Abstract
Chinese hamster ovary (CHO) cells are used as host cells for industrial monoclonal antibody (mAb) production. Cell cycle control is an effective approach to increase mAb production in the cell culture. Violacein, a purple-colored pigment produced by microorganisms, has diverse bioactive properties and has been proposed for various industrial applications. In this study, we evaluated the potency of violacein for cell cycle control and improvement of recombinant immunoglobulin G (IgG) production in CHO cells. Compared with the control, 0.9 μM violacein in a 14-day fed-batch culture increased the maximum IgG concentration by 37.6% via increasing the specific production rate and cell longevity. Cell cycle analysis showed that violacein induced G1 and G2/M phase arrest. However, the G1 arrest was observed only on day 1, while G2/M arrest lasted more than 3 days, suggesting that G2/M arrest mediated the violacein-induced enhanced IgG production. Moreover, in line with the increased protein expression, the expression levels of IgG mRNA and nutrient metabolic rates were also increased. N-Linked glycosylation and charge variant profiles were barely affected by violacein treatment. Our results indicate that violacein affects the cell cycle of CHO cells and increases IgG production without changing product quality, showing promise as a mAb production enhancer in CHO cells. The study provides insight into violacein utilization in industrial mAb manufacturing and can help develop advanced, effective mAb production technologies using CHO cell cultures.
Keywords: CHO cells, Violacein, Cell culture, Cell cycle, Recombinant IgG
Introduction
Monoclonal antibodies (mAbs) are used therapeutically because of their high target specificity, fewer side effects, and long half-life in the blood. Chinese hamster ovary (CHO) cells are the most widely used host cells for therapeutic antibody production because of their ability to perform appropriate post-translational modifications, such as glycosylation (Xu et al. 2011). Moreover, CHO cells can adapt to serum-free media and suspension culture, therefore are suitable for large-scale industrial culture.
Cell cycle control is a potent way to increase mAb production in CHO cell cultures. There are various approaches to cell cycle control and enhanced protein production, such as using mild hypothermic cultures, genetic manipulation, and adding a chemical agent (Kumar et al. 2007). Total production is directly correlated with viable cell density (VCD), specific production rate (Qp), and cell culture time. Arresting the cell cycle decreases VCD but increases Qp. Total production can be increased when the positive effects of Qp improvement exceed the negative effects of lowering VCD.
Cell cycle control is effective for the improvement of productivity in both fed-batch and perfusion cultures. Although fed-batch culture has been common for the production of therapeutic proteins on an industrial-scale, perfusion culture is gaining prevalence because of its high volumetric productivity (Lin et al. 2017; Shukla et al. 2017). In perfusion culture, cell cycle control technology is expected to reduce product loss because of reducing cell bleeding rate (Wang et al. 2018; Wolf et al. 2019). Besides that, cell cycle control can increase the product concentration in the harvest stream by increasing the Qp. Therefore, the cell cycle control technology is useful not only in the current fed-batch culture method but also in the perfusion culture method which may be widely used in the future.
Chemical agent addition is a convenient way to increase protein production because of its simplicity. Sodium butyrate (NaBu) has been used to achieve high-level production of recombinant proteins including antibodies in CHO cells (Damiani et al. 2013; Mimura et al. 2001; Oh et al. 2005; Yin et al. 2018). However, NaBu has side effects, including the induction of apoptosis and effects on glycosylation (Chung et al. 2001; Kim et al. 2009; Oh et al. 2005; Sung et al. 2004; Yin et al. 2018). Thus, there is a need for novel compounds that effectively improve mAb production in CHO cells without inducing cell death and impairing mAb quality.
In our previous study, we screened new compounds from marine-derived bacterium culture extracts to improve mAb production in CHO cells (Kido et al. 2020a). One of the compounds in the extracts, staurosporine was indicated to have effects on mAb productivity and cell cycle in CHO cells without affecting the glycosylation pattern of mAbs (Kido et al. 2020b). Although not yet demonstrated, staurosporine analogs might also affect the cell cycle and improve mAb production in CHO cells.
In the present study, we focused on violacein, a purple-colored pigment produced by microorganisms. Violacein and staurosporine are structurally similar bis-indole compounds. They are biosynthesized from two molecules of l-tryptophan, using a common biosynthetic pathway (Hirano et al. 2008). The structural similarity suggests that violacein may be useful in improving mAb production. In addition, we focused on three characteristics of violacein, which would improve the efficacy and safety of violacein as mAb production enhancer in CHO cells, and the possibility for further development. These characteristics are as follows:
High potential as a bioactive compound: Violacein has diverse biological activities, including antimicrobial, antiparasitic, antitumoral, and antioxidant activities (Choi et al. 2015; Durán et al. 2012). Because of the biological and pharmacological activities, violacein has been proposed for use in pharmaceutical, textile, cosmetic, and food industries (Durán et al. 2012). Although G1 and G2/M arrest in murine fibrosarcoma cells and G1 arrest in colon cancer cells by treating violacein has been reported (Kodach et al. 2006; Mojib et al. 2011), there is no report using CHO cells.
Low toxicity to mammalian organisms: Although violacein shows cytotoxicity to some types of cancer cells, this is cell-dependent, and it shows less cytotoxicity to normal cells (Bromberg et al. 2010; Ferreira et al. 2004). Bromberg et al. reported that intraperitoneal administration of violacein, up to 1,000 μg kg−1 for 35 days in mice, did not cause toxicity in the blood, kidney, and liver (Bromberg et al. 2010). The low toxicity to normal mammalian cells and organs is desirable as it reduces safety concerns regarding its use in the biopharmaceutical manufacturing process.
Abundant scheme for derivative production: The biosynthetic pathway of violacein has been well studied. Violacein is biosynthesized using l-tryptophan as a substrate and requires the expression of five genes (vioA, vioB, vioC, vioD, and vioE) (Choi et al. 2015; Jiang et al. 2012). The substrate specificity of enzymes involved in violacein biosynthesis is not strict. The violacein analogues, such as deoxyviolacein or oxyviolacein, can be produced using genetic engineering techniques or l-triptophan analogues as substrate (Bilsland et al. 2018; Jiang et al. 2012; Wang et al. 2012). The differences in the number of hydroxyl groups of violacein affect its toxicity (Choi et al. 2015; Wang et al. 2012). Considering that structural modifications might be required to optimize future violacein applications, derivative production is useful.
In this study, we investigated the effects of violacein on the CHO cell cycle and its effectiveness as a mAb production enhancer. To date, there have been no reports on the effects of violacein on foreign gene-derived protein production in any host cells, including CHO cells. This study shows the possibility of a new application of violacein and provides new findings on the effects of violacein on the mammalian cell cycle and recombinant protein expression. The obtained findings will contribute to the development of advanced, effective mAb production technologies in the future.
