Abstract
Background
Coronavirus disease 2019 (COVID‐19) is a serious disease caused by severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2). The primary manifestation is respiratory insufficiency that can also be related to diffuse pulmonary microthrombosis in people with COVID‐19. This disease also causes thromboembolic events, such as pulmonary embolism, deep venous thrombosis, arterial thrombosis, catheter thrombosis, and disseminated intravascular coagulopathy. Recent studies have indicated a worse prognosis for people with COVID‐19 who developed thromboembolism.
Anticoagulants are medications used in the prevention and treatment of venous or arterial thromboembolic events. Several drugs are used in the prophylaxis and treatment of thromboembolic events, such as heparinoids (heparins or pentasaccharides), vitamin K antagonists and direct anticoagulants. Besides their anticoagulant properties, heparinoids have an additional anti‐inflammatory potential, that may affect the clinical evolution of people with COVID‐19. Some practical guidelines address the use of anticoagulants for thromboprophylaxis in people with COVID‐19, however, the benefit of anticoagulants for people with COVID‐19 is still under debate.
Objectives
To assess the effects of prophylactic anticoagulants versus active comparator, placebo or no intervention, on mortality and the need for respiratory support in people hospitalised with COVID‐19.
Search methods
We searched CENTRAL, MEDLINE, Embase, LILACS and IBECS databases, the Cochrane COVID‐19 Study Register and medRxiv preprint database from their inception to 20 June 2020. We also checked reference lists of any relevant systematic reviews identified and contacted specialists in the field for additional references to trials.
Selection criteria
Randomised controlled trials (RCTs), quasi‐RCTs, cluster‐RCTs and cohort studies that compared prophylactic anticoagulants (heparin, vitamin K antagonists, direct anticoagulants, and pentasaccharides) versus active comparator, placebo or no intervention for the management of people hospitalised with COVID‐19. We excluded studies without a comparator group. Primary outcomes were all‐cause mortality and need for additional respiratory support. Secondary outcomes were mortality related to COVID‐19, deep vein thrombosis (DVT), pulmonary embolism, major bleeding, adverse events, length of hospital stay and quality of life.
Data collection and analysis
We used standard Cochrane methodological procedures. We used ROBINS‐I to assess risk of bias for non‐randomised studies (NRS) and GRADE to assess the certainty of evidence. We reported results narratively.
Main results
We identified no RCTs or quasi‐RCTs that met the inclusion criteria. We included seven retrospective NRS (5929 participants), three of which were available as preprints. Studies were conducted in China, Italy, Spain and the USA. All of the studies included people hospitalised with COVID‐19, in either intensive care units, hospital wards or emergency departments. The mean age of participants (reported in 6 studies) ranged from 59 to 72 years. Only three included studies reported the follow‐up period, which varied from 8 to 35 days. The studies did not report on most of our outcomes of interest: need for additional respiratory support, mortality related to COVID‐19, DVT, pulmonary embolism, adverse events, and quality of life. Anticoagulants (all types) versus no treatment (6 retrospective NRS, 5685 participants)
One study reported a reduction in all‐cause mortality (adjusted odds ratio (OR) 0.42, 95% confidence interval (CI) 0.26 to 0.66; 2075 participants). One study reported a reduction in mortality only in a subgroup of 395 people who required mechanical ventilation (hazard ratio (HR) 0.86, 95% CI 0.82 to 0.89). Three studies reported no differences in mortality (adjusted OR 1.64, 95% CI 0.92 to 2.92; 449 participants; unadjusted OR 1.66, 95% CI 0.76 to 3.64; 154 participants and adjusted risk ratio (RR) 1.15, 95% CI 0.29 to 2.57; 192 participants). One study reported zero events in both intervention groups (42 participants). The overall risk of bias for all‐cause mortality was critical and the certainty of the evidence was very low. One NRS reported bleeding events in 3% of the intervention group and 1.9% of the control group (OR 1.62, 95% CI 0.96 to 2.71; 2773 participants; low‐certainty evidence). Therapeutic‐dose anticoagulants versus prophylactic‐dose anticoagulants (1 retrospective NRS, 244 participants)
The study reported a reduction in all‐cause mortality (adjusted HR 0.21, 95% CI 0.10 to 0.46) and a lower absolute rate of death in the therapeutic group (34.2% versus 53%). The overall risk of bias for all‐cause mortality was serious and the certainty of the evidence was low. The study also reported bleeding events in 31.7% of the intervention group and 20.5% of the control group (OR 1.8, 95% CI 0.96 to 3.37; low‐certainty evidence). Ongoing studies
We found 22 ongoing studies in hospital settings (20 RCTs, 14,730 participants; 2 NRS, 997 participants) in 10 different countries (Australia (1), Brazil (1), Canada (2), China (3), France (2), Germany (1), Italy (4), Switzerland (1), UK (1) and USA (6)). Twelve ongoing studies plan to report mortality and six plan to report additional respiratory support. Thirteen studies are expected to be completed in December 2020 (6959 participants), eight in July 2021 (8512 participants), and one in December 2021 (256 participants). Four of the studies plan to include 1000 participants or more.
Authors' conclusions
There is currently insufficient evidence to determine the risks and benefits of prophylactic anticoagulants for people hospitalised with COVID‐19. Since there are 22 ongoing studies that plan to evaluate more than 15,000 participants in this setting, we will add more robust evidence to this review in future updates.
Plain language summary
Do blood thinners prevent people who are hospitalised with COVID‐19 from developing blood clots?
COVID‐19 typically affects the lungs and airways, however, in addition to respiratory problems, about 16% of people hospitalised with COVID‐19 experience problems with their blood and blood vessels, leading to blood clots forming in the arteries, veins and lungs. These blood clots can break loose and travel to other parts of the body, where they may cause blockages leading to heart attacks or strokes. Nearly half of all people with severe COVID‐19, in intensive care units, may develop clots in their veins or arteries.
What are blood thinners?
Blood thinners are medicines that prevent harmful blood clots from forming. However, they may cause unwanted effects such as bleeding. Some guidelines recommend giving blood thinners when people are first admitted to hospital with COVID‐19, to prevent blood clots from developing, rather than waiting to see if blood clots develop and then treating them with blood thinners.
What did we want to find out?
We wanted to know whether giving people hospitalised with COVID‐19 blood thinners as a preventive measure, reduced the number of deaths compared to people who received no treatment or who received a placebo treatment. We also wanted to know whether these people needed less support with breathing, whether they still developed harmful blood clots, whether they experienced bleeding and whether they experienced any other unwanted events (for example, nausea, vomiting, kidney problems and amputations).
What did we do?
We searched for studies that assessed blood thinners given to people hospitalised with COVID‐19 to prevent blood clots. Studies could be of any design as long as they compared a blood thinner with another blood thinner, no treatment or a placebo (sham). Studies could take place anywhere in the world and participants could be any age as long as they were in hospital with confirmed COVID‐19 disease.
Search date: 20 June 2020
What we found
We hoped to find randomised controlled trials (RCTs). RCTs allocate participants at random to receive either the treatment under investigation or the comparison treatment (another treatment, no treatment or placebo). RCTs give the best evidence.
We did not find any RCTs, so we included seven non‐randomised ‘retrospective’ studies that looked back at treatments given to 5929 people. These studies took place in intensive care units, hospital wards and emergency departments in China, Italy, Spain and the USA. They provided evidence on deaths and bleeding but no evidence on respiratory support, blood clotting and other unwanted effects. The studies were very different from each other, so we were not able to pool their results.
Blood thinners compared with no treatment (6 studies) ‐ One study reported a reduction in mortality and another study reported a reduction in mortality in severely ill people only. Three studies reported no difference in mortality and the remaining study reported no deaths in either group. ‐ One study reported major bleeding in 3% of participants who received blood thinners and 1.9% of participants who did not receive blood thinners.
Treatment dose of blood thinners compared with preventive dose (1 study) All the participants were in the intensive care unit on mechanical ventilators. They may or may not have had blood clots but were given either blood thinners in a dose usually used to treat clots (higher dose), or a dose used to prevent clots (lower dose). ‐ This study reported a lower rate of death in people who received the treatment dose (34.2%) compared with the preventive dose (53%). ‐ This study reported major bleeding in 31.7% of participants who received the treatment dose compared with 20.5% of those who received the preventive dose.
Reliability of the evidence
We do not know whether blood thinners are a useful preventive treatment for people with COVID‐19 because we are very uncertain about the evidence. None of the studies randomised participants and all were retrospective. Also, they reported different results from each other and did not report their methods fully. This means our confidence (certainty) in the evidence is very low.
What happens next?
Our searches found 22 ongoing studies, 20 of which are RCTs, with 14,730 people. We plan to add the results of these studies to our review when they are published. We hope that these better quality studies will provide a conclusive answer to our review question.
Summary of findings
Background
See Table 3 for a glossary of terms.
1. Glossary of terms.
| Term | Definition |
| Anticoagulants | Drugs that suppress, delay or prevent blood clots |
| Antiplatelet agents | Drugs that prevent blood clots by inhibiting platelet function |
| Arterial thrombosis | An interruption of blood flow to an organ or body part due to a blood clot blocking the flow of blood |
| Body mass index (BMI) | Body mass divided by the square of the body height, universally expressed in units of kg/m2 |
| Catheters | Medical devices (tubes) that can be inserted in the body for a broad range of functions, such as to treat diseases, to perform a surgical procedure, and to provide medicine, fluids and food. |
| COVID‐19 | An infectious disease caused by SARS‐CoV‐2 virus |
| Deep vein thrombosis (DVT) | Coagulation or clotting of the blood in a deep vein, i.e. far beneath the surface of the skin |
| Disseminated intravascular coagulopathy | A severe condition in which blood clots form throughout the body, blocking small blood vessels and that may lead to organ failure. As clotting factors and platelets are used up, bleeding may occur, throughout the body (e.g. in the urine, in the stool, or bleeding into the skin) |
| Duplex ultrasound | Non‐invasive evaluation of blood flow through the arteries and veins by ultrasound devices |
| Heparin (also known as unfractionated heparin (UFH)) | A drug used to prevent blood clotting (anticoagulant, blood thinner) |
| Hypercoagulability | An abnormality of blood coagulation that increases the risk of blood clot formation in blood vessels (thrombosis) |
| Low molecular weight heparin | A drug used to prevent blood clotting (anticoagulant) |
| Obesity | Amount of body fat beyond healthy conditions (BMI > 30 kg/m2) |
| Placebo | Substance or treatment with no active effect, like a sugar pill |
| Platelet | Colourless blood cells that help blood clot by clumping together |
| Pulmonary embolism (PE) | Blood clot in the lung or blood vessel leading to the lung. The clot originates in a vein (e.g. deep vein thrombosis) and travels to the lung |
| Quasi‐randomised controlled trial (Quasi‐RCT) | A study in which participants are divided by date of birth or by hospital register number, i.e. not truly randomly divided into separate groups to compare different treatments |
| Randomised controlled trial (RCT) | A study in which participants are divided randomly into separate groups to compare different treatments |
| Respiratory failure | An abnormality that results from inadequate gas exchange by the respiratory system |
| SARS‐CoV‐2 | The virus (coronavirus 2) that causes COVID‐19 |
| Thrombosis | Local coagulation of blood (clot) in a part of the circulatory system |
| Vascular | Relating to blood vessels (arteries and veins) |
| Venous | Relating to a vein |
| Venous thromboembolism (VTE) | A condition that involves a blood clot that forms in a vein and may migrate to another location (e.g. the lung) |
Description of the condition
The novel coronavirus disease strain, coronavirus disease 2019 (COVID‐19), is caused by severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2). COVID‐19 emerged in Wuhan, China and rapidly spread worldwide (Lai 2020). SARS‐CoV‐2 is a highly transmittable virus, and up to 16% of people hospitalised may develop a severe form of the disease (Giannis 2020). Pulmonary effects are typical, but due to high inflammation, hypoxia, immobilisation and diffuse intravascular coagulation, COVID‐19 may predispose patients to both arterial and venous thromboembolism (Ackermann 2020; Dolhnikoff 2020; Fox 2020; Long 2020). Venous and arterial thromboembolic complications affect 16% of people hospitalised with COVID‐19 and 31% to 49% of people with COVID‐19 in intensive care units (ICUs), with 90% of such cases being venous thromboembolism (Bilaloglu 2020; Klok 2020a; Klok 2020b). Viral infections induce an imbalance between anticoagulant and procoagulant mechanisms and raise the systemic inflammatory response. Indeed, people with COVID‐19 commonly present with both elevated D‐dimer (fibrin degradation product) and reductions of factors related to clot formation (Giannis 2020). Excessive activation of the coagulation cascade and platelets can explain these haematological findings (Giannis 2020). Coagulopathy and vascular endothelial dysfunction have been proposed as complications of COVID‐19. Emerging data support that asymptomatic people with COVID‐19 are at risk of developing pathologic thrombosis. The association between large‐vessel stroke and COVID‐19 in young asymptomatic people requires further investigation (Oxley 2020), but Li 2020 found the incidence of stroke among people hospitalised with COVID‐19 was approximately 5% in a retrospective cohort. Activation of the coagulation system seems to be important in the development of acute respiratory distress syndrome, one of the most typical complications of COVID‐19 infection and it can be related to pulmonary microthrombosis (Ackermann 2020; Dolhnikoff 2020; Fox 2020; Marini 2020).
Description of the intervention
Anticoagulants are pharmacological interventions used in reducing hypercoagulability (Amaral 2020). The decision to use, or not use, thromboprophylaxis, depends on the risk stratification of each patient (NHS 2020).
Anticoagulants are medications used in the prevention and treatment of venous or arterial thromboembolic events (Amaral 2020; Biagioni 2020; Clezar 2020). When used for a prophylactic purpose, the dose of anticoagulants is usually half or significantly lower than that given for therapeutic purposes (Alquwaizani 2013). Even so, adverse events, such as bleeding may occur, and can have a significant impact on patient care (Amaral 2020; AVF 2020; Biagioni 2020; Clezar 2020).
How the intervention might work
D‐dimers are a reflection of the pathophysiology in COVID‐19, which is highly associated with increased mortality in people with COVID‐19 infection (Becker 2020). The elevated D‐dimer levels seen are most likely a reflection of the overall clot burden and critically ill people with COVID‐19 have lower levels of fibrinolytic system activation than the reference population (Panigada 2020). Tang 2020 reported decreased mortality after use of heparin in people with COVID‐19 (40.0% versus 64.2%, P = 0.029). Long 2020 reported that anticoagulation (mainly low molecular weight heparin), may reduce mortality in people with severe COVID‐19 infection or those with higher levels of D‐dimer (e.g. greater than six times the upper limit).
Some authors had also correlated this effect with the anti‐inflammatory effect of heparinoids, for instance, binding and neutralising a wide variety of mediators released from inflammatory cells, reducing IL‐6 and as potent inhibitors of the complement system, which may have effects on the clinical evolution of people with COVID‐19 (Liu 2019; Shi 2020; Tang 2020; Young 2008). It can attenuate ongoing tissue damage (Liu 2019; Young 2008). Practical guidelines and specialist consensus are addressing the management of thromboprophylaxis and anticoagulation in people with COVID‐19 infection (Bikdeli 2020; NHS 2020; Obe 2020; Ramacciotti 2020). However, the effects of anticoagulants on people with COVID‐19 is still under debate.
Objectives
To assess the effects of prophylactic anticoagulants versus active comparator, placebo or no intervention, on mortality and the need for respiratory support in people hospitalised with COVID‐19.
Methods
Criteria for considering studies for this review
Types of studies
The protocol for this review was prospectively registered with the Open Science Framework on 7 August 2020 (Flumignan 2020).
We considered parallel or cluster‐randomised controlled trials (RCTs), quasi‐RCTs, and cohort studies. Cohort studies may be useful for rare adverse events and clinical decisions if there is a lack of controlled studies. We did not consider studies without a comparator group. Although cohort studies (non‐randomised) were considered, we planned to limit our primary analyses to specific studies, that is, RCTs and quasi‐RCTs. We did not perform a meta‐analysis of non‐randomised studies (NRS), and we analysed their data narratively. In future updates of this review, when at least 400 participants are included from RCTs, we will no longer consider NRS for inclusion. We considered all other types of studies irrelevant for this review. Please find further explanations in Appendix 1.
In order to minimise selection bias for NRS, we planned to include only studies that used statistical adjustment for baseline factors using multivariate analyses for at least these confounding factors:
participants already using anticoagulants (e.g. atrial fibrillation)
participants who underwent surgery during the hospitalisation
active cancer treatment
concomitant antiplatelet use
history of venous thromboembolism
We considered only studies with a minimum duration of two weeks.
Types of participants
We included all participants eligible for prophylactic anticoagulation, both male and female of all ages, hospitalised with the diagnosis of COVID‐19. Any hospitalised participants with confirmed COVID‐19 infection were eligible, independently of the disease severity (e.g. patients hospitalised in ICUs or wards). We had also considered participants with the previous history of venous thromboembolism for inclusion in this review. However, the participants with COVID‐19 treated out of the hospital, i.e. those who were not hospitalised were not eligible for our review.
In future updates of this review, if we find studies with mixed populations, that is, hospitalised and non‐hospitalised participants, and only a subset of the participants meets our inclusion criteria, we will attempt to obtain data for the subgroup of interest from the study authors in order to include the study. For studies with mixed populations for which we cannot get the subgroup of interest's data but at least 50% of the study population are of interest, we will include all participants in our analysis. Moreover, we will explore the effect of this decision in a sensitivity analysis. Studies in which less than 50% of the population are of interest and the subgroup of interest data are not available will be excluded.
Types of interventions
We considered the following pharmacological interventions.
Heparinoids, that is, both unfractionated heparin and low molecular weight heparin, and pentasaccharides (synthetic and selective anticoagulant drugs similar to low molecular weight heparin)
Vitamin K antagonists
Direct anticoagulants, both factor Xa inhibitors and direct thrombin inhibitors, that is, direct oral anticoagulants and non‐oral direct anticoagulants (e.g. bivalirudin).
We considered studies comparing different formulations, doses and schedules of the same intervention (e.g. heparinoids).
Some commonly applicable prophylactic doses of the interventions of interest are low molecular weight heparin 30 mg twice a day or 40 mg daily, and unfractionated heparin 5000 IU three times a day. However, we considered all doses of anticoagulants, when used for primary or secondary prophylaxis of thromboembolism, eligible for our review.
Types of comparisons
We included studies that compared one pharmacological intervention (agent or drug) versus another active comparator, or placebo or no treatment with any combination of interventions, provided that co‐treatments were balanced between the treatment and control arms. We allowed other potential interventions (e.g. antiplatelet agents, elastic stockings, intermittent pneumatic compression) as comparators or additional interventions. We also included studies that compared different doses of drugs. We pooled the studies that addressed the same comparisons.
Anticoagulant versus placebo or no treatment (we planned to pool all anticoagulants together – heparinoids, vitamin K antagonists, direct anticoagulants, etc. – if possible)
Anticoagulant versus a different anticoagulant
Anticoagulant versus a different dose, formulation, or schedule of the same anticoagulant
Anticoagulant versus other pharmacological interventions such as antiplatelet agents
Anticoagulant versus non‐pharmacological interventions
Types of outcome measures
We evaluated core outcomes as pre‐defined by the Core Outcome Measures in Efectiveness Trials Initiative for people with COVID‐19 (COMET 2020). We also considered the outcomes after hospital discharge. We intended to present the outcomes at two different time points following the start of the intervention if data were available:
early outcomes (at hospital discharge or before);
long‐term outcomes (after hospital discharge).
Our time point of primary interest is early; we, therefore, intended to produce related 'Summary of findings' tables only for this time point but we also planned to report the long‐term outcomes at the longest possible time of follow‐up.
Primary
All‐cause mortality
-
Necessity for additional respiratory support:
oxygen by non‐invasive ventilators or high flow
intubation and mechanical ventilation
extracorporeal membrane oxygenation
Secondary
Mortality related to COVID‐19
Deep vein thrombosis (DVT), symptomatic or asymptomatic, first episode or recurrent confirmed by ultrasonography or angiography (e.g. by computed tomography (CT), magnetic resonance imaging (MRI) or by digital subtraction) from any site (e.g. lower limbs, upper limbs, abdominal).
Pulmonary embolism (symptomatic or asymptomatic, first episode or recurrent, fatal or non‐fatal): a diagnosis had to be confirmed by angiography (e.g. by CT, MRI or digital subtraction) and ventilation‐perfusion scan, or both. We also considered post mortem examination as an objective confirmation of DVT and pulmonary embolism.
Major bleeding: defined by a haemoglobin concentration decrease of 2 g/dL or more, a retroperitoneal or intracranial bleed, a transfusion of two or more units of blood, or fatal haemorrhagic events, as defined by International Society on Thrombosis and Haemostasis (Schulman 2010).
Adverse events. We will consider all possible adverse events separately, as individual outcomes, such as minor bleeding, gastrointestinal adverse effects (e.g. nausea, vomiting, diarrhoea, abdominal pain), allergic reactions, renal failure and amputations
Hospitalisation time in days
Quality of life: participant's subjective perception of improvement (yes or no) as reported by the study authors or using any validated scoring system such as the Short Form‐36 Health Survey (SF‐36) (Ware 1992).
We planned to include studies in the review irrespective of whether measured outcome data were reported in a ‘usable’ way.
Search methods for identification of studies
An information specialist (LLA) designed and conducted all searches on 20 June 2020, which were informed and verified by a content expert (RLGF) and independently peer reviewed.
Electronic searches
We identified eligible study references through systematic searches of the following bibliographic databases.
Cochrane Central Register of Controlled Trials (CENTRAL; 2020, Issue 6) in the Cochrane Library (searched 20 June 2020; Appendix 2)
MEDLINE PubMed (1946 to 20 June 2020; Appendix 3)
Embase Wiley (1974 to 20 June 2020; Appendix 4)
LILACS Virtual Health Library (Latin American and Caribbean Health Sciences Literature database; 1982 to 20 June 2020; Appendix 5)
IBECS Virtual Health Library (Indice Bibliográfico Español de Ciencias de la Salud; 2015 to 20 June 2020; Appendix 5)
We adapted the preliminary search strategy for MEDLINE (PubMed; Appendix 3) for use in the other databases. We did not apply any RCT filters for any databases, but we selected the study design manually because we also considered NRS for inclusion in this review. We searched all databases from their inception to the present, and we did not restrict the language of publication or publication status. We considered the adverse effects described in the included studies only.
Searching other resources
We also conducted a search of the Cochrane COVID-19 Study Register (Appendix 6), and medRxiv (Appendix 7), for ongoing or unpublished studies (both searched 20 June 2020). We checked reference lists of all included studies and any relevant systematic reviews identified for additional references to studies. We examined any relevant retraction statements and errata for included studies. We contacted the authors of the included studies for any possible unpublished data. Furthermore, we contacted field specialists to enquire about relevant ongoing or unpublished studies.
Data collection and analysis
Inclusion of non‐English language studies
We considered abstracts and full texts in all languages for inclusion. All potentially eligible non‐English language abstracts progressed to full‐text review, with methods translated for eligibility, and full text translated for data extraction.
Selection of studies
Two review authors (JDST, LCUN) independently screened titles and abstracts of all the potential studies we identified as a result of the search and coded them as 'retrieve' (eligible or potentially eligible/unclear) or 'do not retrieve', using the Covidence tool. If there were any disagreements, we asked a third review author to arbitrate (RLGF). We retrieved the full‐text study reports/publications, and two review authors (JDST, LCUN) independently screened the full text and identified studies for inclusion, and identified and recorded reasons for exclusion of the ineligible studies. We resolved any disagreement through discussion or, if required, we consulted a third person (RLGF). We identified and excluded duplicates and collated multiple reports of the same study so that each study, rather than each report, is the unit of interest in the review. We recorded the selection process in sufficient detail to complete a PRISMA flow diagram and ‘Characteristics of excluded studies’ table (Liberati 2009). We considered studies reported as full text, those published as abstract only, and unpublished data. We considered abstracts and conference proceedings if they were eligible and had usable data.
Data extraction and management
We managed and synthesised the available data using Review Manager 5 (Review Manager 2020). If there was a conflict between data reported across multiple sources for a single study (e.g. between a published article and a trial registry record), we planned to use the article published for numerical analysis, and we planned to report the differences and consider it on the certainty of evidence (GRADE approach; Schünemann 2013).
