Abstract
Objective
Recently, a novel trial design has been proposed to overcome challenges with traditional placebo‐controlled trials of antiepileptic drugs in infants and young children (≥1 month of age) (Auvin S, et al. Epilepsia Open 2019;4:537‐43). The proposed time‐to‐event trial design involves seizure counting by caregivers and allows adjustment of the duration of the baseline period and duration of exposure to placebo or potentially ineffective treatment based on the patient's seizure burden and response. We performed post hoc analyses to mimic this trial design and evaluate its viability. As these analyses required trials with prolonged baseline and treatment periods and diary data, which is not a typical design of trials in infants and young children (1 month to <4 years of age), data from two trials in pediatric patients (4‐16 years of age) were used.
Methods
We performed post hoc analyses of two randomized, double‐blind, placebo‐controlled trials of adjunctive levetiracetam (N159; NCT00615615) and lacosamide (SP0969; NCT01921205) in children and adolescents (4‐16 years of age) with focal‐onset seizures. In these analyses, patients were followed until they completed the 10‐week maintenance period, discontinued during the maintenance period, or reached their “nth” seizure (n = number of seizures patient had during baseline). Efficacy was assessed by determining time to nth seizure.
Results
In the analyses of both trials, patients on levetiracetam or lacosamide had a 34% lower risk of reaching their baseline seizure count during their 10‐week maintenance period than patients on placebo. The previously published primary results of these trials also demonstrated efficacy of adjunctive levetiracetam and lacosamide.
Significance
Although these were post hoc analyses of trials in older children (4‐16 years of age), our results provide supportive evidence for the utility of the novel time‐to‐event trial design for future trials in infants and young children (1 month to <4 years of age).
Keywords: clinical trials, drug development, infants, time‐to‐event
Key Points.
Recently, a novel trial design was suggested to overcome issues with traditional placebo‐controlled trials in young children with epilepsy
We performed post hoc analyses of seizure responses in pediatric levetiracetam and lacosamide trials to evaluate this novel trial design
Efficacy was assessed by determining time to nth seizure (time to reach patient's individual baseline seizure count during maintenance)
Patients on levetiracetam or lacosamide had a 34% lower risk of reaching baseline seizure count during 10‐week maintenance versus placebo
These post hoc analyses of pediatric trials support the viability of the novel time‐to‐event trial design proposed recently
1. INTRODUCTION
In the past few decades, several new antiepileptic drugs (AEDs) have been approved for use in adult patients with focal (partial‐onset) seizures. It is important to determine whether these AEDs could also be valuable for use in pediatric patients, particularly those whose seizures are resistant to available AEDs.
While efficacy of AEDs, if demonstrated in adults, can be extrapolated to pediatric patients 4 years of age and older 1 or 2 years of age and older, 2 double‐blind, placebo‐controlled trials in infants and young children (≥1 month of age) are required to investigate the safety and efficacy of AEDs for this population. However, traditional placebo‐controlled trials of AEDs in this age group are difficult to perform because of eligibility constraints and their impractical nature. 3 These trial designs generally require frequent seizures over a short baseline period, two hospitalizations for video‐electroencephalography (video‐EEG) monitoring, and a willingness to accept potential exposure to placebo or potentially ineffective therapy for a predetermined duration regardless of seizure frequency at baseline. Therefore, these trials are not reflective of how patients are treated in clinical practice. As a result, there are often issues with recruitment into pediatric trials, leading to a lack of adequate efficacy and safety data and resulting in delays of regulatory approvals of AEDs for this vulnerable population. Consequently, many children with epilepsy are prescribed off‐label medications without adequate evidence of efficacy and safety. 3 Therefore, redesign of trials in infants and young children is needed to improve efficiency and feasibility and to make them more acceptable to clinicians, investigators, families, and regulatory agencies, while also ensuring that the data can be analyzed in a statistically appropriate manner.
A recent consensus document from the regulatory task force and the pediatric commission of the International League Against Epilepsy, in collaboration with the Pediatric Epilepsy Research Consortium, proposed a novel trial design to overcome these challenges. 3 The proposed time‐to‐event trial design involves seizure counting by caregivers based on previous video‐EEG/video validation of specific seizure semiologies and was designed to adjust the duration of the baseline period and duration of exposure to placebo or potentially ineffective treatment based on the patient's seizure burden and response or lack of response. Although patients with a low seizure burden would not be eligible for traditional trials because of the requirement of video‐EEG‐recorded seizures within the 2‐3 days of allotted video‐EEG monitoring, they may be included using this novel trial design. This would allow the inclusion of more infants and young children with focal seizures than in trials using traditional designs and would more closely reflect clinical practice.
Our objective was to perform post hoc analyses of previous AED trials in pediatric patients to mimic the conduct and analysis of the proposed novel trial design and evaluate its viability. These efficacy analyses required prolonged baseline and treatment periods and diary data, which are not typically aspects of the design of trials enrolling infants and young children (1 month to <4 years of age). Therefore, we used data from previous randomized, double‐blind, placebo‐controlled trials of levetiracetam and lacosamide in children and adolescents (4‐16 years of age).
