Abstract
Objective:
To assess the impact of team structure composition and degree of collaboration among various providers on process and outcomes of primary care.
Method:
We conducted social network analysis (SNA) using data from 20% randomly selected primary care service areas in the 2015 Medicare claims. We identified primary care practices and then selected patients with diabetes, heart failure, or chronic obstructive pulmonary disease cared for by these practices.
Results:
When compared to practices with MDs and nurse practitioners (NPs) or/and physicians assistants (PAs), the practices with MDs only had lower degree of centralization and higher MD-to-MD connectedness. Within the primary care practices comprising MDs, NPs or/and PAs, the non-physician providers were more connected (measured as edge density) to all providers in the practice but with higher degree of centralization compared to the MDs in the practice. After adjusting for patient characteristics and type of practice, higher edge density was associated with lower odds of hospitalization (odds ratio [OR]: 0.89, 95% Confidence Interval [CI]: 0.79-0.99), emergency department (ER) admission (OR: 0.80, 95% CI: 0.70-0.92), and total spending (cost ratio [CR]: 0.86, standard error of the mean [SE]: 0.038). Conversely, higher degree centralization was associated with higher rates of hospitalization (OR: 1.15, 95% CI: 1.03-1.28), ER admission (OR: 1.23, 95% CI: 1.08-1.40), and total spending (CR: 1.14, SE: 0.037). However, higher degree centralization was associated with lower rates of potentially inappropriate medications (OR: 0.90, 95% CI: 0.81, 0.99). Team leadership by an NP versus an MD were similar in the rate of ER admissions, hospitalizations, or total spending.
Conclusion:
Our findings showed that highly connected primary care practices with high collaborative care and less top-down MD-centered authority have lower odds of hospitalization, fewer ER admissions, and less total spending; findings likely reflecting better communication and more coordinated care of older patients.
Keywords: Primary care, Medicare, Nurse practitioners, Social network analysis
Introduction
Several studies have found the team-based primary care model to be associated with improved health care outcomes, greater efficiency, and reduced patient health care utilization.1,2 In a study of 312,377 patients discharged from the hospital, Riverin and colleagues showed that patients cared for under team-based primary care models were less likely to have an emergency department (ER) visit or death within 30 days of being discharged from the hospital, compared with patients cared for under traditional primary care models.3 The 30-day post-discharge mortality difference varied by patient morbidity such that, the sicker the patient, the bigger the mortality advantage conferred by the team-based model.3 A systematic review of 26 Randomized Controlled Trials (n=15,526 participants) showed an association of the team-based care model with significantly higher odds of high patient satisfaction compared with the traditional care model (odds ratio (OR), 2.09; 95% Confidence Interval [CI], 1.54-2.84).4
Nurse practitioners (NPs) and Physician assistants (PAs) have become increasingly important members of the primary care team model.5-6 Team-based primary care delivered by a collaboration between primary care physicians and advanced practice providers (NPs or PAs) has been linked to better healthcare outcomes and lower cost of care compared with the traditional primary care model of physician-only practices (solo or group practice).7-10 However, few studies have examined the degree of team collaboration, team-connectedness, and the dynamics of NP or PA interactions with other providers within the team, and how such interactions affect care outcomes. We hypothesized the high collaborative care provided by physicians and NPs/PAs will be associated with clinically relevant communication, effective coordination of care, and better health outcomes. Understanding the impact of team structure composition and degree of collaboration among various providers on the process and outcomes of primary care can guide the development of programs to improve care team dynamics and the quality of primary care delivery.
Social Network Analysis (SNA) is one method that can be used to understand the interaction dynamics and roles of NPs or PAs in a team-based care. SNA uses graph theory to visualize and quantify a network of participants (such as providers) through mapping information and communication pathways.11,12 As a visualization example, figure 1 demonstrated the preliminary SNA results of 16 primary care teams identified in a county using Medicare data. SNA measures, such as edge density (degree of collaborative care or connectedness), degree centrality (degree to which decision-making process is centralized), and betweenness centralization (degree to which information sharing is centralized) have been used to measure healthcare provider networks of patients with diabetes.13 SNA has also previously been used to demonstrate a link between the characteristics of physicians’ networks and overall spending on and utilization of health care services among Medicare beneficiaries,14 and to identify the type and degree of collaboration between primary care providers, NPs, and PAs using Medicare data.15 Two previous studies have examined physician collaboration using SNA.16,17 However, these studies did not include NPs or PAs. A limited number of studies have examined the link between team-based primary care and specific outcomes using population-based data and SNA. Therefore, the purpose of our study was to examine how team-based primary care can impact patient outcomes, specifically hospitalizations, ER admissions, use of potentially inappropriate medications (PIMs), and Medicare cost using SNA.