We planned to use a data collection form, which we piloted on at least one study in the review, for study characteristics and outcome data. We planned that one review author (RLGF) would extract study characteristics from included studies. We planned to extract the following study characteristics.
Methods: study design, total duration of the study, number of study centres and location, study setting, and date of the study
Participants: comorbidities, ventilation support, pregnancy, number randomised, number lost to follow‐up/withdrawn, number analysed, number of interest, mean age, age range, gender, the severity of the condition, inclusion criteria, and exclusion criteria
Interventions: intervention and comparison characteristics (e.g. manufacture, dosage, additional procedures, method of administration), concomitant medications, and excluded medications
Outcomes: primary and secondary outcomes specified and collected (e.g. how outcomes are measured), and time points reported. For NRS: confounding factors controlled for each relevant analysis presented
Notes: funding for the trial, and notable conflicts of interest of study authors
We planned for one review author (RLGF) to extract outcome data from included studies independently, which would be verified by the other two review authors (CM, BT). We planned to resolve disagreements by discussion. We planned for one review author (RLGF) to transfer data into Review Manager 5 (RevMan 5; Review Manager 2020). We planned to double‐check that data were entered correctly by comparing the data presented in the systematic review with the data extraction form. We planned for two review authors (CM, BT) to spot‐check study characteristics for accuracy against the study report.
Assessment of risk of bias in included studies
For data from RCTs we planned to use the 'Risk of bias' 1.0 tool to analyse the risk of bias in the underlying study results (Higgins 2017). For data from quasi‐RCTs or prospective NRS, we planned to use the Risk Of Bias in Non‐randomised Studies of Interventions (ROBINS‐I) tool (Sterne 2016). We also planned to use ROBINS‐I to assess the risk of bias in retrospective NRS. Please refer to Appendix 1 for detailed information regarding how we planned to assess the risk of bias of RCTs, quasi‐RCTs, and NRS.
We considered the following confounders for the assessment of ROBINS‐I domain on 'confounding' and used the Robvis tool to create the 'risk of bias' graphs for NRS (McGuinness 2020).
Participants already using anticoagulants (e.g. atrial fibrillation)
Participants who underwent surgery during hospitalisation
Active cancer treatment
Concomitant antiplatelet use
History of venous thromboembolism
Measures of treatment effect
Please refer to Appendix 1 for information regarding how we had planned to measure the treatment effects of RCTs, quasi‐RCTs and NRS.
Unit of analysis issues
As we included NRS only, meta‐analysis was not appropriate. Instead, we narratively described and presented results per study also using tables.
Please refer to Appendix 1 for information regarding how we had planned to combine studies with multiple treatment groups.
Dealing with missing data
We planned to contact investigators or study sponsors in order to verify key study characteristics and obtain missing numerical outcome data where possible (e.g. when a study is identified as abstract only). Where possible, we planned to use the RevMan 5 calculator to calculate missing standard deviations using other data from the trial, such as confidence intervals. Where this was not possible, and the missing data were thought to introduce serious bias, we planned to explore the impact of including such studies in the overall assessment of results by a sensitivity analysis. For all outcomes, we planned to follow intention‐to‐treat (ITT) principles to the highest degree possible: that is, we planned to analyse participants in their randomised group regardless of what intervention they received. We planned to use available‐case data for the denominator if ITT data were not available. We estimated the mean difference (MD) using the method reported by Wan 2014 to convert median and interquartile range (IQR) into MD and confidence intervals (CI). When it was not possible, we narratively described skewed data reported as medians and IQRs.
Dealing with sparse data
We planned to adjust comparisons (e.g. grouping broader categories of participants (all ages), grouping broader of variations of intervention (all types of anticoagulants) accordingly, regardless of sparse data.
Assessment of heterogeneity
As we identified NRS only, meta‐analysis was not appropriate. Instead, we narratively described and presented results per study in tables.
Please refer to Appendix 1 for information regarding how we had planned to assess heterogeneity.
Assessment of reporting biases
If we were able to pool more than 10 studies, we planned to create and examine a funnel plot to explore possible small‐study biases for the primary outcomes.
Data synthesis
Please refer to Appendix 1 for information regarding how we had planned to synthesise data from RCTs, quasi‐RCTs and NRS. We did not meta‐analyse data from NRS. We reported outcome data of each included study narratively and using tables.
Synthesis without meta‐analysis
We planned to synthesise the data using RevMan 5 (Review Manager 2020). We planned to report data narratively if it was not appropriate to combine in a meta‐analysis. We planned to undertake meta‐analyses only where this was meaningful, that is, if the treatments, participants and the underlying clinical question were similar enough for pooling to make sense. We planned to analyse data from NRS separately in a spreadsheet with the exposure of the sample number and the quantitative and qualitative variables relevant to the review. We planned to describe skewed data reported as medians and interquartile ranges narratively.
If a meta‐analysis was not possible, we explored the possibilities above to show data of all relevant outcomes considered in this review. Where there was substantial clinical, methodological, or statistical heterogeneity across studies that prevented the pooling of data, we used a narrative approach to data synthesis. We planned to describe narratively skewed data reported as medians and interquartile ranges.
Subgroup analysis and investigation of heterogeneity
We planned to explore the following subgroups related to participants or interventions, if heterogeneity was substantial.
Different doses of drugs
Duration of prophylaxis (e.g. until 30 days after the start of intervention or more)
Age (e.g. children (up to 18 years), adults (18 years to 64 years) and seniors (65 years and over))
Gender
Comorbidities
-
Type of ventilator support:
oxygen by non‐invasive ventilators or high flow
intubation and mechanical ventilation
extracorporeal membrane oxygenation
Sensitivity analysis
We planned to carry out the following sensitivity analyses to test whether critical methodological factors or decisions have affected the main result. We planned to group according to study design (RCTs or cluster‐RCTs, quasi‐RCTs, NRS).
Only including studies with a low risk of bias, as previously specified ('Assessment of risk of bias in included studies').
We planned to examine both the fixed‐effect model and random‐effects model meta‐analyses, and we planned to explore the differences between the two estimates.
We planned to explore the decision to include all participants when at least 50% were of interest in a study with a mixed population.
We planned to explore the impact of missing data. If we identified studies with missing data that were unobtainable, we planned to repeat analyses excluding these studies to determine their impact on the primary analyses.
We also planned to carry out sensitivity analyses considering cluster‐RCTs. We planned to investigate the effect of variation in the intracluster correlation coefficient (ICC), and we also planned to acknowledge heterogeneity in the randomisation unit and perform a sensitivity analysis to investigate the effects of the randomisation unit. We planned to present these results and compare them with the overall findings. We planned to justify any post hoc sensitivity analyses that arose during the review process in the final report.
Summary of findings and assessment of the certainty of the evidence
We created a 'Summary of findings' table for the early time point using the following outcomes.
All‐cause mortality
Necessity for additional respiratory support
Mortality related to COVID‐19
DVT
Pulmonary embolism
Major bleeding
We used the five GRADE considerations (study limitations; consistency of effect; imprecision; indirectness; and publication bias) to assess the certainty of a body of evidence as it relates to the studies that contribute data to the analyses for the prespecified outcomes. We used methods and recommendations described in Chapter 12 of the Cochrane Handbook for Systematic Reviews of Interventions (Schünemann 2019) using GRADEpro software (GRADEpro GDT 2015). We made a separate 'Summary of findings' table for each of the following comparisons with available data.
Anticoagulant (all types) versus no treatment
Anticoagulants (therapeutic dose) versus anticoagulants (prophylactic dose)
We justified all decisions to downgrade the certainty of studies using footnotes, and we made comments to aid the reader's understanding of the review where necessary.
Two review authors (RLGF, LCUN) made judgements about the certainty of the evidence, with disagreements resolved by discussion or by involving a third review author (CM, BT). We justified, documented and incorporated judgements into reporting of results for each outcome. We plan to extract study data, format our comparisons in data tables and prepare a 'Summary of findings' table with meta‐analysis before writing the results and conclusions of future updates of our review.
Results
Results of the search
We retrieved a total of 1148 records from our searches. After excluding 103 duplicate records, we screened 1045 unique records. We considered a total of 991 records not relevant at this stage and selected 54 for full‐text reading. We excluded 12 studies (11 reports) (see Characteristics of excluded studies). Twenty‐two studies are ongoing (see Characteristics of ongoing studies). We considered another 13 studies not relevant after a full‐text analysis. For this review, we found seven non‐randomised studies (NRS) with available data for inclusion. See Figure 1 for the study flow diagram (Liberati 2009).
1.
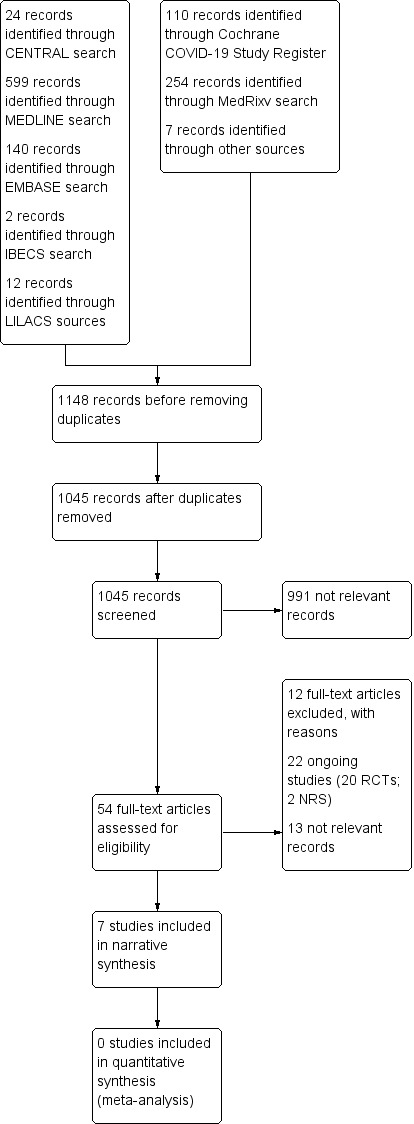
Study flow diagram RCTs: randomised controlled trials; NRS: non‐randomised studies
Included studies
See Table 4 for the summarised characteristics of included studies.
2. Summary of characteristics of included studies.
| Study (design) | Country | Participant age (mean) | Setting | Intervention type (dose) | Comparator | All‐cause mortality | Necessity for additional respiratory support | Follow‐up time (mean days) | Total participants allocated | Intervention group participants (anticoagulant) |
| Ayerbe 2020 (Retrospective cohort) | Spain | 67 | Hospitala | Heparin (NR) | NA | OR 0.42 (95% CI 0.26 to 0.66) P < 0.001, in favour of intervention group | NR | 8 | 2075 | 1734 |
| Liu 2020 (Retrospective cohort) | China | 72 | ICU (intervention) vs hospital ward (comparator) | Heparin (NR) | NA | Unadjusted OR 1.66, 95% CI 0.76 to 3.64 | NR | NR | 154 | 61 |
| Paranjpe 2020 (Retrospective cohort) | USA | NR | Hospitala | Treatment dose anticoagulation | NA | In‐hospital mortality: intervention 22.5% versus comparator 22.8% In subgroup who required mechanical ventilation: intervention 29.1% versus comparator 62.7% (adjusted HR 0.86, 95% CI 0.82 to 0.89; 395 participants, P < 0.001) |
NR | NR | 2773 | 786 |
| Russo 2020 (Retrospective cohort) | Italy | 67 | Hospitala | DOACS (NR) in 18 participants and VKA (NR) in 8 participants | NA | RR 1.15 (95% CI 0.29 to 2.57), P = 0.995 | NR | NR | 192 | 26 |
| Shi 2020 (Retrospective cohort) | China | 69 | Hospitala | LMWH | NA | Reported no deaths in both groups | NR | NR | 42 | 21 |
| Tang 2020 (Retrospective cohort) | China | 65 | Hospitala | UFH (10,000 to 15,000 IU/d in 5 participants and LMWH (40 mg/d to 60 mg/d) in 94 participants | NA | No difference (general mortality): (adjusted OR 1.64, 95% CI 0.92 to 2.92; 449 participants) Subgroup analysis: participants with SIC score of ≥ 4(unadjusted OR 0.37, 95% CI 0.15 to 0.90; 97 participants) Participants with D‐dimer > 6 times the ULN (unadjusted OR 0.44, 95% CI 0.22 to 0.86; 161 participants) |
NR | 28 | 449 | 99 |
| Trinh 2020 (Retrospective cohort) | USA | 59 | ICU | UFH 15 IU/kg/h; or enoxaparin 1 mg/kg twice or once daily; or apixaban 10 mg (if no prior anticoagulation) or 5 mg (if prior anticoagulation) twice dailyb | UFH 5000 IU two to three times daily; or enoxaparin 40 mg twice or once daily; or apixaban 2.5 mg or 5 mg twice dailyb |
Reduction in all‐cause mortality (adjusted HR 0.21, 95% CI 0.10 to 0.46) and a lower absolute rate of death in the therapeutic group (34.2% versus 53%) | NR | 35 | 244 | 161 |
| Total | China: 3 Italy: 1 Spain: 1 USA: 2 |
‐ | ‐ | ‐ | ‐ | 6 studies considered mortality; 1 study did not report mortality data |
No study considered additional respiratory support | 8 to 35 (3 studies) | 5929 | 2888 |
| CI: confidence interval; DOACS: direct oral anticoagulants; GFR: glomerular filtration rate;HR: hazard ratio; ICU: intensive care units;LMWH: low molecular weight heparin; NA: no anticoagulation; NR: not reported; NRS: non‐randomised study;OR: odds ratio; RR: risk ratio; SIC: sepsis‐induced coagulopathy; UFH: unfractionated heparin; VKA: vitamin K antagonist | ||||||||||
aHospital: includes intensive care unit, hospital wards or emergency department. bAnticoagulation used twice daily if glomerular filtration rate (GFR) was greater than 30 mL/min, or once daily if GFR was 30 mL/min or less.
We included seven studies describing 5929 participants in this review, of whom at least 2888 received anticoagulants (Ayerbe 2020; Liu 2020; Paranjpe 2020; Russo 2020; Shi 2020; Tang 2020; Trinh 2020). The seven included studies were all non‐randomised studies (NRS) of interventions, with a comparator group. Of the seven included studies, four originated from China (Liu 2020; Shi 2020; Tang 2020; Trinh 2020), one from Italy (Russo 2020), one from Spain (Ayerbe 2020), and one from the USA (Paranjpe 2020).
Trinh 2020 compared different doses of anticoagulant (prophylactic versus therapeutic) and the six other included studies compared anticoagulation versus no anticoagulation (Ayerbe 2020; Liu 2020; Paranjpe 2020; Russo 2020; Shi 2020; Tang 2020). Only three included studies reported the follow‐up period that varied from 8 to 35 days (Ayerbe 2020; Tang 2020; Trinh 2020). Liu 2020 compared participants from the ICU (intervention group) with participants in hospital wards (comparator group). Trinh 2020 included only participants from the ICU in both groups. The five other studies considered participants from all settings (ICU, hospital wards and emergency departments; Ayerbe 2020; Paranjpe 2020; Russo 2020; Shi 2020; Tang 2020). Paranjpe 2020 did not report data regarding age of participants. The mean age of the other six studies' participants varied from 59 to 72 years (Ayerbe 2020; Liu 2020; Russo 2020; Shi 2020; Tang 2020; Trinh 2020). Six studies reported data on mortality (Ayerbe 2020; Liu 2020; Paranjpe 2020; Russo 2020; Tang 2020; Trinh 2020), and none reported data for necessity for additional respiratory support.
Paranjpe 2020 did not describe the type or dose of anticoagulation. Ayerbe 2020 and Liu 2020 used heparin in the intervention group, but they did not report details about the type of heparin or dose. Shi 2020 used low molecular weight heparin and Russo 2020 used direct oral anticoagulants in 18 participants and vitamin K antagonist in eight other participants, but neither reported more details. Tang 2020 used unfractionated heparin 10,000 IU/day to 15,000 IU/day in five participants and low molecular weight heparin (enoxaparin) 40 mg/day to 60 mg/day in 94 participants. Trinh 2020 used unfractionated heparin 15 IU/kg/hour or enoxaparin 1 mg/kg twice daily if glomerular function rate (GFR) was greater than 30 mL a minute, or once daily if GFR was 30 mL a minute or less. In addition to these anticoagulants, the comparator group in Trinh 2020 also used apixaban 2.5 mg or 5 mg twice daily.
Please refer to the Characteristics of included studies for detailed information.
Excluded studies
We excluded 12 studies for at least one reason (Characteristics of excluded studies). Eleven of the studies had an irrelevant study design because of at least one of the following reasons (Al‐Samkari 2020; Artifoni 2020; EudraCT2020‐001823‐15; Helms 2020; Khider 2020; NCT04354155; NCT04359212; NCT04368377; NCT04394000; NCT04427098; Zhang 2020):
retrospective cases series without a consistent comparator group;
prospective cohort study without a comparator group (single‐arm study);
prospective cohort study without an intervention purpose;
prospective before‐after cohort study without a parallel comparator group;
prospective cohort study without a parallel comparator group of intervention.
One study had an irrelevant intervention, that is, it is a RCT of aspirin for COVID‐19, and there was no difference between the intervention groups regarding anticoagulants (NCT04365309).
Ongoing studies
Twenty‐two ongoing studies met our inclusion criteria, which plan to evaluate 15,727 participants. We tried to contact study authors; we also searched by study registration number and by title of the study on all databases of interest for this review. However, there are no additional data for all these ongoing studies. See the Characteristics of ongoing studies table for further details.
Four of the ongoing studies plan to include 1000 participants or more (NCT04333407; NCT04359277; NCT04366960; NCT04372589). NCT04333407 plans to compare aspirin, clopidogrel, rivaroxaban, atorvastatin, and omeprazole with no treatment in 3170 participants to assess mortality at 30 days. NCT04359277 plans to compare higher‐dose versus low‐dose prophylactic heparin to assess composite outcomes that include mortality in 1000 participants. NCT04366960 plans to compare 40 mg subcutaneous enoxaparin twice daily versus 40 mg subcutaneous enoxaparin once daily to assess venous thromboembolism in 2712 participants. NCT04372589 plans to compare therapeutic anticoagulation using heparin for 14 days with prophylactic anticoagulation to assess intubation and mortality in 3000 participants. See Table 5 for a summary of the characteristics of ongoing studies.
3. Summary of characteristics of ongoing studies.
| Study | Country | Design | Primary outcomes | Estimated number of participants | Estimated primary completion date |
| ACTRN12620000517976 | Australia | RCT | Time to separation from invasive ventilation | 172 | 25 July 2021 |
| ChiCTR2000030700 | China | RCT | Time to virus eradication | 60 | 30 September 2020 |
| ChiCTR2000030701 | China | RCT | Time to virus eradication | 60 | 30 September 2020 |
| ChiCTR2000030946 | China | Prospective cohort | Biochemical indicators | 120 | 24 April 2020 |
| Marietta 2020 | Italy | RCT | Clinical worsening (includes death and necessity for additional respiratory support) | 300 | June 2021 |
| NCT04333407 | UK | RCT | All‐cause mortality at 30 days after admission | 3170 | 30 March 2021 |
| NCT04344756 | France | RCT | Survival without ventilation | 808 | 31 July 2020 |
| NCT04345848 | Switzerland | RCT | Composite outcome of arterial or venous thrombosis, disseminated intravascular coagulation and all‐cause mortality | 200 | 30 November 2020 |
| NCT04352400 | Italy | RCT | Time to clinical improvement | 256 | December 2021 |
| NCT04359277 | USA | RCT | Composite incidence of: all‐cause mortality, cardiac arrest, symptomatic deep venous thrombosis, PE, arterial thromboembolism, myocardial infarction, stroke, or shock | 1000 | 21 April 2021 |
| NCT04360824 | USA | RCT | Risk of all‐cause mortality | 170 | 16 April 2021 |
| NCT04362085 | Canada | RCT | Composite outcome of ICU admission (yes/no), non‐invasive positive pressure ventilation (yes/no), invasive mechanical ventilation (yes/no), or all‐cause death (yes/no) up to 28 days | 462 | November 2020 |
| NCT04366960 | Italy | RCT | Incidence of VTE detected by imaging | 2712 | August 2020 |
| NCT04367831 | USA | RCT | Total number of patients with clinically relevant venous or arterial thrombotic events in ICU | 100 | November 2020 |
| NCT04372589 | Canada | RCT | Intubation and mortality | 3000 | January 2021 |
| NCT04373707 | France | RCT | VTE | 602 | September 2020 |
| NCT04377997 | USA | RCT |
|
300 | 1 January 2021 |
| NCT04393805 | Italy | Retrospective cohort |
|
877 | December 2020 |
| NCT04394377 | Brazil | RCT | Hierarchical composite endpoint composed of mortality, number of days alive, number of days in the hospital and number of days with oxygen therapy at the end of 30 days | 600 | December 2020 |
| NCT04397510 | USA | RCT | Mean daily PaO2:FiO2 | 50 | 31 December 2020 |
| NCT04401293 | USA | RCT | Composite outcome of arterial thromboembolic events, venous thromboembolic events and all‐cause mortality at day 30 ± 2 days | 308 | 22 October 2020 |
| NCT04416048 | Germany | RCT | Composite endpoint of VTE (DVT and/or fatal or non‐fatal PE), arterial thromboembolism, new myocardial infarction, non‐haemorrhagic stroke, all‐cause mortality or progression to intubation and invasive ventilation | 400 | 30 April 2021 |
| Total number of studies | Australia: 1 Brazil: 1 Canada: 2 China: 3 France: 2 Germany: 1 Italy: 4 Switzerland: 1 UK: 1 USA: 6 |
Prospective cohort: 1 RCT: 20 Retrospective cohort: 1 |
12 studies considered mortality Six studies considered additional respiratory support |
15,727 participants
|
13 studies to December 2020 8 studies to July 2021 1 study to December 2021 |
| DVT: deep vein thrombosis; FiO2: fraction of inspired oxygen; ISTH: International Society on Thrombosis and Haemostasis; NRS: non‐randomised studies; PaO2: arterial oxygen pressure;PE: pulmonary embolism; RCT: randomised controlled trial; VTE: venous thromboembolism | |||||
Risk of bias in included studies
We assessed the risk of bias at the result level, in each comparison, using ROBINS‐I tool (Sterne 2016). The specific judgements ('critical risk', 'serious risk', 'moderate risk', 'low risk', or 'no information') by available outcomes, in each comparison, are presented in Figure 2, Figure 3, Figure 4, Figure 5, Figure 6 and Figure 7. The support for judgement is explained in the related 'Risk of bias' tables (Table 6, Table 7, Table 8, Table 9, Table 10 and Table 11). The overall risk of bias for all‐cause mortality and for hospitalisation in the comparison 'anticoagulants (all types) versus no treatment' was critical and in the comparison 'anticoagulants (therapeutic dose) versus anticoagulants (prophylactic dose)' was serious. The overall risk of bias for major bleeding was serious for both comparisons.
2.
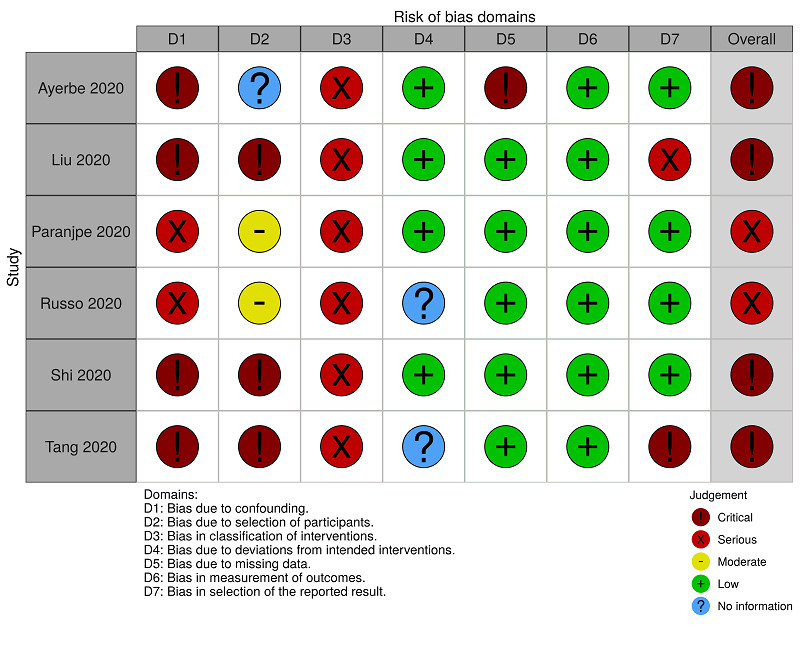
ROBINS‐I assessments: anticoagulants (all types) versus no treatment for people hospitalised with COVID‐19 (all‐cause mortality)
3.
