Abstract
Objective
Compared the outcomes between lenvatinib plus camrelizumab therapy and lenvatinib monotherapy as post-progression treatment for advanced hepatocellular carcinoma (HCC) with progressive disease (PD).
Patients and Methods
A total of 48 advanced HCC patients were included in this retrospective study between June 2019 and March 2020. The patients were divided into the lenvatinib plus camrelizumab group (n=21) and the lenvatinib group (n=27). Primary endpoints were overall survival (OS) and progression-free survival (PFS), and secondary endpoints were the objective response rate (ORR) and adverse events (AEs).
Results
The median follow-up time was 8.4 months. The median OS was not obtained. The median PFS of lenvatinib plus camrelizumab group was significantly longer than that of lenvatinib group (8.0 months vs 4.0 months, p=0.011). Compared with lenvatinib group, lenvatinib plus camrelizumab group had higher ORR (28.57% vs 7.41%) and disease control rate (DCR) (71.43% vs 51.85%). The most common adverse events (AEs) included hand-foot skin reaction, hypertensions and abnormal hepatic function damage. Overall, 23.81% and 25.93% of patients experienced grade ≥3AEs in the lenvatinib plus camrelizumab group and the lenvatinib group, respectively.
Conclusion
Lenvatinib plus camrelizumab as post-progression treatment is effective and safe for advanced hepatocellular carcinoma with PD.
Keywords: advanced hepatocellular carcinoma, lenvatinib, PD-1
Introduction
Hepatocellular carcinoma (HCC) is the fourth common cause of cancer-related deaths worldwide.1 More than 50% of patients with HCC are diagnosed with advanced-stage disease at the first visit.2 Radical resection is considered as the major curative treatment for HCC patients. However, the recurrence rate remains as high as 70% within five years even received radical surgery.3 Hence, post-progression treatment for patients with advanced hepatocellular carcinoma is important to prolong post-progression survival. Based on the Phase III REFLECT trial,4 lenvatinib was shown to be comparable to sorafenib, and has been widely used as the first-line standard systemic therapy for advanced unresectable HCC since 2018. In recent years, programmed cell death protein 1 (PD-1) and programmed cell death ligand 1 (PD-L1) checkpoint inhibitors have emerged as promising treatment strategies for HCC. In a randomized Phase 2 trial,5 camrelizumab, a humanised monoclonal antibody against PD-1, has been shown to block the binding of PD-1 to PD-L1, and consequently inhibit the immune escape of tumor cells. It showed high affinity for PD-1 (KD=3·31 nmol/L) and high receptor occupancy on circulating T lymphocytes (85% at a dose of 200 mg). The binding epitope of camrelizumab is different from that of nivolumab and pembrolizumab. Combined therapies have achieved good results in the treatment of liver cancer, especially the combination of lenvatinib and pembrolizumab.6 However, the efficacy of combination therapy of lenvatinib and camrelizumab in the treatment of advanced HCC has not been reported. This study investigated the clinical efficacy and safety of combination therapy of lenvatinib plus camrelizumab in the treatment of advanced HCC with progressive disease (PD), in comparison with lenvatinib monotherapy.
Patients and Methods
Patient Selection
Between June 2019 and March 2020, the medical records of consecutive advanced HCC patients with PD were reviewed. Advanced HCC who received at least one first-line treatment and diagnosed with PD were eligible for inclusion in this study. First-line treatment includes surgery, transarterial chemoembolization (TACE), systematic chemotherapy and sorafenib. Other key inclusion criteria were age ≥18 years, at least one measurable lesion as defined by modified Response Evaluation Criteria in Solid Tumors version (mRECIST), Child-Pugh scores of ≤7, an Eastern Cooperative Oncology Group performance score (ECOG-PS) of 0 or 1, a predicted life expectancy >12 weeks, patients with hepatitis B virus (HBV) chronic infections, with a viral load <500 IU/mL and were required to continue or start a full course of standardized antiviral therapy during the study.7 Patients were excluded if they had Child-Pugh scores of ≥8, received previous anti-PD-1 or anti-PD-L1 immunotherapy for HCC, pregnant, cholangiocarcinoma or fibrolamellar and mixed hepatocellular subtypes, other active malignancies, symptomatic ascites, and gastrointestinal bleeding in the past six months. This study was conducted according to the ethical guidelines of the 1975 Declaration of Helsinki and was approved by the Ethics Committee of Mengchao Hepatobiliary Hospital of Fujian Medical University (Approval Number: 2020_105_01). The data are anonymous and the requirement for informed consent was therefore waived.
