Abstract
Background: Impaired cognitive flexibility is the core manifestation of schizophrenia (SZ). Previous literature raised a claim against the effect of atypical antipsychotic drugs (AAD) on cognitive and executive functions whose cause needs further investigation. Attention set-shifting task (ASST) tests the prefrontal cortex’s (PFC) executive and flexibility functions. Goals: To examine Olanzapine (OLZ) effect on ASST, expression of N-methyl-D-aspartate receptor 1 (NMDR-NR1) in prefrontal cortex (PFC), and metabolic comorbidity in ketamine (KET) model of SZ. Methods: Sixty-two male rats were divided into three groups: 8 for ASST and 30 for open field, ELISA and immunohistochemistry sub-chronic study, and 24 for regular serological and histopathological examination. Rats treated with V: vehicle; K: KET and KO: OLZ plus KET. Results: KET caused significant increase in time, trials, and errors to reach criterion. OLZ co-administration reversed effects of KET in ASST with no reduction of locomotor activity. OLZ normalized KET-induced rise of NR1 expression and protected against KET-induced degenerative changes in hippocampus and PFC. Significant increase in serum liver enzymes, total bilirubin, and lipids with chronic compared to sub-chronic OLZ administration. In contrast, insignificant difference between sub-chronic OLZ and vehicle was found. Conclusions: Current study demonstrated the efficacy of OLZ to reverse KET-induced cognitive deficits in ASST with neither reduction in NR1 expression in PFC nor metabolic malfunction in the sub-chronic study. It also showed the protective effect of OLZ on KET induced neuronal degeneration and necrosis. We suggest that chronic OLZ treatment-induced-metabolic malfunction might be the cause of time-dependent cognitive deterioration.
Keywords: Schizophrenia, olanzapine (OLZ), ketamine (KET), attention set shifting task (ASST), N methyl-D-aspartate receptors (NMDARs)
Introduction
Schizophrenia (SZ) is chronic cognitive disease whose etiology is multifactorial and not well understood. The implication of glutamate hypothesis in pathogenesis of SZ has been proved based on the ability of glutamatergic N-methyl-D-aspartate receptors (NMDARs) antagonists such as ketamine (KET) and phencyclidine (PCP) to precipitate psychotic symptoms in humans and rats [1]. NMDARs is made up of two subunits: two obligatory as well as essential NR1 and two NR2 (A-D) subunits [2]. Atypical antipsychotic drugs (AAD) such as Olanzapine (OLZ) represents the corner stone in treatment of SZ. Therefore, their effects on the cortical expression of NMDARs are an important mechanism that may explain the effect of those drugs on cognitive function. Malfunction of prefrontal cortex (PFC) that is responsible for executive function is a major contributor to cognitive impairment in SZ [3]. It has been reported that treatment with both OLZ and clozapine (CLZ) decreased expression of NMDARs in the hippocampus that accounted for their poor outcome on some aspects of cognition [1]. Furthermore, literature review of both animal and clinical studies reported controversy and raised claim concerning the effect of AADs on executive and flexibility functions [4].
Cognitive flexibility is the ability to shift from one task to another to appropriately respond to changing environmental requirement [5]. Rat attention set shifting task (ASST) is considered as good and reliable test for fronto-executive function, discrimination and reversal learning [6]. Previous studies reported improvement of rat performance in ASST using CLZ in KET model of SZ [3] and using CLZ and risperidone in PCP model of SZ in rodents [7].
It has been reported that OLZ decreased locomotor activity of rats that may account for its effect on adiposity [8-10]. Previous studies reported that disturbed lipid profile and impaired glucose tolerance with or without increased body mass index induced by AADs considered as major mechanism of cognitive deterioration in SZ that is duration dependent [11,12]. However, the interaction of metabolic comorbidity of short- or long-term administration of AADs and cognition does not establish clear causal relation and needs further studies [4]. The main goal of the current study is to investigate the effect of OLZ administration on the expression of NR1 subunit of NMDAR and how this is related to histopathology of dentate gyrus and PFC, cognitive flexibility using ASST, and locomotor activity in KET induced rat model of SZ. Moreover, we will compare sub chronic and chronic OLZ effects on liver enzymes, lipid profile, serum sugar and liver histopathology.
Material and methods
Drugs and chemicals
Olanzapine (OLZ 10 mg tablet purchased from S.A.E., Badr City, Egypt 3018010101). The tab of OLZ mashed and dissolved in 10 ml distilled water and given at dose of 1 mg/kg/day orally by gavage either 1 hr before the behavioral test in ASST or daily for 6-12 days in sub chronic study or daily for 30 days in the chronic study [13]. Ketamine hydrochloride ampoule (KET 10 ml at 50 mg/ml injection; Batch No. 10194, USP Rotex Medica, Trittau) was given as single IP injection of 10-20 mg/Kg half an hour before behavioral test or daily for 6-12 days in the sub chronic study [14].
Animal grouping and experimental approach
Sixty-two young adult male Albino rats weighing (100-220 gm) purchased from and kept at Assiut University Animal Core Facility, Assiut, Egypt. Rats were transported to Physiology lab one week before start of behavioral testing. They were kept under temperature of 25°C, 12 hr normal light/dark cycle except for ASST it was inverted, normal rat chow and water Ad Libitum. All applicable National and International Guidelines were followed strictly. The Protocol was approved by the Local Experimental Ethical Committee at Deanship of Scientific Research of Assiut University, Assiut, Egypt.
Rats divided into three study groups; acute study: 8 rats for ASST; sub-chronic study: 30 rats divided into three groups 10 rats each; V: vehicle group treated with half ml distilled water orally and 0.1 ml saline by intraperitoneal (IP) injection, K: treated with KET (20 mg/Kg) and KO: treated with KET (20 mg/Kg) plus OLZ (1 mg/Kg) by gavage daily for 6-12 days. The sub-chronic group of rats tested for; open field after 3 days of treatment, expression of NR1 subunit of NMDARs in PFC using ELISA after 6 days using 6 rats in each group, and NR1 immunoreactive cells as well as histopathology in PFC and dentate gyrus of hippocampus using immunohistochemistry after 12 days using 4 rats in each group; 24 rats intended for serological study and liver histopathology divided into two groups 12 for sub-chronic treatment and 12 for chronic treatment; each divided into two subgroups V: vehicle group treated with half ml distilled water orally and O: OLZ (1 mg/Kg) by gavage orally daily for 12 days or 30 days.
