To the editor:
The mRNA-1273 (Moderna) vaccine is a lipid nanoparticle–encapsulated mRNA-based vaccine that encodes the prefusion stabilized full-length spike protein of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the cause of the current coronavirus disease 2019 (COVID-19) pandemic. In a randomized placebo-controlled phase 3 trial, the mRNA-1273 (Moderna) vaccine showed high efficacy at preventing COVID-19. Aside from transient local and systemic reactions, no safety concerns were identified.1
Here we report 2 patients who developed de novo vasculitis shortly after receiving the mRNA-1273 (Moderna) vaccine.
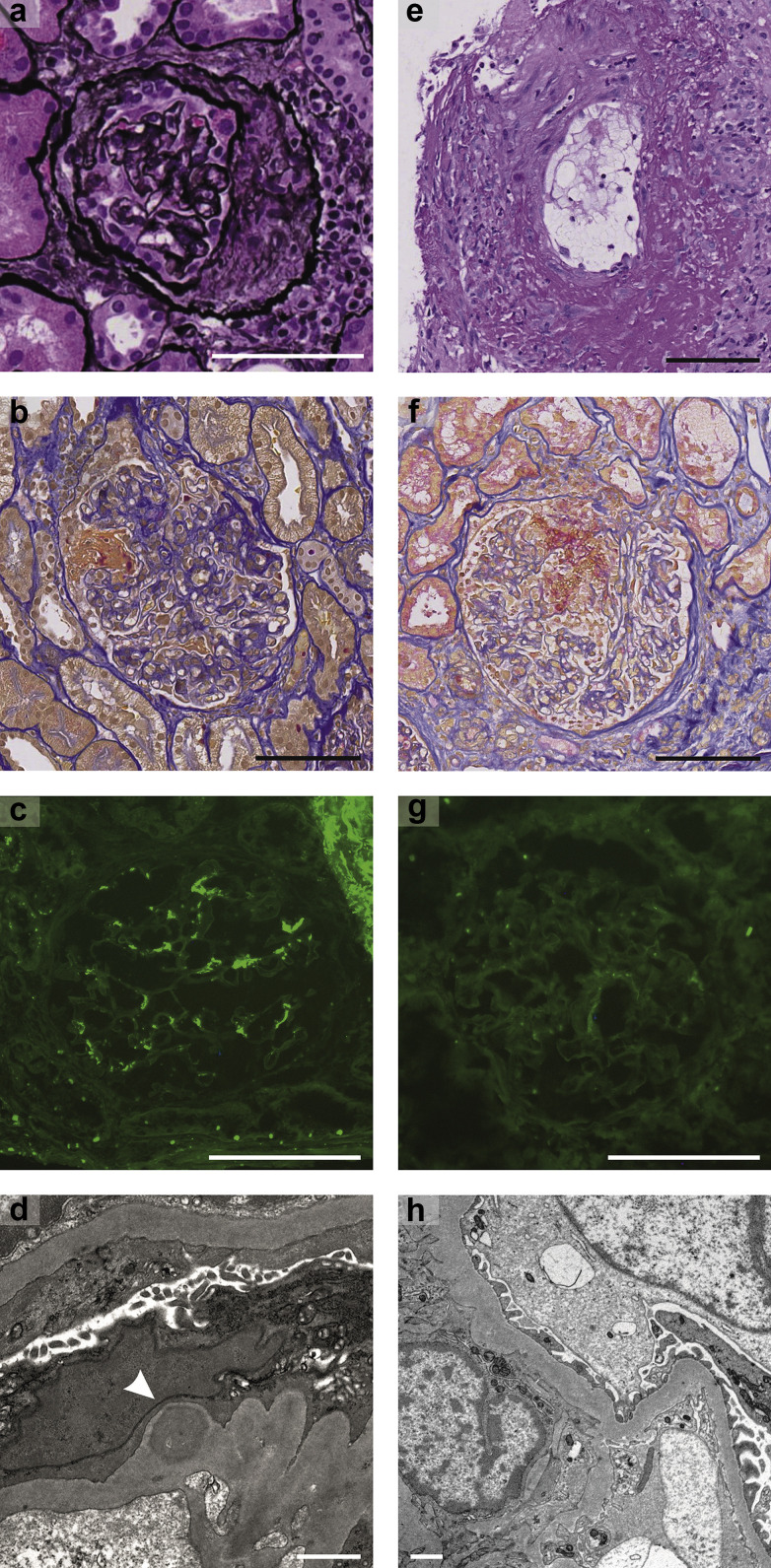
Patient 1 was a 39-year-old man with a history of treated arterial hypertension. After a well-tolerated first dose of mRNA-1273 (Moderna) vaccine, he had severe fever, flu-like symptoms, and macrohematuria immediately after the second dose. Diagnostic workup showed acute kidney injury (AKI) with nephritic syndrome. Repeat reverse transcription polymerase chain reaction (RT-PCR) testing for SARS-CoV-2 from nasopharyngeal swabs was negative. A kidney biopsy revealed severe crescentic IgA nephritis (Figure 1 a–d). Treatment with high-dose glucocorticoids and cyclophosphamide was initiated. Over the following weeks, serum creatinine normalized and proteinuria significantly decreased, but microhematuria persisted.
Figure 1.
