The presence of cytosolic DNA at tumor sites and the activation of the cGAS-STING pathway have been implicated in the activation of antitumor immune responses.1–3 Manganese (Mn) is a necessary trace element for intracellular activities. It plays important roles in development, digestion, reproduction, antioxidant defense, energy production, immunity, and neuronal activity regulation.4 Mn was reported to enhance cGAS-STING activation through increased sensitivity to dsDNA during viral infections.5 However, it is unclear whether Mn can enhance antitumor immune functions. Here, we revealed that Mn2+ could inhibit the development of murine hepatocellular carcinoma (HCC) through immune modulations. It upregulated IFN-γ and TNF-α production in splenic and tumor-infiltrating CD8+ T cells. Moreover, Mn2+ significantly increased type I interferon (IFN) production and type I IFN-stimulated gene (ISG) expression in tumor-bearing mice. Further analysis revealed that myeloid cells could upregulate type I IFN production and increase costimulatory molecule expression upon Mn2+ stimulation. In vivo depletion of CD8+ T cells or treatment of IFNAR KO mice diminished the antitumor activity of Mn2+, suggesting that the antitumor function of Mn2+ is dependent on both CD8+ T cells and type I IFN signaling. Our results provide evidence to support Mn2+ as a novel agent that can be incorporated into cancer immunotherapy.
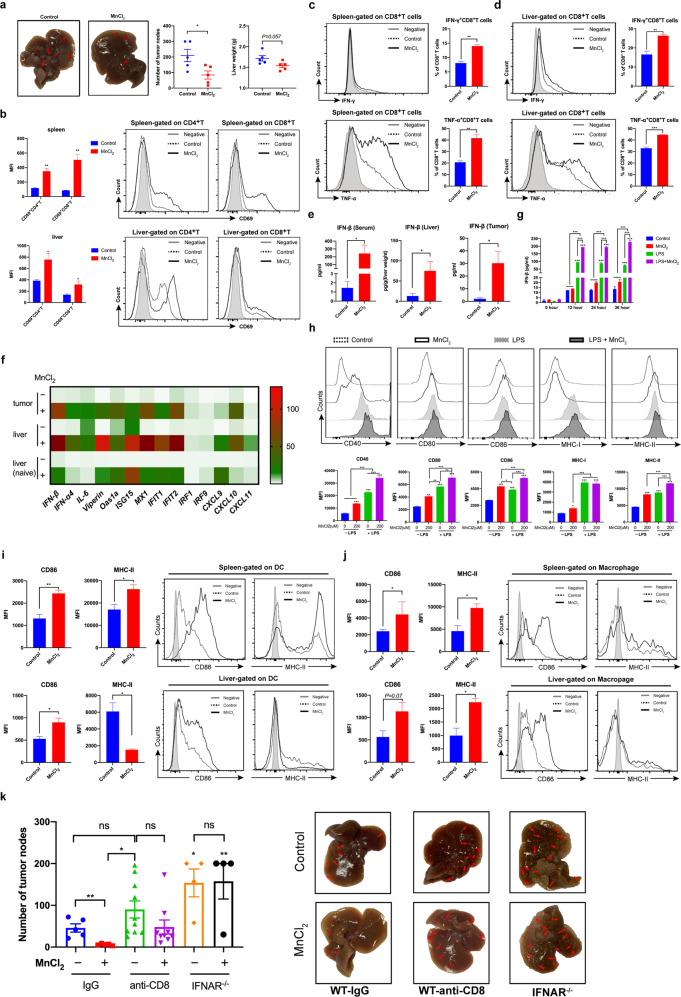
To investigate the effect of Mn2+ treatment on tumor development, we established an orthotopic murine HCC model by hydrodynamic cell delivery. The mice were then treated with MnCl2 or PBS intravenously every other day beginning on day 1. On day 21, the tumor-bearing mice were sacrificed, and the number of tumor nodes in the liver was counted (Fig. 1a). We found that the number of tumor nodes in the liver of MnCl2-treated mice was significantly decreased compared with that in the liver of mice in the control group. The liver weight of tumor-bearing mice also showed a decreasing trend with MnCl2 treatment. To investigate whether MnCl2 treatment can directly inhibit tumor growth, we cultured hepa1–6 cells in the presence or absence of different concentrations of MnCl2 (Supplementary Fig. 1). Tumor cell growth was examined using a Cell Counting Kit-8 assay at 24, 48, and 72 h post treatment. No difference was observed in cell growth at any time point, suggesting that Mn2+ does not directly affect tumor growth and that its tumor-suppressing function is immunomodulatory.
