Abstract
Selenium (Se) deficiency and poor plasma Se levels can cause cardiovascular diseases by decreasing selenoprotein levels. Neutrophil extracellular traps (NETs) may be the vicious cycle center of inflammation in vasculitis. Here, we show that Se deficiency induced arteritis mainly by reducing selenoprotein S (SelS), and promoted the progression of arteritis by regulating the recruitment of neutrophils and NET formation. Silencing SelS induced chicken arterial endothelial cells (PAECs) to secrete cytokines, and activated neutrophils to promote NET formation. Conversely, scavenging DNA-NETs promoted cytokine secretion in PAECs. The NET formation regulated by siSelS was dependent on a reactive oxygen species (ROS) burst. We also found that the PPAR pathway was a major mediator of NET formation induced by Se-deficient arteritis. Overall, our results reveal how Se deficiency regulates NET formation in the progression of arteritis and support silencing-SelS worsens arteritis.
Keywords: Selenium deficiency, Arteritis, Selenoprotein S, Neutrophil extracellular traps, Reactive oxygen species, PPAR pathway
Graphical abstract
Highlights
-
•
Se deficiency induces arteritis and NET formation in broilers.
-
•
PMA-induced NET-DNAs promote PAECs inflammatory in vitro.
-
•
Silencing SelS expression in PAECs induce NET formation.
-
•
Silencing-SelS mediated-NET formation is ROS-dependent.
-
•
PPAR pathway regulates NET formation induced by Se-deficient arteritis.
1. Introduction
Selenium (Se) is an essential micronutrient that plays a crucial role in development and in a wide variety of physiological processes, including antagonist inflammation and oxidative stress [1,2]. Inadequate Se intake leads to the development of several pathologies [3]. Vascular tissue is one of the main target organs of Se deficiency, and studies on the relationship between Se deficiency and vascular injury mainly involve necrosis [4], apoptosis [5], and inflammation [6]. Low Se status increases endothelial cell-derived adhesion molecule expression and microvascular permeability [7], leading to vessel wall thickening and inflammatory cell infiltration, which aggravates aortic vascular oxidative damage [8]. The anti-inflammatory properties of Se can also provide cardiovascular protection. The mRNA expression of cytokines in venous tissue of chickens with Se deficiency is in a high expression state, and the venous tissue has inflammatory damage [5]. In vascular endothelial cells, increased selenoprotein activities may play a protective role by reducing the abnormal adhesion between cells induced by proinflammatory cytokines [9]. Se deficiency downregulates multiple selenoproteins in venous tissues and reduces vascular antistress ability, which in turn triggers vascular injury [10,11], while Se supplementation can protect vascular endothelial cells [12]. More than one third of the 25 selenoproteins expressed in blood vessels are related to redox reaction, such as glutathione peroxidase (GPX), SelK, SelW, thioredoxin reductase (Txnrd), etc. Selenoproteins can destroy the cholesterol accumulated in the blood vessel wall, increase the level of coenzyme A in the myocardium, and increase the production of energy, thus protect the heart [13]. GPX can affect the activities of cyclooxygenases and lipoxygenases and effectively prevent the development of cardiovascular diseases such as atherosclerosis and hypertension [14]. Txnrd can regulate the cardiovascular system through both intra- and extracellular molecular signals, affecting vascular remodeling [15]. Studies on selenoprotein S (SelS) in recent years also explained its inhibitory effects on vascular inflammation and oxidative stress [16,17]. Thus, Se deficiency is able to cause oxidative stress, apoptosis, inflammation, and structural abnormalities in vascular tissues, which are unable to maintain the angioarchitecture and resist multiple cardiovascular injuries, but the specific mechanisms need to be further elucidated.
Neutrophil extracellular traps (NETs) are network structures composed of neutrophils with DNA in the nucleus or mitochondria as the skeleton. The formation of NET is closely related to reactive oxygen species (ROS) and intracellular calcium ion flow. The level of intracellular ROS determines whether autophagy can induce NET formation, and when the intracellular ROS level reaches a certain concentration, it will activate cell autophagy through related signaling pathways, and then promote NET formation [18]. ROS leads to citrullination of histones by elevating intracellular Ca2+ levels, which activates histone peptidyl arginine deiminase 4 (PAD4), neutrophil elastase (NE) and myeloperoxidase (MPO) [19]. Palić, D., et al. found that ROS and Ca2+ were involved in the formation of renal NET induced by exogenous stimulation [20]. Nowadays, evidence suggests that NETs can participate in gouty arthritis [21], promote acute pancreatitis, and lead to local and distal organ damage in severe acute pancreatitis [22], and aggravate the necrotizing inflammation in tissue [23]. NETs can be used as the precursor information for cardiovascular diseases [24], to suggest the occurrence and development of vascular diseases. Patients with vasculitis have high concentrations of antineutrophil cytoplasmic antibodies (ANCAs) that can induce a proinflammatory feedback loop, and continuously released NETs combine with the vascular wall to further aggravate vasculitis [25]. The interaction between activated platelets and neutrophils leads to the activation of local vascular NETs and tissue factors, which is related to arterial thrombosis [26,27]. Among the noninfectious trigger factors, cholesterol crystals can induce atherosclerosis-related NETs, NE and proteinase 3 (PR3) knockout apolipoprotein E (ApoE)−/− mice showed less atherosclerosis damage, indicating that NETs and/or these proteases have potential roles in this process [28]. NET formation is triggered by innate immune receptors through downstream intracellular mediators, including ROS generated by NADPH oxidase or mitochondria, which activate MPO, NE, and PAD4 to promote chromatin decondensation [29]. As demonstrated in another study, exosomes from oxidized low-density lipoprotein-treated macrophages promote the formation of NETs by inducing oxidative stress, which in turn promotes atherosclerosis progression [30]. Excessive NET formation aggravates acute myocardial infarction injury in ApoE−/- mice via the ROS-dependent pathway [31]. However, Eghbalzadeh, K., et al. found that PAD4−/− neutrophils are unable to release NETs, but acute inflammation is exacerbated after myocardial infarction, and produces significantly higher amounts of ROS [32].
To date, Se deficiency has been shown to cause vascular injury [33] and decrease neutrophil function [34], accompanied by oxidative stress and decreased selenoprotein expression [35], and low levels of Se (0.01 mg/L) can significantly induce NETs formation in cows [36], but little is known about the mechanisms by which selenoproteins regulate the formation of NETs involved in the pathogenesis of Se-deficient arteritis. Here, we replicated a chicken Se-deficient arteritis model and found the increased formation of NETs. The most significantly differentially expressed SelS and PPAR pathway were further screened by qRT-PCR and proteomics, respectively, and their interrelationships with arteritis and NET formation were explored in vitro. In addition, the present experiment also determined the role of ROS in the formation of NETs induced by Se-deficient arteritis. This experiment delineates the mechanism of Se-deficient arteritis in chickens that is dependent on the formation of NETs and suggests that targeting SelS and the PPAR pathway may be a therapeutic strategy to prevent Se-deficient arteritis.
2. Material and methods
2.1. Preparation of animals and sample collection
The animal procedures in this study were guided and approved by the Institutional Animal Care and Use Committee of Northeast Agricultural University (SRM-11). Three hundred and sixty one-day-old Ross 308 male broilers (Weiwei Co. Ltd., Harbin, China) were randomly divided into two groups (180 chickens per group), and the broilers were fed a full price diet (the basic diet composition is shown in Table S1). The dietary Se level was 0.2 mg/kg in the control group and less than 0.03 mg/kg in the Se deficiency group. After 42 days of feeding, 200 mg/kg LPS (Sigma-Aldrich, China) was injected intraperitoneally. After 2 h, the peripheral blood and aortas were collected, processed and preserved as needed for subsequent experiments.
2.2. Detection of Se content in plasma
To determine the Se status of the experimental broilers, we extracted broiler plasma and performed an assay for Se content. The collected peripheral blood was centrifuged at 3500 rpm for 10 min to separate the plasma. The content of Se in plasma was determined by an AFS-9750 atomic fluorescence spectrometer. Before the determination of Se content, 100 μL of blood sample was placed into a glass test tube, and mixed acid (nitric acid + hydrogen peroxide) was added, followed by incubation at 37 °C for 15–20 min; then, a 1:1 hydrochloric acid solution was added, which was incubated at 37 °C for 5 min, followed by addition of perchloric acid after the temperature dropped, and then the mixture was incubated for 1 h prior to determination.
2.3. Isobaric tags for relative and absolute quantification (iTRAQ)-based proteomics
In order to screen out the selenoproteins/interacting proteins and signal transduction pathways that are closely involved in the formation of NET, and to elucidate the mechanisms by which selenoproteins regulate the formation of NET and affect the development of Se-deficient arteritis, we applied proteomics to analyze protein expression in aortic tissue of broilers based on the successful establishment of a chicken Se-deficient arteritis model. Protein extraction, digestion, and labeling with iTRAQ of broiler aortas were performed as Zhang et al. described [1]. LC-MS/MS analysis, peptide identification and quantification were analyzed using the Proteome Discoverer Software (Thermo Fisher Scientific, Waltham, MA, USA) as described by Jiang et al. [37]. Compared with the control group, a log2|fold change (FC)| > 1.5 and a false discovery rate (FDR) < 0.05 in the Se-deficient tissues were used as criteria for selection of the differentially expressed proteins.
