Abstract
Background
The world is currently struggling with the Coronavirus disease 2019 (COVID-19) pandemic. Dietary supplements (DSs) and herbal medicine provide a potentially convenient and accessible method for its recovery, but direct evidence is limited.
Objective
This study aims to investigate the effectiveness of DSs and herbs in patients with COVID-19.
Methods
A systematic literature search was conducted in multiple electronic English and Chinese databases. Randomized controlled trials (RCTs) involving DSs or herbal medicine interventions on patients with COVID-19 from November 2019 to February 2021 were included. Data was extracted, summarized and critically examined.
Results
Out of 9402 records identified in the initial search, twelve RCTs were included in this review. Risk of bias of these RCTs was deemed high. Most of the trials were of low methodologic quality. Nine studies showed herbal supplements were beneficial to the recovery of COVID-19 patients; zinc sulfate could shorten the duration of loss of smell but not total recovery from COVID-19. No severe adverse events were reported.
Conclusion
Herbal supplements may help patients with COVID-19, zinc sulfate is likely to shorten the duration of olfactory dysfunction. DS therapy and herbal medicine appear to be safe and effective adjuvant therapies for patients with COVID-19. These results must be interpreted with caution due to the overall low quality of the included trials. More well-designed RCTs are needed in the future.
Keywords: COVID-19, Herbs, Dietary supplement, Diet, Systematic review, Virus
1. Introduction
Coronavirus disease 2019 (COVID-19), which is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is frequently associated with atypical pneumonia [1]. This virus can spread through direct means via droplet transmission from human-to-human and by indirect contact with contaminated objects [2]. Ease of transmission has led to alarming numbers of COVID-19 cases. According to data from the World Health Organization (WHO), as of March 6, 2021 there were over 115 million confirmed cases and 2.5 million deaths [3]. Patients affected by COVID-19 usually suffer from severe viral infection, suppression of the immune system and finally may experience progressive cytokine storm, lung fibrosis, and multi-organ failure, which may ultimately lead to death [[4], [5], [6], [7]]. Vulnerable and immunocompromised individuals seem to be more susceptible to serious COVID-19 complications. Safe, effective, and early interventions are urgently needed to prevent, reduce susceptibility, and lessen the severity of COVID-19. At present, there is no specific conventional medicine proven to effectively manage symptoms of COVID-19. Healthier dietary habits could modulate the individual risk of serious COVID-19 symptoms. In individuals infected with COVID-19, the nutritional status of the host plays a pivotal role to determine the clinical severity and prognosis of COVID-19 [8]. Therefore, optimal diets may be an effective approach in influencing the severity of COVID-19.
Dietary supplement (DS) refers to a commercially available product that is consumed orally to supplement the usual diet [9]. According to the Dietary Supplement Health and Education Act of 1994, DSs comprise a broad array of products, with the ingredients such as amino acids, enzymes, herbs or other botanicals, minerals, and vitamins in the forms of capsules, gelcaps, liquids, powders, tablets, and softgels [10]. DS may be used as an important complementary and alternative medicine therapy to conventional therapies to reduce infection risk and strengthen immunity. Although herbal medicine is an independent medical profession in China, it is usually classified as a form of complementary and alternative therapy in the West [11]. Nowadays, herbal medicine has been widely used globally, in the USA, more than one-third of adults reported using at least 1 herbal medicine [12]. It has been considered as a promising potential supplement for the management of healthcare.
As an adjuvant therapy, specific DSs have been reported to boost the immune system and reduce inflammation and oxidative stress during the COVID-19 crisis [[13], [14], [15]]. A recent US consumer survey showed that over 43% of DS users changed their dietary regimens during the pandemic crisis by increasing their supplement routine for enhancing their immune system function and wellness benefits [16]. In a recent survey conducted in China during the COVID-19 pandemic, 37.7% of the participants reported using certain DSs to cope with COVID-19 [17]. In the wake of COVID-19, the global DS market value amount was approximately USD 220.3 billion in 2020 [18]. Therefore, clarifying and consolidating reported DSs guidelines for widespread dissemination among medical care professionals and the general public during the pandemic crisis is crucial.
Though there has been a large number of relevant articles published on DS use, such as vitamin A, D, Zn, and selenium [19], probiotics and dietary fibers [20], and omega-3 fatty acids [21], they present hypothetical treatment data generated based on previous publications for COVID-19. To date, we did not find any review addressing current RCTs on this topic specifically. Consequently, the medical community has not been provided with efficacious diet guidelines related to the COVID-19 pandemic. Therefore, this research aims to synthesize the available evidence from current existing RCTs and evaluate the impacts of DSs in people with COVID-19. Findings from this review will help identify knowledge gaps and aid DS intervention designs to fight against COVID-19 and provide comprehensive information on the effective use of DS.
2. Materials and methods
2.1. Literature search strategy
This study was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [22]. A comprehensive electronic literature search of multiple Chinese and English databases from November 2019 to February 2021, was conducted. The English databases included Ovid Embase, Ovid Cochrane Database of Systematic Reviews, Ovid MEDLINE(R) Ovid Cochrane Central Register of Controlled Trials, and Epub Ahead of Print, In-Process & Other Non-Indexed Citations and Daily, Scopus, and Web of Science. The Chinese databases included China National Knowledge Infrastructure (CNKI), China Biology Medicine disc (CBM), WanFang, and China Science and Technology Journal Database (VIP). The search strategy was designed and performed by experienced librarians with input from the study's principal investigators. Controlled vocabulary supplemented with keywords was used to search for studies describing DSs or herbs for COVID-19. Since RCTs provide strong evidence for the efficacy of healthcare interventions [23], to best investigate the potential effect of DSs and herbs on patients with COVID-19, only RCT with human subjects were searched. Relevant trials and reviews for further eligible studies were searched synchronously for potentially eligible studies. All the screenings were implemented by two authors independently, and any discrepancy was resolved by the discussion with the third author.
2.2. Eligibility and selection criteria
Articles published in English or Chinese between November 2019 and February 2021 were included using the following inclusion criteria: human subjects relevant to COVID-19; RCTs; peer-reviewed journal articles; DSs or herbs involved in observational groups. Abstracts, surveys, editorials, commentaries, correspondence, case reports, literature reviews, and DSs or herbs vis extraoral administration or in the control group trials were excluded.
2.3. Date extraction
Data were extracted using a prepared excel table from each relevant trial by two reviewers independently: 1) RCT characteristics - first author, publication year, country, setting, design, registration, manufacture, and provider; 2) sample characteristics - population and sample size; 3) intervention characteristics - design, session, and follow up; 4) outcome characteristics - efficacy, main outcomes, follow up and safety monitoring. Safety was monitored by the reporting of adverse events (AEs). Data were extracted by two authors independently and revised by the third author.
