Abstract
This paper addresses the absence of an international diagnostic taxonomy for cognitive disorders in patients with epilepsy. Initiated through the 2020 Memorandum of Understanding between the International League Against Epilepsy and the International Neuropsychological Society, neuropsychological representatives from both organizations met to address the problem and consequences of the absence of an international diagnostic taxonomy for cognitive disorders in epilepsy, overview potential solutions, and propose specific solutions going forward. The group concluded that a classification of cognitive disorders in epilepsy, including an overall taxonomy and associated operational criteria, was clearly lacking and sorely needed. This paper reviews the advantages and shortcomings of four existing cognitive diagnostic approaches, including taxonomies derived from the US National Neuropsychology Network, DSM‐V Neurocognitive Disorders, the Mild Cognitive Impairment classification from the aging/preclinical dementia literature, and the Research Domain Criteria Initiative. We propose a framework to develop a consensus‐based classification system for cognitive disorders in epilepsy that will be international in scope and be applicable for clinical practice and research globally and introduce the International Classification of Cognitive Disorders in Epilepsy (IC‐CODE) project.
Keywords: cognitive diagnosis, IC‐Code, neuropsychology
Key Points.
No consensus‐based taxonomy exists for the diagnosis of cognitive disorders in adults with epilepsy
Through the 2020 Memorandum of Understanding between the ILAE and INS, efforts are underway to develop an internationally applicable taxonomy of cognitive disorders in epilepsy
This initiative is described including overall goals, pertinent processes and action steps, and deliverables to the international community
1. INTRODUCTION
Cognitive impairment is a major comorbidity of the epilepsies representing a worldwide concern that is recognized by the International League Against Epilepsy (ILAE) and the International Neuropsychological Society (INS). In February 2020, leaders from ILAE and INS signed and adopted a Memorandum of Understanding (MOU) designed to facilitate and advance epilepsy‐specific clinical, educational, and research efforts on a global scale. The first collaborative initiative emanating from the MOU addresses the taxonomy of neuropsychological impairment in adults with epilepsy.
On 9 February 2020, representatives from the ILAE and INS met to begin a process designed to determine the feasibility of defining a neuropsychological classification of cognitive disorders with associated operational criteria that would be applicable internationally, providing the foundation for a taxonomy of cognitive disorders in adults with epilepsy that would influence global neuropsychological research and clinical practice. Reviewed at that meeting were the overall mission, structure and activities of the ILAE Neuropsychology Task Force (Sallie Baxendale, UK) and the INS International Liaison Committee (Alberto Fernandez, Argentina), clinical assessment practices in contemporary neuropsychology (William Barr, US), core competences in international neuropsychology (Erik Hessen, Norway), cognitive diagnostic outcomes in aging research (Carrie McDonald, US), cognitive diagnostic outcomes in DSM‐V and ICD‐10 (Bruce Hermann, US), and an overview of the National Neuropsychology Network project in the US aimed at evaluating neuropsychological contributions diagnostic outcomes (David Loring, US). Additional participants and discussants included Sarah Wilson (Australia, Chair of ILAE Diagnostic Methods Commission), Cady Block (US), Robyn Busch (US), and Marc Norman (US, Executive Director of INS). Based on that meeting, this report (a) summarizes the need for this initiative, (b) overviews possible approaches, and (c) establishes recommendations for moving forward.
2. THE PROBLEM: LACK OF A CONSENSUS‐BASED TAXONOMY OF COGNITIVE DIAGNOSES IN EPILEPSY
2.1. Taxonomies
A taxonomy is a scheme well used in science to classify things, such as organisms or concepts, with the principles that underlie the classification forming part of the science. Sophisticated taxometric methods have been developed to determine whether variations in a particular human trait or ability are best understood as differences of degree (dimensional taxonomy) or of kind (categorical taxonomy). For instance, in mental health research dimensional features have been recently incorporated into the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM‐5) 1 classification following significant challenges to categorical models of psychopathology. 2 Whether a disorder is better characterized along a spectrum or in discrete categories has significant implications for assessment and diagnosis, clinical predictions of outcome, and causal explanations such as being multifactorial or due to specific etiology. In other words, for problems of international significance, taxonomies are essential and provide a framework for advancing research and clinical practice, with issues of categorical and dimensional categorization requiring consideration.