Lenvatinib and Camrelizumab Treatment
Lenvatinib was orally administered at a starting dose of 12 mg/day for patients with ≥60 kg body weight or 8 mg/day for patients with <60 kg body weight in the lenvatinib monotherapy group.4 The combined treatment group received the same doses of lenvatinib as the lenvatinib monotherapy group combined with camrelizumab 200 mg/2w.8 In cases that developed drug-related adverse events (AEs), the dose was reduced, or the drug was temporarily stopped until the symptoms resolved to grade 1 or 2, according to the guidelines provided by the manufacturer. All patients continued treatment until disease progression, unacceptable toxicity, death, or discontinuation for any reason.
Primary and Second Endpoints
Primary endpoints were overall survival (OS) and progression-free survival (PFS), and secondary endpoints were the objective response rate (ORR) and adverse events.
Evaluation of Anti-Tumor Responses
Anti-tumor response was evaluated according to the mRECIST.9 The first on-study radiographic examination was conducted at week 8, and subsequently every 3 weeks during the treatment course until disease progression or treatment discontinuation. Complete or partial responses were required to be confirmed at least 4 weeks after the first response. After disease progression or treatment discontinuation, the patients were monitored for OS every 30 days until death, loss to follow-up, or study completion.5
Assessment of Adverse Events
AEs were assessed according to the Common Terminology Criteria for Adverse Events, version 4.0. Hand-foot skin reaction, reactive cutaneous capillary endothelial proliferation, fever, appetite loss, hypertension and diarrhea were routinely assessed, while bone marrow suppression, liver function, renal function, heart function and thyroid function were monitored every 2–4 weeks.
Statistical Analysis
Statistical analyses were performed using R version 3.6.1 (http://www.r-project.org/). Continuous variables were analyzed using the Mann–Whitney U-test, and categorical variables were analyzed using the Fisher’s exact probability test. OS was measured from the date of treatment initiation until the date of death or last visit. PFS after treatment was measured from the date of treatment initiation until the date of confirmation of the first radiologic progressive disease (PD). PFS and OS were calculated using the Kaplan–Meier method, and differences in survival were evaluated by the Log rank test. The forward method of the univariate and multivariate Cox proportional hazard model was used to identify the prognostic factors. Potentially relevant variables were considered for generating the multivariable Cox model. A p-value <0.05 was considered statistically significant.
Results
Baseline Characteristics of Patients
Table 1 shows the baseline characteristics of all 48 patients in the lenvatinib plus camrelizumab group (n=21) and lenvatinib group (n=27). The number of patients who received first-line, second-line and third-line were 6, 10 and 11 in the lenvatinib plus camrelizumab group, and 3, 11 and 7 in the lenvatinib group, respectively. There was no statistically significant difference between the two groups (all p > 0.05, Table 1).
Table 1.
Baseline Characteristics of Patients
| Lenvatinib Group (n=27) | Lenvatinib Plus Camrelizumab Group (n=21) | P | |
|---|---|---|---|
| Age,years | |||
| >45 | 19 | 17 | 0.401 |
| ≤45 | 8 | 4 | |
| Gender | |||
| Male | 24 | 19 | 0.858 |
| Female | 3 | 2 | |
| ECOG-PS | |||
| 0 | 18 | 17 | 0.269 |
| 1 | 9 | 4 | |
| AFP (ng/mL) | |||
| <400 | 11 | 12 | 0.259 |
| ≥400 | 16 | 9 | |
| HBsAg | |||
| - | 2 | 4 | 0.226 |
| + | 25 | 17 | |
| TBil (mean±SD (umol/L)) | 19.90 (16.39–23.88) | 15.47 (12.35–18.42) | 0.363 |
| ALB (mean±SD (g/L)) | 39.37 (37.91–41.26) | 39.90 (37.58–42.07) | 0.328 |
| PT (mean±SD (s)) | 13.52 (13.04–14.01) | 13.02 (12.3–13.83) | 0.522 |
| Child-pugh score | |||
| 5~6 | 25 | 19 | 0.792 |
| 7 | 2 | 2 | |
| Cirrhosis | |||
| No | 3 | 2 | 0.858 |
| Yes | 24 | 19 | |
| Tumor type | |||
| Primary | 11 | 8 | |
| Relapse | 16 | 13 | 0.853 |
| Portal vein thrombus | |||
| No | 15 | 10 | 0.225 |
| I/II | 1 | 4 | |
| III/IV | 11 | 7 | |
| Extrahepatic metastasis | |||
| No | 10 | 11 | 0.288 |
| Yes | 17 | 10 | |
| Previous treatment | |||
| First-line | 6 | 3 | 0.547 |
| Second-lines | 10 | 11 | |
| Third-lines or more | 11 | 7 |
Abbreviations: ECOG-PS, Eastern Cooperative Oncology Group Performance Status; AFP, α‐fetoprotein; ALB, albumin, PT, prothrombin time.