ROBINS‐I assessments: anticoagulants (all types) versus no treatment for people hospitalised with COVID‐19 (major bleeding)
4.
ROBINS‐I assessments: anticoagulants (all types) versus no treatment for people hospitalised with COVID‐19 (hospitalisation)
5.
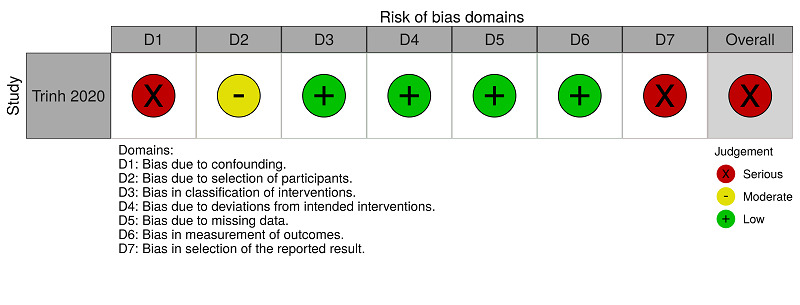
ROBINS‐I assessments: anticoagulants (therapeutic dose) versus anticoagulants (prophylactic dose) for people hospitalised with COVID‐19 (all‐cause mortality)
6.
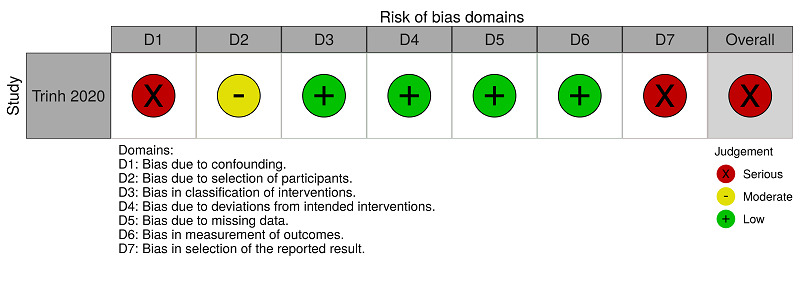
ROBINS‐I assessments: anticoagulants (therapeutic dose) versus anticoagulants (prophylactic dose) for people hospitalised with COVID‐19 (majorbleeding)
7.
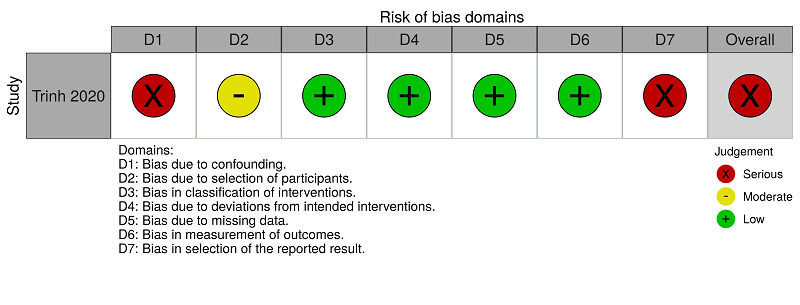
ROBINS‐I assessments: anticoagulants (therapeutic dose) versus anticoagulants (prophylactic dose) for people hospitalised with COVID‐19 (hospitalisation)
4. ROBINS‐I assessments: anticoagulants (all types) versus no treatment for people hospitalised with COVID‐19 (all‐cause mortality).
| Study | Bias due to confounding | Bias in selection of participants into the study | Bias in classification of interventions | Bias due to deviations from the intended intervention | Bias due to missing data | Bias in measurement of outcomes | Bias in selection of the reported result | Overall risk of bias |
| Ayerbe 2020 | Critical risk | No information | Serious risk | Low risk | Critical risk | Low risk | Low risk | Critical risk |
| Judgement | One or more prognostic variables are likely to be unbalanced between the compared groups. There is no baseline characteristics table comparing the two groups. Essential characteristics, such as participants already using anticoagulants, participants who underwent surgery during the hospitalisation, concomitant antiplatelet use, and history of venous thromboembolism, were not considered. | Participants included in both groups were selected from 17 hospitals, and the study was retrospective, therefore it is not possible to know whether the selection was free from bias. | As this was a retrospective study, there is a high risk that the interventions received by participants in the same group were not standardised. The type and doses of heparin in the intervention group were not described. | No deviations from the intended intervention were reported in the study, and if any deviation occurred from usual practice, it was unlikely to impact on the outcome. | There were missing outcome data for 56 participants with no specific information or appropriate analyses. These missing data could cause a critical impact on the estimates. | It is unlikely that the outcome assessment (death) was influenced by the knowledge of the intervention received by the study participants. | The study protocol was not identified but all reported results corresponded to the intended outcome. | The study is too problematic to provide useful evidence. |
| Liu 2020 | Critical risk | Critical risk | Serious risk | Low risk | Low risk | Low risk | Serious risk | Critical risk |
| Judgement | One or more prognostic variables are likely to be unbalanced between the compared groups. There is no baseline characteristics table comparing the two groups. Essential characteristics, such as participants already using anticoagulants, participants who underwent surgery during the hospitalisation, concomitant antiplatelet use, and history of venous thromboembolism, were not considered. | Participants included in both groups were selected from a single hospital, and the study was retrospective, therefore it is not possible to know whether the selection was free from bias. The selection for the study was strongly related to both the intervention and the outcome of interest. We cold not adjust the analyses for this selection bias. | As this was a retrospective study, there is a high risk that the interventions received by participants in the same group were not standardised. The type and doses of heparin in the intervention group were not described. | No deviations from the intended intervention were reported in the study, and if any deviation occurred from usual practice, it was unlikely to impact on the outcome. | No missing data was reported for this outcome. | It is unlikely that the outcome assessment (death) was influenced by the knowledge of the intervention received by the study participants. | The study protocol was not identified or was not available or both (only a preprint was available), and it is not possible to exclude bias in selection of reported effect estimate, based on the results, from different subgroups analyses. | The study is too problematic to provide useful evidence. |
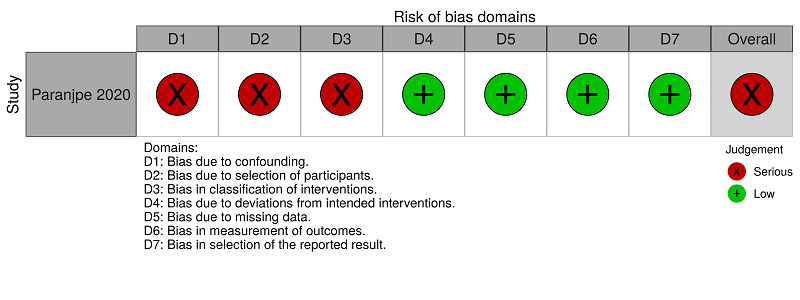
| Paranjpe 2020 | Serious risk | Moderate risk | Serious risk | Low risk | Low risk | Low risk | Low risk | Serious risk |
| Judgement | To minimise the impact of the absence of randomisation, an adjusted analysis with propensity scores was performed considering confounding demographic, clinical, and medication use. However, the confounding factors 'participants who underwent surgery during the hospitalisation', 'active cancer treatment', 'concomitant antiplatelet use' and 'history of venous thromboembolism' were not considered. | The included participants in both groups were selected from the same hospital, and selection may have been related to intervention and outcome, but the study authors used appropriate methods to adjust for selection bias. | There is a high risk that the interventions received by participants in the same group were not standardised. There is a high risk of differential classification errors because the information on the status of the interventions was obtained retrospectively. | No deviations from the intended intervention were reported in the study, and if any deviation occurred from usual practice, it was unlikely to impact on the outcome. | No missing data were reported for this outcome. | It is unlikely that the outcome assessment (death) was influenced by the knowledge of the intervention received by the study participants. | The study protocol was not identified but all reported results corresponded to the intended outcome. | The study has some important problems |
| Russo 2020 | Serious risk | Moderate risk | Serious risk | No information | Low risk | Low risk | Low risk | Serious risk |
| Judgement | To minimise the impact of the absence of randomisation, we performed an analysis with propensity scores, considering confounding demographic and clinical factors, and medication use. However, the study did not consider confounding factors 'participants who underwent a surgery during the hospitalisation', 'active cancer treatment' and 'history of venous thromboembolism'. | The included participants in both groups were selected from the same hospital, and selection may have been related to intervention and outcome, but the study authors used appropriate methods to adjust for selection bias. | There is a high risk that the interventions received by participants in the same group were not standardised. There is a high risk of differential classification errors because the information on the status of the interventions was obtained retrospectively. | Insufficient information to judge. No information is reported on whether there was deviation from the intended intervention. | No missing data were reported for this outcome. | It is unlikely that the outcome assessment (death) was influenced by the knowledge of the intervention received by the study participants. | The study protocol was not identified but all reported results corresponded to the intended outcome. | The study has some important problems |
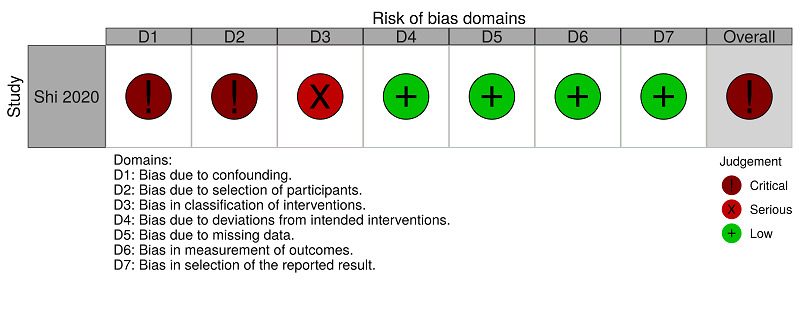
| Shi 2020 | Critical risk | Critical risk | Serious risk | Low risk | Low risk | Low risk | Low risk | Critical risk |
| Judgement | One or more prognostic variables are likely to be unbalanced between the compared groups. There is a baseline characteristics table comparing the two groups with limited items. However, the study did not compare essential characteristics, such as participants already using anticoagulants, participants who underwent surgery during the hospitalisation, concomitant antiplatelet use, and history of venous thromboembolism. | The participants of the two groups (intervention and comparator) were selected from the same hospital, but as the study was retrospective, it is not possible to know if the selection was free from bias. The selection for the study was strongly related to both the intervention and the outcome of interest. We could not adjust the analyses for this selection bias. | There is a risk that the interventions received by participants in the same group were not standardised. There is a high risk of differential classification errors because the information on the status of the interventions was obtained retrospectively. | No deviations from the intended intervention were reported in the study, and if any deviation occurred from usual practice, it was unlikely to impact on the outcome. | No missing data were reported for this outcome. | It is unlikely that the outcome assessment (death) was influenced by the knowledge of the intervention received by the study participants. | The study protocol was not identified but all reported results corresponded to the intended outcome. | The study is too problematic to provide useful evidence. |
| Tang 2020 | Critical risk | Critical risk | Serious risk | No information | Low risk | Low risk | Critical risk | Critical risk |
| Judgement | One or more prognostic variables are likely to be unbalanced among the compared groups. There was no table comparing the characteristics of the two groups at baseline. The comparator group included participants who used heparin for less time or did not use heparin. These participants may be less severely ill than those in the intervention group. | Participants included in both groups were selected from the same hospital, but as the study was retrospective, it is not possible to know whether the selection was free from bias. The selection for the study was strongly related to both the intervention and the outcome of interest. We could not adjust the analyses for this selection bias. | As this was a retrospective study, there is a high risk that the interventions received by participants in the same group were not standardised. Besides, the comparator group also included participants who used heparin for less than seven days. This proximity to the case definition for the intervention group increases the risk of error in the classification of participants. Also, the comparator group considered two very different types of intervention. | Insufficient information to judge. No information is reported on whether there was deviation from the intended intervention. | No missing data were reported for this outcome. | It is unlikely that the outcome assessment (death) was influenced by the knowledge of the intervention received by the study participants. | The study protocol was not identified or was not available or both, and it is not possible to exclude bias in selection of reported effect estimate, based on the results, from multiple measurements within the outcome domain, multiple analyses of the intervention‐outcome relationship, and different subgroups analyses. | The study is too problematic to provide useful evidence. |
5. ROBINS‐I assessments: anticoagulants (all types) versus no treatment for people hospitalised with COVID‐19 (major bleeding).
| Study | Bias due to confounding | Bias in selection of participants into the study | Bias in classification of interventions | Bias due to deviations from the intended intervention | Bias due to missing data | Bias in measurement of outcomes | Bias in selection of the reported result | Overall risk of bias |
| Paranjpe 2020 | Serious risk | Serious risk | Serious risk | Low risk | Low risk | Low risk | Low risk | Serious risk |
| Judgement | To minimise the impact of the absence of randomisation, we performed an adjusted analysis with propensity scores considering confounding demographic and clinical factors, and medication use. However, the study did not consider confounding factors 'participants who underwent surgery during the hospitalisation', 'active cancer treatment', 'concomitant antiplatelet use' and 'history of venous thromboembolism'. | The included participants in both groups were selected from the same hospital, and selection may have been related to intervention and outcome. For this outcome, the authors did not use appropriate methods to adjust for selection bias. | There is a high risk that the interventions received by participants in the same group were not standardised. There is a high risk of differential classification errors because the information on the status of the interventions was obtained retrospectively. | No deviations from the intended intervention were reported in the study, and if any deviation occurred from usual practice, it was unlikely to impact on the outcome. | No missing data were reported for this outcome. | It is unlikely that the outcome assessment (major bleeding) was influenced by the knowledge of the intervention received by the study participants. | The study protocol was not identified but all reported results corresponded to the intended outcome. | The study has some important problems |
6. ROBINS‐I assessments: anticoagulants (all types) versus no treatment for people hospitalised with COVID‐19 (hospitalisation).
| Study | Bias due to confounding | Bias in selection of participants into the study | Bias in classification of interventions | Bias due to deviations from the intended intervention | Bias due to missing data | Bias in measurement of outcomes | Bias in selection of the reported result | Overall risk of bias |
| Shi 2020 | Critical risk | Critical risk | Serious risk | Low risk | Low risk | Low risk | Low risk | Critical risk |
| Judgement | One or more prognostic variables are likely to be unbalanced between the compared groups. There is a baseline characteristics table comparing the two groups with limited items. However, the study did not compare essential characteristics, such as participants already using anticoagulants, participants who underwent surgery during the hospitalisation, concomitant antiplatelet use, and history of venous thromboembolism. | The participants of the two groups (intervention and comparator) were selected from the same hospital, but as the study was retrospective, it is not possible to know if the selection was free from bias. The selection for the study was strongly related to both the intervention and the outcome of interest. We could not adjust the analyses for this selection bias. | There is a risk that the interventions received by participants in the same group have not been standardised. There is a high risk of differential classification errors because the information on the status of the interventions was obtained retrospectively. | No deviations from the intended intervention were reported in the study, and if any deviation occurred from usual practice, it was unlikely to impact on the outcome. | No missing data were reported for this outcome. | It is unlikely that the outcome assessment (length of hospital stay) was influenced by the knowledge of the intervention received by the study participants. | The study protocol was not identified but all reported results corresponded to the intended outcome. | The study is too problematic to provide useful evidence. |
7. ROBINS‐I assessments: anticoagulants (therapeutic dose) versus anticoagulants (prophylactic dose) for people hospitalised with COVID‐19 (all‐cause mortality).
| Study | Bias due to confounding | Bias in selection of participants into the study | Bias in classification of interventions | Bias due to deviations from the intended intervention | Bias due to missing data | Bias in measurement of outcomes | Bias in selection of the reported result | Overall risk of bias |
| Trinh 2020 | Serious risk | Moderate risk | Low risk | Low risk | Low risk | Low risk | Serious risk | Serious risk |
| Judgement | To minimise the impact of the absence of randomisation, we performed an analysis with propensity scores considering confounding demographic, clinical and laboratory factors, and medication use. However, the study did not consider confounding factors 'participants who underwent a surgery during the hospitalisation', 'concomitant antiplatelet use' and 'history of venous thromboembolism'. | The included participants in both groups were selected from the same hospital. The study authors considered for inclusion all patients who met the inclusion criteria and who were treated in each period. | Intervention status was well defined based on information collected at the time of intervention. | No deviations from the intended intervention were reported in the study, and if any deviation occurred from usual practice, it was unlikely to impact on the outcome. | No missing data were reported for this outcome. | It is unlikely that the outcome assessment (death) was influenced by the knowledge of the intervention received by the study participants. | The study protocol was not identified or was not available or both (only a preprint was available), and it is not possible to exclude bias. | The study has some important problems |
8. ROBINS‐I assessments: anticoagulants (therapeutic dose) versus anticoagulants (prophylactic dose) for people hospitalised with COVID‐19 (major bleeding).
| Study | Bias due to confounding | Bias in selection of participants into the study | Bias in classification of interventions | Bias due to deviations from the intended intervention | Bias due to missing data | Bias in measurement of outcomes | Bias in selection of the reported result | Overall risk of bias |
| Trinh 2020 | Serious risk | Moderate risk | Low risk | Low risk | Low risk | Low risk | Serious risk | Serious risk |
| Judgement | To minimise the impact of the absence of randomisation, we performed an analysis with propensity scores considering confounding demographic, clinical and laboratory factors, and medication use. However, the study did not consider confounding factors 'participants who underwent surgery during the hospitalisation', 'concomitant antiplatelet use' and 'history of venous thromboembolism'. | The included participants in both groups were selected from the same hospital. The study authors considered for inclusion all patients who met the inclusion criteria and who were treated in each period. | Intervention status was well defined based on information collected at the time of intervention. | No deviations from the intended intervention were reported in the study, and if any deviation occurred from usual practice, it was unlikely to impact on the outcome. | No missing data were reported for this outcome. | It is unlikely that the outcome assessment (major bleeding) was influenced by the knowledge of the intervention received by the study participants. | The study protocol was not identified or was not available or both (only a preprint was available), and it is not possible to exclude bias. | The study has some important problems |
9. ROBINS‐I assessments: anticoagulants (therapeutic dose) versus anticoagulants (prophylactic dose) for people hospitalised with COVID‐19 (hospitalisation).
| Study | Bias due to confounding | Bias in selection of participants into the study | Bias in classification of interventions | Bias due to deviations from the intended intervention | Bias due to missing data | Bias in measurement of outcomes | Bias in selection of the reported result | Overall risk of bias |
| Trinh 2020 | Serious risk | Moderate risk | Low risk | Low risk | Low risk | Low risk | Serious risk | Serious risk |
| Judgement | To minimise the impact of the absence of randomisation, we performed an analysis with propensity scores considering confounding demographic, clinical and laboratory factors, and medication use. However, the study did not consider confounding factors 'participants who underwent a surgery during the hospitalisation', 'concomitant antiplatelet use' and 'history of venous thromboembolism'. | The included participants in both groups were selected from the same hospital. The study authors considered for inclusion all patients who met the inclusion criteria and who were treated in each period. | Intervention status was well defined based on information collected at the time of intervention. | No deviations from the intended intervention were reported in the study, and if any deviation occurred from usual practice, it was unlikely to impact on the outcome. | No missing data were reported for this outcome. | It is unlikely that the outcome assessment (length of hospital stay) was influenced by the knowledge of the intervention received by the study participants. | The study protocol was not identified or was not available or both (only a preprint was available), and it is not possible to exclude bias. | The study has some important problems |
Bias due to confounding
All‐cause mortality
Six studies reported mortality for the comparison 'anticoagulants (all types) versus no treatment'. We rated four of them as critical risk because one or more prognostic variables are likely to be unbalanced between the compared groups (Ayerbe 2020; Liu 2020; Shi 2020; Tang 2020). There is not a baseline characteristics table comparing the two groups in Ayerbe 2020, Liu 2020 and Tang 2020. There is a baseline characteristics table, with limited items, comparing the two groups in Shi 2020. However, they did not consider essential characteristics, such as participants already using anticoagulants, participants who underwent surgery during hospitalisation, concomitant antiplatelet use, and history of venous thromboembolism. In Tang 2020, the comparator group included participants who used heparin for less time or did not use heparin. These participants may be less severely ill than those in the intervention group.
We rated the other two studies as serious risk because, to minimise the impact of the absence of randomisation, the studies authors performed an adjusted analysis with propensity scores, considering confounding demographic and clinical factors, and medication use. However, neither Paranjpe 2020 nor Russo 2020 considered the confounding factors 'participants who underwent surgery during hospitalisation', 'active cancer treatment', and 'history of venous thromboembolism'. Paranjpe 2020 also did not consider 'concomitant antiplatelet use' as a confounder. See Figure 2 and Table 6.
Trinh 2020 reported mortality for the comparison 'anticoagulants (therapeutic dose) versus anticoagulants (prophylactic dose)' and we rated this study as serious risk because, to minimise the impact of the absence of randomisation, we performed an analysis with propensity scores, considering confounding demographic, clinical, and laboratory factors, and medication use. However, Trinh 2020 did not consider the confounding factors 'participants who underwent surgery during hospitalisation', 'concomitant antiplatelet use' and 'history of venous thromboembolism'. See Figure 5 and Table 9.
Major bleeding
Paranjpe 2020 reported major bleeding for the comparison 'anticoagulants (all types) versus no treatment'. We rated this study as serious risk because, to minimise the impact of the absence of randomisation, we performed an adjusted analysis with propensity scores, considering confounding demographic and clinical factors, and medication use. However, Paranjpe 2020 did not consider the confounding factors 'participants who underwent surgery during hospitalisation', 'active cancer treatment', 'concomitant antiplatelet use' and 'history of venous thromboembolism'. See Figure 3 and Table 7.
Trinh 2020 reported major bleeding for the comparison 'anticoagulants (therapeutic dose) versus anticoagulants (prophylactic dose)'. We rated this study as serious risk because, to minimise the impact of the absence of randomisation, we performed an analysis with propensity scores, considering confounding demographic, clinical, laboratory factors and medication use. However, Trinh 2020 did not consider the confounding factors 'participants who underwent surgery during hospitalisation', 'concomitant antiplatelet use' and 'history of venous thromboembolism'. See Figure 6 and Table 10.
Hospitalisation
Shi 2020 reported hospitalisation for the comparison 'anticoagulants (all types) versus no treatment'. We rated this study as critical risk because one or more prognostic variables are likely to be unbalanced between the compared groups. There is a baseline characteristics table, with limited items, comparing the two groups. However, Shi 2020 did not compare essential characteristics, such as participants already using anticoagulants, participants who underwent surgery during hospitalisation, concomitant antiplatelet use, and history of venous thromboembolism. See Figure 4 and Table 8.
Trinh 2020 reported hospitalisation for the comparison 'anticoagulants (therapeutic dose) versus anticoagulants (prophylactic dose)' and we rated this study as serious risk because, to minimise the impact of the absence of randomisation, we performed an analysis with propensity scores, considering confounding demographic, clinical, laboratory factors and medication use. However, Trinh 2020 did not consider the confounding factors 'participants who underwent surgery during hospitalisation', 'concomitant antiplatelet use' and 'history of venous thromboembolism'. See Figure 7 and Table 11.
Bias in selection of participants into the study
All‐cause mortality
Six studies reported mortality for the comparison 'anticoagulants (all types) versus no treatment'. We rated three of them as critical risk (Liu 2020; Shi 2020; Tang 2020). These studies selected participants included in both groups (intervention and comparator) from a single hospital, and the studies were retrospective, so it is not possible to know whether the selection was free from bias. The selection for the studies was strongly related to both the intervention and the outcome of interest. We could not adjust the analyses for this selection bias (Liu 2020; Shi 2020; Tang 2020).