Materials and methods
Cell culture and culture medium supplementation
A serum-free-adapted CHO-DP12 clone, ATCC® CRL-12445™ (ATCC, Manassas, VA, USA), that produces human-anti-IL-8 immunoglobulin G (Ig G) (Gonzalez et al. 2000) was used in this study. The cell culture medium used was BalanCD® Growth A medium (Fujifilm Irvine Scientific, Santa Ana, CA, USA), supplemented with 200 nM methotrexate (Sigma-Aldrich, St. Louis, MO, USA), 20 μg mL−1 kanamycin sulfate (Fujifilm Wako Pure Chemical, Osaka, Japan), 5 mM l-glutamine (Thermo Fisher Scientific, Waltham, MA, USA), and 1 mg mL−1 Pluronic™ F-68 (Thermo Fisher Scientific). The feed medium used was BalanCD® Feed 1 (Fujifilm Irvine Scientific), supplemented with 200 nM methotrexate (Sigma-Aldrich) and 20 μg mL−1 kanamycin sulfate (Fujifilm Wako Pure Chemical).
Violacein preparation
The marine bacteria producing violacein, Pseudoalteromonas sp. 520P1 (NBRC 107703, NITE Biological Resource Center, Tokyo, Japan), were obtained from Professor Enomoto (Kochi University of Technology, Kochi, Japan). Violacein was produced by Pseudoalteromonas sp. 520P1 and purified according to the method described by Yada et al. (2008). The violacein concentration was measured by measuring the absorbance at 570 nm (Momen and Hoshino 2000). The composition of the purified product was violacein 84%, deoxyviolacein 7%, and others 9%, those were measured by quantitative nuclear magnetic resonance method performed at Japan Food Research Laboratories (Tokyo, Japan).
Batch culture
Cryopreserved CHO cells were subcultured three times, and the culture at the logarithmic growth phase was mixed with fresh medium and adjusted at a concentration of 1.0 × 106 cells mL−1. The cell mixture was dispensed in 30 mL aliquots into 125 mL Corning® Erlenmeyer flasks (Corning, NY, USA). Violacein was dissolved in 100% dimethyl sulfoxide (DMSO) (Fujifilm Wako Pure Chemical), then added to the culture medium at a final violacein concentration of 0.3–2.4 μM and a final DMSO concentration of 0.1% (v/v) at the start of culture. For the control condition (0 μM violacein), DMSO was added to a final concentration of 0.1% (v/v). Flasks were incubated at 37 ˚C in a shaking CO2 incubator (Adolf Kuhner AG, Birsfelden, Switzerland) at 120 rpm, with 5% CO2 and 80% humidity for 9 days. The experiments were performed in triplicates per condition (n = 3).
Fed-batch culture
CHO cells were incubated with or without 0.9 μM violacein (DMSO was added as vehicle control) as described. The flasks were incubated under the same conditions as described, for 14 days. Fed-batch culture was performed by bolus feeding. From day 4 until the end of the culture period, feed medium (5% of the culture volume) was added to the flasks every other day. To prevent a decrease in violacein and DMSO concentrations with feeding, feed media with the same violacein and DMSO concentrations as those in the culture media were used. The experiments were performed in triplicates per condition (n = 3).
VCD, cell viability, and IgG assay
VCD and cell viability were measured using Vi-CELL XR (Beckman Coulter, Brea, CA, USA), while IgG concentration was measured using the Octet Qke system (Fortebio, Fremont, CA, USA) according to the manufacturer’s protocol. Qp was calculated from the plot of the IgG concentration against time integral values of VCD. The slope of the approximate straight line in the area where the linearity was maintained, was taken as Qp. The specific growth rate (μ) was calculated using VCD at day 0 and 2, according to Eq. 1:
| 1 |
where LN denotes the natural logarithm; t1 and t2 represent the cultivation times; VCDt1 and VCDt2 represent viable cell densities at time points t1 and t2, respectively.
Caspase 3/7 activity assay
A caspase 3/7 activity assay was performed using a representative flask for each condition of fed-batch culture. The Cell Event™ Caspase 3/7 Green Detection Reagent (Thermo Fisher Scientific) was used according to the manufacturer’s instructions. The fluorescent peptide contained in the reagent is cleaved by activated caspase-3 or caspase-7 in apoptotic cells, and this produces a fluorescence response with an absorption/emission maximum of ~ 502/530 nm. The reagents were added to cells and incubated according to the manufacturer’s protocol. Aliquots (10 μL) of fluorescently stained cell solution were added to a hemocytometer and observed with EVOS FL Color imaging system (Thermo Fisher Scientific). Each specimen was observed in fluorescence mode using a 470 nm excitation wavelength, and an image was obtained by converting 525 nm emission wavelengths to a green color. Images were obtained in transmission mode overlaying the fluorescence image of the same specimen. Three view fields were captured per specimen. Green pixels and total pixels in the image were counted using ImageJ v. 1.52a software (National Institutes of Health, Bethesda, MD, USA). The ratio of caspase 3/7 active cells to total cells was evaluated as the ratio of the number of green pixels / the number of total pixels.
Culture medium component analysis
The glucose, lactate, glutamine, glutamate, and ammonium levels of the fed-batch culture medium were measured using a YSI Model 2700 (YSI Life Sciences, Yellow Springs, OH, USA) according to the manufacturer’s protocol. The uptake or production rate of the component was calculated by dividing the difference between day 0 and 4 by the time integral values of the VCD.
N-linked glycan analysis
CHO cells were batch-cultured with or without 0.9 μM violacein (DMSO was added as vehicle control) and IgG was purified from the culture medium on day 4 and 7, using a Protein A column antibody purification kit (Kyoto Monotech, Kyoto, Japan). Glycan excision and fluorescence staining were performed using an EZGlyco® mAb-N kit (Sumitomo Bakelite, Tokyo, Japan). Subsequently, glycan analysis was performed under the following conditions: Apparatus, high-performance liquid chromatography (HPLC) LC20-AD (Shimadzu, Kyoto, Japan); column, XBridge Glycan BEH Amide Column, 130A, 2.5 μm, 3.0 × 150 mm (Waters, Milford, MA, USA); mobile phase, eluent A (50 mM ammonium formate pH 4.4) and eluent B (acetonitrile), 56 min gradient from 75 to 54% of eluent B; flow rate 0.7 mL min−1; injection 2 μL; fluorescence detection, excitation 330 nm and emission 420 nm. The glycan structure of each peak was estimated by comparing with the retention time of the 2-AB Glycan Performance Test Standard (Waters, Milford, MA, USA).