Figure 1:
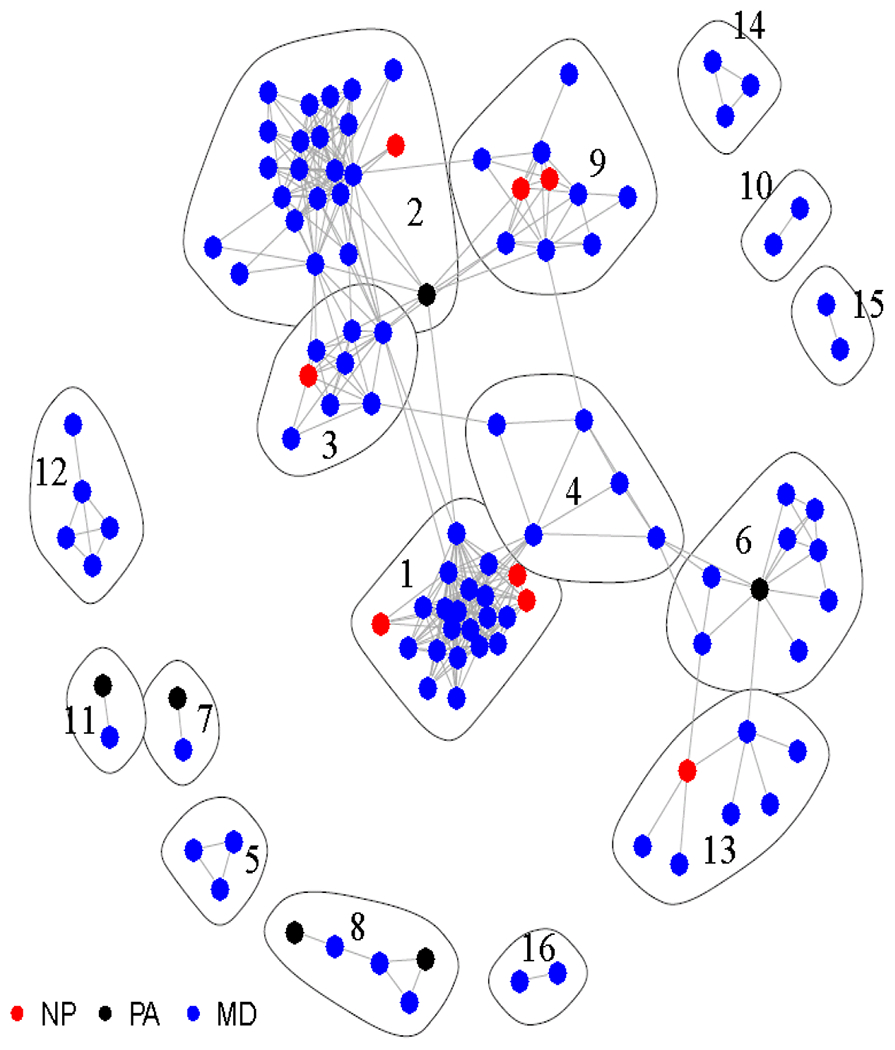
Example on the Visualization of Primary Care Teams Identified through Social Network Analysis. Analysis based on Medicare Data at county in Texas
Methods
Data Source
We first used Medicare outpatient claims from the 20% randomly selected primary care service areas (PCSAs, n=1,400) in 2015 to identify primary care practices. We choose 20% sample because researchers can only request samples including up to 20% of Medicare beneficiaries. A 100% data sample can only be requested if it is for beneficiaries within a certain geographic region or is for specific diseases18. We used PCSAs because they were developed for measuring primary care resources, utilization, and outcomes19. Beneficiaries’ characteristics and outcome measurement were determined from 2015 Medicare claims for 100% Medicare beneficiaries with diabetes, heart failure (HF), or chronic obstructive pulmonary disease (COPD), from the Chronic Conditions Data Warehouse in the same year. We focused on older patients with these three common diseases because these conditions require higher levels of coordinated care and have established guidelines for health care. The data sources included Master Beneficiary Summary Files (MBSF) containing member demographics and enrollment information, Medicare Provider Analysis and Review (MedPAR) file containing claims for inpatient stay, Outpatient statistical analysis file (OutSAF) containing claims from outpatient facilities, Carrier file containing claims from professional services, and Prescription Drug Event (PDE) records. The aforementioned data files were located at the Centers for Medicare & Medicaid Services (CMS) Virtual Research Data Center (VRDC). The University of Texas Medical Branch Institutional Review Board approved this study.
Study Population
We first identified primary care practices within each PCSA via SNA. Primary care visits were determined by Current Procedural Terminology (CPT) code in outpatient claims and those visits billed by primary care providers (primary care physician [MD], NP, and PA) were selected to determine the number of shared patients for each provider pair (Supplementary Table S1). Only providers with at least 30 patients shared with another provider were included in SNA.15 Using the Walktrap community finding algorithm, with four steps as the length of random walk and weighted by the number of patients shared between primary care providers, we identified primary care practices within each PCSA.20-22 In this algorithm, random walks are used to compute distances between providers; then providers are assigned to groups with small intra and larger inter-community distances. Five hundred fifty-nine PCSAs with a modularity ≥ 0.4, indicating well defined modules within the network of PCSAs, were selected.23 We further limited analysis to four types of practice according to type of providers identified at each practice (MD only, MD-NP, MD-PA, or MD-NP-PA), resulting in 4,648 primary care practices (first step in Supplementary Table S2).
Among all patients with diabetes, HF, or COPD, there were 1,004,506 patients with at least two office visits to any of our studied primary care practices. After selecting patients aged 66 or above, who were continuously enrolled in Medicare in 2014 and 2015, and then excluding those cared for by multiple practices, residing in nursing homes, or having unknown residential information, there were 449,460 patients included in the study (second step in Supplementary Table S2). This study cohort was cared for by 17,185 primary care providers in 4,453 primary care practices in 556 PCSAs.
Patient characteristics and outcome
Demographic factors (age, gender, race/ethnicity), Medicare-Medicaid dual eligibility, Medicare original entitlement, and chronic conditions (hypertension, hyperlipidemia, ischemic heart disease, arthritis, atrial fibrillation, cancer, osteoporosis, chronic kidney disease, depression, asthma, Alzheimer’s disease/dementia, and stroke) were obtained from MBSF. Beneficiary residence location was classified as metropolitan, urban, or rural, according to 2013 rural-urban continuum codes from the United States Department of Agriculture.
Hospitalizations were determined, for those with acute hospital and critical access hospital stays, from the MedPAR file. Emergency room (ER) admissions were identified as any positive ER charge amount in the MedPAR file, or any OutSAF claims with ER revenue center code (0450, 0451, 0452, 0456, 0459, 0981). The PDE report was used to identify any potentially inappropriate use of medication(s), and was determined using the algorithm proposed in the Healthcare Effectiveness Data and Information Set (HEDIS) 2016 measures on Use of High-Risk Medications in the Elderly.24 Total Medicare costs were estimated by sum of the paid amount in MedPAR, OutSAF, and Carrier files.