Behavioral testing
Attentional set-shifting task
Procedure
Eight rats were housed individually, handled, and weighed daily on the first week. Food restriction started on the fourth day to 3 gm of food pellet per day divided in two ramekins to maintain them on 85% of their free feeding body weight throughout the experiment with free access to water. Change of home cage bedding done on the seventh day and not changed after that until the completion of testing. Acclimation started on the second week during which weighing, handling and food restriction continued. On the last two days of second week rats transferred to test cage to explore for one hour. Habituation done on day fifteen. Testing started on the third week on two sessions done on two consecutive days; first day (session 1 composed of five phases; SD, CD, CDR, IDS, IDSR) and second day (session 2 composed of four phases; IDS2, IDS2R, EDS, EDSR) according to [15] as discussed in (Tables 1 and 2). Rats were tested in groups of two rats per day. The two sessions of testing were repeated to the same group of rats after giving KET injection (10 mg/Kg) as single IP injection and after giving KET (10 mg/Kg) as single IP injection plus OLZ (1 mg/Kg) by gavage as single oral dose. Ketamine was given 30 min before testing and OLZ was given 1 hr before testing. We allowed 6 days recovery between repetitions (Figure 1).
Table 1.
Types of digging media and odors used in attention set shifting task (ASST)
| Set 1 | Set 2 | Set 3 | Set 4 | |
|---|---|---|---|---|
| Digging medium | Saw dust vs sand | Plastic balls vs cubes | Cardboard vs beads | Dawmina vs gravels |
| Odor | Lemon vs garlic | Vanilla vs coffee | Cinnamon vs cumin | Cocoa vs coriander |
Table 2.
Description of attentional set-shift task (ASST) phases
| First test day | |
|
| |
| SD | Discrimination on digging media only; 1 medium is correct, e.g. sand |
| CD | Correct digging medium is the same as SD, e.g. sand; additional dimension is introduced but irrelevant (e.g. lemon and garlic) |
| CDR | Correct digging medium is the other medium, e.g. Saw dust; odor is irrelevant |
| IDS | Relevant dimension is still the digging medium and odor is irrelevant; the correct medium in the next set (2) of digging medium, e.g. plastic balls |
| IDSR | Correct digging medium is the other medium of the same pair of medium, e.g. cubes and odor is irrelevant |
|
| |
| Second test day | |
|
| |
| IDS2 | Relevant dimension is still the digging medium and odor is irrelevant; the correct medium in the next set (3) of digging medium, e.g. cardboard |
| IDS2R | Correct digging medium is the other medium of the same pair of medium, e.g. beads and odor is irrelevant |
| EDS | Relevant dimension is odor and digging medium is irrelevant; the correct scent is from set (4) of e.g. cocoa |
| EDSR | Correct scent is the other odor of the same pair of odors, e.g. coriander and digging medium is irrelevant |
Direction of shift from medium to odor. SD: simple discrimination, CD: compound discrimination, CDR: compound discrimination reversal, IDS: intra dimensional shift, IDSR: intra dimensional shift reversal, IDS2: intra dimensional shift day 2, IDS2R: intra dimensional shift day 2 reversal, EDS: extra-dimensional shift, EDR: extra-dimensional shift reversal.
Figure 1.

Schematic diagram of experimental workflow used in the attention set shifting task. KET: ketamine; OLZ: Olanzapine.
Apparatus
A plastic cage that was divided into three compartments; a larger waiting compartment and two choice compartments of equal size accessed through sliding doors. There was one ramekin of water in the waiting compartment and two similar ramekins of food rewards in the choice compartments. Food rewards were covered by different digging medium and different odors on their outer walls according to (Table 1).
Two days before habituation on day 13; rats were transferred individually to test cage for one hr. after spreading small amount of dirty bedding from the rat home cage. One ramekin of clear water put in the waiting compartment and two ramekins of 20 mg of uncovered food reward in other two compartments. The start gate was removed, and the rat left in test cage for 1 hr to explore.
Habituation
On day fifteen, rats were transferred to testing cage and allowed to explore the cage for 5 min. Then, the rat was allowed nine trials divided into four phases; each phase composed of two sequential trials and last phase composed of three trials. The food reward was uncovered in the 1st phase, on the top of 0.5 cm of sand in the 2nd, covered by thin layer of sand in the 3rd and covered with 2 cm of sand in the 4th one. Once the food reward successfully retrieved, the rat left to consume it before being returned into the waiting area.
Testing
The rat was placed in the waiting compartment and the sliding door removed to allow an access to the two ramekins in two compartments. Food reward was present in one of the ramekins only buried under 2 cm of different digging media. Once the animal made a choice the door for the other compartment closed. If the trial was correct the rat left to consume the food reward before going to the next trial. If the rat chose the un-baited compartment, the trial was terminated, and the rat returned to the waiting compartment. The intertrial interval was 5 s. and interphase interval was 1 min. in each session. In each trial we calculated whether correct, time of retrieval and number of errors to reach criterion. The criterion is eight correct trials out of ten successive trials. The rat was trained to discriminate between the two ramekins based on digging media in 7 phases (SD, CD, CDR, IDS, IDSR, IDS2, IDS2R). The odor was introduced and irrelevant starting from the CD phase. In the last two phases (EDS and EDSR) discrimination was based on the scent while the media is irrelevant.
Open field test (OFT)
The apparatus was rectangular wooden arena with grid floor and walls (125 cm × 125 cm × 20 cm). The floor divided into 25 equal squares by woody lines fixed on the floor (25 cm × 25 cm). The central nine squares represented as the central area while the rest 16 squares were the peripheral area. We used three rat groups ten animals each; V: vehicle, K: KET 20 mg/Kg and KO: KET 20 mg/Kg IP injection plus OLZ (1 mg/Kg) by gavage orally daily for 3 days before testing. OFT test was done 30 min after ketamine injection and 1 hr after oral OLZ administration. All experimental groups were submitted to 6 min period to the OFT. Rats were brought to the test room in their home cage 30 min prior to testing for acclimation. Rats were tested individually between 11 am to 2 pm by placing into the center of the OFT arena in a standard lit room with a video camera (iPhone 6, full HD 1080 and video recording 30 fps) installed above the cage to record the activity of the rats. Data were evaluated for each of the following parameters: total distance walked, number of rears, time spent in the center, entries into center and peripheral areas. After each test session, the OFT arena was carefully cleaned with 95% ethanol solution and wiped with clean towel [16].