2.4. Risk of bias and quality
The Cochrane Risk of Bias Tool for Randomized Controlled Trials [24] was used to evaluate the potential risk of bias of the retrieved RCTs. The risk of bias tool covers six domains as the following: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other bias. Results were entered in the Revman software (Review Manager (RevMan) [Computer program]. Version 5.4.1, The Cochrane Collaboration, 2020) for the presentation of bias in a comprehensive manner. Additionally, the Jadad scale [25] was used to evaluate the quality of each eligible study. The evaluation was determined in three domains: randomization, blinding and the fate of all participants. Studies with a total quality assessment score of 3–5 were categorized as high quality and 0–2 as low quality. All the assessments were conducted by two authors independently, and any discrepancy was settled through a discussion or consultation with the third review author.
2.5. Data analysis
A descriptive analysis was completed for each study in excel spreadsheets. Meta-analysis was not conducted due to methodological and outcomes heterogeneity.
3. Results
3.1. Study selection
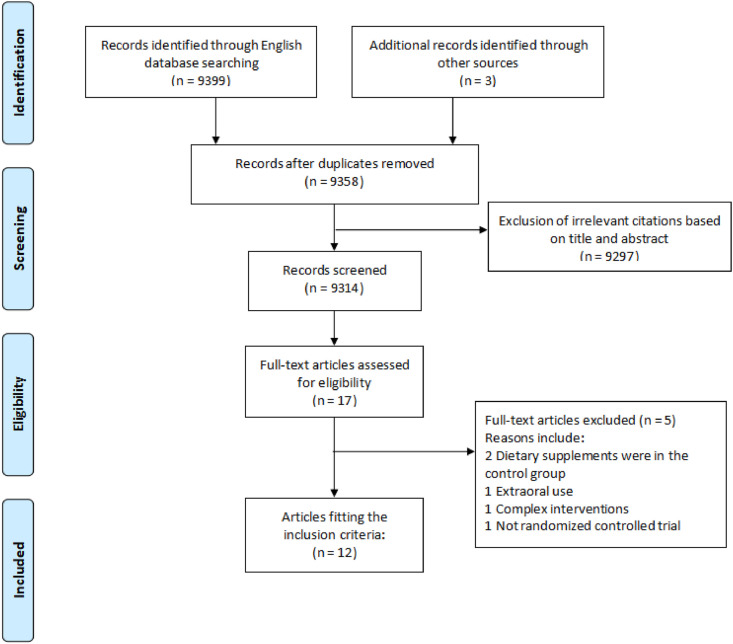
Our search yielded 9402 records. After screening the titles and abstracts, 44 duplicates and 8395 irrelevant results were removed. 17 potentially relevant studies were further investigated for a full-text review, 5 RCTs were excluded due to the following reasons: 2 RCTs [26,27] of which DSs were in the control group, 1 RCT [28] with supplement through extraoral use, 1 RCT [29] with complex interventions and 1 non-RCT [30]. Finally, 12 RCTs [[31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42]] were included in this review (Fig. 1 ).
Fig. 1.
Flow diagram for study selection process.
3.2. Study characteristics
Twelve studies with 1952 COVID-19 patients were evaluated in our review. Among them, eleven studies [[32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42]]were published in English and one [31] in simplified Chinese, between 2020 and 2021. Four of them are multicenter RCTS [36,38,40,42]. Included studies were conducted in five different countries, including 6 RCTs [[31], [32], [33], [34],39,40] from China, two [35,37] from Iran, two [41,42] from Egypt, and one from the USA [36] and Brazil [38] each. Eight RCTs [[32], [33], [34],[36], [37], [38],40,42] were registered on the clinical trial registry website, four [34,36,38,42] registered on the Clinical Trials.gov, three [32,33,40] registered on the China Clinical Trial Registry website, and one [37] registered on the Iranian Registry of Clinical Trials. Two trials involved both suspected and confirmed COVID-19 cases [16], and the other one [34] and nine [32,[35], [36], [37], [38], [39], [40], [41], [42]] suspected or confirmed cases only. Eight articles [[31], [32], [33], [34], [35],37,39,40] were about herbal supplements, two about Zinc therapy [41,42], one with both zinc and ascorbic acid supplement [36], and one for Vitamin D3 [38]. Except for one study with outpatients [36], the other eleven involved hospitalized participants [[31], [32], [33], [34], [35],[37], [38], [39], [40], [41], [42]]. Among all the supplements evaluated, only herbal supplements and zinc sulfate showed significant beneficial effects on COVID-19 patients.
Five studies [34,36,38,41,42] reported follow-up observations, three studies [36,37,42] with a 28-day follow-up, one study with 41-day follow-up [38], and one study [32] until complete recovery of COVID-19 patients and complete recovery of their olfactory function [41](Table 1, Table 2 ).
Table 1.
Characteristics of included studies.
| Author | Arm | Country | Study design | Setting | Registration | Population | N(IG/CG) | Dietary supplement | Follow-up | Results | Safety monitoring (N) | Quality level |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hu 2020 [40] | 2 | China | Prospective multicenter open-label RCT | Hospitalization | ChiCTR2000029434 | Confirmed cases | 284 (142/142) | Herbal formulation | NR | Ameliorate clinical symptoms and shorten the duration of viral shedding. | Abnormal liver function (32), Renal dysfunction (8), Headache (1), Nausea (6), Vomiting (2), Loss of appetite (8), Diarrhea (8); Elevated alanine aminotransferase levels or aspartate aminotransferase levels | Low (1/5) |
| Wang 2020 [34] | 2 | China | RCT | Hospitalization | NCT04251871 | Confirmed cases | 48 (24/24) | Herbal formulation | 28 days | Safe and superior to the standard therapy in suppressing the development of ARDS. | Diarrhea (n = 9), Anorexia (n = 4), Nausea (n = 2), Stomach pain (n = 2) | Low (1/5) |
| Xiao 2020 [33] | 3 | China | RCT | Hospitalization | ChiCTR2000029601 | Suspected cases + Confirmed cases | 283(94/95/94) | Herbal formulation | NR | Combined with western medicine may have clinical advantages in improving clinical symptoms, reducing utilization rate of anti-infective drugs, and improving patient prognosis. | Yes, but not in detail | Low (0/5) |
| Xiong 2020 [32] | 2 | China | Pilot RCT | Hospitalization | ChiCTR2000034795 | Confirmed cases | 42 (22/20) | Herbal formulation | NR | Combined with conventional medicine may significantly improve clinical symptoms, increase WBC and LYM, and reduce CRP and ESR. | Yes, no adverse events reported. | Low (1/5) |
| Yu 2020 [31] | 2 | China | RCT | Hospitalization | / | Suspected cases + Confirmed cases (mild + common) | 295 (147/148) | Herbal formulation | NR | Combined with arbidol can relieve the clinical symptoms, adjust the inflammatory factors, increase the curative effects and reduce the severe transfer rate | Yes, no adverse events reported. | Low (1/5) |
| Liu 2020 [39] | 2 | China | RCT | Hospitalization | / | Confirmed cases (common or severe) | 80 (44/36) | Herbal formulation | NR | Effectively shorten the duration of nucleic acid detection and promote the absorption of pneumonia inflammatory exudate without obvious adverse reactions | Yes, no adverse events reported. | Low (1/5) |
| Sard ari 2021 [37] | 2 | Iran | RCT | Hospitalization | IRCT20200506047319N1 | Confirmed cases | 83 (40/43) | Herb | NR | Containing antioxidant properties, can strengthen the immune system, and induce antiviral effect. | NR | Low (1/5) |
| Valizadeh 2020 [35] | 3 | Iran | RCT | Hospitalization | / | Confirmed cases | 80 (20/20/40) | Herb | NR | May improve clinical manifestation and overall recovery by increase anti-inflammatory cytokines especially IL-1β and IL-6 mRNA expression and cytokine secretion | NR | High (3/5) |
| Abdelmaksoud 2021 [41] | 2 | Egypt | RCT | Hospitalization | / | Confirmed cases (mild, common, severe, and extremely severe) with Olfactory Disturbances | 105 (49/56) | Zinc therapy | Until complete recovery of COVID-19 (pharyngeal swap becomes negative) and complete recovery of olfactory symptoms | Not exhibit a significant role in development of anosmia and/or hyposmia or disease severity but may shorten the duration of smell recovery without affecting the total recovery duration. | NR | Low (1/5) |
| Abd-Elsalam 2020 [42] | 2 | Egypt | Multicenter RCT | Hospitalization | NCT04447534 | Confirmed cases (mild, moderate, severe, and critical) | 191 (96/95) | Zinc therapy | 28 days | Not enhance the clinical efficacy of hydroxychloroquine | NR | Low (0/5) |
| Thomas 2021 [36] | 4 | USA | Multicenter, single health system RCT | Outpatient care | NCT04342728 | Ambulatory confirmed cases | 214(58/48/58/50) | Zinc therapy, Ascorbic acid supplement | 28 days. | Not significantly decrease the duration of symptoms compared with standard of care | Headache (5), nausea (15), vomiting (2), tingling (2), stomach pains/cramps (11), diarrhea (15), dizziness/faintness (2), other (7) | Low (1/5) |
| Murai2021 [38] | 2 | Brazil | Multicenter RCT | Hospitalization | NCT04449718 | Confirmed cases (moderate to severe) | 237 (119/118) | Vitamin D3 | 41days | Not reduce hospital length of stay. | Yes, no adverse events, but episode of vomiting (1) | High (3/5) |
Table 2.
