Abstract
Objective
Hyperventilation (HV) is one of the main and basic activation methods during ambulatory electroencephalogram (EEG), unless medical reasons contraindicate it. During the COVID‐19 pandemic, with the high risk of human‐to‐human infection, local guidelines and recommendations have been developed that suggest not to perform the HV maneuver routinely. Our objective was to characterize patients who present positive HV in an epilepsy center.
Methods
We analyzed retrospectively all the ambulatory EEGs performed during one year in our specialized ambulatory child and adolescent epilepsy center, and describe patients with positive maneuver.
Results
A total of 305 EEGs were performed. Patients under 3 years and 11 months were excluded as well as all patients that did not fill up the criteria for epilepsy diagnosis. From the 252 EEGs that were included in the study, 194 EEGs (77%) were classified as abnormal and 58 (23%) as normal. From these same 252 EEGs, 150 EEG finished correctly the HV maneuver. Physiological slowing response was found in 54 EEGs (36%), no changes (negative) in 83 (55%), and abnormal response (positive) in 13 EEGs (9%). The 13 HV‐positive EEGs showed 4 patients with an increase of epileptiform activity, 3 patients experienced an increase of basal preregistered abnormal slowing, and 6 EEGs showed trigger of bilaterally synchronous and symmetric 2‐4 Hz spike‐and‐slow wave discharges and absences. None of these last 6 patients needed more than 3 minutes to elicit the paroxysmal discharge.
Significance
Based on these findings and according with other studies, the low positivity and high specificity of the HV maneuver support the idea that HV could be excluded during the COVID‐19 pandemic situation, and also reevaluate whether it could be changed to a complementary maneuver, restricted only for cases where absence epilepsy is suspected. Larger studies will be needed to reaffirm this proposal.
Keywords: absence childhood epilepsy, clinical seizure; generalized epileptiform discharges, COVID‐19; SARS‐CoV‐2; EEG activation, hyperventilation
Key Points.
We analyzed the ambulatory EEGs performed during the first‐year opening of our ambulatory child epilepsy center before COVID‐19 pandemic situation.
From the 150 EEG with HV maneuver finished in confirmed epilepsy patients: Physiological response was found in 54 EEGs (36%); negative in 83 (55%) and abnormal positive in 13 (9%). From these 13 abnormal positive responses, only 6 (4%) EEGs were an important key for the epilepsy diagnosis, specifically for absence epilepsy. Also, in these 6 patients, the abnormalities were triggered in 100% of the patients during the first 3 minutes of HV.
The low positivity and high specificity of the HV maneuver support the idea that this maneuver could be excluded during COVID‐19 pandemic situation and could be restricted to patients where absence seizures are suspected with a maximum duration length of 3 minutes.
1. INTRODUCTION
Hyperventilation (HV) is one of the main and basic activation methods during an ambulatory electroencephalogram (EEG), unless medical or other justifiable reasons are contraindicated (eg, a recent intracranial hemorrhage, significant cardiopulmonary disease, sickle cell disease or trait, patient's inability to perform it or unwillingness to cooperate). The same can be said about intermittent photic stimulation, both recommended by the world reference guidelines from the American Clinical Neurophysiology Society. 1
HV can induce physiological slowing of the brain rhythms, usually more sudden and intense in children from 8 to 12 years. Although this effect can also be observed in normal individuals, it is, however, more prevalent and pronounced in patients with epilepsy 2 . HV is frequently used as a trigger procedure for clinical and electrographic seizures 2 , 3 , mainly in generalized epilepsies, but has also shown an impact in some focal ones. 4 , 5
Precipitation of seizures by HV was described before EEGs on humans were performed, 6 even though it was Nims in 1940 7 the one that showed the efficacy of this procedure in inducing generalized synchronous paroxysmal discharges and absence seizures during the test.