Practice and provider measurement
For each practice, the number of providers, broken down by MD, NP, and PA, were reported and summarized by type of practice. SNA network measures were constructed to show how well each practice was connected, measured by edge density; how centralized the practice was, using degree centralization (degree to which decision-making process is centralized); and whether certain providers were more popular as conduits of information than others within the practice, using betweenness centralization (degree to which information sharing is centralized).25 For each provider within the practice, both normalized degree centrality and normalized betweenness centrality were calculated to show whether connections and centrality differed between types of provider. Among practices with NP involvement, the average of provider’s degree centralization and betweenness centralization measures were calculated for NP and MD separately within the practice, and then the provider type specific averages were compared to determine the practice as MD-led (MD average > NP average), NP-led (NP average > MD average), or equal (MD average = NP average).
Statistical model:
Multi-level generalized linear models were constructed to account for the clustering effect of a practice. The estimation of the OR of hospitalization, ER admission, and PIM use was performed with binary distribution and logit link function. The estimation of cost ratio (CR) on Medicare cost was performed with the lognormal distribution. SNA was performed using the igraph package in R 3.4.4. All other analyses were performed using SAS Enterprise Guide version 7.15 at the CMS VRDC (SAS Inc., Cary, NC).
Results
Table 1 displays the number of primary care providers, census regions, and the SNA network measures of the practice (edge density, degree centralization, and betweenness centralization) by type of primary care practice. Overall, among 4,453 primary care practices included in this study, 40% were MD only, 34% were MD/NP, 11% were MD/NP/PA, and 15% were MD/PA. The median number of providers per practice was larger in MD/NP/PA practices, the same for MD/NP and MD/PA practices, and smallest in MD practices (6, 3, 3, and 2 providers per practice type, respectively). Among all types of practices, on average, MD practices had the highest edge density and the lowest degree centralization. Conversely, MD/NP/PA practices had higher degree centralization and lower edge density than others. The differences in betweenness centralization followed a similar pattern.
Table 1.
Practice Network Structure Measurement Stratified by Type of Practice
| Variable | All | Type of Practice | |||
|---|---|---|---|---|---|
| MD only | MD/NP | MD/NP/PA | MD/PA | ||
| Number of practice (N) | 4453 | 1773 | 1513 | 495 | 672 |
| Edge Density | |||||
| Mean (SD) | 0.40 (0.12) | 0.44 (0.10) | 0.40 (0.12) | 0.29 (0.11) | 0.41 (0.11) |
| Median (Q1-Q3) | 0.50 (0.33-0.50) | 0.50 (0.33-0.50) | 0.50 (0.33-0.50) | 0.29 (0.20-0.36) | 0.50 (0.33-0.50) |
| Degree centralization | |||||
| Mean (SD) | 0.10 (0.12) | 0.07 (0.11) | 0.11 (0.13) | 0.19 (0.11) | 0.10 (0.12) |
| Median (Q1-Q3) | 0.00 (0.00-0.22) | 0.00 (0.00-0.13) | 0.00 (0.00-0.25) | 0.20 (0.12-0.25) | 0.00 (0.00-0.23) |
| Betweenness centralizationa | |||||
| N | 2565 | 735 | 934 | 495 | 401 |
| Mean (SD) | 0.09 (0.14) | 0.10 (0.16) | 0.09 (0.14) | 0.08 (0.11) | 0.10 (0.15) |
| Median (Q1-Q3) | 0.00 (0.00-0.14) | 0.00 (0.00-0.15) | 0.00 (0.00-0.14) | 0.06 (0.00-0.11) | 0.00 (0.00-0.17) |
| Number of providers | |||||
| Mean (SD) | 3.9 (3.0) | 3.0 (2.1) | 3.9 (2.7) | 7.2 (4.7) | 3.6 (2.0) |
| Median (Q1-Q3) | 3.0 (2.0-4.0) | 2.0 (2.0-3.0) | 3.0 (2.0-5.0) | 6.0 (4.0-9.0) | 3.0 (2.0-4.0) |
| Percent of provider as MD | |||||
| Mean (SD) | 73 (25) | 100 (0) | 56.7 (15.1) | 46.6 (17.0) | 57.6 (14.9) |
| Median (Q1-Q3) | 72.7 (50-100) | 100 (100-100) | 50 (50-66.7) | 46.2 (33.3-60.0) | 50 (50-66.7) |
| Percent of provider as NP | |||||
| Mean (SD) | 39.6 (16.1) | NA | 43.3 (15.1) | 28.3 (13.7) | NA |
| Median (Q1-Q3) | 40 (25-50) | NA | 50 (33.3-50) | 25 (17.4-33.3) | NA |
| Percent of provider as PA | |||||
| Mean (SD) | 35.1 (15.9) | NA | NA | 25.2 (11.2) | 42.2 (14.9) |
| Median (Q1-Q3) | 33.3 (22.2-50) | NA | NA | 25 (16.7-33.3) | 50 (33.3-50) |
| Region of the practice, n(%) | |||||
| Midwest | 1210 (27.2) | 461 (26.0) | 465 (30.7) | 111 (22.4) | 173 (25.7) |
| Northeast | 890 (20.0) | 318 (17.9) | 319 (21.1) | 115 (23.2) | 138 (20.5) |
| South | 1568 (35.2) | 587 (33.1) | 566 (37.4) | 184 (37.2) | 231 (34.4) |
| West | 785 (17.6) | 407 (23.0) | 163 (10.8) | 85 (17.2) | 130 (19.3) |
Abbreviations: MD, primary care physician; NA, not applicable; NP, nurse practitioner; PA, physician assistant; Q1, quartile 1; Q3, quartile 3; SD: standard deviation
The measurement is only eligible for a practice with at least 3 providers
Figure 2 presents the SNA network analysis measures at the provider level (normalized degree centrality and normalized betweenness centrality) by type of primary care practice. In general, MD practices had the highest average degree centrality for all provider types in the practice, and MD/NP/PA practices had the lowest average betweenness centrality for all provider types in the practice. Comparing MD to NP (or PA) in the practices with NP (or PA) involvement, NPs (or PAs) tended to have both higher degree centrality and greater betweenness centrality than MDs. There were very few differences in patient characteristics including age, gender, Medicare original entitlement, or comorbidity across type of practices, except for a higher proportion of minority patients in MD practices and a higher proportion of MD/NP and MD/NP/PA practices located in non-metropolitan areas (Supplemental Table S3).
Figure 2.