Characteristics of included studies Cont.
| Author/Year | Intervention | Dietary Supplement Ingredient | Session | Manufacture | Main outcomes |
|---|---|---|---|---|---|
| Hu 2020 [40] | IG: Lianghua Qingwen capsules + usual treatment CG: Usual treatment |
Forsythia suspensa, Lonicera japonica, Ephedra sinica, Isatis indigotica, Pogostemon cablin, Rheum palmatum, Glycyrrhiza uralensis, Dryopteris crassirhizoma, Rhodiola crenulata, Houttuynia cordata, Prunus sibirica, gypsum and 1-menthol | 4 capsules thrice daily for 2 weeks | Shijiazhuang Yiling Pharmaceutical Co. Ltd., Shijiazhuang, China Z20100040 | Primary outcome: Clinical symptoms (fever, fatigue, and cough) recovery Secondary outcome: Symptom recovery time, Chest CT improvement, clinical cure rate, conversion time and rate of SARS-CoV-2 viral assay |
| Wang 2020 [34] | IG: Keguan-1 decoction + antiviral treatment and the other supportive treatments CG: Antiviral treatment + the other supportive treatments |
Derived from 3 different formulae, Yinqiao Powder, Sangju Drink, and Sanren Decoction (Lonicera japonica Thunb30 g, Forsythia suspensa 30 g, Morus alba L 15 g, Chrysanthemum morifolium Ramat10 g, Coix lacryma-jobi L. var. mayuen 30 g, Fritillaria thunbergii Miq 15 g, and Prunus armeniaca L. var. ansu Maxim 9 g) | 19.4 g twice daily for 2 weeks | Beijing Tcmages Pharmaceutical Co. Ltd. (Beijing, China) | Primary outcome: ARDS development incidence Secondary outcome: Fever resolution time, lung injury recovery (evaluated by X-ray or CT). |
| Xiao 2020 [33] | IG: Huoxiang Zhengqi dropping pills + Lianhua Qingwen granules CG: Lianhua Qingwen granules + conventional medicine; or conventional medicine | Huoxiang Zhengqi dropping pill (Pogostemon cablin (Blanco) Benth, Atractylodes lancea (Thunb.) DC., Magnolia officinalis Cortex, Angelicae dahurica Radix, Poria cocos (Schw.) Wolf, Areca catechu L., Pinellia ternate (Thunb.) Breit., Glycyrrhizae Radix et Rhizoma, Perilla frutescens, and Citrus reticulata) and Lianhua Qingwen granule (Forsythia suspensa (Thunb.) Vahl, Ephedra sinica Stapf, Lonicera japonica Thunb., Isatis indigotica Fortune, Mentha haplocalyx Briq., Dryopteris crassirhizoma Nakai, Rhodiola rosea L., Gypsum Fibrosum, Pogostemon cablin (Blanco) Benth., Rheum palmatum L., Houttuynia cordata Thunb., and Glycyrrhiza uralensis Fisch. Armeniaca sibirica (L.) Lam) | Lianhua Qingwen granules: 1 bag(6g), thrice daily for 2 weeks; Huoxiang Zhengqi dropping pills: 1 bag (2.6g), twice daily for 2 weeks. | Huo Xiang Zhengqi dropping pills (Chinese medicine Z20000048, Tianjin Tasly Pharmaceutical Group Co., Ltd.) Lianhua Qingwen granules (Chinese medicine Z20100040, Beijing Yiling Pharmaceutical Co., Ltd., specification.) | Primary outcome: clinical symptom improvement and disappearance rates after 14 days of treatment Secondary outcome: the proportion of patients who progressed to severe status. |
| Xiong 2020 [32] | IG: Xuanfei Baidu decoction: + conventional medicine CG: Conventional medicine | Ephedrae Herba 8 g, Armeniacae Semen Amarum 15 g, Gypsum Fibrosum 30 g, Atractylodis Rhizoma 10 g, Semen Coicis 30 g, Agastachis Herba 15 g, Polygoni Cuspidati Rhizoma et Radix 20 g, Lepidii seu Descurainiae Semen 15 g, Verbenae Herba 30 g, Phragmitis Rhizoma 30 g, Artemisiae Annuae Herba 25 g, Citri Grandis Rubrum Exocarpium 20 g, Glycyrrhizae Radix et Rhizoma 10 g | 1 pouch of 200 ml each time, 2 times daily for 1 week | NR | Primary outcome: the main symptoms (fever, fatigue, and cough) disappearance rate after 1-week treatment Secondary outcome: the disappearance rate of secondary symptoms, and the changes in WBC, LYM, CRP, ESR |
| Yu 2020 [31] | IG: Lianhua Qingwen granules: + arbidol + other medicine CG: Arbidol + other medicine |
Forsythia suspensa, Lonicera japonica, Ephedra sinica, Isatis indigotica, Pogostemon cablin, Rheum palmatum, Glycyrrhiza uralensis, Dryopteris crassirhizoma, Rhodiola crenulata, Houttuynia cordata, Prunus sibirica, gypsum and 1-menthol | 6g, 3 times daily for 1 week | Shijiazhuang Yiling Pharmaceutical Co. Ltd., Shijiazhuang, China Z20100040 | Clinic cure rate, severe transfer rate, TCM symptom scores, changes in WBC, LYM, CRP, PCT and effective cure rate based on CT review. |
| Liu 2020 [39] | IG: Jinhua Qinggan granules + oxygen inhalation, and symptomatic and supportive treatment CG: Oxygen inhalation, and symptomatic and supportive treatment + Not take Jinhua Qinggan granules/take less than 2 d | Flos Lonicerae, Herba Ephedra Sinica, Gypsum Fibrosum, Semen Armeniacae Amarum, Radix Scutellariae Baicalensis, Fructus Forsythiae Suspensae, Bulbus Fritillariae Thunbergii, Rhizoma Anemarrhenae, Fructus Arctii, Herba Artemisiae Annuae, Herba Menthae Haplocalycis, Radix Glycyrrhizae | 1 bag (6 g) twice daily (morning and evening) for adult, for 7 consecutive days | Juxiechang (Beijing) Pharmaceutical Co., Ltd., National Medicine Permission No. Z20160001 | Average duration of viral nucleic acid detection and the recovery time indicated by chest CT, the 7-day viral clearance rate |
| Sardari 2021 [37] | IG: Thyme essential oil + routine medications CG: Syrup placebo + routine medications | Thyme (Thymus Vulgaris) Essential Oil | 5 ml every 8 h for 1 week | Pharmacy company | Frequency distribution of different symptoms (fever, cough, coriza, dyspnea, dizziness, muscular pain, headache, sore throat, sputum, anorexia, weakness and lethargy, fatigue, chest wall pain, diarrhea); changes in BUN, N, Ca, LYM |
