Nanovaccines are used as delivery platforms for antigens and adjuvants, which activate antigen-presenting cells (APCs) and enhance anticancer immune responses.1,2 Researchers have recently developed a self-assembled nanocomplex using a polysorbitol-co-PEI (PSPEI) polymer complexed with poly(I:C) (PIC). By binding to different surface proteins, this nanocomplex enhances the intracellular delivery of cargos and induces potent anticancer immune responses against melanoma cells.3 We have previously demonstrated that PD-1/PD-L1 blockade enhanced the efficacy of DC-based cancer immunotherapy.4,5 Moreover, the combination of nanomedicines and PD-L1 blockade has been reported to enhance CD8+ T cell activation and inhibit immunosuppressive cells within the tumor microenvironment.6 In the present study, we investigated the therapeutic efficacy of a combinatorial treatment comprising the immunoadjuvant nanocomplex PSPEI-PIC, a DC vaccine, and PD-L1 blockade in a murine colon cancer model.
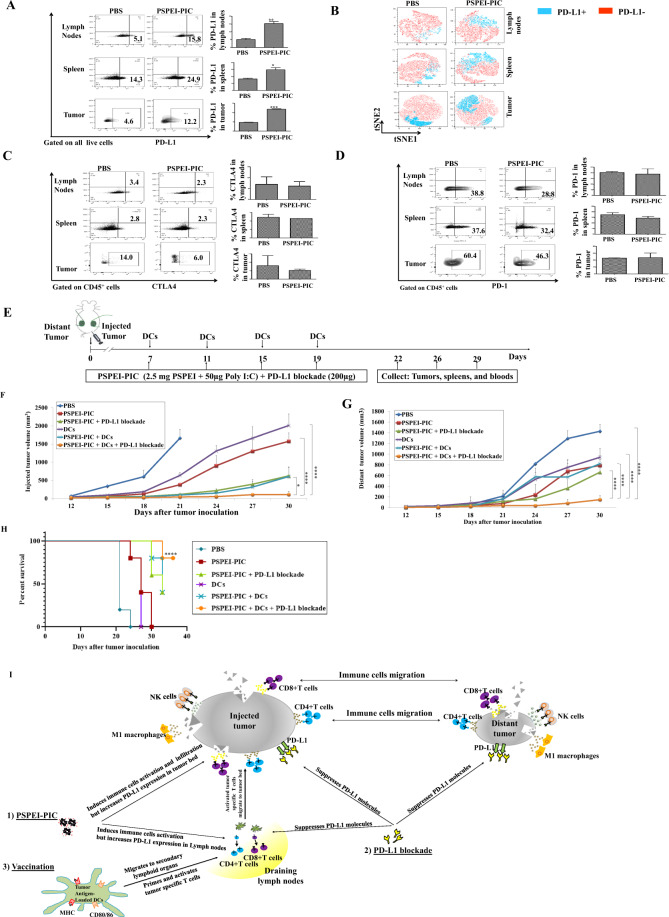
We first explored the effects of PSPEI-PIC injection on the tumor microenvironment. Mice treated with PSPEI-PIC expressed significantly higher levels of PD-L1 in the spleen, lymph nodes, and tumor microenvironment than PBS-control mice (Fig. 1A, B). However, we found no significant differences in the expression levels of CTLA4 (Fig. 1C) and PD-1 (Fig. 1D) between the PSPEI-PIC and PBS-control groups. These data suggest that the administration of PSPEI-PIC enhances the infiltration and activation of innate and adaptive immune cells in the tumor microenvironment. Cancer cells may upregulate PD-L1 to evade antitumor immune responses. Thus, we hypothesized that PD-L1 blockade could augment the antitumor effects of PSPEI-PIC. To test this hypothesis, we treated mice bearing MC-38 tumors with PSPE-PIC combined with PD-L1 blockade. The combination of PSPEI-PIC with PD-L1 blockade significantly suppressed tumor growth to a greater extent than PD-L1 blockade alone or the PBS control (Fig. S1A, B). We also observed that BM-derived CD11c+ DCs exposed to PSPEI-PIC expressed higher levels of maturation molecules than imDCs or DCs matured with LPS (Fig. S2A, B). We therefore tested the additional effects of DC vaccination on the antitumor effects of PSPEI-PIC plus PD-L1 blockade in vivo (Fig. 1E). Treatment with PSPEI-PIC alone, PSPEI-PIC + PD-L1 blockade, DCs alone, or PSPEI-PIC + DCs significantly inhibited tumor growth in the right flank (Fig. 1F, Fig. S3A) but only slightly delayed the progression of the nontreated tumor in the left flank (Fig. 1G, Fig. S3B). However, the combination of PSPEI-PIC, DC vaccination, and PD-L1 blockade strongly inhibited the growth of both injected and distant noninjected tumors. In addition, mice treated with PSPEI-PIC + DCs + PD-L1 blockade exhibited the longest survival among all treatment groups (Fig. 1H). These results suggest that DC vaccination and PD-L1 blockade augment the antitumor effects of PSPEI-PIC and that the combination of these treatments can induce long-term and systemic antitumor immune responses in tumor-bearing mice (Fig. 1I).
Fig. 1.