Charge variant analysis
CHO cells were batch-cultured with or without 0.9 µM violacein (DMSO was added as vehicle control) and IgG was purified from the culture medium on day 4 and 7, using a Protein A column antibody purification kit (Kyoto Monotech). Charge variant analysis was performed using capillary isoelectric focusing (cIEF) (PA800 plus System, Beckman Coulter) according to the manufacturer’s protocol. The main species was defined as the peak with the largest area in the cIEF chart. Peaks with a lower isoelectric point (pI) than the main species were acidic variants, and those with a higher pI were basic variants.
Real-time PCR for IgG mRNA
CHO cells were batch-cultured with or without 0.9 μM violacein (DMSO was added as vehicle control), in triplicates per condition (n = 3). Total RNA was purified from the cells at day 4, 7, and 9, using the RNeasy® Mini kit (Qiagen, Venlo, Netherlands). Reverse transcription and real-time PCR were performed using the Reverse Transcription Master Mix (Fluidigm, South San Francisco, CA, USA) and SYBR® Premix Ex Taq™ II (Takara Bio, Kyoto, Japan), respectively. The primer sets for the IgG heavy and light chains were designed according to Haredy et al. (2013). β-Actin was as an endogenous control. The primer set for β-actin was: Forward 5′-TGACCCTGAAGTACCCCATTG-3′ and reverse 5′-TGGTGCCAGATCTTCTCCATATC-3′. The relative levels of the IgG heavy and light chains were compared with the mRNA expression levels of the control on day 4, using the ΔΔCt method.
Cell cycle analysis
CHO cells were batch-cultured with or without 0.9 μM violacein (DMSO was added as vehicle control) for 5 days, in triplicates per condition (n = 3). Cells were sampled on days 1, 3, and 5, and the cell cycle was analyzed using BD Cycletest Plus DNA Kit, FACSMelody cytometer, and FlowJo software (Becton, Dickinson and Company, Franklin Lakes, NJ, USA).
Statistical analysis
Results were expressed as mean ± standard deviation and analyzed using a two-tailed Student’s t-test. The differences between the means were significant at a p < 0.05 and highly significant at a p < 0.01.
Results
Violacein dose-dependency in CHO cells
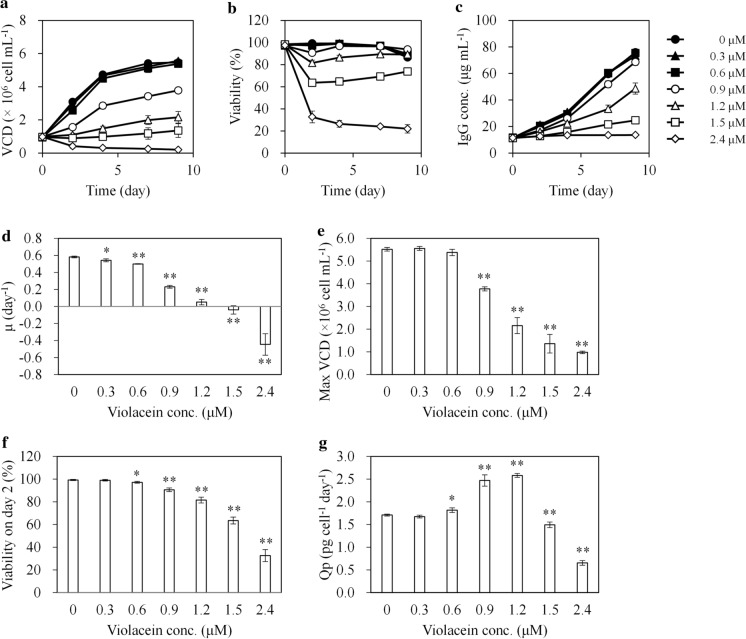
Increasing concentrations of violacein (0–2.4 μM) inhibited CHO cell growth in a dose-dependent manner (Fig. 1). The specific growth rate (μ) and maximum viable cell density (max VCD) decreased at concentrations ≥ 0.3 and ≥ 0.9 μM, respectively (Fig. 1d and e). The cell viability on day 2 decreased in a dose-dependent manner at concentrations ≥ 0.6 μM, indicating that these concentration ranges are cytotoxic to CHO cells (Fig. 1f). The cell viability in cells treated with 0.6–1.5 μM violacein tended to increase over time after day 4. On the other hand, the cell viability in cells treated with 2.4 μM violacein sharply dropped on day 2 and continued to further decrease until the end of the culture.
Fig. 1.
CHO cell profiles during batch culture with different concentrations of violacein. CHO cells were batch-cultured with 0–2.4 μM violacein in triplicates for 9 days. a VCD, b cell viability, c IgG concentration, d μ, e maximum VCD, f cell viability on day 2, and g Qp. The average value is indicated by a solid line or column, and the standard deviation is indicated by an error bar. For d–g, *p < 0.05 and **p < 0.01 compared with the control (0 μM). VCD viable cell density, IgG immunoglobulin G, μ specific growth rate, Qp specific production rate
The Qp increased in cells treated with 0.6–1.2 μM violacein compared to the control (Fig. 1g). However, the maximum IgG (max IgG) concentrations under these conditions were lower than the control because of VCD reduction (max IgG concentration at 0 μM; 76.0 ± 2.3 μg mL−1, 0.6 μM; 75.4 ± 1.3, non-significant difference, 0.9 μM; 68.6 ± 2.4 μg mL−1, p < 0.05, and 1.2 μM; 48.7 ± 4.2 μg mL−1, p < 0.01. The p-value was calculated against 0 μM condition).
Although the max IgG concentration lowered by 9.7% with 0.9 μM violacein compared to the control, it may surpass that of the control by extending the culture period. The Qp in cells treated with 0.9 μM violacein was as high as those under 1.2 μM treatment (no significant difference between the Qp of 0.9 and 1.2 μM treatment). The cell viability at the end of the culture in cells treated with 0.9 μM violacein was higher than in controls or 1.2 μM treated cells (viability on day 9 at 0 μM; 86.5 ± 0.3, 0.9 μM; 93.7 ± 0.6, p < 0.01, and 1.2 μM; 89.2 ± 0.9, p < 0.01. The p-value was calculated against 0 μM condition). Furthermore, 0.9 μM treatment had a less pronounced effect on μ, and the negative effect of VCD reduction on the total production was smaller than 1.2 μM treatment. As described, total production is generally correlated with VCD, Qp, and cell culture time, and these results suggest a potential of productivity improvement with 0.9 μM treatment. Therefore, in the next experiment, the effect of 0.9 μM violacein treatment on CHO cells was further investigated using the fed-batch culture method.
