Abstract
Zinc (Zn2+) is important in cellular processes. In the cell, free zinc is tightly regulated and found in minuscule amounts. However, in an unhealthy cellular environment, such as hypoxia, zinc increases in the cell and zinc overload may occur. Studies have shown that zinc overload causes cellular and mitochondrial stress. Mitochondrial stress affects mitochondrial morphology. In normal cells, mitochondrial morphology resembles a long, tubular shape. In unhealthy cells, mitochondrial morphology resembles fragmented, circular shape. To address whether zinc overload contributes directly to the abnormal changes of mitochondrial morphology, we imaged and analyzed mitochondria that were treated with the application of exogenous zinc. In the first part of the study, exogenous zinc was applied to HeLa cells at 1 µM, 10 µM, 50 µM, 100 µM, or 200 µM zinc chloride along with 10 µM pyrithione. Mitochondrial morphology was analyzed with Mito-Morphology micro in ImageJ. Mitochondrial morphology changed from a healthy tubular shape to an unhealthy circular shape and fragmentation. Mitochondrial morphology changes were observed in a dose-dependent fashion. The second part of the study involved applying the metal ion chelator TPEN after applying 50 µM zinc chloride along with 10 µM pyrithione. TPEN reduced zinc-induced abnormal mitochondrial morphology after zinc treatment. This present study supports that zinc overload may cause morphology changes induced by mitochondrial stress that may lead to cell death.
Keywords: Zinc, cytotoxicity, organelles, mitochondria
Introduction
Zinc has many physiological functions in the cell [1-3]. In healthy cells, zinc is tightly regulated [4,5]. The balance and imbalance of zinc in the cell holds implications for cellular biology. In healthy cells, zinc concentration is measured in the picomolar range intracellularly and extracellularly [6,7]. When zinc rises in concentration, it is toxic to the cell [8-10]. When the levels of zinc is around the high nanomolar to micromolar range, the cell undergoes zinc overload and zinc induced cytotoxicity [11,12]. Zinc disregulation in the cell causes pathological changes [5]. Membrane bound organelles, such as mitochondria, store zinc in healthy cells [5,13-17]. During pathological states, such as ischemia, zinc regulation in the cell and mitochondria becomes skewed which eventually causes a high generation of ROS and cytotoxicity [10,18,19].
Mitochondrial morphology changes hold great significance to cellular health. In healthy cells, high amount of fusion occurs along with low amounts of fission [20-22]. Mitochondria appear to have connected tubular morphology with few circular mitochondria [20,21]. Cells with high amounts of fusion and tubular morphology are less prone to undergo cell death and have less mitophagy [20]. In unhealthy cells, high amounts of fission occurs causing fragmentation and circular mitochondria [20,21]. Cells with high amounts of fission and circular mitochondria are more prone to undergo cell death and have mitophagy occurring in the cell [20,21,23]. It has been shown that mitochondrial stress may cause abnormal mitochondrial morphology [20,21]. These types of morphological changes are seen in medical conditions such as metabolic disorders, stroke, and disorders of aging [22-25]. In metabolic disorders, such as diabetes, the mitochondria in pancreatic β-cells appear to be swollen [24]. Fragmented mitochondria or altered morphology has been observed in aging disorders, such as Alzheimer’s disease, Parkinson’s disease, and ischemic stroke [22,25-27].
Increases of zinc concentration through zinc overload and zinc induced cytotoxicity may cause mitochondrial stress through mitochondrial damage and ROS generation [10,28]. This type of stress may impact mitochondrial morphology, which is an indicator of mitochondrial and cellular health [20,21,23]. In this study, mitochondria morphology was observed and analyzed after varying concentrations of zinc application to address whether zinc overload contributes directly to the abnormal changes of mitochondrial morphology. Our results show that moderate and high concentrations of zinc caused morphological changes in a dose-dependent fashion and that TPEN, a zinc chelator, reduced mitochondrial morphology changes after zinc treatment was applied.
Materials and methods
Preparation and staining of the HeLa cell line
The HeLa cell line (ATCC, Manassas, VA, USA) was cultured on a petri dish with a 35 mm diameter glass bottom (MatTek Corporation, Ashland, MA, USA). The cell line was cultured in EMEM (Gibco) with 4% FBS at 37°C in 5% CO2, 95% air [5]. The dye used to label the mitochondria was Mitotracker Red FM (Invitrogen). The cells were incubated for 30 minutes in 1 mL of dye solution in HBSS (100 nM of Mitotracker Red FM) at 37°C, 5% CO2. After incubation, the cells were washed three times with HBSS buffer [5].