We rated Paranjpe 2020 and Russo 2020 as moderate risk because they selected the included participants in both groups from the same hospital, and selection may have been related to intervention and outcome, but the study authors used appropriate methods to adjust for selection bias.
We rated Ayerbe 2020 as 'no information' because they selected participants included in both groups from 17 hospitals, and the study was retrospective, therefore it is not possible to know whether the selection was free from bias. See Figure 2 and Table 6.
Trinh 2020 reported mortality for the comparison 'anticoagulants (therapeutic dose) versus anticoagulants (prophylactic dose)' and we rated this study as moderate risk because they selected the included participants in both groups from the same hospital. Trinh 2020 considered for inclusion all patients who met the inclusion criteria, and who were treated in each period. See Figure 5 and Table 9.
Major bleeding
Paranjpe 2020 reported major bleeding for the comparison 'anticoagulants (all types) versus no treatment'. We rated this study as serious risk because they selected the included participants in both groups from the same hospital, and selection may have been related to intervention and outcome. For this outcome, the study authors did not use appropriate methods to adjust for selection bias. See Figure 3 and Table 7.
Trinh 2020 reported major bleeding for the comparison 'anticoagulants (therapeutic dose) versus anticoagulants (prophylactic dose)'. We rated this study as moderate risk because they selected the included participants in both groups from the same hospital. Trinh 2020 considered for inclusion all patients who met the inclusion criteria, and who were treated in each period. See Figure 6 and Table 10.
Hospitalisation
Shi 2020 reported hospitalisation for the comparison 'anticoagulants (all types) versus no treatment'. We rated this study as critical risk because they selected the participants of the two groups (intervention and comparator) from the same hospital, but as the study was retrospective, it is not possible to know if the selection was free from bias. The selection for the study was strongly related to both the intervention and the outcome of interest. We could not adjust the analyses for this selection bias. See Figure 4 and Table 8.
Trinh 2020 reported hospitalisation for the comparison 'anticoagulants (therapeutic dose) versus anticoagulants (prophylactic dose)' and we rated this study as moderate risk because they selected the included participants in both groups from the same hospital. Trinh 2020 considered for inclusion all patients who met the inclusion criteria, and who were treated in each period. See Figure 7 and Table 11.
Bias in classification of interventions
All‐cause mortality
Six studies reported mortality for the comparison 'anticoagulants (all types) versus no treatment' (Ayerbe 2020; Liu 2020; Paranjpe 2020; Russo 2020; Shi 2020; Tang 2020). We rated them as serious risk because there is a high risk that these studies did not standardise interventions received by participants in the same group. Ayerbe 2020 and Liu 2020 did not describe the type and doses of heparin in the intervention group. There is a high risk of differential classification errors because the information on the status of the interventions was obtained retrospectively (Paranjpe 2020; Russo 2020; Shi 2020). Besides, in Tang 2020, the comparator group also included participants who used heparin for under seven days. This proximity to the case definition for the intervention group increases the risk of error in the classification of participants. Also, the comparator group in Tang 2020 considered two very different types of intervention. See Figure 2 and Table 6.
Trinh 2020 reported mortality for the comparison 'anticoagulants (therapeutic dose) versus anticoagulants (prophylactic dose)' and we rated this study as low risk because intervention status was well defined based on information collected at the time of intervention. See Figure 5 and Table 9.
Major bleeding
Paranjpe 2020 reported major bleeding for the comparison 'anticoagulants (all types) versus no treatment'. We rated this study as serious risk because there is a high risk that they did not standardise the interventions received by participants in the same group. There is a high risk of differential classification errors because the information on the status of the interventions was obtained retrospectively. See Figure 3 and Table 7.
Trinh 2020 reported major bleeding for the comparison 'anticoagulants (therapeutic dose) versus anticoagulants (prophylactic dose)'. We rated this study as low risk because intervention status was well defined based on information collected at the time of intervention. See Figure 6 and Table 10.
Hospitalisation
Shi 2020 reported hospitalisation for the comparison 'anticoagulants (all types) versus no treatment'. We rated this study as serious risk because there is a risk that the interventions received by participants in the same group were not standardised. There is a high risk of differential classification errors because the information on the status of the interventions was obtained retrospectively. See Figure 4 and Table 8.
Trinh 2020 reported hospitalisation for the comparison 'anticoagulants (therapeutic dose) versus anticoagulants (prophylactic dose)' and we rated this study as low risk because intervention status was well defined based on information collected at the time of intervention. See Figure 7 and Table 11.
Bias due to deviations from the intended intervention
All‐cause mortality
Six studies reported mortality for the comparison 'anticoagulants (all types) versus no treatment'. We rated four of them as low risk because they did not report any deviations from the intended intervention, and if any deviation occurred from usual practice, it was unlikely to impact on the outcome (Ayerbe 2020; Liu 2020; Paranjpe 2020; Shi 2020). We rated Russo 2020 and Tang 2020 as 'no information' because there is insufficient information to judge. They did not report any information on whether there was deviation from the intended intervention. See Figure 2 and Table 6.
Trinh 2020 reported mortality for the comparison 'anticoagulants (therapeutic dose) versus anticoagulants (prophylactic dose)' and we rated this study as low risk because they did not report any deviations from the intended intervention in the study, and if any deviation occurred from usual practice, it was unlikely to impact on the outcome. See Figure 5 and Table 9.
Major bleeding
Paranjpe 2020 reported major bleeding for the comparison 'anticoagulants (all types) versus no treatment'. We rated this study as low risk because they did not report any deviations from the intended intervention in the study, and if any deviation occurred from usual practice, it was unlikely to impact on the outcome. See Figure 3 and Table 7.
Trinh 2020 reported major bleeding for the comparison 'anticoagulants (therapeutic dose) versus anticoagulants (prophylactic dose)'. We rated this study as low risk because they did not report any deviations from the intended intervention in the study, and if any deviation occurred from usual practice, it was unlikely to impact on the outcome. See Figure 6 and Table 10.
Hospitalisation
Shi 2020 reported hospitalisation for the comparison 'anticoagulants (all types) versus no treatment'. We rated this study as low risk because they did not report any deviations from the intended intervention in the study, and if any deviation occurred from usual practice, it was unlikely to impact on the outcome. See Figure 4 and Table 8.
Trinh 2020 reported hospitalisation for the comparison 'anticoagulants (therapeutic dose) versus anticoagulants (prophylactic dose)' and we rated this study as low risk because they did not report any deviations from the intended intervention in the study, and if any deviation occurred from usual practice, it was unlikely to impact on the outcome. See Figure 7 and Table 11.
Bias due to missing data
All‐cause mortality
Six studies reported mortality for the comparison 'anticoagulants (all types) versus no treatment' (Ayerbe 2020; Liu 2020; Paranjpe 2020; Russo 2020; Shi 2020; Tang 2020). We rated Ayerbe 2020 as critical risk because there were missing outcome data for 56 participants with no specific information or appropriate analyses. These missing data could cause a critical impact on the estimates. We rated the other five studies as low because there were no missing data for this outcome (Liu 2020; Paranjpe 2020; Russo 2020; Shi 2020; Tang 2020). See Figure 2 and Table 6.
Trinh 2020 reported mortality for the comparison 'anticoagulants (therapeutic dose) versus anticoagulants (prophylactic dose)' and we rated this study as low risk because there were no missing data for this outcome. See Figure 5 and Table 9.
Major bleeding
Paranjpe 2020 reported major bleeding for the comparison 'anticoagulants (all types) versus no treatment'. We rated this study as low risk because there were no missing data for this outcome. See Figure 3 and Table 7.
Trinh 2020 reported major bleeding for the comparison 'anticoagulants (therapeutic dose) versus anticoagulants (prophylactic dose)'. We rated this study as low risk because there were no missing data for this outcome. See Figure 6 and Table 10.
Hospitalisation
Shi 2020 reported hospitalisation for the comparison 'anticoagulants (all types) versus no treatment'. We rated this study as low risk because there were no missing data for this outcome. See Figure 4 and Table 8.
Trinh 2020 reported hospitalisation for the comparison 'anticoagulants (therapeutic dose) versus anticoagulants (prophylactic dose)' and we rated this study as low risk because there were no missing data for this outcome. See Figure 7 and Table 11.
Bias in measurement of outcomes
All‐cause mortality
Six studies reported mortality for the comparison 'anticoagulants (all types) versus no treatment'. We rated them as low risk because it is unlikely that the outcome assessment (death) was influenced by the knowledge of the intervention received by the study participants (Ayerbe 2020; Liu 2020; Paranjpe 2020; Russo 2020; Shi 2020; Tang 2020). See Figure 2 and Table 6.
Trinh 2020 reported mortality for the comparison 'anticoagulants (therapeutic dose) versus anticoagulants (prophylactic dose)' and we rated this study as low risk because it is unlikely that the outcome assessment (death) was influenced by the knowledge of the intervention received by the study participants. See Figure 5 and Table 9.
Major bleeding
Paranjpe 2020 reported major bleeding for the comparison 'anticoagulants (all types) versus no treatment'. We rated this study as low risk because it is unlikely that the outcome assessment (major bleeding) was influenced by the knowledge of the intervention received by the study participants. See Figure 3 and Table 7.
Trinh 2020 reported major bleeding for the comparison 'anticoagulants (therapeutic dose) versus anticoagulants (prophylactic dose)'. We rated this study as low risk because it is unlikely that the outcome assessment (major bleeding) was influenced by the knowledge of the intervention received by the study participants. See Figure 6 and Table 10.
Hospitalisation
Shi 2020 reported hospitalisation for the comparison 'anticoagulants (all types) versus no treatment'. We rated this study as low risk because it is unlikely that the outcome assessment (length of hospital stay) was influenced by the knowledge of the intervention received by the study participants. See Figure 4 and Table 8.
Trinh 2020 reported hospitalisation for the comparison 'anticoagulants (therapeutic dose) versus anticoagulants (prophylactic dose)' and we rated this study as low risk because it is unlikely that the outcome assessment (length of hospital stay) was influenced by the knowledge of the intervention received by the study participants. See Figure 7 and Table 11.
Bias in selection of the reported result
All‐cause mortality
Six studies reported mortality for the comparison 'anticoagulants (all types) versus no treatment' (Ayerbe 2020; Liu 2020; Paranjpe 2020; Russo 2020; Shi 2020; Tang 2020). We rated Tang 2020 as critical risk because we did not identify the study protocol or it was not available, and it is not possible to exclude bias in selection of reported effect estimates, based on the results, from multiple measurements within the outcome domain, multiple analyses of the intervention‐outcome relationship, and analyses of different subgroups. We rated Liu 2020 as serious risk because we did not identify the study protocol or it was not available (only a preprint was available), and it is not possible to exclude bias in selection of reported effect estimates, based on the results, from analyses of different subgroups. We rated the other four studies as low because we did not identify the study protocol but all reported results corresponded to the intended outcome (Ayerbe 2020; Paranjpe 2020; Russo 2020; Shi 2020). See Figure 2 and Table 6.
Trinh 2020 reported mortality for the comparison 'anticoagulants (therapeutic dose) versus anticoagulants (prophylactic dose)' and we rated this study as serious risk because we did not identify the study protocol or it was not available (only a preprint was available), and it is not possible to exclude bias. See Figure 5 and Table 9.
Major bleeding
Paranjpe 2020 reported major bleeding for the comparison 'anticoagulants (all types) versus no treatment'. We rated this study as low risk because we did not identify the study protocol but all reported results corresponded to the intended outcome. See Figure 3 and Table 7.
Trinh 2020 reported major bleeding for the comparison 'anticoagulants (therapeutic dose) versus anticoagulants (prophylactic dose)'. We rated this study as serious risk because we did not identify the study protocol or it was not available (only a preprint was available), and it is not possible to exclude bias. See Figure 6 and Table 10.
Hospitalisation
Shi 2020 reported hospitalisation for the comparison 'anticoagulants (all types) versus no treatment'. We rated this study as low risk because we did not identify the study protocol but all reported results corresponded to the intended outcome. See Figure 4 and Table 8.
Trinh 2020 reported hospitalisation for the comparison 'anticoagulants (therapeutic dose) versus anticoagulants (prophylactic dose)' and we rated this study as serious risk because we did not identify the study protocol or it was not available (only a preprint was available), and it is not possible to exclude bias. See Figure 7 and Table 11.
Effects of interventions
Since we included seven NRS of interventions and no RCTs, or quasi‐RCTs, we did not perform any quantitative data analysis (meta‐analysis). Otherwise, we restricted our analysis on the qualitative aspects of the results reported by the study authors.
1. Anticoagulants (all types) versus no treatment
Four studies compared heparins (Ayerbe 2020; Liu 2020; Shi 2020; Tang 2020), and Russo 2020 compared oral anticoagulants (direct oral anticoagulants or vitamin K antagonists) to no treatment. Paranjpe 2020 compared 'therapeutic anticoagulation' (including oral, subcutaneous, or intravenous forms) to no treatment, but did not describe the type or dose of the pharmacological intervention. See Table 1.
Summary of findings 1. Anticoagulants (all types) compared to no treatment for people hospitalised with COVID‐19.
| Anticoagulants (all types) compared to no treatment for people hospitalised with COVID‐19 | |||
| Patient or population: people hospitalised with COVID‐19 Setting: hospital (ICU and ward) Intervention: anticoagulants (all types) Comparison: no treatment | |||
| Outcomes | Impact |
№ of participants (studies) |
Certainty of the evidence (GRADE) |
|
All‐cause mortality Follow‐up: range 8 to 28 days |
One study reported reduction of mortality by OR adjusted for confounding (reduction of 58% on chance of death; 2075 participants).
One study reported reduction of mortality only in a subgroup of severely ill participants (HR 0.86, 95% CI 0.82 to 0.89; 395 participants).
Three studies reported no differences by adjusted OR (1.64, 95% CI 0.92 to 2.92; 449 participants), unadjusted OR (1.66, 95% CI 0.76 to 3.64; 154 participants) or adjusted RR (1.15, 95% CI 0.29 to 2.57; 192 participants). One study reported zero events in both intervention groups. |
5685 (6 retrospective NRS) | ⊕⊝⊝⊝ Very lowa,b,c |
| Necessity for additional respiratory support | No study measured this outcome | ||
| Mortality related to COVID‐19 | No study measured this outcome | ||
| Deep vein thrombosis | No study measured this outcome | ||
| Pulmonary embolism | No study measured this outcome | ||
|
Major bleeding Follow‐up: not reported |
One study reported 24 (3%) bleeding events in the intervention group and 38 (1.9%) bleeding events in the control group (OR 1.62, 95% CI 0.96 to 2.71). | 2773 (1 retrospective NRS) | ⊕⊕⊝⊝ Lowc,d |
| CI: confidence interval; HR: hazard ratio; ICU: intensive care unit; NRS: non‐randomised studies; OR: odds ratio; RR: risk ratio | |||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | |||
aDowngraded one level due to study limitations. Overall critical/serious risk of bias across studies, especially related to confounding. bDowngraded one level due to inconsistency. We decided not to pool data due to the heterogeneity of studies (especially due to differences in interventions). cDowngraded one level due to imprecision. Narrative synthesis was conducted with imprecise estimates. dDowngraded one level due to study limitations. Overall serious risk of bias, especially related to confounding.
Primary outcomes
All‐cause mortality
Ayerbe 2020 reported all‐cause mortality as the proportion of participants and as odds ratio (OR) after adjusting for some covariates (e.g. age, gender, saturation of oxygen < 90% and temperature > 37 °C). They found 242 (13.9%) deaths in the intervention group and 44 (15.4%) deaths in the comparator group (adjusted OR 0.42, 95% confidence interval (CI) 0.26 to 0.66; P < 0.001; 2075 participants), in favour of the intervention group after all adjustments.
Liu 2020 evaluated all‐cause mortality in the context of the use or not of substitutive dialysis therapy, but not comparing the use or not of heparin. In this setting, mortality in the intervention group was 22.5% and in the comparator group was 22.8%. Extracting data from the reported tables, irrespective of setting (ICU or ward), we found 35 deaths in 106 participants with anticoagulants and 11 deaths in 48 participants without anticoagulants (unadjusted OR 1.66, 95% CI 0.76 to 3.64; 154 participants).
Paranjpe 2020 reported 22.5% in‐hospital mortality for the intervention group and 22.8% for the comparator group. However, in participants who required mechanical ventilation (n = 395), in‐hospital mortality was 29.1% in the intervention group and 62.7% in the comparator group. In this subgroup, after a multivariate adjustment, the hazard ratio (HR) was 0.86 (95% CI 0.82 to 0.89; P < 0.001; 395 participants).
Russo 2020 reported all‐cause mortality, after regression adjustment, as: risk ratio (RR) 1.15 (95% CI 0.29 to 2.57; P = 0.995; 192 participants).
Shi 2020 did not foresee this outcome but reported that no deaths occurred during the follow‐up period.
Tang 2020 reported no difference in all‐cause mortality between the intervention group (30.3%) and the comparator group (29.7%; P = 0.910) in general (adjusted OR 1.64, 95% CI 0.92 to 2.92; 449 participants). Among participants with a sepsis‐induced coagulopathy (SIC) score of 4 or more (n = 97), mortality was 40% in the intervention group and 64.2% in the comparator group (P = 0.029). The unadjusted OR was 0.37 (95% CI 0.15 to 0.90; 97 participants). Besides, mortality among participants with high levels of D‐dimer (e.g. greater than 6 times the upper limit) was 32.8% in the intervention group and 52.4% in the comparator group (P = 0.017). The unadjusted OR was 0.44 (95% CI 0.22 to 0.86; 161 participants).
It is very uncertain whether anticoagulants (all types) reduce all‐cause mortality compared with no treatment because the certainty of evidence is very low.
Necessity for additional respiratory support
There were no available data for this outcome.
Secondary outcomes
Mortality related to COVID‐19
There were no available data for this outcome.
Deep vein thrombosis (DVT)
There were no available data for this outcome.
Pulmonary embolism
There were no available data for this outcome.
Major bleeding
Liu 2020 did not define their bleeding criteria and reported bleeding in lung tissues of one participant. They did not clarify if this diagnosis was made on necropsies or clinically. Therefore, we did not consider this information as an available datum.
Paranjpe 2020 defined 'major bleeding' as:
haemoglobin less than 7 g/dL and any red blood cell transfusion;
at least 2 units of red blood cell transfusion within 48 hours; or
a diagnosis code for major bleeding including intracranial haemorrhage, haematemesis, melena, peptic ulcer with haemorrhage, colon, rectal, or anal haemorrhage, haematuria, ocular haemorrhage, and acute haemorrhagic gastritis.
They reported 24 (3%) events in the intervention group and 38 (1.9%) events in the comparator group (P = 0.2). Of the 24 participants who had bleeding events in the intervention group, 15 (63%) had bleeding events after starting anticoagulation and 9 (37%) had bleeding events before starting anticoagulation. Bleeding events were more common among intubated participants (30 of 395; 7.5%) than among non‐intubated participants (32 of 2378; 1.35%).
Ayerbe 2020, Russo 2020, Shi 2020 and Tang 2020 did not report data for this outcome.
Anticoagulants (all types) may make no difference in major bleeding compared with no treatment, but the certainty of evidence is low.
Adverse events (minor bleeding, gastrointestinal adverse effects (e.g. nausea, vomiting, diarrhoea, abdominal pain), allergic reactions, renal failure and amputations)
Tang 2020 reported that "the prophylactic dose of low molecular weight heparin was used in most of our heparin users, bleeding complications were unusual and commonly mild, and it is not known if higher doses would have been better." However, the trial authors did not report any related number of events or comparison between the groups. Therefore, we did not consider this information as an available datum.
Ayerbe 2020, Liu 2020, Paranjpe 2020, Russo 2020 and Shi 2020 did not report data for this outcome.
Hospitalisation time in days
Paranjpe 2020 reported 5 days (interquartile range (IQR) 3 to 8) as their median hospitalisation time, but they did not compare this outcome among the intervention and comparator groups. Therefore, we did not consider this information as an available datum.
Shi 2020 reported a median of 29 days (IQR 17 to 42) as hospitalisation time in the intervention group and 27 days (IQR 24 to 31) in the comparator group (P = 0.41). We estimated the mean difference (MD) 2 days (95% CI −0.80 to 4.80) using the method reported by Wan 2014 to convert median and IQR into MD and CI.
Tang 2020 used hospital stay of less than seven days as an exclusion criterion, but they did not report data for analysis.
Ayerbe 2020, Liu 2020 and Russo 2020 did not report data for this outcome.
It is very uncertain whether anticoagulants (all types) have any effect on hospitalisation time compared with no treatment because the certainty of evidence is very low (42 participants, 1 retrospective NRS). We downgraded the certainty of evidence by one level due to study limitations because the overall risk of bias was critical, especially related to confounding. We downgraded the certainty of evidence by two levels due to imprecision because the narrative synthesis was conducted with imprecise estimates based on few participants.
Quality of life
There were no available data for this outcome.
2. Anticoagulants (therapeutic dose) versus anticoagulants (prophylactic dose)
Trinh 2020 compared heparins (unfractionated heparin or low molecular weight heparin) or direct oral anticoagulants (apixaban) in therapeutic doses (161 participants) versus heparins (unfractionated heparin or low molecular weight heparin) or direct oral anticoagulants (apixaban) in prophylactic doses (83 participants). See Table 2.
Summary of findings 2. Anticoagulants (therapeutic dose) compared to anticoagulants (prophylactic dose) for people hospitalised with COVID‐19.
| Anticoagulants (therapeutic dose) compared to anticoagulants (prophylactic dose) for people hospitalised with COVID‐19 | |||
| Patient or population: people hospitalised with COVID‐19 Setting: hospital (ICU and ward) Intervention: anticoagulants (therapeutic dose) Comparison: anticoagulants (prophylactic dose) | |||
| Outcomes | Impact |
№ of participants (Studies) |
Certainty of the evidence (GRADE) |
| All‐cause mortality Follow‐up: 35 days | One study reported an absolute rate of death lower in the therapeutic group (34.2% versus 53%) and an HR adjusted for confounding of 0.21 (95% CI 0.10 to 0.46). | 244 (1 retrospective NRS) | ⊕⊕⊝⊝ Lowa,b |
| Necessity for additional respiratory support | No study measured this outcome | ||
| Mortality related to COVID‐19 | No study measured this outcome | ||
| Deep vein thrombosis | No study measured this outcome | ||
| Pulmonary embolism | No study measured this outcome | ||
| Major bleeding Follow‐up: 35 days | One study reported 51 (31.7%) bleeding events in the intervention group and 17 (20.5%) bleeding events in the control group (OR 1.80, 95% CI 0.96 to 3.37). | 244 (1 retrospective NRS) | ⊕⊕⊝⊝ Lowa,b |
| CI: confidence interval; HR: hazard ratio; ICU: intensive care unit; NRS: non‐randomised studies; OR: odds ratio | |||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | |||
aDowngraded one level due to study limitations. Overall serious risk of bias, especially related to selection bias. bDowngraded one level due to imprecision. Narrative synthesis was conducted with imprecise estimates based on fewer than 400 participants.
Primary outcomes
All‐cause mortality
Trinh 2020 reported in‐hospital mortality with a follow‐up of 35 days in 43.5% of the intervention group and 74.8% of the control group (P = 0.0003). Participants in the intervention group experienced reduced mortality: HR 0.43, (95% CI 0.23 to 0.78). In the subgroup of intubated participants with mechanical ventilation, mortality was 34.2% in the intervention group and 53% in the comparative group: adjusted HR 0.21, 95% CI 0.10 to 0.46.
Anticoagulants (therapeutic dose) may reduce all‐cause mortality compared with anticoagulants (prophylactic dose), but the certainty of evidence is low.
Necessity for additional respiratory support
There were no available data for this outcome.
Secondary outcomes
Mortality related to COVID‐19
There were no available data for this outcome.
Deep vein thrombosis (DVT)
There were no available data for this outcome.
Pulmonary embolism
There were no available data for this outcome.
Major bleeding
Trinh 2020 did not define major and minor bleeding, therefore, we considered their reported bleeding as major bleeding. They reported 51 (31.7%) events of bleeding in the intervention group and 17 (20.5%) in the comparator group (OR 1.80, 95% CI 0.96 to 3.37, P = 0.07).