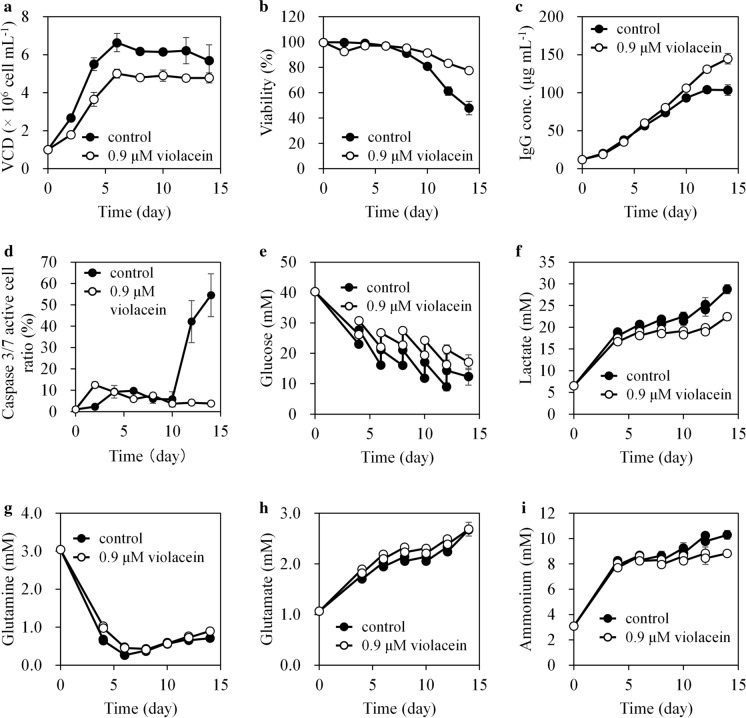
Effect of violacein on cell growth and productivity
The max IgG concentration increased by 37.6% with 0.9 μM violacein compared to the control (without violacein) in 14 days of fed-batch culture, because of Qp and cell longevity improvements. Figure 2 and Table 1 show the profiles of CHO cells during the fed-batch culture. The μ and max VCD in cells treated with 0.9 μM violacein were 41.2 and 24.4% lower than the control, respectively. In contrast, Qp increased by 70.2%. Cell viability on day 2 decreased by violacein treatment but recovered after day 4 and surpassed the control after day 8. Caspase 3/7 activity was associated with cell viability, as caspase 3/7 active cell ratio temporarily increased on day 2 but decreased after day 4 (Fig. 2d). This indicated that the induction of apoptosis in the latter culture stage was suppressed by violacein treatment.
Fig. 2.
Time courses of CHO cell culture profiles during fed-batch culture. CHO cells were fed-batch cultured with or without 0.9 μM violacein in triplicates for 14 days. Time courses of a VCD, b cell viability, c IgG concentration, d caspase 3/7 active cell ratio, e glucose, f lactate, g glutamine, h glutamate, and i ammonium in the culture. For graphs other than (d), the average value from three flasks is indicated by a solid line, and the standard deviation is indicated by an error bar. For (d), caspase 3/7 activity assays were performed in a representative flask for each culture condition. The average value of three field images is indicated by a solid line, and the standard deviation is indicated by an error bar. VCD viable cell density, IgG immunoglobulin G
Table 1.
Metabolic profiles of CHO cells during fed-batch culture
| Without violacein (control) | With 0.9 μM violacein | |
|---|---|---|
| μ (day−1) | 0.490 ± 0.011 | 0.288 ± 0.038** |
| Max VCD (× 106 cell mL−1) | 6.64 ± 0.48 | 5.02 ± 0.22** |
| Qp (pg cell−1 day−1) | 1.51 ± 0.06 | 2.57 ± 0.03** |
| Max IgG conc. (μg mL−1) | 105.1 ± 5.5 | 144.6 ± 7.0** |
| Qglc (pmol cell−1 day−1) | 1.46 ± 0.04 | 1.72 ± 0.08** |
| Qlac (pmol cell−1 day−1) | 1.05 ± 0.04 | 1.34 ± 0.12* |
| Qgln (pmol cell−1 day−1) | 0.257 ± 0.005 | 0.370 ± 0.021** |
| Qglu (pmol cell−1 day−1) | 0.060 ± 0.003 | 0.101 ± 0.003** |
| Qamm (pmol cell−1 day−1) | 0.437 ± 0.017 | 0.605 ± 0.046** |
CHO cells were fed-batch cultured in triplicates. Values are represented as means ± standard deviations. *p < 0.05 and **p < 0.01 compared with the control. The Qp was calculated using time integral values of VCD and IgG concentrations between day 0 and day 12. μ specific growth rate, Max maximum, Qp specific production rate, VCD viable cell density, IgG immunoglobulin G, conc. concentration; uptake or production rate for Glucose (Qglc), Lactate (Qlac), Glutamine (Q gln), Glutamate (Qglu), and Ammonium (Qamm)
Increased nutrient metabolic rate and reduced lactate and ammonium accumulation
The nutrient metabolic rates of CHO cells significantly increased by violacein treatment (production rate of lactate, p < 0.05, uptake or production rates of glucose, glutamine, glutamate, and ammonium, p < 0.01) (Table 1). The uptake rates of glucose and glutamine increased by 17.8 and 44.0% compared to the control, respectively. Moreover, the production rates of lactate, glutamate, and ammonium increased by 27.6, 68.3, and 38.4%, respectively. The increased metabolic rates might be related to the observed Qp improvement by violacein treatment. The total accumulation of lactate and ammonium, harmful metabolites for mammalian cells, were lower in the violacein treatment group than in the control because of the lower VCD (Fig. 2f and i).
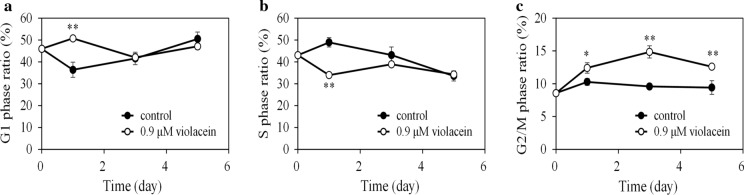
Time-course effects of violacein on the CHO cell cycle
Figure 3 shows the time-course effect on the cell cycle in cells treated with 0.9 μM violacein. The G1 phase ratio in violacein-treated cells on day 1 significantly increased compared to the control, (control; 36.4 ± 3.5%, with 0.9 μM violacein; 50.7 ± 0.8%, p < 0.01), yet there was no difference in the G1 phase ratio after day 3. The G2/M phase ratio in violacein-treated cells, however, always significantly surpassed that of the control during 5 days of culture (day 1, p < 0.05, day 3 and 5, p < 0.01). These results indicate that violacein treatment induced cell cycle arrest in the G1 and G2/M phases. However, the arrest in the G1 phase was temporary, whereas the G2/M phase arrest lasted for an extended period.
Fig. 3.