Zinc and TPEN treatments
Fluorescent microscopy was used to randomly image cells in culture. The different concentrations of zinc used in the study was 1 µM, 10 µM, 50 µM, 100 µM, and 200 µM zinc chloride. Low concentrations of zinc were 1 µM and 10 µM zinc chloride. The moderate concentration of zinc was 50 µM zinc chloride. High concentrations of zinc were 100 µM and 200 µM zinc chloride. The zinc ionophore used in the study was 10 µM pyrithione. Zinc was applied for 30 minutes. After the incubation period, cells were imaged in a random fashion. After imaging, cells were processed and were statistically analyzed. The TPEN posttreatment consisted of the addition of 50 µM zinc for 15 minutes then the removal of zinc solution. After the removal of zinc solution, 50 µM TPEN was added for five minutes. The TPEN had a 0.15% final concentration of DMSO. After the incubation period, cells were imaged in a random fashion. After imaging, cells were processed and were statistically analyzed.
Fluorescent microscopy and mitochondrial morphology analysis
Fluorescent signals were detected using customized fluorescent microscope. ImagePro Plus software (Media Cybernetics, Silver Spring, MD, USA) was used to capture the image at exposures around 1 s (For excitation: blue filter: 470/70 nm, green filter: 517/30 nm; for emission: green filter-517/30 nm, red filter-620/40 nm; Filters were purchased from Chroma) [5]. The area of the cell that was evaluated was the process of the cell. The process of the cell was appropriate for mitochondrial morphology analysis for several reasons. First of all, there are other organelles surrounding the nucleus. This could make it difficult to distinguish the mitochondrial network. Secondly, dye saturation around the nucleus may make it difficult to distinguish the mitochondrial network. Finally, the mitochondrial network has clearer boundaries and is easier to establish in the process of the cell [29]. There was a cut-off of the process from the nuclear area. The region from the nucleus that was not saturated was the cut-off in the cell. A clear boundary of the mitochondrial network must also be present in the cell process [29].
Mitochondrial morphology and analysis
The ImageJ macro called Mito-Morphology was used to analyze the images [29,30]. The area of the cell that was going to be evaluated was transferred to a blank image with a black background. The same threshold was used to cover the mitochondrial network in all of the cells. Then, the mitochondrial network was measured using the Mito-Morphology macro. There was a default size cut-off for Mito-Morphology. The cut-off was 30 pixels to 100000000 pixels [29,30]. The parameters determined by Mito-Morphology were the aspect ratio, circularity, and major axis. The aspect ratio is the ratio of the major axis to the minor axis (Aspect ratio = Major Axis/Minor Axis) [25]. The circularity is how closely the mitochondria represent a perfect circle. Circularity lies between the value of 0 and 1 (Circularity = 4π (mean area/mean perimeter2)). The value of 1.0 is a mitochondrion that represents a perfect circle [29]. The major axis is the longest axis of the mitochondrion.
Statistical analysis
A one-way ANOVA was used to assess the effects of different zinc concentrations on the three mitochondrial parameters. Post hoc comparison was conducted using Tukey HSD test. P-values under 0.05 were considered significant. The statistical software used to analyze the data was Prism.
Results
To study the effect of zinc on mitochondrial morphology changes, different concentrations of exogenous zinc along with a pyrithione vehicle was applied to the cell culture, and the cell culture was imaged with fluorescent microscopy. If zinc affected mitochondrial morphology, the mitochondria would have a fragmented and circular shape rather than a tubular and elongated shape. Before treatment, the mitochondria appeared to be a connected tubular shape in all groups, and to have elongated and tubular mitochondria. As shown in Figure 1, zinc treatment caused mitochondrial morphological changes. In the presence of low concentration of zinc treatments (Figure 1B, 1C), the cells in this group also appeared to have elongated and tubular mitochondria. With the increases of zinc concentration, the cells appeared to have fragmented and circular mitochondria (Figure 1D-F), indicating zinc induced cytotoxicity. These results suggest that moderate and large concentrations of zinc cause visible changes to mitochondrial morphology.
Figure 1.