Anticoagulants (therapeutic dose) may lead to no difference in major bleeding compared with anticoagulants (prophylactic dose), but the certainty of evidence is low.
Adverse events (minor bleeding, gastrointestinal adverse effects (e.g. nausea, vomiting, diarrhoea, abdominal pain), allergic reactions, renal failure and amputations)
Trinh 2020 reported stroke events (intervention: 6 (3.7%) and comparator: 5 (6%), P = 0.41); renal failure requiring dialysis (intervention: 67 (42.7%) and comparator: 25 (30.9%), P = 0.08); and liver failure (intervention: 3 (1.9%) and comparator: 2 (2.4%), P = 1.00). However, they did not report any of the adverse events of interest for this review.
Hospitalisation time in days
Trinh 2020 reported hospitalisation time as mean ± SD for the intervention (23.3 ± 7.7 days) and comparator (15.7 ± 8.9 days) groups (MD 7.6 days, 95% CI 5.35 to 9.85; P < 0.001).
There was low‐certainty evidence (244 participants, one retrospective NRS) that anticoagulants (therapeutic dose) may increase hospitalisation time compared with anticoagulants (prophylactic dose). We downgraded the certainty of evidence by one level due to study limitations because the overall risk of bias was serious, especially related to selection bias. We downgraded the certainty of evidence by one level due to imprecision. Narrative synthesis was conducted with imprecise estimates based on fewer than 400 participants.
Quality of life
There were no available data for this outcome.
Discussion
This review aimed to assess the effects of prophylactic anticoagulants versus active comparator, placebo or no intervention on mortality and need for additional respiratory support for people hospitalised with COVID‐19.
Summary of main results
We found no RCTs, no quasi‐RCTs, and no prospective NRS with available data assessing the effects of prophylactic anticoagulants compared to active comparator, placebo or no intervention on mortality and need for additional respiratory support for people hospitalised with COVID‐19.
We found 22 ongoing studies (from Australia (1), Brazil (1), Canada (2), China (3), France (2), Germany (1), Italy (4), Switzerland (1), UK (1), and USA (6)) that plan to evaluate 15,727 participants in this setting, of whom 14,730 are from 20 RCTs, and 997 are from one prospective NRS (120 estimated participants) and one retrospective NRS (877 estimated participants). See Table 5.
Twelve ongoing studies plan to report data for mortality. Six ongoing studies plan to report data for necessity for additional respiratory support. Thirteen ongoing studies are expected to be completed in December 2020 (6959 estimated participants), eight in July 2021 (8512 estimated participants), and one in December 2021 (256 estimated participants). Four of these ongoing studies plan to include 1000 participants or more.
One of the studies plans to compare aspirin, clopidogrel, rivaroxaban, atorvastatin, and omeprazole with no treatment in 3170 participants to assess mortality at 30 days of follow‐up. One study plans to compare a higher dose versus lower dose of prophylactic heparin to assess composite outcomes that include mortality in 1000 participants. One study plans to compare different doses of enoxaparin to assess venous thromboembolism in 2712 participants. Another study plans to compare therapeutic anticoagulation using heparin for 14 days with prophylactic anticoagulation to assess intubation and mortality in 3000 participants.
We found six retrospective NRS (5685 participants) with limited evidence of anticoagulants (all types) versus no treatment for people hospitalised with COVID‐19 (Table 4). The overall risk of bias for all‐cause mortality and for hospitalisation was critical and for major bleeding was serious in this comparison. Two studies reported reduction of mortality by odds ratio (reduction of 58% on chance of death) or hazard ratio (HR 0.86, 95% CI 0.82 to 0.89; 395 participants), both adjusted for confounding. Another study reported reduction of mortality only in a subgroup of severely ill participants, two studies reported no differences by unadjusted OR 1.66 (95% confidence interval (CI) 0.76 to 3.64) or adjusted risk ratio (RR) 1.15 (95% CI 0.29 to 2.57), and another study reported zero events in both intervention groups. One study reported 3% of bleeding events in the intervention group and 1.9% in the control group (OR 1.62, 95% CI 0.96 to 2.71). One study reported a median of 29 days of hospitalisation (IQR 17 to 42) in the intervention group and 27 days (IQR 24 to 31) in the control group (MD 2 days, 95% CI −0.80 to 4.80). See Table 1.
We found one retrospective NRS (244 participants) with limited evidence about anticoagulants (therapeutic dose) versus anticoagulants (prophylactic dose) for people hospitalised with COVID‐19. One study reported an absolute rate of death lower in the intervention group (34.2% versus 53%) and an adjusted HR 0.21 (95% CI 0.10 to 0.46) for confounding. One study reported 31.7% of bleeding events in the intervention group and 20.5% in the control group (OR 1.8, 95% CI 0.96 to 3.37). One study reported a mean increase of 7.6 days of hospitalisation (95% CI 5.35 to 9.85) in length of hospital stay. See Table 2.
Overall completeness and applicability of the evidence
While most of the studies reported our primary outcome of all‐cause mortality, we identified very little evidence relating to adverse effects of anticoagulants. It is also noteworthy that none of the studies measured our other primary outcome (necessity for additional respiratory support) or our secondary outcomes such as mortality related to COVID‐19, DVT, pulmonary embolism and quality of life.
There was substantial heterogeneity in the methods of the included studies and many of them did not provide complete and clear information about their data. This hindered the qualitative analyses and the assessment of the risk of bias of many outcomes in many studies.
The number of studies for each of the possible comparisons was small, ranging from one to six studies. Moreover, the included studies had small primary sample sizes, except for only two included studies that evaluated more than 2000 participants. The largest study involved 2773 participants treated with anticoagulation, but did not provide details about the type or dose of the pharmacological interventions. Another issue is the poor reporting quality of most of these studies, which directly affects data extraction and judgement of risk of bias.
There was considerable variation in the use of the same intervention (e.g. dosages, type, method of application). The variation of assessment for the confounding factor in NRS also impaired the results.
It is noteworthy that the studies included in this review were conducted in four different countries, most of which (75%) were high‐income countries. Social and cultural aspects of the evaluated interventions can also interfere with their acceptability and effectiveness for the treatment of people hospitalised with COVID‐19. Therefore, the external validity of the overall evidence presented in this review should be considered with caution.
We acknowledge that designing and conducting an appropriate study with available data for this topic is difficult. The new approach regarding prophylactic anticoagulants for people hospitalised with COVID‐19 has been used to provide high levels of anticoagulants for these people, although there is no available evidence based on RCTs or quasi‐RCTs to support their use. This reinforces the importance of this review and serves as an incentive for further investigation.
Certainty of the evidence
We found no RCTs, quasi‐RCTs or prospective NRS with available data that were eligible for this review, and we included only seven retrospective NRS.
Despite the increasing number of studies on prophylactic anticoagulants for people hospitalised with COVID‐19 in the past months, the overall risk of bias for all‐cause mortality and for hospitalisation in the comparison 'anticoagulants (all types) versus no treatment' was critical and in the comparison 'anticoagulants (therapeutic dose) versus anticoagulants (prophylactic dose)' was serious. The overall risk of bias for major bleeding was serious for the both comparisons with available data. We judged the bias domains due to confounding, selection of participants into the study, classification of interventions, deviations from the intended intervention, measurement of outcomes, and selection of the reported results from low to critical risk of bias. There was no information from three included studies for the all‐cause mortality assessment in the comparison 'anticoagulants (all types) versus no treatment'.
The certainty of evidence is low to very low. We downgraded the certainty of evidence due to risk of bias, particularly with regard to overall critical/serious risk of bias across studies, especially related to confounding or selection bias. We downgraded the certainty of evidence due to inconsistency and we decided not to pool data due to heterogeneity of studies (especially due to differences in the interventions). We also downgraded the certainty of evidence by one or two levels due to imprecision because the narrative synthesis was conducted with an imprecise estimate based on fewer than 400 participants (in some cases in very few participants).
It is very uncertain if anticoagulants (all types) compared with no treatment, reduce all‐cause mortality at 28 days after the intervention (5685 participants, 6 retrospective NRS), or have any effect on hospitalisation time (42 participants, 1 retrospective NRS, follow‐up not reported) because the certainty of evidence is very low for both outcomes. Anticoagulants (all types) may make no difference in major bleeding compared with no treatment, but the certainty of evidence is low (2773 participants, 1 retrospective NRS, follow‐up not reported). See Table 1.
Anticoagulants (therapeutic dose), compared with anticoagulants (prophylactic dose), may reduce all‐cause mortality, may make no difference in major bleeding or may increase hospitalisation time, but the certainty of evidence is low (244 participants, 1 retrospective NRS, follow‐up 35 days) for all these outcomes. See Table 2.
Potential biases in the review process
We performed a comprehensive search of the literature, and we performed study selection according to the Cochrane Handbook for Systematic Reviews of Interventions (Lefebvre 2020). We believe that we identified all the relevant studies that met our inclusion criteria. However, the possibility remains that we may have missed some studies, particularly in the grey literature. We adhered to the inclusion and exclusion criteria prespecified in the protocol in order to limit subjectivity (Flumignan 2020). We made efforts to obtain additional relevant data from study authors but were unable to do so. If we can source supplementary data, we will consider them in future updates. Two review authors selected studies in duplicate, independently, to reduce potential bias of the review process. One review author extracted data and assessed risk of bias of the included studies, and another review author checked the data extraction and 'Risk of bias' judgements, to accelerate the process and also to reduce potential bias of the review process.
Agreements and disagreements with other studies or reviews
A systematic review of 'potential rapid diagnostics, vaccine and therapeutics for COVID‐19' searched for published articles in PubMed, Embase and Cochrane Library, and found 27 studies for inclusion, but none of them regarding anticoagulants (Pang 2020).
A systematic review of 'therapeutic management of patients with COVID‐19' searched for studies published in English in Embase, MEDLINE and Google Scholar between 1 December 2019 and 31 March 2020, without any criteria regarding study design. Tobaiqy 2020 included 41 studies (three clinical studies, seven case reports, 10 case series, and 11 retrospective and 10 prospective observational studies) in their review. However, none of their included studies evaluated anticoagulants.
A systematic review of 'hypercoagulation and antithrombotic treatment in coronavirus 2019' searched for studies published in English in PubMed, ISI Web of Science, SCOPUS, and Cochrane Library on 28 March 2020, without any restrictions on publication date or publication status (Violi 2020). They excluded studies without a control group, animal studies, case reports, editorials, commentaries, letters, review articles, and guidelines from their analysis. No additional criteria for the included studies were described. Violi 2020 included nine NRS, which reported measures of clotting activation and their relationship with COVID‐19 clinical severity. However, no included study evaluated prophylactic anticoagulants for people hospitalised with COVID‐19.
Two narrative reviews regarding 'pharmacologic treatments for COVID‐19' and 'management of critically ill adults with COVID‐19' analysed several pharmacological interventions for the management of these people, but neither addressed prophylactic anticoagulants directly (Poston 2020; Sanders 2020).
In order to prevent microvascular thrombosis, some clinicians use higher‐dose anticoagulation rather than prophylactic dosing for inpatients with COVID‐19 (AVF 2020; Bikdeli 2020; Obe 2020). However, this practice is not supported by robust evidence. Although some practical guidelines address the management of prophylactic anticoagulation in people with COVID‐19, all of these recommendations are based on non‐COVID‐19 populations or low‐quality COVID‐19‐related evidence (AVF 2020; Bikdeli 2020; NHS 2020; Obe 2020; Ramacciotti 2020).
Authors' conclusions
Implications for practice
We found no randomised controlled trials (RCTs), no quasi‐RCTs, and no prospective non‐randomised studies (NRS) with available data addressing the effects of prophylactic anticoagulants on mortality and need for additional respiratory support for people hospitalised with COVID‐19. There is currently insufficient evidence to determine the risks and benefits of prophylactic anticoagulants for people hospitalised with COVID‐19; we found low‐ to very low‐certainty evidence from seven retrospective NRS.
Implications for research
High‐quality RCTs that compare prophylactic anticoagulants for people hospitalised with COVID‐19 are needed. Since there are 22 ongoing studies (20 RCTs) that plan to evaluate 15,727 participants in this setting, robust evidence may be available soon. Thirteen ongoing studies with an estimated 6959 participants, including one large RCT with 2712 participants comparing different doses of enoxaparin, are planned to be completed by the end of 2020. Other large RCTs, with an estimated 1000, 3000 and 3170 participants are planned to be completed by July 2021. From these three additional RCTs, two compare different doses of heparin (total of 4000 participants), and one compares oral anticoagulants and other drugs to no treatment (3170 participants). There is a need for RCTs with high methodological quality, that is, adequate reporting of randomisation, allocation concealment, blinding, assessing the effects on this population prospectively in an unconfounded randomised study of prophylactic anticoagulants for people hospitalised with COVID‐19.
The most notable outcomes to be measured are death and necessity for additional respiratory support. Other important issues to be considered are deep vein thrombosis, pulmonary embolism, major bleeding, adverse events, hospitalisation time, and quality of life.
History
Review first published: Issue 10, 2020
Acknowledgements
This review was published in collaboration with the Cochrane Editorial and Methods Department. We particularly thank Sarah Hodgkinson and Liz Bickerdike (Associate Editors), Clare Dooley (Managing Editor), Denise Mitchell (Copy Editor), Theresa Moore (Cochrane Methods Support Unit), Robin Featherstone (Information Specialist) and Leticia Rodrigues (Cochrane Editorial and Methods Department) for their methodological and editorial support. Many thanks to Teo Aminah Wasteneys Quay (Managing Editor, Cochrane Emergency and Critical Care), Harald Harkner (Co‐ordinating Editor, Cochrane Emergency and Critical Care) and Mike Brown (Network Senior Editor, Cochrane Acute and Emergency Care) for their support and contributions at various stages of the editorial process. Thanks to Analysis of Review Group Output (ARGO) for their comments on the Abstract and Plain Language Summary. Thanks to Vinicius T Civile (Cochrane Brazil), Division of Vascular and Endovascular Surgery, Universidade Federal de São Paulo, Brazil, for methodological support.
We would also like to thank Christopher D Barrett (Koch Institute, Massachusetts Institute of Technology, Cambridge MA, USA/Department of Surgery, Beth Israel Deaconess Medical Center, Harvard Medical School, Boston MA, USA) and Dimitrios Giannis (Institute of Health Innovations and Outcomes Research, The Feinstein Institutes for Medical Research, Manhasset NY, USA) for their peer review comments.
Appendices
Appendix 1. Planned methodology for randomised controlled trials (RCTs) and non‐randomised studies (NRS) of interventions
Types of studies
We planned to use the Cochrane Handbook for Systematic Reviews of Interventions to guide whole this review process (Higgins 2020a). To assess the effects of prophylactic anticoagulants for people hospitalised with COVID‐19 we had planned to include randomised controlled trials (RCTs) only, as such studies, if performed appropriately, currently give the best evidence for experimental therapies in highly controlled therapeutic settings.
In case of insufficient evidence (very low‐certainty evidence or no evidence) available from RCTs to answer this review's questions we had planned to include prospective controlled non‐randomised studies (NRS) of interventions, including quasi‐randomised controlled trials (e.g. assignment to treatment by alternation, medical register or by date of birth).
In case of insufficient evidence (very low‐certainty evidence or no evidence) available from RCTs, quasi‐RCTs, and prospective NRS, we planned to include retrospective observational studies with a control group.
As there was no evidence from RCTs, quasi‐RCTs, and prospective NRS, we included retrospective NRS and followed the methodology as specified in the protocol (Flumignan 2020).
Data extraction and management
Assessment of risk of bias in included studies
Randomised controlled trials
We planned for one review author (RLGF) to assess the risk of bias for each study, and another review author (LCUN) to check all judgements, using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2017) for RCTs (RoB1 tool). We planned to resolve any disagreements by consensus or by involving other review authors (CM, BT). For RCTs, we planned to assess the risk of bias according to the following domains.
Random sequence generation
Allocation concealment
Blinding of participants and personnel
Blinding of outcome assessment
Incomplete outcome data
Selective outcome reporting
Other bias
In cluster‐randomised trials, we planned to consider particular biases as recommended by section 8.15.1.1 of the Cochrane Handbook for Systematic Reviews of Interventions: 1) recruitment bias; 2) baseline imbalance; 3) loss of clusters; 4) incorrect analysis; and 5) comparability with individually randomised trials (Higgins 2017). We planned to grade each potential source of bias as high, low or unclear and provide a quote from the study report together with a justification for our judgement in the 'Risk of bias' table. We planned to summarise the 'Risk of bias' judgements across different studies for each of the domains listed. Where information on the risk of bias relates to unpublished data or correspondence with a study author, we planned to note this in the 'Risk of bias' table.
When considering treatment effects, we planned to take into account the risk of bias for the studies that contributed to that outcome.
We planned to base the overall bias judgement of included RCTs on the following three domains of RoB1 tool: 1) adequate sequence generation, 2) blinding of outcome assessors, and 3) selective outcome reporting. An RCT at low risk on all of these domains we planned to label as a low‐risk study. An RCT at high risk on one of these domains we planned to label as a high‐risk study. If there is no clear information on the risk of bias for one or more key domains, but the RCT is not at high risk for any domain, we planned to indicate that the risk of bias in the study is unclear.
Non‐randomised studies
Using the ROBINS‐I tool, we planned to assess the risk of bias of quasi‐RCTs and NRS based on the following seven domains (Sterne 2016).
Bias due to confounding
Bias in selection of participants into the study
Bias in classification of interventions
Bias due to deviations from the intended intervention
Bias due to missing data
Bias in measurement of outcomes
Bias in selection of the reported result
We planned to use our 'Risk of bias' judgements for quasi‐RCTs and NRS to label the outcomes, for each comparison, on these domains as 'critical risk', 'serious risk', 'moderate risk', 'low risk', or 'no information'. We planned to judge the overall risk of bias (across domains) as the worst judgment across all the domains.
Measures of treatment effect
Dichotomous data
For dichotomous variables, we planned to calculate the risk ratio (RR) and 95% confidence intervals (CIs).
Continuous data
For continuous data, we planned to calculate mean differences (MD) and 95% CIs between treatment groups where studies reported the same outcomes. Where similar outcomes are reported on different scales, we planned to calculate the standardised mean difference (SMD) and 95% CI. To interpret SMD, we planned to use the following thresholds.
SMD less than 0.2 = trivial or no effect
SMD equal to or greater than 0.2 and less than 0.5 = small effect
SMD equal to or greater than 0.5 and less than 0.8 = medium effect
SMD equal to or greater than 0.8 = large effect
Unit of analysis issues
We planned to seek advice from a statistician (Adriana Sanudo, Federal University of Sao Paulo, Brazil) to address issues relating to double‐counting, correlation or unit of analysis posed by the following.
Cluster‐RCTs
Episodes of disease
Multi‐arm studies
We planned for individuals to be our unit of analysis. If studies included multi‐arm interventions, we planned to consider only the arms relevant to the scope of our review.
Cluster‐randomised trials
We planned to include cluster‐randomised trials in the analyses along with individually RCTs. We planned to adjust their sample sizes using the methods described in Section 23.1.5 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2020b), using an estimate of the intracluster correlation coefficient (ICC) derived from the trial (if possible), from a similar trial, or a study of a similar population. If we used ICCs from other sources, we planned to report this and conduct sensitivity analyses to investigate the effect of variation in the ICC. If we identified both cluster‐randomised trials and individually randomised trials, we planned to synthesise the relevant information. We planned to consider it reasonable to combine the results from both types of studies if there is little heterogeneity between the study designs, and we consider the interaction between the effect of the intervention and the choice of randomisation unit to be unlikely. We also planned to acknowledge heterogeneity in the randomisation unit and perform a sensitivity analysis to investigate the effects of the randomisation unit.
Assessment of heterogeneity
We planned to inspect forest plots visually to consider the direction and magnitude of effects and the degree of overlap between confidence intervals. We planned to use the I² statistic (Higgins 2003), to measure heterogeneity among the studies in each analysis, but acknowledge that there is substantial uncertainty in the value of I² when there is only a small number of studies: we therefore also planned to consider the P value from the Chi² test. If we identify substantial heterogeneity, we planned to report it and explore possible causes by prespecified subgroup analysis.
As strict thresholds for interpretation of I² are not recommended, we intend to follow the rough guide to interpretation in the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2020).
0% to 40%: might not be important
30% to 60%: may represent moderate heterogeneity
50% to 90%: may represent substantial heterogeneity
75% to 100%: considerable heterogeneity
When I² lies in an area of overlap between two categories (e.g. between 50% and 60%), we planned to consider differences in participants and interventions among the studies contributing data to the analysis (Deeks 2020).
Data synthesis
We planned to use a fixed‐effect model for meta‐analysis when included studies are homogeneous (considering population, interventions, comparators and outcomes characteristics). We planned to use a random‐effects model if at least substantial heterogeneity is identified, or if significant clinical differences regarding participants and interventions exist among included studies.
In preparation for synthesis (either meta‐analyses or synthesis without meta‐analysis), we planned to assess how much data are available for each of our comparisons by the following.
