Abstract
The aim of this study is to detect the diagnosis value of neutrophil lymphocyte ratio (NLR) and fibrinogen (FIB) in type B aortic dissection (TBAD) patients. This retrospective observation study consisted patients with TBAD, aortic aneurysm and physical examination between January 1, 2016 and December 31, 2019. Demographic and clinical information after the first admission were collected. Multivariate logistic regression analysis was performed to explore the correlational relationship between NLR, FIB and TBAD. Receiver Operating Characteristic Curve (ROC) was performed to evaluate the diagnostic implication of NLR and FIB in TBAD patients. Six hundred and six patients who were first diagnosed with TBAD were included. Control groups were 202 aortic aneurysm and 140 physical examination subjects. The level of NLR and FIB in aortic dissection patients was significantly higher than aortic aneurysm patients and healthy group (P < 0.001). According to the results of multivariate logistic regression analysis, NLR and FIB were independent risk factors of aortic dissection, and the odds ratio (OR) and 95% confidence interval (CI) value of NLR and FIB were 1.499 (1.126–1.738) and 1.914 (1.475–2.485), respectively. The area under the curve (AUC) was 0.836 of NLR and 0.756 of FIB. NLR and FIB showed high specificity, 89% and 83% respectively. This is the first study provided information on the diagnosis performance of NLR and FIB in TBAD patients. NLR and FIB showed high specificity, which may be a valuable tool for the diagnosis of TBAD.
Subject terms: Biomarkers, Diseases
Introduction
Aortic dissection (AD) is the most dangerous vascular disease that requires early recognition to achieve better clinical management1. AD was classified as type A and type B according to Stanford criteria2,3. Stanford type B AD (TBAD) accounted for 25% to 40% of all aortic dissections4. TBAD lacks of typical symptoms and always share similar clinical presentations with aortic aneurysm, myocardial infarction or pulmonary embolism5. As to auxiliary examination, the electrocardiogram (ECG) and chest x-ray lacks of sensitivity and specificity for it. Computed tomography angiography (CTA) and magnetic resonance angiography (MRA) need long appointment time. Diagnosis is therefore time consuming, as a result, a large fraction of patients in whom the diagnosis may be mistaken or overlooked6–9.
Biomarkers can provide valuable information to help distinguishing aortic dissection patients. Preliminary evidence suggested a possible diagnostic role of D-dimer, Smooth muscle myosin and so on10–12. However, these biomarkers were still not yet routinely used in clinical practice, which indicated that available biomarkers were not enough for recognition and management of aortic dissection. Therefore, and additional useful biomarkers are urgently needed. Inflammation played a key role in AD initiate and progression13,14. AD involves different inflammatory mediators, which act alone or together to cause damage to the blood vessel wall. For example, S100A12 is a pro-inflammatory protein specific to early recruited phagocytes, which can enhance the migration of activated monocytes to the blood vessel wall, plays a key role in systemic inflammation15, and may be a promising biomarker for predicting the occurrence and prognosis of AD16. IL-22 is an inflammatory mediator, which can affect the process of AD by regulating vascular smooth muscle contraction and macrophage infiltration17,18. Macrophages can release metalloproteinases and pro-inflammatory cytokines to cause extracellular matrix degradation and trigger AD, in which MMP-12 is considered to be a specific marker of aortic wall disease19,20. In addition, the continuous increase of C-reactive protein, IL-6 and TNF- α is also related to the development of AD21.
Neutrophil lymphocyte ratio (NLR) and Fibrinogen (FIB) have been found to be higher in clinical practice. They has been assessed as a valuable diagnostic biomarker involved in the process of coagulation and hemostasis and different in type A aortic dissection (TAAD)22–24. The role of NLR and FIB in predictor of type B aortic dissection (TBAD) is unknown. In this study, data of plasma NLR and FIB in TBAD patients, aneurysm patients, and health people was collected to evaluate its diagnostic performance.