2.4. Bioinformatics analysis
To take a more contextual view of the functions of these differentially expressed proteins, we summarized the differential proteins by enrichment analysis into molecular mechanisms that may be involved. The Gene Ontology (GO) database and the Kyoto Encyclopedia of Genes and Genomes (KEGG) database were used to analyze the GO and pathway enrichment annotations, respectively. The results were defined as significant if the GO terms or pathways had a P value < 0.05 and a FDR <0.05.
2.5. Isolation of neutrophils from chicken peripheral blood
Chicken peripheral blood neutrophils were isolated by a chicken peripheral blood neutrophil isolation kit (Beijing Solarbio Science & Technology Co., Ltd.) according to the instructions. Briefly, 10 mL of liquid A and 5 mL of liquid C were added to a new sterile 50 mL centrifuge tube (NEST Biotechnology. Co., Ltd.), and then 10 mL of chicken peripheral blood was added. After 15 min of centrifugation at 2000 rpm, the neutrophil layer was pipetted into a new 50 mL centrifuge tube, 5 mL of red blood cell lysate was added, and the contents of the tube were lysed for 5 min at room temperature. After 10 min of centrifugation at 300g, 20 mL PBS was added to wash the cells, and the cells were centrifuged at 250 g for 10 min, which was repeated twice. The isolated cells were resuspended in RPMI-1640 (Procell Life Science & Technology Co., Ltd.) supplemented with 10% FBS (Procell Life Science & Technology Co., Ltd.) and 2% CBS (Gibco, USA) for further experiments.
2.6. PAECs culture
PAECs were purchased from Otwo Biotechnology (Shenzhen) Co., Ltd. PAECs were cultured in RPMI-1640 supplemented with 10% FBS and 2% CBS and maintained at 37 °C in a humidified atmosphere containing 5% CO2. The cells were fed every day and subcultured once in a 25 cm2 cell culture flask (NEST Biotechnology Co., Ltd.) until reaching 90–100% confluence.
2.7. NET formation and cell treatment assays
Neutrophils were seeded at a density of 105 cells on poly-l-lysine (PLL)-coated coverslips in 24-well plates and cultured at 37 °C and 5% CO2 for 2.5 h. For NET formation in vitro, cells were treated with 0 nM, 50 nM, 100 mM, 150 mM, 200 mM, and 250 mM phorbol 12-myristate 13-acetate (PMA, Dalian Meilun Biotechnology. Co., Ltd.) for 3 h, and NETs were subsequently digested with or without DNase Ⅰ (50 μg/mL, 100 μg/mL, and 200 μg/mL) for 30 min. Following treatment, cells were stained with 0.25 μM SYTOX Green (Thermo Scientific, USA) and Hoechst 33258 (Leagene Biotechnology) for 30 min. After staining, cells were fixed, permeabilized, and washed with PBS, and images were acquired on a fluorescence microscope (Olympus, Japan).
2.8. Scanning electron microscopy
To observe the area size of NET formation in neutrophils, we performed scanning electron microscopy on neutrophils. Neutrophils were seeded on PLL-coated coverslips in 24-well plates and cultured at 37 °C and 5% CO2 for 2.5 h. Then the samples were fixed with 2.5% glutaraldehyde overnight. After fixation, the samples were washed with PBS, dehydrated with a graded ethanol series and displaced with a graded TERT butanol series. Then, critical-point drying was performed and coated with a metal film of 10 nm. Next, the samples were analyzed using a Hitachi SU8010 scanning electron microscope.
2.9. Cell transfection
To determine the effect of silencing SelS on PAECs, we silenced SelS expression in PAECs by cell transfection. PAECs were first seeded in a 12-well plate. Cells were transfected with Lipofectamine RNAiMAX (Invitrogen, USA) as previously described [38]. In brief, 40 nM RNAi duplexes (Table S5) were diluted with serum-free medium, to which 1 μL Lipofectamine RNAiMax was added, which was mixed gently and incubated at room temperature for 20 min. The medium was removed, and the PAECs were washed once with PBS. The mixture was added to the cells, which were incubated at 37 °C and 5% CO2 for 24 h. After that, complete medium was added and cultured for 24 h.
2.10. Coculture of PAECs and neutrophils
To determine the association of DNA-NETs with arteritis, we cocultured neutrophils with PAECs by the following treatments. For NET-induced PAEC inflammation, after the generation of NETs induced by 3 h-PMA stimulation in neutrophils treated with or without DNase Ⅰ, the medium was removed, and neutrophils were washed once with PBS. PAECs (5 × 104) in serum-free RPMI-1640 were added to the PLL-coated coverslips in 6-well plates and cultured at 37 °C and 5% CO2 for 20 h. For PAECs inflammatory-induced NET formation, after growth to subconfluency, 5 × 104 PAECs were seeded on PLL-coated coverslips in 6-well plates, and 24 h after transfection, were treated with or without NAC (Abmole Bioscience Inc.) for 12 h. PAECs were washed once with PBS, and then 2.5 × 105 neutrophils were seeded on the PLL-coated coverslips in 6-well plates with serum-free RPMI-1640. After 20 h, the cells were washed by PBS, and were stained with 0.25 μM SYTOX Green or 10 μM 2,7-dichlorodihydrofluorescein diacetate (DCFH-DA, NJJCBIO, China). Images were acquired on a fluorescence microscope.
2.11. Histological staining
To determine the pathological changes in the aortic tissues of Se-deficient broilers and determine the etiology of the disease, we performed the following histological examinations. The aortas were washed in precooled 0.9% saline to remove the blood and then dried with filter paper. Then, the tissues were fixed in 4% paraformaldehyde (PFA) for 2 h at 4 °C. H&E staining was performed as previously described [38]. For immunofluorescence (IF), the tissues were fixed in 4% PFA for 24 h at 4 °C, washed with PBS, embedded in paraffin and sectioned at 4-μm thickness. Antigen retrieval was performed using target retrieval solution, pH 9.0 (Dako), in a pressure cooker for 15–20 min. Nonspecific binding was then blocked with 5% BSA for 25 min at room temperature. For IF, cells were fixed with 4% PFA for 25 min at room temperature, washed with PBS and permeabilized with or without 0.2% Triton X-100 in PBS for 20 min. Cells were then blocked in PBS with 2% BSA for 30 min at room temperature. Subsequently, the samples were incubated with primary antibodies overnight at 4 °C. The antibodies, dilution factors, sources and other information are presented in Table S2. The samples were incubated with Alexa Fluor-conjugated secondary antibodies (Invitrogen) in 1% BSA for 1 h at room temperature. DAPI was then used to counterstain the nuclei, and images were obtained by a Leica TCS SP2 laser confocal microscope.
2.12. Lactate dehydrogenase (LDH) release
To examine the degree of integrity of the cell membrane, we examined the LDH release level in cells. An LDH Assay Kit (Beyotime Biotechnology) was used according to the manufacturer's instructions as previously described [38]. Briefly, broiler peripheral blood neutrophils and PAECs were seeded in 96 well plates at a density of 105/mL, add 20 μL LDH release reagent per well until cells were 80% confluence and incubated for 30 min, centrifuged at 400 g for 5 min, 120 μL supernatant was collected from each well and was added to a new 96 well plate. Absorbance was then determined at 490 nm by a microplate reader (BioTek, USA).
2.13. Total RNA isolation and qRT-PCR
Samples for total RNA extraction included broiler aortas, PAECs, and cocultured cells of neutrophils and PAECs. An RNA Extraction Kit (Accurate Biotechnology (Hunan) Co., Ltd.) was used to extract the total RNA according to the manufacturer's instructions as previously described [38]. NanoDrop ND-2000 (Thermo Fisher Scientific) was used to detect the extracted RNA, when A260/A280 was between 1.9 and 2.1, the concentration was >350 ng/μL, the extracted RNA can be used for subsequent first strand cDNA synthesis. First-strand cDNA was synthesized using a cDNA First Strand Synthesis Kit (Bioer Technology, China). qRT-PCR (Bioer Technology, China) was performed on a Light Cycler@480 System (Roche, Switzerland). The sequences of mRNAs (25 selenoproteins, cytokines, NET formation-related genes, chemokines, antioxidant genes, and PPARs) are detailed in Tables S3 and S4. Relative expression of the target genes was calculated according to the 2−ΔΔCt method by comparing them to the expression of the housekeeping gene β-actin.
2.14. Protein extraction and Western blot analysis
Samples for protein extraction included broiler aortas, PAECs, and cocultured cells of neutrophils and PAECs. Total protein extraction and Western blot analysis were performed as previously described [39]. Briefly, protein samples were separated on 12% SDS-PAGE gels and transferred onto PVDF membranes. The PVDF membranes were blocked with 5% nonfat milk for 2 h at 37 °C, incubated with primary antibodies (SelS, cytokines, NET formation-related proteins, and PPARs) for 16 h at 4 °C, and further incubated with secondary antibodies for 1 h at 37 °C. The antibodies, dilution factors, sources and other information are presented in Table S2. The signal was detected by a FluorChem R FR1045 (ProteinSimple, USA). The relative expression levels were calculated by comparing them to the expression of the β-actin.