Table to compare PICO elements/study design features
Conversion of numerical data for meta‐analysis
Forest plots
Qualitative synthesis
Synthesis without meta‐analysis
Appendix 2. CENTRAL (Cochrane Library) search strategy
#1(2019 novel coronavirus infection) or (COVID‐19 pandemic) or (coronavirus disease‐19) or (COVID19) or (2019 novel coronavirus disease) or (coronavirus disease 2019) or COVID‐19
#2MeSH descriptor: [Severe Acute Respiratory Syndrome] explode all trees
#3(Wuhan coronavirus) or (Wuhan seafood market pneumonia virus) or (COVID19 virus) or (COVID‐19 virus) or (coronavirus disease 2019 virus) or (SARS‐CoV‐2) or (SARS2) or (2019 novel coronavirus)
#4MeSH descriptor: [Coronavirus] explode all trees
#5Coronavirus* or Deltacoronavirus* or Deltacoronavirus*
#6#1 OR #2 OR #3 OR #4 OR #5
#7MeSH descriptor: [Antithrombins] explode all trees
#8(Direct Thrombin Inhibitor*) or (Direct Antithrombin*) or (thrombin inhibitor)
#9MeSH descriptor: [Coumarins] explode all trees
#10Coumarin* or (Benzopyran 2 ones) or (Coumarin Derivative*)
#11MeSH descriptor: [Dabigatran] explode all trees
#12Pradaxa or (Dabigatran Etexilate) or (Dabigatran Etexilate Mesylate)
#13MeSH descriptor: [Anticoagulants] explode all trees #14(Anticoagulation Agent*) or (Anticoagulant Drug*) or Anticoagulant* or (Indirect Thrombin Inhibitor*) #15MeSH descriptor: [Heparin] explode all trees #16(Unfractionated Heparin) or (Heparinic Acid) or Liquaemin or (Sodium Heparin) or alpha‐Heparin or (alpha Heparin) or UFH or heparin* #17MeSH descriptor: [Fondaparinux] explode all trees #18(Fondaparinux Sodium) or Quixidar or Arixtra #19MeSH descriptor: [Hirudin Therapy] explode all trees #20Leeching or Hirudin* #21MeSH descriptor: [Phenindione] explode all trees #22Phenylindanedione or Phenyline or Pindione or Fenilin or Dindevan #23MeSH descriptor: [Polysaccharides] explode all trees #24Glycans #25MeSH descriptor: [Rivaroxaban] explode all trees #26Xarelto or Rivaroxaban #27MeSH descriptor: [Warfarin] explode all trees #28Apo‐Warfarin or Aldocumar or Gen‐Warfarin or Warfant or Coumadin* or Marevan or Tedicumar or warfarin* #29MeSH descriptor: [Factor Xa Inhibitors] explode all trees #30(factor Xa inhibitor*) #31MeSH descriptor: [Enoxaparin] explode all trees #32Enoxaparin* or Lovenox or Clexane #33reviparin* or Clivarine or reviparin‐sodium or (reviparin sodium) or Clivarin #34MeSH descriptor: [Dalteparin] explode all trees #35Tedelparin or (Dalteparin Sodium) or Fragmin or Fragmine #36danaproid or Orgaran or Lomoparan or (danaparoid sodium) or (danaproid sodium) or danaparoid* or DOAC or embolex or Liquemine or (oral anticoagulants) or Pentasaccharide* or (vitamin k antagonist) or Savaysa or (edoxaban tosylate) or edoxaban or xi‐melagatran or Exanta #37MeSH descriptor: [Phenprocoumon] explode all trees #38Phenylpropylhydroxycumarinum or Phenprocoumalol or Phenprocoumarol or Phenprogramma or Marcoumar or Marcumar or Falithrom or Liquamar or Oligosaccharides or (idraparinux sodium) #39MeSH descriptor: [Tinzaparin] explode all trees #40(Tinzaparin Sodium) or Innohep #41MeSH descriptor: [Heparin, Low‐Molecular‐Weight] explode all trees #42(Heparin Low Molecular Weight) or LMWH or (Low‐Molecular‐Weight Heparin) or parnaparin or Azetidines or Benzylamines #43MeSH descriptor: [Nadroparin] explode all trees #44Nadroparin* or Fraxiparin*#45MeSH descriptor: [Acenocoumarol] explode all trees #46Nicoumalone or Acenocoumarin or Sinthrome or Synthrom or Syncoumar or Syncumar or Sinkumar or Sintrom or Mini‐Sintrom or (Mini Sintrom) or MiniSintrom or Lactones or Pyridines #47#7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28 OR #29 OR #30 OR #31 OR #32 OR #33 OR #34 OR #35 OR #36 OR #37 OR #38 OR #39 OR #40 OR #41 OR #42 OR #43 OR #44 OR #45 OR #46 #48#6 AND #47 #49#48 AND trials
Appendix 3. MEDLINE (PubMed) search strategy
1
"COVID‐19" [Supplementary Concept] or (2019 novel coronavirus infection) or (2019‐nCoV infection) or (COVID‐19 pandemic) or (coronavirus disease‐19) or (2019‐nCoV disease) or (COVID19) or (2019 novel coronavirus disease) or (coronavirus disease 2019) or COVID‐19
2
"severe acute respiratory syndrome coronavirus 2" [Supplementary Concept] or (Wuhan coronavirus) or (Wuhan seafood market pneumonia virus) or (COVID19 virus) or (COVID‐19 virus) or (coronavirus disease 2019 virus) or (SARS‐CoV‐2) or (SARS2) or (2019‐nCoV) or (2019 novel coronavirus)
3
"Coronavirus"[Mesh] or Coronavirus* or Deltacoronavirus*
4
1‐3 / OR
5
"Antithrombins"[Mesh] or (Direct Thrombin Inhibitor*) or (Direct Antithrombin*) or (thrombin inhibitor)
6
"Coumarins"[Mesh] or Coumarin* or (1,2‐Benzopyrone Derivative*) or (1,2 Benzopyrone Derivative*) or Benzopyran‐2‐ones or (Benzopyran 2 ones) or (Coumarin Derivative*) or (1,2‐Benzopyrones) or (1,2 Benzopyrones) or (1,2‐Benzo‐Pyrones) or (1,2 Benzo Pyrones)
7
"Dabigatran"[Mesh] or Pradaxa or Dabigatran*
8
"Anticoagulants"[Mesh] or Anticoagulant* or (Indirect Thrombin Inhibitor*)
9
"Heparin"[Mesh] or (Unfractionated Heparin) or (Heparinic Acid) or Liquaemin or (Sodium Heparin) or alpha‐Heparin or (alpha Heparin) or UFH or heparin*
10
"Fondaparinux"[Mesh] or (Fondaparinux Sodium) or Quixidar or Arixtra
11
"Hirudin Therapy"[Mesh] or Leeching or Hirudin*
12
"Phenindione"[Mesh] or Phenylindanedione or Phenyline or Pindione or Fenilin or Dindevan
13
"Polysaccharides"[Mesh] or Glycans
14
"Rivaroxaban"[Mesh] or Xarelto or Rivaroxaban
15
"Warfarin"[Mesh] or Apo‐Warfarin or Aldocumar or Gen‐Warfarin or Warfant or Coumadin* or Marevan or Tedicumar or warfarin*
16
"Factor Xa Inhibitors" [Pharmacological Action] or (factor Xa inhibitor*)
17
"Enoxaparin"[Mesh] or Enoxaparin* or Lovenox or Clexane
18
"reviparin" [Supplementary Concept] or reviparin* or Clivarine or reviparin‐sodium or (reviparin sodium) or Clivarin
19
"Dalteparin"[Mesh] or Tedelparin or (Dalteparin Sodium) or Fragmin*
20
"danaparoid" [Supplementary Concept] or danaproid* or Orgaran or Lomoparan or danaparoid*
21
DOAC or embolex or Liquemine or (oral anticoagulants) or Pentasaccharide* or (vitamin k antagonist)
22
"edoxaban" [Supplementary Concept] or Savaysa or (edoxaban tosylate) or edoxaban
23
"ximelagatran" [Supplementary Concept] or xi‐melagatran or Exanta
24
"Phenprocoumon"[Mesh] or Phenylpropylhydroxycumarinum or Phenprocoumalol or Phenprocoumarol or Phenprogramma or Marcoumar or Marcumar or Falithrom or Liquamar
25
"idrabiotaparinux" [Supplementary Concept] or (Biotin/analogs and derivatives) or Oligosaccharides
26
"idraparinux" [Supplementary Concept] or (idraparinux sodium)
27
"Tinzaparin"[Mesh] or (Tinzaparin Sodium) or Innohep
28
"Heparin, Low‐Molecular‐Weight"[Mesh] or (Heparin Low Molecular Weight) or LMWH or (Low‐Molecular‐Weight Heparin) or parnaparin
29
"melagatran" [Supplementary Concept] or Azetidines or Benzylamines
30
"Nadroparin"[Mesh] or Nadroparin* or Fraxiparin or Fraxiparine
31
"Acenocoumarol"[Mesh] or Nicoumalone or Acenocoumarin or Sinthrome or Synthrom or Syncoumar or Syncumar or Sinkumar or Sintrom or Mini‐Sintrom or (Mini Sintrom) or MiniSintrom
32
"vorapaxar" [Supplementary Concept] or Lactones or Pyridines
33
5‐32 / OR
34
4 AND 33
Appendix 4. Embase (Wiley) search strategy
1 ('coronavirus disease 2019'/exp or (2019 novel coronavirus infection) or (COVID‐19 pandemic) or (coronavirus disease‐19) or (COVID19) or (2019 novel coronavirus disease) or (coronavirus disease 2019) or COVID‐19 OR 'Severe acute respiratory syndrome coronavirus 2'/exp OR (Wuhan coronavirus) or (Wuhan seafood market pneumonia virus) or (COVID19 virus) or (COVID‐19 virus) or (coronavirus disease 2019 virus) or (SARS‐CoV‐2) or (SARS2) or (2019 novel coronavirus) OR 'Coronavirus infection'/exp OR Coronavirus* or Deltacoronavirus* or Deltacoronavirus*) AND ('antithrombin'/exp OR (Direct Thrombin Inhibitor*) or (Direct Antithrombin*) or (thrombin inhibitor) OR 'coumarin derivative'/exp OR Coumarin* or (Benzopyran 2 ones) or (Coumarin Derivative*) OR 'dabigatran'/exp OR Pradaxa or (Dabigatran Etexilate) or (Dabigatran Etexilate Mesylate) OR 'anticoagulant agent'/exp OR (Anticoagulation Agent*) or (Anticoagulant Drug*) or Anticoagulant* or (Indirect Thrombin Inhibitor*) OR 'heparin derivative'/exp OR (Unfractionated Heparin) or (Heparinic Acid) or Liquaemin or (Sodium Heparin) or alpha‐Heparin or (alpha Heparin) or UFH or heparin* OR 'fondaparinux'/exp OR (Fondaparinux Sodium) or Quixidar or Arixtra OR 'anticoagulant therapy'/exp OR Hirudins or Leeching or Hirudin* OR 'phenindione'/exp OR Phenylindanedione or Phenyline or Pindione or Fenilin or Dindevan OR 'polysaccharide'/exp OR Glycans OR 'rivaroxaban'/exp OR Xarelto or Rivaroxaban OR 'warfarin'/exp OR Apo‐Warfarin or Aldocumar or Gen‐Warfarin or Warfant or Coumadin* or Marevan or Tedicumar or warfarin* OR 'blood clotting factor 10a inhibitor'/exp OR (factor Xa inhibitor*) OR 'enoxaparin'/exp OR Enoxaparin* or Lovenox or Clexane OR reviparin* or Clivarine or reviparin‐sodium or (reviparin sodium) or Clivarin OR 'dalteparin'/exp OR Tedelparin or (Dalteparin Sodium) or Fragmin* OR danaproid or Orgaran or Lomoparan or or danaparoid* or DOAC or embolex or Liquemine or (oral anticoagulants) or Pentasaccharide* or (vitamin k antagonist) or Savaysa or (edoxaban tosylate) or edoxaban or xi‐melagatran or Exanta OR 'phenprocoumon h 3'/exp OR Phenylpropylhydroxycumarinum or Phenprocoumalol or Phenprocoumarol or Phenprogramma or Marcoumar or Marcumar or Falithrom or Liquamar or Oligosaccharides or (idraparinux sodium) OR 'tinzaparin'/exp OR (Tinzaparin Sodium) OR 'low molecular weight heparin'/exp OR (Heparin Low Molecular Weight) or LMWH or (Low‐Molecular‐Weight Heparin) or parnaparin or Azetidines or Benzylamines OR 'nadroparin'/exp OR Nadroparin* or Fraxiparin or Fraxiparine OR 'acenocoumarol'/exp OR Nicoumalone or Acenocoumarin or Sinthrome or Synthrom or Syncoumar or Syncumar or Sinkumar or Sintrom or Mini‐Sintrom or (Mini Sintrom) or MiniSintrom or Lactones or Pyridines) 2 #1 AND [embase]/lim NOT ([embase]/lim AND [medline]/lim)
Appendix 5. LILACS and IBECS (Virtual Health Library) search strategy
tw:((tw:(mh: "Coronavirus Infections" OR mh: "Infecciones por Coronavirus" OR mh: "Infecções por Coronavirus" OR covid‐19 OR (coronavirus infection*) OR mers OR (middle east respiratory syndrome) OR (novel coronavirus pneumonia) OR (wuhan seafood market pneumonia) OR (brote por el nuevo coronavirus 2019) OR (brote por el coronavirus de wuhan) OR (epidemia de neumonía por coronavirus de wuhan) OR (síndrome respiratório de oriente medio) OR (síndrome respiratorio de oriente medio por coronavirus) OR (epidemia de pneumonia por coronavirus de wuhan) OR (epidemia de pneumonia por coronavírus de wuhan) OR (epidemia de pneumonia por coronavírus de wuhan de 2019‐2020) OR mh: betacoronavirus OR (2019 new coronavirus) OR (2019 novel coronavirus) OR betacoronavirus* OR sars‐cov‐2 OR (severe acute respiratory syndrome coronavirus 2) OR (wuhan coronavirus) OR (wuhan seafood market pneumonia virus) OR (coronavirus de wuhan) OR (coronavirus del síndrome respiratorio agudo grave 2) OR (nuevo coronavirus 2019) OR (virus de la neumonía del mercado de pescado y marisco de wuhan) OR (wuhan coronavirus) OR (coronavírus da síndrome respiratória aguda grave 2) OR (coronavírus de wuhan) OR (vírus de pneumonia no mercado de frutos do mar de wuhan) OR mh: coronavirus OR (coronavirus* rabbit) OR coronavirus* OR deltacoronavirus* OR (coronavirus del conejo) OR (coronavirus do coelho))) AND (tw:(tw:((tw:(mh: antithrombins OR mh: antitrombinas OR (direct antithrombins) OR (direct thrombin inhibitors) OR (antitrombinas directas) OR (antitrombinas diretas) OR d27.505.519.389.745.800.449 OR d27.505.954.502.119.500)) OR (tw:(mh: coumarins OR mh: cumarinas OR mh: cumarínicos OR (coumarin derivative*) OR coumarin* OR cumarina* OR d03.383.663.283.446 OR d03.633.100.150.446)) OR (tw:(mh: dabigatran OR mh: dabigatrán OR mh: dabigatrana OR (dabigatran* etexilat*) OR (dabigatran etexilate mesylate) OR pradaxa OR (etexilato de dabigatrana) OR d03.383.725.192 OR d03.633.100.103.280)) OR (tw:(mh: anticoagulants OR mh: anticoagulantes OR (agent* anticoagulant*) OR anticoagulant* OR (anticoagulant drug*) OR (anticoagulation agents) OR (indirect thrombin inhibitor*) OR (agentes anticoagulantes) OR (agentes de anticoagulación) OR anticoagulante*)) OR (tw:(mh: heparin OR mh: heparina OR (heparin sodium) OR (heparin unfractionated) OR (heparinic acid) OR liquaemin OR (alpha heparin) OR alpha‐heparin OR alfa‐heparina OR (ácido heparínico) OR (heparina alfa) OR heparina‐alfa)) OR (tw:(mh: fondaparinux OR arixtra OR (fondaparinux sodium) OR quixidar OR (fondaparinux sódico))) OR (tw:(mh: "Hirudin Therapy" OR mh: "Terapia con Hirudina" OR mh: "Terapia com Hirudina")) OR (tw:(mh: phenindione OR mh: fenindiona OR dindevan OR fenilin OR phenylindanedione OR phenyline OR pindione OR d02.455.426.559.847.486.487.750 OR d04.615.486.487.750)) OR (tw:(mh: polysaccharides OR mh: polisacáridos OR mh: polissacarídeos OR glycans OR glican*)) OR (tw:(mh: rivaroxaban OR mh: rivaroxabán OR mh: rivaroxabana OR xarelto OR d02.886.778.727 OR d03.383.533.640.713 OR d03.383.903.727)) OR (tw:(mh: warfarin OR mh: warfarina OR mh: varfarina OR aldocumar OR apo‐warfarin OR coumadin OR coumadine OR gen‐warfarin OR marevan OR tedicumar OR warfant OR (warfarin potassium) OR (warfarin sodium) OR d03.383.663.283.446.520.914 OR d03.633.100.150.446.520.914)) OR (tw:(mh: "Factor Xa Inhibitors" OR mh: "Inhibidores del Factor Xa" OR mh: "Inibidores do Fator Xa" OR (anticoagulant* direct‐acting oral) OR (direct acting oral anticoagulant*) OR (direct factor xa inhibitor*) OR d27.505.519.389.745.800.449.500 OR d27.505.954.502.119.500.500 OR (anticoagulantes orales de acción directa) OR (inhibidor del factor xa) OR (inhibidores directos del factor xa) OR (anticoagulantes orais de ação direta) OR (inibidor do fator xa) OR (inibidores diretos do fator xa))) OR (tw:(mh: enoxaparin OR mh: enoxaparin* OR clexane OR lovenox)) OR (tw:(mh: dalteparin OR mh: dalteparina OR (dalteparin sodium) OR fragmin* OR tedelparin*)) OR (tw:(doac OR embolex OR liquemine OR (oral anticoagulants) OR pentasaccharide* OR (vitamin k antagonist) OR savaysa OR (edoxaban tosylate) OR edoxaban OR xi‐melagatran OR exanta OR danaproid* OR orgaran OR lomoparan OR danaparoid* OR reviparin* OR clivarine OR reviparin‐sodium OR (reviparin sodium) OR clivarin OR azetidines OR benzylamines OR lactones OR pyridines)) OR (tw:(mh: phenprocoumon OR mh: fenprocumón OR mh: femprocumona OR falithrom OR liquamar OR marcoumar OR marcumar OR phenprocoumalol OR phenprocoumarol OR phenprogramma OR phenylpropylhydroxycumarinum OR d03.383.663.283.446.520.750 OR d03.633.100.150.446.520.750 OR fenilpropilhidroxicumarina OR fenprocumalol OR fenprocumarol OR femprocumalol OR femprocumarol OR fenilpropilidroxicumarina OR (feno procumarol) OR fenoprocumalol OR fenoprocumona)) OR (tw:(mh: tinzaparin OR mh: tinzaparina OR innohep OR (tinzaparin sodium) OR (tinzaparina sódica))) OR (tw:(mh: "Heparin, Low‐Molecular‐Weight" OR mh: "Heparina de Bajo‐Peso‐Molecular" OR mh: "Heparina de Baixo Peso Molecular" OR (heparin low molecular weight) OR lmwh OR (low molecular weight heparin) OR (low‐molecular‐weight heparin) OR hbpm)) OR (tw:(mh: nadroparin OR mh: nadroparina OR (calcium nadroparin) OR fraxiparin* OR nadroparin*)) OR (tw:(mh: acenocoumarol OR mh: acenocumarol OR acenocoumarin OR (mini sintrom) OR mini‐sintrom OR minisintrom OR nicoumalone OR sinkumar OR sinthrome OR sintrom* OR syncoumar OR syncumar OR synthrom OR d03.383.663.283.446.520.079 OR d03.633.100.150.446.520.079 OR acenocumarina OR nicumalon*)))))) AND ( db:("LILACS" OR "IBECS"))
Appendix 6. Cochrane COVID‐19 search strategy
Anticoagulant* or Heparin* or Rivaroxaban or Warfarin or Enoxaparin or DOAC or LMWH
Appendix 7. medRxiv search strategy
Anticoagulant OR anticoagulants OR Heparin OR Rivaroxaban OR Warfarin OR Enoxaparin OR DOAC OR LMWH
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ayerbe 2020.
| Study characteristics | ||
| Methods |
|
|
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
|
|
| Outcomes | There is no differentiation between primary and secondary outcomes.
|
|
| Notes |
|
|
Liu 2020.
| Study characteristics | ||
| Methods |
|
|
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
|
|
| Outcomes | There is no differentiation between primary and secondary outcomes.
|
|
| Notes |
|
|
Paranjpe 2020.
| Study characteristics | ||
| Methods |
|
|
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
|
|
| Outcomes | There is no differentiation between primary and secondary outcomes.
|
|
| Notes |
|
|
Russo 2020.
| Study characteristics | ||
| Methods |
|
|
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
|
|
| Outcomes | There is no a differentiation between primary and secondary outcomes.
|
|
| Notes |
|
|
Shi 2020.
| Study characteristics | ||
| Methods |
|
|
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
|
|
| Outcomes | There is no differentiation between primary and secondary outcomes.
|
|
| Notes |
|
|
Tang 2020.
| Study characteristics | ||
| Methods |
|
|
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
|
|
| Outcomes | There is no differentiation between primary and secondary outcomes.
|
|
| Notes |
|
|
Trinh 2020.
| Study characteristics | ||
| Methods |
|
|
| Participants |
Inclusion criteria
Exclusion criteria
|
|
| Interventions |
|
|
| Outcomes | There is no a differentiation between primary and secondary outcomes.
|
|
| Notes |
|
|
ARDS: acute respiratory distress syndrome; COI: conflict of interest; CPR: cardiopulmonary resuscitation; DOACS: direct oral anticoagulants; FiO2: fractional inspired oxygen; GFR: glomerular filtration rate; HIT: heparin‐induced thrombocytopenia; ICU: intensive care unit; IQR: interquartile range; IU: international unit; IV: intravenous(ly); LMWH: low molecular weight heparin; NR: not reported; PaO2: arterial blood oxygen partial pressure; PCR: polymerase chain reaction; SC: subcutaneous(ly); SD: standard deviation; UFH: unfractionated heparin; ULN: upper limit of normal; VKA: vitamin K antagonists; VTE: venous thromboembolism
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Al‐Samkari 2020 | Irrelevant study design. Retrospective cohort study without a parallel comparator group of intervention |
| Artifoni 2020 | Irrelevant study design. Retrospective cohort study without a comparator group (single‐arm study) |
| EudraCT2020‐001823‐15 | Irrelevant study design. Prospective cohort study without a comparator group (single‐arm study). |
| Helms 2020 | Irrelevant study design. Prospective cohort study without an intervention purpose |
| Khider 2020 | Irrelevant study design. Prospective cohort study without a parallel comparator group of intervention |
| NCT04354155 | Irrelevant study design. Prospective cohort study without a comparator group (single‐arm study) |
| NCT04359212 | Irrelevant study design. Prospective cohort study without a parallel comparator group of intervention |
| NCT04365309 | Irrelevant intervention. RCT of aspirin for COVID‐19. There is no difference between the intervention groups regarding anticoagulants. |
| NCT04368377 | Irrelevant study design. Prospective cohort study without a comparator group (single‐arm study). |
| NCT04394000 | Irrelevant study design. Prospective before‐after cohort study without a parallel comparator group |
| NCT04427098 | Irrelevant study design. Prospective cohort study without a comparator group (single‐arm study) |
| Zhang 2020 | Irrelevant study design. Retrospective cases series. Description of 7 participants without a consistent comparator group |
RCT: randomised controlled trial
Characteristics of ongoing studies [ordered by study ID]
ACTRN12620000517976.
| Study name | A randomised controlled trial of nebulised heparin in critically ill mechanically ventilated patients with COVID‐19 to assess the effect on the duration of mechanical ventilation |
| Starting date | 21 May 2020 |
| Contact information | Barry Dixon St Vincent’s Hospital, Melbourne, Australia +613439618815 | barry.dixon@svha.org.au |
| Methods | Multicenter, prospective, randomised controlled, 2‐armed, parallel assignment study |
| Participants | 172 participants, ≥ 18 years, female and male Inclusion criteria
Exclusion criteria
|
| Interventions | Experimental: nebulised (vibrating mesh nebuliser) heparin sodium 25,000 IU in 5 mL 6‐hourly to day 10 while invasively ventilated in addition to standard care. The medication will be prescribed and administration documented in the medical record. Comparator: standard care represents the treatments routinely provided by the medical team managing the patient. Standard care will be at the discretion of the medical team. |
| Outcomes | Primary
Secondary
|
| Notes | ACTRN12620000517976p | No data provided |
ChiCTR2000030700.
| Study name | An evaluative clinical study: efficacy and safety of Prolongin (enoxaparin sodium injection) in treatment of hospitalized adult patients with common novel coronavirus pneumonia (COVID‐19) |
| Starting date | 09 March 2020 |
| Contact information | Zhang Yu Union Hospital affiliated to Tongji Medical College of Huazhong University of Science and Technology, Wuhan, Hubei, China +86 13901849660 | whxhzy@163.com |
| Methods | Prospective RCT; open label, 1:1; 2‐armed, parallel‐assignment study |
| Participants | 60 participants, ≥ 18 years, female and male Inclusion criteria
Exclusion criteria
|
| Interventions | Experimental: based on the standard treatment recommended in the guidelines, a combination of Prolongin (enoxaparin sodium injection) was used Comparator: follow the guidelines for standard treatment |
| Outcomes | Primary
Secondary
|
| Notes | ChiCTR2000030700 | No data provided |
ChiCTR2000030701.
| Study name | A randomized, parallel controlled open‐label trial to evaluate the efficacy and safety of Prolongin (enoxaparin sodium injection) in adult hospitalized patients with novel coronavirus pneumonia (COVID‐19) |
| Starting date | 10 March 2020 |
| Contact information | Cai Qingxian The Third People's Hospital of Shenzhen, Shenzhen, Guangdong, China +86 13901849660 | 41180423@qq.com |
| Methods | Single‐centre, open‐label, 2‐armed, parallel assignment, RCT |
| Participants | 60 participants, ≥ 18 years, female and male Inclusion criteria
Exclusion criteria
|
| Interventions | Experimental: based on the standard treatment recommended in the guidelines, a combination of Prolongin (enoxaparin sodium injection) was used Comparison: follow the guidelines for standard treatment |
| Outcomes | Primary
Secondary
|
| Notes | ChiCTR2000030701 | No data provided |
ChiCTR2000030946.
| Study name | Effects of different VTE prevention methods on the prognosis of hospitalized patients with novel coronavirus pneumonia (COVID‐19) |
| Starting date | 10 February 2020 |
| Contact information | Chunli Liu The First Affiliated Hospital of Guangzhou Medical University, Guangzhou, Guangdong, China +86 13560158649 | chunli@gird.cn |
| Methods | Prospective cohort, non‐randomised, open‐label, two parallel and comparative arms |
| Participants | 120 participants, 18‐80 years, female and male Inclusion criteria
Exclusion criteria
|
| Interventions | Experimental: 7/5000 LMWH therapy Comparison: mechanical prevention |
| Outcomes | Primary: biochemical indicators Secondary: not described |
| Notes | ChiCTR2000030946 | No data provided |
Marietta 2020.