Cell-cycle distribution of CHO cells treated with violacein. CHO cells were batch-cultured with or without 0.9 μM violacein in triplicates for 5 days. Time courses of a G1, b S, and c G2/M phase ratios. The average value is indicated by a solid line, and the standard deviation is indicated by an error bar. *p < 0.05 and **p < 0.01 compared with the control on the corresponding days
Effects on IgG mRNA levels
The mRNA expression levels of IgG heavy and light chains significantly increased in cells treated with 0.9 μM violacein by 47.3–71.1% and 44.4–46.4% compared to the control during 9 days of batch culture, respectively (p-values of the heavy chain and light chain on days 4, 7 and 9 were all < 0.01) (Fig. 4). The consistent effect indicates that violacein treatment led to a stable increase in the IgG mRNA levels.
Fig. 4.
Effect of violacein on IgG mRNA. CHO cells were batch-cultured with or without 0.9 μM violacein in triplicates, and IgG mRNA levels were evaluated on day 4, 7, and 9. a Heavy chain mRNA. b Light chain mRNA. The relative levels compared to the amount of heavy chain or light chain mRNA on day 4 of the control are shown. The average value from three flasks is indicated by a column, and the standard deviation is indicated by an error bar. **p < 0.01 compared with the control on the corresponding days. IgG immunoglobulin G, Hc heavy chain, Lc light chain
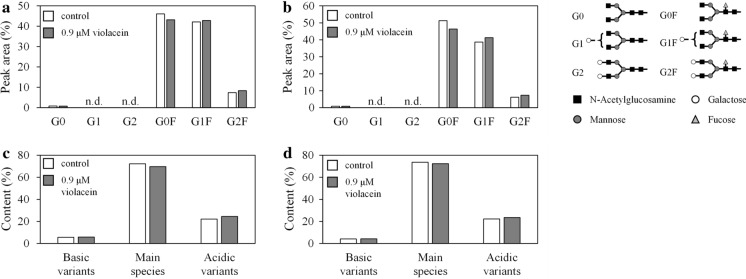
Effects of violacein on IgG N-linked glycosylation and charge variant levels
Violacein treatment had a negligible effect on the N-linked glycosylation and charge variant profiles of IgG (Fig. 5). Regardless of violacein treatment, the N-linked glycan contained core fucose and various terminal galactose residues (G0F, G1F, and G2F), with G0F being the most dominant. The total peak area of G0F, G1F, and G2F was ≥ 94.3% in all conditions, and these three structures occupied most N-glycans. The G0F content of the control and violacein-treated samples on day 4 were 46.1 and 43.1%, increasing to 51.3 and 46.3% on day 7, respectively. The G0F ratio decreased, while the ratio of terminal galactose-added structures (G1F and G2F) increased by violacein treatment; yet it is unclear whether this difference is meaningful. Reportedly, the terminal galactose addition to the N-glycan of IgG improves complement-dependent cytotoxicity (Peschke et al. 2017). Although the HPLC charts are not shown in this manuscript, the chart shapes were similar under all conditions, suggesting the glycan distribution was not affected by violacein treatment.
Fig. 5.
Effect of violacein on N-linked glycosylation and charge variant distribution. N-linked glycosylation profiles of IgG obtained from batch culture with or without 0.9 μM violacein on a day 4 and b day 7. Charge variation profiles of IgG obtained from batch culture with or without 0.9 μM violacein on c day 4 and d day 7. The data were obtained from a single experiment. n.d. not detected, IgG immunoglobulin G
Figure 5c and d show the composition ratio of charge variants and the main species of IgG. Regardless of violacein treatment, there was almost no difference in the ratio of the main species, acidic variants, and basic variants. Also, the variant levels between days 4 and 7 were almost the same, and a time-course difference was not observed.
Discussion
Our results show that violacein treatment improved IgG production and suppressed CHO cell growth, without changing the product quality (N-linked glycan and charge variant profiles). Moreover, our findings indicate that violacein arrests CHO cell cycle at G1 and G2/M. The cell growth suppression and improved IgG production might be because of violacein’s effects on the cell cycle. The cell cycle arrest in the G1 phase by violacein was temporarily observed on day 1, whereas G2/M arrest lasted for a longer time. Therefore, arresting the cells at the G2/M phase might be the reason for the improved IgG production in the batch and fed-batch cultures. The increased Qp was because of the higher mRNA expression levels, and the increased nutrient metabolic rate might apply to upregulated protein production.
Many of the existing cell cycle control methods to improve protein production have been applied to G1 phase arrest (Kumar et al. 2007). To the best of our knowledge, an improvement of recombinant protein production by arresting CHO cells in the G2/M phase was only reported by Ha et al., using lithium chloride (LiCl) (Ha et al. 2014), a specific inhibitor of glycogen synthase kinase-3β, which arrests the cell cycle in the G2/M phase and regulates apoptosis. This study provided new findings regarding the contribution to improved recombinant protein production in CHO cells via G2/M arrest.
Regarding the use of cell cycle inhibiting compounds, the decrease in cell longevity because of cytotoxicity is an issue of concern. To make NaBu useful as an additive, combining genetic engineering approaches, such as effector caspase genes silencing, have been studied to prevent NaBu-induced apoptosis (Mohan et al. 2009). However, genetic engineering approaches require substantial time and effort and cannot be applied to existing cell lines. Violacein significantly improves long-term culture, although there may be temporary lower cell viability. A great advantage of violacein may be its simplicity, as violacein does not require combination with other complicated agents to prevent apoptosis.
Regarding the industrial manufacturing process application, safety issues need to be addressed. Using chemical inhibitors is of concern for downstream clearance and product safety (Wang et al. 2018). Therapeutic antibodies are successfully separated from impurities through a multi-step purification process, including affinity and ion-exchange chromatography. Multi-step purification might be sufficient to remove violacein from the final product. Nevertheless, the actual removal capacity of this method should be fully verified in advance. It is also necessary to prepare an appropriate addition method, as DMSO might not be acceptable for the industrial manufacturing process of biopharmaceuticals. Violacein dissolves in ethanol (Yada et al. 2007), which is generally considered safe; therefore, it can be used as a solvent instead of DMSO. Alternatively, as a solvent-free addition scheme, solubilization techniques can also be used. Attempts to increase the solubility and the biological activity of violacein using violacein-inclusion complexes with β-cyclodextrin have been reported (De Azevedo et al. 2000; Durán et al. 2012; Melo et al. 2003).
In addition, considering the application to industrial manufacturing processes, it is necessary to verify the applicability to industrial high-production CHO cells. Generally, mAb industrial manufacturing CHO cells show about 1–10 g L−1 productivity in fed-batch culture (Kunert and Reinhart 2016). The productivity is much higher than the cells used in this study. These industrial production cells are variously designed in gene transcription, translation, and secretion in order to efficiently produce the target recombinant protein. Since the action mechanism of violacein has not been fully elucidated, it is unclear whether it is effective to these high-producing strains. As a further study, it is necessary to elucidate the action mechanism and investigate the effect on industrial high production cells.