Methods
Study population
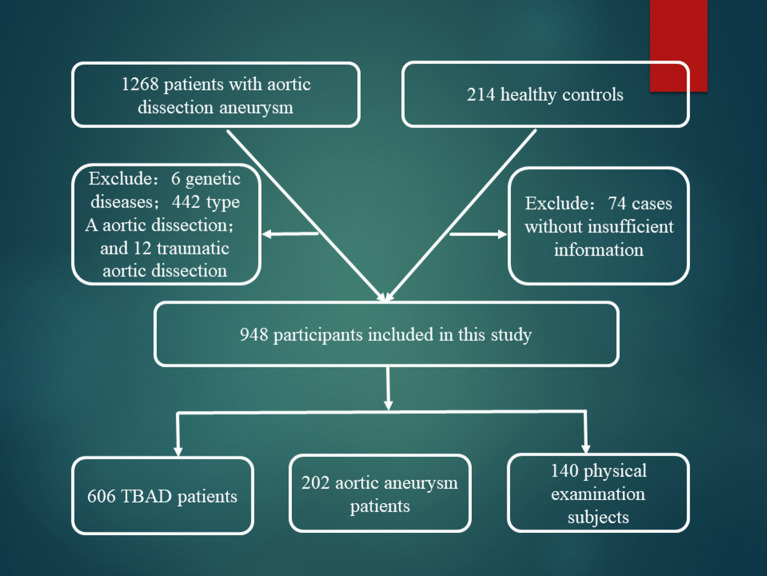
TBAD and aortic aneurysm was diagnosed by clinician according to physical examination and image examination (CTA or MRA). Demographic and clinical variables were retrospectively collected from medical charts and electronic medical records (code as 171.001—aortic dissection aneurysm). Then 1268 aortic dissection aneurysm and 214 healthy controls were retrieved between January, 1, 2016 and December 31, 2019. After screening, 442 type A aortic dissection, 12 traumatic aortic dissection, 6 genetic diseases patients, and 74 cases without insufficient information were excluded. Finally, 948 participants were included in this study, 606 TBAD patients, 202 aortic aneurysm and 140 physical examination subjects. Then we named the three cohorts as dissection group, aneurysm group and healthy group, respectively. Flowchart of inclusion of the patients can been seen in Fig. 1. All methods in this study were carried out in accordance with the guidelines Reporting of Observational Studies in Epidemiology (STROBE). The Written consent was obtained from all the participants and the study was approved by the Changhai Hospital medical ethics committee.
Figure 1.
Flowchart of inclusion of the patients.
Data collection
Blood samples were obtained immediately after admission, then measured by laboratory of hospital. Demographic and blood examination information were collected by hospital medical records, including age, sex, blood pressure, blood routine, inflammation, coagulation and lipid related indicators. NLR was calculated as the ration of neutrophils divided by lymphocytes.
Statistical analysis
Continuous variable was expressed as mean ± SD, median and interquartile range according to its distribution. One-way ANOVA or Kruskal–Wallis H test were used to examine the differences among groups. Categorical variable was shown as percentage, and the Chi-square or Fisher’s exact test was used to examine the differences. Multiple logistic regression analysis was preformed to examine associations with outcome, controlling for potential confounders. Receiver operating characteristic curve analysis was performed to determine the diagnostic performance of NLR and FIB in three groups. Statistical analyses were performed using IBM SPSS (version 26; SPSS Inc, Chicago, Ill) and GraphPad Prism. The reported P-value was two-tailed, and P < 0.05 was considered to be statistically significant.
Results
Clinical and blood biochemistry indicators in the three groups were listed in Table 1.There was no significant difference in age, sex among the groups. Hypertension, the most frequency risk factors for dissection/aneurysm, didn’t differ between the three groups (P = 0.319). WBC count, PLR, D-dimer and FDP were higher in dissection group than the other two groups. Cholesterol, LDL, Eosinophil (EO), BNP and CKMB were little higher in aneurysm group.
Table 1.
Basic and laboratory date among the three group.