| Valizadeh 2020 [35] | IG: Nano-curcumin capsule + conventional medications CG: Placebo capsules + conventional medications | Medicinal part of turmeric | 160 mg of Nano-curcumin in four 40 mg capsules daily for 2 weeks | Exir Nano Sina Company, Iran (IRC:1228225765) | The mRNA expression and cytokine secretion levels of IL-1β, IL-6, TNF-α and IL-18 |
| Abdelmaksou 2021 [41] | IG: Zinc therapy + Egyptian protocol of treatment CG: Egyptian protocol of treatment | Zinc sulfate | 220 mg zinc sulfate (50 mg elemental zinc), twice daily | NR | Serum zinc levels regarding disease severity or the presence or absence of olfactory and/or gustatory dysfunction, the median duration of complete recovery |
| Abd-Elsalam 2020 [42] | IG: Zinc therapy + hydroxychloroquine + standard treatment CG: Hydroxychloroquine + standard treatment | Zinc sulfate | Zinc sulfate 220 mg (50 mg of elemental zinc) twice daily for 6 days | NR | The recovery within 28 days, the need for mechanical ventilation, and death |
| Thomas 2021 [36] | IG: (1) Zinc gluconate; (2) Ascorbic acid; (3) Zinc gluconate + ascorbic acid CG: Standard care | Zinc gluconate (50 mg), ascorbic acid (8000 mg), both agents | Zinc gluconate: 50 mg at bedtime for 10 days; ascorbic acid: 8000 mg (to be divided over 2–3 times per day with meals) for 10 days. | NR | Primary outcome: the number of days required to reach a 50% reduction in symptom severity score from peak symptom score. Secondary outcome: the number of days required to reach a total symptom severity score of 0, cumulative severity score at day 5, hospitalizations, deaths, adjunctive prescribed medications, and adverse effects of the study supplements. |
| Murai 2021 [38] | IG: Vitamin D3+ conventional care CG: Placebo peanut oil solution + conventional care | Vitamin D3 | 200 000IU dissolved in a 10-ml peanut oil solution | NR | Primary outcome: hospital length of stay Secondary outcome: mortality; the intensive care unit administration number; the number of patients who needed mechanical ventilation and the duration of mechanical ventilation; and serum levels of 25-hydroxyvitamin D, total calcium, creatinine, CRP, D-dimer |
Note: RCT = Randomized controlled trial; IG = Interventional group; CG = Control group; NR = Not reported; WBC = White blood cell; LYM = Lymphocytes; CRP = C-reactive protein; ESR = Erythrocyte sedimentation rate; ARDS = Acute respiratory distress syndrome; BUN = Blood urea nitrogen; IL-1β = Interleukin 1 beta; IL-6 = Interleukin 6, TNF-α = Tumor necrosis factor α, and IL-18 = Interleukin 18.
3.3. Evaluation of methodological quality and bias
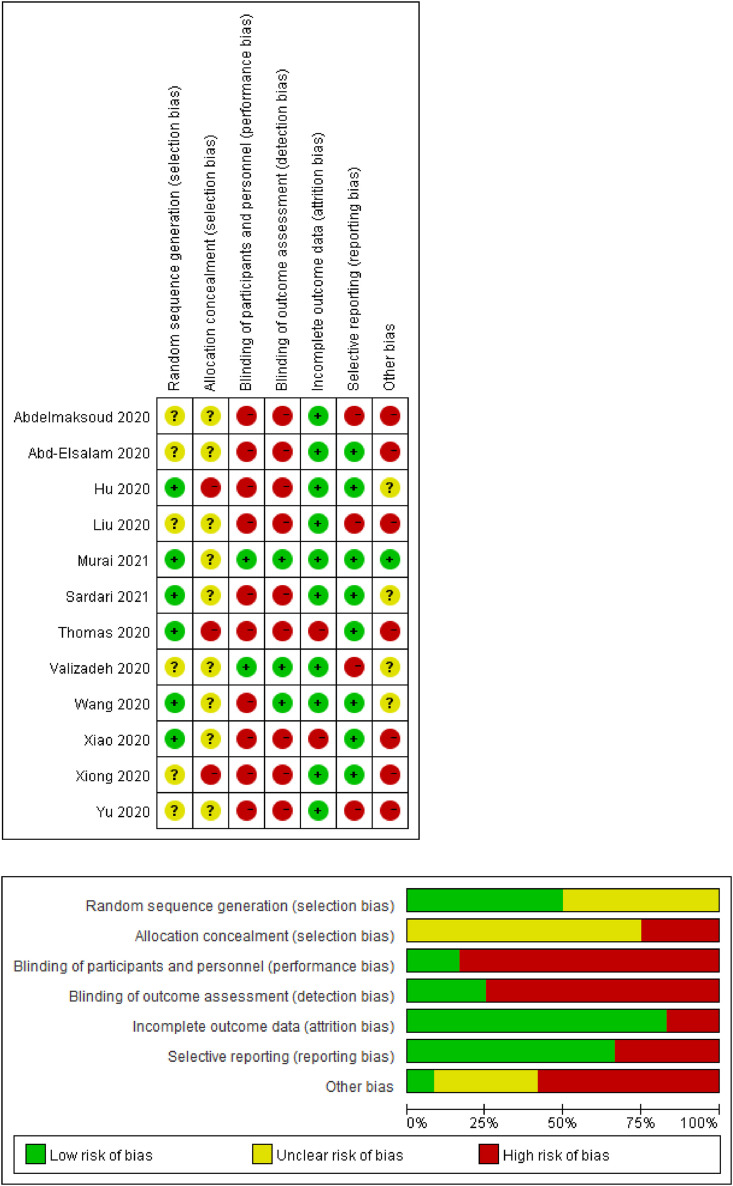
Six of the twelve RCTs [33,34,[36], [37], [38],40] had a lower risk of bias of randomization allocation due to specified reporting of random-sequence generation. Two trials used SAS software [33,40], two referred to computer-generated random locks [37,38], one used random number table [34], and one used REDCap database [36] for setting the random variable seeds. The other six trials [31,32,35,39,41,42] had an unclear risk for randomization allocation due to a lack of detailed information. Three trials [32,36,40] had a high-risk bias of allocation concealment, and the other nine [31,[33], [34], [35],[37], [38], [39],41,42] had unclear allocation concealment without description in enough detail. Two trials [35,38] had a lower risk of performance bias for double-blinding participants and personals as well as an outcome assessment. One trial [34] was blinded only in outcome assessment, without in participants and personals. The other nine trials were at high risk of bias due to insufficient data. Ten trials [31,32,34,35,[37], [38], [39], [40], [41], [42]] had a low risk of bias with complete outcome data, except one trial [36] had a high risk of bias due to insufficient data of dropping patients, and the other one [33] without detailed safety monitoring information. Eight trials [[32], [33], [34],[36], [37], [38],40,42] had a low risk of bias due to their registration online with available pre-specified outcomes, whereas the other four [31,35,39,41] had a high risk of bias without pre-specified outcomes had been reported. One RCT [38] was estimated at low risk because of its good study design. The other trials were assessed at high [[31], [32], [33],36,39,41,42] or unknown risk of bias [34,35,37,40] in the other resource. For both poor unknown design of randomization number generation and poor design allocation, three trials were estimated as high risk of bias in the other resource. Two RCTs [35,38] were evaluated as high quality because of their low jaded scores, and the other ten [[31], [32], [33], [34],36,37,[39], [40], [41], [42]] as poor quality. The total methodological quality of the twelve RCTs was low. The main reasons for downgrading the quality of included studies were the high risk of selection bias and performance bias. Detailed information on the evaluation of the methodological quality and risk of bias of original studies was summarized with Revman software (Fig. 2 ).
Fig. 2.
Risk of bias. Judgments about each risk of bias across all included studies.
3.4. Summary of findings
3.4.1. Herbs