Although the reason for the violacein-induced enhanced cell longevity was unclear, there are two probable explanations, listed as follows:
The cell populations or epigenetics changed because of drug selection pressure. CHO cells are aneuploid; they are prone to gene mutations and are genetically diverse (Bandyopadhyay et al. 2019; Scarcelli et al. 2018). Besides, many genes are in a functionally hemizygous state (Siminovitch 1976), and gene mutations are likely to change the phenotype. The genetic variation occurs as the culture progresses after cloning. Therefore, CHO cells in the culture medium represent a cell population with a certain degree of genetic and phenotypic diversity, and these cells may have varying sensitivity to the compound. Our results show that the viability decreased and the ratio of caspase 3/7 active cells increased temporarily on day 2 in cells treated with 0.9 μM violacein (Fig. 2b and d). At that time, the death of cells prone to apoptosis because of higher violacein sensitivity may have preferentially occurred. Moreover, the population of cells with high apoptosis tolerance became dominant because of the drug selection pressure, and this might have led to more extended cell longevity in this population compared to cells with a low apoptosis tolerance. Furthermore, epigenetic modifications of apoptosis-related genes may have occurred because of the drug selection pressure. Feichtinger et al. reported that the histone modification pattern continuously changed during CHO cells batch culture, while only slight DNA methylation was observed (Feichtinger et al. 2016). Their results indicated that gene transcription regulation in response to short-term environmental changes is primarily controlled by histone modification alterations.
The violacein-induced enhanced cell longevity might be because violacein affected the kinase activity in CHO cells. Queiroz et al. reported that violacein affected the kinome profile of TF1 leukemia progenitor cells (Queiroz et al. 2012). In a study performed by Queiroz et al., violacein increased protein kinase A (PKA), Akt, and phosphoinositide-dependent protein kinase (PDK) activity in TF1 cells. These activated kinases affect apoptosis suppression and pro-survival. PKA phosphorylates cAMP response element-binding protein (CREB), a transcription factor, and phosphorylated CREB promotes the transcription of various genes. Activated CREB mediates upregulation of the anti-apoptosis factor B-cell lymphoma 2 (Bcl-2) (Lonze et al. 2002), while PDK and Akt are kinases located downstream of the phosphoinositide 3-kinase (PI3K) pathway. PDK activates Akt, an important pro-survival kinase, and Akt inhibits the function or expression of several Bcl-2 homology domain 3 (BH3)-only proteins, such as Bad, which bind and inactivate pro-survival Bcl-2 family members (Manning and Cantley 2007). Moreover, Akt phosphorylates and decreases the activity of caspase-9, which is an initiator caspase in the mitochondrial pathway (Cardone et al. 1998). In addition, Akt phosphorylates forkhead box O (FOXO), which is the transcription factor of apoptosis-promoting genes, and blocks FOXO-mediated transcription (Manning and Cantley 2007).
Furthermore, the effects of harmful metabolite accumulation are also possible. Lactate and ammonium are well known harmful metabolic by-products of mammalian cells (Cruz et al. 2000; Wagner 1997). As shown in Figs. 2f and 2i, accumulation of lactate and ammonium were both suppressed by 0.9 μM violacein treatment. It is undeniable that the difference in lactate and ammonium accumulation might have affected cell viability. However, the difference in lactate and ammonium accumulation on day 8 when the difference in cell viability began to occur was small between violacein-treated conditions and controls. The concentration of lactate and ammonium on day 8 was reduced by 11.0 and 3.9% by violacein treatment compared to the control, respectively. Therefore, the impact of the accumulation of harmful metabolites in this study might not be significant.
In this study, there was a difference between the cell viability and caspase activity in the logarithmic and early/middle stationary phase. Programmed cell death other than apoptosis have not been evaluated in this study, however, there is a possibility that violacein might not affect other programmed cell death. Both in violacein-treated and control conditions, a gradual cell viability decrease was observed throughout the culture period (Fig. 2b). The viability of cells under control conditions was the highest at day 2 (99.8 ± 0.1%) and then decreased over time. In violacein treated condition, it was temporary decreased at day 2 (92.4 ± 0.6%) and increased at day 4 (97.3 ± 0.6%), then decreased. The trends were different from those of caspase 3/7 active cell ratios (Fig. 2d). Although the reason is unknown, slight but continuous necrosis might have occurred preceded apoptosis because of shear stress or rapid increase of osmolality by the bolus feed strategy. Therefore, there is a possibility that violacein is not effective in suppressing other programmed cell death such as necrosis. To evaluate this, further experiments are needed.
Elucidation of the mechanism underlying violacein-induced cell longevity may lead to future effective techniques to avoid apoptosis induction during the production process. Programmed cell death in cell culture is an issue that needs to be addressed because it affects VCD and product quality (Mohan et al. 2009). Charge variants or host cell protein (HCP) in therapeutic antibodies can affect their efficacy or cause immunogenic reactions. Because cell viability reduction causes the increasing acidic variants or affects HCP profile in the culture process (Hossler et al. 2015; Jin et al. 2010), the prevention of apoptosis will lower the residual risks of impurities in final products and contribute to a robust manufacturing process. Transcriptome, epigenomic, and kinome analyses will help to evaluate the effects of violacein on CHO cells, and the results will provide useful information for CHO cell apoptosis suppression. For example, ways to improve the addition method to avoid apoptosis induction by other cell cycle inhibiting compounds, such as NaBu, might be obtained. Furthermore, genes, expressions of which are increased/decreased by violacein, could be related to apoptosis tolerance, while overexpression/knockout of these genes may allow the construction of new apoptotic tolerant cell lines.
This study indicated the effectiveness of violacein as a mAb production enhancer in CHO cells. The obtained findings will help researchers develop more efficient mAb production technologies. In addition to the analysis of the effect of violacein on several cellular mechanisms, such as cell longevity, improvement of the chemical structure or its usage are other potential approaches. The violacein analogs and violacein complexes of other compounds, such as β-cyclodextrin complex, might show a desirable effect (high efficacy, high safety, and easy to use) on mAb production in the industrial-manufacturing process. Furthermore, although the effects in perfusion cultures were not evaluated in this study, violacein and its analogs might contribute to the stable long-term high productivity in perfusion culture. Further studies for violacein analogs, other compound complexes, and combinations of other production technologies will lead to more practical and effective technology development.
In conclusion, the findings obtained in this study on CHO cells will help expand the range of violacein utilization in the future. Although violacein is a promising bioactive compound in a wide range of industrial fields, including pharmaceuticals, its action on mammalian cells has not been fully elucidated and therefore it is still underutilized. Our study will serve as a reference to fully elucidate violacein effects on mammalian cells and provide important information for further effective utilization.
Acknowledgements
We thank Professor Enomoto for providing Pseudoalteromonas sp. 520P1. This research was supported by the Japan Agency for Medical Research and Development (AMED) under Grant Number JP17ae0101003. The authors would like to thank Editage for English language editing. The authors declare no conflicts of interest.