| Dissection group (n = 606) | Aneurysm group (n = 202) | Healthy group (n = 140) | P value | |
|---|---|---|---|---|
| Age (year) | 59.88 ± 13.05 | 60.47 ± 17.72 | 57.44 ± 11.04 | 0.112 |
| Male | 485 (80) | 164 (81.19) | 113 (80.71) | 0.932 |
| Hypertension | 449 (74.1) | 140 (69.3) | 100 (71.4) | 0.391 |
| Cholesterol (mmol/l) | 4.42 (3.82–4.98) | 4.52 (3.83–5.31) | 4.42 (3.82–4.97) | 0.040 |
| Triglyceride (mmol/l) | 1.27 (0.93–1.73) | 1.36 (0.92–1.93) | 1.30 (0.92–1.7) | 0.221 |
| HDL (mmol/l) | 1.16 (0.96–1.36) | 1.16 (1.01–1.22) | 1.22 (1.04–1.42) | 0.035 |
| LDL (mmol/l) | 2.54 (2.09–3.02) | 2.78 (2.31–2.97) | 2.64 (2.15–3.09) | 0.011 |
| WBC (× 109/l) | 8.05 (6.25–10.81) | 6.37 (5.28–7.92) | 6.07 (5.12–7.21) | < 0.001 |
| NLR (mg/l) | 4.52 (2.7–8.13) | 2.43 (1.75–3.94) | 2.03 (1.57–2.49) | < 0.001 |
| PLR (mg/l) | 149.77 (109.82–209.89) | 120.19 (91.78–160.71) | 112.9 (87.94–140.6) | < 0.001 |
| EO (× 109/l) | 0.07 (0.01–0.16) | 0.14 (0.06–0.22) | 0.11 (0.07–0.18) | < 0.001 |
| d-Dimer (mg/ml) | 2.12 (0.82–3.95) | 1.66 (0.5–4.35) | 0.27 (0.22–0.32) | < 0.001 |
| FIB (g/ml) | 4.03 (3.1–5.4) | 3.44 (2.9–4.11) | 2.97 (2.58–3.39) | < 0.001 |
| FDP (ug/ml) | 8.09 (3.54–16.26) | 5.74 (2.49–15.06) | 1.99 (1.7–2.27) | < 0.001 |
| APTT (s) | 38.6 (35–42.5) | 38.15 (34.7–41.03) | 35.85 (33.9–38.55) | < 0.001 |
| BNP (pg/ml) | 66.29 (4.09–202.81) | 101.66 (26.79–207.22) | 23.6 (11.38–32.04) | 0.013 |
| CKMB (ng/ml) | 3.65 (0.8–10.74) | 5.08 (1.1–9.68) | 1.73 (1–2.76) | 0.045 |
HDL High density lipoprotein, LDL Low density lipoprotein, WBC White blood cell, NLR Neutrophil/lymphocyte ratio, PLR Platelet/lymphocyte ratio, EO Eosinophil, FIB Fibrinogen, FDP Fibrinogen degradation product, APTT Activated partial thromboplastin time, BNP Brain natriuretic peptide, CKMB Creatine kinase isoenzyme MB.
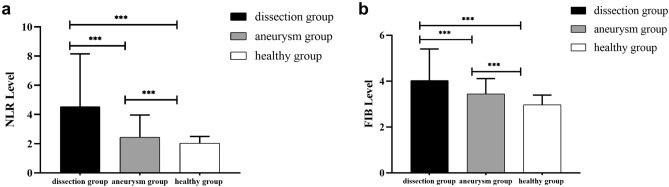
The level of NLR in dissection group [4.52 (2.7–8.13)] was significantly higher than aneurysm group [2.43 (1.75–3.94)] and healthy group [2.03 (1.57–2.49)] [P < 0.001, Fig. 2a]. The FIB level in dissection group [4.03 (3.1–5.4)] was also significantly higher than that both in aneurysm group [3.44 (2.9–4.11)] and healthy group [2.97 (2.58–3.39)] [P < 0.001, Fig. 2b].
Figure 2.
The NLR and FIB levels among three groups. (a) NLR levels; (b) FIB levels. ***P < 0.001.
