of the flexor carpi radialis tendon (arrowhead) is seen anterior to the scaphoid. Reprinted with permission from Senall et al. (2004).
Herneth et al. in 2001 performed US in 7 patients with scaphoid fractures, describing a hyperechogenic line parallel to the scaphoid cortex in 3 patients (43 %), which was initially published as periosteal changes (Fig. 9) [3]; however, Hauger et al. in 2002 established another hypothesis by detecting this sign in 2 patients with no scaphoid fracture in the follow-up, for that reason, those changes were interpreted as positive echo signal of the anterior interface of the cartilage induced by a high gain level, they supported this hypothesis by the high amount of cartilage covering the scaphoid bone and the particularity of not being surrounded by periosteum; nevertheless, it continues to be unknown the true representation of this sign [2].
Fig. 9.
Longitudinal high-spatial-resolution sonogram obtained in the dorsal direction in a 21-year-old man with a scaphoid fracture after acute trauma of the left wrist clearly shows a hyperechogenic line parallel to the scaphoid cortex (solid arrows) and cortical discontinuity (open arrows). Reprinted with permission from Herneth et al. (2001).
Fusetti et al. published a prospective double-blinded study in which the mean distance between the skin and the scaphoid bone was measured; no statistical correlation was found between an increased skin-scaphoid distance and the presence of a fracture [18].
According to multiple prospective studies, US for the diagnosis of scaphoid fractures has an overall sensitivity of 84.75 % (50–100 %), specificity 85.5 % (71–100 %), PPV 72 % (46–100 %), and NPV 87.5 % (58–100 %) [2,3,12,19,23,24,26]; nevertheless, most of the studies evaluated were old, and due to the fast improvement of technology is expected that those results have improved [2].
On the other hand, each US sign has its own sensitivity, specificity, PPV, and NPV to diagnose scaphoid fractures, as described in Table 1 [2,18].
Table 1.
Reported US sensitivity, specificity, and predictive values in the diagnosis of scaphoid fractures.
| US signs | Sensitivity | Specificity | PPV | NPV | References |
|---|---|---|---|---|---|
| Scaphoid cortical disruption | 100 % | 96.5 % (95−98%) | 83 % | 100 % | (218) |
| Radio carpal effusion | 100 % | 53 % (42−65%) | 27 (23−31%) | 100 % | (218) |
| Scapho-trapezium-trapezoid effusion | 100 % | 74.5 % (65−84%) | 42.5 % (23−62%) | 100 % | (218) |
| Cortical disruption and Articular effusion | 100 % | 99 % (98−100%) | 99 % (98−100%) | 100 % | (218) |
The key diagnostic sign for scaphoid fractures is the cortical disruption, which has a sensitivity of 100 %, specificity of 96.5 %, PPV of 83 %, and NPV 100 %, being an indispensable sign for the diagnosis. The presence of cortical disruption alone is not enough to perform the diagnosis because it leaves space for a considerable amount of false positives; therefore, cortical disruption must be associated with articular effusion to increase sensitivity, specificity, and predictive values (Fig. 7, Fig. 8) [18,2,21]. Isolated soft tissue findings lack specificity, and sensitivity may reflect ligament sprain or tears [2,20,21].
Wrist US is an accurate imaging technique for the detection of soft tissue lesions; in 2017 Oguz et al. evaluated 80 patients with suspected wrist injury, in which US was performed by an emergency physician trained in musculoskeletal US, they compared the soft tissue (tendons and ligaments) findings on US and MRI examinations, concluding that there was not any clinically relevant difference between what was detected by the wrist US and MRI [6].
Most patients with normal X-rays upon the initial presentation have undisplaced or unicortical scaphoid fractures on US or MRI. If undisplaced or unicortical scaphoid fracture is presented, it can be appropriately managed with a thumb spica cast for six weeks. Nevertheless, patients with >1 mm displacement, comminuted fractures, radio lunate angle >15 grades, and scaphoid-lunate angle >60 grades seen on images are considered to be candidates for internal fixation [17,28]. Cast immobilization has several disadvantages, such as requiring multiple office visits and prolonged time to healing. Therefore, some surgeons recommend early internal fixation even for non-displaced fractures when early return to work is needed, but this remains controversial [28].