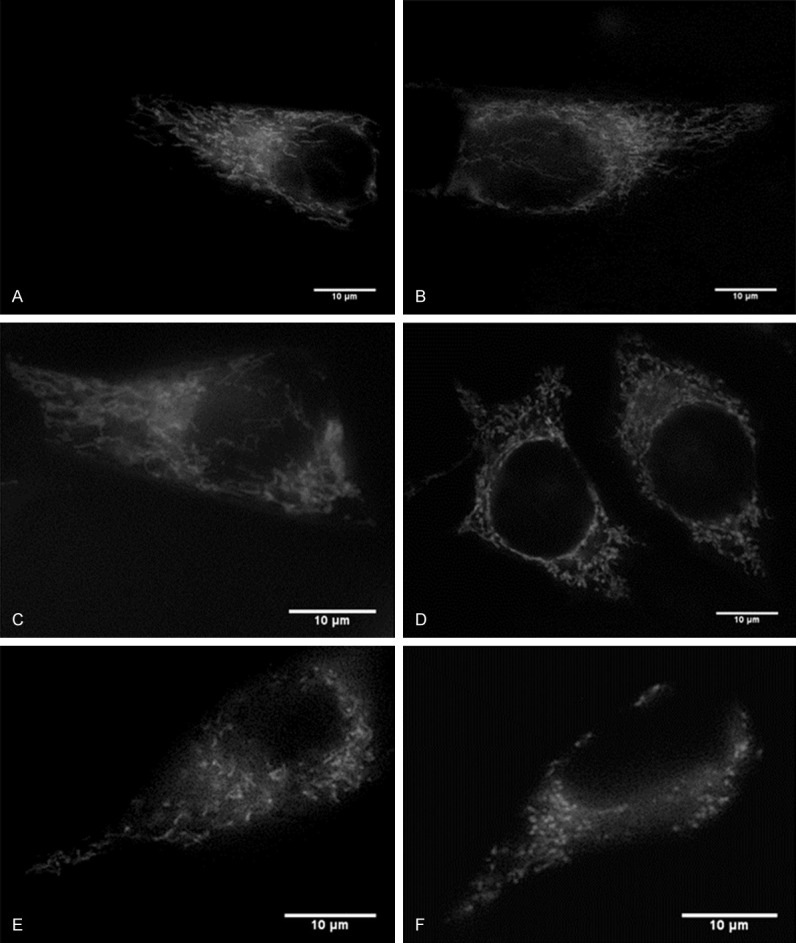
Effects of zinc on mitochondrial morphology in the HeLa Cell Line. (A) Shows a representative cell of control (no treatment) with mostly tubular mitochondria. (B) Shows a representative cell with 1 μM ZnCl2 treatment with mostly tubular mitochondria. (C) Shows a representative cell with 10 μM ZnCl2 with mostly tubular mitochondria, (D) Shows a representative cell with 50 μM ZnCl2 with mostly circular and fragmented mitochondria, (E) Shows a representative cell with 100 μM ZnCl2 with mostly circular and fragmented mitochondria, and (F) Shows a representative cell with 200 μM ZnCl2 with mostly circular and fragmented mitochondria. Scale bars: 10 μm. Pyrithione (10 µM) was presented with all zinc treatments. The cells were observed under fluorescent microscopy at 100X.
We further analyzed zinc application-induced abnormal mitochondrial morphology or cytotoxicity. The software used was ImageJ macro called Mito-Morphology (see Methods) to measure the changes of mitochondrial morphology. If zinc affected mitochondrial morphology, the mitochondria would have a fragmented and circular shape rather than a tubular and elongated shape. The data are summarized in Figure 2. Zinc treatment caused higher circularity (Figure 2A). In Figure 2A1, the histogram shows that the moderate and high concentration groups were grouped together with significant increase in circularity ([F(5,1059) = 14.34, P<0.0001]) than the other treatment groups. Figure 2B shows that moderate and high concentration groups were grouped together with a shorter major axial length than the other treatment groups, as indicated by significant decreases in the major axis ([F(5,1059) = 10.86, P<0.0001]) (Figure 2B1). Moderate and high concentrations of zinc also caused a significant decrease in the aspect ratio ([F(5,1059) = 17.04, P<0.0001]) (Figure 2C).
Figure 2.