(a–d) Histopathologic findings of patient 1 with (a,b) crescentic IgA nephritis and (c,d) mesangial IgA deposition on immunofluorescence and electron microscopy, and histopathologic findings of patient 2 with (e,f) severe necrotizing vasculitis, but (g,h) without deposition of Igs on immunofluorescence and electron microscopy. (a) Hematoxylin-eosin and silver staining (original magnification ×20). (b) Acid fuchsin–orange G stain (original magnification ×20). (c) Immunofluorescence against IgA (original magnification ×20). (d) Transmission electron microscopy. The arrowhead shows mesangial IgA depot. (e) Periodic acid–Schiff stain (original magnification ×20). (f) Acid fuchsin–orange G stain (original magnification ×20). (g) Immunofluorescence against IgA (original magnification ×20). (h) Transmission electron microscopy. Bar = 100 μm for light microscopy and immunofluorescence, and bar = 1 μm for electron microscopy. To optimize viewing of this image, please see the online version of this article at www.kidney-international.org.
Patient 2 was a healthy 81-year-old man. After the first dose of the mRNA-1273 (Moderna) vaccine, he had sustained flu-like symptoms, which significantly worsened after the second dose. Laboratory workup showed AKI, proteinuria in the nonnephrotic range, and an elevated proteinase 3 (PR3) anti–neutrophil cytoplasmic antibody (ANCA) titer. A pulmonary computed tomography scan demonstrated bilateral necrotic masses of the lung parenchyma and slight pleural effusion, without evidence of tumor or lymphadenopathy. Repeat RT-PCR testing for SARS-CoV-2 from nasopharyngeal swabs was negative; serologic testing for SARS-CoV-2 showed a positive anti-spike IgG and negative anti-nucleocapsid IgG. A kidney biopsy performed at day 22 after the second vaccine dose showed severe pauci-immune crescentic glomerulonephritis with capillary necrosis and vasculitis present in the renal vessel walls (Figure 1e–h). The patient was treated with high-dose glucocorticoids, cyclophosphamide, and plasmapheresis. Over the course of 3 weeks, the patient’s symptoms disappeared, and renal function improved along with a significant decrease of PR3-ANCA and anti-spike IgG titer. Immunohistochemical staining for the SARS-CoV-2 spike protein was negative in both patients.
Appearance of AKI concurrently with serious systemic symptoms shortly after the second dose strongly suggests a causal mechanism. Isolated cases of SARS-CoV-2–induced IgA vasculitis and ANCA-associated vasculitis have been reported.2 , 3 In contrast, 2 patients with preexisting IgA nephropathy have been reported to experience gross hematuria after receiving the mRNA-1273 (Moderna) vaccine, with spontaneous resolution after 3 days.4 Two cases of minimal change nephropathy associated with the BNT162b2 mRNA (Pfizer-BioNTech) vaccine have also been described.5 , 6
To our knowledge, these are the first 2 cases of de novo vasculitis after vaccination with an mRNA-based vaccine.
The mechanism remains to be elucidated but is likely due to aberrant immune response to the spike protein or mRNA of SARS-CoV-2 in predisposed individuals.
We hope that this correspondence will prompt clinicians to consider vasculitis workup in the case of protracted systemic reactions, new-onset macrohematuria, or worsening kidney function after vaccination with mRNA-based SARS-CoV-2 vaccines. Given the massive scale-up of vaccination efforts worldwide, it is very likely that additional cases of vaccination-induced vasculitis will emerge. We strongly encourage additional reporting and communication for this rare, albeit severe, side effect of the mRNA-1273 (Moderna) vaccine.
Disclosure
DGF reports unrestricted research grants from Otsuka and Boehringer Ingelheim and consulting fees from Otsuka and Alnylam. All the other authors declared no competing interests.
Acknowledgments
Author Contributions
All authors contributed to the study design. MAA, ML, CS, and MM performed the data analysis. UH-D and DGF verified the data. All authors contributed to the data interpretation. MAA and ML wrote the first draft of the manuscript, which was subsequently revised by the remaining authors. All authors approved the final version of the manuscript before submission.
References
- 1.Baden L.R., El Sahly H.M., Essink B. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384:403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Suso A.S., Mon C., Onate Alonso I. IgA vasculitis with nephritis (Henoch-Schonlein purpura) in a COVID-19 patient. Kidney Int Rep. 2020;5:2074–2078. doi: 10.1016/j.ekir.2020.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Uppal N.N., Kello N., Shah H.H. De novo ANCA-associated vasculitis with glomerulonephritis in COVID-19. Kidney Int Rep. 2020;5:2079–2083. doi: 10.1016/j.ekir.2020.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Negrea L., Rovin B.H. Gross hematuria following vaccination for severe acute respiratory syndrome coronavirus 2 in 2 patients with IgA nephropathy. Kidney Int. 2021;99:1487. doi: 10.1016/j.kint.2021.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lebedev L., Sapojnikov M., Wechsler A. Minimal change disease following the Pfizer-BioNTech COVID-19 vaccine. Am J Kidney Dis. 2021;78:142–145. doi: 10.1053/j.ajkd.2021.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.D'Agati V.D., Kudose S., Bomback A.S. Minimal change disease and acute kidney injury following the Pfizer-BioNTech COVID-19 vaccine. Kidney Int. 2021;100:461–463. doi: 10.1016/j.kint.2021.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]