The physiological explanation of this slowing, as well as the induction of seizures, is still not completely understood. It has been partly explained due to secondary alkalosis and decrease of the cerebral blood flow. 3 , 8
HV should not be performed with a mask, it produces the opposite effect because it increases the pCO2, thus resulting in cerebral vasodilatation. 9
During the COVID‐19 pandemic, a high risk of human‐to‐human infection with SARS‐CoV‐2 by aerosols has been described. This happens mainly through contaminated small droplets from the nose or mouth, which are expelled when a person coughs, sneezes, or speaks (symptomatic or asymptomatic). Because of this high risk of contagion, guidelines and recommendations have been developed by different neurophysiological societies on whether or not HV should be performed, suggesting not to perform it routinely, unless there is the concern or suspicion of childhood absence epilepsy (CAE) or other primary generalized epilepsies that will most likely benefit from HV to confirm diagnosis. 1 , 2 , 3 , 4 , 5 , 6 , 7 , 8 , 10
Based on these facts, we decided to analyze retrospectively all the ambulatory pediatric EEG performed during one year in our specialized ambulatory epilepsy center just before COVID‐19 pandemic situation. Considering the various expert recommendations, we suspended the performance of the hyperventilation maneuver when the pandemic arrived in Chile. Our objective was to characterize patients with positive HV in an epilepsy center before COVID‐19 situation.
2. METHODS
We analyzed all ambulatory pediatric EEGs performed in our outpatient clinic between March 2019 and February 2020, immediately before the arrival of COVID‐19 to our country. The routine 45‐minute recording, sleep‐deprived recording (45 minutes), video‐EEG recording, and prolonged EEG monitoring (24 hours) were included.
We included only patients with confirmed diagnosis of epilepsy and a good effort on HV according to the EEG technician.
Hyperventilation maneuver consisted in 3 minutes with good effort on voluntary HV. If an absence diagnosis was suspected and the first attempt was unsuccessful, laboratory protocol indicated that the procedure should be extended to 5 minutes or repeat 3 minutes more HV at least 10 minutes after the first attempt.
All video‐EEG recordings were done with 21 scalp electrodes placed according to the international 10‐20 system and using 32‐channel EEG.
The effects of HV were classified as: (a) physiological response: normal generalized slowing for the age of the child; (b) negative response: no changes on basal background rhythm; (c) abnormal response: clinical epileptic seizure induction, increase of interictal epileptiform discharges and/ or focal abnormal slowing; (d) not correctly or completely performed: due to no cooperation because of intellectual disability or behavioral problems.
3. RESULTS
A total of 305 EEGs were performed during the selected time frame. Age range was between 5 months and 18 years old. Patients under 4 years were excluded from the analysis, because HV maneuver is not routinely done under this age.
From the 252 EEGs that applied for the study, 135 of them were female patients (F) and 117, male (M). From that same total of 252 EEGs, 194 (77%) were qualified as abnormal because of epileptic activity or abnormal slowing and 58 (23%) as normal.
Not correctly or completely performed maneuver was reported in 102 hyperventilation tests (F: 62 – M: 40). These were children with mental retardation and/or behavior problems that was not possible to get cooperation for a good HV maneuver effort.
Hence, from the 150 EEG with HV procedure effectively done, 13 (8,6%) were positive for abnormalities (Figure 1). Physiological response was found in 54 EEGs (F: 28 – M: 26) and negative response in 83 (F: 38 – M: 45). Operational classification of seizure types, syndromic classification, and etiology were established according to the ILAE proposal to these 150 EEG patients retrospectively (Table 1). 11 , 12
FIGURE 1.