OS and PFS
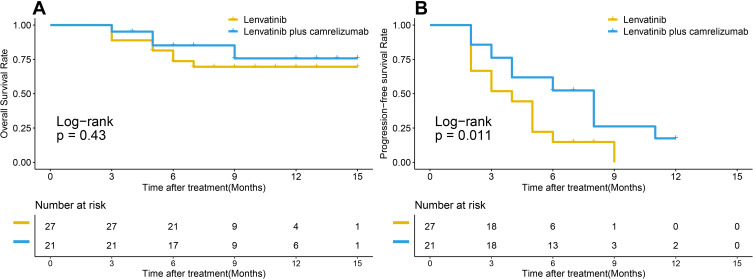
In all 48 patients, the median OS was not obtained, the 6-month and 12-month OS rates were 85.2% and 75.7% for the lenvatinib plus camrelizumab group, 73.7% and 69.6% for the lenvatinib group (P=0.43) (Figure 1A), respectively. The median PFS was significantly longer for the lenvatinib plus camrelizumab group than for the lenvatinib group (8.0 months vs 4.0 months, p=0.011) (Figure 1B). The 6-month and 12-month PFS rates were 52.4% and 17.5% for the lenvatinib plus camrelizumab group, 14.8% and 0% for the lenvatinib group, respectively.
Figure 1.
Survival curves of all patients with advanced hepatocellular carcinoma who underwent lenvatinib plus camrelizumab treatment and lenvatinib monotherapy. (A) cumulative overall survival (OS) curves and, (B) cumulative progression-free survival (rPFS) curves.
Subgroup analysis showed that no subgroup there was no statistical difference in OS between the two groups in each subgroup (Supplementary Figure 1), and patients who was >45 years old, or male, or AFP< 400 ng/mL, or HBsAg positive, or Child-Pugh A grade, or received third-lines and more previous treatment could benefit from lenvatinib plus camrelizumab combined therapy (Supplementary Figure 2).
Prognostic Factors Associated with Good PFS
In the univariate analysis of all 48 patients, the prognostic factors that were significantly associated with good OS were ECOG-PS score of 0, Child-Pugh score of 5–6, no portal vein thrombus and 1st-line previous treatment (Table 2). In the multivariate analysis, ECOG-PS score of 0 and Child-Pugh score of 5–6 remained significant independent predictors of good OS (HR=13.403, 95% CI=1.766–30.171, p=0.027; HR=5.981, 95% CI=1.208–29.609, p=0.040, respectively). The prognostic factors associated with good PFS in all 48 patients showed ECOG-PS score of 0, HbsAg positive, and lenvatinib plus camrelizumab treatment as the significant variables (Table 3). In the multivariate analysis, ECOG-PS score of 0 and lenvatinib plus camrelizumab treatment remained significant independent predictors of good PFS (HR=10.685, 95% CI=2.888–35.529, p<0.001; HR=0.365, 95% CI=0.148–0.897, p=0.028, respectively).
Table 2.