6. The role of ultrasound in the diagnostic algorithm
Klauser et al. in 2012 and Sconfienza et al. in 2018 published the European Society of Musculoskeletal Radiology consensus regarding the clinical indications of musculoskeletal US. They concluded that wrist US to diagnose scaphoid fractures is indicated when other image modalities are not appropriate (level of evidence C) [29,30]. This statement is supported by multiple prospective studies, which suggest that the initial approach to diagnose scaphoid fractures should be based on wrist radiography due to its low cost and health services availability. When the X-ray reveals a fracture, the treatment must be established immediately. Nevertheless, radiography shows 20–25 % of false negatives results during the acute phase of the injury [[2], [3], [4]], is in here when US plays its role as an intermediary diagnostic tool, with the capacity to rule out or to support the diagnosis of scaphoid fracture (algorithm 1) [2,3,7,31]. The gold standard tests to diagnose scaphoid fractures are MRI and CT; however, these tools' limited availability and costs reduce their usage in most cases, especially in developing countries.
Taking into account the relative frequency of scaphoid fractures, the sensitivity and specificity of radiography as a diagnostic tool [7,32], and the fact that health centers with low complexity in low or moderate incomes countries do not have specialized diagnostic images such as MRI and CT, it is mandatory to rely on other diagnostic methods with higher availability and lower prices, such as US. Also, it is important to know in which health centers the medical personnel has training in bedside US, since this factor will determine its implementation for the diagnosis of scaphoid fractures [6,24,33].
The proposed algorithm is a valuable tool in emergency departments where physicians are well-trained in bedside US, by this way disposing of an available, reliable, fast, irradiation-free, and cost-effective diagnostic method for scaphoid fractures that can reduce overtreatment [2,6,24,25].
Algorithm 1
Diagnosis of suspected scaphoid fracture in emergency departments. Adapted from Hauger et al.
*High clinical suspicion: When three clinical criteria were presented. Moderate clinical suspicion: When two criteria were presented. Low clinical suspicion: If just one sign was presented.
**Treatment may be thumb spica cast for six weeks (if undisplaced or unicortical scaphoid fracture) or internal fixation (if >1mm displacement, comminuted fracture, radio lunate angle >15 grades, and scaphoid-lunate angle >60 grades).
Diagnosis of scaphoid fracture begins with the clinical suspicion through an adequate anamnesis, followed by a physical examination that, as we have mentioned previously, will lead us to a high, moderate, or low clinical suspicion of scaphoid fracture. The first radiological tool to confirm the diagnosis in the emergency department should be radiography due to its low price and accessibility. Consequently, if the initial radiograph is negative, we propose performing ultrasonography in the emergency department, allowing us to promptly rule out or confirm the diagnosis, leading to a reduction in patients' morbidity and overall healthcare system expenses [19,28,34].
Suppose the sonography only shows soft tissue abnormalities and the physician has a high clinical suspicion of scaphoid fracture; therefore, a supplementary MRI, or CT, should be carried out within three to five days. In that case, the patient should be immobilized, give discharge with thumb spica cast and perform the MRI or CT on the assigned date. It is essential to know that MRI is the gold standard for diagnosing scaphoid fracture, but CT is better to evaluate displacement and assess for fracture healing [19,35].
7. Differential diagnosis according to ultrasound findings
There are some situations in which false-positive results in US can occur. For example, the presence of arthritic deformity of the wrist, which is a condition presented primarily in older adults, may generate confusion due to cortical irregularities that can simulate interruption of the scaphoid contour; furthermore, the fluid associated with degenerative changes can not be differentiated from fluid related to fracture [20]. The scaphoid tubercle area may appear quite irregular, sometimes mimicking cortical disruption; however, such irregularities usually occur in older adults, which are less subjected to scaphoid fractures [2]. For all above, positives US results must be interpreted with caution in elderly populations [2,20].