2.2. Taxonomies in epilepsy
Through the ILAE, the 2017 Classification of the Epilepsies by the Commission for Classification and Terminology 3 represents the latest advance in a long evolving taxonomic system. 4 , 5 This has provided the international clinical and research communities with a “common language”, a basis for organization of knowledge, and a framework for diagnosis, clinical care, and research. Other epilepsy‐specific taxonomies have been developed within the ILAE, examples being the operational criteria for classifying and diagnosing major epilepsy‐related neuropathologies, including hippocampal sclerosis 6 and focal cortical dysplasias 7 that are now used in international clinical practice and research. 8 , 9 ILAE‐based consensus criteria for the diagnosis of psychogenic nonepileptic seizures have been advanced 10 while, in other instances, existing taxonomies have been imported from other fields into epilepsy care and research, best exemplified by the application of the DSM for the characterization of psychiatric diagnoses. 11 , 12 Notwithstanding criticisms of the DSM system, it has facilitated understanding of the psychiatric complications of the epilepsies compared with the antecedent use of a myriad of behavioral measures and rating scales, inconsistent nomenclature, and competing views. 13 , 14
2.3. Neuropsychological taxonomies in epilepsy
Unlike neurology, neuropathology, and psychiatry, neuropsychology has yet to develop a consensus taxonomy of cognitive disorders in epilepsy, which is a somewhat startling state of affairs. Furthermore, neuropsychology has yet to reach international consensus regarding “gold standard” tests and their most useful metrics as well as consensus‐based definitions of abnormality. This has been a chronic state of affairs that several groups, including prior ILAE Neuropsychology Task Force teams, have addressed through surveys of national and international test selection and utilization practices in the field of epilepsy, with an eye toward standardizing administered test batteries. 15 , 16 , 17 , 18 These goals continue to remain aspirational and are complicated by a number of factors including their application to patients from diverse cultures and languages, underdevelopment of the field of neuropsychology in regions of the world, lags in the incorporation of digital technology, and variability in the availability of specific tests and appropriate norms. Contemporary efforts to standardize test and assessment practices with recommended clinical practice guidelines within and across national boundaries continue, 19 , 20 albeit with persisting variable recommendations.
To begin to address these limitations and needs, the current ILAE Neuropsychology Task Force recently published guidelines for the general content and use of neuropsychological evaluations in clinical and surgical settings. 21 , 22 While these guidelines set out the general principles for the role of neuropsychology in the assessment and treatment of people with epilepsy in both routine and surgical settings, they stopped short of prescribing specific tests or advising on test interpretation for the reasons described above. However, the imperative for clear operational criteria to characterize cognitive impairment in epilepsy remains strong, particularly as we move into the era of big data and clinical/research collaborations that span geographical and cultural boundaries. Big data and international collaborations are priorities for the ILAE. If neuropsychology is to keep pace with other diagnostic methods in epilepsy, the development of universally applicable operational criteria that instantiate a taxonomy of cognitive impairment must now be a priority for the field.
Therefore, the fundamental thesis reflected in this ILAE‐INS initiative is that a new direction in neuropsychology is needed. Required is a taxonomy of cognitive diagnoses informed by operational criteria, which if appropriately structured and detailed would guide clinical diagnostics internationally while permitting flexibility in clinical practice and research. The emphasis here is on cognitive diagnostics, not specific test prescription.
3. THE CONSEQUENCES: IMPLICATIONS OF DIAGNOSTIC IMPRECISION
The current state of affairs has implications for epilepsy research and care. To give but one example, in the current “test‐driven model” from an administered comprehensive test battery, a specific cognitive construct may be of interest (eg, memory) that is related to other variables of interest (eg, a clinical characteristic such as age of onset, or imaging metric). The implicit assumption is that the presence, nature, and even combination of abnormalities in other cognitive domains (language, executive function, processing speed, perception) are of little import on identified associations of memory with imaging or clinical variables of interest—an assumption now shown to be incorrect. 23 Clinically, there is a lack of, or at best imprecision, in cognitive diagnoses that are applied to epilepsy patients across the world. In some ways, this resembles the state of affairs regarding epilepsy classification prior to the initiation of the ILAE’s first international classification in the 1960s. 5
4. POTENTIAL SOLUTIONS: EXISTING COGNITIVE TAXONOMIES
At a minimum, there are four potential contemporary approaches to cognitive diagnostics. See Table 1.
TABLE 1.
Contemporary approaches to cognitive diagnostics: Key characteristics
| Model | Cognitive domains specified? | Operational criteria for impairment defined? | Beta tested in epilepsy? | Incorporates clinical judgment into diagnosis? | Incorporates information regarding everyday functioning? | Classification of symptoms (diagnostic system) |
|---|---|---|---|---|---|---|
| DSM‐5 | Y | Y | N | Y | Y | Categorical |
| Cognitive Aging/MCI | Y | Y | Y | Y | Y | Categorical |
| NNN | Y | Y | N a | Y | Y | Hybrid b |
| RDoC | Y | N | N | N c | N | Dimensional |
Plans in place for testing with epilepsy patients.
Elements of both categorical and dimensional.
Preferentially based on biological basis of disorder.
4.1. DSM‐5
According to DSM‐5, the primary diagnostic divisions of neuropsychological impairment are Mild and Major Neurocognitive Disorders that, by themselves, do not speak to the specific nature of the detected cognitive anomalies (eg, memory, language, executive function, mixed) nor their course over time. 24 Mild and Major (dementia) Neurocognitive Disorders have variable meaning for clinical colleagues, and the use of these diagnostic terms has not entered into the epilepsy research literature in a meaningful way. In this context, it is important to note that the official publication of the DSM‐5 Working Group for Neurocognitive Disorders 25 described specific cognitive domains for assessment and potential operational criteria for cognitive diagnoses (Figure 1).
FIGURE 1.