Univariate and Multivariate Analyses of the Prognostic Factors for OS
| Variables | Univariate Analysis | Multivariate Analysis | ||||
|---|---|---|---|---|---|---|
| Hazard Ratio | 95% CI | P value | Hazard Ratio | 95% CI | P value | |
| Age, years (>45 vs ≤45) | 1.029 | 0.278–3.804 | 0.996 | 0.030 | 0.001–1.017 | 0.051 |
| Gender (male vs female) | 0.728 | 0.094–5.639 | 0.761 | |||
| ECOG-PS (0 vs 1) | 5.801 | 1.798–18.712 | 0.003* | 13.403 | 1.766–30.171 | 0.027* |
| AFP (≥400 vs <400ng/mL) | 3.503 | 0.937–13.099 | 0.062 | 2.432 | 0.509–11.613 | 0.265 |
| HBsAg (- VS +) | 0.804 | 0.174–3.719 | 0.780 | |||
| TBil (umol/L) | 1.047 | 0.991–1.017 | 0.098 | |||
| ALB (g/L) | 0.908 | 0.791–1.042 | 0.169 | |||
| PT (s) | 1.212 | 0.902–1.627 | 0.202 | |||
| Child-Pugh score (5~6 vs 7) | 4.927 | 1.302–18.642 | 0.019* | 5.981 | 1.208–29.609 | 0.040* |
| Cirrhosis (no vs yes) | 1.230 | 0.159–9.534 | 0.843 | |||
| Tumor type (primary vs relapse) | 0.370 | 0.115–1.197 | 0.097 | 0.069 | 0.001–4.951 | 0.220 |
| Portal vein thrombus (no vs I/II vs III/IV) | 1.924 | 1.008–3.671 | 0.047* | 0.147 | 0.007–3.133 | 0.219 |
| Extrahepatic metastasis (no vs yes) | 1.112 | 0.353–3.506 | 0.856 | 5.159 | 0.612–43.454 | 0.131 |
| Previous treatment (first-line vs second-lines vs third-lines or more) | 0.334 | 0.148–0.756 | 0.008* | |||
| Treatment (lenvatinib group vs lenvatinib plus camrelizumab group) | 0.625 | 0.188–2.077 | 0.443 | 5.785 | 0.221–8.998 | 0.292 |
Note: *P<0.05.
Abbreviations: ECOG-PS, Eastern Cooperative Oncology Group Performance Status; AFP, α‐fetoprotein; ALB, albumin, PT, prothrombin time.
Table 3.
Univariate and Multivariate Analyses of the Prognostic Factors for PFS
| Variables | Univariate Analysis | Multivariate Analysis | ||||
|---|---|---|---|---|---|---|
| Hazard Ratio | 95% CI | P value | Hazard Ratio | 95% CI | P value | |
| Age, years (>45 vs ≤45) | 1.013 | 0.984–1.044 | 0.379 | |||
| Gender (male vs female) | 1.401 | 0.490–4.008 | 0.529 | |||
| ECOG (0 vs 1) | 3.236 | 1.535–6.821 | 0.002* | 10.685 | 2.888–39.529 | <0.001* |
| AFP (≥400 vs <400ng/mL) | 1.300 | 0.667–2.532 | 0.441 | 1.464 | 0.569–3.765 | 0.429 |
| HBsAg (- VS +) | 0.345 | 1.336–3.181 | 0.035* | 0.378 | 0.090–1.582 | 0.183 |
| TBil (umol/L) | 1.026 | 0.991–1.063 | 0.152 | |||
| ALB (g/L) | 1.032 | 0.961–1.108 | 0.387 | |||
| PT (s) | 1.112 | 0.899–1.376 | 0.329 | |||
| Child-Pugh score (5~6 vs 7) | 1.031 | 0.310–3.423 | 0.960 | 2.757 | 0.631–12.040 | 0.178 |
| Cirrhosis (no vs yes) | 0.643 | 0.190–2.182 | 0.479 | |||
| Tumor type (primary vs relapse) | 1.058 | 0.533–2.099 | 0.873 | 0.637 | 0.170–2.391 | 0.504 |
| Portal vein thrombus (no vs I/II vs III/IV) | 1.015 | 0.781–1.434 | 0.934 | 0.449 | 0.194–1.038 | 0.061 |
| Extrahepatic metastasis (no vs yes) | 0.832 | 0.433–1.596 | 0.579 | 0.435 | 0.148–1.278 | 0.130 |
| Previous treatment (first-line vs second-lines vs third-lines or more) | 0.812 | 0.528–1.249 | 0.344 | 0.755 | 0.387–1.471 | 0.409 |
| Treatment (lenvatinib group vs Lenvatinib plus camrelizumab group) | 0.434 | 0.217–0.867 | 0.018* | 0.365 | 0.148–0.897 | 0.028* |
Note: *P<0.05.