2. METHODS
2.1. Design of levetiracetam and lacosamide trials in pediatric patients
N159 (NCT00615615) was a randomized, double‐blind, placebo‐controlled trial that demonstrated the efficacy and tolerability of adjunctive levetiracetam in children and adolescents (4‐16 years of age) with uncontrolled focal (partial‐onset) seizures. 4 This trial consisted of an 8‐week baseline period and 14‐week treatment period (4‐week titration period and 10‐week maintenance period [referred to as “Evaluation Period” in the primary publication 4 ]). Each patient's 10‐week maintenance period may have been shorter or longer based on visit windows. At the conclusion of the treatment period, patients could either withdraw trial medication over 6 weeks or enter a blinded conversion period leading to an open‐label extension trial. To be eligible, patients were required to have ≥4 focal seizures during the 4 weeks before screening and ≥4 focal seizures during each 4‐week interval of the 8‐week baseline period. During the titration period, patients were up‐titrated from a starting dose of 20 mg/kg/d to levetiracetam 60 mg/kg/d or placebo. If a patient could not tolerate 60 mg/kg/d, the dose could be reduced to 40 mg/kg/d and maintained at that dose for the remainder of the maintenance period. Throughout the baseline and treatment periods, patients or their parents or legal guardians maintained a daily diary of seizure activity (type and frequency) that was used to assess efficacy outcomes. The primary efficacy outcome was focal seizure frequency per week during the treatment period. Focal seizure frequency per week during the maintenance period was analyzed as a secondary outcome.
SP0969 (NCT01921205) was a randomized, double‐blind, placebo‐controlled trial that demonstrated the efficacy and safety of adjunctive lacosamide in children and adolescents (4‐16 years of age) with uncontrolled focal seizures. 5 This trial consisted of an 8‐week baseline period, 16‐week treatment period (6‐week titration period and 10‐week maintenance period), 4‐week taper/transition period, and a 30‐day safety follow‐up for patients not entering the open‐label extension trial. Each patient's 10‐week maintenance period may have been shorter or longer based on visit windows. To be eligible, patients were required to have an average of ≥2 focal seizures per 28 days, with no more than 21 consecutive days without seizures in the 8‐week period before entering the baseline period, and ≥2 focal seizures during the baseline period. Patients initiated lacosamide or placebo at a dose of 2 mg/kg/d (patients <50 kg; oral solution) or 100 mg/d (patients ≥50 kg; tablets). Patients who reached the target dose range for their weight during the titration period (<30 kg: 8‐12 mg/kg/d oral solution; ≥30 to <50 kg: 6‐8 mg/kg/d oral solution; ≥50 kg: 300‐400 mg/d tablets) entered the maintenance period. Throughout the trial, patients and/or their caregivers completed a daily diary of seizure activity (type and frequency) that was used to assess efficacy outcomes. The primary efficacy outcome was change in focal seizure frequency per 28 days from baseline to the maintenance period.
2.2. Proposed novel trial design
In the novel time‐to‐event trial design proposed by Auvin et al, 3 seizures are counted by caregivers based on previous video‐EEG/video validation of specific seizure semiologies. Patients who meet initial entry criteria enter a prospective baseline period. The baseline period has a variable duration based on the patient's seizure frequency. When the patient returns at the week 1 baseline visit, the number of seizures reported by the patient (considering only observable focal seizures as identified by the parent or guardian, ie, focal aware with motor symptoms, focal impaired awareness, and focal to bilateral tonic‐clonic) is assessed. If the count exceeds a certain threshold (determined based on the specifics of the trial medication, patient population, and statistical considerations, including expected seizure threshold distributions), the patient is randomized to either placebo or test therapy. If the patient does not meet this threshold, then they continue until the week 2 baseline visit. At the week 2 visit, the number of seizures is compared with a threshold (possibly different from the week 1 threshold). If the count exceeds this threshold, the patient is randomized. If the patient does not meet the threshold, they continue until the week 4 baseline visit. At the week 4 visit, the number of seizures is compared with a threshold (possibly different from the week 1 and week 2 thresholds). If the count exceeds this threshold, the patient is randomized. If a patient does not meet the minimum seizure count requirement for randomization for week 1, weeks 1‐2, or weeks 1‐4, the patient does not qualify for randomization into the trial.
Randomized patients enter the titration period and patients who complete titration enter the maintenance period. Patients who enter the maintenance period exit the trial if the cumulative number of seizures (of seizure types of interest) reaches the number of seizures observed during their baseline.
The proposed primary efficacy outcome is the number of days to the “nth” seizure, where n is the number of seizures observed during baseline (week 1, weeks 1‐2, or weeks 1‐4). The number of seizures is specific to each patient.