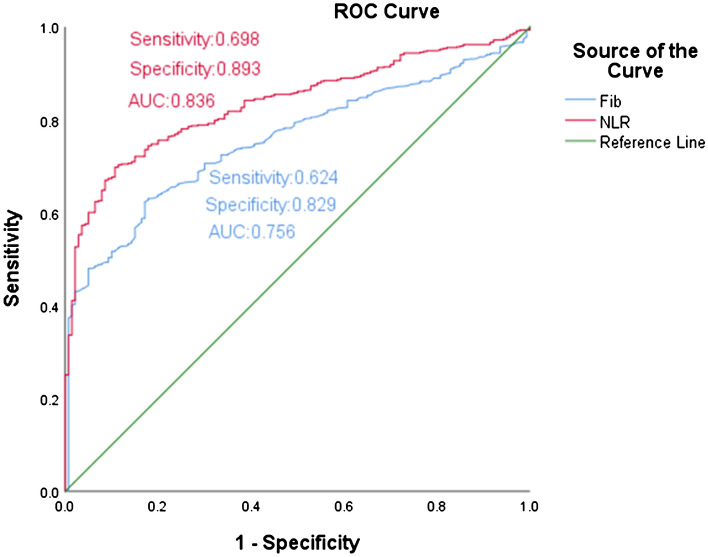
According to univariate and multivariate logistic regression analysis, NLR and FIB were independent risk factors of the dissection group, and the OR and 95% CI value of NLR and FIB were 1.499 (1.126–1.738) and 1.914 (1.475–2.485), respectively (Table 2). ROC analysis was performed to evaluate the diagnostic implication of NLR and FIB in dissection group. The area under the curve (AUC) was 0.836 of NLR and 0.756 of FIB. NLR and FIB showed high specificity, 89% and 83% respectively (Fig. 3).
Table 2.
Univariate and multivariate analysis among the three group.
| Univariate | Multivariate | |||
|---|---|---|---|---|
| OR and 95% CI | P value | OR and 95% CI | P value | |
| Cholesterol (mmol/l) | 1.147 (0.955–1.377) | 0.143 | NA | NA |
| HDL (mmol/l) | 0.607 (0.361–1.021) | 0.06 | NA | NA |
| LDL (mmol/l) | 0.991 (0.781–1.258) | 0.941 | NA | NA |
| WBC (× 109/l) | 1.408 (1.285–1.543) | < 0.001 | 1.096 (0.953–1.260) | 0.197 |
| NLR (mg/l) | 2.013 (1.695–2.392) | < 0.001 | 1.499 (1.126–1.738) | < 0.001 |
| PLR (mg/l) | 1.013 (1.009–1.016) | < 0.001 | 1.004 (0.998–1.009) | 0.182 |
| EO (× 109/l) | 0.462 (0.131–1.634) | 0.231 | NA | NA |
| d-Dimer (mg/ml) | 1.941 (1.23–1.892) | < 0.000 | 1.276 (1.034–1.133) | 0.005 |
| FIB (g/ml) | 2.115 (1.753–2.553) | < 0.001 | 1.914 (1.475–2.485) | < 0.001 |
| FDP (ug/ml) | 1.348 (1.257–1.446) | < 0.001 | 1.215 (1.207–1.432) | 0.008 |
| APTT (s) | 1.094 (1.055–1.134) | < 0.001 | 0.998 (0.950–1.049) | 0.994 |
| BNP (pg/ml) | 1.001 (1–1.002) | 0.001 | 1.0 (0.99–1.001) | 0.785 |
| CKMB (ng/ml) | 1.017 (1.003–1.031) | 0.02 | 1.048 (1.014–1.083) | 0.015 |
NA represented non data available.
Figure 3.
The ROC curves of NLR and FIB to distinguish TBAD patients.
Discussion
This study found that preoperative NLR and FIB were higher in patients with TBAD compared to both aortic aneurysm and healthy participants. NLR and FIB showed excellent specificity, which was different from previous published diagnostic biomarkers. Just as we suspected NLR and FIB maybe a powerful indicator in improving diagnostic accuracy for TBAD.
Biomarkers were widely used in cardiovascular diseases because of their low cost, easy availability and less waiting time. Previous studies presented that D-dimer had excellent sensitivity but modest specificity diagnostic performance. Troponin T can be used for early risk stratification of patients with acute type A aortic dissection25. White blood cells and N-terminal pro-brain natriuretic peptide were suggested for early diagnosis of aortic dissection26. In this study, NLR and FIB showed wonderful specificity, that maybe a powerful complementary with the existing biomarkers to improve the diagnostic value.