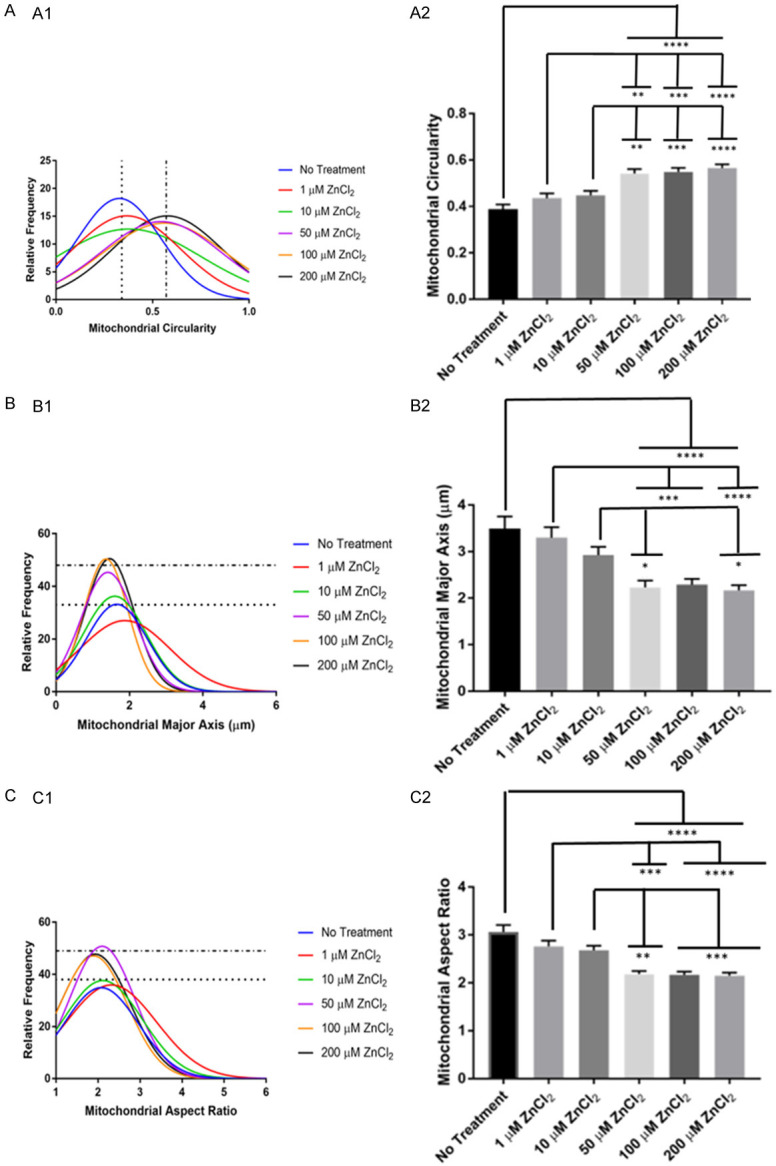
Analysis of zinc induced abnormalities of mitochondrial morphology. Histograms (A1, B1, C1) and bar graphs (A2, B2, C2) of the parameters of No Treatment, 1 µM ZnCl2, 10 µM ZnCl2, 50 µM ZnCl2, 100 µM ZnCl2, and 200 µM ZnCl2. (A) Represents the mitochondrial circularity, (B) Represents the mitochondrial major axis, (C) Represents the aspect ratio. Histogram relative frequency is in percentages. Bar graph data represent means + SE, *P<0.05, **P≤0.01, ***P≤0.001, ****P≤0.0001. No treatment - n = 135 mitochondria from 8 cells, 8 trials; 1 µM ZnCl2 - n = 151 mitochondria from 9 cells, 8 trials; 10 µM ZnCl2 - 199 mitochondria from 8 cells, 8 trials; 50 µM ZnCl2 - 170 mitochondria from 7 cells, 6 trials; 100 µM ZnCl2 - 210 mitochondria from 9 cells, 8 trials; 200 µM ZnCl2 - 200 mitochondria from 9 cells, 7 trials.
We investigated further if the application of zinc chelator, such as TPEN, would reduce zinc induced-abnormal mitochondrial morphology or cytotoxicity. If the TPEN application reduced mitochondrial morphology changes, the mitochondria would have a tubular and elongated shape rather than a fragmented and circular shape. The data are summarized in Figure 3. TPEN mitigated zinc-induced mitochondrial morphology changes as shown in Figure 3A. The mitochondria appeared to be elongated and tubular in both the control and TPEN posttreatment cells. TPEN posttreatment group was significantly lower in circularity compared to the 50 µM ZnCl2 group ([F(2,461) = 16.27, P<0.0001]) (Figure 3B1). In Figure 3B2, the bar graph shows that the TPEN posttreatment group has a significantly lower mean circularity than the 50 µM ZnCl2 group. TPEN posttreatment group had a significantly larger major axis than 50 µM ZnCl2 group ([F(2,461) = 8.652, P = 0.0002]) (Figure 3C1), and a larger average major axis than 50 µM ZnCl2 group (Figure 3C2). TPEN posttreatment had a significantly larger aspect ratio than the 50 µM ZnCl2 group ([F(2,461) = 12.47, P<0.0001]) (Figure 3D1), and a larger average aspect ratio than 50 µM ZnCl2 group (Figure 3D2). These results suggest that TPEN posttreatment reduces abnormal mitochondrial morphology changes.
Figure 3.