Compliance with ethical standards
Conflict of interest
The authors declare no conflicts of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Masahide Kido, Email: mkido@osaka-soda.co.jp.
Hideaki Idogaki, Email: hidogaki@osaka-soda.co.jp.
Kouji Nishikawa, Email: knishika@osaka-soda.co.jp.
Takeshi Omasa, Email: omasa@bio.eng.osaka-u.ac.jp.
References
- Bandyopadhyay AA, O'Brien SA, Zhao L, et al. Recurring genomic structural variation leads to clonal instability and loss of productivity. Biotechnol Bioeng. 2019;116:41–53. doi: 10.1002/bit.26823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilsland E, Tavella TA, Krogh R, et al. Antiplasmodial and trypanocidal activity of violacein and deoxyviolacein produced from synthetic operons. BMC Biotechnol. 2018;18:22. doi: 10.1186/s12896-018-0428-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromberg N, Dreyfuss JL, Regatieri CV, et al. Growth inhibition and pro-apoptotic activity of violacein in Ehrlich ascites tumor. Chem Biol Interact. 2010;186:43–52. doi: 10.1016/j.cbi.2010.04.016. [DOI] [PubMed] [Google Scholar]
- Cardone MH, Roy N, Stennicke HR, et al. Regulation of cell death protease caspase-9 by phosphorylation. Science. 1998;282:1318–1321. doi: 10.1126/science.282.5392.1318. [DOI] [PubMed] [Google Scholar]
- Choi SY, Yoon KH, Lee JI, et al. Violacein: properties and production of a versatile bacterial pigment. Biomed Res Int. 2015;2015:465056. doi: 10.1155/2015/465056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung B, Jeong Y, Choi O, et al. Effect of sodium butyrate on glycosylation of recombinant erythropoietin. In: Lindner-Olsson E, Chatzissavidou N, Lüllau E, et al., editors. Animal cell technology: from target to market, ESACT proceedings. Dordrecht: Springer; 2001. pp. 207–209. [Google Scholar]
- Cruz HJ, Freitas CM, Alves PM, et al. Effects of ammonia and lactate on growth, metabolism, and productivity of BHK cells. Enzyme Microb Technol. 2000;27:43–52. doi: 10.1016/S0141-0229(00)00151-4. [DOI] [PubMed] [Google Scholar]
- Damiani R, Almeida BE, Oliveira JE, et al. Enhancement of human thyrotropin synthesis by sodium butyrate addition to serum-free CHO cell culture. Appl Biochem Biotechnol. 2013;171:1658–1672. doi: 10.1007/s12010-013-0467-9. [DOI] [PubMed] [Google Scholar]
- De Azevedo MBM, Alderete J, Rodriguez JA, et al. Biological activities of violacein, a new antitumoral indole derivative, in an inclusion complex with β-cyclodextrin. J Incl Phenom Macrocycl Chem. 2000;37:93–101. doi: 10.1023/A:1008138807481. [DOI] [Google Scholar]
- Durán M, Ponezi AN, Faljoni-Alario A, et al. Potential applications of violacein: a microbial pigment. Med Chem Res. 2012;21:1524–1532. doi: 10.1007/s00044-011-9654-9. [DOI] [Google Scholar]
- Feichtinger J, Hernandez I, Fischer C, et al. Comprehensive genome and epigenome characterization of CHO cells in response to evolutionary pressures and over time. Biotechnol Bioeng. 2016;113:2241–2253. doi: 10.1002/bit.25990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira CV, Bos CL, Versteeg HH, et al. Molecular mechanism of violacein-mediated human leukemia cell death. Blood. 2004;104:1459–1464. doi: 10.1182/blood-2004-02-0594. [DOI] [PubMed] [Google Scholar]
- Gonzalez T, Leong SR, Presta LG (2000) Nucleic acids encoding humanized anti-IL-8 monoclonal antibodies. US Patent 6025158
- Ha TK, Kim YG, Lee GM. Effect of lithium chloride on the production and sialylation of Fc-fusion protein in Chinese hamster ovary cell culture. Appl Microbiol Biotechnol. 2014;98:9239–9248. doi: 10.1007/s00253-014-6012-0. [DOI] [PubMed] [Google Scholar]
- Haredy AM, Nishizawa A, Honda K, et al. Improved antibody production in Chinese hamster ovary cells by ATF4 overexpression. Cytotechnology. 2013;65:993–1002. doi: 10.1007/s10616-013-9631-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano S, Asamizu S, Onaka H, Shiro Y, Nagano S. Crystal structure of VioE, a key player in the construction of the molecular skeleton of violacein. J Biol Chem. 2008;283:6459–6466. doi: 10.1074/jbc.M708109200. [DOI] [PubMed] [Google Scholar]
- Hossler P, Wang M, McDermott S, et al. Cell culture media supplementation of bioflavonoids for the targeted reduction of acidic species charge variants on recombinant therapeutic proteins. Biotechnol Prog. 2015;31:1039–1052. doi: 10.1002/btpr.2095. [DOI] [PubMed] [Google Scholar]
- Jiang PX, Wang HS, Xiao S, et al. Pathway redesign for deoxyviolacein biosynthesis in Citrobacter freundii and characterization of this pigment. Appl Microbiol Biotechnol. 2012;94:1521–1532. doi: 10.1007/s00253-012-3960-0. [DOI] [PubMed] [Google Scholar]
- Jin M, Szapiel N, Zhang J, et al. Profiling of host cell proteins by two-dimensional difference gel electrophoresis (2D-DIGE): Implications for downstream process development. Biotechnol Bioeng. 2010;105:306–316. doi: 10.1002/bit.22532. [DOI] [PubMed] [Google Scholar]
- Kido M, Idogaki H, Nishikawa K, Motoishi K, Omasa T. Screening of new cell cycle suppressive compounds from marine-derived microorganisms in Chinese hamster ovary cells. J Biosci Bioeng. 2020;130:106–113. doi: 10.1016/j.jbiosc.2020.03.001. [DOI] [PubMed] [Google Scholar]
- Kido M, Idogaki H, Nishikawa K, Omasa T. Low-concentration staurosporine improves recombinant antibody productivity in Chinese hamster ovary cells without inducing cell death. J Biosci Bioeng. 2020;130:525–532. doi: 10.1016/j.jbiosc.2020.07.005. [DOI] [PubMed] [Google Scholar]
- Kim YG, Kim JY, Lee GM. Effect of XIAP overexpression on sodium butyrate-induced apoptosis in recombinant Chinese hamster ovary cells producing erythropoietin. J Biotechnol. 2009;144:299–303. doi: 10.1016/j.jbiotec.2009.09.016. [DOI] [PubMed] [Google Scholar]
- Kodach LL, Bos CL, Duran N, et al. Violacein synergistically increases 5-fluorouracil cytotoxicity, induces apoptosis and inhibits Akt-mediated signal transduction in human colorectal cancer cells. Carcinogenesis. 2006;27:508–516. doi: 10.1093/carcin/bgi307. [DOI] [PubMed] [Google Scholar]