Preparation of prefrontal tissue extract
Animals were injected with the daily dose of KET (20 mg/Kg) IP and within 5 min were euthanized by humane cervical dislocation at atlantooccipital region and rapid decapitation done by experienced Staff member who strictly adhere to IACUC-approved protocols and institutional policies to avoid disturbance of brain tissue according to guidelines for the Use of Cervical Dislocation for Rodent Euthanasia The University of Texas at Austin Institutional Animal Care and Use Committee and all efforts were made to minimize suffering [17]. The skull was opened, and the brain was removed and washed with ice-cold physiologic saline solution (0.9%), PFC was sectioned and immediately dipped in liquid nitrogen and stored at -80°C for further processing. About half gm of PFC tissues was homogenized in PBS (PH 7.4). The PFC tissue proteins were then sonicated for 10 s. bursts, centrifuged for 20 min at the speed of 2000-3000 rpm and supernatant obtained and kept at -30°C for detection of NR1 protein concentration using Rat NMD-R1 ELISA Kit following manufacturer’s instruction.
Biochemical parameters
At the end of 12 and 30 days of OLZ treatment, rats intended for serological study fasted for twelve hours and anaesthetized with KET (50 mg/Kg). Blood sampling and serum isolation done and stored at -20°C for further analysis. Serum liver enzymes; alanine aminotransferase (ALT/GPT)-Liquizyme (4+1) E.C.2.6.1.2. (Cat No: 292 000), aspartate aminotransferase (AST/GOT)-Liquizyme (4+1) E.C.2.6.1.1. (Cat No: 291 000), Alkaline phosphatase (ALP) Liquizyme (9+1) IFCC E.C.3.1.3.1. (Cat No: 214 001) were detected using ELISA kits following manufacturer’s instruction. Serum Bilirubin (Total and direct) Jendrassik Grof (Cat No: 222 001); Total protein Biuret Reagent (Cat No: 310 001); Albumin-BCG (Cat No: 211 001). Serum lipid profile detected using chemical methods; Total Cholesterol Liquizyme CHOD-PAP (Single Reagent) (TC; Cat No. 230 001); Low density lipoproteins Direct Enzymatic colorimetric, Liquid (LDL-c: 280 001); High density lipoproteins Direct Enzymatic colorimetric, Liquid (HDL-c: Cat. No. 267 001); Triglycerides-Liquizyme GPO-PAP (Single Reagent) (TGs: Cat No. 314 001) and glucose Liquizyme GOD-PAP (Single Reagent) (Cat No. 250 001) all purchased from Egyptian Company for Biotechnology (S.A.E.). Rat N-Methyl-D-aspartate receptor 1 (NMD-R1) ELISA Kit (Cat No. SG-21707) For the quantitative determination of Rat NMD-R1 concentrations purchased from SinoGeneClon Biotech Co., Ltd. (E-mail: tech@sinogeneclon.com). Absorbance was read using Automated ELIZA plate reader (Robonite-Readwell-India) and all biochemical analysis done following Manufacturer’s instruction and read using Biochemistry analyzer (Robonite Prietest-touch-India).
Histopathology and immunohistochemistry
After animal sacrifice samples from liver, hippocampus and prefrontal cortex were taken from four animals per group and immediately fixed in 10% of neutral buffered formalin at room temperature for 24 h. The samples were then dehydrated in increasing concentrations of ethanol and routinely processed for paraffin sectioning. Sections (4 µm thick) were deparaffinized, stained with hematoxylin and eosin (H&E) according to standard protocol and examined using an Olympus light microscope. All liver sections were evaluated using a scale of 0-3 [0 (>5%), 1 (5-33%), 2 (33-66%) and 3 (>66%)] for the following parameters: steatosis degree and pattern (micro or macrovacuolar), venous congestion, hepatocyte necrosis, portal and lobular inflammation according to [13].
Immunohistochemistry was performed using Avidin-Biotin Immunoperoxidase method. Sections (4 µm thick) were prepared from the paraffin-embedded blocks and mounted on positively charged slides. The sections were deparaffinized and rehydrated with a descending concentration of ethanol to distilled water. Endogenous peroxidase activity was blocked using 3% hydrogen peroxide treatment. For epitope retrieval, sections were microwaved in citrate buffer (pH 6) for a total of 12 min. Sections were incubated with a primary antibody against glutamate receptor (NR1 subunit of N-methyl-D-aspartate receptor) polyclonal antibody (Elabscience Biotechnology Inc., E-AB-15806, diluted at 1/50) overnight at 4°C for 24 h using Ventana Benchmark GX autostainer and Ventana Ultra view DAB detection system according to manufacturer instructions. Negative controls were obtained by omitting the primary antibody. The sections were examined by light microscope. In each sample, ten microscopic fields are examined under magnification (× 400) and the number of immunoreactive cells were counted and averaged in each group [2].
Statistical analysis
GraphPad Prism 5 (GraphPad Software Inc., La Jolla, CA, USA) was used for data analysis. Data were presented as mean ± SEM. Data were compared among groups using Kruskal-Wallis test with Dunn’s Multiple Comparison as posthoc test or One way-ANOVA and Two-way ANOVA with Bonferroni Multiple Comparison test as appropriate. A (P) value of less than 0.05 was considered to represent a statistically significant difference. Neuronal count and area were calculated using Image J (IJ-1.46r software).
Results
Olanzapine antagonizes the effect of ketamine in attention set shifting task
Effect of ketamine and olanzapine on time to reach criterion
Ketamine administration caused significant increase in time to reach criterion compared to vehicle in the following phases; SD (P<0.001), CD (P<0.001), EDS (P<0.01) and EDSR (P<0.05). Co administration of KET and OLZ caused tendency toward decrease in time to reach criterion in all the phases that reached significant level in CDR (P<0.05), IDS (P<0.001), IDS2 (P<0.01) and EDS (P<0.05) phases when compared with KET alone. We found significant difference between vehicle and KO in SD (P<0.01) and CD (P<0.001) (Figure 2A).
Figure 2.