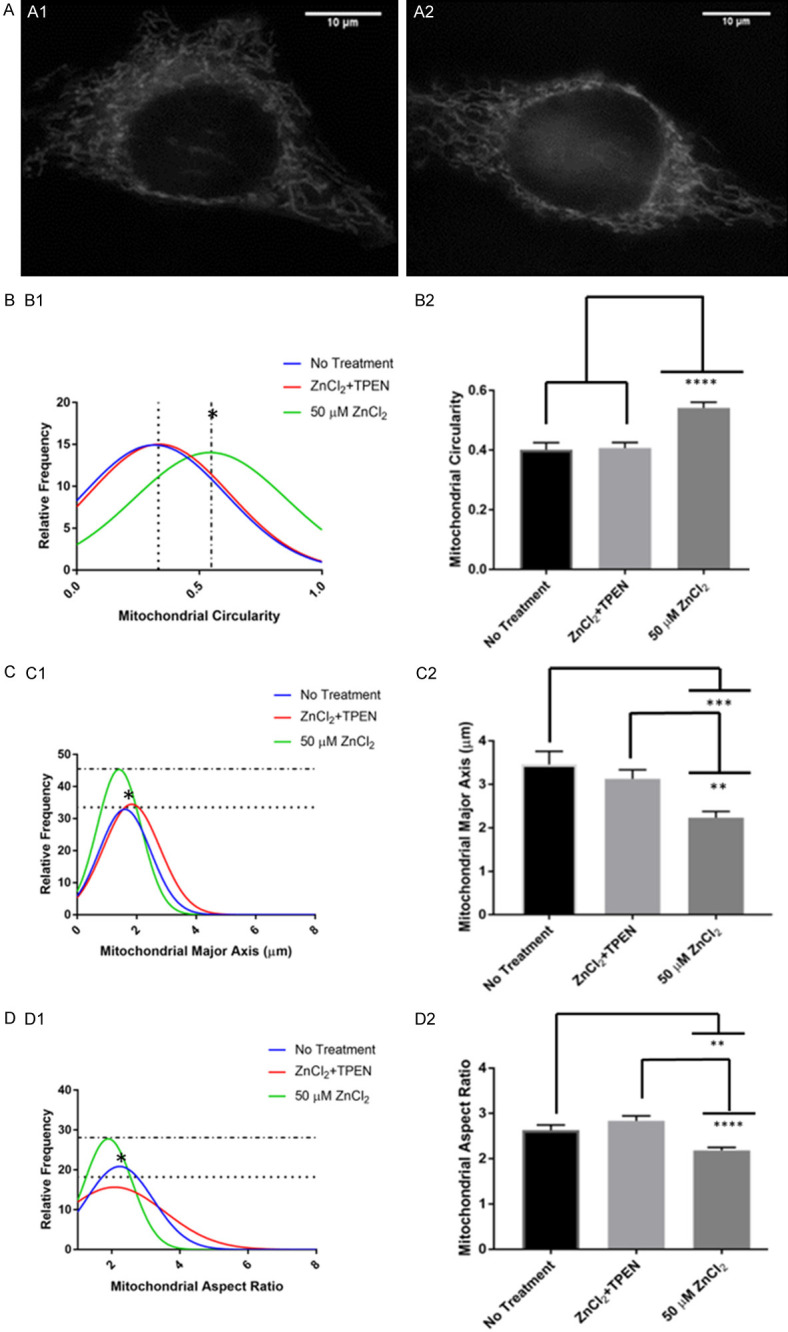
TPEN Posttreatment mitigated zinc-induced mitochondrial morphology changes. (A1) Shows a representative cell with no treatment with mostly tubular mitochondria, (A2) Shows a representative cell with 50 μM ZnCl2 and with TPEN Posttreatment with mostly tubular mitochondria. The cells were observed under fluorescent microscopy at 100X. The histogram (B1, C1, D1) and bar graphs (B2, C2, D2) of the parameters of No Treatment, ZnCl2 + TPEN, and 50 µM ZnCl2 treatments. (B) Represents the mitochondrial circularity, (C) Represents the mitochondrial major axis, (D) Represents the aspect ratio. Histogram relative frequency is in percentages. The * on histogram signifies significance. Bar graph data represent means + SE, *P<0.05, **P≤0.01, ***P≤0.001, ****P≤0.0001. ZnCl2 + TPEN represents TPEN post-treatment. No Treatment - n = 112 mitochondria from 8 cells, 6 trials; 50 µM ZnCl2 - 170 mitochondria from 7 cells, 6 trials; TPEN post-treatment - n = 182 mitochondria from 8 cells, 6 trials.