8. Conclusions
Scaphoid fractures represent the most common carpal bone fracture. US acts as an intermediary diagnostic tool with the capacity to rule out or to support the diagnosis of scaphoid fracture in the emergency department when initial radiography is inconclusive. US detects direct signs (cortical disruption) and indirect signs (radiocarpal effusion and scapho-trapezium-trapezoid effusion) of scaphoid fracture. The diagnosis is performed when both direct and indirect signs are presented. The presence of isolated direct or indirect signs is not enough to make the diagnosis, for which more advanced imaging modalities such as magnetic resonance imaging and computed tomography are usually required. In the elderly population, positive US results must be interpreted with caution due to several conditions that may mimic scaphoid fractures, such as arthritic deformity of the wrist and fluid associated with degenerative changes. The scaphoid tubercle area's normal ultrasonographic appearance may seem quite irregular, sometimes inducing false positives results if isolated direct signs are interpreted as pathological. Until now, the studies that evaluated scaphoid fracture by US were done by physicians with experience (either a musculoskeletal radiologist or emergency physician with special training in wrist US); therefore, this technique could only be recommended to be performed by experienced observers. Studies that evaluate its reliability with different levels of expertise are required.
Ethical statement
This article does not involve human experimentation.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
CRediT authorship contribution statement
Andrés Felipe Herrera Ortiz: Conceptualization, Writing - original draft, Writing - review & editing, Supervision. Stephani Zoe Guevara: Writing - original draft, Conceptualization. Sandra Milena Ramírez: Writing - review & editing. Julian Cubillos Rojas: Writing - original draft, Conceptualization. Rubén Giraldo Malo: Writing - review & editing. Lorena Fernández Beaujon: Writing - review & editing. María Mónica Ochoa: Writing - review & editing. Juan Felipe Zarate: Writing - review & editing. María Fernanda Niño: Writing - review & editing. Manuela Ochoa Aguilar: Writing - review & editing.
Declaration of Competing Interest
The authors report no declarations of interest.
Acknowledgments
Thanks to Dr. Ana María Fernández for reading the manuscript and giving her opinion.
References
- 1.Bäcker H.C., Wu C.H., Strauch R.J. Systematic review of diagnosis of clinically suspected scaphoid fractures. J. Wrist Surg. 2020;09(01):081–089. doi: 10.1055/s-0039-1693147. [Internet] Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hauger O., Bonnefoy O., Moinard M., Bersani D.D.F. Occult fractures of the waist of the scaphoid: early diagnosis by high-spatial-resolution sonography. Am. J. Roentgenol. 2002;178(5):1239–1245. doi: 10.2214/ajr.178.5.1781239. [DOI] [PubMed] [Google Scholar]
- 3.Herneth A.M., Siegmeth A., Bader T.R., Ba-Ssalamah A., Lechner G., Metz V.M. Scaphoid fractures: evaluation with high-spatial-Resolution US-Initial results. Radiology. 2001;220(1):231–235. doi: 10.1148/radiology.220.1.r01jl15231. [DOI] [PubMed] [Google Scholar]
- 4.Yildirim A., Ünlüer E.E., Vandenberk N., Karagöz A. The role of bedside ultrasonography for occult scaphoid fractures in the emergency department. Ulus Travma ve Acil Cerrahi Derg. 2013;19(3):241–245. doi: 10.5505/tjtes.2013.64927. [Internet] Available from: [DOI] [PubMed] [Google Scholar]