Effect of ketamine and olanzapine on attention set shifting task (ASST). A: Time to reach criterion; B: Number of trials to reach criterion; C: Number of errors to reach criterion; V: vehicle; K: KET; KO: KET+OLZ; One way ANOVA Kruskal-Wallis test with Dunn’s Multiple Comparison posthoc test; (a) V vs K of the same phase; (b) KO vs K of same phase; (c) KO vs V of same phase; (1P<0.05; 2P<0.01; 3P<0.001); P<0.05 is considered significant; N = 8.
Effect of ketamine and olanzapine on number of trials to reach criterion
Ketamine administration caused significant increase in number of trials to reach criterion compared to vehicle in the following phases; SD (P<0.05), IDS (P<0.01) and IDSR (P<0.01). Co administration of KET and OLZ caused tendency toward decrease in trials to reach criterion in some of the phases that reached significant level in CDR (P<0.05) and EDS (P<0.05) phases only when compared with ketamine alone. We found significant difference between vehicle and KO in SD (P<0.01) and IDSR (P<0.001) (Figure 2B).
Effect of ketamine and olanzapine on number of errors to reach criterion
Ketamine administration caused significant increase in number of errors to reach criterion compared to vehicle in the following phases; SD (P<0.05), IDS (P<0.01), IDSR (P<0.001), IDS2 (P<0.05), IDS2R (P<0.05) and EDS (P<0.05). Co administration of KET and OLZ caused tendency toward decrease in errors to reach criterion in all trials that reached significant level in EDS (P<0.05) phase only. We found significant difference between vehicle and KO in IDS (P<0.05) and IDSR (P<0.001) (Figure 2C).
Lack of the effect of olanzapine on ketamine-induced hyperlocomotion in open field test
KET caused significant increase in total distance walked (P<0.05) and insignificant effect on entries into center or the peripheral area, time spent in the center or number of rears compared to vehicle. Co administration of OLZ caused insignificant effect in total distance walked and significantly decreased rearing behavior (P<0.01) and time spent in the center (P<0.05) compared to KET alone. We found significant increase in distance walked (P<0.05), increase in entries into the peripheral area (P<0.05), decrease in time spent in the center (P<0.01) and rearing behavior (P<0.01) in KO group compared to vehicle group (Figure 3).
Figure 3.
Effect of ketamine and olanzapine on rat performance in open field (OF). Data represent box blot; V: vehicle; K: KET; KO: KET+OLZ; One way ANOVA with Newman-Keuls Multiple Comparison Test; (*) K or KO vs V group: *P<0.05; **P<0.01; (+) KO vs K group: +P<0.05; ++P<0.01; N = 10 in each group.
Olanzapine antagonizes ketamine-induced histopathological damage and normalize the over-expression of NMDARs-NR1 subunit in dentate gyrus and prefrontal cortex
Effect of olanzapine and ketamine on the number of NR1 immuno-reactive cells in hippocampus and prefrontal cortex
The results of the current study demonestrated significant increase in the number of the NR1 immunoreactive cells in the PFC and dentate gyrus (DG) of hippocampus of rats treated with KET 20 mg/Kg for 12 days (P<0.01) when compared with vehicle. Co administration of OLZ 1 mg/Kg orally for 12 days caused insignificant change in number of NR1 immunoreactive cells in PFC and DG in KO group when compared with V or K groups (Figure 4A-C) and significant increase in NR1 integrated optical density in the DG in KO group when compared to V group (Figure 4D).
Figure 4.

Effect of olanzapine and ketamine on NR1 protein expression in hippocampus and prefrontal cortex (PFC). Immunohistochemical expression of NR1 after 12 days of treatment in (A) dentate gyrus (DG) (× 400); (B) prefrontal cortex (PFC) (× 400); (C) number of immunoreactive cells in DG and PFC; (D) results based on the mean values of integrated optical density (IOD); (E) NR1 protein concentration in PFC after 6 days of treatment; V: Vehicle, K: KET, KO: KET+OLZ. One Way ANOVA with Bonferroni Multiple Comparison posthoc Test for ELISA and Kruskal-Wallis test with Dunn’s Multiple Comparison Test for immunohistochemistry; P<0.05 is considered significant; (*) K group vs V group; **P<0.01; N = 6 (ELISA) and N = 4 (immunohistochemistry) in each group.
Effect of olanzapine and ketamine on NR1 protein concentration in prefrontal cortex
We found insignificant change of NR1 protein concentration in the PFC with KET 20 mg/Kg for 6 days (P>0.05) when compared with vehicle. Co administration of OLZ 1 mg/Kg orally for 6 days caused insignificant change of NR1 protein concentration in PFC (P>0.05) when compared to both V and K groups (Figure 4E).
Effect of olanzapine and ketamine on prefrontal cortex and dentate gyrus histopathology
Treatment with KET at a dose of 20 mg/Kg for 12 days caused widespread degeneration and necrosis in the dentate gyrus granular neurons and perineuronal edema. Sections of hippocampus from rats treated with OLZ and KET showed proliferation of the granular cells, and increased thickness of the granular cell layer (GL) and decreased degenerated cells and edema (Figure 5A). Co administration of OLZ caused significant increase in granular cell count (P<0.001) (Figure 5C) and mean percent area (P<0.001) when compared with V and KO groups (Figure 5D). We found insignificant difference between K and V group concerning granular cell count and mean percent area (Figure 5C, 5D).
Figure 5.
Photomicrographs of rat hippocampus and prefrontal cortex. (A) Dentate gyrus; V: vehicle; ML: molecular layer, GL: granular layer, PL: polymorphic layer, SGZ: subgranular zone, H: hilus; K: KET treated group showing degeneration/necrosis in granular neurons (arrow), perineuronal edema (arrowhead); KO: KET+OLZ treated group showing proliferation of granular cells, few degenerated cells (arrow) and edema (arrow head). (B) PFC of V: vehicle showing normally looking pyramidal (p), satellite (s) cells, microglia (m); K: KET treated group showing multiple neuronal necrosis (nc), vacuolation of the neuropil (*) and multiple neurons exhibiting pyknotic nuclei and acidophilic cytoplasm (arrowheads); KO: KET+OLZ showing neuronal cell degeneration (nd), neuronophagia of the degenerated neurons, vacuolation of the neuropil (*); Statistical analysis of (C) average cell count and (D) mean percent area of neurons in PFC and DG; H&E (400 ×); n = 4 in each group.