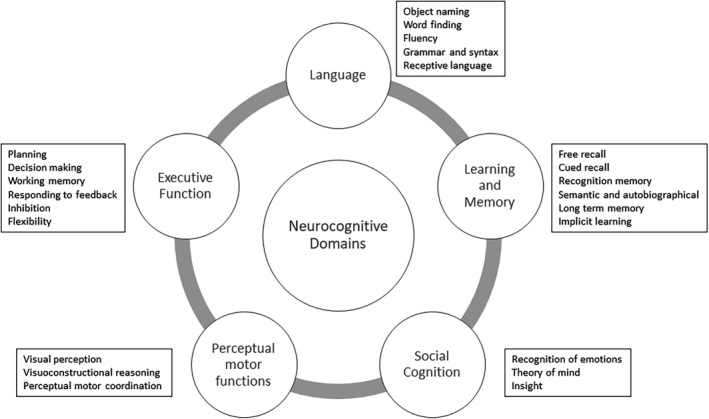
DSM‐5 defined key domains of cognitive function and their subdomains
They stopped short of recommending this system for clinical practice, but it is detailed and to a significant degree based on developments in the field of aging that has arguably served as a leader for cognitive diagnostics. To date, we are not aware of any systematic attempts to apply the DSM‐5 system to epilepsy and, in fact, epilepsy was omitted as one of the potential etiologies of cognitive impairment despite it being one of the most common neurological disorders. 26 Nonetheless, the potential applicability of this taxonomy to epilepsy can be appreciated but remains to be tested empirically.
4.2. Cognitive diagnostics in aging
Research in the areas of cognitive aging, dementia, and preclinical dementia is pertinent. In part to detect the earliest signs of a progressive neurodegenerative process, the diagnosis of mild cognitive impairment (MCI) was established and refined over decades in national and international initiatives. 27 , 28 , 29 , 30
As shown in Figure 2, MCI encompasses several distinct diagnostic phenotypes (single domain amnestic or nonamnestic [language, visuospatial, dysexecutive] or multidomain amnestic or nonamnestic MCI). The original classifications included clear operational criteria, that is performance −1.5 SD below the mean on at least one cognitive measure and with otherwise intact functional status. 28 , 29 However, alternative thresholds 31 , 32 , 33 and more comprehensive criteria 34 have been proposed, the latter of which will be described in detail below. In addition to serving as a taxonomy of cognitive abnormality, its operational definitions implicitly force assessment of specific cognitive domains, in a sense driving clinical practice by the intrinsic diagnostic algorithms. This approach has had enormous impact and has served to identify cognitive phenotypes internationally that have filtered to specialty (neurology, geriatrics) and general medicine memory clinics, 35 as well as unifying the language and taxonomy used by medical, neuropsychological, and other healthcare providers. The operational criteria are such that while the exact test may not be identical across centers, countries and languages, the type of procedure (eg, verbal list learning, retention after 30‐minute delay) can be specified and nomenclature and taxonomy achieved across international borders, thereby advancing clinical care and research on a global scale. In addition, there is now updated nomenclature and operational criteria for characterization of phenotypes of progressive cognitive course, namely cognitively impaired—progressive, cognitively impaired—stable, and cognitively intact—declining. 36
FIGURE 2.
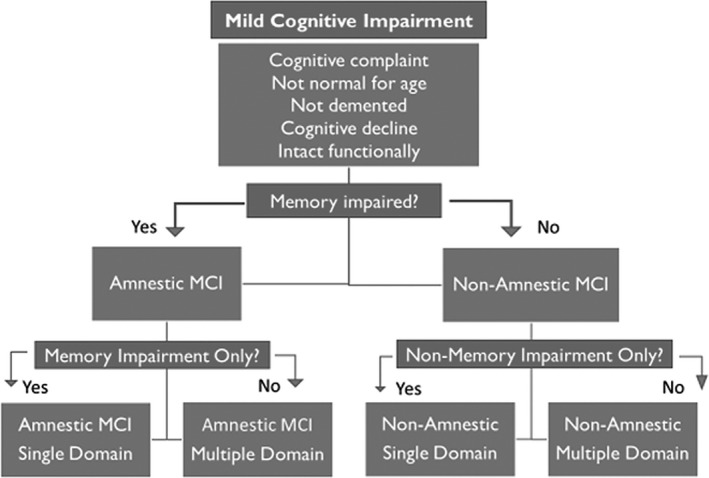
Contemporary MCI classification
However, problems in this system have been identified that impact diagnostic stability, calling predictive accuracy into question. 37 , 38 , 39 , 40 In particular, both in clinical trials and memory clinics, conventional diagnostic criteria for MCI are often based on impairment on a single test measure (typically of memory), subjective cognitive complaints, and clinical judgment of mild impairment based on a semi‐structured clinical interview. 41 Contemporary research has shown that this approach is quite vulnerable to false‐positive diagnostic errors 37 , 42 as many patients given an MCI diagnosis demonstrate intact performance on more extensive neuropsychological evaluation, harbor few AD biomarkers 37 with normal cortical thickness profiles, 38 and show low rates of progression to AD. 40 Furthermore, patients in this “false‐positive” group tend to over‐report subjective cognitive complaints, 43 likely contributing to their MCI diagnosis, but they remain cognitively normal and functionally independent over time.