- 5.Krastman P., Mathijssen N.M., Bierma-Zeinstra S.M.A., Kraan G., Runhaar J. Diagnostic accuracy of history taking, physical examination and imaging for phalangeal, metacarpal and carpal fractures: a systematic review update. BMC Musculoskelet. Disord. 2020;21(1):1–24. doi: 10.1186/s12891-019-2988-z. [Internet] Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oguz A.B., Polat O., Eneyli M.G., Gulunay B., Eksioglu M., Gurler S. The efficiency of bedside ultrasonography in patients with wrist injury and comparison with other radiological imaging methods: a prospective study. Am. J. Emerg. Med. 2017;35(6):855–859. doi: 10.1016/j.ajem.2017.01.043. [Internet]. Available from: [DOI] [PubMed] [Google Scholar]
- 7.Simard R. Ultrasound imaging of orthopedic injuries. Emerg. Med. Clin. North Am. 2020;38(1):243–265. doi: 10.1016/j.emc.2019.09.009. [DOI] [PubMed] [Google Scholar]
- 8.Rodríguez Ramirez D., Eduardo Ruiz Moreno C., Ángel Nieto Bayona M., Alejandro Leuro Torres S., Ángel Gómez Rueda M. Aspectos anatómicos I. Generalidades, osteología y artrología. Morfolia. 2020;12(1):11–30. [Google Scholar]
- 9.Schmitt R., Rosenthal H. Imaging of scaphoid fractures according to the new S3 guidelines. RoFo. 2016;188(5):459–469. doi: 10.1055/s-0042-104660. [DOI] [PubMed] [Google Scholar]
- 10.Patterson R.M., Moritomo H., Yamaguchi S., Mitsuyasu H., Buford W.L., Viegas S.F. Scaphoid anatomy and mechanics: update and review. Oper Tech orthapaedics. 2003;12(1):2–10. [Google Scholar]
- 11.Duckworth A.D., Ring D., Mcqueen M.M. Assessment of the suspected fracture of the scaphoid. J. Bone Jt Surg. 2011;93(6):93–713. doi: 10.1302/0301-620X.93B6.26506. [DOI] [PubMed] [Google Scholar]
- 12.Langer M.F., Unglaub F., Breiter S., Ueberberg J., Wieskötter B., Oeckenpöhler S. Anatomy and pathobiomechanics of the scaphoid. Unfallchirurg. 2019;122(3):170–181. doi: 10.1007/s00113-018-0597-1. [DOI] [PubMed] [Google Scholar]
- 13.Senall J.A., Failla J.M., Bouffard J.A., Van Holsbeeck M. Ultrasound for the early diagnosis of clinically suspected scaphoid fracture. J. Hand Surg. Am. 2004;29(3):400–405. doi: 10.1016/j.jhsa.2003.12.012. [DOI] [PubMed] [Google Scholar]
- 14.Carpenter C.R., Pines J.M., Schuur J.D., Muir M., Calfee R.P., Raja A.S. Adult scaphoid fracture. Acad. Emerg. Med. 2014;21(2):101–121. doi: 10.1111/acem.12317. [DOI] [PubMed] [Google Scholar]
- 15.Mallee W.H., Henny E.P., Van Dijk C.N., Kamminga S.P., Van Enst W.A., Kloen P. Clinical diagnostic evaluation for scaphoid fractures: a systematic review and meta-analysis. J. Hand Surg Am. 2014;39(9):1683–1691. doi: 10.1016/j.jhsa.2014.06.004. [DOI] [PubMed] [Google Scholar]
- 16.Grant Phillips T., Andrew M., Reibach W.P.S. Diagnosis and management of scaphoid fractures. Am. Fam. Phys. 2004;70(5):879–884. [PubMed] [Google Scholar]
- 17.Rhemrev S.J., Ootes D., Beeres F.J.P., Meylaerts S.A.G., Schipper I.B. Current methods of diagnosis and treatment of scaphoid fractures. Int. J. Emerg. Med. 2011;4(1):4. doi: 10.1186/1865-1380-4-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bergh T.H., Lindau T., Soldal L.A., Bernardshaw S.V., Behzadi M., Steen K. Clinical scaphoid score (CSS) to identify scaphoid fracture with MRI in patients with normal x-ray after a wrist trauma. Emerg. Med. J. 2014;31(8):659–664. doi: 10.1136/emermed-2012-202219. [DOI] [PubMed] [Google Scholar]
- 19.Clementson M., Björkman A., Thomsen N.O.B. Acute scaphoid fractures: guidelines for diagnosis and treatment. EFORT Open Rev. 2020;5(2):96–103. doi: 10.1302/2058-5241.5.190025. [Internet]. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Platon A., Poletti P.A., Van Aaken J., Fusetti C., Della Santa D., Beaulieu J.Y. Occult fractures of the scaphoid: the role of ultrasonography in the emergency department. Skeletal Radiol. 2011;40(7):869–875. doi: 10.1007/s00256-010-1086-y. [DOI] [PubMed] [Google Scholar]