Treatment with KET caused neuronal degeneration, neuronophagia of the degenerated neurons, necrosis and vacuolation of the neuropil in PFC (Figure 5B-K). Moreover, it caused significant decrease in neuronal cell count (P<0.05) (Figure 5C) and mean percent area (P<0.001) when compared with V group (Figure 5D). Sections of PFC from rats treated with both OLZ and KET showed less neuronal degeneration, less necrosis, multiple neurons with acidophilic cytoplasm, darkly stained nuclei and vacuolation of the neuropil (Figure 5B-KO). Co administration of OLZ caused significant increase in neuronal count (P<0.05) when compared with both K and V groups (Figure 5C) and mean percent area (P<0.05) compared to K group (Figure 5D).
Metabolic comorbidity and liver histopathology induced by olanzapine with chronic compared to its sub-chronic administration
Effect of sub-chronic and chronic olanzapine administration on serum lipoproteins and serum glucose
Two-way ANOVA (Treatment × Time) showed significant effects of treatment duration (F6055 = 25, P<0.0001), serum variables (F1952 = 8, P<0.0001), and a significant interaction between the two factors (F5235 = 21, P<0.001) on serum lipoproteins. We found insignificant change in serum lipoproteins and glucose with OLZ administration for 12 days when compared to V-12 group. Administration of OLZ 1 mg/Kg for 30 days caused significant rise in percent change of serum TC (P<0.05) and LDL-c (P<0.001) in O-30 group when compared with OLZ administration for 12 days O-12 group. Insignificant difference in percent change of serum TGs was found between O-30 compared to O-12 groups. Insignificant difference in serum glucose and HDL-c were found between treated groups (Figure 6A).
Figure 6.
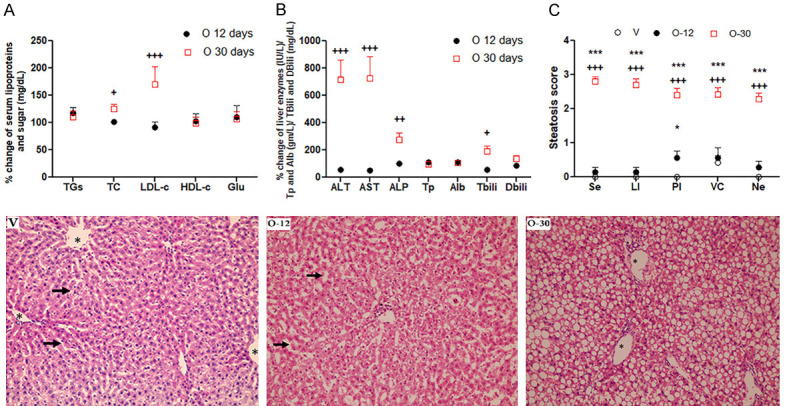
Changes of serum lipoproteins, glucose, liver function and histopathology with sub-chronic and chronic treatment with olanzapine. Interaction of treatment time and (A) serum lipoproteins; (B) liver function; (C) steatosis score; TC: total cholesterol; TGs: triglycerides; LDL-c: low density lipoproteins; HDL-c: high density lipoproteins; Glu: glucose; ALT: alanine transaminase; AST: aspartate transaminase; ALP: alkaline phosphatase; Tp: total protein; Alb: albumin; Tbili: total bilirubin; Dbili: direct bilirubin; Photomicrograph of rat liver tissue of sub chronic and chronic OLZ treated rats (lower panel). V: liver tissue of control group showing normal polygonal appearance of hepatocytes radiating from the central vein (asterisk) towards the portal tract, normal sized nuclei and normal size of blood sinusoids (thick arrows); O-12: liver tissue from OLZ group treated for 12 days showing dilated blood sinusoids (thick arrows) with inflammatory cell infiltration (thin arrows) and mild vacuolation of cytoplasm of hepatocytes; O-30 liver tissue from OLZ group treated for 30 days showing severe steatosis, ballooning, flattened pyknotic nuclei and inflammatory cellular infiltration (thin arrow); Two Way ANOVA with Bonferroni multiple comparison posthoc test (+) O-30 vs O-12: +++P<0.001, ++P<0.01, +P<0.05; (*) O-12 or O-30 vs V groups: ***P<0.001, *P<0.05; N = 6 in each group.
Effect of sub-chronic and chronic olanzapine administration on liver function tests
Two-way ANOVA (Treatment × Time) showed significant effects of treatment duration (F2.4e+006 = 53, P<0.0001), serum variables (F406491 = 9.1, P<0.0001), and a significant interaction between the two factors (F541891 = 12, P<0.001) on liver function tests. We found insignificant change in serum liver enzymes, total protein, albumin, total and direct bilirubin with OLZ administration for 12 days when compared to V-12 group. Administration of OLZ 1 mg/Kg for 30 days caused significant rise in serum liver enzymes; ALT (P<0.001), AST (P<0.001) and ALP (P<0.01) compared with both V-30 days and O-12 days groups. Significant rise in serum total bilirubin (TBili) (P<0.05) in O-30 compared to O-12 and V-30. Insignificant difference in serum direct bilirubin and albumin between treated groups (Figure 6B).
Effect of sub-chronic and chronic olanzapine administration on liver histopathology
Control rat liver tissue of sub chronic and chronic OLZ treated rats showed normal polygonal appearance of hepatocytes radiating from the central vein towards the portal tract, normal sized nuclei and normal size of blood sinusoids. Liver tissue from OLZ group treated for 12 days showed dilated blood sinusoids, focal areas of congestion, inflammatory cell infiltration specially in the portal area and mild vacuolation of cytoplasm of hepatocytes. Liver tissue from OLZ group treated for 30 days showing severe micro and macro-vesicular steatosis, flattened pyknotic nuclei with ballooning of the hepatocytes and inflammatory cellular infiltration (Figure 6 lower panel). Two-way ANOVA (Treatment × Time) showed significant effects of treatment duration (F63 = 575, P<0.0001), insignificant effect steatosis score criteria (F0.22 = 2, P = 0.097), and a significant interaction between the two factors (F0.53 = 3.2, P = 0.0033) on liver histopathology (Figure 6C).