2.3. Post hoc analyses to mimic proposed trial design
Post hoc analyses based on daily seizure count data (observable focal seizures only) were performed separately for the levetiracetam and lacosamide trials. In both trials, the efficacy populations included randomized patients who received at least one dose of trial medication and who had at least one postbaseline assessment of seizure frequency data. 4 , 5 The post hoc efficacy population included patients from the respective efficacy populations who met the minimum baseline seizure count requirements for the post hoc analyses. The baseline periods of N159 and SP0969 were used to mimic the baseline period of the novel design, with only the first 4 weeks of the baseline periods being used. The baseline seizure thresholds were set so that all patients qualified for these analyses except for patients with no observable focal seizures in the first 4 weeks of baseline. For the main analysis, the baseline seizure threshold was set so that 25% of patients qualified based on week 1, 50% of patients qualified based on weeks 1‐2, and the remaining ~25% of patients qualified based on weeks 1‐4. Since predicting the exact seizure threshold distribution for a future trial may be difficult, other seizure threshold distributions (25%/25%/50%, 50%/25%/25%, 33%/33%/33%) were also assessed to determine the robustness of the results in comparison with alternative distributions. This was necessary to establish whether or not the trial is put at undue risk due to this uncertainty.
The efficacy outcome of time to nth seizure was determined relative to the start of the maintenance period. Patients were followed until the earliest of the following occurred: The patient completed the 10‐week maintenance period, discontinued during the maintenance period, or had a timepoint during the maintenance period when the cumulative number of seizures over the maintenance period equaled or exceeded the number of seizures in the baseline period (ie, the patient reached their nth seizure). Patients who discontinued during titration were analyzed as efficacy failures on day 1 of the maintenance period. For the primary analysis, patients who discontinued during the maintenance period without reaching their nth seizure were censored at the end of their maintenance period (which may have been longer than 10 weeks based on visit windows). For the secondary analysis, patients who discontinued during their maintenance period without reaching their nth seizure were analyzed as efficacy failures.
The efficacy outcomes were analyzed with proportional hazards regression with an effect for treatment and stratification factor of duration of baseline category. For these analyses, the baseline period is not the full baseline from the levetiracetam or lacosamide trial as it was conducted, but a subset of the baseline period reflecting the time until a patient reached the seizure threshold. The remaining weeks of the baseline period after a patient reached this threshold were disregarded. Strata were defined based on patients' baseline seizure frequency using the seizure threshold distributions defined above.
3. RESULTS
3.1. Reanalysis of efficacy in the levetiracetam trial
The efficacy population in the levetiracetam trial comprised 198 patients (levetiracetam: 101; placebo: 97). Of these, 193 patients were included in the post hoc efficacy population (levetiracetam: 98; placebo: 95). Five patients were excluded from these analyses because they had no observable focal seizures in the first 4 weeks of baseline.
Baseline demographics were comparable between patients in the placebo and levetiracetam groups (Table 1). Patients had a mean age of 10.0 years, and 50.8% of patients were male. Overall, 50 (25.9%) patients qualified based on week 1, 104 (53.9%) based on weeks 1‐2, and 39 (20.2%) based on weeks 1‐4. The majority of patients in all strata had focal seizures with impaired awareness.
TABLE 1.
Baseline demographics (post hoc efficacy population)
| Levetiracetam trial N159 | Lacosamide trial SP0969 | |||||
|---|---|---|---|---|---|---|
|
Placebo (N = 95) |
Levetiracetam (N = 98) |
All patients (N = 193) |
Placebo (N = 166) |
Lacosamide (N = 167) |
All patients (N = 333) |
|
| Patient demographics | ||||||
| Age, mean (SD), years | 9.8 (3.5) | 10.2 (3.2) | 10.0 (3.3) | 11.0 (3.5) | 10.6 (3.6) | 10.8 (3.5) |
| <4 years, n (%) | 2 (2.1) | 0 | 2 (1.0) | 0 | 0 | 0 |
| ≥4 to < 12 years, n (%) | 70 (73.7) | 69 (70.4) | 139 (72.0) | 87 (52.4) | 88 (52.7) | 175 (52.6) |
| ≥12 to < 17 years, n (%) | 20 (21.1) | 29 (29.6) | 49 (25.4) | 79 (47.6) | 79 (47.3) | 158 (47.4) |
| ≥17 years, n (%) | 3 (3.2) | 0 | 3 (1.6) | 0 | 0 | 0 |
| Male, n (%) | 45 (47.4) | 53 (54.1) | 98 (50.8) | 96 (57.8) | 89 (53.3) | 185 (55.6) |
| Seizure characteristics by baseline strata | ||||||