Discussion
Mitochondrial morphology indicates the overall health of the cell [20-22]. In unhealthy cells undergoing apoptosis and high ROS, mitochondria appear to have fragmentation and circular mitochondria from fission [20]. The results in the present study support previous morphological studies concerning apoptosis and ROS generation. In this study, we report that zinc overload and zinc cytotoxicity led to abnormal morphological changes in the cell, in which mitochondria had shortened in length and appeared circular. There was an overall increase in the circularity, decrease in major axis, and a decrease in the aspect ratio. When TPEN was applied after applying moderate amount of zinc, abnormal mitochondrial morphology was reduced. This implicates that zinc application causes high ROS production and may even cause apoptosis which is in general agreement with previous observations [10,28,31,32].
Past studies have shown that intracellular zinc is transported and stored in the mitochondria [5,13-17]. Then, high amounts of zinc in the mitochondria causes intramitochondrial zinc overload and ROS generation [10]. Eventually, zinc is released from the mitochondria and other zinc-storing organelles. This causes zinc overload and zinc cytotoxicity [5,10]. Furthermore, zinc also induces NADPH oxidase which contributes to ROS production in the cell [33]. This stress effects mitochondrial structure and function through apoptosis and increased ROS generation [10,34,35].
Studies using zinc chelators have also been done to observe zinc effects on mitochondrial functioning [36-38]. Zinc chelators reduce morphology changes, thus decreasing ROS production and preventing apoptosis [10,18,39]. In summary, the present study supports that zinc overload leads to mitochondrial stress in the cell. This stress may cause mitochondrial morphology changes that could eventually lead to cell death. The present study is unique from past studies because it observed morphological changes and measured the parameters of these changes after zinc application. This is consistent with cellular stress and increased cellular and mitochondrial ROS, which supports the findings of previous studies. Applying the zinc chelator TPEN after zinc treatment prevented zinc overload and the associated changes to mitochondrial morphology.
Disclosure of conflict of interest
None.
References
- 1.Frederickson C. Zinc signal-secreting cells. In: Kretsinger R, Uversky V, Permyakov E, editors. Encyclopedia of metalloproteins. New York: Springer; 2013. pp. 2506–2514. [Google Scholar]
- 2.Li Y, Frederickson C. Zinc-secreting neurons, gluzincergic and zincergic neurons. In: Kretsinger R, Uversky V, Permyakov E, editors. Encyclopedia of Metalloproteins. New York: Springer; 2013. pp. 2565–2571. [Google Scholar]
- 3.Li M, Inoue K, Branigan D, Kratzer E, Hansen JC, Chen JW, Simon RP, Xiong ZG. Acid-sensing ion channels in acidosis-induced injury of human brain neurons. J Cereb Blood Flow Metab. 2010;30:1247–1260. doi: 10.1038/jcbfm.2010.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Krezel A, Maret W. The biological inorganic chemistry of zinc ions. Arch Biochem Biophys. 2016;611:3–19. doi: 10.1016/j.abb.2016.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lu Q, Haragopal H, Slepchenko KG, Stork C, Li YV. Intracellular zinc distribution in mitochondria, ER and the Golgi apparatus. Int J Physiol Pathophysiol Pharmacol. 2016;8:35–43. [PMC free article] [PubMed] [Google Scholar]
- 6.Li Y, Maret W. Transient fluctuations of intracellular zinc ions in cell proliferation. Exp Cell Res. 2009;315:2463–2470. doi: 10.1016/j.yexcr.2009.05.016. [DOI] [PubMed] [Google Scholar]
- 7.Shen Z, Haragopal H, Li YV. Zinc modulates synaptic transmission by differentially regulating synaptic glutamate homeostasis in hippocampus. Eur J Neurosci. 2020;52:3710–3722. doi: 10.1111/ejn.14749. [DOI] [PubMed] [Google Scholar]
- 8.Stork CJ, Li YV. Elevated cytoplasmic free zinc and increased reactive oxygen species generation in the context of brain injury. Acta Neurochir Suppl. 2016;121:347–353. doi: 10.1007/978-3-319-18497-5_60. [DOI] [PubMed] [Google Scholar]