2.15. Statistical analysis
Statistical analysis of all data was conducted using GraphPad Prism version 8.0 software. All results were expressed as the mean ± SEM. Statistical significance was obtained by one-way ANOVA or unpaired Student's t-test using Tukey's post hoc test. P < 0.05 was considered to be statistically significant. The software showed a normal distribution. For the in vivo data, the bars represent the mean ± SEM of 10 individuals (N = 10). For the in vitro data, the bars represent the mean ± SEM of triplicate cells (N = 3).
3. Results
3.1. Se deficiency induces arteritis in broilers
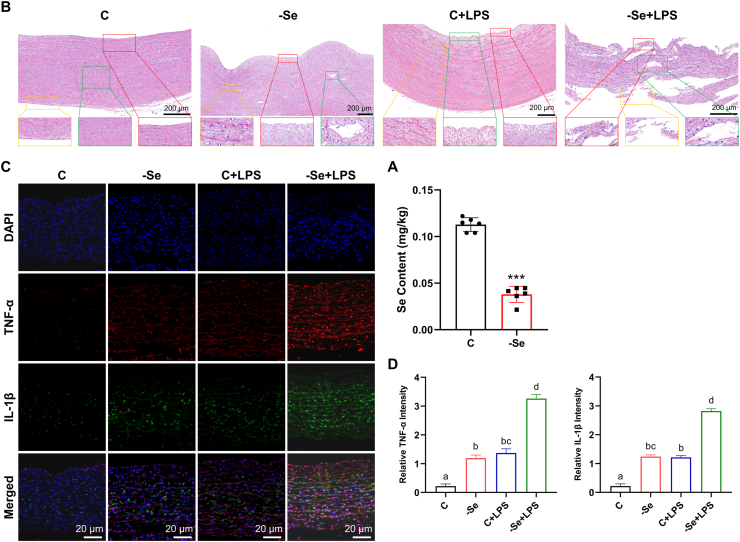
To determine the correlation between Se deficiency and arteritis in broilers, we first examined the Se content in broiler plasma and found that the Se content in broiler plasma in the Se deficiency group was significantly decreased compared with that in the control group (Fig. 1A) (P < 0.001), which illustrated the successful establishment of the chicken Se deficiency model. H&E staining was further used to observe the pathological changes in the broiler aortas (Fig. 1B). Compared with the normal aorta morphology of the control group, the broiler aortas in the -Se group showed infiltration of lymphocytes, plasma cells and monocytes, hyperplasia of collagen fibers in the intima and adventitia (yellow box), partial rupture of elastic fibers in the media (green box), and slight uplift of the intima (red box). This suggested that the model of Se-deficient arteritis was established successfully. For the broiler aortas stimulated by LPS, their histopathological structures also showed obvious inflammatory characteristics, such as the destruction and interruption of the aortic elastic plate, the replacement of scar tissue (yellow box), the uneven surface of the intima, a large amount of plaque uplift (green box), a large amount of collagen fiber hyperplasia in the adventitia, thickening of the vascular wall, and obvious inflammatory cell infiltration. Pathological changes in the aortas in the -Se + LPS group were observed, and the most significant characteristics of arteritis were found, including degeneration and necrosis of arterial smooth muscle cells, destruction of elastic fibers and infiltration of inflammatory cells (green box), and rupture and destruction of internal and external elastic membranes, which caused granulomatous inflammatory reactions, including multinucleated giant cells, epithelial-like cells and inflammatory cell lymphocyte and monocyte infiltration (red box and yellow box).
Fig. 1.
Se deficiency induces arteritis in broilers. (A) Se content in plasma of broilers (N = 10; ***P < 0.001). (B) Histopathological changes of aortas of broilers (scale bar, 200 μm). Fields from one representative experiment of three are shown. (C) IF analyses of TNF-α (red) and IL-1β (green) in broiler aortas (scale bar, 20 μm). Fields from one representative experiment of three are shown. (D) Quantification of relative TNF-α and IL-1β intensity in broiler aortas (N = 3; Bars that do not share the same letters are significantly different (P < 0.05) from each other). Results are presented as mean ± SEM. Statistical significance was obtained by one-way ANOVA or unpaired Student's t-test. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Furthermore, the expression of cytokines was detected (Figs. S1A–S1C). It was found that -Se and LPS could significantly increased the mRNA and protein expression levels of cytokines (P < 0.05). Under the combined treatment of -Se and LPS, except for interferon-γ (IFN-γ), the expression levels of other cytokines in the aortas reached the highest level, even significantly higher than those in the -Se and LPS groups (P < 0.05). The expression of tumor necrosis factor-α (TNF-α) and interleukin-1β (IL-1β) in broilers aortas detected by IF showed the same trend in changes (Fig. 1C and D) (P < 0.05). The above results show that -Se can promote the development of arteritis and reflect the successful replication of the broiler arteritis model.
3.2. Se deficiency increases NET formation in the aortas and neutrophils of broilers
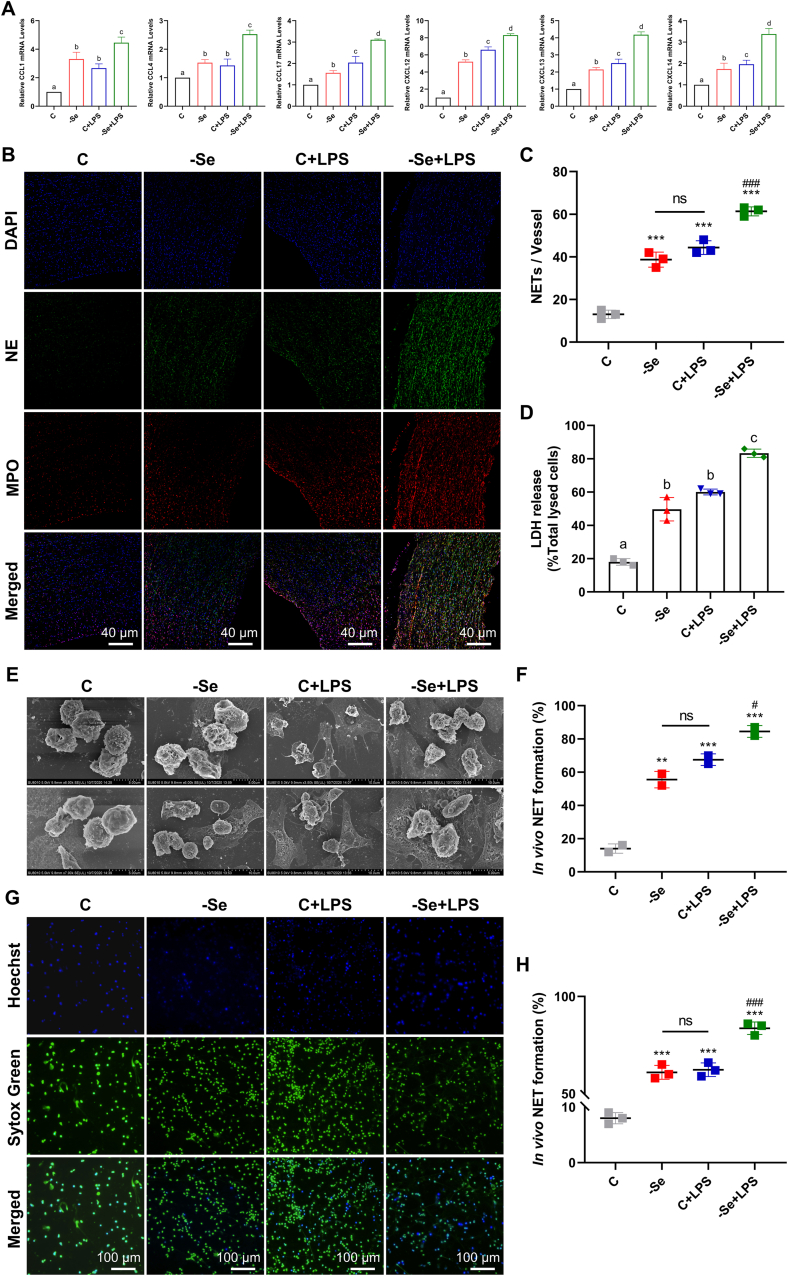
We next explored whether NET was formed in Se-deficient arteritis. First, we examined the mRNA expression levels of neutrophil chemokines (CCL1, CCl4, CCL17, CXCL12, CXCL13, and CXCL14) in the aortas and found that they were all significantly expressed under the pathological conditions of Se-deficient arteritis compared with the C group (P < 0.05); In the aortas of the C + LPS group, the mRNA expression levels of chemokines also exhibited an elevation similar to that of the -Se group (P < 0.05); whereas in the -Se + LPS group, the mRNA expression levels of chemokines were all significantly higher than those of the other groups (Fig. 2A) (P < 0.05), suggesting that neutrophils are recruited to Se-deficient aortas. Next, examination of the expression profiles of genes and proteins involved in the formation of NET (MPO, NE, H3, NOX2, PKCα, PKCβ, PKCζ, and PLCγ) revealed that compared with the control group, the mRNA and protein expression levels of NET formation-related genes in the aortas of the other groups were significantly increased, and that their expression peaked in the -Se + LPS group (Figs. S2A–S2C) (P < 0.05), indicating that Se deficiency may induce the formation of NET in the aortas to the same extent as LPS. We further detected the colocalization of MPO and NE (marker proteins for NET formation) in the aortas by IF (Fig. 2B and C) (P < 0.001), which was strong evidence that Se deficiency could induce increased NET formation in the aortas of broilers. LDH assays on neutrophils extracted from the peripheral blood of broilers in each group revealed that LDH release from neutrophils in the other groups was significantly increased compared with that of the control group (Fig. 2D) (P < 0.05), indicating that Se deficiency can induce neutrophil rupture, suggesting the possibility of NET release. Next, we observed directly that -Se induced the extrusion of NET from neutrophils by SEM and SYTOX Green staining (Fig. 2E-H) (P < 0.01). All the above results illustrate that Se deficiency can induce the extrusion of NET and that Se-deficient arteritis can lead to increased formation of NET in the aorta.