| Stratum 1: 1‐week baseline, n (%) | n = 27 | n = 23 | n = 50 | n = 41 | n = 46 | n = 87 |
| Number of seizures a during 1‐week baseline, median (range) | 30.0 (14.0‐117.0) | 28.0 (14.0‐745.0) | 30.0 (14.0‐745.0) | 21.0 (11.0‐168.0) | 21.0 (11.0‐143.0) | 21.0 (11.0‐168.0) |
| Seizure types at baseline b , n (%) | ||||||
| Focal aware with motor symptoms | 8 (29.6) | 4 (17.4) | 12 (24.0) | 18 (43.9) | 17 (37.0) | 35 (40.2) |
| Focal impaired awareness | 21 (77.8) | 17 (73.9) | 38 (76.0) | 26 (63.4) | 36 (78.3) | 62 (71.3) |
| Focal to bilateral tonic‐clonic | 10 (37.0) | 7 (30.4) | 17 (34.0) | 15 (36.6) | 15 (32.6) | 30 (34.5) |
| Stratum 2: 2‐week baseline, n (%) | n = 48 | n = 56 | n = 104 | n = 79 | n = 85 | n = 164 |
| Number of seizures a during 2‐week baseline, median (range) | 9.0 (4.0‐118.0) | 8.5 (4.0‐96.0) | 8.5 (4.0‐118.0) | 6.0 (2.0‐26.0) | 4.0 (2.0‐227.0) | 5.0 (2.0‐227.0) |
| Seizure types at baseline b , n (%) | ||||||
| Focal aware with motor symptoms | 9 (18.8) | 3 (5.4) | 12 (11.5) | 23 (29.1) | 32 (37.6) | 55 (33.5) |
| Focal impaired awareness | 37 (77.1) | 43 (76.8) | 80 (76.9) | 43 (54.4) | 47 (55.3) | 90 (54.9) |
| Focal to bilateral tonic‐clonic | 10 (20.8) | 20 (35.7) | 30 (28.8) | 27 (34.2) | 27 (31.8) | 54 (32.9) |
| Stratum 3: 4‐week baseline, n (%) | n = 20 | n = 19 | n = 39 | n = 46 | n = 36 | n = 82 |
| Number of seizures a during 4‐week baseline, median (range) | 6.0 (1.0‐15.0) | 5.0 (1.0‐14.0) | 5.0 (1.0‐15.0) | 2.0 (1.0‐21.0) | 2.0 (1.0‐21.0) | 2.0 (1.0‐21.0) |
| Seizure types at baseline b , n (%) | ||||||
| Focal aware with motor symptoms | 3 (15.0) | 1 (5.3) | 4 (10.3) | 14 (30.4) | 15 (41.7) | 29 (35.4) |
| Focal impaired awareness | 16 (80.0) | 15 (78.9) | 31 (79.5) | 23 (50.0) | 19 (52.8) | 42 (51.2) |
| Focal to bilateral tonic‐clonic | 5 (25.0) | 3 (15.8) | 8 (20.5) | 14 (30.4) | 6 (16.7) | 20 (24.4) |
Abbreviation: SD, standard deviation.
Number of observable focal seizures (ie, focal aware with motor symptoms, focal impaired awareness, and focal to bilateral tonic‐clonic).
Patients may have more than one seizure type; percentages are relative to the number of patients in the baseline stratum.
Overall, 157 (81.3%) patients had an event (ie, reached their baseline seizure count). Of these, 11 patients discontinued during titration and were analyzed as efficacy failures on day 1 of the maintenance period, and 146 patients reached their nth seizure during the maintenance period (Table 2). Four patients discontinued during the maintenance period before they reached their nth seizure and were censored for the primary analysis. For the secondary analysis, these four patients were analyzed as efficacy failures.
TABLE 2.
Patient disposition (post hoc efficacy population)
| Patients, n (%) | Levetiracetam trial N159 | Lacosamide trial SP0969 | ||||
|---|---|---|---|---|---|---|
| Placebo (N = 95) | Levetiracetam (N = 98) | All patients (N = 193) | Placebo (N = 166) | Lacosamide (N = 167) | All patients (N = 333) | |
| Discontinued during titration | 7 (7.4) | 4 (4.1) | 11 (5.7) | 8 (4.8) | 10 (6.0) | 18 (5.4) |
| Reached nth seizure during maintenance | 74 (77.9) | 72 (73.5) | 146 (75.6) | 130 (78.3) | 111 (66.5) | 241 (72.4) |
| Completed maintenance without reaching nth seizure | 13 (13.7) | 19 (19.4) | 32 (16.6) | 27 (16.3) | 44 (26.3) | 71 (21.3) |
| Discontinued during maintenance without reaching nth seizure | 1 (1.1) | 3 (3.1) | 4 (2.1) | 1 (0.6) | 2 (1.2) | 3 (0.9) |
| Patients with event (primary analysis a ) | 81 (85.3) | 76 (77.6) | 157 (81.3) | 138 (83.1) | 121 (72.5) | 259 (77.8) |
| Patients with event (secondary analysis b ) | 82 (86.3) | 79 (80.6) | 161 (83.4) | 139 (83.7) | 123 (73.7) | 262 (78.7) |
Patients who discontinued during titration were analyzed as efficacy failures on day 1 of the maintenance period, and patients who discontinued during the maintenance period without reaching their nth seizure were censored at the end of their maintenance period.
Patients who discontinued during titration were analyzed as efficacy failures on day 1 of the maintenance period, and patients who discontinued during their maintenance period without reaching their nth seizure were analyzed as efficacy failures.
Separation between the Kaplan‐Meier curves for time to nth seizure for levetiracetam and placebo was seen early on and continued to the end of the maintenance period (Figure 1). In the primary analysis, the hazard ratio (HR) for levetiracetam vs placebo was 0.661, reflecting a 34% reduction in risk of reaching nth seizure in patients on levetiracetam vs placebo (Table 3). The results for the secondary analysis were comparable with those from the primary analysis. Analysis of other distributions of baseline seizures (25%/25%/50%, 50%/25%/25%, and 33%/33%/33%) showed similar results (HRs: 0.597, 0.639, and 0.606, respectively).