Abbreviations: ECOG-PS, Eastern Cooperative Oncology Group Performance Status; AFP, α‐fetoprotein; ALB, albumin, PT, prothrombin time.
Anti-Tumor Responses
Among all 48 patients, the best anti-tumor responses were partial response (PR) in six, stable disease (SD) in nine, and PD in six patients in the lenvatinib plus camrelizumab group, and PR in two, SD in 12, and PD in 13 patients in the lenvatinib group (Table 4). Compared with the lenvatinib group, the lenvatinib plus camrelizumab group had higher ORR (28.57% vs 7.41%) and disease control rate (DCR) (71.43% vs 51.85%).
Table 4.
Best Anti-Tumor Response According to the mRECIST
| Lenvatinib Group (n=27) | Lenvatinib Plus Camrelizumab Group(n=21) | |
|---|---|---|
| Complete response | 0 (0%) | 0 (0%) |
| Partial response | 2 (7.41%) | 6 (28.57%) |
| Stable disease | 12 (44.44%) | 9 (42.86%) |
| Progressive disease | 13 (38.15%) | 6 (28.57%) |
| Objective Response Rate | 7.41% | 28.57% |
| Disease Control Rate | 51.85% | 71.43% |
Note: Responses were evaluated according to modified Response Evaluation Criteria in Solid Tumors version by investigators.
Adverse Events
Table 5 shows the frequency of AEs within six weeks after the initiation of treatment in all 48 patients. The most common AEs in the two groups were hand-foot skin reaction (n=7), hypertension (n=13) and abnormal hepatic function damage (n=12). For AEs of any grade, abnormal cardiac function, hypothyroidism and reactive cutaneous capillary endothelial proliferation (RCCEP) occurred only in the lenvatinib plus camrelizumab group. Overall, AEs of grade ≥3 had similar frequencies in both groups. However, one patient needed to discontinue lenvatinib treatment due to severe AEs (grade 3 hypertension) and one patient needed to discontinue lenvatinib plus camrelizumab treatment due to severe AEs (grade 3 decreased platelet count).
Table 5.
Treatment-Related Adverse Events
| Lenvatinib Group (n=27) | Lenvatinib Plus Camrelizumab Group (n=21) | |||
|---|---|---|---|---|
| All Grade | Grade3/4 | All Grade | Grade3/4 | |
| Hand-foot skin reaction | 2 (7.41%) | 1 (3.70%) | 5 (23.81%) | 1 (4.76%) |
| Hypertension | 6 (22.22%) | 4 (14.81%) | 7 (33.33%) | 3 (14.29%) |
| RECCP | 0 | 0 | 3 (14.29%) | 0 |
| Weight loss | 0 | 0 | 0 | 0 |
| Decreased appetite | 1 (3.70%) | 0 | 0 | 0 |
| Nausea | 4 (14.81%) | 0 | 0 | 0 |
| Diarrhea | 2 (7.41%) | 1 (3.70%) | 2 (9.52%) | 0 |
| Alopecia | 2 (7.41%) | 0 | 1 (4.76%) | 0 |
| Weakness | 1 (3.70%) | 0 | 0 | 0 |
| Rash | 2 (7.41%) | 0 | 0 | 0 |
| Hemorrhage | 2 (7.41%) | 0 | 0 | 0 |
| Hoarseness | 0 | 0 | 1 (4.76%) | 0 |
| Mucositis | 1 (3.70%) | 0 | 0 | 0 |
| Pain | 3 (11.11%) | 0 | 1 (4.76%) | 0 |
| Myelosuppression | ||||
| Decreased white blood cell | 1 (3.70%) | 1 (3.70%) | 1 (4.76%) | 0 |
| Decreased red blood cell | 1 (3.70%) | 0 | 0 | 0 |
| Decreased platelet count | 1 (3.70%) | 0 | 2 (9.52%) | 1 (4.76%) |
| Abnormal hepatic function | ||||
| Increased blood bilirubin | 4 (14.81%) | 0 | 1 (4.76%) | 0 |
| Increased alanine aminotransferase | 6 (22.22%) | 0 | 2 (9.52%) | 0 |
| Increased aspartate aminotransferase | 7 (25.93%) | 0 | 5 (23.81%) | 0 |
| Abnormal renal function | ||||
| Proteinuria | 4 (14.81%) | 0 | 3 (14.29%) | 0 |
| Increased creatinine | 0 | 0 | 0 | 0 |
| Increased urea nitrogen | 0 | 0 | 0 | 0 |
| Abnormal cardiac function | ||||
| Increased creatine kinase | 0 | 0 | 4 (19.05%) | 0 |
| Increased creatine kinase isoenzyme | 0 | 0 | 3 (14.29%) | 0 |
| Hypothyroidism | 0 | 0 | 2 (9.52%) | 0 |
Abbreviation: RECCP, reactive cutaneous capillary endothelial proliferation.