Compared with neutrophil count, lymphocyte count, NLR is more stable in the prediction of aortic disease. Studies have shown that there is a significant correlation between NLR and systemic inflammation27. Accumulating evidence has shown the value of NLR in evaluating the prognosis of aortic diseases28–31. Elevated NLR is also positively associated with the development of hypertension, stroke, and acute myocardial infarction (AMI)32–34. Aurelian et al. found that NLR is significantly higher among patients with infrarenal abdominal aortic aneurysm (rAAA) and that an NLR > 5 indicates a 5 times greater possibility of AAA being ruptured35. However, the prognostic capacity of NLR on admission is yet to be clarified in patients with type B AAD. Fibrinogen is associated with preoperative hypoxemia in patients with aortic dissection36. Low preoperative fibrinogen level is a risk factor for neurological complications and in-hospital mortality in patients with acute aortic dissection37,38. In addition, studies have shown that fibrinogen-fibrin degradation product level at admission can be used as a predictor of aortic growth and poor 1-year outcome in uncomplicated type B aortic dissection39,40. This is the first study that evaluated the potential diagnosis performance of FIB in patients with TBAD.
The function and mechanism of NLR in TBAD morbidity are still unclear. Elevated neutrophils interaction with endothelial cells leading to vascular intima injury. In addition, inflammatory cells can increase matrix metalloproteinases, which can degrade collagen and elastin, resulting in structural destruction of the aortic wall, resulting in aortic dissection41,42. Fibrinogen is an important protein in the process of coagulation and hemostasis. It can be abnormally elevated during infection, trauma, inflammation, surgery or tumor as a direct coagulation factor involved in the coagulation process. The coagulation disorder of patients with acute aortic dissection can be improved by further increasing the level of fibrinogen before operation43. When vascular endothelial cells are damaged in patients with aortic dissection, the increase of fibrinogen can be caused by endogenous and exogenous coagulation pathways44. This study shows that the level of fibrinogen in patients with aortic dissection is significantly higher than that in other groups, suggesting that the coagulation function of patients with aortic dissection is enhanced, which is helpful for pseudocavitary thromboembolization45.
This study is the first to combine NLR and FIB in the diagnosis of type B aortic dissection, which has a high clinical application value. However, there are still several disadvantages in this study: (1) the sample size of patients with non-aortic dissection is small; (2) This study is retrospective observational study; (3) Further studies are needed to understand the diagnostic role of NLR and FIB in type B aortic dissection, including combined use with other biomarkers; (4) Since many inflammatory and malignant diseases other than AD were associated with the research parameters, the patients in the study without excluding co-founder diseases.
Conclusion
NLR levels are significantly higher among patients with Type B aortic dissection than those in abdominal aneurysm and healthy controls. Elevated NLR levels may contribute to the pathogenesis of aortic dissection. Further large clinical studies to investigate whether NLR levels are an independently risk factor for aortic dissection are warranted.
Acknowledgements
All methods in this study were carried out in accordance with the guidelines Reporting of Observational Studies in Epidemiology (STROBE). The Written consent was obtained from all the participants and the study was approved by the Changhai Hospital medical ethics committee.
Abbreviations
- AAD
Acute aortic dissection
- AMI
Acute myocardial infarction
- APE
Acute pulmonary embolism
- APTT
Activated partial thromboplastin time
- BNP
Brain Natriuretic Peptide
- CT
Computed tomography
- CTA
Computed tomography angiography
- CKMB
Creatine kinase isoenzyme MB
- DSA
Digital subtraction angiography
- EO
Eosinophil
- FDP
Fibrinogen degradation product
- FIB
Fibrinogen
- HDL
High-density lipoprotein
- INR
International normalized ratio
- LDL
Low-density lipoprotein
- Lymp
Lymphocyte
- NLR
Neutrophil to lymphocyte ratio
- PLT
Platelet
- WBC
White blood cell
Author contributions
S.S.L, J.Y., and R.L.G. conceived and wrote the main manuscript text; J.D., S.C., and H.C.Z. prepared figures and tables; J.Z., Z.H.L., and Z.J. designed the study and modification. All authors reviewed the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China [81370441], Shanghai Health System Diseases Important Joint Research Project [2014ZYJB0401], and Military Medical talent program of the PLA Naval Medical University [2019-QH-23].
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Shuangshuang Li, Jin Yang, and Jian Dong.
Contributor Information
Zhaohui Li, Email: 1187510153@qq.com.
Jian Zhou, Email: zhoujian1-2@163.com.
Zaiping Jing, Email: xueguanky@163.com.