FIGURE 1.
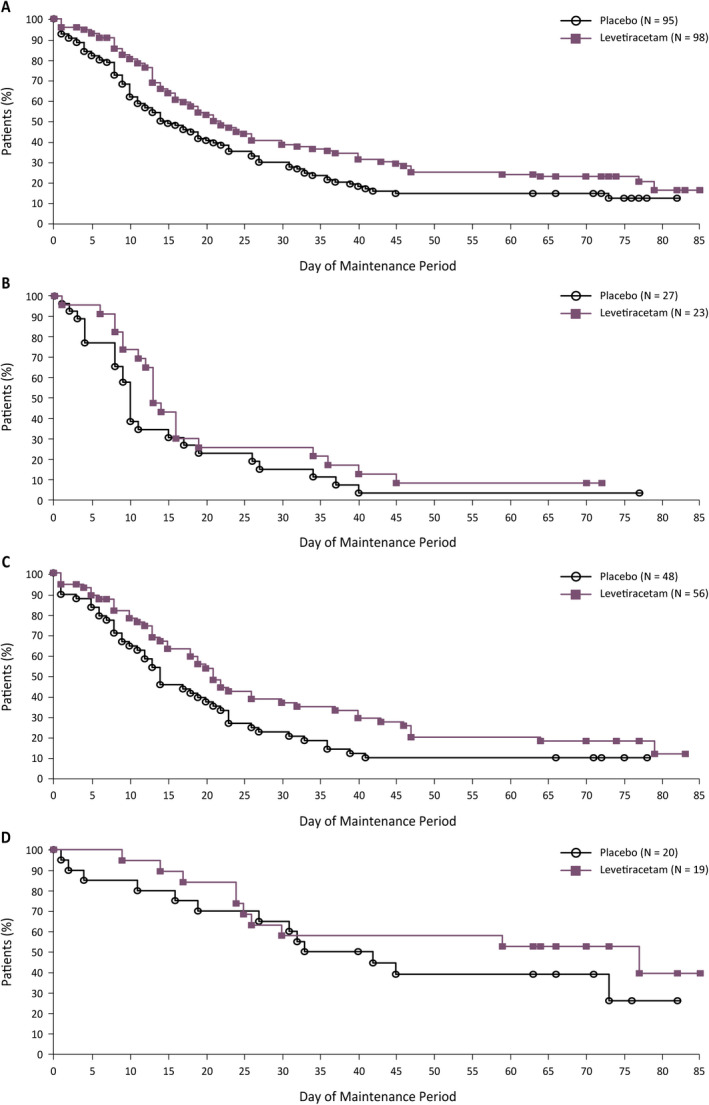
Time to nth seizure during the maintenance period of the levetiracetam trial (post hoc efficacy population). A, Overall. B, Stratum 1: 1‐week baseline. C, Stratum 2: 2‐week baseline. D, Stratum 3: 4‐week baseline. Kaplan‐Meier estimates of the percentage of patients yet to reach their nth seizure. This analysis is based on the full 10‐week maintenance period of the levetiracetam trial; each patient's 10‐week maintenance period may have been shorter or longer based on visit windows. Patients who discontinued during titration were analyzed as efficacy failures on day 1 of the maintenance period, and patients who discontinued during the maintenance period without reaching their nth seizure were censored
TABLE 3.
Risk of reaching nth seizure during maintenance period (post hoc efficacy population)
| Levetiracetam trial N159 | Lacosamide trial SP0969 | |||
|---|---|---|---|---|
| Placebo (N = 95) | Levetiracetam (N = 98) | Placebo (N = 166) | Lacosamide (N = 167) | |
| Patients with event a , n (%) | 81 (85.3) | 76 (77.6) | 138 (83.1) | 121 (72.5) |
| Patients without event, n (%) | 14 (14.7) | 22 (22.4) | 28 (16.9) | 46 (27.5) |
| Overall test of treatment effect b | HR levetiracetam vs placebo (95% CI) | HR lacosamide vs placebo (95% CI) | ||
|---|---|---|---|---|
| All patients | 0.661 (0.481‐0.908); P = .0107 | 0.660 (0.515‐0.845); P = .0010 | ||
| Stratum 1: 1‐week baseline | 0.676 (0.376‐1.214); P = .1895 | 0.590 (0.376‐0.925); P = .0214 | ||
| Stratum 2: 2‐week baseline | 0.652 (0.427‐0.996); P = .0477 | 0.662 (0.469‐0.935); P = .0194 | ||
| Stratum 3: 4‐week baseline | 0.669 (0.290‐1.541); P = .3445 | 0.784 (0.445‐1.382); P = .4001 | ||
Abbreviations: CI, confidence interval; HR, hazard ratio.
Patients who discontinued during titration were analyzed as efficacy failures on day 1 of the maintenance period, and patients who discontinued during the maintenance period without reaching their nth seizure were censored at the end of their maintenance period.
The overall test of treatment effect tests the null hypothesis of no treatment effect in any stratum; rejecting the null hypothesis supports the alternative that there is a treatment effect in at least one stratum; all reported P‐values are nominal and can only be interpreted in an exploratory manner.