A second limitation of the conventional MCI classification system is that it may combine patients with very different cognitive profiles. Recent studies using comprehensive neuropsychological criteria (ie, Jak/Bondi criteria) 34 have identified considerable heterogeneity beyond the amnestic/nonamnestic distinction with respect to neuropsychological performance, identifying amnestic, dysnomic, and dysexecutive/mixed MCI profiles as well as a sizable group of patients who are cognitively normal (ie, the aforementioned “false‐positive” group). 42 This cognitive phenotyping in MCI using comprehensive criteria (ie, typically impairment of greater than −1.0 SDs below the normative mean on at least two tests per cognitive domain; no subjective ratings of impairment) has led to the emergence of MCI subtypes with unique AD biomarkers, imaging profiles, 38 , 44 and better risk stratification of patients for progression to AD than conventional criteria. 40
Despite the increasing popularity of this comprehensive neuropsychological approach for cognitive subtyping in MCI, a similar method has only recently been applied to patients with epilepsy. 23 , 45 , 46 Investigating patients with temporal lobe epilepsy, this diagnostic approach yielded distinct groups of patients (memory‐impaired, language‐impaired, multidomain, cognitively normal) with unique clinical, cognitive, and neuroimaging profiles. 23 More generally, there have been a modest number of empirical attempts to identify underlying latent cognitive groups in patients with adult epilepsies that have revealed associated neuroimaging correlates and varying prospective cognitive trajectories associated with the identified groups. 23 , 47 , 48 Moreover, cognitive phenotyping in elders with epilepsy is now underway 49 which may shed additional light on the intersection between epilepsy, aging, and MCI, including identification of subsets of older adults with epilepsy who are at risk for progression to dementia. However, whether cognitive phenotyping in epilepsy is clinically feasible at most centers (ie, it requires at least two tests per neuropsychological domain) and whether this approach can predict patient outcomes within or across epilepsy syndromes remains to be established.
4.3. National Neuropsychology Network
In general clinical practice, neuropsychologists are often tasked with establishing whether cognitive decline is present and exceeds that expected with normal aging and, if so, whether the pattern is consistent with a specific underlying etiology. As previously described, DSM‐5 nomenclature characterizes patients based upon their pattern of cognitive performance: No Neurocognitive Disorder (No NCD), Mild NCD, or Major NCD. MCI cognitive phenotyping provides a means of capturing the complexity of mild presentations. In a large multi‐center study funded by the US National Institute of Mental Health (National Neuropsychology Network, NNN), the DSM‐5 classification is being applied to all patients regardless of their medical or psychiatric diagnoses, not simply patients referred due to age‐related cognitive or memory concerns. Characterization of cognitive patterns for all diagnostic referrals will further our understanding of cognitive profiles and change in non‐neurodegenerative conditions and elucidate the relations of psychopathology to mild NCD. There is also limited understanding of the relationships between neuropsychological classification, levels of independent activities of daily living (iADL), and disease comorbidities including mood and anxiety disorders to cognitive performance across domains. Data will be analyzed by including estimated premorbid level of function, neuropsychological performance across cognitive domains, psychopathology ratings from selective NIH Patient‐Reported Outcomes Measure Information System (PROMIS) measures, and DSM‐5 classification. Importantly, the level of everyday functioning is being systematically evaluated using the WHODAS 2.0 and Neuro‐QOL. Thus, the NNN will characterize how quantitative neurocognitive evidence, evidence of disruption in instrumental activities of daily living, and evidence of noncognitive psychopathology (particularly mood and anxiety symptoms) all contribute to the ultimate diagnosis of Mild and Major NCD. While initially relying primarily on DSM‐5 diagnostic classification of neurocognitive function, the long‐term strategy is agnostic about the validity of different diagnostic taxonomies and will evaluate alternative systems for dimensional or categorical representation after sufficient clinical samples have been obtained.
4.4. Research Domain Criteria Initiative (RDoc)
The RDoc is a research framework for integrating multiple levels of information in the investigation of mental disorders. It is expressly not a diagnostic guide nor is it intended to replace diagnostic systems, but as a framework, it is designed to aid the understanding of mental health and illness in the wider context of psychological and biological systems. As a research tool primarily designed to examine mental health, the RDoc framework cannot be directly applied to the clinical diagnosis of cognitive dysfunction in epilepsy. However, the overall model is attractive, particularly with respect to the emphasis on specific cognitive constructs and subconstructs, and some parallels with contemporary models of cognitive function can be drawn. In the creation of a clinical taxonomy, it may be helpful to consider incorporation of elements of the RDoc framework to facilitate subsequent research. (See Table 1).
5. THE FUTURE: NEXT STEPS
Classification of cognitive disorders in epilepsy, including an overall taxonomy and associated operational criteria, is clearly lacking and sorely needed. Possible remedies have been briefly overviewed, and the question of how best to move forward is key. Several issues relate to this endeavor.