The effect of herbs on COVID-19 patients was assessed by eight studies [[31], [32], [33], [34], [35],37,39,40] in our review, including six RCTs [[31], [32], [33], [34],39,40] with herbal formulations and two RCTs with single herb alone. Three RCTs [31,33,40] evaluated the effect of Lianhua Qinwen formula on COVID-19. Hu et al. [40] in 2020 suggested that Lianhua Qingwen capsule has the potential to improve the rate of symptom recovery in patients with COVID-19. The authors performed an RCT lasting 2 weeks investigating the effect of Lianhua Qingwen capsules (4 capsules thrice daily) combined with routine treatment based on the Diagnosis and Treatment Protocol for Corona Pneumonia 2020 versus the effect of the routine treatment only in the control group. Lianhua Qinwen capsules combined with routine treatment according to the Treatment Protocol for Corona Pneumonia showed a significant improvement of the recovery rate, the rate of improvement in chest CT manifestations and clinical cure. At the same time, the median time to symptom recovery and the time to recovery of fever, fatigue, and coughing also significantly shortened (all P < 0.05). However, both groups did not differ in the rate of conversion to severe cases or viral assay findings (both P > 0.05). Xiao et al. [33] conducted a 3-arm RCT aimed to confirm the effects of Lianhua Qingwen granules and Huoxiang Zhengqi dropping pills combined with western medicine on COVID-19 patients. 61 participants were randomly assigned to receive Lianhua Qingwen granules combined with Huoxiang Zhengqi dropping pills and western medicine. 58 participants were randomly assigned to receive Lianhua Qingwen granules and western medicine. Meanwhile, 63 participants were allocated to receive western medicine. After 14 days of treatment, the 3 groups showed no significant difference in the improvement of clinical symptoms (P > 0.05). However, the progression to severe disease was lowest in the Lianhua qingwen combined with Huoxiang Zhengqi group. Yu et al. [31] verified the effect of Lianhua qingwen granules combined with arbidol on patients with COVID-19. An RCT was performed with a total of 295 subjects who received 6g, 3 times/day Lianhua Qingwen granules combined with arbidol and other medicine or a control group of arbidol and other medicine for 1 week. As a result, the effective rate, and the level of WBC and LYM significantly improved; and the severe transfer rate, as did the traditional Chinese medicine syndromes score (fever, weakness, cough, throat dryness and sore, and chest discomfort), the level of CRP and PCT (all P < 0.05); whereas, effective cure rates based on CT reviews showed no significant difference in the comparison of two groups (P > 0.05).
Three other herbal formulations for COVID-19 management were reported in the studies reviewed. Wang et al. [34] examined the effects of Keguan-1 decoction in 2-week RCT. 48 patients with COVID-19 were assigned to either a Keguan-1decoction group (Keguan-1decoction 19.4 g twice/day) combined with antiviral treatment and other supportive treatments or a control group of antiviral treatment and other supportive treatments. Keguan-1 decoction group showed that time to fever resolution was significantly shorter and the development of ARDS was significantly reduced (both P < 0.05). Xiong et al. [32] designed an RCT with 42 subjects to assess the effect of Xuanfei Baidu decoction (1 pouch of 200 ml each time, 2 times/day) combined with conventional medicine or a control group of conventional medicine with a 1-week treatment period. The disappearance rate of clinical symptoms significantly lessened, the number of white blood cells, C-reactive protein, lymphocytes, and erythrocyte sedimentation rate significantly reduced (P < 0.05) in the treatment group. In another study patients suffering with COVID-19 received Jinhua Qinggan granules (containing 6g, thrice daily) combined with arbidol or arbidol for 1 week, the results suggested that the average duration of viral nucleic acid detection and the recovery time were significantly shortened, and the 7-day viral clearance rate was improved significantly in the treatment group (P < 0.05) [39].
The effect of herbal supplements on COVID-19 was assessed by two RCTs [35,37]. Sardari et al. [37] examined the effects of thyme essential oil on patients with COVID-19, 83 participants were divided to take in the thyme essential oil 5 ml every 8 h combined with routine medications or syrup placebo with the same dosage and administration combined with routine medications for one week. A questionnaire was provided before and after treatment to confirm the effect. Symptoms significantly lessened, the level of blood urea nitrogen, neutrophil count, and calcium were significantly lower, but lymphocyte count was significantly higher in the thyme essential oil group compared to the control group (P < 0.05). Valizadeh et al. [35] reported on 40 patients with COVID-19 treated for 2 weeks. Participants were randomly assigned to take nano-curcumin capsules 160 mg, 4 capsules a day combined with western medicine or a control group of placebo capsules combined with conventional medicine, mRNA expression and cytokine secretion of IL-6 and IL-1β in serum and in the supernatant were significantly lower (both P<0.05) in the treatment group compared to the control group. However, difference was not found significantly in IL-18 mRNA expression and TNF-αconcentration (P>0.05).