A PSPEI-PIC treatment enhanced PD-L1 expression. B PD-L1 expression was assessed via t-distributed stochastic neighbor embedding (t-SNE) using FlowJo. PSPEI-PIC did not affect CTLA4 (C) or PD-1 (D) expression levels. E Schematic representation of the combined PSPEI-PIC + DCs + PD-L1 blockade treatment. The growth rates of the primary (F) (*P < 0.05) and distant (G) tumors (****P < 0.0001) were monitored. H Survival time of tumor-bearing mice (****P < 0.0001). I Immune cascade underlying the systemic antitumor immune responses initiated by PSPEI-PIC combined with DCs and PD-L1 blockade. Data are presented as the mean ± standard error of the mean (SEM) and are representative of three independent experiments
We found that ~97% of MC-38 cells and ~6% of BM-derived DCs expressed PD-L1 (Fig. S2C, D). To gain further insight into the immunological mechanisms underlying the potent antitumor effects of PSPEI-PIC + DCs + PD-L1 blockade, we evaluated the effects of the combination therapy on the immune response on days 22, 26, and 29 post treatment. Mice treated with PSPEI-PIC alone, DCs alone, and PSPEI-PIC + DCs expressed higher levels of PD-L1 in the spleens and tumors than mice treated with anti-PD-L1 alone or the PBS control. By contrast, the spleens and tumors of mice treated with PSPEI-PIC + PD-L1 blockade and PSPEI-PIC + DCs + PD-L1 blockade exhibited the lowest percentage of PD-L1-expressing cells among all groups (Fig. S4A–F). In addition, restimulated splenocytes and serum from mice treated with PSPEI-PIC + DCs + PD-L1 blockade increased IFN-γ levels and decreased IL-10 and TGF-β levels (Fig. S5A–E). Importantly, mice in the PSPEI-PIC + DCs + PD-L1 blockade group exhibited the highest proportions of splenic M1 macrophages (Fig. S6A, B), CD4+CD44+ T cells (Fig. S7A, B), CD8+CD44+ T cells (Fig. S7C, D), effector NK cells (Fig. S8A, B), CD4+ TEM cells (Fig. S9A, B), and CD8+ TEM cells (Fig. S9C, D) among all groups. Moreover, mice in the PSPEI-PIC + DCs + PD-L1 blockade group displayed a trend toward higher proportions of tumor-infiltrating CD8+CD69+ T cells (Fig. S10A–D), CD8+CD44+ T cells (Fig. S10E–H), CD8+ TCM cells (Fig. S9E–H), CD4+CD44+ T cells (Fig. S7E–H), effector NK cells (Fig. S8C–F), and M1 macrophages (Fig. S6D, E, G, H) among all treatment groups, in both the injected and distant tumors. Interestingly, treatment with PSPEI-PIC + DCs + PD-L1 blockade promoted the activation of CD62L(lo)CD44(hi) splenic effector cells (Fig. S9A–D), which is necessary for their efficient activation and migration to injected and distant tumors. Upon migration into the tumor microenvironment, effector cells become CD62L(hi)CD44(hi) (Fig. S9E–H), allowing them to reside in the tumor microenvironment to kill cancer cells. In addition, mice treated with PSPEI-PIC + DCs + PD-L1 blockade showed a trend toward lower percentages of M2 macrophages (Fig. S6A, C, D, F; Fig. 6G, I), Tregs (Fig. S11A–F), and MDSCs (Fig. S12A–S12) in the spleen and in both the injected and distant tumors. Furthermore, mice treated with PSPEI-PIC + DCs + PD-L1 blockade produced the lowest levels of IL-10 (Fig. S13A), VEGF (Fig. S13B), and TGF-β (Fig. S13C) among all groups. These results indicate that the combination of PSPEI-PIC, DCs, and PD-L1 blockade promotes the infiltration of effector immune cells, inhibits immunosuppressive cells and strongly suppresses the production of angiogenic factors (VEGF) and inhibitory cytokines (TGF-β and IL-10) in the spleen and tumor microenvironment of treated mice, which showed a clear correlation with tumor suppression at both injected and distant sites.
In conclusion, this study demonstrated that PSPEI-PIC plus DC vaccine and PD-L1 blockade induce potent antitumor immunity by targeting the immunosuppressive tumor microenvironment, activating effector cells, and polarizing Th1/Th2 responses to enhance antitumor immunity.
Supplementary information
Acknowledgements
This study was supported by grants from the Basic Science Research Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Education, Science and Technology (2018R1A5A2024181, 2020R1A2C2010098).
Competing interests
The authors declare no competing interests.
Footnotes
These authors contributed equally: Manh-Cuong Vo, Seo-Yeon Ahn, Tan-Huy Chu
Contributor Information
In-Kyu Park, Email: pik96@jnu.ac.kr.
Je-Jung Lee, Email: drjejung@chonnam.ac.kr.
Supplementary information
The online version contains supplementary material available at 10.1038/s41423-021-00666-z.
References
- 1.Fang RH, et al. Nanoparticle-based manipulation of antigen-presenting cells for cancer immunotherapy. Small. 2015;11:5483–5496. doi: 10.1002/smll.201501284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shao K, et al. Nanoparticle-based immunotherapy for cancer. ACS Nano. 2015;9:16–30. doi: 10.1021/nn5062029. [DOI] [PubMed] [Google Scholar]
- 3.Santhosh KR, et al. Self-assembled, adjuvant/antigen-based nanovaccine mediates anti-tumor immune response against melanoma tumor. Polymers. 2018;10:1063. doi: 10.3390/polym10101063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chu TH, et al. Potent anti-myeloma efficacy of dendritic cell therapy in combination with pomalidomide and programmed death-ligand 1 blockade in a preclinical model of multiple myeloma. Cancer Immunol. Immunother. 2021;70:31–45. doi: 10.1007/s00262-020-02654-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vo MC, et al. Lenalidomide and programmed death-1 blockade synergistically enhances the effects of dendritic cell vaccination in a model of murine myeloma. Front Immunol. 2018;9:1370. doi: 10.3389/fimmu.2018.01370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xiaopin D, et al. Immunostimulatory nanomedicines synergize with checkpoint blockade immunotherapy to eradicate colorectal tumors. Nat. Commun. 2019;10:1899. doi: 10.1038/s41467-019-09221-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.