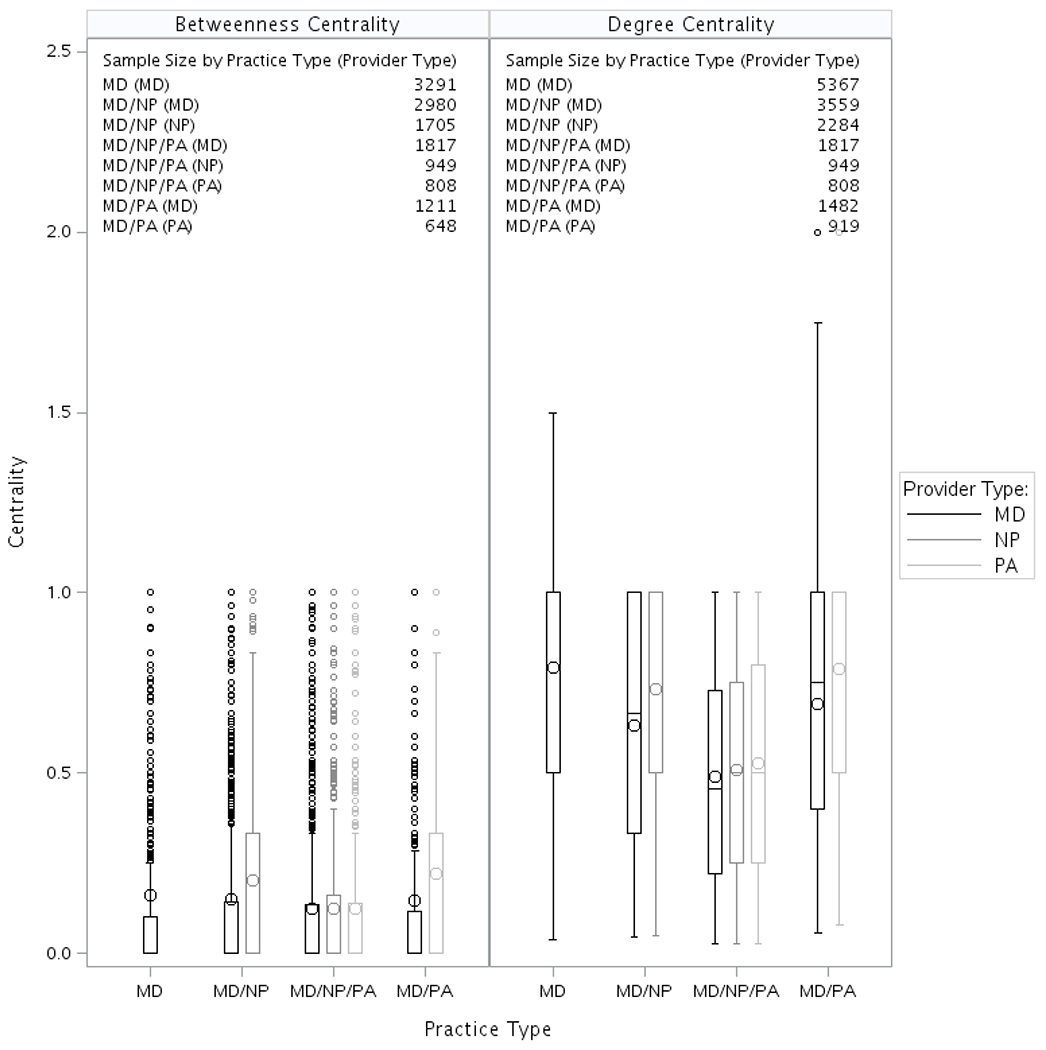
Provider Network Measurement Stratified by Type of Practice for Each Type of Provider. Each box plot presents the interquartile range. The line inside the box represents the median, the large circle represents the mean, and the small circles represents those with value outside 3 times of IQR (interquartile range). The betweenness measurement is only eligible for a practice with at least 3 providers. Abbreviations: MD, primary care physician; NA, not applicable; NP, nurse practitioner; PA, physician assistant
Table 2 presents the rates of hospitalization, ER visits, PIMs, and Medicare cost by type of practice. The rate of hospitalization varied from 21.8% in MD/PA practices to 23.2% in MD/NP practices; the rate of ER visits varied from 16.0% in MD/PA practices to 17.5% in MD practices; and the rate of PIMs varied from 16.5% in MD practices to 17.3% in MD/PA practices. Overall, the average total Medicare costs were lower in MD/NP practices and MD/NP/PA practices than in MD practices and MD/PA practices. None of the observed differences across types of practice were significantly different after adjusting for patient characteristics (Figure 3 Panel A and supplemental Table S4). However, we found significant associations between SNA network measures and outcomes. After adjusting for patient characteristics and type of practice, higher edge density (high collaborative care) was associated with lower odds of hospitalization (OR: 0.89, 95% CI: 0.79-0.99), ER admission (OR: 0.80, 95% CI: 0.70-0.92), and total spending (CR: 0.86, 95% CI: 0.80-0.93). Conversely, higher degree centralization was associated with higher rates of hospitalization (OR: 1.15, 95% CI: 1.03-1.28), ER admission (OR: 1.23, 95% CI: 1.08-1.40), and total spending (CR: 1.14, 95% CI: 1.07-1.23). The results for PIMs were different. Higher edge density was associated with higher rates of PIMs (OR: 1.14, 95% CI: 1.03-1.26) and higher degree centralization was associated with lower rates of PIMs (OR: 0.90, 95% CI: 0.81, 0.99). Betweenness centralization (highly centralized information sharing) was associated only with increased total spending (CR: 1.10, SE: 0.037). When we compared the differences in SNA network measures between MD-led and NP-led practices, among practices with NP involvement, there were no significant differences in health outcomes or cost, regardless of whether the practices were led by an MD or an NP (Figure 3 Panel B).
Table 2.