| Study name | Randomised controlled trial comparing high versus low LMWH dosages in hospitalized patients with severe COVID‐19 pneumonia and coagulopathy not requiring invasive mechanical ventilation |
| Starting date | 1 June 2020 |
| Contact information | Marco Marietta, MD Azienda Ospedaliero‐Universitaria di Modena, Italy 0594224640 ext +39 | marco.marietta@unimore.it |
| Methods | Multicentre, open‐label, investigator‐sponsored, two arms, parallel‐assignment, RCT |
| Participants | 300 participants, 18‐80 years, female and male Inclusion criteria (all required)
Exclusion criteria
|
| Interventions | Experimental: high‐dose LMWH: 70 IU/kg twice daily, other name: Inhixa Comparator: low‐dose LMWH: enoxaparin 4000 IU daily |
| Outcomes | Primary
Secondary
|
| Notes | NCT04408235 | EudraCT 2020‐001972‐13 | No data provided |
NCT04333407.
| Study name | Preventing cardiac complication of COVID‐19 disease with early acute coronary syndrome therapy: a randomised controlled trial |
| Starting date | 3 April 2020 |
| Contact information | Alena Marynina Charing Cross Hospital, London, UK 07776 224520 | alena.marynina@nhs.net |
| Methods | Multicentre RCT with 2 parallels arms, 1:1, open label |
| Participants | 3170 participants, ≥ 18 years, female and male Inclusion criteria
Exclusion criteria
|
| Interventions | Experimental: active arm
Comparator: no intervention |
| Outcomes | Primary
Secondary
|
| Notes | NCT04333407 | No data provided |
NCT04344756.
| Study name | Cohort multiple randomized controlled trials open‐label of immune modulatory drugs and other treatments in COVID‐19 patients CORIMUNO‐COAG trial |
| Starting date | 20 April 2020 |
| Contact information | Tristan Mirault Assistance Publique ‐ Hôpitaux de Paris, France 1 56 09 50 41 ext 33 | tristan.mirault@aphp.fr |
| Methods | Randomised clinical trial with 2 parallel arms, 1:1, stratified on disease severity (ventilation or not) |
| Participants | 808 participants, ≥ 18 years, female and male Inclusion criteria
Exclusion criteria
|
| Interventions | Experimental: tinzaparin or UFH
Comparator: standard of care
|
| Outcomes | Primary
Secondary
|
| Notes | NCT04344756 | APHP200389‐6 | No data provided |
NCT04345848.
| Study name | Preventing COVID‐19‐associated thrombosis, coagulopathy and mortality with low‐ and high‐dose anticoagulation: a randomized, open‐label clinical trial |
| Starting date | 28 April 2020 |
| Contact information | Marc Blondon University Hospital, Geneva, Switzerland +41.22.372.92.92 | marc.blondon@hcuge.ch |
| Methods | Multicenter, prospective, single‐blind (outcomes assessor), 2‐armed, parallel‐assignment, RCT |
| Participants | 200 participants, ≥ 18 years, female and male Inclusion criteria Adult patient with COVID‐19 infections, admitted to:
Exclusion criteria
|
| Interventions | Experimental: therapeutic anticoagulation Participants will be treated with therapeutic doses of SC LMWH (enoxaparin) or IV UFH, from admission until the end of hospital stay or clinical recovery. Comparator: prophylactic anticoagulation Participants will be treated with prophylactic doses of SC LMWH (enoxaparin) or UFH, from admission until the end of hospital stay or clinical recovery. If hospitalised in the ICU, they will receive an augmented thromboprophylaxis regimen as standard of care. |
| Outcomes | Primary
Secondary
Other outcome
|
| Notes | NCT04345848 | No data provided |
NCT04352400.
| Study name | RAndomized clinical trial in COvid19 patients to assess the efficacy of the transmembrane protease serine 2 (TMPRSS2) inhibitor NAfamostat (RACONA Study) |
| Starting date | 1 April 2020 |
| Contact information | Gian Paolo Rossi University Hospital Padova, Italy 00390498217821 | gianpaolo.rossi@unipd.it |
| Methods | Multicentre, double‐blind, 2‐armed, parallel‐assignment RCT |
| Participants | 256 participants, 18‐85 years, female and male Inclusion criteria
Exclusion criteria
|
| Interventions | Experimental: nafamostat mesilate, administered IV as a continuous infusion Comparator: placebo, administered IV as a continuous infusion |
| Outcomes | Primary
Secondary
|
| Notes | NCT04352400 | No data provided |
NCT04359277.
| Study name | A randomized trial of anticoagulation strategies in COVID‐19 |
| Starting date | 21 April 2020 |
| Contact information | Jeffrey Berger NYU Langone Health, New York, USA 212‐263‐4004 | PROTECT.COVID19@nyulangone.org |
| Methods | Open‐label, 2‐armed, parallel‐assignment, RCT |
| Participants | 1000 participants, ≥ 18 years, female and male Inclusion criteria
Exclusion criteria
|
| Interventions | Experimental: higher‐dose anticoagulation Drug: enoxaparin higher dose
For enoxaparin, antiXA testing will be done after fourth injection only for participants with BMI > 40 or weight > 150 kg as per institutional policy Comparator: lower‐dose prophylactic anticoagulation Drug: lower‐dose prophylactic anticoagulation
For enoxaparin, antiXA testing will be done after fourth injection only for participants with BMI > 40 or weight > 150 kg as per institutional policy. For participants who develop AKI, and received enoxaparin, transition to IV UFH by checking antiXa when next dose of enoxaparin would be due and initiating IV heparin when antiXa < 0.7 IU/mL |
| Outcomes | Primary
Secondary
|
| Notes | NCT04359277 | No data provided |
NCT04360824.
| Study name | COVID‐19‐associated coagulopathy: safety and efficacy of prophylactic anticoagulation therapy in hospitalized adults with COVID‐19 |
| Starting date | 6 May 2020 |
| Contact information | Usha Perepu University of Iowa, Iowa City, Iowa, USA 319‐356‐2195 | usha‐perepu@uiowa.edu |
| Methods | Multicentre, open‐label, 2‐armed, parallel‐assignment RCT |
| Participants | 170 participants, ≥ 18 years, female and male Inclusion criteria
Exclusion criteria
|
| Interventions | Interventional: intermediate‐dose enoxaparin (1 mg/kg SC daily if BMI < 30 kg/m2 or 0.5 mg/kg SC twice daily if BMI ≥ 30 kg/m2) Comparator: standard of care. Standard prophylactic dose enoxaparin (40 mg SC daily if BMI < 30 kg/m2 and 30 mg SC twice daily or 40 mg SC twice daily if BMI ≥ 30 kg/m2) |
| Outcomes | Primary
Secondary
Other outcomes
|
| Notes | NCT04360824 | No data provided |
NCT04362085.
| Study name | Coagulopathy of COVID‐19: a pragmatic randomized controlled trial of therapeutic anticoagulation versus standard care as a rapid response to the COVID‐19 pandemic (RAPID COVID COAG) |
| Starting date | 11 May 2020 |
| Contact information | Michelle Sholzberg St. Michael's Hospital, Toronto, Ontario, Canada 416‐864‐5389 | Michelle.Sholzberg@unityhealth.to |
| Methods | Multicentre, quadruple masking (participant, care provider, investigator, outcomes assessor), investigator‐sponsored, 2‐armed, parallel‐assignment RCT |
| Participants | 462 participants, ≥ 18 years, female and male Inclusion criteria
Exclusion criteria
|
| Interventions | Experimental: therapeutic anticoagulation Therapeutic anticoagulation with LMWH or UFH (high‐dose nomogram). The choice of LMWH versus UFH will be at the clinician's discretion and dependent on local institutional supply. Therapeutic anticoagulation will be administered until discharged from hospital, 28 days or death. If the participant is admitted to the ICU or requiring ventilatory support, we recommend continuation of the allocated treatment as long as the treating physician is in agreement. Comparison: standard care In Canada and the USA, administration of LMWH, UFH or fondaparinux at thromboprophylactic doses for acutely ill hospitalised medical patients, in the absence of contraindication, is considered standard care. |
| Outcomes | Primary
Secondary
|
| Notes | NCT04362085 | No data provided |
NCT04366960.
| Study name | Enoxaparin for thromboprophylaxis in hospitalized COVID‐19 patients: comparison of 40 mg o.d. versus 40 mg b.i.d. a randomized clinical trial |
| Starting date | 14 May 2020 |
| Contact information | Nuccia Morici Azienda Socio Sanitaria Territoriale Grande Ospedale Metropolitano Niguarda, Milano, Italy +396444 ext 2565 | nuccia.morici@ospedaleniguarda.it |
| Methods | Multicentre, prospective, open‐label, 1:1, 2‐armed, parallel‐assignment RCT |
| Participants | 2712 participants, ≥ 18 years, female and male Inclusion criteria
Exclusion criteria
|
| Interventions | Experimental: 40 mg SC enoxaparin twice a day Comparator: 40 mg SC enoxaparin once a day |
| Outcomes | Primary
Secondary
|
| Notes | NCT04366960 | No data provided |
NCT04367831.
| Study name | Intermediate or prophylactic‐dose anticoagulation for venous or arterial thromboembolism in severe COVID‐19: a cluster based randomized selection trial (IMPROVE‐COVID) |
| Starting date | 2 May 2020 |
| Contact information | Sahil A. Parikh Columbia University, New York, New York, USA 212‐305‐7060 | sap2196@cumc.columbia.edu |
| Methods | Single‐centre, prospective, single‐blinded (outcomes assessor), 2‐armed, cluster, parallel‐assignment RCT |
| Participants | 100 participants, ≥ 18 years, female and male Inclusion criteria
Exclusion criteria
|
| Interventions | Experimental: intermediate‐dose anticoagulation UFH infusion at 10 units/kg/h with goal anti‐Xa 0.1 ‐0.3U/mL If estimated GFR ≥ 30 mL/min: enoxaparin 1 mg/kg SC daily Comparator: enoxaparin prophylactic dose following local guideline If estimated GFR ≥ 30 mL/min (stable kidney function):
UFH at 5000‐7500 units SC every 8 h |
| Outcomes | Primary
Secondary
|
| Notes | NCT04367831 | No data provided |
NCT04372589.
| Study name | Antithrombotic therapy to ameliorate complications of COVID‐19 |
| Starting date | 20 May 2020 |
| Contact information | Ryan Zarychanski University of Manitoba, Canada 204‐787‐2993 | rzarychanski@cancercare.mb.ca |
| Methods | Multicentre, prospective, open‐label, 1:1. 2‐armed, parallel‐assignment RCT |
| Participants | 3000 participants, ≥ 18 years, female and male Inclusion criteria
Exclusion criteria
|
| Interventions | Experimental: therapeutic heparin Therapeutic anticoagulation for 14 days (or until hospital discharge or liberation from supplemental oxygen > 24 h if previously required, whichever comes first) with heparin, with preference for SC LMWH (enoxaparin preferred, although dalteparin or tinzaparin are also acceptable, as available) if no contraindication is present; alternatively, IV UFH infusion may be used. Comparator: prophylactic anticoagulation Participants will receive usual care of thromboprophylactic dose anticoagulation according to local practice. |
| Outcomes | Primary
Secondary
|
| Notes | NCT04372589 | No data provided |
NCT04373707.
| Study name | Effectiveness of weight‐adjusted prophylactic low molecular weight heparin doses compared with lower fixed prophylactic doses to prevent venous thromboembolism in COVID‐2019. The multicenter randomized controlled open‐label trial COVI‐DOSE |
| Starting date | 13 May 2020 |
| Contact information | Yohann Bernard Central Hospital, Nancy, France +33.3.83.15.52.72 | y.bernard@chru‐nancy.Fr |
| Methods | Multicenter, open‐label, 2‐armed, parallel‐assignment RCT; stratified on disease severity (admission to ICU or not) |
| Participants | 602 participants, ≥ 18 years, female and male Inclusion criteria
Exclusion criteria
|
| Interventions | Experimental: weight‐adjusted prophylactic dose LMWH For example (enoxaparin):
Other names: tinzaparin, nadroparin, dalteparin Comparator: low prophylactic dose of LMWH For example (enoxaparin): from 4000 IU once a day in participants admitted in medical ward to 4000 IU twice a day in participants admitted in the ICU. In participants with severe renal insufficiency (GFR = 15‐30 mL/min/1.73 m²), LMWH doses will be reduced by 50%. Other names: tinzaparin, nadroparin, dalteparin |
| Outcomes | Primary
Secondary
|
| Notes | NCT04373707 | 2020‐001709‐21 | No data provided |
NCT04377997.
| Study name | A randomized, open‐label trial of therapeutic anticoagulation in COVID‐19 patients with an elevated D‐dimer |
| Starting date | 15 May 2020 |
| Contact information | Mazen Albaghdadi Massachusetts General Hospital, USA 617‐726‐7400 | MALBAGHDADI@mgh.harvard.edu |
| Methods | Open‐label, 2‐armed, parallel‐assignment RCT |
| Participants | 300 participants, ≥ 18 years, female and male Inclusion
Exclusion
|
| Interventions | Experimental: therapeutic anticoagulation group Higher dose (not described) of heparin (LMWH for most participants but UFH for those with morbid obesity or moderate to severe renal dysfunction) Comparator: standard of care anticoagulation group There is no dose or drug description. |
| Outcomes | Primary
Secondary There is no description. |
| Notes | NCT04377997 | No data provided |
NCT04393805.
| Study name | Heparins for thromboprophylaxis in COVID‐19 patients: HETHICO study in Veneto |
| Starting date | 1 June 2020 |
| Contact information | Paolo Simioni Department of Medicine, University of Padua, Italy +39 0498212667 | paolo.simioni@unipd.it |
| Methods | Multicentre, retrospective cohort, open label, investigator‐sponsored, two hospitalised population arms (ICU and wards). A comparison of anticoagulant types and doses is foreseen as secondary analysis. |
| Participants | 877 participants, ≥ 18 years, female and male Inclusion criteria
Exclusion criteria
|
| Interventions | ICU group: thromboprophylaxis with LMWH, mostly enoxaparin Ward group: thromboprophylaxis with LMWH, mostly enoxaparin |
| Outcomes | Primary
Secondary
|
| Notes | NCT04393805 | No data provided |
NCT04394377.
| Study name | Randomized clinical trial to evaluate a routine full anticoagulation strategy in patients with coronavirus (COVID‐19) ‐ COALIZAO ACTION Trial |
| Starting date | 21 June 2020 |
| Contact information | Renato Delascio Lopes, MD, PhD Brazilian Clinical Research Institute, Sao Paulo, Brazil +55 11 5904 7339 | renato.lopes@duke.edu |
| Methods | Multicentre, quadruple masking (participant, care provider, investigator, outcomes assessor), investigator‐sponsored, 2‐armed, parallel‐assignment RCT |
| Participants | 600 participants, ≥ 18 years, female and male Inclusion
Exclusion
|
| Interventions | Experimental: routine full anticoagulation strategy. Rivaroxaban 20 mg/d followed by enoxaparin/UFH when needed Comparator: usual standard of care and currently have no indication of full anticoagulation. Control group with enoxaparin 40 mg/d |
| Outcomes | Primary
Secondary
|
| Notes | NCT04394377 | No data provided |
NCT04397510.
| Study name | Nebulized heparin vs. placebo for the treatment of COVID‐19 induced lung injury |
| Starting date | 1 June 2020 |
| Contact information | Thomas Smoot Frederick Health Hospital, Frederick, Maryland, USA |
| Methods | Multicentre, single masking (outcomes assessor), investigator‐sponsored, 2‐armed, parallel‐assignment RCT |
| Participants | 50 participants, ≥ 18 years, female and male Inclusion criteria
Exclusion criteria
|
| Interventions | Experimental: nebulised heparin 5000 units/mL IV formulation diluted with 3 mL of 0.9% sodium chloride Dose: 10,000 units. Frequency: every 4 h. Duration: 10 days Comparator: placebo. 0.9% sodium chloride. Dose: 5 mL. Frequency: every 4 h. Duration: 10 days |
| Outcomes | Primary
Secondary
|
| Notes | NCT04397510 | FHHep518 | No data provided |
NCT04401293.
| Study name | Systemic anticoagulation with full dose low molecular weight heparin (LMWH) vs. prophylactic or intermediate dose LMWH in high risk COVID‐19 patients (HEP‐COVID Trial) |
| Starting date | 26 April 2020 |
| Contact information | Damian N Inlall Northwell Health, USA (516) 600‐1482 | dinlall@northwell.edu |
| Methods | Multicenter, prospective, triple blinded, 2‐armed, parallel‐assignment RCT |
| Participants | 308 participants, ≥ 18 years, female and male Inclusion criteria
Exclusion criteria
|
| Interventions | Experimental: full‐dose LMWH anticoagulation therapy Participants in this study arm will be treated with therapeutic doses of SC LMWH (enoxaparin). Enoxaparin 1 mg/kg SC twice a day for creatinine clearance ≥ 30 mL/min (or enoxaparin 0.5 mg/kg SC twice a day for creatinine clearance ≥ 15 mL/min and < 30 mL/min) during the course of their hospitalisation. Comparator: prophylactic/intermediate‐dose LMWH or UFH therapy Participants in this study arm will be treated with local institutional standard of care for prophylactic‐dose or intermediate‐dose UFH or LMWH. Regimens allowed are UFH up to 22,500 IU daily in twice daily or three times daily doses (i.e. UFH 5000 IU SC twice a day/three times a day or 7500 IU twice a day/three times a day), enoxaparin 30 mg and 40 mg SC daily or twice daily (the use of weight‐based enoxaparin i.e. 0.5 mg/kg SC twice a day for this arm is acceptable but strongly discouraged), dalteparin 2500 IU or 5000 IU a day |
| Outcomes | Primary
Secondary
|
| Notes | NCT04401293 | No data provided |
NCT04416048.
| Study name | Effect of anticoagulation therapy on clinical outcomes in moderate to severe coronavirus disease 2019 (COVID‐19) |
| Starting date | 15 June 2020 |
| Contact information | Ulf Landmesser Charite University, Berlin, Germany +49 30 450 513 702 | ulf.landmesser@charite.de |
| Methods | Multicenter, prospective, event‐driven, 2‐armed, parallel‐assignment RCT |
| Participants | 400 participants, ≥ 18 years, female and male Inclusion criteria
Exclusion criteria:
|
| Interventions | Experimental: rivaroxaban Treatment with rivaroxaban 20 mg (15 mg for participants with an estimated GFR ≥ 30 mL/min/1.73 m2 and < 50 mL/min/1.73 m2) once daily for at least 7 days. In case of hospitalisation for > 7 days, the therapeutic treatment with rivaroxaban will be continued for the duration of the hospital stay until discharge. After at least 7 days of therapeutic treatment with rivaroxaban or after hospital discharge, the study dose of rivaroxaban will be adjusted as follows:
Other Name: XARELTO Comparator: standard care Participants will receive standard care treatment including prophylactic LMWH or UFH, when considered appropriate according to the judgment of the treating physician. |
| Outcomes | Primary
Secondary
|
| Notes | NCT04416048 | 2020‐002282‐33 | No data provided |
APTT: activated partial thromboplastin time; ACS: acute coronary syndrome; AKI: acute kidney injury; ARDS: acute respiratory distress syndrome; BARC: Bleeding Academic Research Consortium; BMI: body mass index; BP: blood pressure; CKI‐EPI: Chronic Kidney Disease Epidemiology Collaboration; CPAP: continuous positive airway pressure; CPR: cardiopulmonary resuscitation; CT: computed tomography;DBP: diastolic blood pressure; DIC: disseminated intravascular coagulation; DVT: deep vein thrombosis; ECMO: extracorporeal membrane oxygenation; ELISA: enzyme‐linked immunosorbent assay; GFR: glomerular filtration rate; GI: gastrointestinal; HFOV: High‐frequency oscillatory ventilation; HIT: heparin‐induced thrombocytopenia ICU: intensive care unit; INR: international normalised ratio; ISTH: International Society on Thrombosis and Haemostasis; IV: intravenous(ly); LMWH: low molecular weight heparin; NIV: non‐invasive ventilation; PCR: polymerase chain reaction; PE: pulmonary embolism; PRCB: packed red blood cell; RCT: randomised controlled trial; RT‐PCR: reverse transcription polymerase chain reaction; SARS: severe acute respiratory syndrome; SBP: systolic blood pressure; SC: subcutaneous(ly);SIC: sepsis‐induced coagulopathy;SOFA: sequential organ failure assessment; TIA: transient ischaemic attack; UFH: unfractionated heparin; ULN: upper limit of normal; WHO: World Health Organization
Differences between protocol and review
Types of studies
We did not include retrospective non‐randomised studies (NRS) in our protocol. However, as there was no evidence from randomised controlled trials (RCTs), quasi‐RCTs, and prospective NRS, we included retrospective NRS with a control group and followed the methodology as specified in the protocol (Flumignan 2020).
At the protocol stage we had planned to narratively describe skewed data reported as medians and interquartile ranges. However, in our review we estimated the mean difference (MD) using the method reported by Wan 2014 to convert median and interquartile range (IQR) into MD and confidence intervals (CI). When this was not possible, we narratively described the skewed data as originally planned.
Data extraction and management
Assessment of risk of bias in included studies
We planned to include only studies that used statistical adjustment for baseline factors using multivariate analyses for the following confounding factors in our protocol (Flumignan 2020):
participants already using anticoagulants (e.g. atrial fibrillation);
participants who underwent surgery during the hospitalisation;
active cancer treatment;
concomitant antiplatelet use;
history of venous thromboembolism.
However, we included all retrospective NRS that met our inclusion criteria, irrespective of the 'statistical adjustment for baseline factors', and assessed the confounders at the 'bias due to confounding' domain of the ROBINS‐I tool in this review (Sterne 2016).
Contributions of authors
RLGF: clinical and methodological expertise, development of the search strategy and conception and writing of the review JDST: clinical and methodological expertise and advice PP: clinical expertise and advice LLA: development of the search strategy MC: clinical expertise and advice MIF: clinical expertise and advice IC: clinical expertise and advice LS: clinical expertise and advice CM: clinical expertise and advice BT: methodological expertise and advice VT: methodological expertise and advice AA: clinical and methodological expertise and advice LCUN: clinical and methodological expertise and writing of the review
Sources of support
Internal sources
-
Division of Vascular and Endovascular Surgery, Universidade Federal de São Paulo, Brazil
Non‐financial internal sources.
-
Cochrane Brazil, Brazil
Non‐financial internal sources.