Fig. 1. Mn2+ inhibited tumor growth by promoting the antitumor function of CD8+ T cells in a type I IFN-dependent manner in a murine orthotopic HCC model.
Hepa1–6 cells were injected into recipient mice by hydrodynamic cell delivery (1.5 × 106 cells/2 ml/mouse, n = 5 per group). Then, MnCl2 in 200 μl PBS (5 mg/kg body weight) was intravenously administered to the tumor-bearing mice once every other day from day 1 to day 20. PBS was injected as a control. On day 21, the mice were euthanized, the number of tumor nodules in the liver was counted, and the livers harvested from tumor-bearing mice were weighed. Lymphocytes were isolated from the spleen and tumor-bearing liver on day 7 post tumor implantation. a The tumor nodules in livers from MnCl2- or PBS-treated mice are shown. The number of tumor nodules in the liver and the liver weight of tumor-bearing mice were quantified and analyzed. b The expression of CD69 on the surface of CD4+ T and CD8+ T cells derived from the spleen or liver of tumor-bearing mice was analyzed. c, d. IFN-γ and TNF-α production by T cells from the spleen or liver was detected by intracellular staining and flow cytometry. Representative histograms and percentages of IFN-γ- or TNF-α-producing CD8+ T cells in the spleen (c) or liver (d) of tumor-bearing mice are shown. (negative: gray line, control: dotted line, MnCl2: solid line). e A murine orthotopic HCC model was established by hydrodynamic injection of hepa1–6 cells (1.5 × 106 cells/2 ml/mouse, n = 2 mice per group). PBS was injected into naive control mice. MnCl2 in 200 μl PBS (5 mg/kg body weight) was intravenously administered to the tumor-bearing mice once on day 20. PBS was injected as a control. Naive mice (n = 2 mice per group) were also injected with PBS or MnCl2. Twelve hours later, the serum was collected. Normal liver tissues and tumor tissues were isolated from the mice and homogenized in RIPA buffer. The concentrations of IFN-β in the serum, liver tissues, and tumor tissues of tumor-bearing mice were measured by ELISA. f The expression of selected genes was analyzed by qPCR. GAPDH was used as an internal control. Heatmap of the gene expression levels in the tumor and normal liver tissues of MnCl2-treated and PBS-treated mice. The value for the PBS-treated liver of a naive mouse was set as 1. g J774A.1 cells were stimulated with or without MnCl2 (200 μM) in the presence or absence of LPS (1 μg/ml). The concentrations of IFN-β in the supernatant of J774A.1 cells cultured in the absence or presence of LPS were detected by ELISA. h J774A.1 cells were stimulated with or without MnCl2 (200 μM) in the presence or absence of LPS (1 μg/ml). Twenty hours later, the expression of CD40, CD80, CD86, MHC-I, and MHC-II on the cell surface was measured by flow cytometry. The expression of CD86 and MHC-II on the surfaces of DCs (i) and CD11b+F4/80+ macrophages (j) isolated from the spleen or liver of tumor-bearing mice treated with PBS or MnCl2 on day 7 after tumor implantation was analyzed. k A murine orthotopic HCC model was established by hydrodynamic injection of hepa1–6 cells into WT mice or IFNAR−/− mice (1 × 106 cells/2 ml/mouse, n = 4–10 per group). MnCl2 (5 mg/kg body weight) was intravenously administered to the tumor-bearing mice from day 1 to day 20. PBS was injected as a control. WT mice were given an anti-mouse CD8 antibody to deplete CD8+ T cells once a week, and an isotype IgG antibody was injected as a control. On day 21, the mice were euthanized, and the number of tumor nodules in the liver was counted. Data are shown as the mean ± SEM. Data are representative of at least two independent experiments. A two-tailed, unpaired Student’s t test (a–e, i, j) or one-way ANOVA followed by Dunnett’s multiple comparisons test (f–h, k) was used for statistical analysis. *p < 0.05, **p < 0.01, ***p < 0.001