Health Outcomes and Cost Stratified by Type of Practice
| Outcome | All | Type of Practice |
|||
|---|---|---|---|---|---|
| MD only | MD/NP | MD/NP/PA | MD/PA | ||
| Number of subject | 449 460 | 155 565 | 144 300 | 88 600 | 60 995 |
| Health care, n(%) | |||||
| Hospitalization | 102 361 (22.8) | 35 394 (22.8) | 33 471 (23.2) | 20 200 (22.8) | 13 296 (21.8) |
| ER Admission | 76 739 (17.1) | 27 232 (17.5) | 24 756 (17.2) | 14 974 (16.9) | 9 777 (16.0) |
| PIMs, n(%) | |||||
| Criteria A§ | 56 976 (12.7) | 19 186 (12.3) | 18 789 (13.0) | 11 096 (12.5) | 7 905 (13.0) |
| Criteria B§ | 17 139 (3.8) | 5 972 (3.8) | 5 416 (3.8) | 3 318 (3.7) | 2 433 (4.0) |
| Criteria C§ | 7 230 (1.6) | 2 336 (1.5) | 2 364 (1.6) | 1 585 (1.8) | 945 (1.5) |
| Any | 75 989 (16.9) | 25 680 (16.5) | 24 810 (17.2) | 14 961 (16.9) | 10 538 (17.3) |
| Medicare cost, mean(SD) median (Q1-Q3) | |||||
| Professional service | |||||
| Primary care | 768.1 (1065) | 771.2 (1155) | 755.8 (1002) | 784.2 (962.9) | 766.0 (1113) |
| 560.1 (318.4-938.8) | 549.6 (304.0-939.3) | 551.4 (318.3-919.2) | 582.2 (335.7-964.7) | 573.9 (329.2-944.7) | |
| Specialists | 2179 (4660) | 2276 (4682) | 2072 (4347) | 2088 (4424) | 2317 (5558) |
| 1084 (386.8-2402) | 1147 (406.5-2527) | 1033 (369.5-2305) | 1037 (372.1-2291) | 1120 (404.3-2487) | |
| Other | 751.6 (1486) | 798.6 (1716) | 721.2 (1440) | 716.5 (1274) | 754.2 (1224) |
| 385.5 (121.4-944.5) | 408.3 (133.0-996.1) | 361.8 (107.9-903.1) | 375.8 (119.2-911.3) | 401.6 (133.4-962.7) | |
| Outpatient facilities | 2639 (6860) | 2566 (6718) | 2678 (7065) | 2697 (6581) | 2649 (7119) |
| 746.6 (153.9-2303) | 697.6 (142.1-2193) | 769.0 (168.8-2342) | 812.9 (165.3-2456) | 728.0 (135.8-2280) | |
| Hospitalization | 3378 (10339) | 3496 (10819) | 3305 (9832) | 3335 (10356) | 3310 (10229) |
| 0.0 (0.0-0.0) | 0.0 (0.0-0.0) | 0.0 (0.0-0.0) | 0.0 (0.0-0.0) | 0.0 (0.0-0.0) | |
| Other facilities | 459.9 (3613) | 455.0 (3682) | 501.1 (3766) | 422.1 (3360) | 430.2 (3416) |
| 0.0 (0.0-0.0) | 0.0 (0.0-0.0) | 0.0 (0.0-0.0) | 0.0 (0.0-0.0) | 0.0 (0.0-0.0) | |
| Total cost | 10 176 (17 566) | 10 363 (18 110) | 10 033 (17 212) | 10 043 (17 056) | 10 227 (17 713) |
| 3 988 (1 710-10 695) | 4 034 (1 728-10 739) | 3 906 (1 659-10 622) | 4 019 (1 735-10 774) | 4 024 (1 750-10 637) | |
Abbreviations: MD, primary care physician; NP, nurse practitioner; PA, physician assistant; PIM: potentially inappropriate medication; Q1, quartile 1; Q3, quartile 3; SD: standard deviation
Criteria A: high risk medication; Criteria B: high risk medication with days of supply > 90; Criteria C: high risk medication with dosage greater than average daily dose criteria
Figure 3.
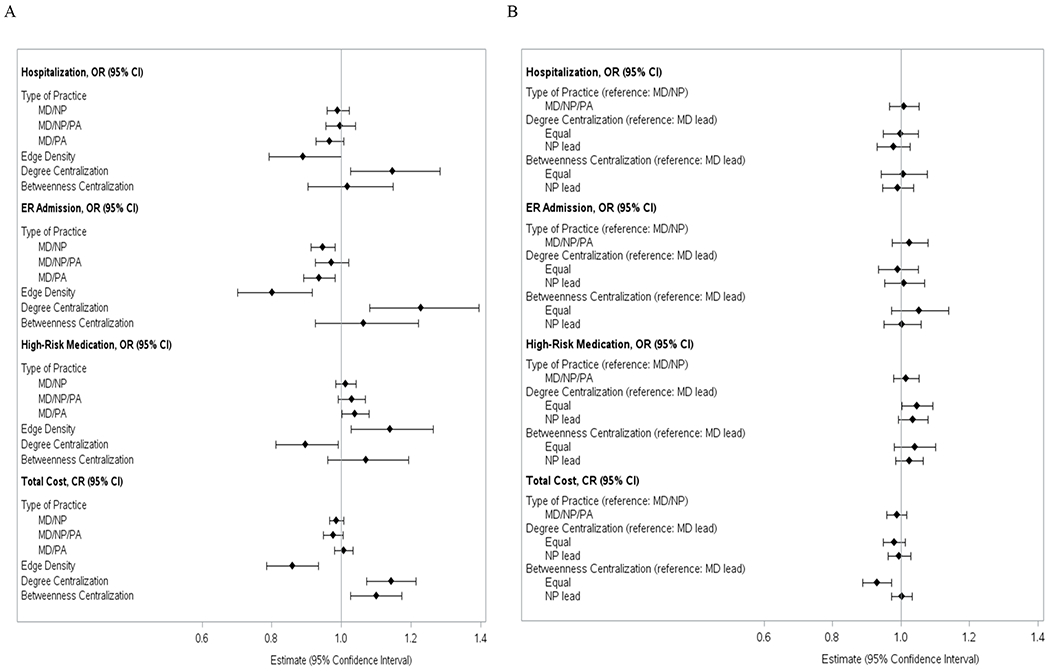
Association between Practice Network Structure and Health Outcome and Cost Panel A: For the entire study cohort (N=449,460). The results on type of practice with the reference group as MD only were estimated from the models with adjustment of subject characteristics listed in Supplemental Table S3, and region of practice. The results for edge density, degree centralization, and betweenness centralization were estimated from the models with adjustment of subject characteristics, type of practice and location of practice. The model for betweenness centralization only excluded subjects cared by practice with only 2 providers (N=348536). Abbreviations: CI, confidence interval; CR, cost ratio; ER, emergency department; MD, primary care physician; NP, nurse practitioner; OR, odds ratio; PA, physician assistant. Panel B: For the sub-cohort of patient cared under the practices with NP involvement to show the association between role of NP and health outcome and cost (N=232900). The results on type of practice with the reference group as MD only were estimated from the models with adjustment of subject characteristics listed in Supplemental Table S3, and region of practice. The results for edge density, degree centralization, and betweenness centralization were estimated from the models with adjustment of subject characteristics, type of practice and location of practice. The model for betweenness centralization only excluded subjects cared by practice with only 2 providers (N=205689). Abbreviations: CI, confidence interval; CR, cost ratio; ER, emergency department; MD, primary care physician; NP, nurse practitioner; OR, odds ratio; PA, physician assistant.