Discussion
The present study compared the outcomes between lenvatinib plus camrelizumab and lenvatinib monotherapy for patients with advanced HCC in the clinical setting. The results showed that both ORR and DCR according to the mRECIST were significantly higher in the lenvatinib plus camrelizumab group than in the lenvatinib group. Moreover, PFS was significantly longer in the lenvatinib plus camrelizumab group than in the lenvatinib group, and lenvatinib plus camrelizumab treatment was a significant independent predictor of better PFS. To the best of our knowledge, this was the first study on the efficacy of lenvatinib plus camrelizumab in the treatment of advanced HCC in clinical practice.
Several reports have described the anti-tumor response to combination therapy in advanced HCC.6,10–14 These reports showed ORRs of 22.7–46.0% and DCRs of 73.6–88.0% according to mRECIST or RECIST 1.1. Similarly, the present study showed that the ORR and DCR were 14.3% and 35.7%, respectively. As for the prognosis, the previous reports showed median OS of 15.9–22.0 months and PFS of 7.8–9.3 months. However, the median OS in this study was not obtained and PFS were only 4.0 months, respectively. The possible reasons for this result were considered as follow: (1) most of these patients received second-line or above treatment and with progressive disease, the prognosis for such patients is usually poor; (2) the follow-up time was not long enough. Even so, these results still suggest that lenvatinib plus camrelizumab treatment is more effective than lenvatinib alone in advanced HCC as post-progression treatment.
The combination therapy including tyrosine kinase inhibitor (TKI) combined with PD-1/PD-L1 checkpoint inhibitors and PD-1/PD-L1 checkpoint inhibitors combined with cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) inhibitor have emerged as a promising treatment strategy for advanced HCC. However, only a minority of advanced HCC patients benefit from these combination therapies. Several reports have described predictive factors for response to TKI or PD-1/PD-L1 checkpoint inhibition in HCC.15–19 Gender, age, ECOG-PS score, AFP, liver function reserve, grade of AEs and levels of PD-L1 may influence the efficacy of above treatments. In this study, ECOG-PS score of 0 was a significant independent predictor of good OS and PFS, and Child-Pugh score of 5–6 was a significant independent predictor of good OS. Future strategies might require predictive factor-based patient selection to identify patients who are likely to respond to the combination strategies in order to enhance anti-tumor efficacy and clinical success.
In the present study, previously known AEs occurred in the lenvatinib plus camrelizumab group and the lenvatinib group.4,5,20–22 One patient needed to discontinue lenvatinib treatment due to grade 3 hypertensions, while one patient needed to discontinue lenvatinib plus camrelizumab treatment due to grade 3 decreased platelet count. Wang et al reported that 66.8% of camrelizumab-treated patients experienced RCCEP,23 but in the present study, RCCEP occurred in only 14.29% of the patients in the combined treatment group. This may be because lenvatinib, a type of VEGFR inhibitor, binds to VEGF and inhibits RCCEP formation by blocking signal transduction. Preclinical data suggested that the immunomodulatory effect of TKI drugs complements PD-1 activity, thereby increasing sensitivity of tumors to combination therapy and reducing the occurrence of AEs.24,25
There were several limitations in the present study. First, it was a retrospective study and therefore selection bias could not be avoided. Second, since lenvatinib and immunotherapy have only been used for advanced hepatocellular carcinoma in recent years and the number of patients receive Lenvatinib treatment as second-line or above treatment was little, the sample size was small. Third, the study was conducted at a single institution, further studies such as random multi-center researches are needed. Fourth, the follow-up duration was short, the long-term outcomes remain further study.
Conclusion
Lenvatinib plus camrelizumab treatment is effective and safe as a post-progression treatment for advanced hepatocellular carcinoma with progressive disease, and may lead to more favorable short-term outcomes compared with lenvatinib monotherapy.