3.4.2. Zinc supplements
Studies about zinc therapy were also frequently reported during this pandemic. Abd-Elsalam et al. [42] performed an RCT aiming to evaluating the effect of combining hydroxychloroquine and zinc on COVID-19 patients. 191 participants were randomized to receive hydroxychloroquine (400 mg twice on the first day, then 200 mg twice a day) combined with 220 mg zinc twice a day (50 mg of elemental zinc) or a control group of hydroxychloroquine only for 6 days. The study indicated that the integration of Zinc supplements did not enhance the effect of hydroxychloroquine for patients with COVID-19 (p>0.05).
Thomas et al. [36] assessed the effect of high-dose zinc and/or high-dose ascorbic acid on the severity or duration of symptoms among ambulatory COVID-19 patients. 214 eligible participants were randomly assigned to four different groups:1) participants were provided zinc gluconate 50 mg at bedtime for 10 days; 2) participants were provided ascorbic acid 8000 mg (divided over 2–3 times daily with meals); 3) participants were provided with the both of the foregoing supplements; and 4) participants received standard care only. The study was finally stopped due to futility with no significant difference among groups. (P<0.05).
Abdelmaksoud et al. [41] completed an RCT lasting for 5 days, investigating the effect of zinc therapy (zinc sulfate 220, twice a daily) combined with hydroxychloroquine or hydroxychloroquine only on COVID-19 patients who suffered from anosmia and/or hyposmia. Study results indicated no significant difference among the two groups regarding disease severity and the median duration of total recovery (p > 0.05). However, recovery of gustatory and/or olfactory function was significantly shorted (p < 0.05) in the group that received zinc therapy. Zinc supplements might shorten smell recovery, without influencing the complete recovery duration from COVID-19.
3.4.3. Vitamin D3
Regarding vitamin supplementation, only one eligible trial specifically examined the effect of vitamin D3 intervention (200000 IU, dissolved in a 10-ml peanut oil solution), compared with placebo in COVID-19 patients. This study demonstrated that Vitamin D3 did not significantly reduce patient hospitalization time (P > 0.05). Moreover, the differences between the observational group and the placebo group were not significant for inpatient mortality, admission to the intensive care unit, or need of mechanical ventilation. (P > 0.05) This RCT showed that high dose of vitamin D3 intervention appears not to be useful for moderate to severe COVID-19 [38].
3.5. Safety
Four studies (33.3%) did not report the data of safety monitoring [35,37,41,42]. Eight studies [[31], [32], [33], [34],36,[38], [39], [40]] (66.7%) reported the outcome of safety monitoring, three of which reported no occurrence of AEs in either trial [31,32,39], one [33] mention safety monitoring without detailed information, and the remaining four [34,36,38,40] reported details. Wang et al. [16] reported mild AEs of diarrhea, anorexia, nausea, stomach pain, and vomiting. Thomas et al. [36], recorded 4 serious AEs, including three deaths and one hospitalization for chronic obstructive pulmonary disease exacerbation, which were not regarded as related to the DS interventions. Murai et al. [38] reported no adverse events, but an episode of vomiting was associated with the intervention. Hu et al. [40] reported non-serious findings of AEs. All the adverse events related to DSs intervention were mild. DSs and herbal therapy appeared to be safe treatments for patients with COVID-19 (Table 1).
4. Discussion
The current research is the first systematic review to investigate recent RCTs regarding the efficacy of DSs and herbal interventions in patients with COVID-19. Overall findings indicated that herbal and zinc supplements might benefit COVID-19 case recovery. However, taking the evaluation results into account, we could not derive a firm conclusion due to the poor-quality evidence this review is based on.
Without a doubt, diet therapies are the most challenging to study to produce high-quality evidence. The overall quality of the eligible studies is poor, which could be attributed to the urgency of the outbreak that entailed a timely treatment, as well as varied intervention modalities, sessions, follow-up, and outcomes across the studies. Therefore, well-design of future high-quality RCTs should pay closer attention to randomization, blinding measures, and complete selective reporting.
Interestingly, although various DSs were reported potentially beneficial for COVID-19, our findings only showed consumption of herbal supplements and Zinc sulfate primarily may significantly benefit COVID-19 recovery, which is inconsistent with most reviews published at this time. This discrepancy may be an artifact of the current existing RCTs. Many other RCTs evaluating ginger [43] vitamin C [44] vitamin D [45], and Ayurvedic formulation [46] are presently being conducted and will provide evidence for coping with COVID-19 in the future.
To our best knowledge, natural substances can be used as either “supplements” to improve health or “medicines” for diseases. The context of usage of herbal medicine varies widely from country-to-country. Herbs are considered nutritional or as food supplements for general health and well-being in the US, while in China, herbs are consumed as medications for medical purposes or health foods for well-being [47]. For synergizing the therapeutic effects, except for the DS therapy, we searched herbal treatments related to COVID-19 from both Chinese and English literature databases. Among all the eligible RCTs, 50% are studies involving herbal formulations. Herbal practitioners generally believe that ingredient herbal prescriptions are usually safe and effective due to their long-term successful use. However, the interactions of the herbal ingredients may result in an efficacy change of the formulations, even enhance or ameliorate their AEs [48]. For example, one of the formulations discussed in this review, Lianhuaqingwen is composed of thirteen herbs, hence, studying the effect of a bioactive compound against SAR-CoV-2 inevitably poses a challenge for most researchers. It should be noted that RCTs have substantial limitations to detect rare AEs or long-term use [49]. In our review, only 33.3% of included studies reported detailed data, and no serious adverse events were demonstrated. However, considering the positive or negative potential interactions among the herbal formulation ingredients in COVID-19 management, the overall safety monitoring warrants further investigation. Future RCT design for COVID-19 management should consider the need to assess the possibility of herbal interactions and AEs associated with long-term use.
4.1. Strengths and limitations
Our systematic review has several strengths. Firstly, our database search strategy covers both English and Chinese databases, which are not available in other relevant published studies. Secondly, we can be confident that the previously relevant reviews were hypothetical treatments based on the literature. This study is the first review to comprehensively assess the extent and range of RCTs undertaken to evaluate dietary supplement interventions that support the COVID-19 epidemic and is now among the most updated direct dietary recommendation facilitating better clinical outcomes.
Nevertheless, important limitations of this research should also be noted. When interpreting findings regarding the impact of DSs and herbs on patients suffering from COVID-19, differences in study design should be considered. To reduce heterogeneity, we limited our analysis to RCTs only, excluding commentaries, cross-over trials, or case reports, which means that we could have missed some potentially important observational studies. It was difficult to compare these RCTs and generalize results because of the wide variation with the targeted population, modes of delivering the DS and herbal interventions, treatment sessions, outcomes, and follow-up periods. Based on evidence from this review, we found some promising effects of DSs and herbs on mitigating symptoms, improving inflammation markers, shortening recovery duration, and intensive care unit use of COVID-19 patients. However, most had a high or unclear risk of bias in many domains such as selection bias, detection bias, and reporting bias. Therefore, it is still premature to recommend those therapies in clinical practice. Further rigorous high-quality trials are required to confirm the efficacy of the DSs and herbs modalities for COVID-19.