5.1. Leveraging international resources: Big data approaches
The ILAE and INS have independent international resources that can be marshaled to facilitate and support the introduction, education, training, and evaluation of a cognitive diagnostic system. In addition, worldwide neuroimaging and genetic resources are now available to participate in this endeavor, enabling large‐scale analysis of well‐harmonized data across many disease states, including epilepsy. 50 The Enhancing NeuroImaging and Genetics through Meta‐Analysis‐Epilepsy (ENIGMA‐Epilepsy) is one example, having collated and analyzed structural MRI 51 and diffusion MRI data 52 on over 2149 patients with common epilepsy syndromes and 1727 healthy controls across 26 centers worldwide. Treatment outcomes and other phenotypic data are now being aggregated and analyzed, and studies are underway to link imaging features to gene expression data in efforts to reveal the underlying biology of regional atrophy in epilepsy. 53 However, cognitive data are not yet included in ENIGMA‐Epilepsy, as efforts to standardize and harmonize cognitive data worldwide have not been established. This is a high priority for ENIGMA‐Epilepsy and other worldwide collaborations and presents a tremendous opportunity for the neuropsychological community. Harmonizing rich datasets would enable the presence of neuropsychology in large consortium efforts and help to reveal neurobiological correlates of cognitive phenotypes in epilepsy that are invariant to first language or geographic location.
5.2. Application
The potential applications of a cognitive taxonomy are many. Comparison of the presence, rate, and distribution of single (eg, memory, executive function) and multiple (memory plus executive function) cognitive diagnoses would inform the shared and syndrome‐specific patterns of abnormality, their natural history, and the impact of treatments on that course, as well as their relationship to common clinical, sociodemographic, psychiatric, and neurobiological variables. A common taxonomy and operational criteria should in theory facilitate international neuropsychological collaborations in this and other ways not possible at present.
5.3. Clinician burden
If a diagnostic system can be developed, would it be a burden to clinical practice? One concern with the actuarial system is the need for two impaired tests within a given domain. This requires an increase in testing compared with single impairment and would increase assessment time. A comprehensive method could be used as the first step to define the phenotypes/diagnostic categories, this being the “optimal” approach. It could then be determined whether a screening process, which might optimally involve digital assessment methodology, could be developed that yields approximately the same groups. The impact of varying operational criteria would also need to be addressed (ie, does >1.0 SD on two measures vs >1.5 SD on one measure or two indices from the same measure yield the same categories).
5.4. Core competencies in international neuropsychology
Another concern is the degree to which such a system could be practically implemented, and whether international neuropsychology is ready to implement it. Relevant here is that careful characterizations of the status of the field across the globe are available. 54 Hessen et al 55 conducted a systematic audit of training and competence in international neuropsychology programs. While varying significantly in content and type, it was concluded that international competence would allow implementation of sophisticated advances such as the type proposed here.
5.5. Process versus tests
A broader consideration for the future is the degree to which neuropsychology begins to focus on cognitive processes rather than the tests per se. By way of example, processes such as arbitrary relational learning and rapid forgetting are those we might expect to see impaired for a given diagnostic classification. There are a range of “test types” or procedures designed to measure these cognitive processes with varying degrees of sensitivity and specificity. By necessity, these procedures must take contextual factors such as culture and language into account to provide a nuanced or “locally informed” assessment of the underlying cognitive process. The aim, then, is to select the most culturally sensitive and specific measures for a given individual to ensure precise phenotyping of the cognitive processes of interest. For instance, in an indigenous population arguably verbal list learning may be a less sensitive measure of arbitrary relational learning than a virtual arbitrary object‐environment location task, as the former is a strongly “Western‐based” educational activity. This way of thinking would move neuropsychological practice away from blindly administering a “one‐size‐fits‐all assessment battery” under the guise that it is psychometrically more robust, as it may not sensitively capture the cognitive process of interest in an individual who falls outside the cultural and linguistic norms of the battery.
Focusing on cognitive processes in a taxonomy of cognitive diagnoses in epilepsy would also facilitate better integration of new discoveries in fields such as cognitive neuroscience, where the imaging and assessment of particular cognitive processes is becoming increasingly sophisticated. For instance, the Cognitive Control Network is now well established to underpin working memory function, including the sensitivity of the n‐back task to assessing this network function. Yet in clinical practice there remains slavish adherence to traditional measures such as Digit Span as the “best” measure of working memory function. But is this justified and would it be better to adopt the n‐back in clinical practice, knowing that it is specifically assessing the function of a well characterized frontoparietal network? A taxonomy built on the alignment of genetic, neuroimaging, and cognitive processes promises high precision for the diagnosis of cognitive disorders in epilepsy and their treatment.
6. FORMAL PROPOSAL FOR THE INTERNATIONAL CLASSIFICATION OF COGNITIVE DISORDERS IN EPILEPSY (IC‐CODE)
Despite multiple attempts to remediate the problem, there remains significant heterogeneity in clinical neuropsychological practice for epilepsy throughout the world. Most inexplicable is the complete absence of a common cognitive diagnostic system that would enhance and advance understanding, communication, and clinical and research efforts in the way that other epilepsy‐based classification systems have advanced the understanding of the epilepsies, their underlying neuropathologies, and psychiatric comorbidities among our collaborating disciplines. The new Memorandum of Understanding between the ILAE and INS is put in place to facilitate international efforts such as these and to advance such a system for neuropsychology in particular.