Acknowledgments
The authors thank the Fujian provincial medical center of hepatobiliary for the subsidy.
Funding Statement
This study was sponsored by the Fujian provincial medical center of hepatobiliary; Key Clinical Specialty Discipline Construction Program of Fuzhou, Fujian, P.R.C (201912002); Synergetic enhancement of photodynamic therapy/chemotherapy of tumor by tumor microenvironment triggered self-supplying oxygen BMSF @ Pt system (2019Y0013).
Abbreviations
AEs, adverse events; DCR, disease control rate; ECOG-PS, Eastern Cooperative Oncology Group performance score; HBV, hepatitis B virus; HCC, hepatocellular carcinoma; OS, overall survival; ORR, objective response rate; PD, progressive disease; PD-1, programmed cell death protein 1; PD-L1, programmed cell death ligand 1; PFS, progression-free survival; TACE, transarterial chemoembolization.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no competing interests.
References
- 1.Park JW, Chen M, Colombo M, et al. Global patterns of hepatocellular carcinoma management from diagnosis to death: the BRIDGE Study. Liver Int. 2015;35(9):2155–2166. doi: 10.1111/liv.12818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang JD, Hainaut P, Gores GJ, Amadou A, Plymoth A, Roberts LR. A global view of hepatocellular carcinoma: trends, risk, prevention and management. Nat Rev Gastroenterol Hepatol. 2019;16(10):589–604. doi: 10.1038/s41575-019-0186-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018;391(10127):1301–1314. doi: 10.1016/S0140-6736(18)30010-2 [DOI] [PubMed] [Google Scholar]
- 4.Kudo M, Finn RS, Qin S, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised Phase 3 non-inferiority trial. Lancet. 2018;391(10126):1163–1173. doi: 10.1016/S0140-6736(18)30207-1 [DOI] [PubMed] [Google Scholar]
- 5.Qin S, Ren Z, Meng Z, et al. Camrelizumab in patients with previously treated advanced hepatocellular carcinoma: a multicentre, open-label, parallel-group, randomised, phase 2 trial. Lancet Oncol. 2020;21(4):571–580. doi: 10.1016/S1470-2045(20)30011-5 [DOI] [PubMed] [Google Scholar]
- 6.Finn RS, Ikeda M, Zhu AX, Sung MW, Llovet JM. Phase Ib study of lenvatinib plus pembrolizumab in patients with unresectable hepatocellular carcinoma. J Clin Oncol. 2020;38(26):2960–2970. doi: 10.1200/JCO.20.00808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hou J, Wang G, Wang F, et al. Guideline of prevention and treatment for chronic hepatitis B (2015 update). J Clin Transl Hepatol. 2017;5(4):297–318. doi: 10.14218/JCTH.2016.00019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang J, Mo H, Wu D, Chen X, Xu B. Phase I study of the anti-PD-1 antibody SHR-1210 in patients with advanced solid tumors. J Clin Oncol. 2017;35(15_suppl):e15572–e15572. doi: 10.1200/JCO.2017.35.15_suppl.e15572 [DOI] [Google Scholar]
- 9.Lencioni R. New data supporting modified RECIST (mRECIST) for hepatocellular carcinoma. Clin Cancer Res. 2013;19(6):1312–1314. doi: 10.1158/1078-0432.CCR-12-3796 [DOI] [PubMed] [Google Scholar]
- 10.Finn RS, Qin S, Ikeda M, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382(20):1894–1905. doi: 10.1056/NEJMoa1915745 [DOI] [PubMed] [Google Scholar]
- 11.Xu J, Zhang Y, Jia R, et al. Anti-PD-1 antibody SHR-1210 combined with apatinib for advanced hepatocellular carcinoma, gastric, or esophagogastric junction cancer: an open-label, dose escalation and expansion study. Clin Cancer Res. 2019;25(2):515–523. doi: 10.1158/1078-0432.CCR-18-2484 [DOI] [PubMed] [Google Scholar]
- 12.Yang X, Shi J, Chen X, Jiang Y, Zhao H. Efficacy of cabozantinib and nivolumab in treating hepatocellular carcinoma with RET amplification, high tumor mutational burden, and PD-L1 expression. Oncologist. 2020;25(6):470–474. doi: 10.1634/theoncologist.2019-0563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kelley RK, Abou-Alfa GK, Bendell JC, Kim TY, Sangro B. Phase I/II study of durvalumab and tremelimumab in patients with unresectable hepatocellular carcinoma (HCC): phase I safety and efficacy analyses. J Clin Oncol. 2017;35(15_suppl):4073. doi: 10.1200/JCO.2017.35.15_suppl.4073 [DOI] [Google Scholar]