Fig. 2.
Se deficiency increases NET formation in the aortas and neutrophils of broilers. (A) The mRNA expression levels of neutrophil chemokines (CCL1, CCL4, CCL17, CXCL12, CXCL13, and CXCL14) in broiler aortas (N = 10; Bars that do not share the same letters are significantly different (P < 0.05) from each other). (B) IF analyses of NE (green) and MPO (red) in broiler aortas (scale bar, 40 μm). Fields from one representative experiment of three are shown. (C) Quantification of relative NE and MPO intensity in broiler aortas (N = 3, ***P < 0.001, compared with the C group; ###P < 0.001, compared with the -Se group and the C + LPS group). (D) LDH release of chicken peripheral blood neutrophils (N = 3; Bars that do not share the same letters are significantly different (P < 0.05) from each other). (E) Scanning electron microscopy images of neutrophils (NETs) isolated from chicken peripheral blood (scale bar, 5 μm and 10 μm). Fields from two representative experiment of three are shown. (F) Quantification of NET formation by scanning electron microscopy (N = 3, ***P < 0.001, compared with the C group; ###P < 0.001, compared with the -Se group and the C + LPS group). (G) Fluorescence microscope for NETs (green) of neutrophils isolated from chicken peripheral blood (scale bar, 100 μm). Fields from one representative experiment of three are shown. (H) Quantification of NET formation by fluorescence microscope (N = 3, ***P < 0.001, compared with the C group; ###P < 0.001, compared with the -Se group and the C + LPS group). Results are presented as mean ± SEM. Statistical significance was obtained by one-way ANOVA or unpaired Student's t-test. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
3.3. DNA-NETs promote arteritis progression
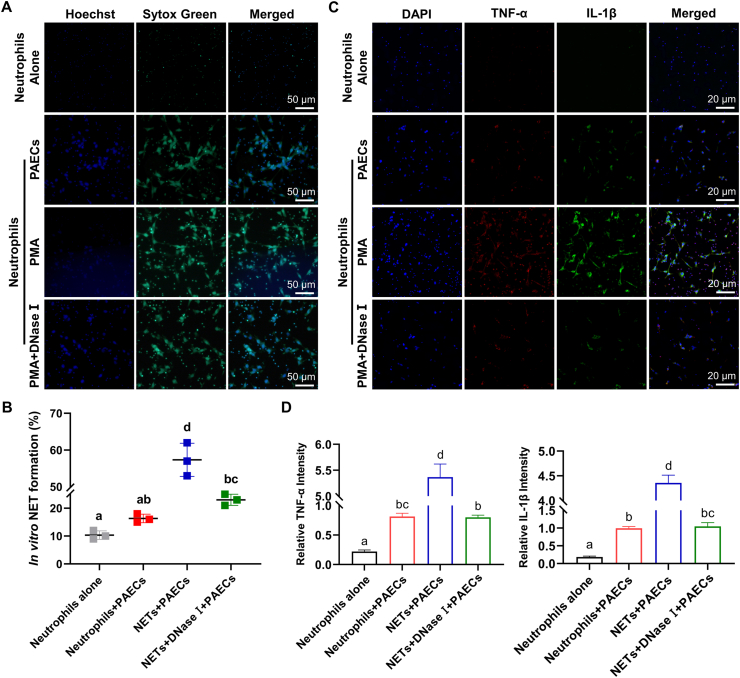
To determine whether NETs could induce arteritis, we extracted chicken peripheral blood neutrophils and stimulated them with different concentrations of PMA to generate NETs. Finally, we determined that the stimulating concentration of PMA required was 200 nM (Figs. S3A and S3B) (P < 0.05). Similarly, DNase I is known to be the most effective method to clear DNA-NETs. In this experiment, neutrophils stimulated with 200 nM PMA were further treated with different concentrations of DNase I, and 100 μg/mL DNase I was determined to have a significant effect on clearing NETs (Figs. S3C and S3D) (P < 0.01). Next, neutrophils were stimulated by PMA to generate NETs, and then PAECs were placed in 6-well plates containing NETs. After coculture for 20 h, the release of NETs in the NET + PAEC group was observed by SYTOX Green staining (Fig. 3A and B) (P < 0.05). IF was used to detect the expression of TNF-α and IL-1β in the cocultured cells. PAECs treated with NETs expressed higher levels of TNF-α and IL-1β, while the expression of these cytokines was decreased significantly in PAECs cocultured with neutrophils after DNase I-scavenged DNA-NETs (Fig. 3C and D) (P < 0.05), indicating that the DNA-NETs can induce an inflammatory response in PAECs.
Fig. 3.
DNA-NETs promote arteritis progression. (A) Fluorescence microscope for NETs (green) of PAECs cocultured with or without PMA-stimulated and with or without DNase Ⅰ-treated neutrophils (scale bar, 50 μm). Fields from one representative experiment of three are shown. (B) Quantification of NET formation by fluorescence microscope in PAECs cocultured with or without PMA-stimulated and with or without DNase Ⅰ-treated neutrophils (N = 3; Groups that do not share the same letters are significantly different (P < 0.05) from each other). (C) IF analyses of TNF-α (red) and IL-1β (green) in PAECs cocultured with or without PMA-stimulated and with or without DNase Ⅰ-treated neutrophils (scale bar, 20 μm). Fields from one representative experiment of three are shown. (D) Quantification of relative TNF-α and IL-1β intensity in PAECs cocultured with or without PMA-stimulated and with or without DNase Ⅰ-treated neutrophils (N = 3; Bars that do not share the same letters are significantly different (P < 0.05) from each other). Results are presented as mean ± SEM. Statistical significance was obtained by one-way ANOVA or unpaired Student's t-test. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
3.4. SelS is a major regulator of NET formation mediated by Se-deficient arteritis
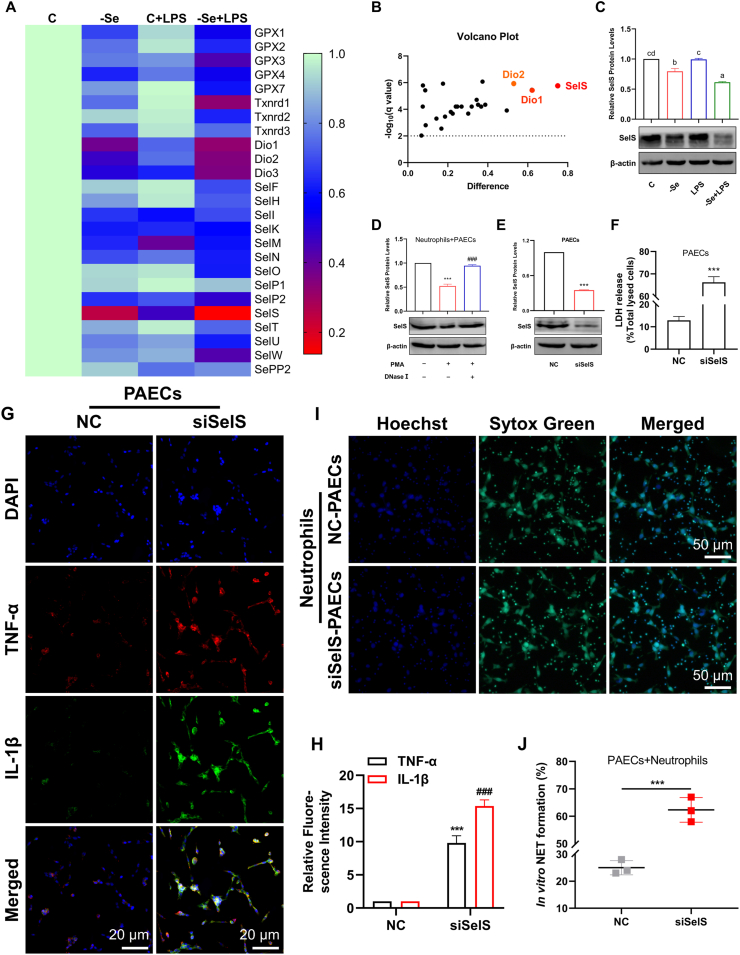
Se, as an essential trace element, mainly exists in proteins in the form of selenocysteine. To explore the specific role of selenoproteins in NET formation mediated by Se-deficient arteritis, we first assessed the mRNA expression of 25 selenoproteins in chicken aortas. The mRNA expression level of SelS was decreased most significantly in the -Se group, and the mRNA expression level of SelS decreased to its lowest level in the -Se + LPS group (Fig. 4A and B). Further assessment of SelS protein expression level in the chicken aortas of each group also showed that compared with the control group, the protein expression levels of SelS in other groups were significantly decreased, and the SelS protein level of the -Se + LPS group was significantly lower than that of the -Se group and the C + LPS group (Fig. 4C) (P < 0.05). Moreover, in the presence of NETs induced in vitro, the level of SelS in PAECs was decreased significantly, and the level of SelS was increased after DNase I cleared the DNA-NETs (Fig. 4D) (P < 0.001). These results suggest that SelS may play an important role in regulating NET formation mediated by Se-deficient arteritis.