Given the cognitive diagnostic system devised within DSM‐V (Figure 1) and a taxonomy informed by years of careful cognitive diagnostic research in the field of aging along with preliminary work suggesting its applicability to epilepsy, we propose to (a) adopt this overall classification as a useful starting point as is or modified by expert opinion using the DELPHI method, (b) determine the operational criteria for each diagnosis focusing on cognitive processes (eg, cut points reflecting abnormality relative to demographically adjusted norms [−1 or −1.5 SD), (c) test the applicability of the taxonomy in large datasets to compare strengths and weakness of various approaches (eg, single vs multiple test definitions) with an eye to insuring international applicability through use of unique resources (eg, ENIGMA‐Epilepsy), and (d) provide preliminary information regarding their relation to important clinical and research outcomes. In this process, also considered will be the impact and appropriate consideration of psychological comorbidities (eg, depression, anxiety) as well as subjective memory complaints, and applicability across diverse epilepsy syndromes. While this initiative is focused on adults with epilepsy, a similar initiative dedicated to children with epilepsy should also be considered. Critically, this will represent an ongoing international effort forged through the cooperative efforts of the ILAE and INS under the auspices of their 2020 Memorandum of Understanding.
CONFLICT OF INTEREST
This report was written by experts selected by the International League Against Epilepsy (ILAE) and was approved for publication by the ILAE. Opinions expressed by the authors, however, do not necessarily represent the policy or position of the ILAE. Similarly, the content does not necessarily represent the policy or position of the INS. All authors are members of the ILAE‐ INS working group for this project and are members of both organizations. No authors have any conflicts of interests to declare with respect to this manuscript. None of the authors have any conflict of interest to disclose. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
ACKNOWLEDGMENTS
The authors are grateful for the support of the International League Against Epilepsy and The International Neuropsychological Society for this project.
Norman M, Wilson SJ, Baxendale S, et al. Addressing neuropsychological diagnostics in adults with epilepsy: Introducing the International Classification of Cognitive Disorders in Epilepsy: The IC CODE Initiative. Epilepsia Open. 2021;6:266–275. 10.1002/epi4.12478
REFERENCES
- 1. American Psychiatric Association . Diagnostic and statistical manual of mental disorders (DSM–5). Washington, DC: APA Press; 2013. [Google Scholar]
- 2. Haslam N, McGrath MJ, Viechtbauer W, Kuppens P. Dimensions over categories: a meta‐analysis of taxometric research. Psychol Med. 2020;50(9):1418–32. [DOI] [PubMed] [Google Scholar]
- 3. Scheffer IE, Berkovic S, Capovilla G, Connolly MB, French J, Guilhoto L, et al. ILAE classification of the epilepsies: position paper of the ILAE commission for classification and terminology. Epilepsia. 2017;58:512–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gastaut H. Clinical and electroencephalographical classification of epileptic seizures. Epilepsia. 1970;11:102–13. [DOI] [PubMed] [Google Scholar]
- 5. Wolf P. History of epilepsy: nosological concepts and classification. Epileptic Disord. 2014;16:261–9. [DOI] [PubMed] [Google Scholar]
- 6. Blümcke I, Thom M, Aronica E, Armstrong DD, Bartolomei F, Bernasconi A, et al. International consensus classification of hippocampal sclerosis in temporal lobe epilepsy: a Task Force report from the ILAE Commission on Diagnostic Methods. Epilepsia. 2013;54:1315–29. [DOI] [PubMed] [Google Scholar]
- 7. Blümcke I, Thom M, Aronica E, Armstrong DD, Vinters HV, Palmini A, et al. The clinicopathologic spectrum of focal cortical dysplasias: a consensus classification proposed by an ad hoc Task Force of the ILAE Diagnostic Methods Commission. Epilepsia. 2011;52:158–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Coras R, Pauli E, Li J, Schwarz M, Rössler K, Buchfelder M, et al. Differential influence of hippocampal subfields to memory formation: insights from patients with temporal lobe epilepsy. Brain. 2014;137:1945–57. [DOI] [PubMed] [Google Scholar]
- 9. Witt J‐A, Coras R, Schramm J, Becker AJ, Elger CE, Blümcke I, et al. Relevance of hippocampal integrity for memory outcome after surgical treatment of mesial temporal lobe epilepsy. J Neurol. 2015;262:2214–24. [DOI] [PubMed] [Google Scholar]
- 10. LaFrance WC Jr, Baker GA, Duncan R, Goldstein LH, Reuber M. Minimum requirements for the diagnosis of psychogenic nonepileptic seizures: a staged approach: a report from the International League Against Epilepsy Nonepileptic Seizures Task Force. Epilepsia. 2013;54:2005–18. [DOI] [PubMed] [Google Scholar]
- 11. Rai D, Kerr MP, McManus S, Jordanova V, Lewis G, Brugha TS. Epilepsy and psychiatric comorbidity: a Nationally Representative Population‐based Study. Epilepsia. 2012;53:1095–103. [DOI] [PubMed] [Google Scholar]