References
- 1.Nienaber CA, Clough RE. Management of acute aortic dissection. Lancet. 2015;385:800–811. doi: 10.1016/S0140-6736(14)61005-9. [DOI] [PubMed] [Google Scholar]
- 2.Daily PO, Trueblood HW, Stinson EB, Wuerflein RD, Shumway NE. Management of acute aortic dissections. Ann. Thorac. Surg. 1970;10:237–247. doi: 10.1016/S0003-4975(10)65594-4. [DOI] [PubMed] [Google Scholar]
- 3.DeBakey ME, Beall AC, Jr, Cooley DA, Crawford ES, Morris GC, Jr, Garrett HE, Howell JF. Dissecting aneurysms of the aorta. Surg. Clin. N. Am. 1966;46:1045–1055. doi: 10.1016/S0039-6109(16)37946-4. [DOI] [PubMed] [Google Scholar]
- 4.Hughes GC, Andersen ND, McCann RL. Management of acute type B aortic dissection. J. Thorac. Cardiovasc. Surg. 2013;145:S202–207. doi: 10.1016/j.jtcvs.2012.11.078. [DOI] [PubMed] [Google Scholar]
- 5.Gawinecka J, Schönrath F, von Eckardstein A. Acute aortic dissection: Pathogenesis, risk factors and diagnosis. Swiss Med. Wkly. 2017;147:14489. doi: 10.4414/smw.2017.14489. [DOI] [PubMed] [Google Scholar]
- 6.Clouse WD, Hallett JW, Jr, Schaff HV, Spittell PC, Rowland CM, Ilstrup DM, Melton LJ., 3rd Acute aortic dissection: Population-based incidence compared with degenerative aortic aneurysm rupture. Mayo Clin. Proc. 2004;79:176–180. doi: 10.4065/79.2.176. [DOI] [PubMed] [Google Scholar]
- 7.Tsai TT, Trimarchi S, Nienaber CA. Acute aortic dissection: Perspectives from the International Registry of Acute Aortic Dissection (IRAD) Eur. J. Vasc. Endovasc. Surg. 2009;37:149–159. doi: 10.1016/j.ejvs.2008.11.032. [DOI] [PubMed] [Google Scholar]
- 8.Baloria KA, Dhir A, Pillai B, Selot N. Aortic dissection: To be or not to be. Ann. Card. Anaesth. 2013;16:126–128. doi: 10.4103/0971-9784.109761. [DOI] [PubMed] [Google Scholar]
- 9.Ranasinghe AM, Bonser RS. Biomarkers in acute aortic dissection and other aortic syndromes. J. Am. Coll. Cardiol. 2010;56:1535–1541. doi: 10.1016/j.jacc.2010.01.076. [DOI] [PubMed] [Google Scholar]
- 10.Dong J, et al. Diagnostic implication of fibrin degradation products and D-dimer in aortic dissection. Sci. Rep. 2017;7:43957. doi: 10.1038/srep43957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Suzuki T, et al. Diagnostic implications of elevated levels of smooth-muscle myosin heavy-chain protein in acute aortic dissection: The smooth muscle myosin heavy chain study. Ann. Intern. Med. 2000;133:537–541. doi: 10.7326/0003-4819-133-7-200010030-00013. [DOI] [PubMed] [Google Scholar]
- 12.Vrsalović M, Vrsalović Presečki A. Admission C-reactive protein and outcomes in acute aortic dissection: A systematic review. Croat. Med. J. 2019;60:309–315. doi: 10.3325/cmj.2019.60.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou Z, et al. Exaggerated autophagy in stanford type a aortic dissection: A transcriptome pilot analysis of human ascending aortic tissues. Genes. 2020;11:1187. doi: 10.3390/genes11101187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chumachenko PV, et al. Thoracic aortic aneurysm and factors affecting aortic dissection. J. Pers. Med. 2020;10:153. doi: 10.3390/jpm10040153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kosaki A, et al. Increased plasma S100A12 (EN-RAGE) levels in patients with type 2 diabetes. J. Clin. Endocrinol. Metab. 2004;89:5423–5428. doi: 10.1210/jc.2003-032223. [DOI] [PubMed] [Google Scholar]
- 16.Jiang W, et al. Highly expressed S100A12 in aortic wall of patients with DeBakey type I aortic dissection could be a promising marker to predict perioperative complications. Ann. Vasc. Surg. 2014;28:1556–1562. doi: 10.1016/j.avsg.2014.03.020. [DOI] [PubMed] [Google Scholar]