Fig. 4.
SelS is a major regulator of NET formation mediated by Se-deficient arteritis. (A)–(B) Heatmap (A) and volcano plot (B) of 25 selenoprotein mRNA expression levels in broiler aortas. (C) The protein expression levels of SelS in broiler aortas (N = 3; Bars that do not share the same letters are significantly different (P < 0.05) from each other). (D) The protein expression levels of SelS in PAECs cocultured with or without PMA-stimulated and with or without DNase Ⅰ-treated neutrophils (N = 3, ***P < 0.001, compared with the normal Neutrophils + PAECs; ###P < 0.001, compared with the PAECs cocultured with PMA-stimulated but without DNase Ⅰ-treated neutrophils). (E) After transfection with siSelS for 24 h, the protein expression of SelS in PAECs. (F) LDH release of PAECs after transfection with siSelS for 24 h (N = 3, ***P < 0.001). (G) IF analyses of TNF-α (red) and IL-1β (green) in PAECs after transfection with siSelS for 24 h (scale bar, 20 μm). Fields from one representative experiment of three are shown. (H) Quantification of relative TNF-α and IL-1β intensity in PAECs after transfection with siSelS for 24 h (N = 3, ***P < 0.001, compared with the NC group for TNF-α; ###P < 0.001, compared with the NC group for IL-1β). (I) Fluorescence microscope for NETs (green) of neutrophils cocultured with PAECs transfected with or without siSelS (scale bar, 50 μm). Fields from one representative experiment of three are shown. (J) Quantification of NET formation by fluorescence microscope in neutrophils co-cultured with PAECs transfected with or without siSelS (N = 3; ***P < 0.001). Results are presented as mean ± SEM. Statistical significance was obtained by one-way ANOVA or unpaired Student's t-test. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
To further investigate the regulatory effect of SelS on NETs in arteritis, we next silenced the expression of SelS in PAECs (Fig. 4E) (P < 0.001) and found that LDH was released significantly in siSelS-PAECs (Fig. 4F) (P < 0.001). The expression levels of TNF-α and IL-1β were significantly higher than those in NC-PAECs (Fig. 4G and H) (P < 0.001), indicating that silencing SelS can significantly induce an inflammatory response in PAECs. Next, PAECs were cocultured with neutrophils for 12 h in the inflammatory state of PAECs induced by silencing SelS, and SYTOX Green staining showed that the generation of NET was increased significantly (Fig. 4I and J) (P < 0.001). These results suggest that SelS may be involved in NET formation mediated by Se-deficient arteritis.
3.5. NET formation in Se-deficient arteritis is ROS-dependent
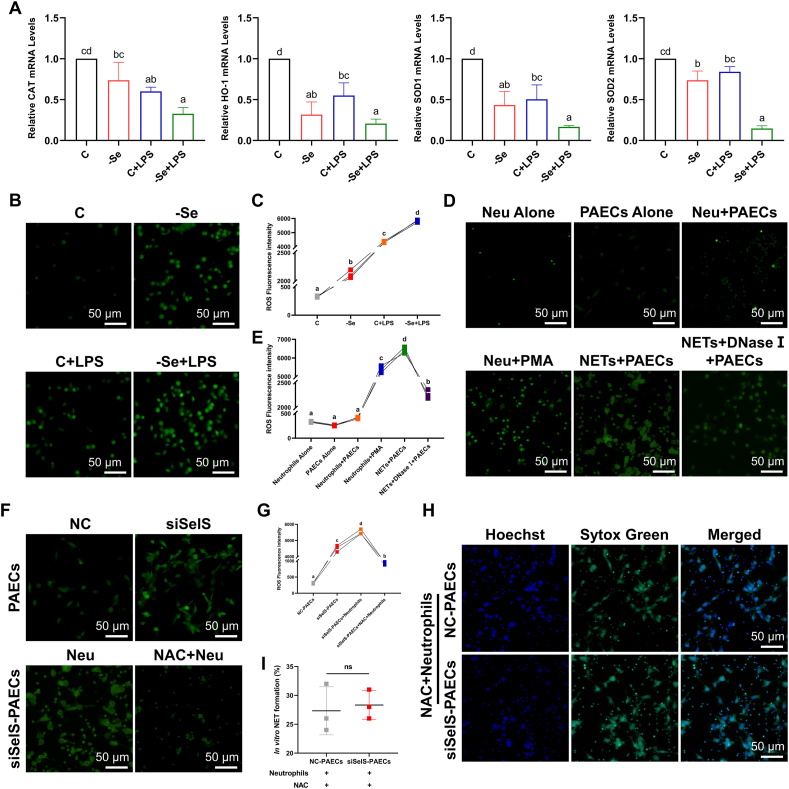
Se deficiency can lead to an inflammatory response in multiple tissues accompanied by the involvement of oxidative stress. Additionally, one of the initiating factors of NET extrusion is ROS. In this experiment, the mRNA expression levels of antioxidant genes (CAT, HO-1, SOD1, and SOD2) in the aortas of broilers were first examined, and we found that the mRNA expression levels of antioxidant genes in the aortas of the other groups were all significantly decreased compared with the control group and that their expression levels reached the lowest levels in the -Se + LPS group (Fig. 5A) (P < 0.05). Likewise, fluorescence microscopy was used to observe the ROS levels in the peripheral blood neutrophils of broilers in each group, and similar changes were also found with the mRNA expression of the antioxidant genes in the aortas (Fig. 5B and C) (P < 0.05). We next stimulated chicken peripheral blood neutrophils with PMA in vitro to induce NET generation, and after coculturing NETs with PAECs, we found that PMA-stimulated neutrophils underwent a significant ROS burst, which was significantly reduced after digestion of the DNA-NETs with DNase I (Fig. 5D and E) (P < 0.05), suggesting that DNA-NETs can induce an ROS burst in PAECs.
Fig. 5.
NET formation in Se-deficient arteritis is ROS-dependent. (A) The mRNA expression levels of antioxidant genes (CAT, HO-1, SOD1, and SOD2) in broiler aortas (N = 10; Bars that do not share the same letters are significantly different (P < 0.05) from each other). (B)–(C) Representative images of fluorescence microscope (B) and quantification (C) of ROS (green) in chicken peripheral blood neutrophils (scale bar, 50 μm, N = 3; Groups that do not share the same letters are significantly different (P < 0.05) from each other). (D)–(E) Representative images of fluorescence microscope (D) and quantification (E) of ROS (green) in neutrophils, and PAECs cocultured with or without PMA-stimulated and with or without DNase Ⅰ-treated neutrophils (scale bar, 50 μm, N = 3; Groups that do not share the same letters are significantly different (P < 0.05) from each other). (F)–(G) Representative images of fluorescence microscope (F) and quantification (G) of ROS (green) in NC-PAECs, siSelS-PAECs, siSelS-PAECs + Neutrophils, and siSelS-PAECs + NAC + Neutrophils (scale bar, 50 μm, N = 3; Groups that do not share the same letters are significantly different (P < 0.05) from each other). (H)–(I) Representative images of fluorescence microscope (H) and quantification (I) of NETs (green) in neutrophils cocultured with siSelS-PAECs pre-treated with NAC (scale bar, 50 μm, N = 3). Results are presented as mean ± SEM. Statistical significance was obtained by one-way ANOVA or unpaired Student's t-test. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
To determine whether NET formation in Se-deficient arteritis was dependent on ROS, we found that siSelS-PAECs underwent an ROS burst, and no reduction was observed in ROS levels after coculture with neutrophils (Fig. 5F and G) (P < 0.05). However, inhibiting the ROS burst in siSelS-PAECs with NAC and then coculturing them with neutrophils revealed no change in NET formation compared with the NC-Control (Fig. 5H and I) (P > 0.05). These results illustrate that the DNA-NETs-induced inflammatory response of arterial endothelial cells by NETs is dependent on ROS.