5. Conclusion
Herbal supplements seem to be an effective adjunctive therapy for patients with COVID-19, zinc sulfate is likely to shorten olfactory recovery. DS and herbal therapy appeared to be safe and effective adjuvant treatments for patients with COVID-19. While this preliminary evidence showed a positive effect, the poor methodological quality of included studies makes it difficult to draw any firm conclusion. Caution is needed to translate these findings into clinical practice and standard care. More well-designed trials regarding interventions using DSs and herbs for COVID-19 are encouraged in the future.
Funding
This study is supported by Shenzhen Special Fund for Introducing High-Level Medical Team Project (SZSM201502044).
Authorship
Brent A. Bauer and Shaoyang Cui conceptualized the study. David C. Patchett and Mingzhu Xu designed the study. Zitong Feng, Run Lin, Huijun Yang, Liting Lai and Yixiao Wang searched, screened the data, Xuan Zhou, Kyung-Min Shin and Zitong Feng identified relevant articles and extracted and analyzed data. Zitong Feng and Juan Yang wrote the original draft. Dietlind L. Wahner-Roedler, Manisha Salinas, Molly J. Mallory, Chunzhi Tang, David C. Patchett, Brent A. Bauer and Mingzhu Xu reviewed and revised the manuscript. All authors approved the final manuscript.
Declaration of competing interest
The authors declare no conflict of interest.
Acknowledgments
The authors would thank The HEAD Foundation, Singapore for their support of this work.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.clnesp.2021.05.018.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lotfi M., Hamblin M.R., Rezaei N. COVID-19: transmission, prevention, and potential therapeutic opportunities. Clin Chim Acta. 2020;508:254–266. doi: 10.1016/j.cca.2020.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.WHO https://www.who.int/emergencies/diseases/novel-coronavirus-2019
- 4.Wang T.B., Du Z., Zhu F.X., Cao Z.L., An Y.Z., Gao Y., et al. Comorbidities and multi-organ injuries in the treatment of COVID-19. Lancet. 2020:395. doi: 10.1016/S0140-6736(20)30558-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mahmudpour M., Roozbeh J., Keshavarz M., Farrokhi S., Nabipour I. COVID-19 cytokine storm: the anger of inflammation. Cytokine. 2020;133:155151. doi: 10.1016/j.cyto.2020.155151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Melenotte C., Silvin A., Goubet A.G., Lahmar I., Dubuisson A., Zumla A., et al. Immune responses during COVID-19 infection. OncoImmunology. 2020;9:1807836. doi: 10.1080/2162402X.2020.1807836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hu B., Huang S., Yin L. The cytokine storm and COVID-19. J Med Virol. 2021;93:250–256. doi: 10.1002/jmv.26232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Laviano A., Koverech A., Zanetti M. Nutrition support in the time of SARS-CoV-2 (COVID-19) Nutrition. 2020;74:110834. doi: 10.1016/j.nut.2020.110834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.NIH . 1994. Dietary supplement health and education Act of.https://ods.od.nih.gov/factsheets/DietarySupplements-HealthProfessional/# [Google Scholar]
- 10.FDA Dietary supplement products & ingredients. https://www.fda.gov/food/dietary-supplements/dietary-supplement-products-ingredients
- 11.Thakkar S., Anklam E., Xu A., Ulberth F., Li J., Li B., et al. Regulatory landscape of dietary supplements and herbal medicines from a global perspective. Regul Toxicol Pharmacol. 2020;114:104647. doi: 10.1016/j.yrtph.2020.104647. [DOI] [PubMed] [Google Scholar]
- 12.Rashrash M., Schommer J.C., Brown L.M. Prevalence and predictors of herbal medicine use among adults in the United States. J Pat Exp. 2017;4:108–113. doi: 10.1177/2374373517706612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fernandez-Quintela A., Milton-Laskibar I., Trepiana J., Gomez-Zorita S., Kajarabille N., Leniz A., et al. Key aspects in nutritional management of COVID-19 patients. J Clin Med. 2020;9 doi: 10.3390/jcm9082589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Calder P.C., Carr A.C., Gombart A.F., Eggersdorfer M. Optimal nutritional status for a well-functioning immune system is an important factor to protect against viral infections. Nutrients. 2020;12:1181. doi: 10.3390/nu12041181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iddir M., Brito A., Dingeo G., Fernandez Del Campo S.S., Samouda H., La Frano M.R., et al. Strengthening the immune system and reducing inflammation and oxidative stress through diet and nutrition: considerations during the COVID-19 crisis. Nutrients. 2020;12:1562. doi: 10.3390/nu12061562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zupo R., Castellana F., Sardone R., Sila A., Giagulli V.A., Triggiani V., et al. Preliminary trajectories in dietary behaviors during the COVID-19 pandemic: a public health call to action to face obesity. Int J Environ Res Publ Health. 2020;17 doi: 10.3390/ijerph17197073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhao A., Li Z., Ke Y., Huo S., Ma Y., Zhang Y., et al. Dietary diversity among Chinese residents during the COVID-19 outbreak and its associated factors. Nutrients. 2020;12:1699. doi: 10.3390/nu12061699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hys K. Springer; 2020. Identification of the reasons why individual consumers purchase dietary supplements. Perspectives on Consumer Behaviour; pp. 193–209. [Google Scholar]
- 19.Jayawardena R., Sooriyaarachchi P., Chourdakis M., Jeewandara C., Ranasinghe P. Enhancing immunity in viral infections, with special emphasis on COVID-19: a review. Diab Metab Synd: Clin Res Rev. 2020;14:367–382. doi: 10.1016/j.dsx.2020.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Conte L., Toraldo D.M. Targeting the gut-lung microbiota axis by means of a high-fibre diet and probiotics may have anti-inflammatory effects in COVID-19 infection. Ther Adv Respir Dis. 2020;14 doi: 10.1177/1753466620937170. 1753466620937170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hathaway D., Pandav K., Patel M., Riva-Moscoso A., Singh B.M., Patel A., et al. Omega 3 fatty acids and COVID-19: a comprehensive review. Infect Chemother. 2020;52:478–495. doi: 10.3947/ic.2020.52.4.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moher D., Liberati A., Tetzlaff J., Altman D.G. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6 [PMC free article] [PubMed] [Google Scholar]
- 23.Altman D.G., Schulz K.F., Moher D., Egger M., Davidoff F., Elbourne D., et al. The revised CONSORT statement for reporting randomized trials: explanation and elaboration. Ann Intern Med. 2001;134:663–694. doi: 10.7326/0003-4819-134-8-200104170-00012. [DOI] [PubMed] [Google Scholar]