Discussion
Deficits in cognitive flexibility tasks such as attention set shifting (ASST) has been evidenced in SZ patients [18]. The current study showed that KET administration caused significant increase in time, errors and trials to reach criterion in ASST compared to vehicle. Co administration of KET and OLZ caused significant decrease in time to reach criterion in CDR, IDS, IDS2 and EDS phases, in trials to reach criterion in CDR and EDS phases and errors to reach criterion in EDS phase when compared with ketamine alone. Up to our knowledge, no previous study tested the effect of OLZ administration on ASST in KET-model of SZ in rats. Inline with us, previous study reported significant increase in trials and errors to reach criterion in EDS phase of ASST with acute and subchronic KET treatment that was reversed by clozapine in mice model [3]. Moreover, It was reported that AADs; quetiapine and sertindole reversed the subchronic KET-induced impairment of EDS phase in male rats [19]. Another study demonstrated that subchronic treatment with CLZ and respridal reversed the PCP-induced increase in trials to reach criterion in EDS phase of ASST in female rats [7].
The current result showed that OLZ co administration reversed KET-induced deficit in ASST. However, the mechanism of this cognitive improvement need to be elucidated specially with the apparent conflict of the previous litrature concerning the effect of AADs on cognitive function. Therefore, the next question was whether OLZ has an effect on NMDAR expression in PFC in KET-induced SZ model. The results of the current study demonestrated significant increase in the number of the NR1 immunopositive cells in both PFC and DG that was associated with neuronal degeneration, necrosis, perineural edema, significant decrease in cell count and area in rats treated with KET 20 mg/Kg IP injection for 12 days when compared with vehicle. In line with us, it was reported that injection of KET 50 mg/Kg upregulated NR1 message and protein expression in frontal cortex of 7 days old rats that was associated with increased apoptotic index [20]. Moreover, MK801 (0.6 mg/Kg IP) injection for 14 days increased the number of immunopositive cells and protein expression of NR1 subunit in hippocampus CA1 area and dentate gyrus of mice that was reversed with single intracerebroventricular injection of 75 ng of NMDA [2]. Along us, it was reported that six injection with KET 20 mg/Kg with 2 hours interval between injections and 6 hours recovery caused significant increase of NR1 mRNA expression that was associted with increased caspase 3 immunopositive cells and apoptosis in rat frontal cortex [21].
The current study showed that co administration of OLZ 1 mg/Kg for 12 days normalized the the KET-induced increase of NR1 immunopositive cells compared to vehicle in the PFC and hippocampus. Moreover, OLZ caused significant increase in granular cell count and percent area in DG when compared with both V and KO groups. It also caused less degenerative changes and significant increase in neuronal count and percent area compared to K group in PFC. Unfortunately, no previous study tested the effect of OLZ on KET-induced histopathological changes or NR1 expression in PFC and DG. However, one study reported that OLZ normalized the PCP-induced alteration of 17 SZ candidate genes including AKT, SYN1 and RGS4 in PFC [22]. Another study reported that CLZ has protective and antiapoptotic effect against KET-induced cytotoxicity of neuronal stem cells isolated from subventricular zone of mouse brain [23]. Moreover, it was reported that risperidone and haloperidol exhibited protective effect on KET induced cell death and helped cell survival, neurogenesis and cell proliferation in hippocampus [24]. Along the current result, it was reported that neither OLZ nor CLZ caused significant change in the NMDAR expression in the neocortx, however they significantly decreased their expression in hippocampus [1,25]. Moreover, an insignificant change in NR1 protein expression in the frontal cortex was found with 14 days of daily IP CLZ injection [26]. However, it was reported that 28 days treatment with OLZ (5 mg/Kg) and risperidone (3 mg/Kg) decreased NMDAR expression in CA1 and CA3 areas of the hippocampus of rat forebrain as well as in caudate nucleus and putamen that accounts for their lower extrapyramidal side effects [27]. Moreover, CLZ used at high dose 45 mg/Kg in the drinking water for 6 months downregulate the expression of NR1 subunit in the dorsolateral PFC only and produced insignificant change in NR1 expression in hippocampus, striatum, nucleus accumbens and other areas of PFC [28]. This apparent conflict may be explained based on different dose, duration, type of AAD used in the previous studies and that they tested OLZ effect per se not in KET model.
We found that KET and OLZ elicited behavioral changes in rats on open field (OF) test. KET caused significant increase in total distance walked in OF test compared to vehicle. In line with this result, previous studies reported hyperlocomotion with KET administration [29,30]. Co administration of OLZ caused insignificant effect concerning locomoter activity compared to KET and significantly increased it compared to vehicle. Moreover, it decreased rearing behaviour and time spent in the center compared to both K or V groups. In line with us, it was reported that both OLZ and resperidone administration didn’t affect motor activity while increasing anxiety and decreasing the exploratory activity in OF test that was antagonized by fluoxetine in male wistar rats [31]. Moreover, another study reported that low dose of CLZ 0.3 mg/Kg antagonized the effects of acute and subchronic KET without affecting the locomotor activity in OF test in mice [3]. In contrast to the current result, it was reported that OLZ decreased hyperactivity induced in mutant mice model of low NR1 expression with minimal effect on the wildtype mice that becomes more significant with time [32]. The apparent conflict may be explained by the short time that we used in the current study 6 min compared to 3 hr of recording in Duncan study and the upregulation of NR1 expression in the KET model we used.
Previous literature reported great controversy and does not establish a causal relation between the adverse metabolic effects of AADs and cognitive function [4]. Chronic treatment with AADs has been linked to an increased risk of metabolic comorbidity [33]. Therefore, in the current study we compared the effect of sub chronic and chronic OLZ administration on liver function and lipid profile. We found that administration of OLZ for 12 days didn’t significantly affect liver function and serum lipids compared with V group. The OLZ-induced rise in serum TGs in chronic study was insignificant when compared with V-30 group. However, 30 days of OLZ administration caused significant elevation of serum TBili, TC, LDL-c, ALT, AST and ALP compared to 12 days treatment. In addition, 30 days OLZ treatment caused severe damage to the liver tissue and steatosis when compared to 12 days treatment. The current result is supported by previous studies of us and others. It was found that 25 days of OLZ treatment (1 mg/Kg) caused significant elevation of liver enzymes, TGs, TC and hepatic steatosis compared to controls in male rats [13]. Moreover, OLZ administration (1 mg/Kg) for 10 weeks caused significant elevation of liver enzymes [34]. In addition, it was reported that both OLZ and CLZ elevated blood lipids and impaired glucose tolerance in time dependent manner [33]. Taken together, we may speculate that the protective effect of OLZ against the KET-induced cognitive deficit was helped by the lack of metabolic deterioration and liver damage with short-term treatment.