- 17.Ye J, et al. Interleukin 22 promotes blood pressure elevation and endothelial dysfunction in angiotensin II-treated mice. J. Am. Heart Assoc. 2017;6:10. doi: 10.1161/JAHA.117.005875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ye J, et al. Increased levels of interleukin-22 in thoracic aorta and plasma from patients with acute thoracic aortic dissection. Clin. Chim. Acta. 2018;486:395–401. doi: 10.1016/j.cca.2017.10.033. [DOI] [PubMed] [Google Scholar]
- 19.Cifani N, Proietta M, Tritapepe L, Di Gioia C, Ferri L, Taurino M, Del Porto F. Stanford-A acute aortic dissection, inflammation, and metalloproteinases: A review. Ann. Med. 2015;47:441–446. doi: 10.3109/07853890.2015.1073346. [DOI] [PubMed] [Google Scholar]
- 20.Del Porto F, et al. The multitasking role of macrophages in Stanford type A acute aortic dissection. Cardiology. 2014;127:123–129. doi: 10.1159/000355253. [DOI] [PubMed] [Google Scholar]
- 21.Gu J, et al. Time-dependent changes of plasma inflammatory biomarkers in type A aortic dissection patients without optimal medical management. J. Cardiothorac. Surg. 2015;10:3. doi: 10.1186/s13019-014-0199-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sbarouni E, Georgiadou P, Analitis A, Voudris V. High neutrophil to lymphocyte ratio in type A acute aortic dissection facilitates diagnosis and predicts worse outcome. Expert Rev. Mol. Diagn. 2015;15:965–970. doi: 10.1586/14737159.2015.1042367. [DOI] [PubMed] [Google Scholar]
- 23.Zindovic I, et al. The coagulopathy of acute type a aortic dissection: A prospective, observational study. J. Cardiothorac. Vasc. Anesth. 2019;33:2746–2754. doi: 10.1053/j.jvca.2019.02.013. [DOI] [PubMed] [Google Scholar]
- 24.de Moerloose P, Boehlen F, Neerman-Arbez M. Fibrinogen and the risk of thrombosis. Semin. Thromb. Hemost. 2010;36:7–17. doi: 10.1055/s-0030-1248720. [DOI] [PubMed] [Google Scholar]
- 25.Li G, et al. High-sensitivity cardiac troponin T: A biomarker for the early risk stratification of type-A acute aortic dissection. Arch. Cardiovasc. Dis. 2016;109:163–170. doi: 10.1016/j.acvd.2015.09.007. [DOI] [PubMed] [Google Scholar]
- 26.Zhang R, et al. Biomarkers investigation for in-hospital death in patients with stanford type A acute aortic dissection. Int. Heart J. 2016;57:622–626. doi: 10.1536/ihj.15-484. [DOI] [PubMed] [Google Scholar]
- 27.Mallappa S, Sinha A, Gupta S, Chadwick SJ. Preoperative neutrophil to lymphocyte ratio >5 is a prognostic factor for recurrent colorectal cancer. Colorectal Dis. 2013;15:323–328. doi: 10.1111/codi.12008. [DOI] [PubMed] [Google Scholar]
- 28.Oz K, Iyigun T, Karaman Z, Çelik Ö, Akbay E, Akınc O, Erkanli K. Prognostic value of neutrophil to lymphocyte ratio and risk factors for mortality in patients with stanford type A aortic dissection. Heart. Surg. Forum. 2017;20:119–123. doi: 10.1532/hsf.1736. [DOI] [PubMed] [Google Scholar]
- 29.Onuk T, et al. Increased neutrophil to lymphocyte ratio is associated with in-hospital mortality in patients with aortic dissection. Clin. Lab. 2015;61:1275–1282. doi: 10.7754/Clin.Lab.2015.150216. [DOI] [PubMed] [Google Scholar]
- 30.Bedel C, Selvi F. Association of platelet to lymphocyte and neutrophil to lymphocyte ratios with in-hospital mortality in patients with type A acute aortic dissection. Braz. J. Cardiovasc. Surg. 2019;34:694–698. doi: 10.21470/1678-9741-2018-0343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Karakoyun S, et al. Neutrophil-lymphocyte ratio may predict in-hospital mortality in patients with acute type A aortic dissection. Herz. 2015;40:716–721. doi: 10.1007/s00059-014-4121-2. [DOI] [PubMed] [Google Scholar]