- 12. Kessler RC, Lane MC, Shahly V, Stang PE. Accounting for comorbidity in assessing the burden of epilepsy among US adults: results from the National Comorbidity Survey Replication (NCS‐R). Mol Psychiatry. 2012;17:748–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jones R, Rickards H, Cavanna AE. The prevalence of psychiatric disorders in epilepsy: a critical review of the evidence. Funct Neurol. 2010;25:191–4. [PubMed] [Google Scholar]
- 14. Devinsky O, Vazquez B. Behavioral changes associated with epilepsy. Neuro Clin. 1993;11:127–49. [PubMed] [Google Scholar]
- 15. Jones‐Gotman M, Smith ML, Risse GL, Westerveld M, Swanson SJ, Giovagnoli AR, et al. The contribution of neuropsychology to diagnostic assessment in epilepsy. Epilepsy Behav. 2010;18:3–12. [DOI] [PubMed] [Google Scholar]
- 16. Rabin LA, Barr WB, Burton LA. Assessment practices of clinical neuropsychologists in the United States and Canada: a survey of INS, NAN, and APA division 40 members. Arch Clin Neuropsychol. 2005;20:33–65. [DOI] [PubMed] [Google Scholar]
- 17. Rabin LA, Paolillo E, Barr WB. Stability in test‐usage practices of clinical neuropsychologists in the United States and Canada over a 10‐year period: a Follow‐up Survey of INS and NAN Members. Arch Clin Neuropsychol. 2016;31:206–30. [DOI] [PubMed] [Google Scholar]
- 18. Helmstaedter CHB, Lassonde M, Kahane P, Arzimanaglou A. Neuropsychology in the care of people with epilepsy. Esher, UK: John Libby Eurotext; 2012:338. [Google Scholar]
- 19. Brissart H, Planton M, Bilger M, Bulteau C, Forthoffer N, Guinet V, et al. French neuropsychological procedure consensus in epilepsy surgery. Epilepsy Behav. 2019;100:106522. [DOI] [PubMed] [Google Scholar]
- 20. Vogt VL, Äikiä M, del Barrio A, Boon P, Borbély C, Bran E, et al. Current standards of neuropsychological assessment in epilepsy surgery centers across Europe. Epilepsia. 2017;58:343–55. [DOI] [PubMed] [Google Scholar]
- 21. Baxendale S, Wilson SJ, Baker GA, Barr W, Helmstaedter C, Hermann BP, et al. Indications and expectations for neuropsychological assessment in epilepsy surgery in children and adults: executive summary of the report of the ILAE Neuropsychology Task Force Diagnostic Methods Commission: 2017–2021. Epilepsia. 2019;60:1794–6. [DOI] [PubMed] [Google Scholar]
- 22. Wilson SJ, Baxendale S, Barr W, Hamed S, Langfitt J, Samson S, et al. Indications and expectations for neuropsychological assessment in routine epilepsy care: report of the ILAE Neuropsychology Task Force, Diagnostic Methods Commission, 2013–2017. Epilepsia. 2015;56:674–81. [DOI] [PubMed] [Google Scholar]
- 23. Reyes A, Kaestner E, Bahrami N, Balachandra A, Hegde M, Paul BM, et al. Cognitive phenotypes in temporal lobe epilepsy are associated with distinct patterns of white matter network abnormalities. Neurology. 2019;92:e1957–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Stokin GB, Krell‐Roesch J, Petersen RC, Geda YE. Mild neurocognitive disorder: an old wine in a new bottle. Harv Rev Psychiatry. 2015;23:368–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sachdev PS, Blacker D, Blazer DG, Ganguli M, Jeste DV, Paulsen JS, et al. Classifying neurocognitive disorders: the DSM‐5 approach. Nat Rev Neurol. 2014;10:634–42. [DOI] [PubMed] [Google Scholar]
- 26. Covanis A, Guekht A, Li S, Secco M, Shakir R, Perucca E. From global campaign to global commitment: the World Health Assembly's Resolution on epilepsy. Epilepsia. 2015;56:1651–7. [DOI] [PubMed] [Google Scholar]
- 27. Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, et al. The diagnosis of mild cognitive impairment due to Alzheimer's disease: recommendations from the National Institute on Aging‐Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:270–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Winblad B, Palmer K, Kivipelto M, Jelic V, Fratiglioni L, Wahlund L‐O, et al. Mild cognitive impairment–beyond controversies, towards a consensus: report of the International Working Group on Mild Cognitive Impairment. J Intern Med. 2004;256:240–6. [DOI] [PubMed] [Google Scholar]
- 29. Petersen RC, Doody R, Kurz A, Mohs RC, Morris JC, Rabins PV, et al. Current concepts in mild cognitive impairment. Arch Neurol. 2001;58:1985–92. [DOI] [PubMed] [Google Scholar]
- 30. Petersen RC. Mild cognitive impairment. Continuum. 2016;22:404–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Busse A, Hensel A, Gühne U, Angermeyer MC, Riedel‐Heller SG. Mild cognitive impairment: long‐term course of four clinical subtypes. Neurology. 2006;67(12):2176–85. [DOI] [PubMed] [Google Scholar]
- 32. Bickel H, Mosch E, Seigerschmidt E, Siemen M, Forstl H. Prevalence and persistence of mild cognitive impairment among elderly patients in general hospitals. Dement Geriatr Cogn Disord. 2006;21:242–50. [DOI] [PubMed] [Google Scholar]
- 33. Zanetti M, Ballabio C, Abbate C, Cutaia C, Vergani C, Bergamaschini L. Mild cognitive impairment subtypes and vascular dementia in community‐dwelling elderly people: a 3‐year follow‐up study. J Am Geriatr Soc. 2006;54:580–6. [DOI] [PubMed] [Google Scholar]