Conclusion
The results of the present study demonstrated the efficacy of OLZ to reverse KET-induced cognitive deficits in ASST. It also showed the lack of the effect of OLZ on hyperlocomotion induced by KET with significant decrease in the exploratory behavior indicating the need for anxietolytic cotherapy. Moreover, it provides further prove for the paradoxical effect of KET on the expression of NR1 subunit of NMDARs. Moreover, the current work demonstrated the protective effect of OLZ on KET induced neuronal degeneration and necrosis in both PFC and DG. The current work provides an evidence for the lack of OLZ-induced metabolic malfunction in the sub chronic study. We suggest that chronic OLZ treatment induced-metabolic malfunction might be the cause of time-dependent cognitive deterioration.
Acknowledgements
The Authors would like to thank Dr Abdelmawla SE (Department of Anesthesia and Intensive Care) for helping with the ASST experiment and Mr. Amer SA (Bachelor of Accounting and Financial Management) for contributing to preparing and embedding the figures.
Disclosure of conflict of interest
None.
Abbreviations
- OLZ
Olanzapine
- KET
Ketamine
- AADs
Atypical antipsychotic drugs
- CLZ
Clozapine
- ASST
Attention set shifting task
- SD
Simple discrimination
- CD
Compound discrimination
- CDR
Compound discrimination reversal
- IDS
Intra-dimensional shift
- IDSR
Intra-dimensional shift reversal
- IDS2
Intra-dimensional shift day 2
- IDSR2
Intra-dimensional shift reversal day 2
- EDS
extra-dimensional shift
- EDSR
extra-dimensional shift reversal
- NMDARs
N methyl-D-aspartate receptors
- ALT
Alanine transaminase
- AST
Aspartate transaminase
- ALP
Alkaline phosphatase
- Tp
Total protein
- Alb
Albumin
- Tbili
Total bilirubin
- Dbili
Direct bilirubin
- TGs
Triglycerides
- TC
Total cholesterol
- LDL-c
Low density lipoproteins
- HDL-c
High density lipoproteins
References
- 1.Krzystanek M, Pałasz A. NMDA receptor model of antipsychotic drug-induced hypofrontality. Int J Mol Sci. 2019;20:1442. doi: 10.3390/ijms20061442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ding J, Zhou HH, Ma QR, He ZY, Ma JB, Liu YM, Zhang YW, He YQ, Liu J. Expression of NR1 and apoptosis levels in the hippocampal cells of mice treated with MK801. Mol Med Rep. 2017;16:8359–8364. doi: 10.3892/mmr.2017.7674. [DOI] [PubMed] [Google Scholar]
- 3.Szlachta M, Pabian P, Kuśmider M, Solich J, Kolasa M, Żurawek D, Dziedzicka-Wasylewska M, Faron-Górecka A. Effect of clozapine on ketamine-induced deficits in attentional set shift task in mice. Psychopharmacology (Berl) 2017;234:2103–2112. doi: 10.1007/s00213-017-4613-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mackenzie NE, Kowalchuk C, Agarwal SM, Costa-Dookhan KA, Caravaggio F, Gerretsen P, Chintoh A, Remington GJ, Taylor VH, Müeller DJ, Graff-Guerrero A, Hahn MK. Antipsychotics, metabolic adverse effects, and cognitive function in schizophrenia. Front Psychiatry. 2018;9:622. doi: 10.3389/fpsyt.2018.00622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dajani DR, Uddin LQ. Demystifying cognitive flexibility: implications for clinical and developmental neuroscience. Trends Neurosci. 2015;38:571–578. doi: 10.1016/j.tins.2015.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tait DS, Chase EA, Brown VJ. Attentional set-shifting in rodents: a review of behavioural methods and pharmacological results. Curr Pharm Des. 2014;20:5046–5059. doi: 10.2174/1381612819666131216115802. [DOI] [PubMed] [Google Scholar]
- 7.McLean SL, Beck JP, Woolley ML, Neill JC. A preliminary investigation into the effects of antipsychotics on sub-chronic phencyclidine-induced deficits in attentional set-shifting in female rats. Behav Brain Res. 2008;189:152–158. doi: 10.1016/j.bbr.2007.12.029. [DOI] [PubMed] [Google Scholar]
- 8.van der Zwaal EM, Luijendijk MC, Evers SS, la Fleur SE, Adan RA. Olanzapine affects locomotor activity and meal size in male rats. Pharmacol Biochem Behav. 2010;97:130–137. doi: 10.1016/j.pbb.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 9.Zhang Q, Lian J, He M, Deng C, Wang H, Huang XF. Olanzapine reduced brown adipose tissue thermogenesis and locomotor activity in female rats. Prog Neuropsychopharmacol Biol Psychiatry. 2014;51:172–180. doi: 10.1016/j.pnpbp.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 10.Chee MJ, Douris N, Forrow AB, Monnard A, Lu S, Flaherty SE, Adams AC, Maratos-Flier E. Melanin-concentrating hormone is necessary for olanzapine-inhibited locomotor activity in male mice. Eur Neuropsychopharmacol. 2015;25:1808–1816. doi: 10.1016/j.euroneuro.2015.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boyer L, Richieri R, Dassa D, Boucekine M, Fernandez J, Vaillant F, Padovani R, Auquier P, Lancon C. Association of metabolic syndrome and inflammation with neurocognition in patients with schizophrenia. Psychiatry Res. 2013;210:381–6. doi: 10.1016/j.psychres.2013.06.020. [DOI] [PubMed] [Google Scholar]
- 12.Chen DC, Du XD, Yin GZ, Yang KB, Nie Y, Wang N, Li YL, Xiu MH, He SC, Yang FD, Cho RY, Kosten TR, Soares JC, Zhao JP, Zhang XY. Impaired glucose tolerance in first-episode drug-naive patients with schizophrenia: relationships with clinical phenotypes and cognitive deficits. Psychol Med. 2016;46:3219–30. doi: 10.1017/S0033291716001902. [DOI] [PubMed] [Google Scholar]
- 13.Mahmoud GS, El-Deek HE. Melatonin modulates inflammatory mediators and improves olanzapine-induced hepatic steatosis in rat model of schizophrenia. Int J Physiol Pathophysiol Pharmacol. 2019;11:64–75. [PMC free article] [PubMed] [Google Scholar]