- 32.Farah R, Samra N. Mean platelets volume and neutrophil to lymphocyte ratio as predictors of stroke. J. Clin. Lab. Anal. 2018;32:22189. doi: 10.1002/jcla.22189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Budzianowski J, Pieszko K, Burchardt P, Rzeźniczak J, Hiczkiewicz J. The role of hematological indices in patients with acute coronary syndrome. Dis. Markers. 2017;2017:3041565. doi: 10.1155/2017/3041565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang S, et al. Predictive value of neutrophil to lymphocyte ratio in patients with acute ST segment elevation myocardial infarction after percutaneous coronary intervention: A meta-analysis. BMC Cardiovasc. Disord. 2018;18:75. doi: 10.1186/s12872-018-0812-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aurelian SV, Adrian M, Andercou O, Bruno S, Alexandru O, Catalin T, Dan B. Neutrophil-to-lymphocyte ratio: A comparative study of rupture to nonruptured infrarenal abdominal aortic aneurysm. Ann. Vasc. Surg. 2019;58:270–275. doi: 10.1016/j.avsg.2018.11.026. [DOI] [PubMed] [Google Scholar]
- 36.Guo Z, Yang Y, Zhao M, Zhang B, Lu J, Jin M, Cheng W. Preoperative hypoxemia in patients with type A acute aortic dissection: A retrospective study on incidence, related factors and clinical significance. J. Thorac. Dis. 2019;11:5390–5397. doi: 10.21037/jtd.2019.11.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guan X, Gong M, Wang X, Zhu J, Liu Y, Sun L, Zhang H. Low preoperative fibrinogen level is risk factor for neurological complications in acute aortic dissection. Medicine. 2018;97:e10830. doi: 10.1097/MD.0000000000010830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu J, Sun LL, Wang J, Ji G. The relationship between fibrinogen and in-hospital mortality in patients with type A acute aortic dissection. Am. J. Emerg. Med. 2018;36:741–744. doi: 10.1016/j.ajem.2017.10.001. [DOI] [PubMed] [Google Scholar]
- 39.van Bogerijen GH, et al. Predictors of aortic growth in uncomplicated type B aortic dissection. J. Vasc. Surg. 2014;59:1134–1143. doi: 10.1016/j.jvs.2014.01.042. [DOI] [PubMed] [Google Scholar]
- 40.Kitada S, Akutsu K, Tamori Y, Yoshimuta T, Hashimoto H, Takeshita S. Usefulness of fibrinogen/fibrin degradation product to predict poor one-year outcome of medically treated patients with acute type B aortic dissection. Am. J. Cardiol. 2008;101:1341–1344. doi: 10.1016/j.amjcard.2007.12.036. [DOI] [PubMed] [Google Scholar]
- 41.Manabe T, Imoto K, Uchida K, Doi C, Takanashi Y. Decreased tissue inhibitor of metalloproteinase-2/matrix metalloproteinase ratio in the acute phase of aortic dissection. Surg. Today. 2004;34:220–225. doi: 10.1007/s00595-003-2683-3. [DOI] [PubMed] [Google Scholar]
- 42.Koullias GJ, Ravichandran P, Korkolis DP, Rimm DL, Elefteriades JA. Increased tissue microarray matrix metalloproteinase expression favors proteolysis in thoracic aortic aneurysms and dissections. Ann. Thorac. Surg. 2004;78:2106–2110. doi: 10.1016/j.athoracsur.2004.05.088. [DOI] [PubMed] [Google Scholar]
- 43.Guan XL, et al. Changes in the hemostatic system of patients with acute aortic dissection undergoing aortic arch surgery. Ann. Thorac. Surg. 2016;101:945–951. doi: 10.1016/j.athoracsur.2015.08.047. [DOI] [PubMed] [Google Scholar]
- 44.Paparella D, et al. Hemostasis alterations in patients with acute aortic dissection. Ann. Thorac. Surg. 2011;91:1364–1369. doi: 10.1016/j.athoracsur.2011.01.058. [DOI] [PubMed] [Google Scholar]
- 45.Nagaoka K, et al. Fibrinogen/fibrin degradation products in acute aortic dissection. Intern. Med. 2010;49:1943–1947. doi: 10.2169/internalmedicine.49.3770. [DOI] [PubMed] [Google Scholar]