- 34. Jak AJ, Bondi MW, Delano‐Wood L, Wierenga C, Corey‐Bloom J, Salmon DP, et al. Quantification of five neuropsychological approaches to defining mild cognitive impairment. Am J Geriatr Psychiatry. 2009;17:368–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jolley D, Benbow SM, Grizzell M. Memory clinics. Postgrad Med J. 2006;82:199–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Jack CR, Bennett DA, Blennow K, Carrillo MC, Dunn B, Haeberlein SB, et al. NIA‐AA research framework: toward a biological definition of Alzheimer's disease. Alzheimers Dement. 2018;14:535–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Edmonds EC, Delano‐Wood L, Clark LR, Jak AJ, Nation DA, McDonald CR, et al. Susceptibility of the conventional criteria for mild cognitive impairment to false‐positive diagnostic errors. Alzheimers Dement. 2015;11:415–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Edmonds EC, Eppig J, Bondi MW, Leyden KM, Goodwin B, Delano‐Wood L, et al. Heterogeneous cortical atrophy patterns in MCI not captured by conventional diagnostic criteria. Neurology. 2016;87:2108–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Edmonds EC, McDonald CR, Marshall A, Thomas KR, Eppig J, Weigand AJ, et al. Early versus late MCI: improved MCI staging using a neuropsychological approach. Alzheimers Dement. 2019;15:699–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bondi MW, Edmonds EC, Jak AJ, Clark LR, Delano‐Wood L, McDonald CR, et al. Neuropsychological criteria for mild cognitive impairment improves diagnostic precision, biomarker associations, and progression rates. J Alzheimers Dis. 2014;42:275–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Petersen RC, Aisen PS, Beckett LA, Donohue MC, Gamst AC, Harvey DJ, et al. Alzheimer's Disease Neuroimaging Initiative (ADNI): clinical characterization. Neurology. 2010;74:201–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Clark LR, Delano‐Wood L, Libon DJ, McDonald CR, Nation DA, Bangen KJ, et al. Are empirically‐derived subtypes of mild cognitive impairment consistent with conventional subtypes? J Int Neuropsychol Soc. 2013;19:635–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Edmonds EC, Weigand AJ, Thomas KR, Eppig J, Delano‐Wood L, Galasko DR, et al. Increasing inaccuracy of self‐reported subjective cognitive complaints over 24 months in empirically derived subtypes of mild cognitive impairment. J Int Neuropsychol Soc. 2018;24:842–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Edmonds EC, Weigand AJ, Hatton SN, Marshall AJ, Thomas KR, Ayala DA, et al. Patterns of longitudinal cortical atrophy over 3 years in empirically derived MCI subtypes. Neurology. 2020;94:e2532–e2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kaestner E, Reyes A, Macari AC, Chang YH, Paul BM, Hermann BP, et al. Identifying the neural basis of a language‐impaired phenotype of temporal lobe epilepsy. Epilepsia. 2019;60:1627–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Reyes A, Kaestner E, Ferguson L, Jones JE, Seidenberg M, Barr WB, et al. Cognitive phenotypes in temporal lobe epilepsy utilizing data‐ and clinically driven approaches: moving toward a new taxonomy. Epilepsia. 2020;61(6):1211–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hermann B, Seidenberg M, Lee EJ, Chan F, Rutecki P. Cognitive phenotypes in temporal lobe epilepsy. J Int Neuropsychol Soc. 2007;13(1):12–20. [DOI] [PubMed] [Google Scholar]
- 48. Elverman KH, Resch ZJ, Quasney EE, Sabsevitz DS, Binder JR, Swanson SJ. Temporal lobe epilepsy is associated with distinct cognitive phenotypes. Epilepsy Behav. 2019;96:61–8. [DOI] [PubMed] [Google Scholar]
- 49. Reyes A, Kaestner E, Macari A, Wang Z, Drane D, Punia V,et al. Diagnosing cognitive disorders associated with aging in temporal lobe epilepsy. Epilepsia. 2021;62:460‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Thompson PM, Jahanshad N, Ching CRK, Salminen LE, Thomopoulos SI, Bright J, et al. ENIGMA and global neuroscience: A decade of large‐scale studies of the brain in health and disease across more than 40 countries. Transl Psychiatry. 2020;10:100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Whelan CD, Altmann A, Botía JA, Jahanshad N, Hibar DP, Absil J, et al. Structural brain abnormalities in the common epilepsies assessed in a worldwide ENIGMA Study. Brain. 2018;141:391–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Hatton SN, Huynh KH, Bonilha L, Abela E, Alhusaini S, Altmann A, et al. White matter abnormalities across different epilepsy syndromes in adults: an ENIGMA‐Epilepsy Study. Brain. 2020;143:2454–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Sisodiya SM, Whelan CD, Hatton SN, Huynh K, Altmann A, Ryten M, et al. The ENIGMA‐Epilepsy working group: mapping disease from large data sets. Hum Brain Mapp. 2020. Epub ahead of print. PMID: 32468614. 10.1002/hbm.25037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Ponsford J. International growth of neuropsychology. Neuropsychology. 2017;31:921–33. [DOI] [PubMed] [Google Scholar]
- 55. Hessen E, Hokkanen L, Ponsford J, van Zandvoort M, Watts A, Evans J, et al. Core competencies in clinical neuropsychology training across the world. Cini Neuropsychol. 2018;32:642–56. [DOI] [PubMed] [Google Scholar]