- 14.Nikiforuk A, Golembiowska K, Popik P. Mazindol attenuates ketamine-induced cognitive deficit in the attentional set shifting task in rats. Eur Neuropsychopharmacol. 2010;20:37–48. doi: 10.1016/j.euroneuro.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 15.Kos T, Nikiforuk A, Rafa D, Popik P. The effects of NMDA receptor antagonists on attentional set-shifting task performance in mice. Psychopharmacology (Berl) 2011;214:911–921. doi: 10.1007/s00213-010-2102-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Seibenhener ML, Wooten MC. Use of the Open Field Maze to measure locomotor and anxiety-like behavior in mice. J Vis Exp. 2015:e52434. doi: 10.3791/52434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guidelines for the Use of Cervical Dislocation for Rodent Euthanasia [Internet 2018] The University of Texas at Austin: Institutional Animal Care and Use Committee; 2018 update. Available at https://research.utexas.edu/wp-content/uploads/sites/3/2018/04/guideline04.pdf. [Google Scholar]
- 18.Waltz JA. The neural underpinnings of cognitive flexibility and their disruption in psychotic illness. Neuroscience. 2017;345:203–217. doi: 10.1016/j.neuroscience.2016.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nikiforuk A, Popik P. Effects of quetiapine and sertindole on subchronic ketamine-induced deficits in attentional set-shifting in rats. Psychopharmacology (Berl) 2012;220:65–74. doi: 10.1007/s00213-011-2487-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu C, Wang J, Guo X, Zhang Y. Ketamine exacerbates cortical neuroapoptosis under hyperoxic conditions by upregulating expression of the N-methyl-D-aspartate receptor subunit NR1 in the developing rat brain. BMC Anesthesiol. 2018;18:52. doi: 10.1186/s12871-018-0511-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zou X, Patterson TA, Sadovova N, Twaddle NC, Doerge DR, Zhang X, Fu X, Hanig JP, Paule MG, Slikker W, Wang C. Potential neurotoxicity of ketamine in the developing rat brain. Toxicol Sci. 2009;108:149–158. doi: 10.1093/toxsci/kfn270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martin MV, Mirnics K, Nisenbaum LK, Vawter MP. Olanzapine reversed brain gene expression changes induced by phencyclidine treatment in non-human primates. Mol Neuropsychiatry. 2015;1:82–93. doi: 10.1159/000430786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lundberg M, Curbo S, Bohman H, Agartz I, Ögren SO, Patrone C, Mansouri S. Clozapine protects adult neural stem cells from ketamine-induced cell death in correlation with decreased apoptosis and autophagy. Biosci Rep. 2020;40:BSR20193156. doi: 10.1042/BSR20193156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Keilhoff G, Grecksch G, Bernstein HG, Roskoden T, Becker A. Risperidone and haloperidol promote survival of stem cells in the rat hippocampus. Eur Arch Psychiatry Clin Neurosci. 2010;260:151–162. doi: 10.1007/s00406-009-0033-1. [DOI] [PubMed] [Google Scholar]
- 25.Krzystanek M, Bogus K, Pałasz A, Wiaderkiewicz A, Filipczyk Ł, Rojczyk E, Worthington J, Wiaderkiewicz R. Extended neuroleptic administration modulates NMDA-R subunit immunoexpression in the rat neocortex and diencephalon. Pharmacol Rep. 2016;68:990–995. doi: 10.1016/j.pharep.2016.05.009. [DOI] [PubMed] [Google Scholar]
- 26.Hanaoka T, Toyoda H, Mizuno T, Kikuyama H, Morimoto K, Takahata R, Matsumura H, Yoneda H. Alterations in NMDA receptor subunit levels in the brain regions of rats chronically administered typical or atypical antipsychotic drugs. Neurochem Res. 2003;28:919–924. doi: 10.1023/a:1023231611616. [DOI] [PubMed] [Google Scholar]
- 27.Tarazi FI, Baldessarini RJ, Kula NS, Zhang K. Long-term effects of olanzapine, risperidone, and quetiapine on ionotropic glutamate receptor types: implications for antipsychotic drug treatment. J Pharmacol Exp Ther. 2003;306:1145–1151. doi: 10.1124/jpet.103.052597. [DOI] [PubMed] [Google Scholar]
- 28.Schmitt A, Zink M, Müller B, May B, Herb A, Jatzko A, Braus DF, Henn FA. Effects of long-term antipsychotic treatment on NMDA receptor binding and gene expression of subunits. Neurochem Res. 2003;28:235–241. doi: 10.1023/a:1022325116309. [DOI] [PubMed] [Google Scholar]
- 29.McDougall SA, Moran AE, Baum TJ, Apodaca MG, Real V. Effects of ketamine on the unconditioned and conditioned locomotor activity of preadolescent and adolescent rats: impact of age, sex, and drug dose. Psychopharmacology (Berl) 2017;234:2683–2696. doi: 10.1007/s00213-017-4660-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tanaka S, Young JW, Halberstadt AL, Masten VL, Geyer MA. Four factors underlying mouse behavior in an open field. Behav Brain Res. 2012;233:55–61. doi: 10.1016/j.bbr.2012.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rogóż Z, Skuza G. Anxiolytic-like effects of olanzapine, risperidone and fluoxetine in the elevated plus-maze test in rats. Pharmacol Rep. 2011;63:1547–1552. doi: 10.1016/s1734-1140(11)70719-8. [DOI] [PubMed] [Google Scholar]
- 32.Duncan GE, Moy SS, Lieberman JA, Koller BH. Typical and atypical antipsychotic drug effects on locomotor hyperactivity and deficits in sensorimotor gating in a genetic model of NMDA receptor hypofunction. Pharmacol Biochem Behav. 2006;85:481–491. doi: 10.1016/j.pbb.2006.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu X, Wu Z, Lian J, Hu CH, Huang XF, Deng C. Time-dependent changes and potential mechanisms of glucose-lipid metabolic disorders associated with chronic clozapine or olanzapine treatment in rats. Sci Rep. 2017;7:2762. doi: 10.1038/s41598-017-02884-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mahmoud GS, Sayed SA, Abdelmawla SN, Amer MA. Positive effects of systemic sodium benzoate and olanzapine treatment on activities of daily life, spatial learning and working memory in ketamine-induced rat model of schizophrenia. Int J Physiol Pathophysiol Pharmacol. 2019;11:21–30. [PMC free article] [PubMed] [Google Scholar]