3.6. The PPAR pathway participates in NET formation induced by Se-deficient arteritis
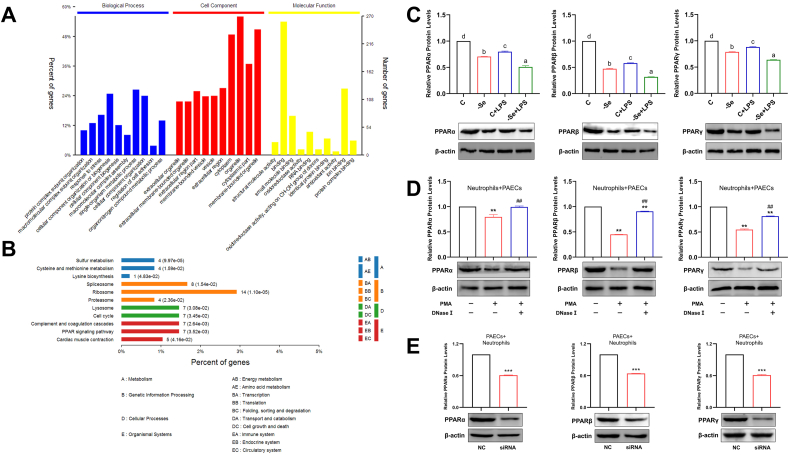
To explore the molecular mechanisms involved in NET formation induced by Se deficiency, we subjected aortas from control and Se-deficient broilers to proteomic analysis. Based on the cutoff assessment, 352 proteins were mapped, and 128 proteins were unmapped. GO enrichment analysis showed that the differentially expressed proteins in the -Se group were significantly enriched in processes such as “energy metabolism”, “cell growth and death”, “immune system”, and “antioxidant activity” (Fig. 6A). KEGG analysis indicated that the differentially expressed proteins in the -Se group were significantly enriched in the “cell cycle”, “complement and coagulation cascades”, and “PPAR pathway” (Fig. 6B). The PPAR pathway is known to be important in cardiovascular diseases, and to clarify whether the PPAR pathway could be involved in regulating NET formation induced by Se deficiency, we first examined the mRNA and protein expression levels of PPARs in the aortas of broilers. As expected, PPARs were significantly downregulated in both the -Se group and the C + LPS group and were decreased to a minimum in the -Se + LPS group (Figs. S4 and 6C) (P < 0.05). The expression levels of PPARs were significantly decreased in PAECs treated with NETs, whereas their expression levels rebounded after elimination of DNA-NETs (Fig. 6D) (P < 0.01). Conversely, we next cocultured siSelS-PAECs with neutrophils, and the expression levels of PPARs were similarly significantly downregulated (Fig. 6E) (P < 0.001). The above results illustrate that the PPAR pathway is involved in the process of NET formation induced by Se-deficient arteritis.
Fig. 6.
The PPAR pathway participates in NET formation induced by Se-deficient arteritis. (A)–(B) The GO analysis (A) with GO database and the pathway analysis (B) with KEGG database were applied for functional annotation. The significant GO terms and pathways were defined as P < 0.05 and FDR <0.05. (C) The protein expression levels of PPARs (PPARα, PPARβ, and PPARγ) in broiler aortas (N = 10; Bars that do not share the same letters are significantly different (P < 0.05) from each other). (D) The protein expression levels of PPARs (PPARα, PPARβ, and PPARγ) in PAECs cocultured with or without PMA-stimulated and with or without DNase Ⅰ-treated neutrophils (N = 3, ***P < 0.001, compared with the normal Neutrophils + PAECs; ###P < 0.001, compared with the PAECs cocultured with PMA-stimulated but without DNase Ⅰ-treated neutrophils). (E) The protein expression levels of PPARs (PPARα, PPARβ, and PPARγ) in neutrophils cocultured with PAECs transfected with or without siSelS (N = 3; ***P < 0.001). Results are presented as mean ± SEM. Statistical significance was obtained by one-way ANOVA or unpaired Student's t-test.
4. Discussion
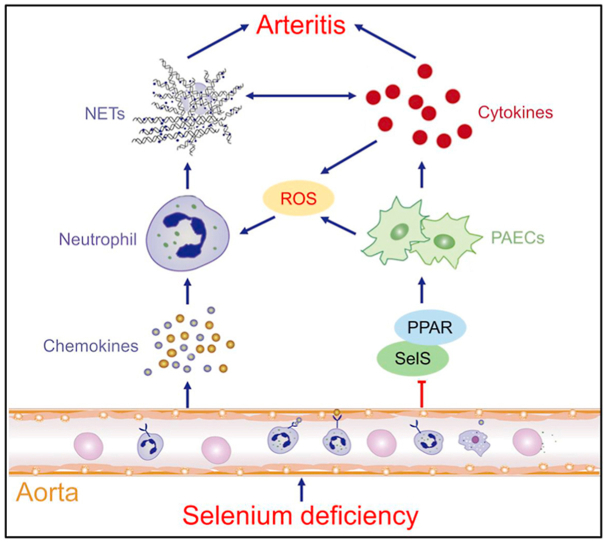
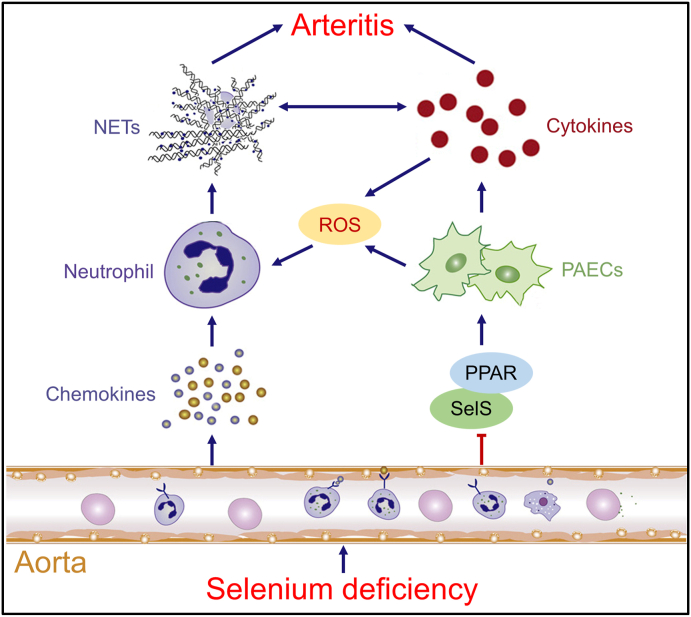
In this study, we report a role for silencing SelS in promoting Se-deficient arteritis by both recruiting and activating neutrophils in Se-deficient aortas. Se deficiency induces chemokine expression, recruitment of neutrophils, and extrusion of NET in the aortic tissue of broilers, inducing the release of inflammatory factors from PAECs and promoting arteritis progression. On the other hand, Se deficiency induces the secretion of proinflammatory cytokines from PAECs by decreasing the expression of SelS and inhibiting the PPAR pathway, inducing NET formation in a ROS-dependent manner, and ultimately promoting arteritis (Fig. 7).
Fig. 7.
The schematic diagram of regulatory of SelS in ROS-dependent NET formation induced by Se-deficient arteritis. Se deficiency induces chemokine expression, recruitment of neutrophils, and extrusion of NET in the aortic tissue of broilers, inducing the release of inflammatory factors from PAECs and promoting arteritis progression. On the other hand, Se deficiency induces the secretion of proinflammatory cytokines from PAECs by decreasing the expression of SelS and inhibiting the PPAR pathway, inducing NET formation in a ROS-dependent manner, and ultimately promoting arteritis.
Se deficiency can induce inflammation and oxidative stress in multiple tissues, cause vascular injury and promote the release of cytokines [40,41]. A 10-year prospective study reported the association between Se and atherosclerosis, arterial stiffness and hypertension, they suggested that Se has long-term vasoprotective effects on arterial stiffness and blood pressure in Africans [42]. Serum Se levels were significantly lower in chronic heart failure patients and Se levels were negatively correlated with left ventricular volume and pulmonary artery pressure [43]. The involvement of NETs in inflammatory diseases has recently received intensive attention. NETs are usually induced by proinflammatory chemokines, immune complexes, ionomycin and nicotine [29]. Deficiencies in the trace elements zinc and copper can also lead to NET formation [44,45]. Recently, the detrimental aspect of DNA-NET, in that NETs generated by the inflammatory microenvironment may be the center of a vicious cycle of inflammation, has gradually been revealed. In a mouse model of ventilator-induced lung injury, intravascular NETs impaired ventilatory function and pulmonary microcirculation [46]. Under acute sterile inflammation, the administration of DNase Ⅰ significantly reduced hepatic damage [47]. However, to date, in cardiovascular diseases, most studies on NETs have focused on their promoting effects on atherosclerosis, thrombosis and acute myocardial infarction [48]. Moreover, NETs have been suggested to directly induce endothelial dysfunction by activating and damaging endothelial cells [49,50]. In this experiment, we found that NET formation was increased in Se-deficient arteritis and that DNA-NET structures induced by PMA in vitro could also sensitize arterial endothelial cells to an inflammatory response. Therefore, exploring the regulation of NET formation by selenoproteins may suggest a new mechanism for the development of Se-deficient arteritis.