- 24.Higgins J.P.T., Altman D.G., Gøtzsche P.C., Jüni P., Moher D., Oxman A.D., et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jadad A.R., Moore R.A., Carroll D., Jenkinson C., Reynolds D.J.M., Gavaghan D.J., et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Contr Clin Trials. 1996;17:1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 26.Barnabas R.V., Brown E.R., Bershteyn A., Stankiewicz Karita H.C., Johnston C., Thorpe L.E., et al. Hydroxychloroquine as postexposure prophylaxis to prevent severe acute respiratory syndrome coronavirus 2 infection : a randomized trial. Ann Intern Med. 2020;8:8. doi: 10.7326/M20-6519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cao Y., Wei J., Zou L., Jiang T., Wang G., Chen L., et al. Ruxolitinib in treatment of severe coronavirus disease 2019 (COVID-19): a multicenter, single-blind, randomized controlled trial. J Allergy Clin Immunol. 2020;146:137–146. doi: 10.1016/j.jaci.2020.05.019. e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhao J., Yang X., Wang C., Song S., Cao K., Wei T., et al. Yidu-toxicity blocking lung decoction ameliorates inflammation in severe pneumonia of SARS-COV-2 patients with Yidu-toxicity blocking lung syndrome by eliminating IL-6 and TNF-a. Biomed Pharmacother. 2020;129:110436. doi: 10.1016/j.biopha.2020.110436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang Y., Yang X., Liu Y., Zhang J., Feng Y., Shang L., et al. Clinical effect of the treatment of novel coronavirus pneumonia by internal administration of traditional Chinese medicine plus fumigation and absorption combined with super dose of vitamin C in treating COVID-19. J Xi'an Jiaot Univ (Med Sci) 2020;41:931–935. [Chinese] [Google Scholar]
- 30.Huang T.T., Yan C.H., Wang L., Hu X. Application of low-molecular-weight collagen peptide in oral nutrition supplements on patients with COVID-19. Food Nutr China. 2020:1–6. [Google Scholar]
- 31.Yu P., Li Y.Z., Wan S.B., Wang Y. Effects of Lianhua qingwen granules plus arbidol on treatment of mild Corona virus disease-19. [Chinese] Chin Pharmaceut J. 2020;55:1042–1045. [Google Scholar]
- 32.Xiong W.-Z., Wang G., Du J., Ai W. Efficacy of herbal medicine (Xuanfei Baidu decoction) combined with conventional drug in treating COVID-19:A pilot randomized clinical trial. Integr Med Res. 2020;9:100489. doi: 10.1016/j.imr.2020.100489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xiao M., Tian J., Zhou Y., Xu X., Min X., Lv Y., et al. Efficacy of Huoxiang Zhengqi dropping pills and Lianhua Qingwen granules in treatment of COVID-19: a randomized controlled trial. Pharmacol Res. 2020;161:105126. doi: 10.1016/j.phrs.2020.105126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang J.B., Wang Z.X., Jing J., Zhao P., Dong J.H., Zhou Y.F., et al. Exploring an integrative therapy for treating COVID-19: a randomized controlled trial. Chin J Integr Med. 2020;26:648–655. doi: 10.1007/s11655-020-3426-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Valizadeh H., Abdolmohammadi-Vahid S., Danshina S., Ziya Gencer M., Ammari A., Sadeghi A., et al. Nano-curcumin therapy, a promising method in modulating inflammatory cytokines in COVID-19 patients. Int Immunopharm. 2020;89:107088. doi: 10.1016/j.intimp.2020.107088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thomas S., Patel D., Bittel B., Wolski K., Wang Q., Kumar A., et al. Effect of high-dose zinc and ascorbic acid supplementation vs usual care on symptom length and reduction among ambulatory patients with SARS-CoV-2 infection: the COVID A to Z randomized clinical trial. JAMA Netw. 2021;4 doi: 10.1001/jamanetworkopen.2021.0369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sardari S., Mobaien A., Ghassemifard L., Kamali K., Khavasi N. Therapeutic effect of thyme (Thymus vulgaris) essential oil on patients with covid19: a randomized clinical trial. J Adv Med Biomed Res. 2021;29:83–91. [Google Scholar]
- 38.Murai I.H., Fernandes A.L., Sales L.P., Pinto A.J., Goessler K.F., Duran C.S.C., et al. Effect of a single high dose of vitamin D3 on hospital length of stay in patients with moderate to severe COVID-19: a randomized clinical trial. Jama. 2021;325:1053–1060. doi: 10.1001/jama.2020.26848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu Z.L., Li X.H., Gou C.Y., Li L., Luo X.L., Zhang C., et al. Effect of Jinhua Qinggan granules on novel coronavirus pneumonia in patients. J Tradit Chin Med. 2020;40:467–472. doi: 10.19852/j.cnki.jtcm.2020.03.016. [DOI] [PubMed] [Google Scholar]
- 40.Hu K., Guan W.-J., Bi Y., Zhang W., Li L., Zhang B., et al. Efficacy and safety of Lianhuaqingwen capsules, a repurposed Chinese herb, in patients with coronavirus disease 2019: a multicenter, prospective, randomized controlled trial. Phytomedicine. 2020:153242. doi: 10.1016/j.phymed.2020.153242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Abdelmaksoud A.A., Ghweil A.A., Hassan M.H., Rashad A., Khodeary A., Aref Z.F., et al. Olfactory disturbances as presenting manifestation among Egyptian patients with COVID-19: possible role of zinc. Biol Trace Elem Res. 2021;7:7. doi: 10.1007/s12011-020-02546-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Abd-Elsalam S., Soliman S., Esmail E.S., Khalaf M., Mostafa E.F., Medhat M.A., et al. Do zinc supplements enhance the clinical efficacy of hydroxychloroquine?: a randomized, multicenter trial. Biol Trace Elem Res. 2020;27:27. doi: 10.1007/s12011-020-02512-1. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 43.Irct20200506047323N. The effects of Ginger in treatment COVID-19. 2020. http://wwwwhoint/trialsearch/Trial2aspx?TrialID=IRCT20200506047323N1 [Google Scholar]
- 44.Ctri . 2020. Can Vitamin C reduces COVID-19 symptoms.http://wwwwhoint/trialsearch/Trial2aspx?TrialID=CTRI 10. [Google Scholar]
- 45.Nct . 2020. Vitamin D and COVID-19 trial.https://clinicaltrialsgov/show/NCT04536298 [Google Scholar]
- 46.Ctri . 2020. Ayurvedic rasayana therapies.http://wwwwhoint/trialsearch/Trial2aspx?TrialID=CTRI 07. [Google Scholar]
- 47.Lu W.I., Lu D.P. Impact of Chinese herbal medicine on american society and health care system: perspective and concern. Evid base Compl Alternative Med. 2014;2014 doi: 10.1155/2014/251891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Enioutina E.Y., Salis E.R., Job K.M., Gubarev M.I., Krepkova L.V., Sherwin C.M. Herbal Medicines: challenges in the modern world. Part 5. status and current directions of complementary and alternative herbal medicine worldwide. Expet Rev Clin Pharmacol. 2017;10:327–338. doi: 10.1080/17512433.2017.1268917. [DOI] [PubMed] [Google Scholar]
- 49.Berlin J.A., Glasser S.C., Ellenberg S.S. Adverse event detection in drug development: recommendations and obligations beyond phase 3. Am J Publ Health. 2008;98:1366–1371. doi: 10.2105/AJPH.2007.124537. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.