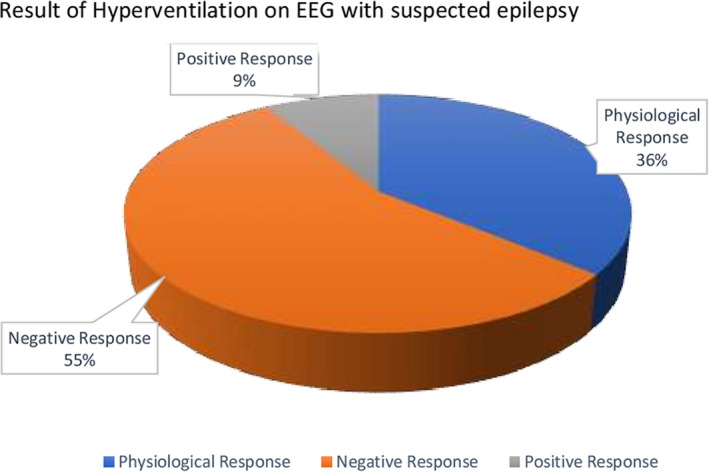
Result of Hyperventilation on EEG with suspected epilepsy
TABLE 1.
Classification of the epilepsies
| Epilepsy | HV result | |||
|---|---|---|---|---|
| Negative | Physiologic | Positive | Total | |
| Not syndromic | ||||
| Focal Seizures | ||||
| Focal Structural | 13 | 11 | 1 | 25 |
| Focal Structural—Genetic | 2 | 0 | 1 | 3 |
| Focal Unknown | 8 | 9 | 0 | 17 |
| Generalized Seizures | ||||
| Generalized Unknown etiology | 1 | 1 | 2 | 4 |
| Syndromic | ||||
| BECTS | 24 | 18 | 1 | 43 |
| CSWS Structural | 9 | 3 | 0 | 12 |
| CSWS Genetic | 4 | 1 | 0 | 5 |
| CSWS unknown etiology | 4 | 0 | 0 | 4 |
| Late‐onset Occipital Epilepsy | 1 | 2 | 0 | 3 |
| Genetic Epilepsy (known mutation) | 6 | 2 | 1 | 9 |
| JME | 10 | 2 | 1 | 13 |
| Generalized Genetic | 1 | 0 | 0 | 1 |
| CAE under Treatment | 0 | 4 | 0 | 4 |
| CAE without Treatment | 0 | 0 | 5 | 5 |
| JAE | 0 | 0 | 1 | 1 |
| Absence under remission | 0 | 1 | 0 | 1 |
| Total | 83 | 54 | 13 | 150 |
Abbreviations: BECTS, epilepsy with centrotemporal spikes; CAE, childhood absence epilepsy; CWS, continuous spikes and waves during slow sleep; JAE, juvenile absence epilepsy; JME, juvenile myoclonic epilepsy.
The analysis of the 13 positive EEGs showed that 4 patients had an increase of basal epileptiform activity, one of them was prolonged EEG monitoring (24 hours). Three patients had their basal focal abnormal slowing increase with the HV maneuver, without preponderance of any specific epilepsy or providing some additional value for its diagnosis (Figure 2). Two of them were prolonged EEG monitoring (24 hours).
FIGURE 2.
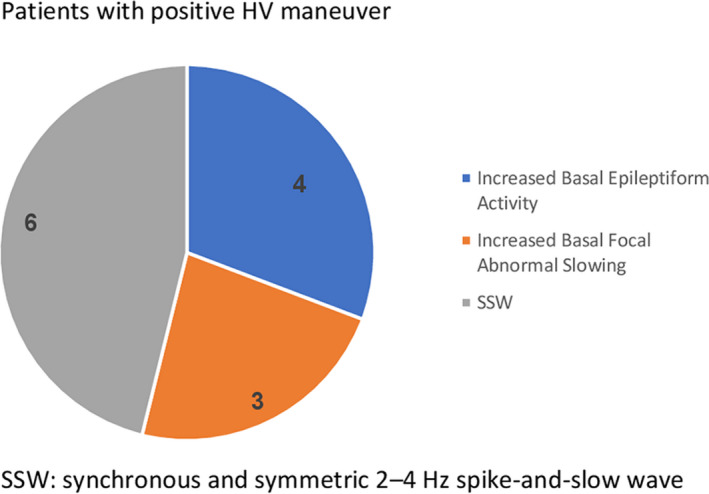
Patients with positive HV maneuver
In the six EEGs left (4%), the hyperventilation maneuver triggered bilaterally synchronous and symmetric 2‐4 Hz spike‐and‐slow wave (SSW) discharges with and without concomitant absences seizures, depending on if they were or not under treatment. None of these patients needed more than 3 minutes to elicit the paroxysmal epileptiform discharge. In this last group of six EEGs, four of them were the patient's first EEG and thus allowed the diagnosis of childhood absence epilepsy. In the remaining 2 patients who presented a SSW pattern, suspicion of absence epilepsy was not specified in the medical report but reported by the technician in the clinical chart. One of them had a history of generalized tonic‐clonic seizures, and the EEG confirmed a juvenile absence epilepsy.