External sources
No sources of support supplied
Declarations of interest
RLGF: none known JDST: none known PP: none known LLA: none known MC: none known MIF: none known IC: none known LS: none known CM: none known BT: none known VT: none known AA: none known LCUN: none known
Edited (no change to conclusions)
References
References to studies included in this review
Ayerbe 2020 {published data only}
- Ayerbe L, Risco C, Ayis S. The association between treatment with heparin and survival in patients with Covid-19. Journal of Thrombosis and Thrombolysis 2020;50(2):298-301. [DOI: 10.1007/s11239-020-02162-z] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayerbe L, Risco C, Ayis S. The association between treatment with heparin and survival in patients with Covid-19. medRxiv. [DOI: 10.1101/2020.05.27.20114694] [DOI] [PMC free article] [PubMed] [Google Scholar]
Liu 2020 {published data only}
- Liu X, Zhang X, Xiao Y, Gao T, Wang G, Wang Z, et al. Heparin-induced thrombocytopenia is associated with a high risk of mortality in critical COVID-19 patients receiving heparin-involved treatment. medRxiv [Preprint]. [DOI: 10.1101/2020.04.23.20076851] [DOI]
Paranjpe 2020 {published data only}
- Paranjpe I, Fuster V, Lala A, Russak AJ, Glicksberg BS, Levin MA, et al. Association of treatment dose anticoagulation with in-hospital survival among hospitalized patients with COVID-19. Journal of the American College of Cardiology 2020;76(1):122-4. [PMID: ] [DOI] [PMC free article] [PubMed]
Russo 2020 {published data only}
- Russo V, Di Maio M, Attena E, Silverio A, Scudiero F, Celentani D, et al. Clinical impact of pre-admission antithrombotic therapy in hospitalized patients with COVID-19: a multicenter observational study. Pharmacological Research 2020;159:104965. [DOI: 10.1016/j.phrs.2020.104965] [DOI] [PMC free article] [PubMed] [Google Scholar]
Shi 2020 {published data only}
- Shi C, Wang C, Wang H, Yang C, Cai F, Zeng F, et al. The potential of low molecular weight heparin to mitigate cytokine storm in severe COVID-19 patients: a retrospective clinical study. medRxiv [Preprint]. [DOI: 10.1101/2020.03.28.20046144] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tang 2020 {published data only}
- Tang N, Bai H, Chen X, Gong J, Li D, Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. Journal of Thrombosis and Haemostasis : JTH 2020;18(5):1094-9. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Trinh 2020 {published data only}
- Trinh M, Chang DR, Govindarajulu US, Kane E, Fuster V, Kohli-Seth R, et al. Therapeutic anticoagulation is associated with decreased mortality in mechanically ventilated COVID-19 patients. medRxiv [Preprint]. [DOI: 10.1101/2020.05.30.20117929] [DOI]
References to studies excluded from this review
Al‐Samkari 2020 {published data only}
- Al-Samkari H, Karp Leaf RS, Dzik WH, Carlson JC, Fogerty AE, Waheed A, et al. COVID-19 and coagulation: bleeding and thrombotic manifestations of SARS-CoV-2 infection. Blood 2020;136(4):489-500. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Artifoni 2020 {published data only}
- Artifoni M, Danic G, Gautier G, Gicquel P, Boutoille D, Raffi F, et al. Systematic assessment of venous thromboembolism in COVID-19 patients receiving thromboprophylaxis: incidence and role of D-dimer as predictive factors. Journal of Thrombosis and Thrombolysis 2020;50(1):211-6. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
EudraCT2020‐001823‐15 {published data only}
- EudraCT2020-001823-15. Evaluation of the concentration-effect relationship of enoxaparin for thromboembolic prevention in COVID-19 resuscitation patients. COV-ENOX study. clinicaltrialsregister.eu/ctr-search/trial/2020-001823-15/FR (first received 15 April 2020).
Helms 2020 {published data only}
- Helms J, Tacquard C, Severac F, Leonard-Lorant I, Ohana M, Delabranche X, et al. High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Medicine 2020;46(6):1089-98. [DOI: 10.1007/s00134-020-06062-x] [DOI] [PMC free article] [PubMed] [Google Scholar]
Khider 2020 {published data only}
- Khider L, Gendron N, Goudot G, Chocron R, Hauw-Berlemont C, Cheng C, et al. Curative anticoagulation prevents endothelial lesion in COVID-19 patients. Journal of Thrombosis and Haemostasis 2020 June 18 [Epub ahead of print]. [DOI: 10.1111/jth.14968] [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT04354155 {published data only}
- NCT04354155. COVID-19 anticoagulation in children - thromboprophlaxis (COVAC-TP) trial. clinicaltrials.gov/ct2/show/NCT04354155 (first received 21 April 2020).
NCT04359212 {published data only}
- NCT04359212. Increased risk of venous thromboembolism and higher hypercoagulable state in patients recovered in intensive care unit and in medical ward for coronavirus disease 2019 (COVID-19). clinicaltrials.gov/ct2/show/NCT04359212 (first received 21 April 2020).
NCT04365309 {published data only}
- NCT04365309. Protective effect of aspirin on COVID-19 patients (PEAC). clinicaltrials.gov/ct2/show/NCT04365309 (first received 28 April 2020).
NCT04368377 {published data only}
- NCT04368377. Platelet inhibition with GP IIb/IIIa inhibitor in critically ill patients with coronavirus disease 2019 (COVID-19). A compassionate use protocol. clinicaltrials.gov/ct2/show/NCT04368377 (first received 29 April 2020).
NCT04394000 {published data only}
- NCT04394000. Impact of implementation of an intensified thromboprofylaxis protocol in critically ill ICU patients with COVID-19: a longitudinal controlled before-after study. clinicaltrials.gov/ct2/show/record/NCT04394000 (first received 19 May 2020).
NCT04427098 {published data only}
- NCT04427098. Intermediate dose enoxaparin in hospitalized patients with moderate-severe COVID19: a pilot phase II single-arm study, INHIXACOVID19. clinicaltrials.gov/ct2/show/study/NCT04427098 (first received 11 June 2020).
Zhang 2020 {published data only}
- Zhang Y, Cao W, Xiao M, Li YJ, Yang Y, Zhao J, et al. Clinical and coagulation characteristics of 7 patients with critical COVID-2019 pneumonia and acro-ischemia. Zhonghua Xue Ye Xue za Zhi 2020;41(0):E006. [PMID: ] [DOI] [PubMed] [Google Scholar]
References to ongoing studies
ACTRN12620000517976 {published data only}
- ACTRN12620000517976. Can nebulised heparin reduce time to extubation in SARS CoV 2. The CHARTER study protocol. apps.who.int/trialsearch/Trial2.aspx?TrialID=ACTRN12620000517976 (first received 27 April 2020).
- ACTRN12620000517976p. A randomised controlled trial of nebulised heparin in critically ill mechanically ventilated patients with COVID-19 to assess the effect on the duration of mechanical ventilation. anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12620000517976 (first posted 27 April 2020).
- Dixon B, Smith RJ, Artigas A, Laffey J, McNicholas B, Schmidt E, et al. Can nebulised heparin reduce time to extubation in SARS CoV 2. The CHARTER study protocol. medRxiv [Preprint]. [DOI: 10.1101/2020.04.28.20082552] [DOI]
ChiCTR2000030700 {published data only}
- ChiCTR2000030700. Study for the efficacy and safety of prolongin (enoxaparin sodium injection) in treatment of novel coronavirus pneumonia (COVID-19) adult common patients. apps.who.int/trialsearch/Trial2.aspx?TrialID=ChiCTR2000030700 (first received 10 March 2020).
- ChiCTR2000030700. Study for the efficacy and safety of prolongin (enoxaparin sodium injection) in treatment of novel coronavirus pneumonia (COVID-19) adult common patients. www.chictr.org.cn/showprojen.aspx?proj=50786 (first posted 10 March 2020).
ChiCTR2000030701 {published data only}
- ChiCTR2000030701. A randomized, parallel controlled open-label trial for the efficacy and safety of prolongin (enoxaparin sodium injection) in the treatment of adult patients with novel coronavirus pneumonia (COVID-19). chictr.org.cn/showprojen.aspx?proj=50795 (first received 10 March 2020).
ChiCTR2000030946 {published data only}
- ChiCTR2000030946. Effects of different VTE prevention methods on the prognosis of hospitalized patients with novel coronavirus pneumonia (COVID-19). chictr.org.cn/showprojen.aspx?proj=51265 (first received 19 March 2020).
Marietta 2020 {published data only}
- Marietta M, Vandelli P, Mighali P, Vicini R, Coluccio V, D'Amico R. Randomised controlled trial comparing efficacy and safety of high versus low molecular weight heparin dosages in hospitalized patients with severe COVID-19 pneumonia and coagulopathy not requiring invasive mechanical ventilation (COVID-19 HD): a structured summary of a study protocol. Trials 2020;21(1):574. [PMID: ] [DOI] [PMC free article] [PubMed]
- NCT04408235. High versus low LMWH dosages in hospitalized patients with severe COVID-19 pneumonia and coagulopathy (COVID-19 HD). clinicaltrials.gov/ct2/show/NCT04408235 (first received 29 May 2020).
NCT04333407 {published data only}
- NCT04333407. Preventing cardiac complication of COVID-19 disease with early acute coronary syndrome therapy: a randomised controlled trial. clinicaltrials.gov/ct2/show/NCT04333407 (first received 3 April 2020).
NCT04344756 {published data only}
- NCT04344756. Trial evaluating efficacy and safety of anticoagulation in patients with COVID-19 infection, nested in the Corimmuno-19 cohort (CORIMMUNO-COAG). clinicaltrials.gov/ct2/show/study/NCT04344756 (first received 14 April 2020).
NCT04345848 {published data only}
- NCT04345848. Preventing COVID-19-associated thrombosis, coagulopathy and mortality with low- and high-dose anticoagulation: a randomized, open-label clinical trial. clinicaltrials.gov/ct2/show/NCT04345848 (first received 15 April 2020).
NCT04352400 {published data only}
- NCT04352400. Efficacy of nafamostat in COVID-19 patients (RACONA Study) (RACONA). clinicaltrials.gov/ct2/show/NCT04352400 (first posted 20 April 2020).
NCT04359277 {published data only}
- NCT04359277. A randomized trial of anticoagulation strategies in COVID-19. clinicaltrials.gov/ct2/show/NCT04359277 (first received 24 April 2020).
NCT04360824 {published data only}
- NCT04360824. COVID-19-associated coagulopathy: safety and efficacy of prophylactic anticoagulation therapy in hospitalized adults with COVID-19. clinicaltrials.gov/ct2/show/NCT04360824 (first received 24 April 2020).
NCT04362085 {published data only}
- NCT04362085. Coagulopathy of COVID-19: a pragmatic randomized controlled trial of therapeutic anticoagulation versus standard care as a rapid response to the COVID-19 pandemic (RAPID COVID COAG). clinicaltrials.gov/ct2/show/NCT04362085 (first received 24 April 2020).
NCT04366960 {published data only}
- NCT04366960. Comparison of two doses of enoxaparin for thromboprophylaxis in hospitalized COVID-19 patients. clinicaltrials.gov/ct2/show/NCT04366960 (first posted 29 April 2020).
NCT04367831 {published data only}
- NCT04367831. Intermediate or prophylactic-dose anticoagulation for venous or arterial thromboembolism in severe COVID-19: a cluster based randomized selection trial (IMPROVE-COVID). clinicaltrials.gov/ct2/show/NCT04367831 (first received 27 April 2020).
NCT04372589 {published data only}
- NCT04372589. Antithrombotic therapy to ameliorate complications of COVID-19. clinicaltrials.gov/ct2/show/NCT04372589 (first received 4 May 2020).
NCT04373707 {published data only}
- NCT04373707. Effectiveness of weight-adjusted prophylactic low molecular weight heparin doses compared with lower fixed prophylactic doses to prevent venous thromboembolism in COVID-2019. The multicenter randomized controlled open-label trial COVI-DOSE. clinicaltrials.gov/ct2/show/record/NCT04373707 (first received 4 May 2020).
NCT04377997 {published data only}
- NCT04377997. A randomized, open-label trial of therapeutic anticoagulation in COVID-19 patients with an elevated D-dimer. clinicaltrials.gov/ct2/show/NCT04377997 (first received 7 May 2020).
NCT04393805 {published data only}
- NCT04393805. Heparins for thromboprophylaxis in COVID-19 patients: HETHICO study in Veneto. clinicaltrials.gov/ct2/show/NCT04393805 (first received 16 May 2020).
NCT04394377 {published data only}
- NCT04394377. Randomized clinical trial to evaluate a routine full anticoagulation strategy in patients with coronavirus (COVID-19) - COALIZAO ACTION Trial. clinicaltrials.gov/ct2/show/NCT04394377 (first received 8 May 2020).
NCT04397510 {published data only}
- NCT04397510. Nebulized heparin vs. placebo for the treatment of COVID-19 induced lung injury. clinicaltrials.gov/ct2/show/NCT04397510 (first received 21 May 2020).
NCT04401293 {published data only}
- NCT04401293. Systemic anticoagulation with full dose low molecular weight heparin (LMWH) vs. prophylactic or intermediate dose LMWH in high risk COVID-19 patients (HEP-COVID Trial). clinicaltrials.gov/ct2/show/NCT04401293 (first received 26 May 2020).
NCT04416048 {published data only}
- NCT04416048. Effect of anticoagulation therapy on clinical outcomes in moderate to severe coronavirus disease 2019 (COVID-19). clinicaltrials.gov/ct2/show/NCT04416048 (first received 4 June 2020).
Additional references
Ackermann 2020
- Ackermann M, Verleden SE, Kuehnel M, Haverich A, Welte T, Laenger F, et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in COVID-19. New England Journal of Medicine 2020;383(2):120-8. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Alquwaizani 2013
- Alquwaizani M, Buckley L, Adams C, Fanikos J. Anticoagulants: a review of the pharmacology, dosing, and complications. Current Emergency and Hospital Medicine Reports 2013;1(2):83-97. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Amaral 2020
- Amaral FC, Baptista-Silva JC, Nakano LC, Flumignan RL. Pharmacological interventions for preventing venous thromboembolism in patients undergoing bariatric surgery. Cochrane Database of Systematic Reviews 2020, Issue 7. Art. No: CD013683. [DOI: 10.1002/14651858.CD013683] [DOI] [PMC free article] [PubMed] [Google Scholar]
AVF 2020
- American Venous Forum. Considerations in prophylaxis and treatment of VTE in COVID-19 patients. www.veinforum.org/wp-content/uploads/2020/04/COVID-19-White-Paper-04-17-2020-FINAL-1.pdf (accessed 3 July 2020).
Becker 2020
- Becker RC. COVID-19 update: COVID-19-associated coagulopathy. Journal of Thrombosis and Thrombolysis 2020;50(1):54-67. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Biagioni 2020
- Biagioni RB, Lopes RD, Agati LB, Sacilotto R, Wolosker N, Sobreira ML, et al. Rationale and design for the study apixaban versus clopidogrel on a background of aspirin in patients undergoing infrapopliteal angioplasty for critical limb ischemia: AGRIPPA trial. American Heart Journal 2020;227:100-6. [DOI: 10.1016/j.ahj.2020.06.010] [DOI] [PubMed] [Google Scholar]
Bikdeli 2020
- Bikdeli B, Madhavan M, Jimenez D, Chuich T, Dreyfus I, Driggin E, et al. COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up: JACC state of the art review. Journal of American College of Cardiology 2020;75(23):2950-73. [DOI: 10.1016/j.jacc.2020.04.031] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bilaloglu 2020
- Bilaloglu S, Aphinyanaphongs Y, Jones S, Iturrate E, Hochman J, Berger JS. Thrombosis in hospitalized patients with COVID-19 in a New York City health system. JAMA 2020;324(8):799-801. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Clezar 2020
- Clezar CN, Cassola N, Flumignan CD, Nakano LC, Trevisani VF, Flumignan RL. Pharmacological interventions for asymptomatic carotid stenosis. Cochrane Database of Systematic Reviews 2020, Issue 4. Art. No: CD013573. [DOI: 10.1002/14651858.CD013573] [DOI] [PMC free article] [PubMed] [Google Scholar]
COMET 2020
- Core outcome set developers’ response to COVID-19 (7th July 2020). comet-initiative.org/Studies/Details/1538 (accessed 04 August 2020).
Covidence [Computer program]
- Veritas Health Innovation Covidence. Version accessed 1 August 2020. Melbourne, Australia: Veritas Health Innovation. Available at www.covidence.org.
Deeks 2020
- Deeks JJ, Higgins JP, Altman DG, editor(s). Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page M, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 6 (updated September 2020). Cochrane, 2020. Available from www.training.cochrane.org/handbook.
Dolhnikoff 2020
- Dolhnikoff M, Duarte-Neto AN, Almeida Monteiro RA, da Silva LF, Oliveira EP, Saldiva PH, et al. Pathological evidence of pulmonary thrombotic phenomena in severe COVID-19. Journal of Thrombosis and Haemostasis : JTH 2020;18(6):1517-9. [PMID: ] [DOI] [PMC free article] [PubMed]
Fox 2020
- Fox SE, Akmatbekov A, Harbert JL, Li G, Quincy-Brown J, Vander-Heide RS. Pulmonary and cardiac pathology in African American patients with COVID-19: an autopsy series from New Orleans. Lancet Respiratory Medicine 2020;8(7):681-6. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Giannis 2020
- Giannis D, Ziogas IA, Gianni P. Coagulation disorders in coronavirus infected patients: COVID-19, SARS-CoV-1, MERS-CoV and lessons from the past. Journal of Clinical Virology 2020;127:104362. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADEpro GDT 2015 [Computer program]
- McMaster University (developed by Evidence Prime) GRADEpro GDT. Version version accessed 1 August 2020. Hamilton (ON): McMaster University (developed by Evidence Prime). Available at gradepro.org.
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;326:557-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2017
- Higgins JP, Altman DG, Sterne JA, editor(s). Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Churchill R, Chandler J, Cumpston MS, editor(s). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.2.0 (updated June 2017). Cochrane, 2017. Available from www.training.cochrane.org/handbook.
Higgins 2020a
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.1 (updated September 2020). Cochrane, 2020. Available from www.training.cochrane.org/handbook.
Higgins 2020b
- Higgins JP, Eldridge S, Li T, editor(s). Chapter 23: Including variants on randomized trials. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.1 (updated September 2020). Cochrane, 2020. Available from www.training.cochrane.org/handbook.
Klok 2020a
- Klok FA, Kruip MJ, Van der Meer NJ, Arbous MS, Gommers DA, Kant KM, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thrombosis Research 2020;191:145-7. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Klok 2020b
- Klok FA, Kruip MJ, Van der Meer NJ, Arbous MS, Gommers D, Kant KM, et al. Confirmation of the high cumulative incidence of thrombotic complications in critically ill ICU patients with COVID-19: an updated analysis. Thrombosis Research 2020;191:148-50. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lai 2020
- Lai CC, Wang CY, Wang YH, Hsueh SC, Ko WC, Hsueh PR. Global epidemiology of coronavirus disease 2019 (COVID-19): disease incidence, daily cumulative index, mortality, and their association with country healthcare resources and economic status. International Journal of Antimicrobial Agents 2020;55(4):105946. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lefebvre 2020
- Lefebvre C, Glanville J, Briscoe S, Littlewood A, Marshall C, Metzendorf M-I, et al. Chapter 4: Searching for and selecting studies. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.1 (updated September 2020). Cochrane, 2020. Available from www.training.cochrane.org/handbook.
Li 2020
- Li Y, Li M, Wang M, Zhou Y, Chang J, Xian Y, et al. Acute cerebrovascular disease following COVID-19: a single center, retrospective, observational study. Stroke and Vascular Neurology 2020 July 2 [Epub ahead of print]. [DOI: 10.1136/svn-2020-000431] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Liberati 2009
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Medicine 2009;6(7):e1000100. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Liu 2019
- Liu Y, Mu S, Li X, Liang Y, Wang L, Ma X. Unfractionated heparin alleviates sepsis-induced acute lung injury by protecting tight junctions. Journal of Surgical Research 2019;238:175-85. [PMID: ] [DOI] [PubMed] [Google Scholar]
Long 2020
- Long B, Brady WJ, Koyfman A, Gottlieb M. Cardiovascular complications in COVID-19. American Journal of Emergency Medicine 2020;38(7):1504-7. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Marini 2020
- Marini JJ, Gattinoni L. Management of COVID-19 respiratory distress. JAMA 2020;323(22):2329-30. [DOI: 10.1001/jama.2020.6825] [PMID: ] [DOI] [PubMed] [Google Scholar]
McGuinness 2020
- McGuinness LA, Higgins JP. Risk-of-bias VISualization (robvis): an R package and Shiny web app for visualizing risk-of-bias assessments. Research Synthesis Methods 2020:1-7. [DOI: 10.1002/jrsm.1411] [DOI] [PubMed] [Google Scholar]
NHS 2020
- NHS England and NHS Improvement. Clinical guide for the management of anticoagulant services during the coronavirus pandemic. www.england.nhs.uk/coronavirus/wp-content/uploads/sites/52/2020/03/C0077-Specialty-guide_Anticoagulant-services-and-coronavirus-v1-31-March.pdf (accessed 3 July 2020).
Obe 2020
- Obe BH, Retter A, McClintock C. Practical guidance for the prevention of thrombosis and management of coagulopathy and disseminated intravascular coagulation of patients infected with COVID-19. thrombosisuk.org/downloads/T&H%20and%20COVID.pdf (accessed 3 July 2020).
Oxley 2020
- Oxley TJ, Mocco J, Majidi S, Kellner CP, Shoirah H, Singh IP, et al. Large-vessel stroke as a presenting feature of COVID-19 in the young. New England Journal of Medicine 2020;382(20):e60. [DOI: 10.1056/NEJMc2009787] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pang 2020
- Pang J, Wang MX, Ang IY, Tan SH, Lewis RF, Chen JP, et al. Potential rapid diagnostics, vaccine and therapeutics for 2019 novel coronavirus (2019-nCoV): a systematic review. Journal of Clinical Medicine 2020;9(623):1-33. [10.3390/jcm9030623] [DOI] [PMC free article] [PubMed] [Google Scholar]
Panigada 2020
- Panigada M, Bottino N, Tagliabue P, Grasselli G, Novembrino C, Chantarangkul V, et al. Hypercoagulability of COVID-19 patients in intensive care unit: a report of thromboelastography findings and other parameters of hemostasis. Journal of Thrombosis and Haemostasis 2020;18(7):1738-42. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Poston 2020
- Poston JT, Patel BK, Davis AM. Management of critically ill adults with COVID-19. JAMA 2020;323(18):1839-41. [DOI: 10.1001/jama.2020.4914] [DOI] [PubMed] [Google Scholar]
Ramacciotti 2020
- Ramacciotti E, Macedo AS, Biagioni RB, Caffaro RA, Lopes RD, Guerra JC, et al. Evidence-based practical guidance for the antithrombotic management in patients with coronavirus disease (COVID-19) in 2020. Clinical and Applied Thrombosis/hemostasis 2020;26:1076029620936350. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Review Manager 2020 [Computer program]
- The Cochrane Collaboration Review Manager 5 (RevMan 5). Version 5.4. Copenhagen: The Cochrane Collaboration, 2020.
Robvis [Computer program]
- Robvis (visualization tool). Version accessed 20 August 2020. Bristol, UK: Luke McGuinness. Available at mcguinlu.shinyapps.io/robvis/.
Sanders 2020
- Sanders JM, Monogue ML, Jodlowski TZ, Cutrell JB. Pharmacologic treatments for coronavirus disease 2019 (COVID-19): a review. JAMA 2020;323(18):1824-36. [DOI: 10.1001/jama.2020.6019] [DOI] [PubMed] [Google Scholar]
Schulman 2010
- Schulman S, Angerås U, Bergqvist D, Eriksson B, Lassen MR, Fisher W. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in surgical patients. Journal of Thrombosis and Haemostasis : JTH 2010;8(1):202-4. [PMID: ] [DOI] [PubMed] [Google Scholar]
Schünemann 2013
- Schünemann H, Brożek J, Guyatt G, Oxman A, editor(s). Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach (updated October 2013). GRADE Working Group, 2013. Available from gdt.gradepro.org/app/handbook/handbook.html.
Schünemann 2019
- Schünemann HJ, Vist GE, Higgins JP, Santesso N, Deeks JJ, Glasziou P, et al. Chapter 15: Interpreting results and drawing conclusions. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. Available from www.training.cochrane.org/handbook.
Sterne 2016
- Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ (Clinical Research Ed.) 2016;355:i4919. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tobaiqy 2020
- Tobaiqy M, Qashqary M, Al-Dahery S, Mujallad A, Hershan AA, Kamal MA, et al. Therapeutic management of patients with COVID-19: a systematic review. Infection Prevention in Practice 2020;2(3):1-26. [DOI: 10.1016/j.infpip.2020.100061] [DOI] [PMC free article] [PubMed] [Google Scholar]
Violi 2020
- Violi F, Pastori D, Cangemi R, Pignatelli P, Loffredo L. Hypercoagulation and antithrombotic treatment in coronavirus 2019: a new challenge. Thrombosis and Haemostasis 2020;120(6):949-56. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wan 2014
- Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC medical research methodology 2014;14:135. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ware 1992
- Ware JE Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Medical Care 1992;30(6):473-83. [PMID: ] [PubMed] [Google Scholar]
Young 2008
- Young E. The anti-inflammatory effects of heparin and related compounds. Thrombosis Research 2008;122(6):743-52. [PMID: ] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Flumignan 2020
- Flumignan RL, Tinôco JD, Pascoal PI, Areias LL, Cossi MS, Fernandes MI et al. Prophylactic anticoagulants for patients hospitalised with COVID-19 (Protocol). available from doi.org/10.17605/OSF.IO/8PRXW (registered 7 August 2020).