3.2. Reanalysis of efficacy in the lacosamide trial
The efficacy population in the lacosamide trial comprised 340 patients (lacosamide: 170; placebo: 170). Of these, 333 patients were included in the post hoc efficacy population (lacosamide: 167; placebo: 166). Seven patients were excluded from these analyses because they had no observable focal seizures in the first 4 weeks of baseline.
Baseline demographics were comparable between patients in the placebo and lacosamide groups (Table 1). Patients had a mean age of 10.8 years, and 55.6% of patients were male. Overall, 87 (26.1%) patients qualified based on week 1, 164 (49.2%) based on weeks 1‐2, and 82 (24.6%) based on weeks 1‐4. More than half of the patients in all strata had focal seizures with impaired awareness.
Overall, 259 (77.8%) patients had an event. Of these, 18 patients discontinued during titration and were analyzed as efficacy failures on day 1 of the maintenance period, and 241 patients reached their nth seizure during the maintenance period (Table 2). Three patients discontinued during the maintenance period before they reached their nth seizure and were censored for the primary analysis. For the secondary analysis, these three patients were analyzed as efficacy failures.
Separation between the Kaplan‐Meier curves for time to nth seizure for lacosamide and placebo was seen early on and continued to the end of the maintenance period (Figure 2). In the primary analysis, the HR for lacosamide vs placebo was 0.660, reflecting a 34% reduction in risk of reaching nth seizure in patients on lacosamide vs placebo (Table 3). The results for the secondary analysis were comparable with those from the primary analysis. Analysis of other distributions of baseline seizures (25%/25%/50%, 50%/25%/25%, and 33%/33%/33%) showed similar results (HRs: 0.643, 0.666, and 0.628, respectively).
FIGURE 2.
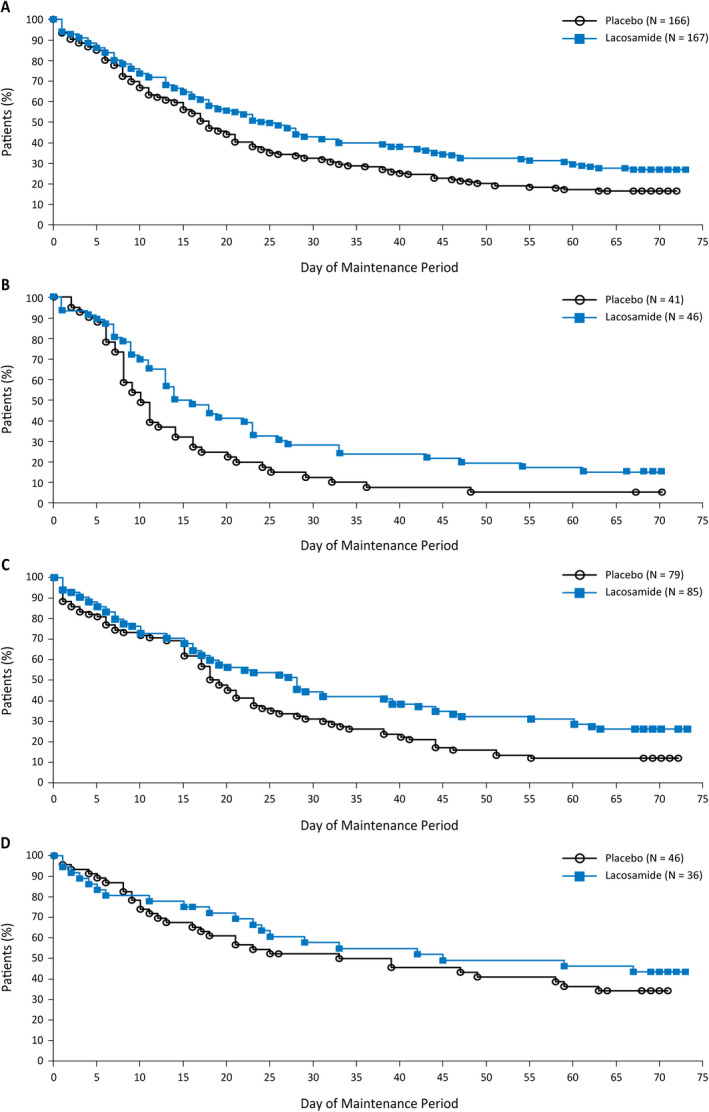
Time to nth seizure during the maintenance period of the lacosamide trial (post hoc efficacy population). A, Overall. B, Stratum 1: 1‐week baseline. C, Stratum 2: 2‐week baseline. D, Stratum 3: 4‐week baseline. Kaplan‐Meier estimates of the percentage of patients yet to reach their nth seizure. This analysis is based on the full 10‐week maintenance period of the lacosamide trial; each patient's 10‐week maintenance period may have been shorter or longer based on visit windows. Patients who discontinued during titration were analyzed as efficacy failures on day 1 of the maintenance period, and patients who discontinued during the maintenance period without reaching their nth seizure were censored
4. DISCUSSION
We performed post hoc analyses of previous trials of adjunctive levetiracetam and lacosamide in children (4‐16 years of age) to mimic the novel trial design proposed by Auvin et al. 3 This novel time‐to‐event trial design was proposed to address challenges with traditional trials in infants and young children (1 month to <4 years of age). 3
In the post hoc analysis of the levetiracetam trial data, patients on levetiracetam had a 34% lower risk of reaching their baseline seizure count during their 10‐week maintenance period than patients on placebo (P = .0107). The previously published primary results of the levetiracetam trial showed that patients on adjunctive levetiracetam had a greater median percentage reduction in focal seizure frequency per week from baseline than patients on placebo (43.8% vs 23.3%; P < .01). 4
In the post hoc analysis of the lacosamide trial data, patients on lacosamide had a 34% lower risk of reaching their baseline seizure count during their 10‐week maintenance period than patients on placebo (P = .0010). The previously published primary results of the lacosamide trial showed that patients on adjunctive lacosamide had a greater median percentage reduction in focal seizure frequency per 28 days from baseline than patients on placebo (51.7% vs 21.7%; percentage reduction vs placebo: 31.7%; P = .0003). 5
These post hoc analyses included stratification by duration of baseline category, and strata were defined based on patients' baseline seizure frequency. While the treatment effect may be more pronounced in patients with a high baseline seizure frequency (strata 1 and 2) than in patients with a low baseline seizure frequency (stratum 3), these data should be interpreted with caution because this was a post hoc analysis with low patient numbers in the strata and the study was not powered to detect differences between the strata.