From the 13 positive EEGs: 3 were prolonged EEG monitoring (24 hours) and 10 were sleep‐deprived recording (45 minutes recording).
4. DISCUSSION
Hyperventilation is globally accepted as a mandatory and basic routine maneuver during an EEG procedure. It is described to be effective in generating interictal and ictal epileptiform discharges in patients with epilepsies. Many studies have reported that HV may elicit clinical seizures in as many as one half of patients with generalized epilepsy, particularly in children with typical absences. 7 , 13 , 14 In the last twenty years, evidence has been raised that disputes the value of HV in routine EEG recordings. 15
Clinical guidelines and standardized protocol involve HV trials with a minimum duration of three minutes, which can be extended to five minutes if absence seizures are suspected. 16 , 17
After 3 minutes of HV, paresthesia on fingers and electrolyte modifications on phosphorus, magnesium, potassium, and chlorine can be evidenced. When HV is prolonged for more than 6 minutes, a hypocalcemia can be triggered, with mild tetany symptoms. 18
HV is usually a difficult and uncomfortable procedure for young patients; in fact, we had to exclude 102 patients from this study because the maneuver could not be correctly performed.
Research done by Baldin and colleagues 19 addressed the contribution of activation procedures to the appearance of epileptiform abnormalities on serial EEGs, in a group of 449 patients with newly diagnosed epilepsy. They found HV had a low impact on the activation of related epileptiform abnormalities, even after serial studies being 10.3% for patients aged 1 to 19% versus 5% for patients ≥20 years, at the first EEG. However, the cumulative incidence of epileptiform abnormalities was greater in those with generalized seizures (20.5%) than in focal (5%) or unclassified seizures.
Another interesting brief communication of Raybarman 20 showed that from 275 recorded pediatric patients with generalized epilepsy, in only a small amount of them (11.6%) the HV maneuver induced an increase of interictal epileptiform discharges, and none of them had clinical seizures induced during HV. The value of hyperventilation as an ‘‘activating’’ procedure in routine clinical EEG studies was questioned in this study even in generalized epilepsies.
Other studies have shown that HV has added information in very few patients, Ahdab and Riachi 21 described that only 8 patients out of 1172 did the HV add additional information.
With the COVID‐19 pandemic, the validity of performing HV as a routine procedure became even more questioned. The discussion poses mostly a scenario in which the health professional, family, and patients are using facial masks.
In our study with selected EEGs performed only in children with highly suspected or confirmed epilepsy, the positivity of the maneuver was significantly low, with an incidence of only 8,6% of all HV procedures done. The low positivity of HV may be due to the fact that most of the patients present focal epilepsy or BECTS and a low rate of GGE and because many patients are with antiepileptic treatment. Only in four EEG records (2,6%), the HV induced ictal activity and allowed the diagnosis of generalized epilepsy, specifically CAE. In the remaining 2 patients who presented a 3 Hz SSW pattern, suspicion of absent epilepsy was not specified in the medical order but registered as the presented type of seizure in the technician's clinical chart.
Considering previous studies 19 , 22 , 23 and that this study was developed in a specialized childhood and adolescent epilepsy center, the low positivity and high specificity of the HV maneuver support the idea that this maneuver could be excluded from pediatric routine EEG examinations usually recommended with a length of 20 to 30 minutes. This would also save time to register a better and prolonged non‐REM sleep that has been described and is well known to increase the yield of epileptiform activity in pediatric patients with idiopathic generalized epilepsy and focal epilepsy. 19 , 23
The role of the EEG technician is fundamental to add the maneuver in case CAE is suspected from the medical history and has not been requested in the medical order.