Discussion
Using Medicare data and SNA measures, we examined the association of team structure and dynamics with health outcomes and cost in primary care. When compared to practices with NPs, PAs, or both, the practices with MDs only had higher connections between physicians (more shared patients among MDs) and lower degrees centralization. Within the practices with NPs, PAs, or both, NPs and PAs were more connected and central than MDs were. Previous studies have not used SNA measures to compare centrality and connectedness between practices with varying composition. However, one study found that physicians tend to share patients with other physicians who have similar personal traits, practice styles, and patient panels.26 This association may explain the high level of connectivity in practices led by MDs vs others.
In the current study, higher edge density (or connectedness—degree of collaborative care) in any care team was associated with lower odds of hospitalization, ER admission, and total spending. Previous research has found similar results. For example, one study found that the density of physician collaboration networks was negatively correlated with hospitalization cost and readmission rate.17 Another study reported an association of higher provider connectedness with better health outcomes, such as fewer hospital readmissions, among heart failure patients.27 Various mechanisms may account for these results, such as better coordination of care, faster sharing of information, timely communication of and response to changes in patient clinical status, and effective delivery of services.28 An unexpected finding is the association of higher edge density with higher rates of PIMs. Although increased connectedness enables faster sharing of data about patients’ conditions and medications, it is possible that the increased number of prescribers involved in the patient’s care may lead to increase in overall number of prescriptions medications and polypharmacy (using>4 drugs), a known risk for exposure to PIMs and adverse drug interactions29.
We also found that higher degree centralization was associated with lower rates of PIMs. Degree centralization indicates that the network is more centered at one provider, and that information and connection flow through that provider. One possible explanation for this phenomenon is that review and reconciliation of prescription medications might be easier to accomplish when one or two main prescribers (as opposed to several prescribers) are centrally responsible for approval and oversight of patients’ medication regimen, especially in older patients needing many drugs for multiple co-existing chronic conditions. One way to achieve the same lower rate of PIM use, even in the team model with less centralization and high connectedness, is to incorporate a process of reminders and warnings into the electronic medical record (EMR) system to reduce or prevent prescribing of PIMs or drugs with potential adverse drug-disease interactions. A randomized clinical trial (vs. usual care) of an EMR medication decision support tool “Tool to Reduce Inappropriate Medications (TRIM)” in persons 65 years old at VA taking 7 or more drugs was associated with more purposeful patient and clinician medication-related communication and a higher rate of correction of discrepancies in prescription medications, although there was no significant effect on PIM reduction.30
Though associated with lower PIMs, higher degree centralization was linked to higher rates of hospitalization, ER admission, and total spending. This result contrasts with a previous study which showed that a high performance network had greater centralization.31 In addition, another prior study found that degree centralization was negatively correlated with both hospitalization cost and readmission rate.17 However, a further study found that higher degree centralization was associated with readmissions, but had no correlation with cost.16 These studies examined only collaboration among physicians, and did not include NPs or PAs, which might explain the differences between our findings and prior studies.
We found practices with higher betweenness centralization (centralized and tight control of information sharing in team) had higher total cost, but there was no significant association of betweenness centralization with hospitalization, ER admission, or PIM. Previous studies have had inconsistent results. One study showed an association of higher betweenness centralization with greater hospitalization and more ER visits,32 but another study found that betweenness centralization was negatively associated with readmission and cost.16 Overall, the inconsistency of our findings with others can be attributed to type of providers studied (MD only vs. MD/NP/PA as in ours), different sources of data (single institution vs. national data), using different payers of claims, or setting in a different country.
Primary care team leadership by an NP versus an MD showed no significant differences in ER admissions, hospitalizations, or total spending. Previous systematic reviews have found similar results, with little to no difference in ER admissions and hospitalizations when comparing primary care led by NPs versus physicians.33,34 Further, several studies have found no differences35 or reduced costs with NP consultations as compared to those from general practitioners.36 No studies to the authors’ knowledge have examined differences in overall spending between NPs and MDs using Medicare data.
This study has several limitations. First, team-care practices were identified using fee-for-service claims from Medicare data, and thus the results may not be generalizable to younger patients, those under Medicare Advantage plans, or those with commercial insurance. Second, the identification of team-care practices by SNA is specific, but with low sensitivity.15 Practices that were not tightly connected, or those located in areas with lower population and lower income, were likely under-identified.37 Third, although we found NPs or PAs played a more central role than MDs in patient care, whether they substituted for MDs or contributed their complementary skills to patient care cannot be distinguished. Lastly, the cross-sectional study design limits the ability to examine the causal relationship between, and temporal patterns within, team structure and health outcomes. Further longitudinal study will help to characterize these relationships.