The efficacy and safety of adjunctive levetiracetam for the treatment of uncontrolled focal seizures in infants and young children (1 month to <4 years of age) was demonstrated in a randomized, double‐blind, placebo‐controlled trial using a 5‐day inpatient treatment period including 48‐hour evaluation video‐EEG in the last 2 days (N01009; NCT00175890). 6 A randomized, double‐blind, placebo‐controlled trial assessing the efficacy, safety, and tolerability of adjunctive lacosamide for the treatment of infants and young children (1 month to <4 years of age) with uncontrolled focal seizures was recently completed (SP0967; NCT02477839). Efficacy outcomes in this trial were based on 72‐hour video‐EEGs that were conducted in an inpatient setting (the first EEG was conducted during baseline; after a 20‐day titration period, the second EEG was conducted in the last 3 days of a 7‐day maintenance period). Both of these trials used the traditional trial design with video‐EEG and short treatment periods. There were no prolonged baseline and treatment periods, and efficacy was not assessed using diary data, making it impossible to perform analyses to mimic the novel trial design. For these reasons, we performed post hoc analyses on randomized, double‐blind, placebo‐controlled trials that had demonstrated efficacy and safety of levetiracetam and lacosamide in children and adolescents (4‐16 years of age). 4 , 5 Both of these trials had an 8‐week baseline period and 10‐week maintenance period, and efficacy was assessed based on diary data.
Interpretation of these data is limited by the post hoc nature of the analyses. To partially compensate for this, we performed alternative analyses for other distributions of baseline seizures, which showed consistent results with the main analysis. All presented P‐values are nominal only. As efficacy was assessed based on diary data, it is also possible that some diary entries were missing. Comparison of the levetiracetam and lacosamide data is limited as the trials were conducted more than a decade apart. 4 , 5 During this timespan, there have been changes in medical practice and the enrolled patient populations may not be comparable. Further, the trials analyzed in these post hoc analyses enrolled children and adolescents (4‐16 years of age) who may have different epilepsy etiologies and syndromes compared with infants and young children (1 month to <4 years of age). In addition, the clinical interpretation of the novel outcome for time to nth seizure requires consideration. The challenges of defining a clinically relevant effect are not unique to this outcome but are a challenge for any clinical outcome in a treatment‐resistant population. Future studies should carefully consider the specific way in which this outcome is defined, and how the numeric magnitude of the hazard ratio translates into a practical understanding of benefit for a treating physician, a parent or caretaker, and the patient.
Trial designs using time to nth seizure as the efficacy outcome have been proposed in the past, 7 where n is set as an arbitrary number and patients exit the trial if they experience this number of seizures. 8 Post hoc analyses of a trial in adult patients have suggested that such a trial design could identify efficacious treatments and shorten the duration of exposure to placebo. 9 However, a large sample size would likely be required, because patients with high seizure frequencies would quickly reach the specified number of seizures and exit the trial, often before there was a chance for improvement. 8 By using an individualized endpoint based on each patient's seizure count during baseline, the novel trial design proposed by Auvin et al 3 would enable enrollment of patients with a wide range of baseline seizure frequencies, which could help address the selection bias of previous time to nth seizure trials. 8 This novel trial design is intended to shorten the trial duration and therefore the duration of exposure to placebo or potentially ineffective treatment. Extension trials would remain essential for the evaluation of long‐term safety outcomes. 8
Our post hoc analyses of previous pediatric AED trials support the viability of the novel time‐to‐event trial design proposed by Auvin et al. 3 This novel trial design will hopefully encourage more clinicians and families to consider enrollment, leading to better recruitment rates for trials in infants and young children, and ultimately improve clinical care. Although this was a post hoc analysis of trials in older children (4‐16 years of age), our results provide supportive evidence for the utility of this design in future trials, including trials in infants and young children (1 month to <4 years of age).