To investigate the immunoregulatory functions of Mn2+ during tumor development, we performed flow cytometry analysis of immune cells from the liver and spleen of tumor-bearing mice. The percentages of CD4+ T and CD8+ T cells were slightly decreased in the spleen after MnCl2 treatment (Supplementary Fig. 2a). However, in the tumor-bearing liver, the percentage of CD8+ T cells showed an increasing trend upon MnCl2 treatment. The percentage of DCs was increased in the spleen and liver upon MnCl2 treatment. The activation (CD69 expression) of CD4+ T cells and CD8+ T cells was also upregulated in the spleen and liver of MnCl2-treated tumor-bearing mice (Fig. 1b). Although the percentage of effector T cells was not affected (Supplementary Fig. 2b, c), IFN-γ and TNF-α production by CD8+ T cells from the spleen or tumor-bearing liver of MnCl2-treated mice displayed significant increases compared with that by CD8+ T cells from the same organs of control mice (Fig. 1c, d). Mn2+ did not affect the TNF-α or IFN-γ production of CD4+ T cells from the spleen or tumor-bearing liver (Supplementary Fig. 3a). A recent study with nanoparticles acting as both drug carriers and Mn2+ generators demonstrated that Mn2+ enhanced cGAS-STING activity and upregulated the production of TNF-α and IL-6.6 Moreover, TNF-α has been found to be essential for the therapeutic efficacy of cyclic dinucleotide STING agonists in nonimmunogenic tumors.7 Therefore, IFN-γ and TNF-α could be the critical effector molecules produced by T cells downstream of type I IFN during the antitumor immune response. To determine whether Mn2+ can directly affect cytokine production by T cells, we isolated T cells from the liver and spleen of tumor-bearing mice and then treated these cells with MnCl2 for 3 days in the presence of anti-CD3 and anti-CD28 antibodies (Supplementary Fig. 3b). There was no significant difference in the production of TNF-α and IFN-γ by CD4+ T cells or CD8+ T cells between the MnCl2-treated and control groups. These results suggest that MnCl2 does not directly affect cytokine production by T cells and that the increased cytokine production by CD8+ T cells induced by MnCl2 is mediated through other immune mechanisms.
Mn2+ was reported to activate the cGAS-STING pathway to induce type I IFN production.5 To investigate whether Mn2+ can enhance type I IFN production in tumor-bearing mice, we detected the production of IFN-β in the serum, normal liver tissue and tumor tissue derived from tumor-bearing mice treated with or without MnCl2 (Fig. 1e). The results showed that the expression levels of IFN-β in the serum, liver tissue, and tumor tissue were all increased after MnCl2 treatment. To confirm the upregulation of type I IFN and ISG expression induced by MnCl2, we examined the transcription of type I IFNs and ISGs in normal liver and tumor tissue from tumor-bearing mice as well as liver tissue from nontumor-bearing mice treated with or without MnCl2 (Fig. 1f). The results showed that the expression of type I IFNs, including IFN-β and IFN-α4, was significantly upregulated in the tumor and liver of tumor-bearing mice as well as in the liver of nontumor-bearing mice after MnCl2 treatment. The expression of ISGs, including Viperin, Oas1a, ISG15, MX1, IFIT1, IFIT2, IRF1, and IRF9, was also upregulated in the tumor and liver of tumor-bearing mice as well as in the liver of nontumor-bearing mice after MnCl2 treatment. Moreover, the levels of chemokines that can be induced by type I IFN, such as CXCL9, CXCL10, and CXCL11, were significantly increased in the presence of MnCl2. A recent study reported that cancer cells could induce STING-dependent type I IFN production in myeloid cells via cGAMP in the tumor microenvironment.8 To investigate whether MnCl2 treatment can enhance the production of type I IFN in myeloid cells, we treated the macrophage cell line J774A.1 with MnCl2 in the presence or absence of LPS to mimic resting and inflammatory conditions in vivo, respectively (Supplementary Fig. 4a). The expression levels of IFN-β, IL-6, and CXCL10 were significantly upregulated with 200 µM MnCl2 treatment in the presence or absence of LPS. We also found that the IFN-β level was significantly increased with MnCl2 treatment in the presence of LPS in the culture supernatant (Fig. 1g). Upon MnCl2 treatment in the absence of LPS, J774A.1 cells also significantly upregulated the expression of CD40, CD80, CD86, MHC I and MHC II. LPS alone could increase the expression of all the molecules examined, and MnCl2 treatment further upregulated the expression of CD40, CD80, CD86, and MHC II, although the expression of MHC I was unchanged (Fig. 1h). In the in vivo HCC model, the expression of CD86 on DCs and CD11b+F4/80+ macrophages derived from the spleen and tumor-bearing liver was significantly upregulated by MnCl2 treatment (Fig. 1i, j). MnCl2 treatment also induced the upregulation of MHC-II expression on microphages from the spleen and liver. These results suggest that Mn2+ can activate myeloid cells to upregulate the expression of costimulatory and MHC molecules and may facilitate their functions as antigen-presenting cells.