Our findings suggest a need to incorporate various approaches to improve medical professional training in ways that emphasize the free flow and sharing of patient information across disciplines and providers, leading to efficient transition, integration, and coordination of care across different care sites. We found the benefits of high connectedness and low centrality are underscored in the efficiency in care and better patient outcomes attributed to daily team huddle, a system where team meets briefly before round or clinic to discuss care priorities, key patient data and updates, and any safety concerns without fear.38-39 Highly centralized practices with concentrated and MD-centered authority may not be as efficient as a system of collaborative management with mutual and open information sharing.
Key strategies to improve safety, quality, and connectedness in team-based care practice include adoption of validated team communication tools39, engagement in daily interdisciplinary team huddles (with a representative from each discipline) before the clinic or work-day starts, and adoption of other communication initiatives that support openness and transparency in communication.38-42 Such strategies would likely have the most impact in a team model, a decrease in centralized care decision making (less centrality) and high connectedness (more collaboration in care). Our study suggests that, as a team moves towards connectedness and away from top-down MD-focused centrality, there is substantial improvement in purposeful communication and in outcomes of care. Such improvement likely reflects timely communication of safety hazards, change in patient status and abnormal laboratory results, and an increase in shared use of best available evidence-based clinical care algorithms.41,43
In conclusion, our results demonstrate that a well-connected and highly collaborative practice with free flow of information and transparency in communication has lower odds of ER/hospital visits and lower cost-of-care than a highly centralized team. Future study of degree of connectedness and centrality of NP, PA, and MD teams in accountable care organizations (ACO), and how these affect process and outcome of care, can provide clinically relevant and policy information to improve the quality of inter-professional communication and interaction among ACO providers in a way that best serves the needs of patients.
Acknowledgments
Funding Sources: This work was supported by grants R01-HS020642 from the Agency for Healthcare Research and Quality, and P30-AG024832 from the National Institutes of Health.
Supplementary Material
Supplemental Table S1. Algorithm to Determine Primary Care Visit and Primary Care Provider.
Supplemental Table S2. Flowchart of study cohort.
Supplemental Table S3. Baseline Characteristics for All Eligible Beneficiaries and Stratified by Type of Practice.
Supplemental Table S4. Impact of Baseline Characteristics on Health Outcome from adjusted models (N=449460)
References
- 1.Goldberg DG, Beeson T, Kuzel AJ, Love LE, Carver MC. Team-based care: a critical element of primary care practice transformation. Population Health Management 2013;16(3):150–156. [DOI] [PubMed] [Google Scholar]
- 2.Meyers DJ, Chien AT, Nguyen KH, Li Z, Singer SJ, Rosenthal MB. Association of Team-Based Primary Care With Health Care Utilization and Costs Among Chronically Ill Patients. JAMA Internal Medicine 2019;179(1):54–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Riverin BD, Li P, Naimi AI, Strumpf E. Team-based versus traditional primary care models and short-term outcomes after hospital discharge. Canadian Medical Association Journal 2017;189(16):E585–E593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wen J, Schulman KA. Can team-based care improve patient satisfaction? A systematic review of randomized controlled trials. PLoS One 2014;9(7):e100603–e100603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xue Y, Goodwin JS, Adhikari D, Raji MA, Kuo YF. Trends in primary care provision to Medicare beneficiaries by physicians, nurse practitioners, or physician assistants: 2008-2014. J Prim Care Community Health 2017. October;8(4):256–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barnes H, Richards MR, McHugh MD, Martsolf G. Rural and nonrural primary care physician practices increasingly rely on nurse practitioners. Health Aff (Millwood) 2018. June;37(6):908–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jackson GL, Lee S-YD, Edelman D, Weinberger M, Yano EM. Employment of mid-level providers in primary care and control of diabetes. Primary care diabetes 2011;5(1):25–31. [DOI] [PubMed] [Google Scholar]
- 8.Litaker D, MION LC, Planavsky L, Kippes C, Mehta N, Frolkis J. Physician-nurse practitioner teams in chronic disease management: the impact on costs, clinical effectiveness, and patients’ perception of care. Journal of interprofessional care 2003;17(3):223–237. [DOI] [PubMed] [Google Scholar]
- 9.Scherpbier-de Haan ND, Vervoort GM, Van Weel C, et al. Effect of shared care on blood pressure in patients with chronic kidney disease: a cluster randomised controlled trial. Br J Gen Pract 2013;63(617):e798–e806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ganz DA, Koretz BK, Bail JK, et al. Nurse practitioner co-management for patients in an academic geriatric practice. The American journal of managed care 2010;16(12):e343. [PMC free article] [PubMed] [Google Scholar]
- 11.Stanley W, Faust K. Social network analysis: methods and applications Cambridge: Cambridge University. 1994. [Google Scholar]
- 12.Scott J Social network analysis: A handbook 2nd edn sage publications. In: London; 2000. [Google Scholar]
- 13.Ostovari M, Yu D, Steele-Morris CJ. Identifying key players in the care process of patients with diabetes using social network analysis and administrative data Paper presented at: AMIA Annual Symposium Proceedings 2018. [PMC free article] [PubMed] [Google Scholar]
- 14.Landon BE, Keating NL, Onnela J- P, Zaslavsky AM, Christakis NA, O’Malley AJ. Patient-Sharing Networks of Physicians and Health Care Utilization and Spending Among Medicare Beneficiaries. JAMA internal medicine 2018;178(1):66–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuo Y-F, Raji MA, Lin Y-L, Ottenbacher ME, Jupiter D, Goodwin JS. Use of Medicare Data to Identify Team-based Primary Care: Is it Possible? Medical care 2019;57(11):905–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Uddin S, Hossain L, Hamra J, Alam A. A study of physician collaborations through social network and exponential random graph. BMC health services research 2013;13(1):234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Uddin S, Hossain L, Kelaher M. Effect of physician collaboration network on hospitalization cost and readmission rate. The European Journal of Public Health 2012;22(5):629–633. [DOI] [PubMed] [Google Scholar]
- 18.Mues KE, Liede A, Liu J, et al. Use of the Medicare database in epidemiology and health services research: a valuable source of real-world evidence on the older and disabled population in the US. Clin Epidemiol 2017;2017(9):267–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goodman DC, Mick SS, Bott D, Stukel T, Chang CH, Marth N, Poage J, Carretta HJ. Primary care service areas: a new tool for the evaluation of primary care services. Health Serv Res 2003; 38(1 pt 1):287–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pons P, Latapy M. Computing Communities in Large Networks Using Random Walks. In Computer and Information Sciences - ISCIS 2005. ISCIS 2005. Lecture Notes in Computer Science, vol 3733. Springer, Berlin, Heidelberg [Google Scholar]
- 21.Chejara P, Godfrey WW. Comparative Analysis of Community Detection Algorithms 2017. Conference on Information and Communication Technology (CICT17). https://ieeexplore.ieee.org/stamp/stamp.jsp?tp=&arnumber=8340627 [Google Scholar]
- 22.Yang Z, Algesheimer R, Tessone CJ. A Comparative Analysis of Community Detection Algorithms on Artificial Networks. Scientific Reports 2016; 6:30750 | DOI: 10.1038/srep30750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Newman MEJ. Modularity and community structure in networks. PNAS 2006; 103(23):8577–8582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.HEDIS 2016. Final NDC Lists https://www.ncqa.org/hedis/measures/hedis-2016-ndc-license/hedis-2016-final-ndc-lists/. Accessed 08/15/2019.