- 14.Floudas CS, Xie C, Brar G, Morelli MP, Greten TF. Combined immune checkpoint inhibition (ICI) with tremelimumab and durvalumab in patients with advanced hepatocellular carcinoma (HCC) or biliary tract carcinomas (BTC). J Clin Oncol. 2019;37(4_suppl):336. doi: 10.1200/JCO.2019.37.4_suppl.33630707056 [DOI] [Google Scholar]
- 15.Macek Jilkova Z, Aspord C, Decaens T. Predictive factors for response to PD-1/PD-L1 checkpoint inhibition in the field of hepatocellular carcinoma: current status and challenges. Cancers. 2019;11(10):1554. doi: 10.3390/cancers11101554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kodama K, Kawaoka T, Namba M, et al. Correlation between early tumor marker response and imaging response in patients with advanced hepatocellular carcinoma treated with lenvatinib. Oncology. 2019;97(2):75–81. doi: 10.1159/000499715 [DOI] [PubMed] [Google Scholar]
- 17.Terashima T, Yamashita T, Takata N, et al. Comparative analysis of liver functional reserve during lenvatinib and sorafenib for advanced hepatocellular carcinoma. Hepatol Res. 2020;50(7):871–884. doi: 10.1111/hepr.13505 [DOI] [PubMed] [Google Scholar]
- 18.Ohki T, Sato K, Kondo M, et al. Impact of adverse events on the progression-free survival of patients with advanced hepatocellular carcinoma treated with lenvatinib: a multicenter retrospective study. Drugs Real World Outcomes. 2020;7(2):141–149. doi: 10.1007/s40801-020-00179-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Herbst RS, Soria JC, Kowanetz M, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515(7528):563–567. doi: 10.1038/nature14011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hatanaka T, Kakizaki S, Nagashima T, et al. Analyses of objective response rate, progression‐free survival, and adverse events in hepatocellular carcinoma patients treated with lenvatinib: a multicenter retrospective study. Hepatol Res. 2019;50(3):382–395. doi: 10.1111/hepr.13460 [DOI] [PubMed] [Google Scholar]
- 21.Finn R, Ryoo B, Merle P, et al. Pembrolizumab as second-line therapy in patients with advanced hepatocellular carcinoma in KEYNOTE-240: a randomized, double-blind, phase III trial. J Clin Oncol. 2020;38(3):193–202. doi: 10.1200/JCO.19.01307 [DOI] [PubMed] [Google Scholar]
- 22.El-Khoueiry AB, Sangro B, Yau TC, Crocenzi TS, Melero I. Phase I/II safety and antitumor activity of nivolumab (nivo) in patients (pts) with advanced hepatocellular carcinoma (HCC): interim analysis of the CheckMate-040 dose escalation study. J Clin Oncol. 2016;34(15_suppl):4012. doi: 10.1200/JCO.2016.34.15_suppl.4012 [DOI] [Google Scholar]
- 23.Wang F, Qin S, Sun X, et al. Reactive cutaneous capillary endothelial proliferation in advanced hepatocellular carcinoma patients treated with camrelizumab: data derived from a multicenter phase 2 trial. J Hematol Oncol. 2020;13(1):47. doi: 10.1186/s13045-020-00886-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kato Y, Tabata K, Kimura T, et al. Lenvatinib plus anti-PD-1 antibody combination treatment activates CD8+ T cells through reduction of tumor-associated macrophage and activation of the interferon pathway. PLoS One. 2019;14(2):e0212513. doi: 10.1371/journal.pone.0212513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kimura T, Kato Y, Ozawa Y, Kodama K, Nomoto K. Immunomodulatory activity of lenvatinib contributes to antitumor activity in the Hepa1‐6 hepatocellular carcinoma model. Cancer Sci. 2018;109(6):3993–4002. doi: 10.1111/cas.13806 [DOI] [PMC free article] [PubMed] [Google Scholar]