To investigate whether the induction of type I IFN production by Mn2+ can promote T-cell functions, we first treated J774A.1 cells with MnCl2 for 24 h in the presence or absence of LPS, and then the culture supernatants were collected and added to T cells stimulated with anti-CD3/anti-CD28 antibodies (Supplementary Fig. 4b). We found that the addition of supernatants from J774A.1 cells treated with MnCl2 significantly promoted the production of IFN-γ and TNF-α by both CD4+ T cells and CD8+ T cells compared with the addition of supernatants from control cells. To further confirm whether the antitumor function of Mn2+ in vivo is mediated by CD8+ T cells, we treated tumor-bearing mice with an anti-CD8 antibody to deplete CD8+ T cells (Supplementary Fig. 4c, Fig. 1k). The results showed that tumor growth was significantly inhibited by MnCl2 treatment in the isotype control group, while this antitumor effect of Mn2+ was diminished when CD8+ T cells were depleted (Fig. 1k). This suggests that CD8+ T cells are essential in mediating the antitumor effect of Mn2+. We also established a murine HCC model using IFNAR−/– mice and found that there was no difference between the MnCl2-treated and control groups in the absence of type I IFN signaling (Fig . 1k). Thus, the antitumor function of Mn2+ is dependent on both type I IFN signaling and CD8+ T cells.
In the current study, we demonstrated that Mn2+ could inhibit tumor development by enhancing CD8+ T-cell function via induction of type I IFN production. These findings provide evidence that Mn2+, as one of the essential nutrients, is a safe and potent agent activating both innate and adaptive immune responses in cancer immunotherapy.
Supplementary information
Acknowledgements
This work was supported by the National Research Foundation Singapore CRP grant (NRF2017NRF-CRP001–034) and the start-up grant of National University of Singapore. We thank Mr. Teo Guohui and Dr. Paul Edward Hutchinson for assistance with flow cytometry.
Author contributions
Y.S., Y.L., and H.L. designed the research. Y.S. and Y.L. performed the experiments. H.Y.T., Z.B.H., Y.M., Y.Z., and C.Y.L. assisted in the experiments. M.L. and Z.J. assisted in the data interpretation. Y.S. and H.L. analyzed the data and wrote the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
These authors contributed equally: Yuan Song, Yonghao Liu
Supplementary information
The online version of this article (10.1038/s41423-020-00524-4) contains supplementary material.
References
- 1.Goubau D, Deddouche S, Reis e Sousa C. Cytosolic sensing of viruses. Immunity. 2013;38:855–869. doi: 10.1016/j.immuni.2013.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ho SS, et al. The DNA structure-specific endonuclease MUS81 mediates DNA sensor STING-dependent host rejection of prostate cancer cells. Immunity. 2016;44:1177–1189. doi: 10.1016/j.immuni.2016.04.010. [DOI] [PubMed] [Google Scholar]
- 3.Yang H, Wang H, Ren J, Chen Q, Chen ZJ. cGAS is essential for cellular senescence. Proc. Natl Acad. Sci. USA. 2017;114:E4612–E4620. doi: 10.1073/pnas.1705499114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen P, Bornhorst J, Aschner M. Manganese metabolism in humans. Front. Biosci. 2018;23:1655–1679. doi: 10.2741/4665. [DOI] [PubMed] [Google Scholar]
- 5.Wang C, et al. Manganese increases the sensitivity of the cGAS-STING pathway for double-stranded DNA and is required for the host defense against DNA viruses. Immunity. 2018;48:675–687. e677. doi: 10.1016/j.immuni.2018.03.017. [DOI] [PubMed] [Google Scholar]
- 6.Hou L, et al. Manganese-based nanoactivator optimizes cancer immunotherapy via enhancing innate immunity. ACS Nano. 2020;14:3927–3940. doi: 10.1021/acsnano.9b06111. [DOI] [PubMed] [Google Scholar]
- 7.Francica BJ, et al. TNFalpha and radioresistant stromal cells are essential for therapeutic efficacy of cyclic dinucleotide STING agonists in nonimmunogenic tumors. Cancer Immunol. Res. 2018;6:422–433. doi: 10.1158/2326-6066.CIR-17-0263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schadt L, et al. Cancer-cell-intrinsic cGAS expression mediates tumor immunogenicity. Cell Rep. 2019;29:1236–1248. e1237. doi: 10.1016/j.celrep.2019.09.065. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.