- 25.Freeman LC. Centrality in social networks conceptual clarification. Social Networks 1978/79 1:215–239. [Google Scholar]
- 26.Landon BE, Keating NL, Barnett ML, et al. Variation in patient-sharing networks of physicians across the United States. Jama 2012;308(3):265–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Geva A, Olson KL, Liu C, Mandl KD. Provider connectedness to other providers reduces risk of readmission after hospitalization for heart failure. Medical Care Research and Review 2019;76(1):115–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schottenfeld L, Petersen D, Peikes D, et al. Creating patient-centered team-based primary care Rockville: Agency for Healthcare Research and Quality. 2016:1–27. [Google Scholar]
- 29.Steinman MA, Landefeld CS, Rosenthal GE, Berthenthal D, Sen S, Kaboli PJ. Polypharmacy and prescribing quality in older people. J Am Geriatr Soc 2006. October;54(10):1516–23. [DOI] [PubMed] [Google Scholar]
- 30.Fried TR, Niehoff KM, Street RL, et al. Effect of the Tool to Reduce Inappropriate Medications on Medication Communication and Deprescribing. J Am Geriatr Soc 2017;65(10):2265–2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brewster AL, Yuan Ct, Tan AX, Tangoren CG, Curry LA. Collaboration in health care and social service networks for older adults: association with health care utilization measrues. Med Care 2019; 57(5): 327–333. [DOI] [PubMed] [Google Scholar]
- 32.Ostovari M, Yu D. Impact of care provider network characteristics on patient outcomes: usage of social network analysis and a multi-scale community detection. PLoS One 2019; 14(9): e0222016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Laurant M, van der Biezen M, Wijers N, Watananirun K, Kontopantelis E, van Vught AJ. Nurses as substitutes for doctors in primary care. The Cochrane Database of Systematic Reviews 2018;2018(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Newhouse RP, Stanik-Hutt J, White KM, et al. Advanced practice nurse outcomes 1990-2008: a systematic review. In: Database of Abstracts of Reviews of Effects (DARE): Quality-assessed Reviews [Internet]. Centre for Reviews and Dissemination; (UK: ); 2011. [Google Scholar]
- 35.Dierick-van Daele AT, Metsemakers JF, Derckx EW, Spreeuwenberg C, Vrijhoef HJ. Nurse practitioners substituting for general practitioners: randomized controlled trial. Journal of advanced nursing 2009;65(2):391–401. [DOI] [PubMed] [Google Scholar]
- 36.Dierick-van Daele AT, Steuten LM, Metsemakers JF, Derckx EW, Spreeuwenberg C, Vrijhoef HJ. Economic evaluation of nurse practitioners versus GPs in treating common conditions. Br J Gen Pract 2010;60(570):e28–e35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kuo YF, Lin YL, Jupiter D. How to identify team-based primary care in the US using Medicare data. Med Care 2021. February 1;59(2):118–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Branda ME, Chandrasekaran A, Tumerman MD, et al. Optimizing huddle engagement through leadership and problem-solving within primary care: A study protocol for a cluster randomized trial. Trials 2018;19(1):536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Daily Huddle Component Kit | Agency for Health Research and Quality; https://www.ahrq.gov/hai/tools/ambulatory-surgery/sections/sustainability/management/huddles-comp-kit.html. Accessed June 19, 2020 [Google Scholar]
- 40.Weaver SJ, Lubomksi LH, Wilson RF, Pfoh ER, Martinez KA, Dy SM. Promoting a culture of safety as a patient safety strategy: a systematic review. Ann Intern Med 2013;158(5 Pt 2):369–374. doi: 10.7326/0003-4819-158-5-201303051-00002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pronovost P, Needham D, Berenholtz S, Sinopoli D, Chu H, Cosgrove S, Sexton B, Hyzy R, Welsh R, Roth G, Bander J, Kepros J, Goeschel C. An intervention to decrease catheter-related bloodstream infections in the ICU. N Engl J Med 2006. December 28; 355(26):2725–32. [DOI] [PubMed] [Google Scholar]
- 42.Provost S, Lanham H, Leykum LK, McDaniel RR Jr, Pugh J. Health care huddles: managing complexity to achieve high reliability. Health Care Manage Rev 2015;40:1–12. [DOI] [PubMed] [Google Scholar]
- 43.Melton L et al. Evaluation of Huddles. A Multisite Study. The Health Care Manager 2017;36(3):282–287. doi: 10.1097/HCM.0000000000000171 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table S1. Algorithm to Determine Primary Care Visit and Primary Care Provider.
Supplemental Table S2. Flowchart of study cohort.
Supplemental Table S3. Baseline Characteristics for All Eligible Beneficiaries and Stratified by Type of Practice.
Supplemental Table S4. Impact of Baseline Characteristics on Health Outcome from adjusted models (N=449460)