- 21.Fusetti C., Poletti P.A., Pradel P.H., Garavaglia G., Platon A., Della Santa D.R. Diagnosis of occult scaphoid fracture with high-spatial-resolution sonography: a prospective blind study. J. Trauma - Inj Infect Crit Care. 2005;59(3):677–681. doi: 10.1097/01.ta.0000177467.79282.40. [Internet]. Available from: [DOI] [PubMed] [Google Scholar]
- 22.Blum A., Sauer B., Detreille R., Zabel J., Pierrucci F., Witte Y. Le diagnostic des fractures récentes du scaphoïde : revue de la littérature. J. Radiol. 2007;88(5):741–759. doi: 10.1016/s0221-0363(07)91342-6. [DOI] [PubMed] [Google Scholar]
- 23.Olchowy C., Łasecki M., Zaleska-Dorobisz U. Wrist ultrasound examination – scanning technique and ultrasound anatomy. Part 1: dorsal wrist. J. Ultrason. 2015;61(1):172–188. doi: 10.15557/JoU.2015.0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dias J.J., Hui A.C.W., Lamont A.C. Real time ultrasonography in the assessment of movement at the site of a scaphoid fracture non-union. J. Hand Surg. Am. 1994;19(4):498–504. doi: 10.1016/0266-7681(94)90216-x. [DOI] [PubMed] [Google Scholar]
- 25.Jain R., Jain N., Sheikh T., Yadav C. Early scaphoid fractures are better diagnosed with ultrasonography than X-rays: a prospective study over 114 patients. Chin. J. Traumatol – Eng. Ed. 2018;21(4):206–210. doi: 10.1016/j.cjtee.2017.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kwee R.M., Kwee T.C. Ultrasound for diagnosing radiographically occult scaphoid fracture. Skeletal Radiol. 2018;47(9):1205–1212. doi: 10.1007/s00256-018-2931-7. [Internet] Available from: [DOI] [PubMed] [Google Scholar]
- 27.Munk B., Bolvig L., Krøner K., Christiansen T., Borris L., Boe S. Ultrasound for diagnosis of scaphoid fractures. J. Hand Surg. 2000;25(4):369–371. doi: 10.1054/jhsb.2000.0432. [DOI] [PubMed] [Google Scholar]
- 28.Kawamura K., Chung K. Treatment of scaphoid fractures and nonunions. J. Hand Surg. 2008;33(6):988–997. doi: 10.1016/j.jhsa.2008.04.026. [Internet]. Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Klauser A.S., Tagliafico A., Allen G.M., Boutry N., Campbell R., Court-Payen M. Clinical indications for musculoskeletal ultrasound: a Delphi-based consensus paper of the European society of musculoskeletal radiology. Eur. Radiol. 2012;22:1140–1148. doi: 10.1007/s00330-011-2356-3. [DOI] [PubMed] [Google Scholar]
- 30.Sconfienza L.M., Albano D., Allen G., Bazzocchi A., Bignotti B., Chianca V. Clinical indications for musculoskeletal ultrasound updated in 2017 by European Society of Musculoskeletal Radiology (ESSR) consensus. Eur. Radiol. 2018;28(12):5338–5351. doi: 10.1007/s00330-018-5474-3. [DOI] [PubMed] [Google Scholar]
- 31.Hannemann P.F.W., Brouwers L., Dullaert K., van der Linden E.S., Poeze M., Brink P.R.G. Determining scaphoid waist fracture union by conventional radiographic examination: an analysis of reliability and validity. Arch. Orthop. Trauma Surg. 2015;135(2):291–296. doi: 10.1007/s00402-014-2147-9. [DOI] [PubMed] [Google Scholar]
- 32.Ring D., Lozano-Calderón S. Imaging for suspected scaphoid fracture. J. Hand Surg. 2008;33(6):954–957. doi: 10.1016/j.jhsa.2008.04.016. [DOI] [PubMed] [Google Scholar]
- 33.Ali M., Ali M., Mohamed A., Mannan S., Fallahi F. The role of ultrasonography in the diagnosis of occult scaphoid fractures. J. Ultrason. 2018;18(75):325–331. doi: 10.15557/JoU.2018.0047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jernigan Edward W., Kyle W., Morse M.G.C. Managing the athlete with a scaphoid fracture. Hand Clin. 2019;35(3):365–371. doi: 10.1016/j.hcl.2019.03.011. [Internet]. Available from: [DOI] [PubMed] [Google Scholar]
- 35.Amrami Kimberly K., Matthew A., Frick J.M.M. Imaging for acute and chronic scaphoid fractures. Hand Clin. 2019;35(3):241–257. doi: 10.1016/j.hcl.2019.03.001. [Internet]. Available from: [DOI] [PubMed] [Google Scholar]