CONFLICT OF INTEREST
ME Johnson was an employee of UCB Pharma at the time of these analyses and is currently employed by Clinipace. C McClung and AM Bozorg are employees of UCB Pharma.
ETHICAL PUBLICATION STATEMENT
We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
ACKNOWLEDGMENTS
The trials and post hoc analyses were funded by UCB Pharma. The authors thank the patients and their caregivers in addition to the investigators and their teams who contributed to these trials. The authors acknowledge Ying Zhang (formerly UCB Pharma, Raleigh, NC, USA) and Cynthia Beller (UCB Pharma, Raleigh, NC, USA) for statistical support with the post hoc analyses, and Michaela Fuchs, PhD, CMPP (Evidence Scientific Solutions, Horsham, UK) for writing assistance, which was funded by UCB Pharma. Publication coordination was provided by Fabien Debailleul, PhD (UCB Pharma, Brussels, Belgium).
Johnson ME, McClung C, Bozorg AM. Analyses of seizure responses supportive of a novel trial design to assess efficacy of antiepileptic drugs in infants and young children with epilepsy: Post hoc analyses of pediatric levetiracetam and lacosamide trials. Epilepsia Open. 2021;6:359–368. 10.1002/epi4.12482
DATA AVAILABILITY STATEMENT
Underlying data from this manuscript may be requested by qualified researchers 6 months after product approval in the US and/or Europe, or global development is discontinued, and 18 months after trial completion. Investigators may request access to anonymized individual patient‐level data and redacted trial documents which may include: analysis‐ready datasets, study protocol, annotated case report form, statistical analysis plan, dataset specifications, and clinical study report. Prior to the use of the data, proposals need to be approved by an independent review panel at www.Vivli.org and a signed data sharing agreement will need to be executed. All documents are available in English only, for a prespecified time, typically 12 months, on a password‐protected portal.
REFERENCES
- 1. European Medicines Agency . Guideline on clinical investigation of medicinal products in the treatment of epileptic disorders. 2018. https://www.ema.europa.eu/en/documents/scientific‐guideline/draft‐guideline‐clinical‐investigation‐medicinal‐products‐treatment‐epileptic‐disorders‐revision‐3_en.pdf. Accessed May 6, 2020.
- 2. Food and Drug Administration . Drugs for treatment of partial onset seizures: full extrapolation of efficacy from adults to pediatric patients 2 years of age and older – guidance for industry. 2019. https://www.fda.gov/media/130449/download. Accessed May 6, 2020.
- 3. Auvin S, French J, Dlugos D, Knupp KG, Perucca E, Arzimanoglou A, et al. Novel study design to assess the efficacy and tolerability of antiseizure medications for focal‐onset seizures in infants and young children: a consensus document from the regulatory task force and the pediatric commission of the International League against Epilepsy (ILAE), in collaboration with the Pediatric Epilepsy Research Consortium (PERC). Epilepsia Open. 2019;4:537–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Glauser TA, Ayala R, Elterman RD, Mitchell WG, Van Orman CB, Gauer LJ, et al. Double‐blind placebo‐controlled trial of adjunctive levetiracetam in pediatric partial seizures. Neurology. 2006;66:1654–60. [DOI] [PubMed] [Google Scholar]
- 5. Farkas V, Steinborn B, Flamini JR, Zhang Y, Yuen N, Borghs S, et al. Efficacy and tolerability of adjunctive lacosamide in pediatric patients with focal seizures. Neurology. 2019;93:e1212–e1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Piña‐Garza JE, Nordli DR Jr, Rating D, Yang H, Schiemann‐Delgado J, Duncan B, et al. Adjunctive levetiracetam in infants and young children with refractory partial‐onset seizures. Epilepsia. 2009;50:1141–9. [DOI] [PubMed] [Google Scholar]
- 7. Pledger GW, Sahlroot JT. Alternative analyses for antiepileptic drug trials. Epilepsy Res Suppl. 1993;10:167–74. [PubMed] [Google Scholar]
- 8. French JA, Gil‐Nagel A, Malerba S, Kramer L, Kumar D, Bagiella E. Time to prerandomization monthly seizure count in perampanel trials: a novel epilepsy endpoint. Neurology. 2015;84:2014–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Leppik IE, Dreifuss FE, Pledger GW, Graves NM, Santilli N, Drury I, et al. Felbamate for partial seizures: results of a controlled clinical trial. Neurology. 1991;41:1785–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Underlying data from this manuscript may be requested by qualified researchers 6 months after product approval in the US and/or Europe, or global development is discontinued, and 18 months after trial completion. Investigators may request access to anonymized individual patient‐level data and redacted trial documents which may include: analysis‐ready datasets, study protocol, annotated case report form, statistical analysis plan, dataset specifications, and clinical study report. Prior to the use of the data, proposals need to be approved by an independent review panel at www.Vivli.org and a signed data sharing agreement will need to be executed. All documents are available in English only, for a prespecified time, typically 12 months, on a password‐protected portal.