- 9.Pan R, Chen C, Liu WL, Liu KJ. Zinc promotes the death of hypoxic astrocytes by upregulating hypoxia-induced hypoxia-inducible factor-1alpha expression via poly(ADP-ribose) polymerase-1. CNS Neurosci Ther. 2013;19:511–520. doi: 10.1111/cns.12098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Slepchenko KG, Lu Q, Li YV. Cross talk between increased intracellular zinc (Zn(2+)) and accumulation of reactive oxygen species in chemical ischemia. Am J Physiol Cell Physiol. 2017;313:C448–C459. doi: 10.1152/ajpcell.00048.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stork CJ, Li YV. Rising zinc: a significant cause of ischemic neuronal death in the CA1 region of rat hippocampus. J Cereb Blood Flow Metab. 2009;29:1399–408. doi: 10.1038/jcbfm.2009.64. [DOI] [PubMed] [Google Scholar]
- 12.Li YV. Zinc overload in stroke. In: Li YV, Zhang JH, editors. Metal Ion in Stroke. 1st. New York: Springer Science+Business Media; 2012. pp. 167–189. [Google Scholar]
- 13.Stork CJ, Li YV. Zinc release from thapsigargin/IP3-sensitive stores in cultured cortical neurons. J Mol Signal. 2010;5:5. doi: 10.1186/1750-2187-5-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marino J, Garcia Vior MC, Furmento VA, Blank VC, Awruch J, Roguin LP. Lysosomal and mitochondrial permeabilization mediates zinc(II) cationic phthalocyanine phototoxicity. Int J Biochem Cell Biol. 2013;45:2553–2562. doi: 10.1016/j.biocel.2013.08.012. [DOI] [PubMed] [Google Scholar]
- 15.Medvedeva YV, Weiss JH. Intramitochondrial Zn2+ accumulation via the Ca2+ uniporter contributes to acute ischemic neurodegeneration. Neurobiol Dis. 2014;68:137–144. doi: 10.1016/j.nbd.2014.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jiang D, Sullivan PG, Sensi SL, Steward O, Weiss JH. Zn(2+) induces permeability transition pore opening and release of pro-apoptotic peptides from neuronal mitochondria. J Biol Chem. 2001;276:47524–47529. doi: 10.1074/jbc.M108834200. [DOI] [PubMed] [Google Scholar]
- 17.Inoue K, O’Bryant Z, Xiong ZG. Zinc-permeable ion channels: effects on intracellular zinc dynamics and potential physiological/pathophysiological significance. Curr Med Chem. 2015;22:1248–1257. doi: 10.2174/0929867322666150209153750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang Y, Xing F, Zheng H, Xi J, Cui X, Xu Z. Roles of mitochondrial Src tyrosine kinase and zinc in nitric oxide-induced cardioprotection against ischemia/reperfusion injury. Free Radic Res. 2013;47:517–525. doi: 10.3109/10715762.2013.796044. [DOI] [PubMed] [Google Scholar]
- 19.Shuttleworth CW, Weiss JH. Zinc: new clues to diverse roles in brain ischemia. Trends Pharmacol Sci. 2011;32:480–486. doi: 10.1016/j.tips.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Picard M, Shirihai OS, Gentil BJ, Burelle Y. Mitochondrial morphology transitions and functions: implications for retrograde signaling? Am J Physiol Regul Integr Comp Physiol. 2013;304:R393–406. doi: 10.1152/ajpregu.00584.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ahmad T, Aggarwal K, Pattnaik B, Mukherjee S, Sethi T, Tiwari BK, Kumar M, Micheal A, Mabalirajan U, Ghosh B, Sinha Roy S, Agrawal A. Computational classification of mitochondrial shapes reflects stress and redox state. Cell Death Dis. 2013;4:e461. doi: 10.1038/cddis.2012.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wiemerslage L, Ismael S, Lee D. Early alterations of mitochondrial morphology in dopaminergic neurons from Parkinson’s disease-like pathology and time-dependent neuroprotection with D2 receptor activation. Mitochondrion. 2016;30:138–147. doi: 10.1016/j.mito.2016.07.004. [DOI] [PubMed] [Google Scholar]
- 23.Osellame LD, Blacker TS, Duchen MR. Cellular and molecular mechanisms of mitochondrial function. Best Pract Res Clin Endocrinol Metab. 2012;26:711–723. doi: 10.1016/j.beem.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yoon Y, Galloway CA, Jhun BS, Yu T. Mitochondrial dynamics in diabetes. Antioxid Redox Signal. 2011;14:439–457. doi: 10.1089/ars.2010.3286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mortiboys H, Thomas KJ, Koopman WJ, Klaffke S, Abou-Sleiman P, Olpin S, Wood NW, Willems PH, Smeitink JA, Cookson MR, Bandmann O. Mitochondrial function and morphology are impaired in parkin-mutant fibroblasts. Ann Neurol. 2008;64:555–565. doi: 10.1002/ana.21492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Solenski NJ, diPierro CG, Trimmer PA, Kwan AL, Helm GA. Ultrastructural changes of neuronal mitochondria after transient and permanent cerebral ischemia. Stroke. 2002;33:816–824. doi: 10.1161/hs0302.104541. [DOI] [PubMed] [Google Scholar]