- Kumar N, Gammell P, Clynes M. Proliferation control strategies to improve productivity and survival during CHO based production culture: a summary of recent methods employed and the effects of proliferation control in product secreting CHO cell lines. Cytotechnology. 2007;53:33–46. doi: 10.1007/s10616-007-9047-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunert R, Reinhart D. Advances in recombinant antibody manufacturing. Appl Microbiol Biotechnol. 2016;100:3451–3461. doi: 10.1007/s00253-016-7388-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin H, Leighty RW, Godfrey S, et al. Principles and approach to developing mammalian cell culture media for high cell density perfusion process leveraging established fed-batch media. Biotechnol Prog. 2017;33:891–901. doi: 10.1002/btpr.2472. [DOI] [PubMed] [Google Scholar]
- Lonze BE, Riccio A, Cohen S, et al. Apoptosis, axonal growth defects, and degeneration of peripheral neurons in mice lacking CREB. Neuron. 2002;34:371–385. doi: 10.1016/s0896-6273(02)00686-4. [DOI] [PubMed] [Google Scholar]
- Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell. 2007;129:1261–1274. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melo PS, Justo GZ, de Azevedo MB, et al. Violacein and its beta-cyclodextrin complexes induce apoptosis and differentiation in HL60 cells. Toxicology. 2003;186:217–225. doi: 10.1016/s0300-483x(02)00751-5. [DOI] [PubMed] [Google Scholar]
- Mimura Y, Lund J, Church S, et al. Butyrate increases production of human chimeric IgG in CHO-K1 cells whilst maintaining function and glycoform profile. J Immunol Methods. 2001;247:205–216. doi: 10.1016/S0022-1759(00)00308-2. [DOI] [PubMed] [Google Scholar]
- Mohan C, Kim Y-G, Lee GM. Apoptosis and autophagy cell engineering. In: Al-Rubeai M, editor. Cell line development, cell engineering. Dordrecht: Springer; 2009. pp. 195–216. [Google Scholar]
- Mojib N, Nasti TH, Andersen DT, et al. The antiproliferative function of violacein-like purple violet pigment (PVP) from an Antarctic Janthinobacterium sp. Ant5-2 in UV-induced 2237 fibrosarcoma. Int J Dermatol. 2011;50:1223–1233. doi: 10.1111/j.1365-4632.2010.04825.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Momen AZ, Hoshino T. Biosynthesis of violacein: intact incorporation of the tryptophan molecule on the oxindole side, with intramolecular rearrangement of the indole ring on the 5-hydroxyindole side. Biosci Biotechnol Biochem. 2000;64:539–549. doi: 10.1271/bbb.64.539. [DOI] [PubMed] [Google Scholar]
- Oh HK, So MK, Yang J, et al. Effect of N-Acetylcystein on butyrate-treated Chinese hamster ovary cells to improve the production of recombinant human interferon-beta-1a. Biotechnol Prog. 2005;21:1154–1164. doi: 10.1021/bp050057v. [DOI] [PubMed] [Google Scholar]
- Peschke B, Keller CW, Weber P, et al. Fc-galactosylation of human immunoglobulin gamma isotypes improves C1q binding and enhances complement-dependent cytotoxicity. Front Immunol. 2017;8:646. doi: 10.3389/fimmu.2017.00646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Queiroz KC, Milani R, Ruela-de-Sousa RR, et al. Violacein induces death of resistant leukaemia cells via kinome reprogramming, endoplasmic reticulum stress and Golgi apparatus collapse. PLoS ONE. 2012;7:e45362. doi: 10.1371/journal.pone.0045362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarcelli JJ, Hone M, Beal K, et al. Analytical subcloning of a clonal cell line demonstrates cellular heterogeneity that does not impact process consistency or robustness. Biotechnol Prog. 2018;34:602–612. doi: 10.1002/btpr.2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla AA, Wolfe LS, Mostafa SS, et al. Evolving trends in mAb production processes. Bioeng Transl Med. 2017;2:58–69. doi: 10.1002/btm2.10061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siminovitch L. On the nature of hereditable variation in cultured somatic cells. Cell. 1976;7:1–11. doi: 10.1016/0092-8674(76)90249-X. [DOI] [PubMed] [Google Scholar]
- Sung YH, Song YJ, Lim SW, et al. Effect of sodium butyrate on the production, heterogeneity and biological activity of human thrombopoietin by recombinant Chinese hamster ovary cells. J Biotechnol. 2004;112:323–335. doi: 10.1016/j.jbiotec.2004.05.003. [DOI] [PubMed] [Google Scholar]
- Wagner R. 2.1 Metabolic control of animal cell culture processes. In: Hauser H, Wager R, editors. Mammalian cell biotechnology in protein production. Berlin: De Gruyter; 1997. pp. 193–232. [Google Scholar]
- Wang H, Wang F, Zhu X, et al. Biosynthesis and characterization of violacein, deoxyviolacein and oxyviolacein in heterologous host, and their antimicrobial activities. Biochem Eng J. 2012;67:148–155. doi: 10.1016/j.bej.2012.06.005. [DOI] [Google Scholar]
- Wang SB, Lee-Goldman A, Ravikrishnan J, et al. Manipulation of the sodium-potassium ratio as a lever for controlling cell growth and improving cell specific productivity in perfusion CHO cell cultures. Biotechnol Bioeng. 2018;115:921–931. doi: 10.1002/bit.26527. [DOI] [PubMed] [Google Scholar]
- Wolf MKF, Closet A, Bzowska M, et al. Improved performance in mammalian cell perfusion cultures by growth inhibition. Biotechnol J. 2019;14:e1700722. doi: 10.1002/biot.201700722. [DOI] [PubMed] [Google Scholar]
- Xu X, Nagarajan H, Lewis NE, et al. The genomic sequence of the Chinese hamster ovary (CHO)-K1 cell line. Nat Biotechnol. 2011;29:735–741. doi: 10.1038/nbt.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yada S, Wang Y, Zou Y, et al. Isolation and characterization of two groups of novel marine bacteria producing violacein. Mar Biotechnol (NY) 2008;10:128–132. doi: 10.1007/s10126-007-9046-9. [DOI] [PubMed] [Google Scholar]
- Yin B, Wang Q, Chung CY, et al. Butyrated ManNAc analog improves protein expression in Chinese hamster ovary cells. Biotechnol Bioeng. 2018;115:1531–1541. doi: 10.1002/bit.26560. [DOI] [PubMed] [Google Scholar]