On the other hand, even though the number of positive patients was small in our sample, all patients presented paroxysmal alteration before 3 minutes. This finding has also been confirmed by other authors 19 , 22 , 23 endorsing the fact that 3 minutes long HV maneuvers should be enough and a sufficient tool. This would also help to diminish the already stressful situation that an EEG already means for the child and the risk of metabolic alkalosis symptoms.
We cannot yet assess the impact of the pandemic on the diagnosis of epilepsy, because it is still ongoing and because the number of EEGs performed was reduced by up to 70% during the first months. 9 Hyperventilation should never be done with mask and we think should be reserved, in this pandemic period, only for CAE.
Based on other studies 24 and our findings that support low positivity and high specificity of the HV maneuver for CAE, we propose that even though our sample is small and larger studies are needed, it would be reasonable to review and update ambulatory EEG pediatric guidelines, 25 changing hyperventilation procedure from a routine activation to a complementary maneuver that could be performed only if absence epilepsy is suspected, with a maximum length of 3 minutes and an additional second 3 minutes trial at least 10 minutes after the first one. We highlight that in the 5 previously untreated patients with CAE, the maneuver was positive in all of them.
DISCLOSURE
Neither of the authors has any conflict of interest to disclose.
Ríos‐Pohl L, Macarena F, Magdalena G. Hyperventilation maneuver during EEG in children with epilepsy after the COVID‐19 pandemic. Is a routine procedure necessary?. Epilepsia Open. 2021;6:437–442. 10.1002/epi4.12493
We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines
REFERENCES
- 1.American Clinical Neurophysiology Society: August 05, 2005: 02‐Guideline 2: Minimal technical standards for pediatric EEG.
- 2. Gibbs FA, El G, Lennox WG. Electroencephalographic response to overventilation and its relation to age. J Pediatr. 1943;23:497–505. [Google Scholar]
- 3. Takahashi T. Activation methods. In: Niedermeyer E, Lopes da Silva FH eds. Electroencephalography. Philadelphia: Lippincott Williams and Wilkins. 2005:281–303. [Google Scholar]
- 4. Guaranha MSB, Garzon E, Buchpiguel CA, Tazima S, Yacubian EMT, Sakamoto AC. Hyperventilation revisited: physiological effects and efficacy on focal seizure activation in the era of video‐EEG Monitoring. Epilepsia. 2005;46(1):69–75. [DOI] [PubMed] [Google Scholar]
- 5. Arain A, Arbogast PG, Abou‐Khalil BW. Utility of daily supervised hyperventilation during long‐term video‐EEG monitoring. J Clin Neurophysiol. 2009;26(1):17–20. [DOI] [PubMed] [Google Scholar]
- 6. Hyperventilationsepilepsie FO. Zbl.ges. Neurol Psychiat. 1924;38:289–93. [Google Scholar]
- 7. Nims LF, Gibbs EL, Lennox WG. Adjustment of Acid –Base balance of patients with petit mal epilepsy to overventilation. Arch Neurol Psychiatry. 1940;43:262–9. [Google Scholar]
- 8. Fisch B, So E. Activation methods. In: Ebersole J, Pedley T, eds. Current Practice of Clinical Electroencephalography. Philadelphia: Lippincott Williams and Wilkins; 2003. p. 246–70. [Google Scholar]
- 9. Assenza G, Lanzone J, Ricci L, Boscarino M, Tombini M, Galimberti CA, et al Electroencephalography at the time of Covid‐19 pandemic in Italy. Neurol Sci. 2020;41:1999–2004. 10.1007/s10072-020-04546-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.The British society for clinical neurophysiology (formerly the EEG society).https://www.bscn.org.uk/data/files/Guidelines/Covid‐19%20‐%20Policy%20for%20Hyperventilation%20during%20EEG.pdf.