- 27.Dagda RK, Zhu J, Chu CT. Mitochondrial kinases in Parkinson’s disease: converging insights from neurotoxin and genetic models. Mitochondrion. 2009;9:289–298. doi: 10.1016/j.mito.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Granzotto A, Sensi SL. Intracellular zinc is a critical intermediate in the excitotoxic cascade. Neurobiol Dis. 2015;81:25–37. doi: 10.1016/j.nbd.2015.04.010. [DOI] [PubMed] [Google Scholar]
- 29.Wiemerslage L, Lee D. Quantification of mitochondrial morphology in neurites of dopaminergic neurons using multiple parameters. J Neurosci Methods. 2016;262:56–65. doi: 10.1016/j.jneumeth.2016.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dagda RK, Cherra SJ 3rd, Kulich SM, Tandon A, Park D, Chu CT. Loss of PINK1 function promotes mitophagy through effects on oxidative stress and mitochondrial fission. J Biol Chem. 2009;284:13843–13855. doi: 10.1074/jbc.M808515200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yu KN, Yoon TJ, Minai-Tehrani A, Kim JE, Park SJ, Jeong MS, Ha SW, Lee JK, Kim JS, Cho MH. Zinc oxide nanoparticle induced autophagic cell death and mitochondrial damage via reactive oxygen species generation. Toxicol In Vitro. 2013;27:1187–1195. doi: 10.1016/j.tiv.2013.02.010. [DOI] [PubMed] [Google Scholar]
- 32.Wu W, Bromberg PA, Samet JM. Zinc ions as effectors of environmental oxidative lung injury. Free Radic Biol Med. 2013;65:57–69. doi: 10.1016/j.freeradbiomed.2013.05.048. [DOI] [PubMed] [Google Scholar]
- 33.Slepchenko KG, Lu Q, Li YV. Zinc wave during the treatment of hypoxia is required for initial reactive oxygen species activation in mitochondria. Int J Physiol Pathophysiol Pharmacol. 2016;8:44–51. [PMC free article] [PubMed] [Google Scholar]
- 34.Dikalov S. Cross talk between mitochondria and NADPH oxidases. Free Radic Biol Med. 2011;51:1289–1301. doi: 10.1016/j.freeradbiomed.2011.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Babu D, Leclercq G, Goossens V, Vanden Berghe T, Van Hamme E, Vandenabeele P, Lefebvre RA. Mitochondria and NADPH oxidases are the major sources of TNF-alpha/cycloheximide-induced oxidative stress in murine intestinal epithelial MODE-K cells. Cell Signal. 2015;27:1141–1158. doi: 10.1016/j.cellsig.2015.02.019. [DOI] [PubMed] [Google Scholar]
- 36.Dong W, Qi Z, Liang J, Shi W, Zhao Y, Luo Y, Ji X, Liu KJ. Reduction of zinc accumulation in mitochondria contributes to decreased cerebral ischemic injury by normobaric hyperoxia treatment in an experimental stroke model. Exp Neurol. 2015;272:181–189. doi: 10.1016/j.expneurol.2015.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhao Y, Pan R, Li S, Luo Y, Yan F, Yin J, Qi Z, Yan Y, Ji X, Liu KJ. Chelating intracellularly accumulated zinc decreased ischemic brain injury through reducing neuronal apoptotic death. Stroke. 2014;45:1139–1147. doi: 10.1161/STROKEAHA.113.004296. [DOI] [PubMed] [Google Scholar]
- 38.Wang WM, Liu Z, Liu AJ, Wang YX, Wang HG, An D, Heng B, Xie LH, Duan JL, Liu YQ. The zinc Ion chelating agent TPEN attenuates neuronal death/apoptosis caused by hypoxia/ischemia via mediating the pathophysiological cascade including excitotoxicity, oxidative stress, and inflammation. CNS Neurosci Ther. 2015;21:708–717. doi: 10.1111/cns.12428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bonanni L, Chachar M, Jover-Mengual T, Li H, Jones A, Yokota H, Ofengeim D, Flannery RJ, Miyawaki T, Cho CH, Polster BM, Pypaert M, Hardwick JM, Sensi SL, Zukin RS, Jonas EA. Zinc-dependent multi-conductance channel activity in mitochondria isolated from ischemic brain. J Neurosci. 2006;26:6851–6862. doi: 10.1523/JNEUROSCI.5444-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]