Se plays an important role in antioxidant defense, redox signaling, and redox homeostasis, and excessive ROS can induce oxidative stress when overwhelming antioxidant defense systems. Thus, most of the body with Se deficiency will develop oxidative stress [51]. Se deficiency is a major regulator of selenoprotein expression, and several of the 25 selenoprotein genes identified to date have important cellular functions in antioxidant defense, cellular signaling, and redox homeostasis [2]. SelS is implicated in oxidative stress and the inflammatory response. SelS is involved in defense against oxidative stress-induced vascular endothelial injury by regulating the PKCα/PI3K/Akt/eNOS pathway [17] and inhibits inflammation-induced vascular smooth muscle cell calcification by inhibiting the activation of the nuclear factor kappa-B (NF-κB) signaling pathway and endoplasmic reticulum stress [16]. Conversely, the knockdown of SelS results in an enhancement of TNF-α-induced injury in human umbilical vein endothelial cells [52]. The activation of neutrophils and their release of cellular contents are strongly regulated by ROS, and the respiratory burst is considered a key early step in NET formation [29]. Although NET formation may also be ROS-independent [53], the premise is to consider Ca2+ influx as an inducer of NETs. Our results showed that siSelS-PAECs induced an increase in ROS burst and NET formation, but when cocultured with neutrophils after NAC treatment of siSelS-PAECs, ROS levels and NET formation were significantly reduced, indicating that siSelS regulated NET generation in a ROS-dependent manner.
In recent years, researchers have widely applied omics analysis to explore the pathogenesis of cardiovascular diseases in humans and animals, which suggests that it is also more important to apply omics analysis to the study of herd diseases. In this study, we used proteomic analysis to determine that the PPAR pathway may be involved in the formation of NET induced by Se-deficient arteritis. More is known about the role of the PPAR pathway in cardiovascular diseases, which is manifested in fat metabolism, as activation of both PPARα and PPARγ prevent foam cell formation and atherosclerosis development in mice [54]. However, PPARs can also regulate inflammatory processes through some inflammatory target genes. Activation of PPARs can inhibit LPS- and cytokine-induced proinflammatory gene expression through inhibition of the NF-κB signaling pathway [55]. PPARα increases the expression of an inhibitor of NF-κB to antagonize the NF-κB signaling pathway [56]. PPARβ induces transforming growth factor β and inhibits the activation of NF-κB, thereby regulating inflammatory processes [57]. Activation of PPARγ also decreases TNF-α expression in the aortic root [58]. In this experiment, whether in the inflammatory response of PAECs induced by DNA-NETs in vitro or in the NET generation induced by siSelS-PAECs, the expression levels of PPARs appeared to be decreased, raising the idea that the activation of the PPAR pathway may also be involved in the inhibition of NET formation.
Overall, our data establish the potential of SelS as a prognostic marker and therapeutic target in ROS-dependent NET-mediated arteritis. Given the potential regulatory role of the PPAR pathway in NET formation, future clinical trials with agonists and ligands of the PPAR pathway could be performed to determine their effectiveness against NET-mediated arteritis. Although the detailed mechanism of NET formation has yet to be defined, these findings will help our understanding of the regulation and functionality of NETs in arteritis.
Funding
This work was supported by the National Natural Science Foundation of China (31872437, 32072811).
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We thank the Key Laboratory of the Provincial Education Department of Heilongjiang for Common Animal Disease Prevention and Treatment, College of Veterinary Medicine, Northeast Agricultural University for collecting samples.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.redox.2021.102003.
Contributor Information
Shiwen Xu, Email: shiwenxu@neau.edu.cn.
Shu Li, Email: lishu@neau.edu.cn.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Zhang Z. The proteomic profiling of multiple tissue damage in chickens for a selenium deficiency biomarker discovery. Food & function. 2020;11:1312–1321. doi: 10.1039/c9fo02861g. [DOI] [PubMed] [Google Scholar]
- 2.Avery J.C., Hoffmann P.R. Selenium, selenoproteins, and immunity. Nutrients. 2018;10:1203. doi: 10.3390/nu10091203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nève J., Vertongen F., Molle L. Selenium deficiency. Clin. Endocrinol. Metabol. 1985;14:629–656. doi: 10.1016/s0300-595x(85)80010-4. [DOI] [PubMed] [Google Scholar]
- 4.Ferrans V.J., Van Vleet J.F. Cardiac lesions of selenium-vitamin E deficiency in animals. Heart Ves. 1985;1:294–297. doi: 10.1007/BF02072413. [DOI] [PubMed] [Google Scholar]
- 5.Zhang Y. MicroRNA-33-3p regulates vein endothelial cell apoptosis in selenium-deficient broilers by targeting E4F1. Oxidative medicine and cellular longevity. 2019 doi: 10.1155/2019/6274010. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cao C. Inflammatory response occurs in veins of broiler chickens treated with a selenium deficiency diet. Biol. Trace Elem. Res. 2018;183:361–369. doi: 10.1007/s12011-017-1145-5. [DOI] [PubMed] [Google Scholar]
- 7.Arthur J.R., McKenzie R.C., Beckett G.J. Selenium in the immune system. J. Nutr. 2003;133:1457s–1459s. doi: 10.1093/jn/133.5.1457S. [DOI] [PubMed] [Google Scholar]
- 8.Cao Y.Z., Reddy C.C., Sordillo L.M. Altered eicosanoid biosynthesis in selenium-deficient endothelial cells. Free Radical Biol. Med. 2000;28:381–389. doi: 10.1016/s0891-5849(99)00251-8. [DOI] [PubMed] [Google Scholar]
- 9.Li S. Se deficiency induces renal pathological changes by regulating selenoprotein expression, disrupting redox balance, and activating inflammation. Metall. 2020;12:1576–1584. doi: 10.1039/d0mt00165a. [DOI] [PubMed] [Google Scholar]
- 10.Branco V., Canário J., Lu J., Holmgren A., Carvalho C. Mercury and selenium interaction in vivo: effects on thioredoxin reductase and glutathione peroxidase. Free Radical Biol. Med. 2012;52:781–793. doi: 10.1016/j.freeradbiomed.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 11.Huang K., Liu H., Chen Z., Xu H. Role of selenium in cytoprotection against cholesterol oxide-induced vascular damage in rats. Atherosclerosis. 2002;162:137–144. doi: 10.1016/s0021-9150(01)00707-9. [DOI] [PubMed] [Google Scholar]
- 12.Liu H., Xu H., Huang K. Selenium in the prevention of atherosclerosis and its underlying mechanisms. Metall. 2017;9:21–37. doi: 10.1039/c6mt00195e. [DOI] [PubMed] [Google Scholar]
- 13.Kiełczykowska M., Kocot J., Paździor M., Musik I. Selenium——a fascinating antioxidant of protective properties. Adv. Clin. Exp. Med. 2018;27:245–255. doi: 10.17219/acem/67222. [DOI] [PubMed] [Google Scholar]
- 14.Thangavel S. Oxidative stress in HIV infection and alcohol use: role of redox signals in modulation of lipid rafts and ATP-binding cassette transporters. Antioxidants Redox Signal. 2018;28:324–337. doi: 10.1089/ars.2016.6830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dunn S., Hilgers R.H., Das K.C. Thioredoxin deficiency exacerbates vascular dysfunction during diet-induced obesity in small mesenteric artery in mice. Microcirculation. 2020 doi: 10.1111/micc.12674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ye Y., Bian W., Fu F., Hu J., Liu H. Selenoprotein S inhibits inflammation-induced vascular smooth muscle cell calcification. J. Biol. Inorg. Chem. 2018;23:739–751. doi: 10.1007/s00775-018-1563-7. [DOI] [PubMed] [Google Scholar]
- 17.Zhong Y. Selenoprotein S attenuates endothelial dysfunction in a diabetic vascular chip. Exp. Gerontol. 2020;137 doi: 10.1016/j.exger.2020.110963. [DOI] [PubMed] [Google Scholar]
- 18.Chargui A., El May M.V. Autophagy mediates neutrophil responses to bacterial infection. APMIS. 2014;122:1047–1058. doi: 10.1111/apm.12271. [DOI] [PubMed] [Google Scholar]
- 19.Muñoz-Caro T., Lendner M., Daugschies A., Hermosilla C., Taubert A. NADPH oxidase, MPO, NE, ERK1/2, p38 MAPK and Ca2+ influx are essential for Cryptosporidium parvum-induced NET formation. Dev. Comp. Immunol. 2015;52:245–254. doi: 10.1016/j.dci.2015.05.007. [DOI] [PubMed] [Google Scholar]
- 20.Palić D., Andreasen C.B., Ostojić J., Tell R.M., Roth J.A. Zebrafish (Danio rerio) whole kidney assays to measure neutrophil extracellular trap release and degranulation of primary granules. J. Immunol. Methods. 2007;319:87–97. doi: 10.1016/j.jim.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 21.Schauer C. Aggregated neutrophil extracellular traps limit inflammation by degrading cytokines and chemokines. Nat. Med. 2014;20:511–517. doi: 10.1038/nm.3547. [DOI] [PubMed] [Google Scholar]