- 11. Fisher RS, Cross JH, French JA, Higurashi N, Hirsch E, Jansen FE, et al Operational classification of seizure types by the international league against epilepsy: position paper of the ILAE commission for classification and terminology. Epilepsia. 2017;58(4):522–30. 10.1111/epi.13670 [DOI] [PubMed] [Google Scholar]
- 12. Berg AT, Berkovic SF, Brodie MJ, Buchhalter J, Cross JH, van Emde BW , et al. Revised terminology and concepts for organization of seizures and epilepsies: report of the ILAE Commission on Classification and Terminology, 2005‐2009. Epilepsia. 2010;51(4):676‐85. 10.1111/j.1528-1167.2010.02522.x. [DOI] [PubMed] [Google Scholar]
- 13. Wirrell EC, Camfield PR, Gordon KE, Camfield CS, Dooley JM, Hanna BD. Will a critical level of hyperventilation‐induced hypocapnia always induce an absence seizure? Epilepsia. 1996;37(5):459–62. [DOI] [PubMed] [Google Scholar]
- 14. Miley C, Forster F. Activation of complex partial seizures by hyperventilation. Arch Neurol. 1977;34(6):371–3. [DOI] [PubMed] [Google Scholar]
- 15. Holmes MD, Dewaraja AS, Vanhatalo S. Does Hyperventilation elicit epileptic Seizures? Epilepsia. 2004;45(6):618–20. [DOI] [PubMed] [Google Scholar]
- 16. ANTA Inc . Non Routine EEG Guideline, 2016. https://www.anta.org.au/wp‐content/uploads/2018/10/ANTA‐Inc‐EEG‐Guideline‐v2‐2016.pdf
- 17. Sinha SR, Lucy S. American Clinical Neurophysiology society guideline 1: minimum technical requirements for performing clinical electroencephalography. J Clin Neurophysiol. 2016;33:303–7. [DOI] [PubMed] [Google Scholar]
- 18. Macefield G, Burke D. Paraesthesiae and tetany induced by voluntary hyperventilation. Increased excitability of human cutaneous and motor axons. Brain. 1991;114(Pt 1B):527‐40. [DOI] [PubMed] [Google Scholar]
- 19. Elisa B, Hauser WA, Buchhalter JR, Hesdorffer DC, Ottman R. Utility of EEG activation procedures in epilepsy: a population‐based study. J Clin Neurophysiol. 2017;34(6):512–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Raybarman C. Is hyperventilation an effective "activating" procedure in routine clinical EEG studies in children. J Child Neurol. 2009;24(10):1294–5. [DOI] [PubMed] [Google Scholar]
- 21. Ahdab R, Riachi N. Reexamining the added value of intermittent photic stimulation and hyperventilation in routine EEG Practice. Eur Neurol. 2014;71(1‐2):93–8. 10.1159/000353650. Epub 2013 Dec 10. PMID: 24335163 [DOI] [PubMed] [Google Scholar]
- 22. Watemberg N, Farkash M. Hyperventilation during routine electroencephalography: are three minutes really necessary? Pediatr Neurol. 2015;52(4):410–3. [DOI] [PubMed] [Google Scholar]
- 23. Klein KM, Susanne K, Hamer HM, Ziegler A, Oertel WH, Rosenow F, et al Sleep but not hyperventilation increases the sensitivity of the EEG in patients with temporal lobe epilepsy. Epilepsy Res. 2003;56:43‐9. [DOI] [PubMed] [Google Scholar]
- 24. Dennis Dlugos MD, Shlomo Shinnar MD, Cnaan A, Dlugos D, Conry J, Hirtz DG, et al Pretreatment EEG in childhood absence epilepsy. Neurology. 2013. ;81(2):150‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Stadler G, Steurer J, Dür P, Binswanger U, Vetter W. Electrolyte changes during and after voluntary hyperventilation. Praxis (Bern 1994). 1995;84(12):328‐34. [PubMed] [Google Scholar]