- 22.Korhonen J.T., Dudeja V., Dawra R., Kubes P., Saluja A. Neutrophil extracellular traps provide a grip on the enigmatic pathogenesis of acute pancreatitis. Gastroenterology. 2015;149:1682–1695. doi: 10.1053/j.gastro.2015.10.027. [DOI] [PubMed] [Google Scholar]
- 23.Pallet N. Neutrophil extracellular traps orchestrate necroinflammation. J. Am. Soc. Nephrol.: JASN (J. Am. Soc. Nephrol.) 2017;28:1670–1672. doi: 10.1681/ASN.2017010064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arroyo A.B. MiR-146a regulates neutrophil extracellular trap formation that predicts adverse cardiovascular events in patients with atrial fibrillation. Arterioscler. Thromb. Vasc. Biol. 2018;38:892–902. doi: 10.1161/ATVBAHA.117.310597. [DOI] [PubMed] [Google Scholar]
- 25.Nakazawa D., Masuda S., Tomaru U., Ishizu A. Pathogenesis and therapeutic interventions for ANCA-associated vasculitis. Nat. Rev. Rheumatol. 2019;15:91–101. doi: 10.1038/s41584-018-0145-y. [DOI] [PubMed] [Google Scholar]
- 26.Gaul D.S. Loss of Sirt3 accelerates arterial thrombosis by increasing formation of neutrophil extracellular traps and plasma tissue factor activity. Cardiovasc. Res. 2018;114:1178–1188. doi: 10.1093/cvr/cvy036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ducroux C. Thrombus neutrophil extracellular traps content impair tPA-induced thrombolysis in acute ischemic stroke. Stroke. 2018;49:754–757. doi: 10.1161/STROKEAHA.117.019896. [DOI] [PubMed] [Google Scholar]
- 28.Warnatsch A., Ioannou M., Wang Q., Papayannopoulos V. Neutrophil extracellular traps license macrophages for cytokine production in atherosclerosis. Science. 2015;349:316. doi: 10.1126/science.aaa8064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Papayannopoulos V. Neutrophil extracellular traps in immunity and disease. Nat. Rev. Immunol. 2018;18:134–147. doi: 10.1038/nri.2017.105. [DOI] [PubMed] [Google Scholar]
- 30.Zhang Y.G. Exosomes derived from oxLDL-stimulated macrophages induce neutrophil extracellular traps to drive atherosclerosis. Cell Cycle. 2019;18:2674–2684. doi: 10.1080/15384101.2019.1654797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhou Z. Excessive neutrophil extracellular trap formation aggravates acute myocardial infarction injury in apolipoprotein E deficiency mice via the ROS-dependent pathway. Oxidative medicine and cellular longevity. 2019 doi: 10.1155/2019/1209307. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eghbalzadeh K. Compromised anti-inflammatory action of neutrophil extracellular traps in PAD4-deficient mice contributes to aggravated acute inflammation after myocardial infarction. Front. Immunol. 2019;10:2313. doi: 10.3389/fimmu.2019.02313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cao C. Impact of exudative diathesis induced by selenium deficiency on LncRNAs and their roles in the oxidative reduction process in broiler chick veins. Oncotarget. 2017;8:20695–20705. doi: 10.18632/oncotarget.14971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang T. Oxidative stress induced by Se-deficient high-energy diet implicates neutrophil dysfunction via Nrf2 pathway suppression in swine. Oncotarget. 2017;8:13428–13439. doi: 10.18632/oncotarget.14550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li X. Effects of selenium-lead interaction on the gene expression of inflammatory factors and selenoproteins in chicken neutrophils. Ecotoxicol. Environ. Saf. 2017;139:447–453. doi: 10.1016/j.ecoenv.2017.02.017. [DOI] [PubMed] [Google Scholar]
- 36.Zhou X., Wang H., Lian S., Wang J., Wu R. 2020. Effect of Copper, Zinc, and Selenium on the Formation of Bovine Neutrophil Extracellular Traps. Biological Trace Element Research. [DOI] [PubMed] [Google Scholar]
- 37.Jiang H. ITRAQ-based quantitative proteomics reveals the first proteome profiles of piglets infected with porcine circovirus type 3. Journal of Proteomics. 2020;212 doi: 10.1016/j.jprot.2019.103598. [DOI] [PubMed] [Google Scholar]
- 38.Chi Q. Regulation of H2S-induced necroptosis and inflammation in broiler bursa of Fabricius by the miR-15b-5p/TGFBR3 axis and the involvement of oxidative stress in this process. J. Hazard Mater. 2020;406 doi: 10.1016/j.jhazmat.2020.124682. [DOI] [PubMed] [Google Scholar]
- 39.Chi Q., Luan Y., Zhang Y., Hu X., Li S. The regulatory effects of miR-138-5p on selenium deficiency-induced chondrocyte apoptosis are mediated by targeting SelM. Metall. 2019;11:845–857. doi: 10.1039/c9mt00006b. [DOI] [PubMed] [Google Scholar]
- 40.Gać P. The importance of selenium and zinc deficiency in cardiovascular disorders. Environ. Toxicol. Pharmacol. 2021;82 doi: 10.1016/j.etap.2020.103553. [DOI] [PubMed] [Google Scholar]
- 41.Sahebari M., Rezaieyazdi Z., Khodashahi M. Selenium and autoimmune diseases: a review article. Curr. Rheumatol. Rev. 2019;15:123–134. doi: 10.2174/1573397114666181016112342. [DOI] [PubMed] [Google Scholar]
- 42.Swart R., Schutte A.E., van Rooyen J.M., Mels C.M.C. Selenium and large artery structure and function: a 10-year prospective study. Eur. J. Nutr. 2019;58:3313–3323. doi: 10.1007/s00394-018-1875-y. [DOI] [PubMed] [Google Scholar]
- 43.Mirdamadi A., Rafiei R., Kahazaipour G., Fouladi L. Selenium level in patients with heart failure versus normal individuals. Int. J. Prev. Med. 2019;10:210. doi: 10.4103/ijpvm.IJPVM_45_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hasan R., Rink L., Haase H. Zinc signals in neutrophil granulocytes are required for the formation of neutrophil extracellular traps. Innate Immun. 2013;19:253–264. doi: 10.1177/1753425912458815. [DOI] [PubMed] [Google Scholar]
- 45.Cichon I., Ortmann W., Bednarz A., Lenartowicz M., Kolaczkowska E. Reduced neutrophil extracellular trap (NET) formation during systemic inflammation in mice with menkes disease and wilson disease: copper requirement for NET release. Front. Immunol. 2019;10:3021. doi: 10.3389/fimmu.2019.03021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rossaint J. Synchronized integrin engagement and chemokine activation is crucial in neutrophil extracellular trap-mediated sterile inflammation. Blood. 2014;123:2573–2584. doi: 10.1182/blood-2013-07-516484. [DOI] [PubMed] [Google Scholar]
- 47.Tohme S. Computational analysis supports IL-17a as a central driver of neutrophil extracellular trap-mediated injury in liver ischemia reperfusion. J. Immunol. 2019;202:268–277. doi: 10.4049/jimmunol.1800454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bonaventura A., Vecchié A., Abbate A., Montecucco F. Neutrophil extracellular traps and cardiovascular diseases: an update. Cells. 2020;9:231. doi: 10.3390/cells9010231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Carmona-Rivera C., Zhao W., Yalavarthi S., Kaplan M.J. Neutrophil extracellular traps induce endothelial dysfunction in systemic lupus erythematosus through the activation of matrix metalloproteinase-2. Ann. Rheum. Dis. 2015;74:1417–1424. doi: 10.1136/annrheumdis-2013-204837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Qi H., Yang S., Zhang L. Neutrophil extracellular traps and endothelial dysfunction in atherosclerosis and thrombosis. Front. Immunol. 2017;8:928. doi: 10.3389/fimmu.2017.00928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yang T. miR-200a-5p regulates myocardial necroptosis induced by Se deficiency via targeting RNF11. Redox Biol. 2018;15:159–169. doi: 10.1016/j.redox.2017.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cui S. Selenoprotein S attenuates tumor necrosis factor-α-induced dysfunction in endothelial cells. Mediat. Inflamm. 2018 doi: 10.1155/2018/1625414. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Douda D.N., Khan M.A., Grasemann H., Palaniyar N. SK3 channel and mitochondrial ROS mediate NADPH oxidase-independent NETosis induced by calcium influx. Proc. Natl. Acad. Sci. Unit. States Am. 2015;112:2817–2822. doi: 10.1073/pnas.1414055112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li A.C. Differential inhibition of macrophage foam-cell formation and atherosclerosis in mice by PPARalpha, beta/delta, and gamma. J. Clin. Invest. 2004;114:1564–1576. doi: 10.1172/JCI18730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fessler M.B. The challenges and promise of targeting the Liver X Receptors for treatment of inflammatory disease. Pharmacol. Ther. 2018;181:1–12. doi: 10.1016/j.pharmthera.2017.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Delerive P. DNA binding-independent induction of IkappaBalpha gene transcription by PPARalpha. Mol. Endocrinol. 2002;16:1029–1039. doi: 10.1210/mend.16.5.0826. [DOI] [PubMed] [Google Scholar]
- 57.Bishop-Bailey D., Bystrom J. Emerging roles of peroxisome proliferator-activated receptor-beta/delta in inflammation. Pharmacol. Ther. 2009;124:141–150. doi: 10.1016/j.pharmthera.2009.06.011. [DOI] [PubMed] [Google Scholar]
- 58.Li A.C. Peroxisome proliferator-activated receptor gamma ligands inhibit development of atherosclerosis in LDL receptor-deficient mice. J. Clin. Invest. 2000;106:523–531. doi: 10.1172/JCI10370. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.